Numerical Study of Heat Transfer Enhancement by Arc-Shaped Fins in a Shell-Tube Thermal Energy Storage Unit
Abstract
1. Introduction
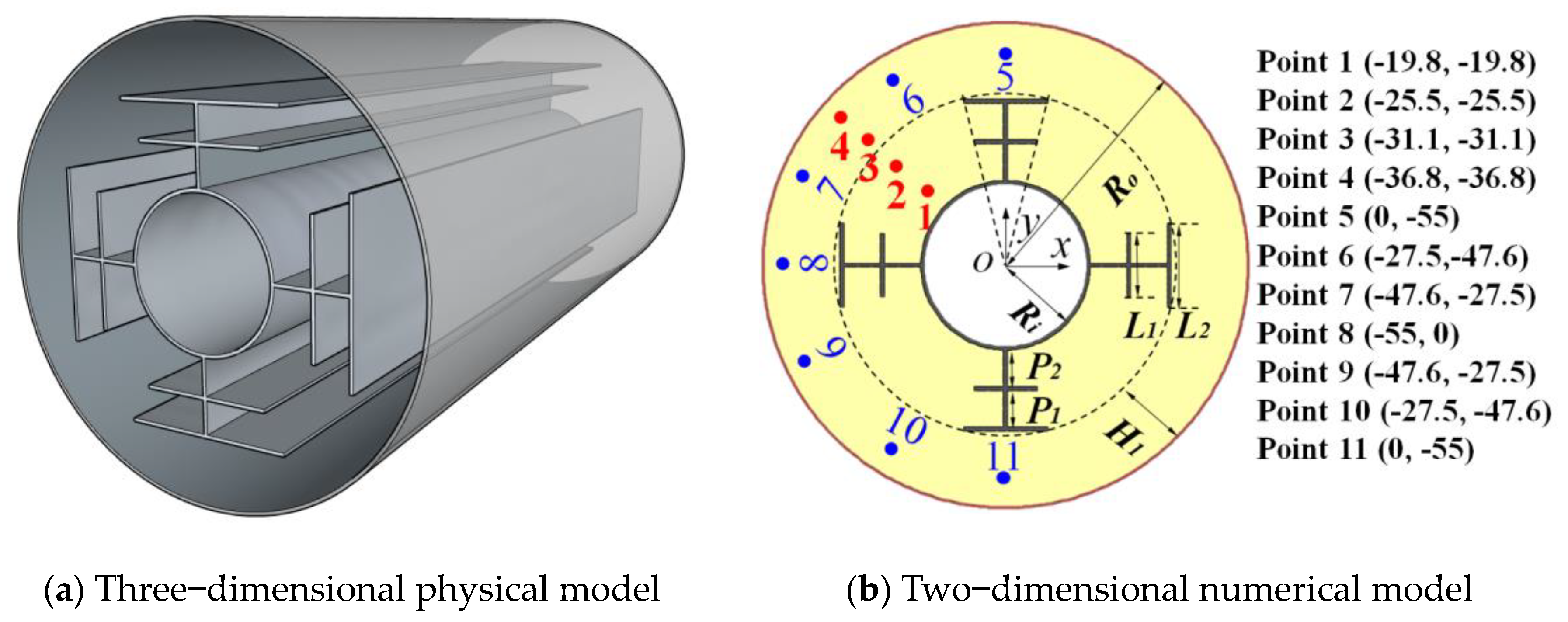
2. Problem Definition and Modeling
2.1. Problem Definition
2.2. Mathematical Modeling
- The PCMs is pure.
