A Reduced Reaction Mechanism for Diesel/2-Methyltetrahydrofuran Dual-Fuel Engine Application
Abstract
1. Introduction
2. Mechanism Development
2.1. Base Diesel Surrogate Mechanism
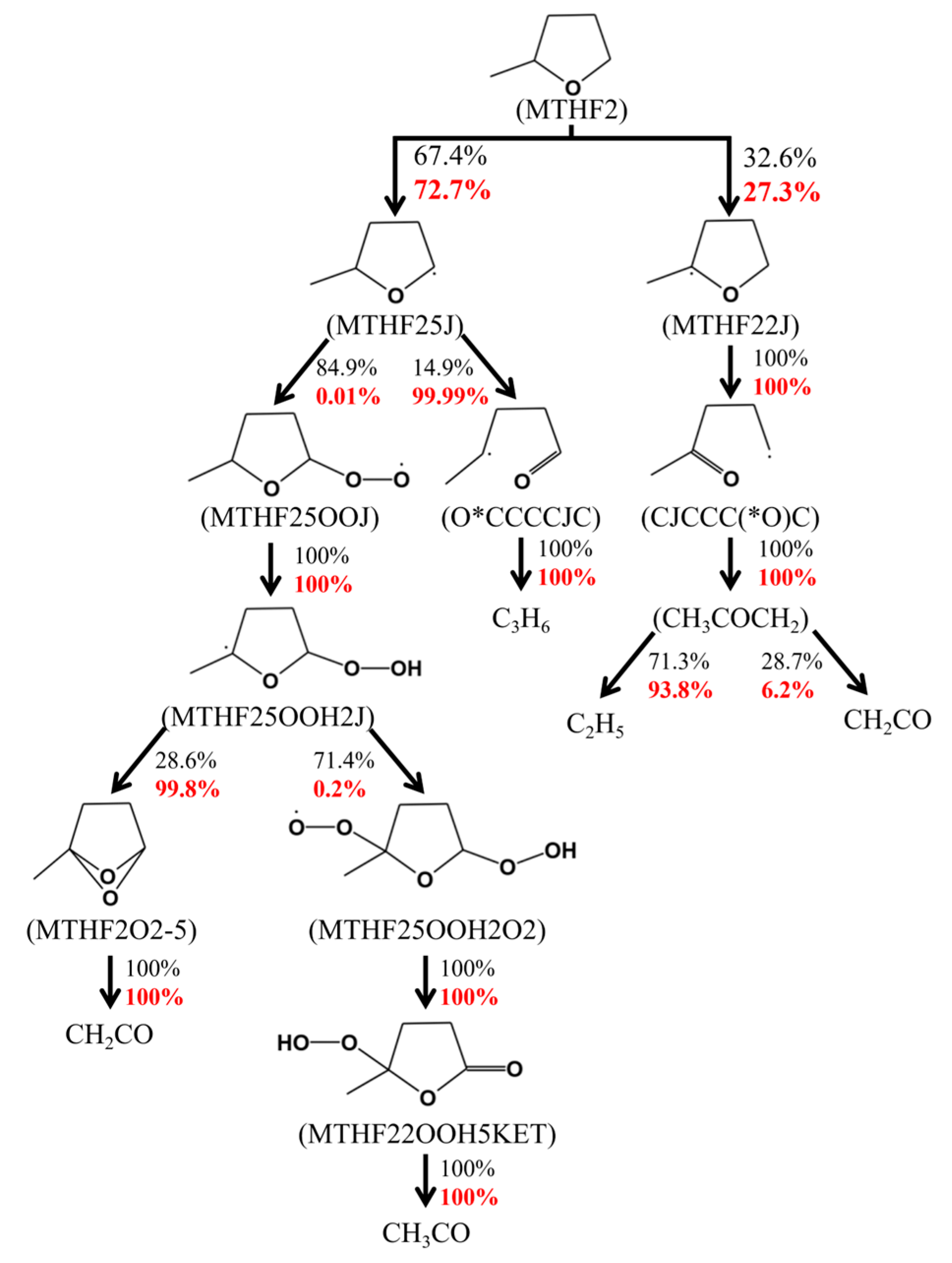
2.2. The Reduced MTHF2 Mechanism
2.3. Formation of the