- The liquid phase of the PCMs is a Newtonian fluid.
- The flow in liquid is two-dimensional, laminar and incompressible.
- The volume changes when phase transition is negligible.
2.3. Model Verification
3. Results and Discussion
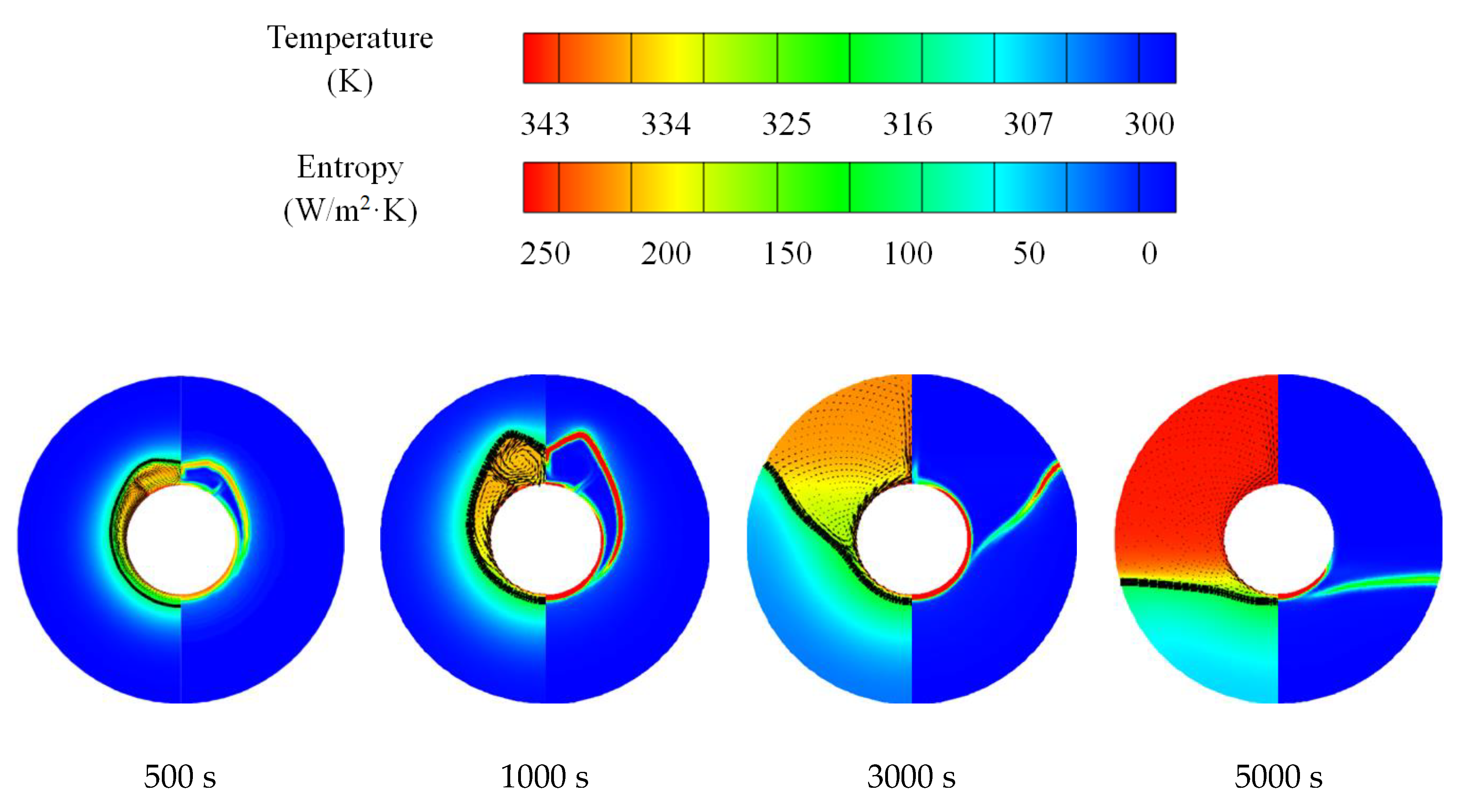
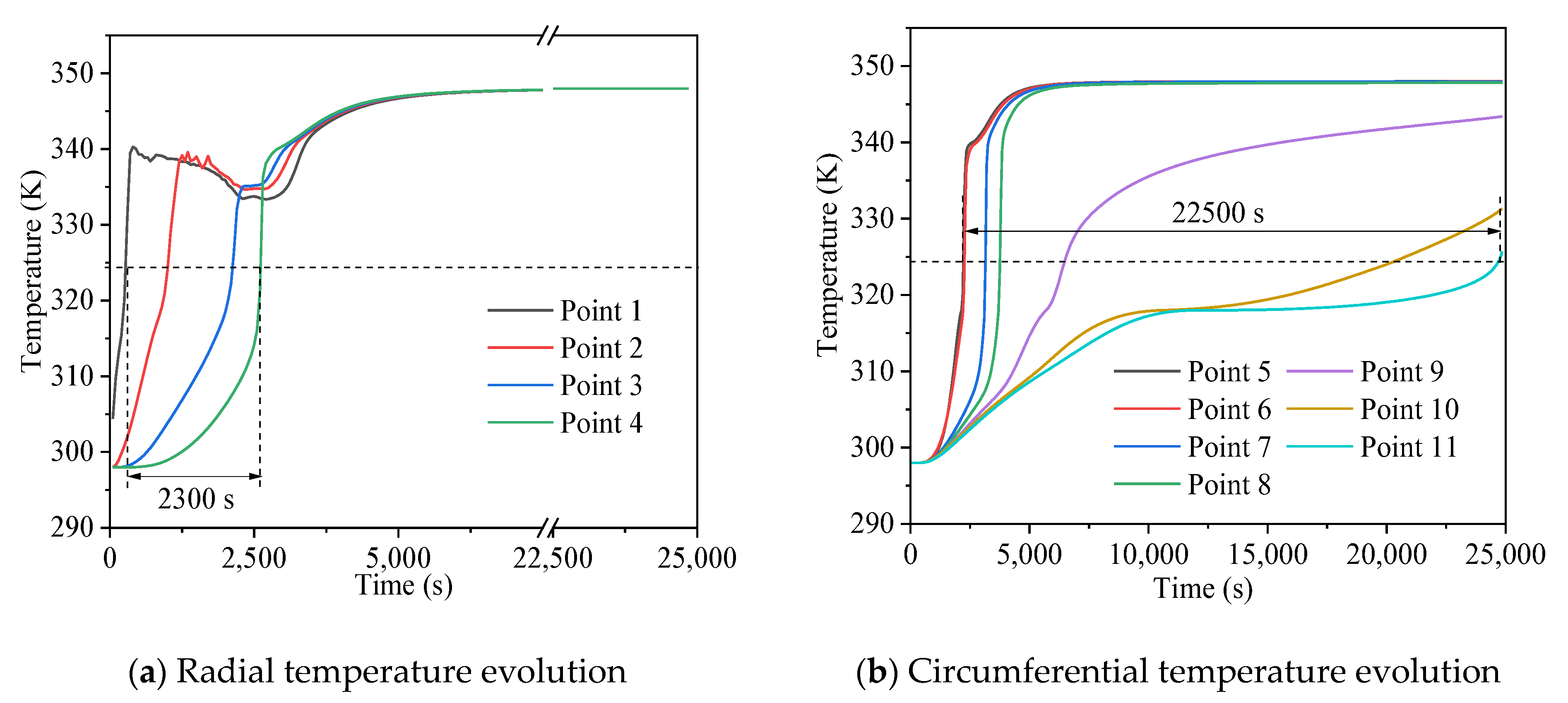
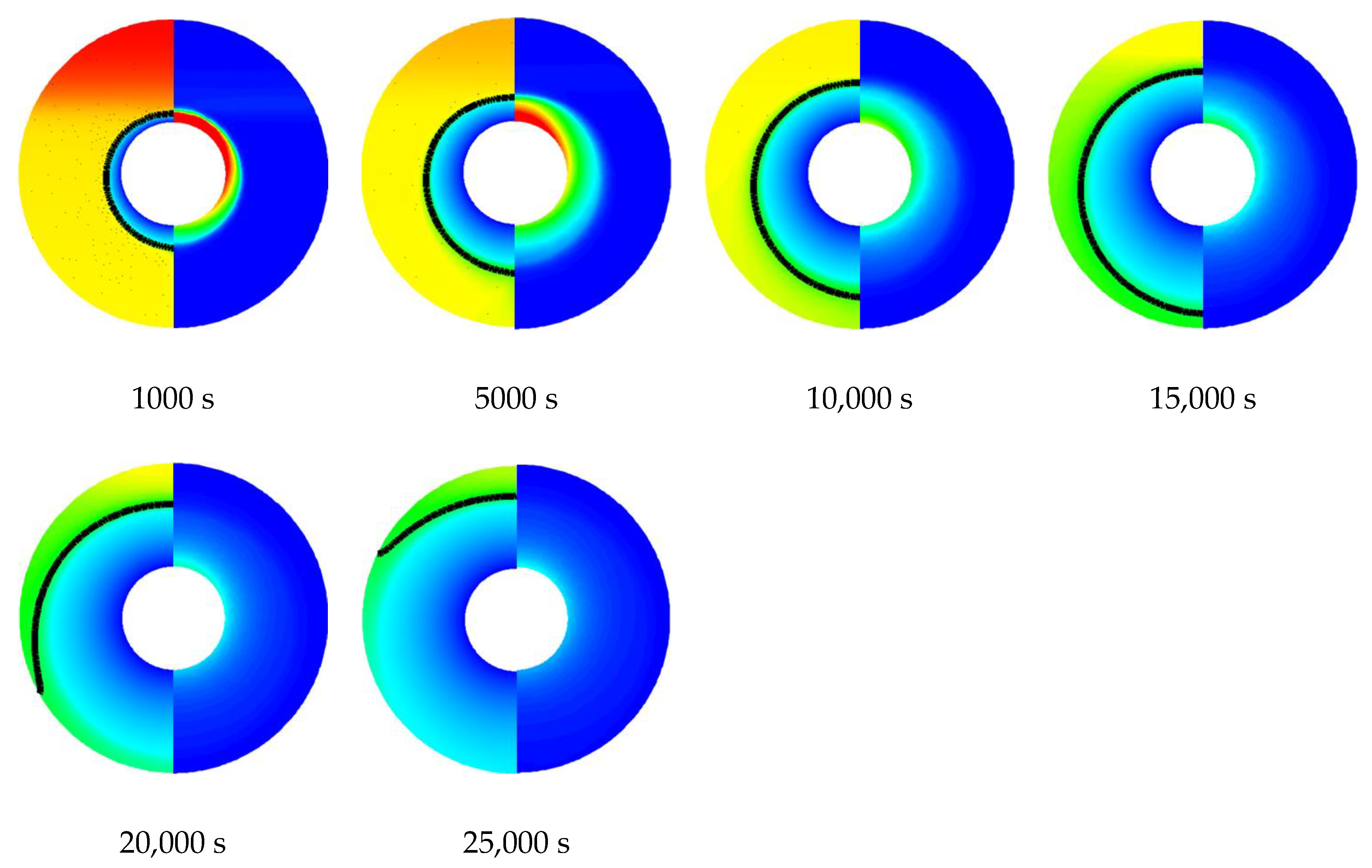
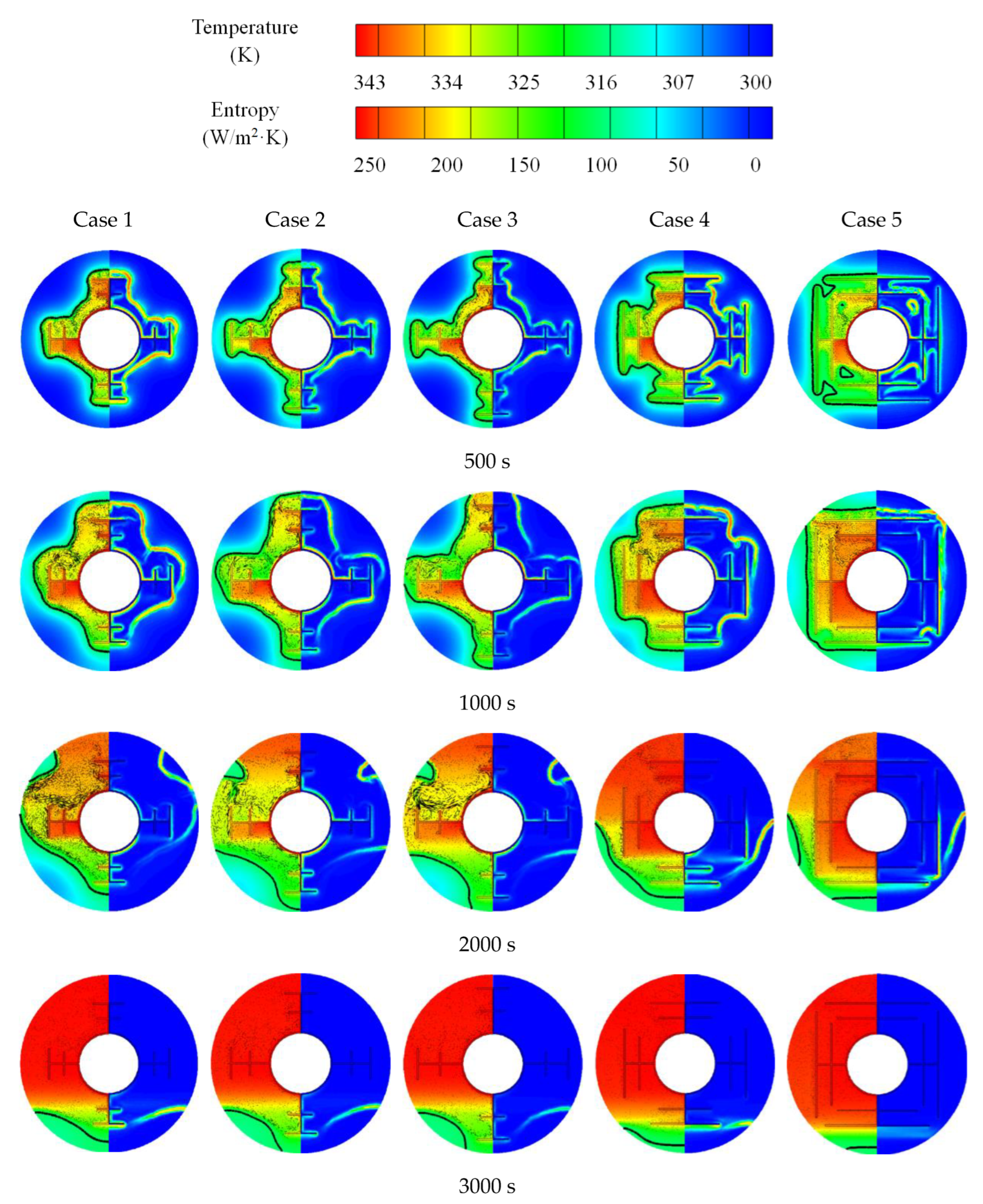
3.1. Melting and Solidification Processes for No-Fin Case
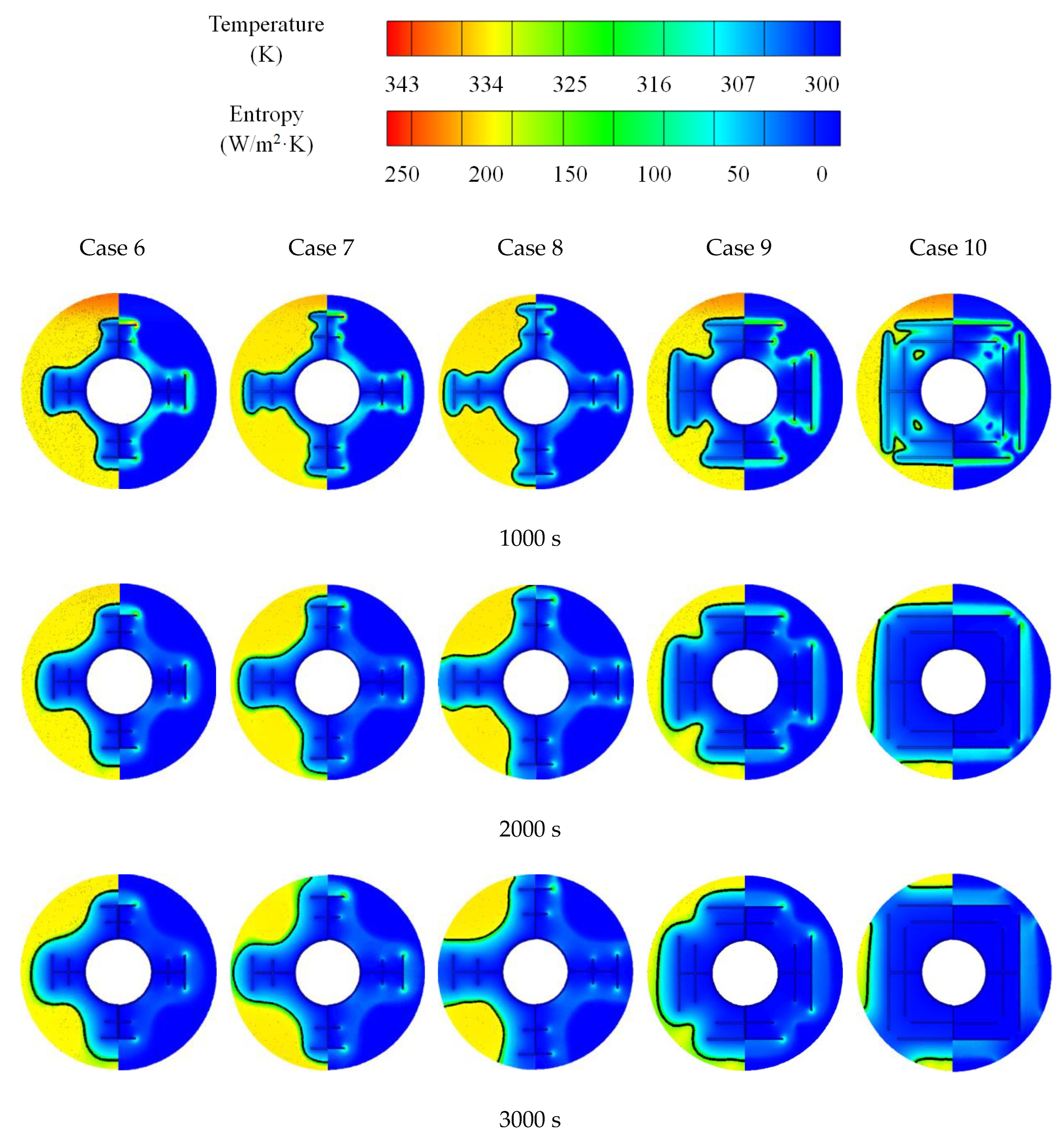
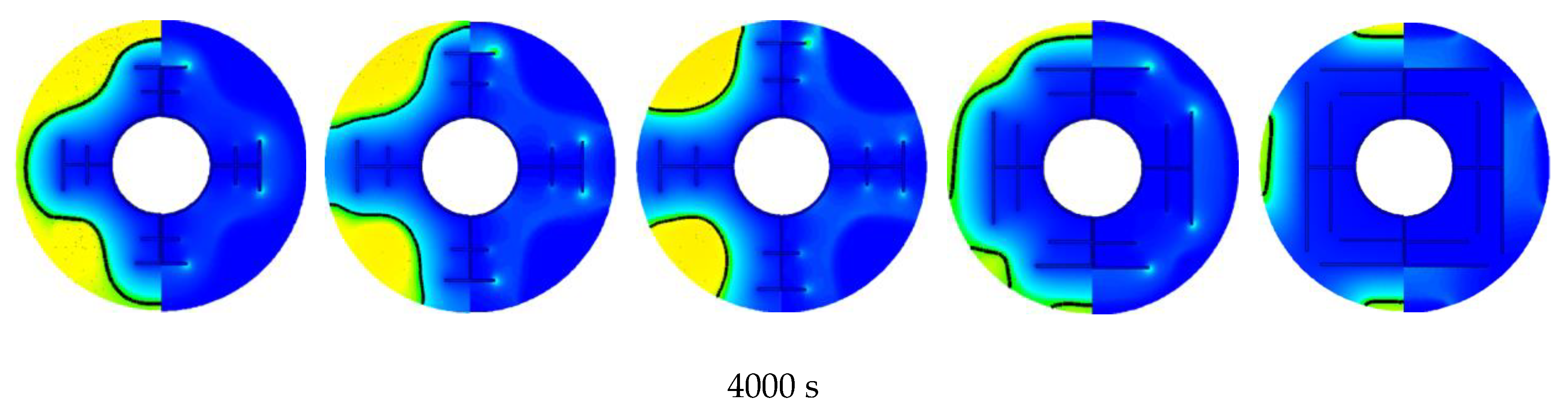
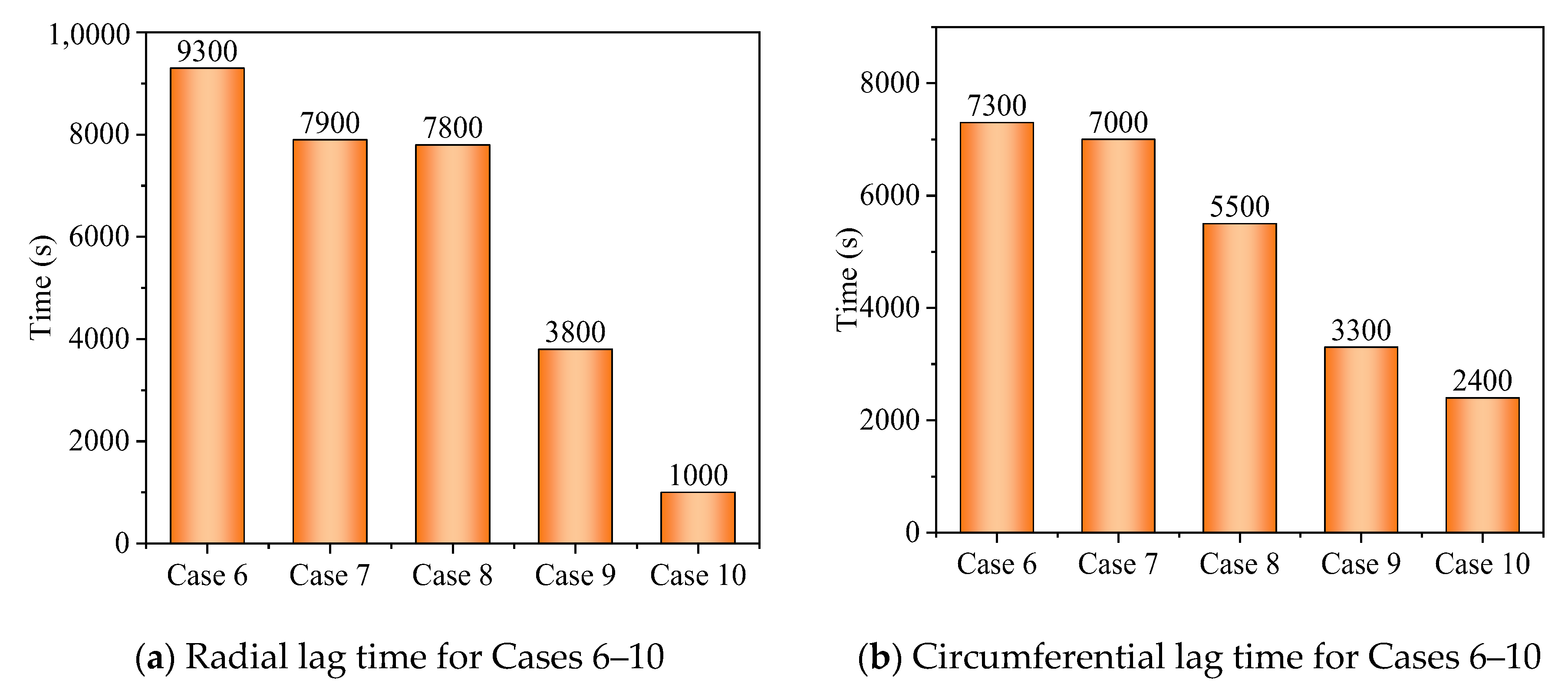
3.2. Promotion of Melting and Solidification by Bifurcated Fins
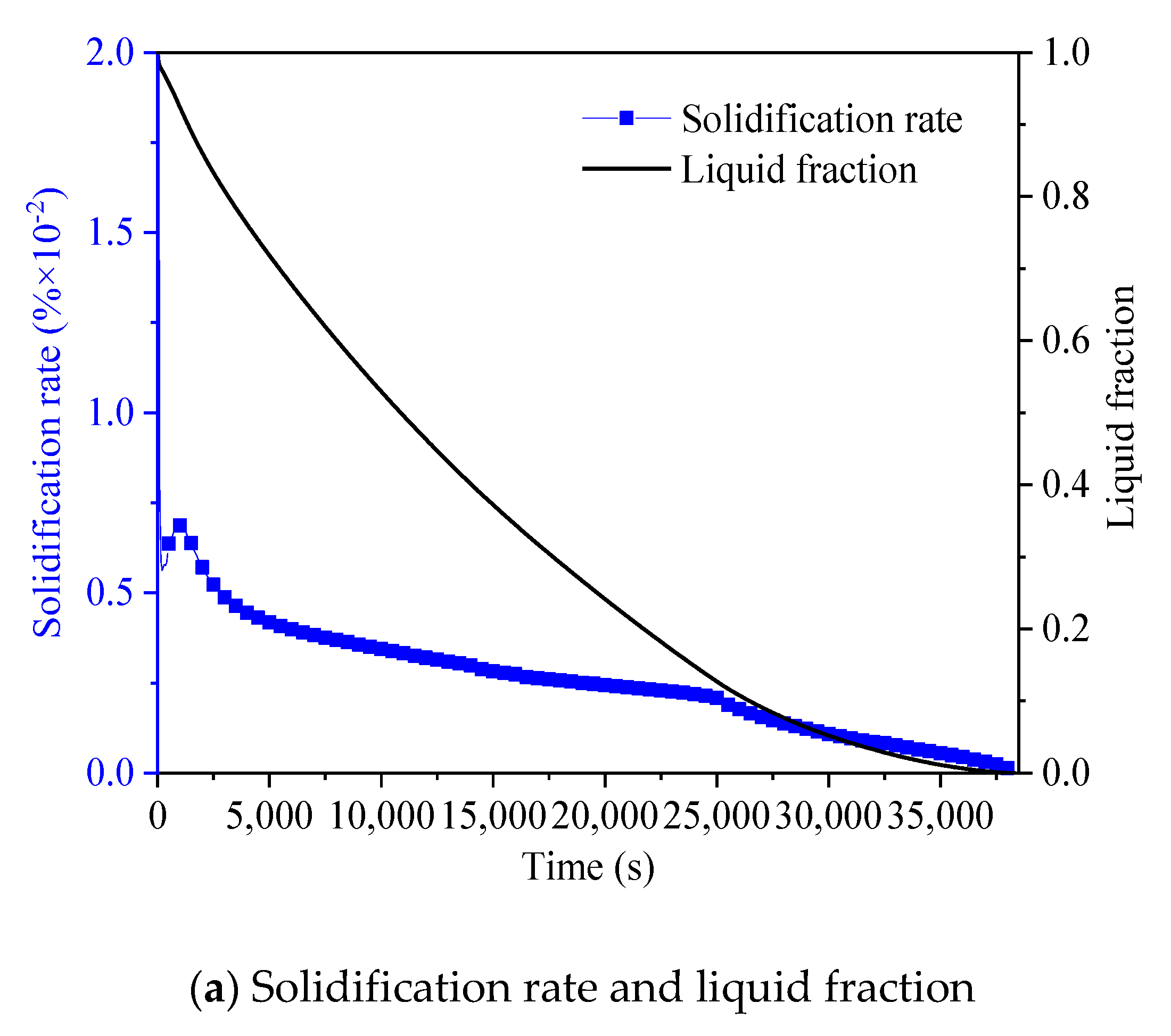
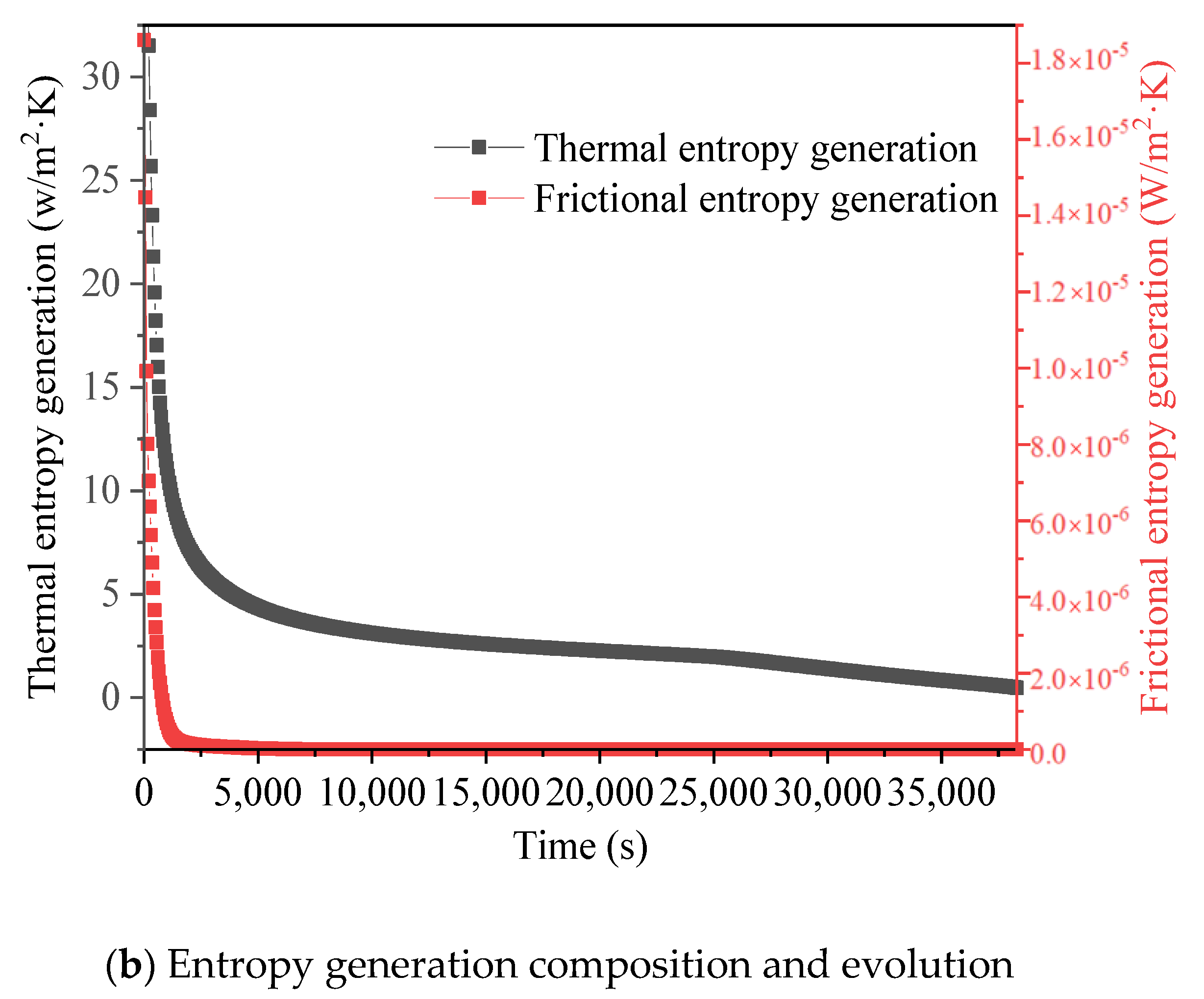
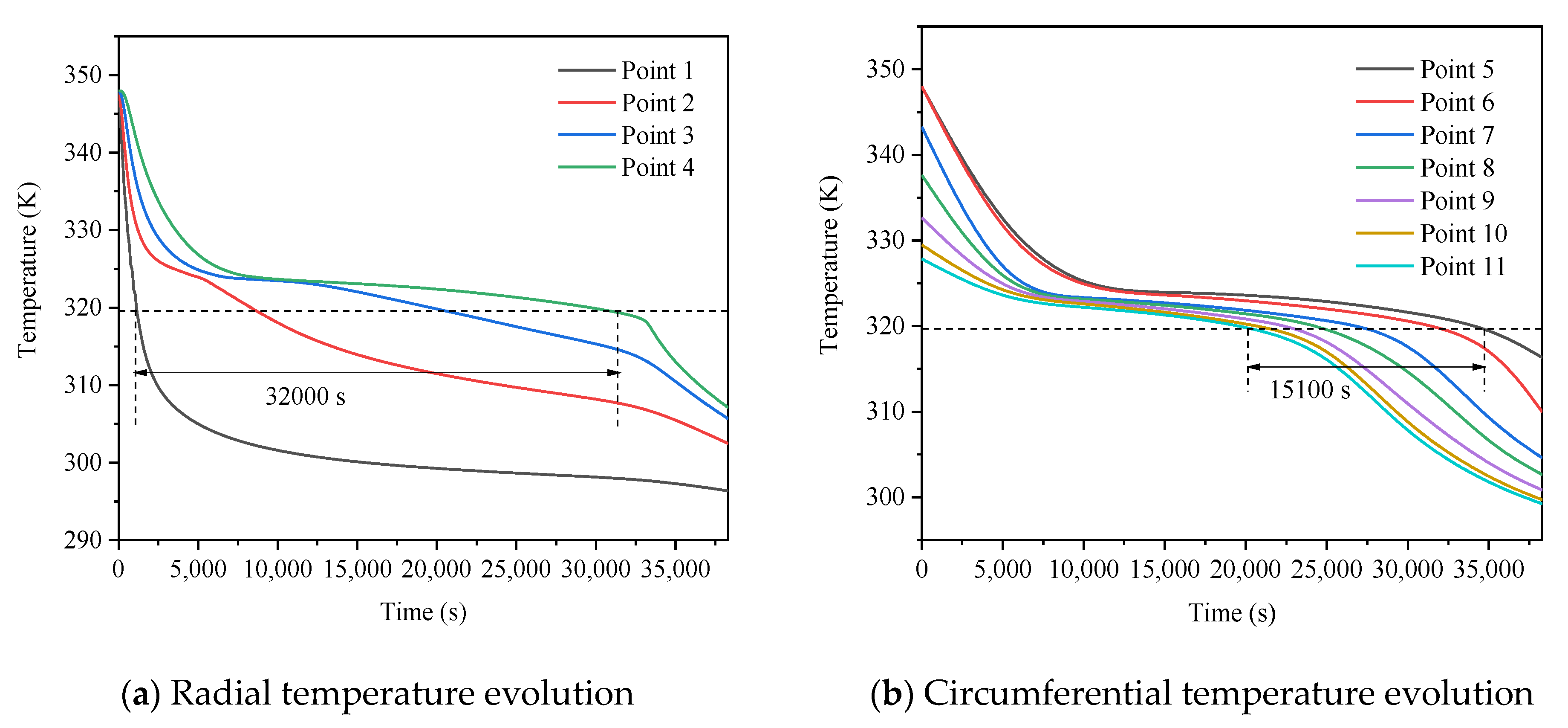
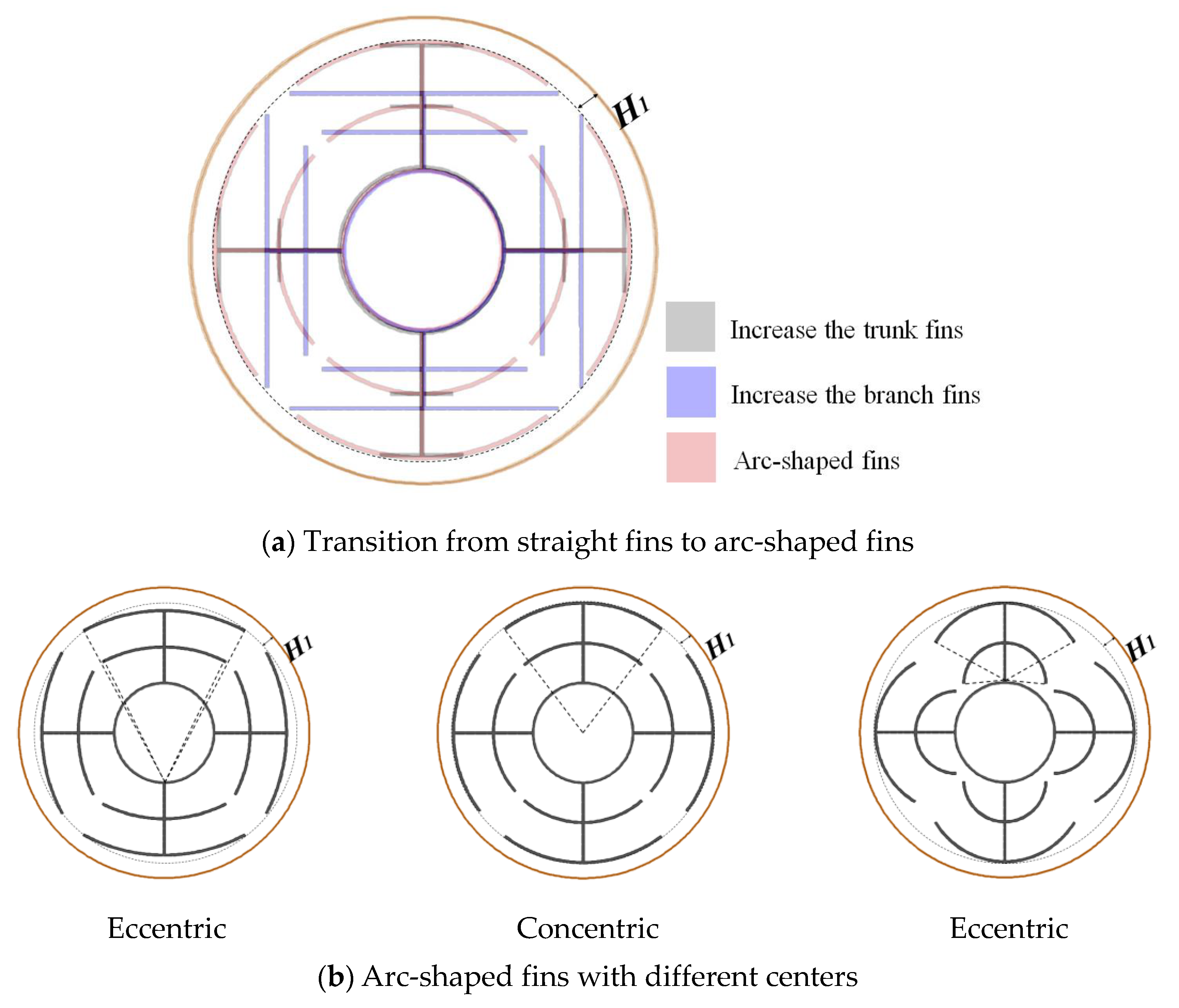
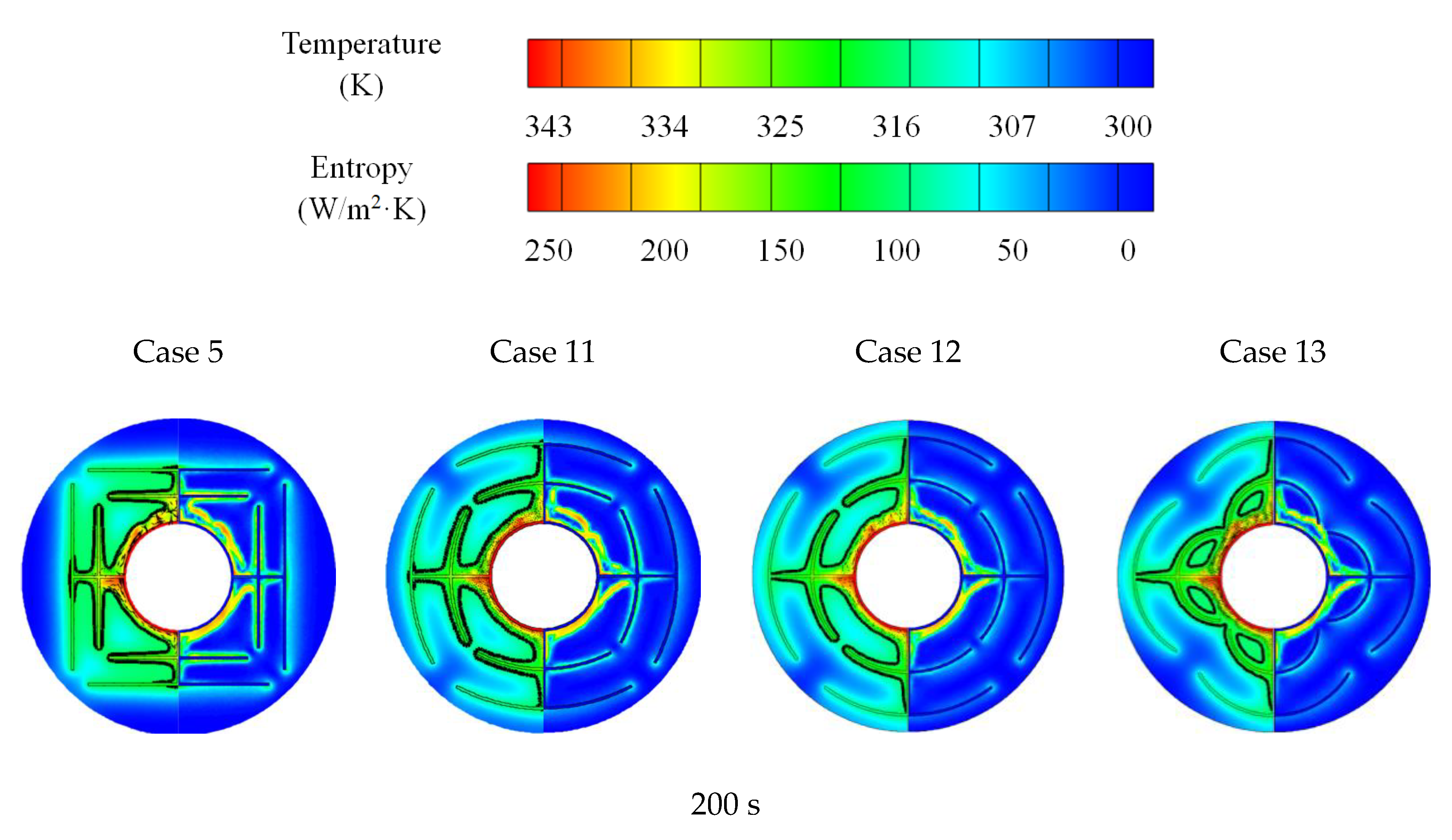
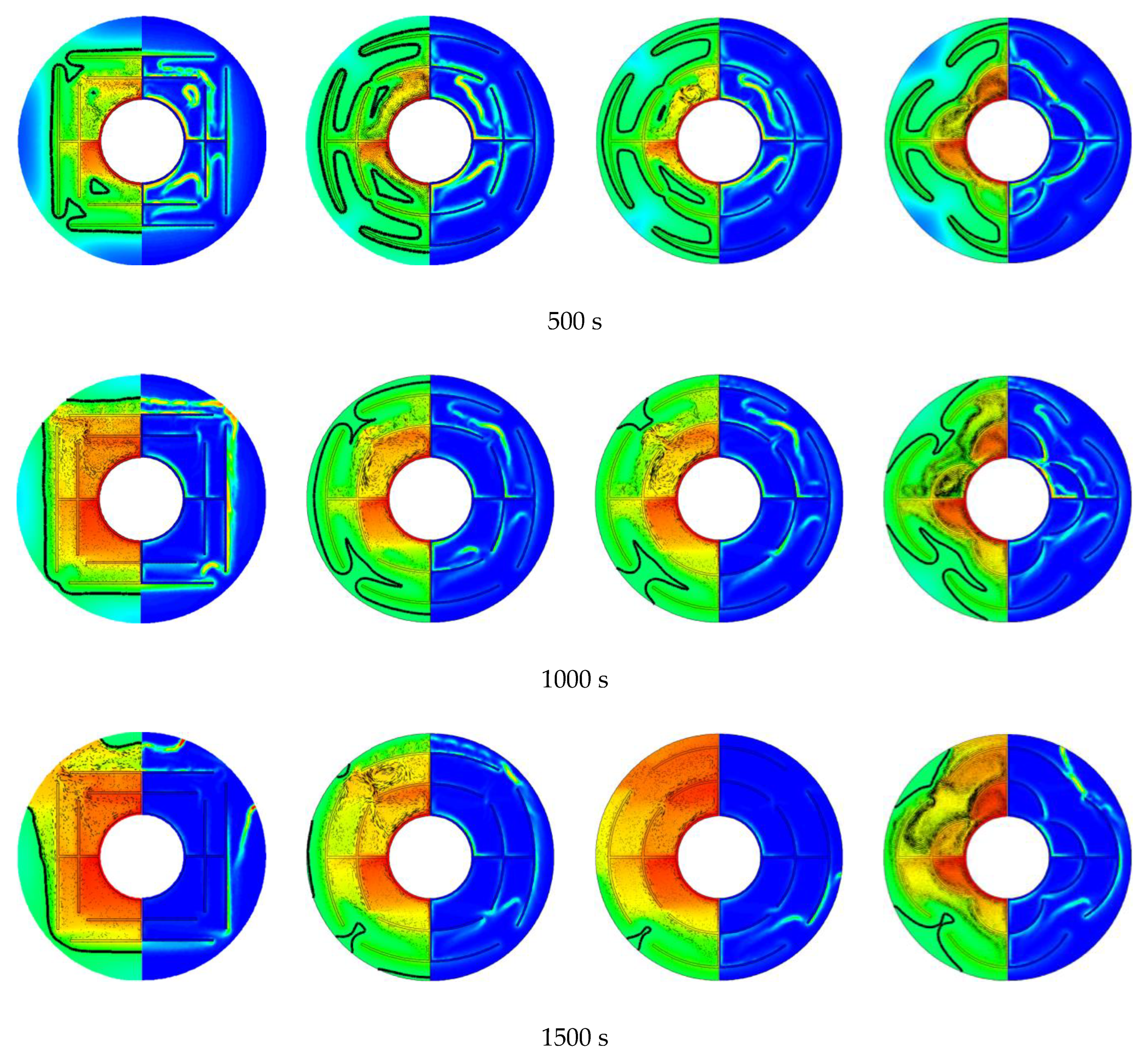
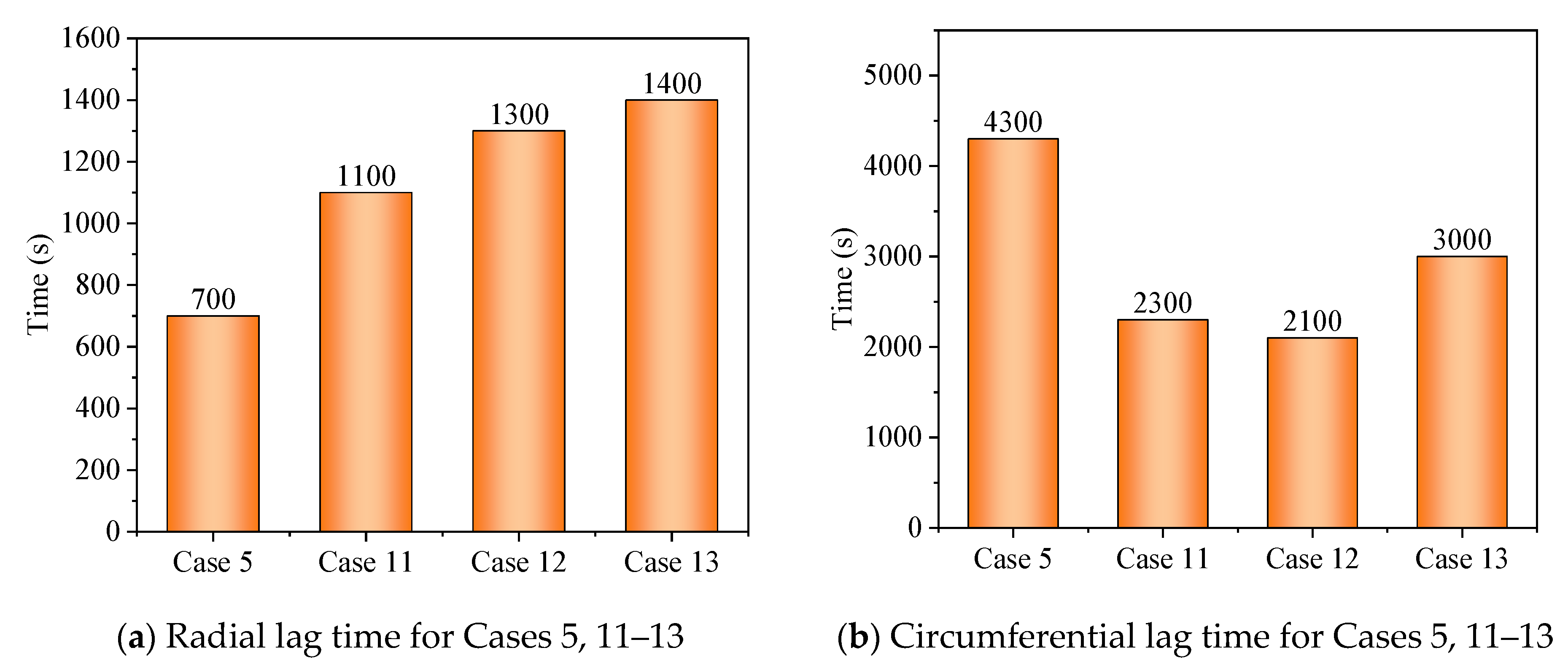
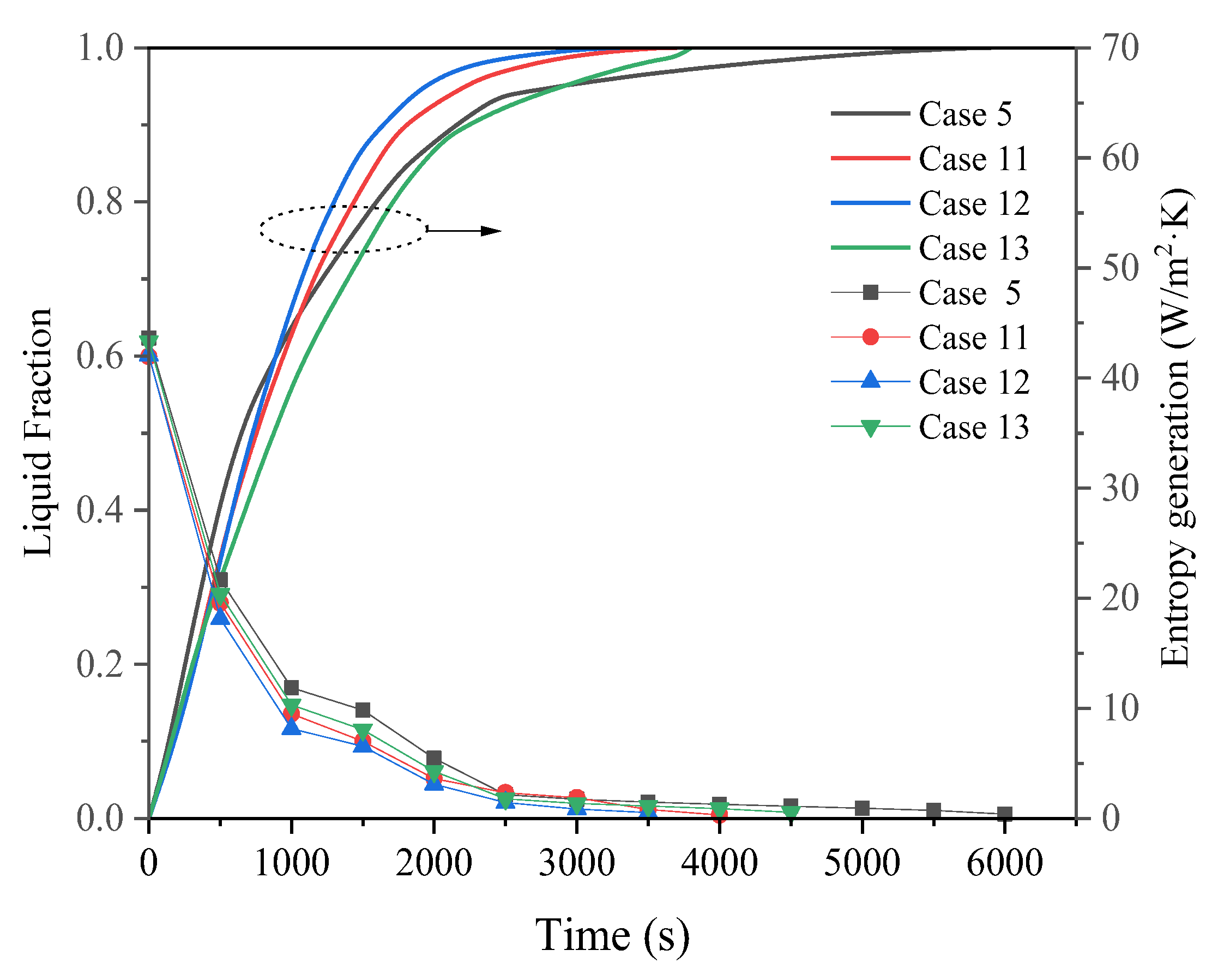
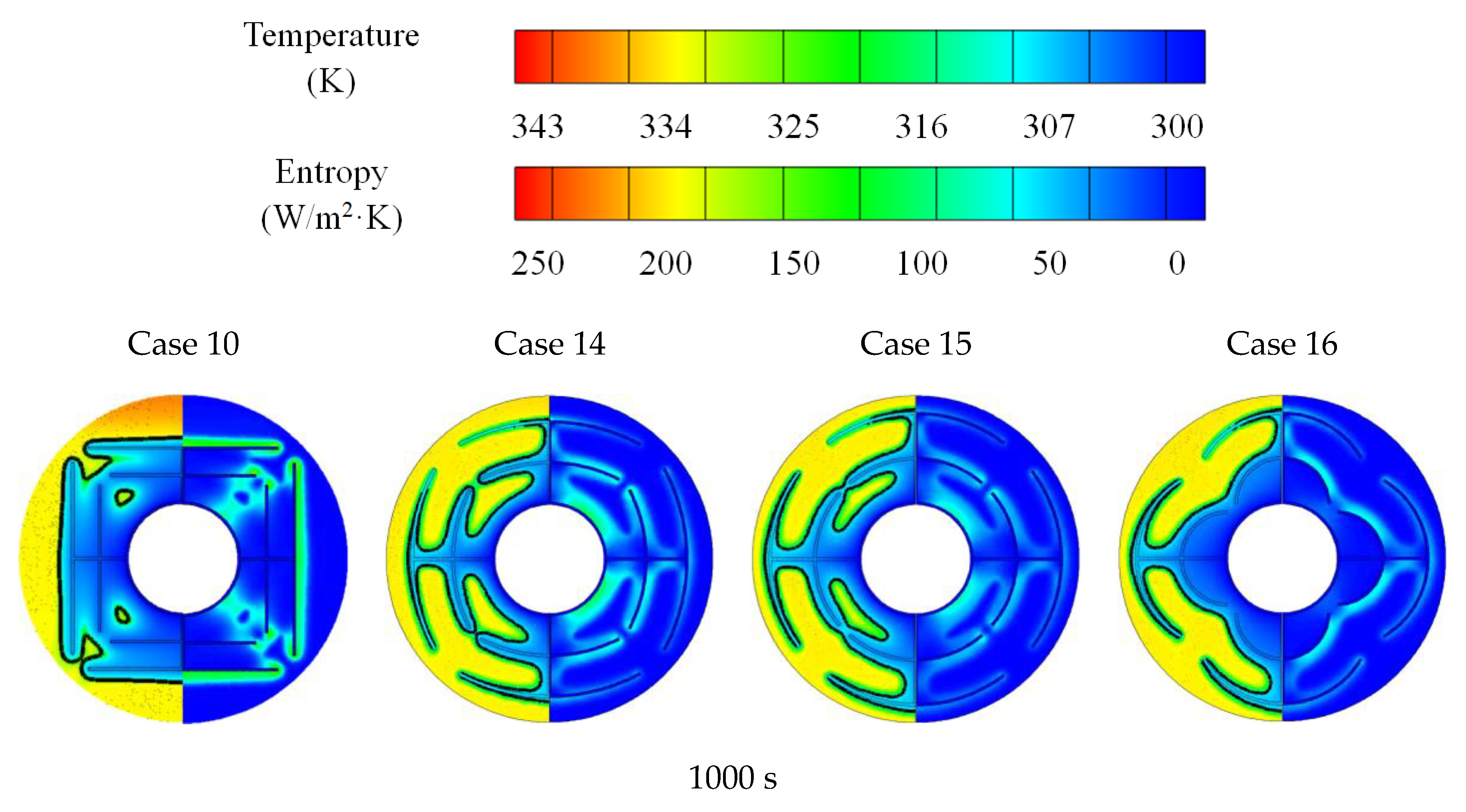
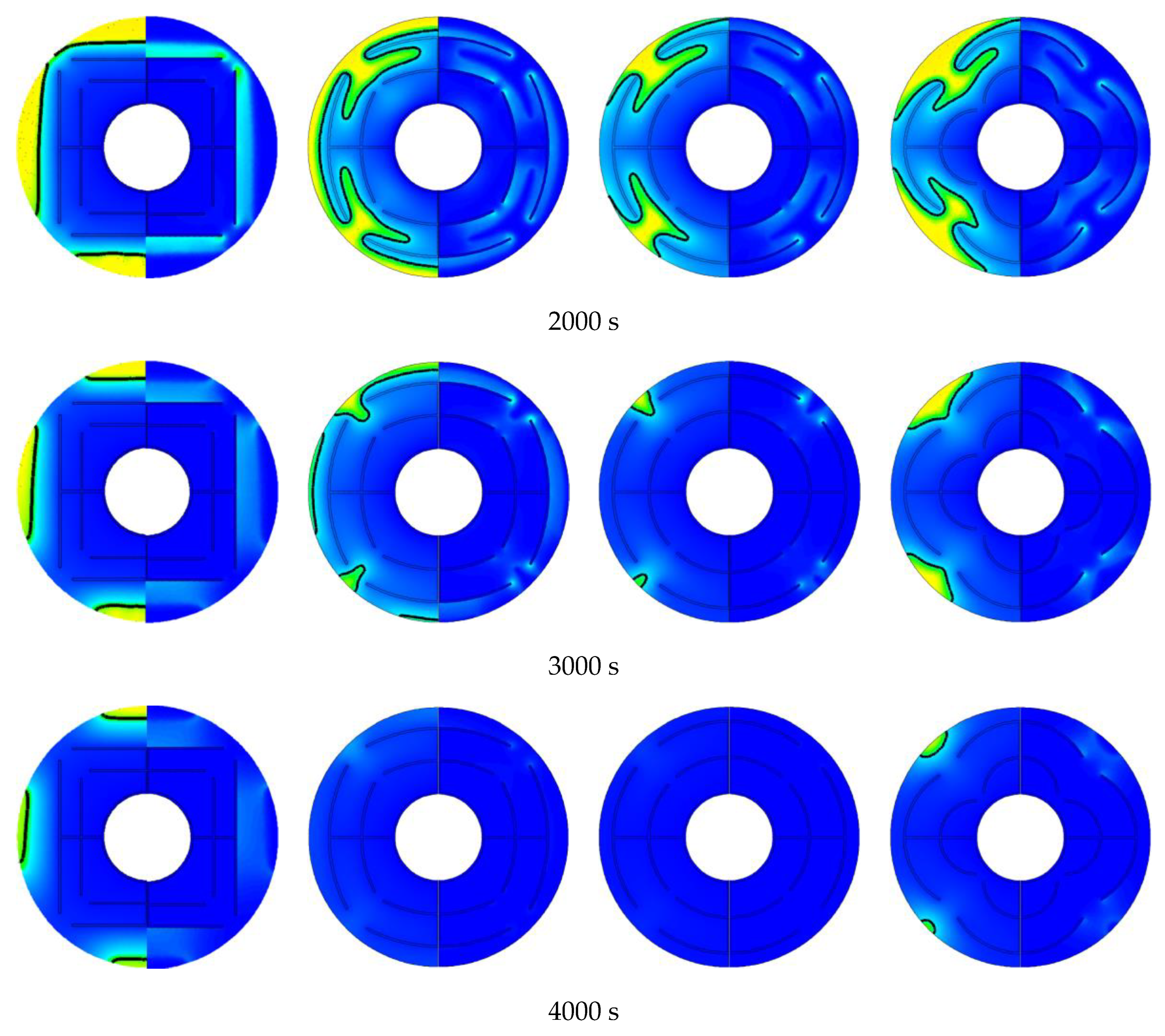
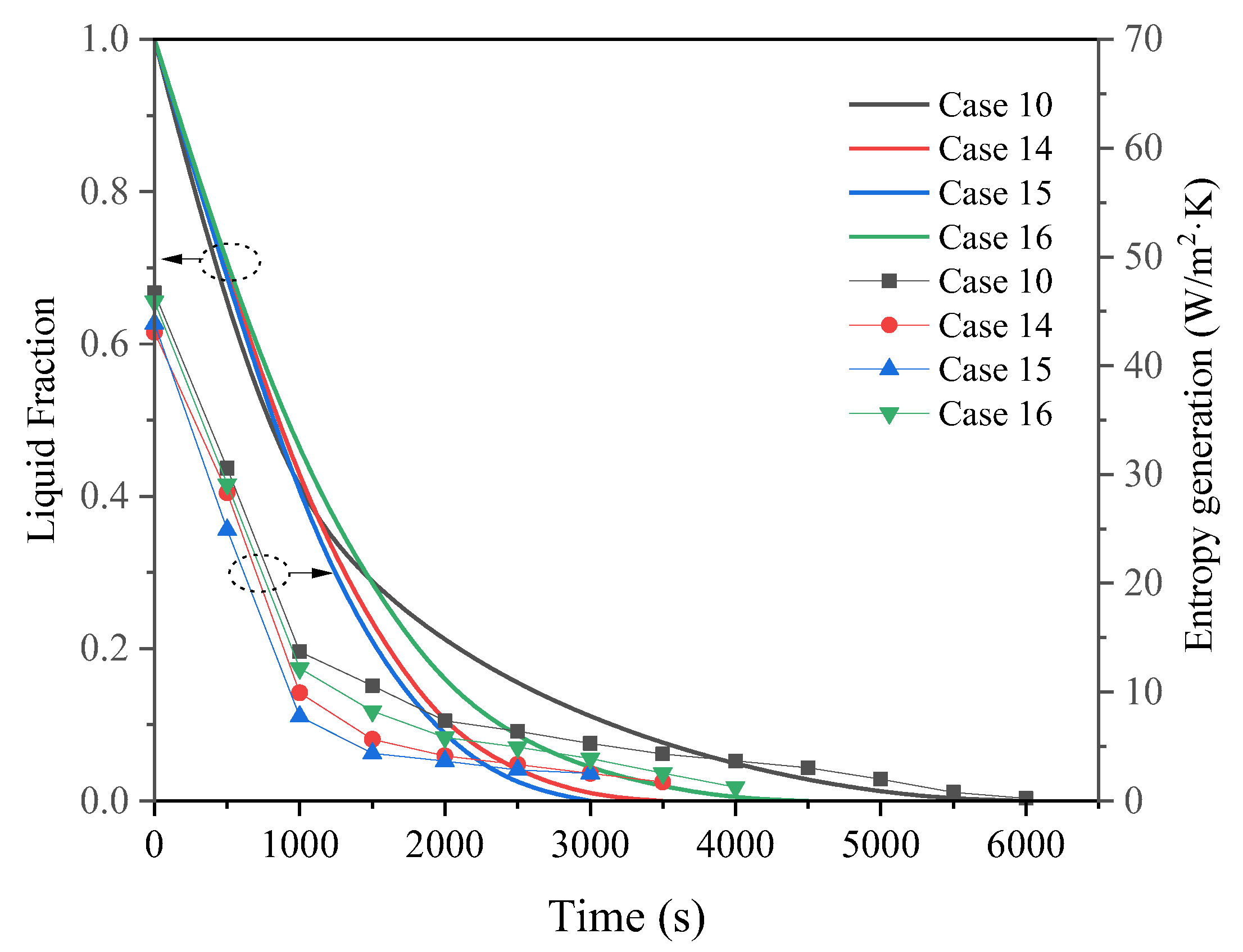
3.3. Promotion of Melting and Solidification by Arc-Shape Fins
4. Conclusions
- (1)
- For bifurcated fins composed of straight fins, increasing trunk fin length was more effective in the melting process in a certain space. For the solidification, however, increasing branch fin length was more effective. The straight branch fins were not compatible in shell-tube LHTES unit.
- (2)
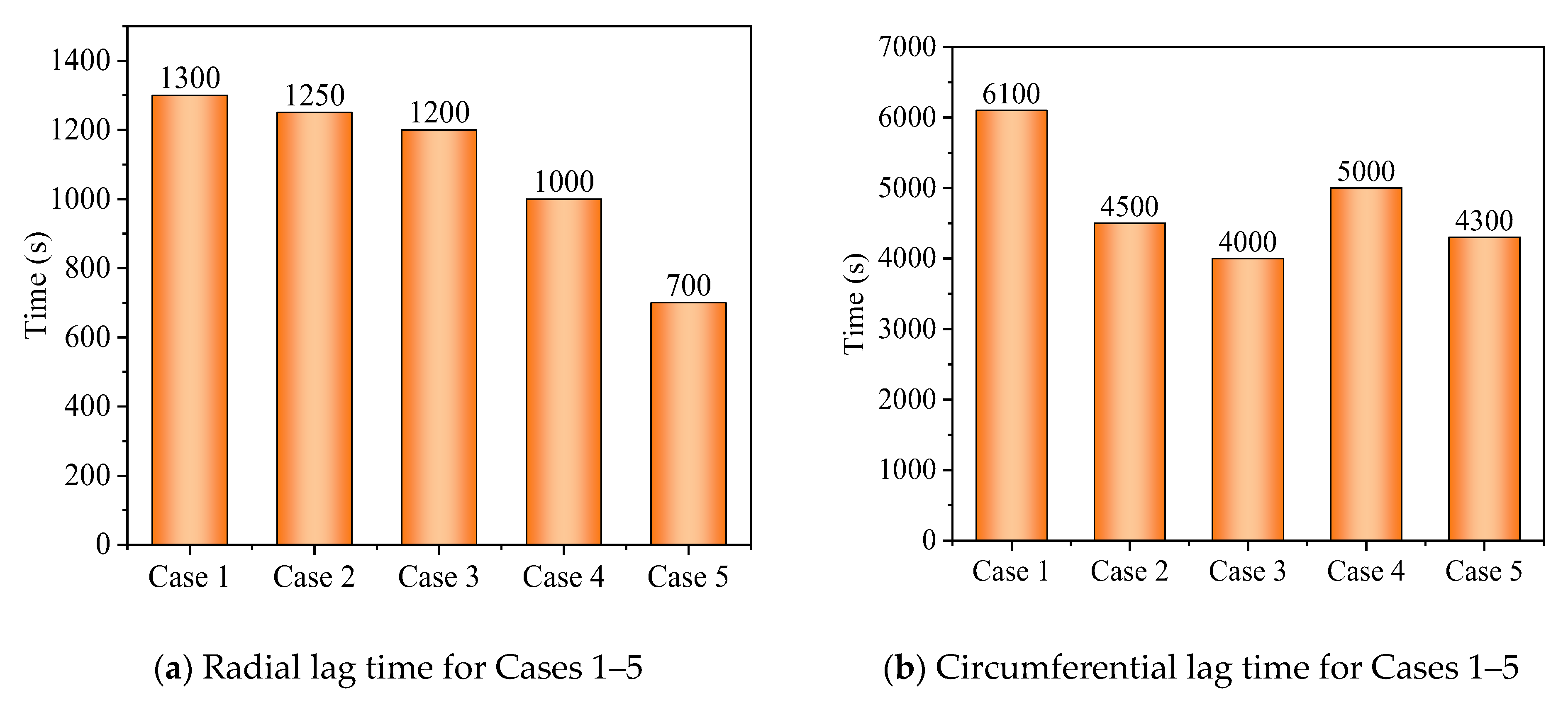
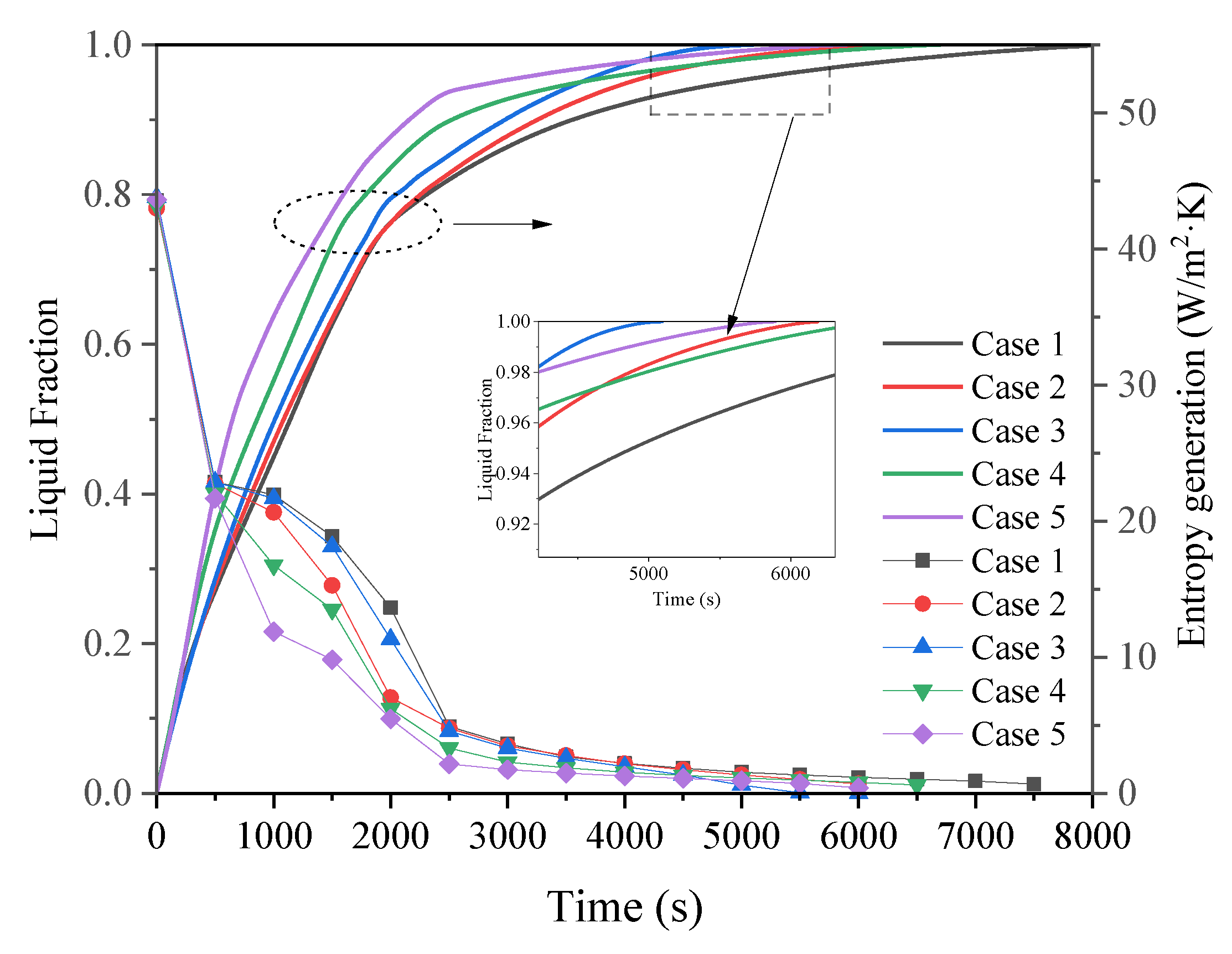
- The arc-shaped fins effectively promoted thermal performance of the LHTES unit. More importantly, the arc-shaped fins concentric with the LHTES unit exhibited the best thermal uniformity the lowest global entropy generation. In the same space, the concentric arc-shaped fins shortened the energy storage time by 52.7% and the energy release time by 51.6%.
- (3)
- The circumferential thermal uniformity directly reflects the phase transition process. In addition, the radial thermal penetration in one direction could not comprehensively reflect the phase transition process because of the existence of the heat transfer hysteresis region. Thermal uniformity is a more important evaluation for shell-tube LHTES units.
- (4)
- The present conclusions are applicable to the LHTES units with single tubes. For technical LHTES units with multiple inner tubes, the applicability of arc fins still needs to be further evaluated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Saha, S.K. Performance study of a novel funnel shaped shell and tube latent heat thermal energy storage system. Renew. Energy 2021, 165, 731–747. [Google Scholar] [CrossRef]
- Thonon, M.; Fraisse, G.; Zalewski, L. Analytical modelling of PCM supercooling including recalescence for complete and partial heating/cooling cycles. Appl. Therm. Eng. 2021, 190, 116751. [Google Scholar] [CrossRef]
- Demirkran, S.G.; Cetkin, E. Emergence of rectangular shell shape in thermal energy storage applications: Fitting melted phase changing material in a fixed space. J. Energy Storage 2021, 37, 102455. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Mohammed, H.I.; Talebizadehsardari, P. A new approach for employing multiple PCMs in the passive thermal management of photovoltaic modules. Sol. Energy 2021, 222, 160–174. [Google Scholar] [CrossRef]
- Li, X.; Duan, J.; Simon, T. Nonuniform metal foam design and pore-scale analysis of a tilted composite phase change material system for photovoltaics thermal management. Appl. Energy 2021, 298, 117203. [Google Scholar] [CrossRef]
- Wang, J.; Yu, K.; Duan, R. Enhanced Thermal Management by Introducing Nanoparticle Composite Phase Change Materials for Cooling Multiple Heat Sources Systems. Energy 2021, 227, 120495. [Google Scholar] [CrossRef]
- Rathore, P.; Shukla, S.K. An experimental evaluation of thermal behavior of the building envelope using macroencapsulated PCM for energy savings. Renew. Energy 2020, 149, 1300–1313. [Google Scholar] [CrossRef]
- Wen, R.; Liu, Y.; Yang, C. Enhanced thermal properties of stearic acid/carbonized maize straw composite phase change material for thermal energy storage in buildings. J. Energy Storage 2021, 36, 102420. [Google Scholar] [CrossRef]
- Peng, W.; Sadaghiani, O.K. Thermal function improvement of phase-change material (PCM) using alumina nanoparticles in a circular-rectangular cavity using Lattice Boltzmann method. J. Energy Storage 2021, 37, 102493. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Montazer, E.; Shafii, M.B. Solidification enhancement in triplex thermal energy storage system via triplets fins configuration and hybrid nanoparticles. J. Energy Storage 2021, 34, 102177. [Google Scholar] [CrossRef]
- Li, W.Q.; Guo, S.J.; Tan, L. Heat transfer enhancement of nano-encapsulated phase change material (NEPCM) using metal foam for thermal energy storage. Int. J. Heat Mass Tran. 2021, 166, 120737. [Google Scholar] [CrossRef]
- Dinesh, B.; Bhattacharya, A. Comparison of energy absorption characteristics of PCM-metal foam systems with different pore size distributions. J. Energy Storage 2020, 28, 101190. [Google Scholar] [CrossRef]
- Zhao, C.; Opolot, M.; Liu, M. Numerical study of melting performance enhancement for PCM in an annular enclosure with internal-external fins and metal foams. Int. J. Heat Mass Tran. 2020, 150, 119348. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Zhou, D. Experimental study on thermal conductivity of paraffin-based shape-stabilized phase change material with hybrid carbon nano-additives. Renew. Energy 2020, 146, 2637–2645. [Google Scholar] [CrossRef]
- Kuziel, A.W.; Dzido, G.; Turczyn, R. Ultra-long carbon nanotube-paraffin composites of record thermal conductivity and high phase change enthalpy among paraffin-based heat storage materials. J. Energy Storage 2021, 6, 102396. [Google Scholar] [CrossRef]
- Sodhi, G.S.; Muthukumar, P. Compound charging and discharging enhancement in multi-PCM system using non-uniform fin distribution. Renew. Energy 2021, 171, 299–314. [Google Scholar] [CrossRef]
- Mao, Q.; Li, Y. Experimental and numerical investigation on enhancing heat transfer performance of a phase change thermal storage tank. J. Energy Storage 2020, 31, 101725. [Google Scholar] [CrossRef]
- Raul, A.; Saha, S.K.; Jain, M. Transient performance analysis of concentrating solar thermal power plant with finned latent heat thermal energy storage. Renew. Energy 2020, 145, 1957–1971. [Google Scholar] [CrossRef]
- Jia, X.; Zhai, X.; Cheng, X. Thermal performance analysis and optimization of a spherical PCM capsule with pin-fins for cold storage. Appl. Therm. Eng. 2018, 148, 929–938. [Google Scholar] [CrossRef]
- Paria, S.; Baradaran, S.; Amiri, A. Performance evaluation of latent heat energy storage in horizontal shell-and-finned tube for solar application. J. Therm. Anal. Calorim. 2016, 123, 1371–1381. [Google Scholar] [CrossRef]
- Deng, S.; Nie, C.; Wei, G. Improving the melting performance of a horizontal shell-tube latent-heat thermal energy storage unit using local enhanced finned tube. Energy Build. 2019, 183, 161–173. [Google Scholar] [CrossRef]
- Al-Abidi, A.; Mat, S.; Sopian, K. Experimental study of melting and solidification of PCM in a triplex tube heat exchanger with fins. Energy Build. 2014, 68, 33–41. [Google Scholar] [CrossRef]
- Zarei, M.J.; Bazai, H.; Sharifpur, M. The Effects of Fin Parameters on the Solidification of PCMs in a Fin-Enhanced Thermal Energy Storage System. Energies 2020, 13, 198. [Google Scholar] [CrossRef]
- Kunal, B.; Mohit, P.; Saha, S.K. Estimation of thermal performance and design optimization of finned multitube latent heat thermal energy storage. J. Energy Storage 2018, 19, 135–144. [Google Scholar]
- Al-Abidi, A.A.; Mat, S.; Sopian, K. Numerical study of PCM solidification in a triplex tube heat exchanger with internal and external fins. Int. J. Heat Mass Tran. 2013, 61, 684–695. [Google Scholar] [CrossRef]
- Yldz, A.; Arc, M.; Nieti, S. Numerical investigation of natural convection behavior of molten PCM in an enclosure having rectangular and tree-like branching fins. Energy 2020, 207, 118223. [Google Scholar] [CrossRef]
- Alizadeh, M.; Khosseinzadeh, H. Solidification acceleration in a triplex-tube latent heat thermal energy storage system using V-shaped fin and nano-enhanced phase change material. Appl. Therm. Eng. 2019, 163, 114436. [Google Scholar] [CrossRef]
- Al-Mudhafar, A.; Nowakowski, A.F.; Nicolleau, F.G. Enhancing the thermal performance of PCM in a shell and tube latent heat energy storage system by utilizing innovative fins. Energy Rep. 2021, 7, 120–126. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Gagliardi, F.; Verda, V. Maximization of performance of a PCM latent heat storage system with innovative fins. Appl. Energy 2015, 137, 707–715. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.H.; Alizadeh, M.; Ganji, D.D. Solidification process of hybrid nano-enhanced phase change material in a LHTESS with tree-like branching fin in the presence of thermal radiation. J. Mol. Liq. 2018, 275, 909–925. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Chen, Y. Improving the energy discharging performance of a latent heat storage (LHS) unit using fractal-tree-shaped fins. Appl. Energy 2020, 259, 114102. [Google Scholar] [CrossRef]
- Cao, X.; Yuan, Y.; Xiang, B. Effect of natural convection on melting performance of eccentric horizontal shell and tube latent heat storage unit. Sustain. Cities. Soc. 2018, 38, 571–581. [Google Scholar] [CrossRef]
- Mallya, N.; Haussener, S. Buoyancy-driven melting and solidification heat transfer analysis in encapsulated phase change materials. Int. J. Heat Mass Tran. 2021, 164, 120525. [Google Scholar] [CrossRef]
- Nie, C.; Liu, J.; Deng, S. Effect of geometry modification on the thermal response of composite metal foam/phase change material for thermal energy storage. Int. J. Heat Mass Tran. 2021, 165, 120652. [Google Scholar] [CrossRef]
- Guelpa, E.; Sciacovelli, A.; Verda, V. Entropy generation analysis for the design improvement of a latent heat storage system. Energy 2013, 53, 128–138. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Zhang, C. Role of tree-shaped fins in charging performance of a latent heat storage unit. Int. J. Energy Res. 2020, 44, 4800–4811. [Google Scholar] [CrossRef]
- Chen, S.B.; Saleem, S.; Alghamdi, M.N. Combined effect of using porous media and nano-particle on melting performance of PCM filled enclosure with triangular double fins. Case Stud. Therm. Eng. 2021, 25, 100939. [Google Scholar] [CrossRef]




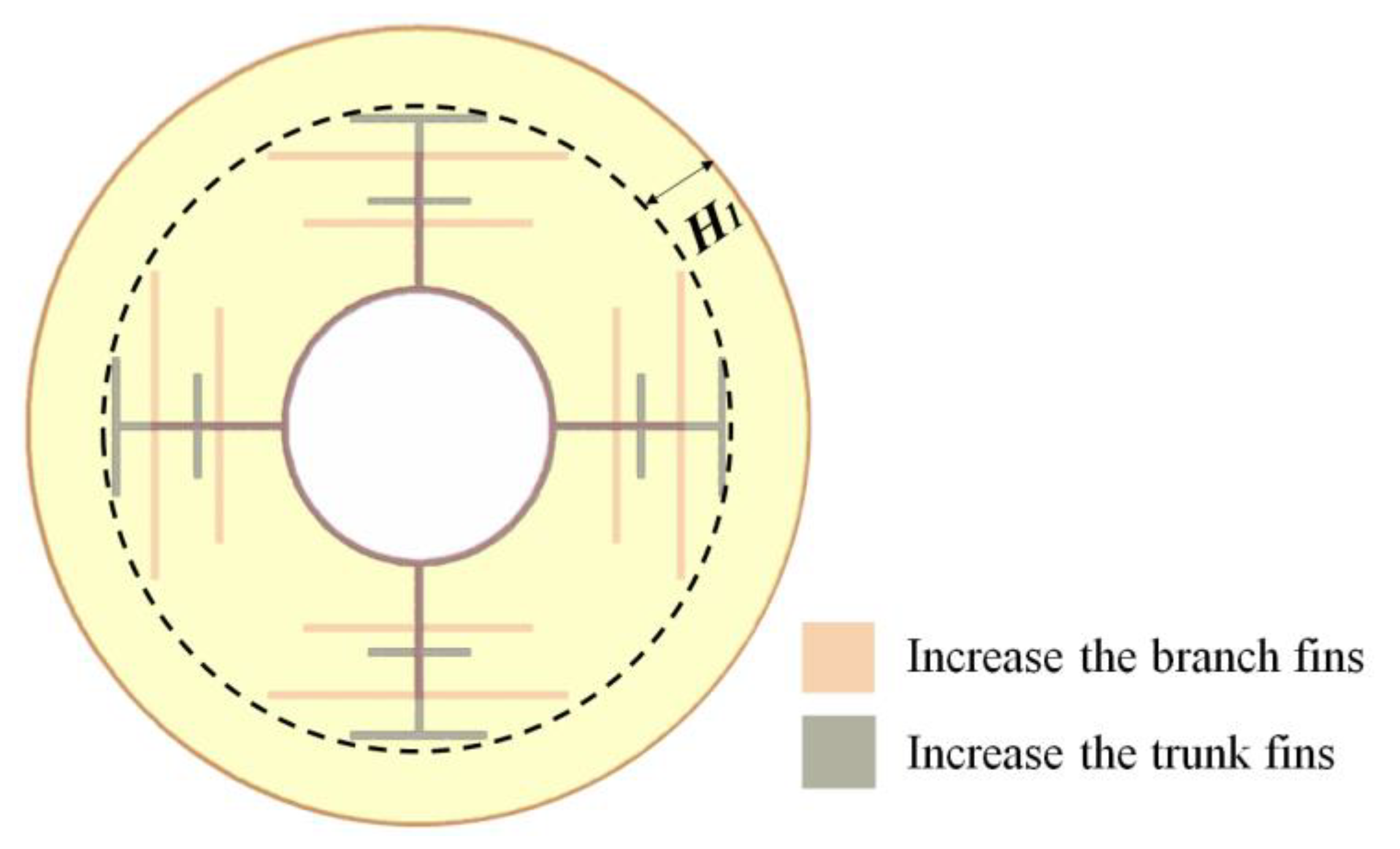


| Parameter | Unit | Paraffin (RT50) | Aluminum |
|---|---|---|---|
| Specific heat capacity | (kJ/kg·K) | 2.0 | 947 |
| Melting temperature | (°C) | 51 | - |
| Solidification temperature | (°C) | 45 | |
| Latent heat | (kJ/kg) | 168 | - |
| Thermal conductivity | (W/m·K) | 0.2 | 237 |
| Density | (kg/m3) | 800 | 2.7 × 103 |
| Thermal expansion coefficient | K−1 | 0.0006 | - |
| Dynamic viscosity | (Pa·s) | 0.004 |
| Case No. | H1 (mm) | L1 (mm) | L2 (mm) | P1 + P2 (mm) | State |
|---|---|---|---|---|---|
| Case 1 | 19 | 7.9 | 10.5 | 20 | Melting |
| Case 2 | 14 | 7.9 | 10.5 | 25 | Melting |
| Case 3 | 9 | 7.9 | 10.5 | 30 | Melting |
| Case 4 | 14 | 17.6 | 23.1 | 20 | Melting |
| Case 5 | 9 | 26.5 | 34.6 | 20 | Melting |
| Case 6 | 19 | 7.9 | 10.5 | 20 | Solidification |
| Case 7 | 14 | 7.9 | 10.5 | 25 | Solidification |
| Case 8 | 9 | 7.9 | 10.5 | 30 | Solidification |
| Case 9 | 14 | 17.6 | 23.1 | 20 | Solidification |
| Case 10 | 9 | 26.5 | 34.6 | 20 | Solidification |
| Case No. | L1 (mm) | L2 (mm) | P1 + P2 (mm) | Center of the Upper arc Fin (mm) | State |
|---|---|---|---|---|---|
| Case 11 | 26.5 | 34.6 | 30 | (0, −40) | Melting |
| Case 12 | 26.5 | 34.6 | 33 | (0, 0) | Melting |
| Case 13 | 26.5 | 34.6 | 33 | (0, 40) | Melting |
| Case 14 | 26.5 | 34.6 | 30 | (0, −40) | Solidification |
| Case 15 | 26.5 | 34.6 | 33 | (0, 0) | Solidification |
| Case 16 | 26.5 | 34.6 | 33 | (0, 40) | Solidification |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wu, J.; Sun, K.; Zhang, Y. Numerical Study of Heat Transfer Enhancement by Arc-Shaped Fins in a Shell-Tube Thermal Energy Storage Unit. Energies 2022, 15, 7799. https://doi.org/10.3390/en15207799
Chen Q, Wu J, Sun K, Zhang Y. Numerical Study of Heat Transfer Enhancement by Arc-Shaped Fins in a Shell-Tube Thermal Energy Storage Unit. Energies. 2022; 15(20):7799. https://doi.org/10.3390/en15207799
Chicago/Turabian StyleChen, Qicheng, Junting Wu, Kanglong Sun, and Yingjin Zhang. 2022. "Numerical Study of Heat Transfer Enhancement by Arc-Shaped Fins in a Shell-Tube Thermal Energy Storage Unit" Energies 15, no. 20: 7799. https://doi.org/10.3390/en15207799
APA StyleChen, Q., Wu, J., Sun, K., & Zhang, Y. (2022). Numerical Study of Heat Transfer Enhancement by Arc-Shaped Fins in a Shell-Tube Thermal Energy Storage Unit. Energies, 15(20), 7799. https://doi.org/10.3390/en15207799