Reduced Diesel/MTHF2 Mechanism
3. Mechanism Validation
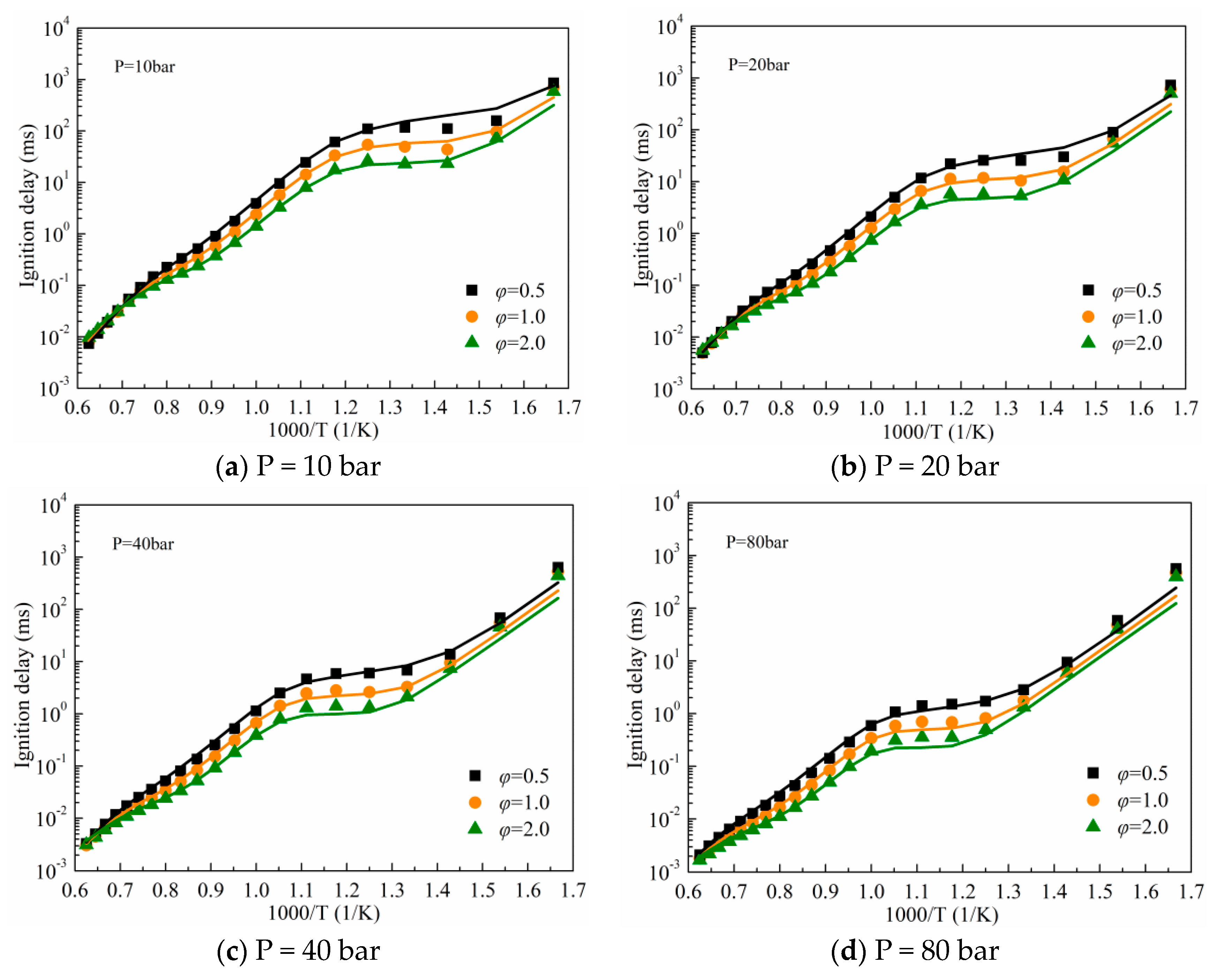
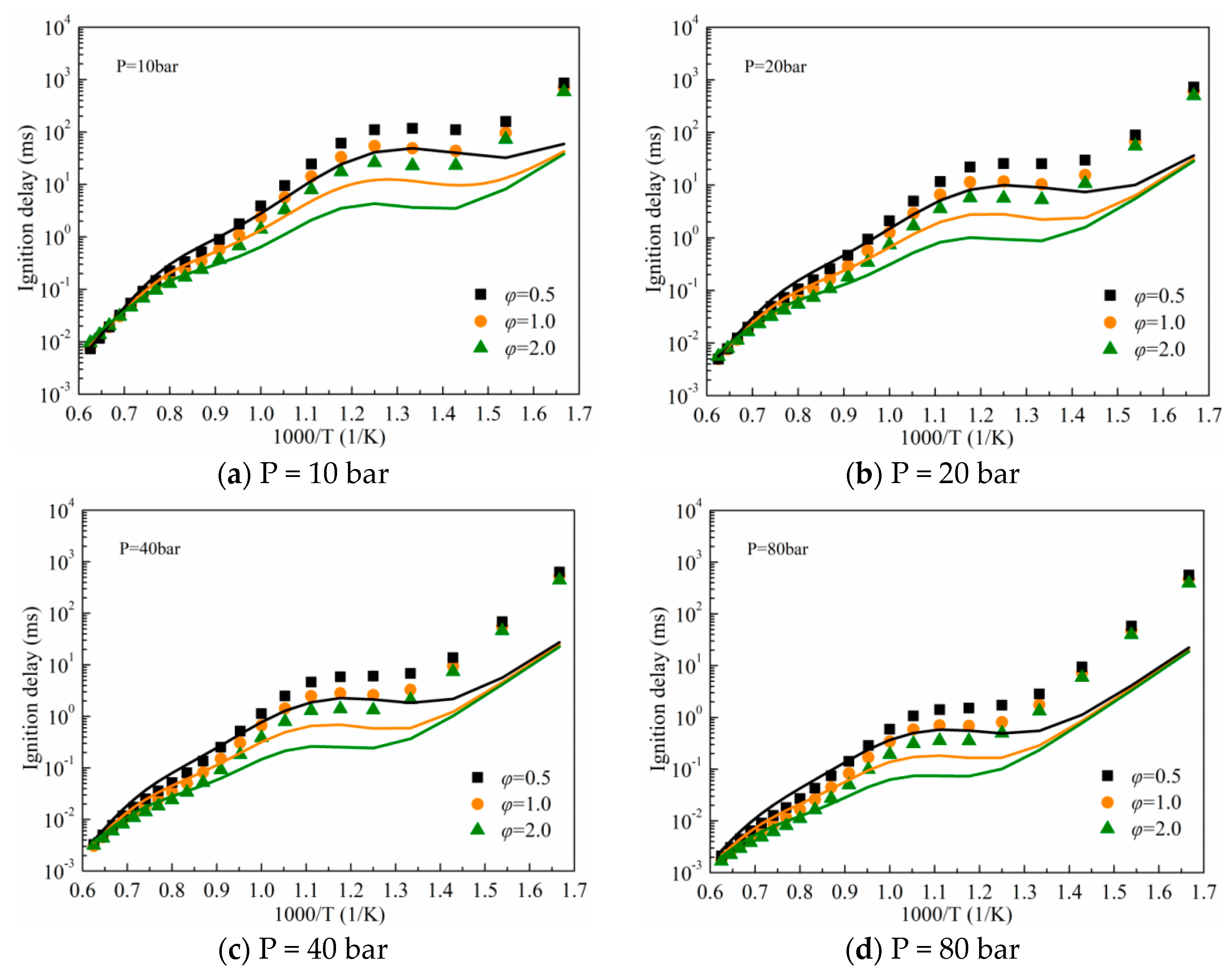
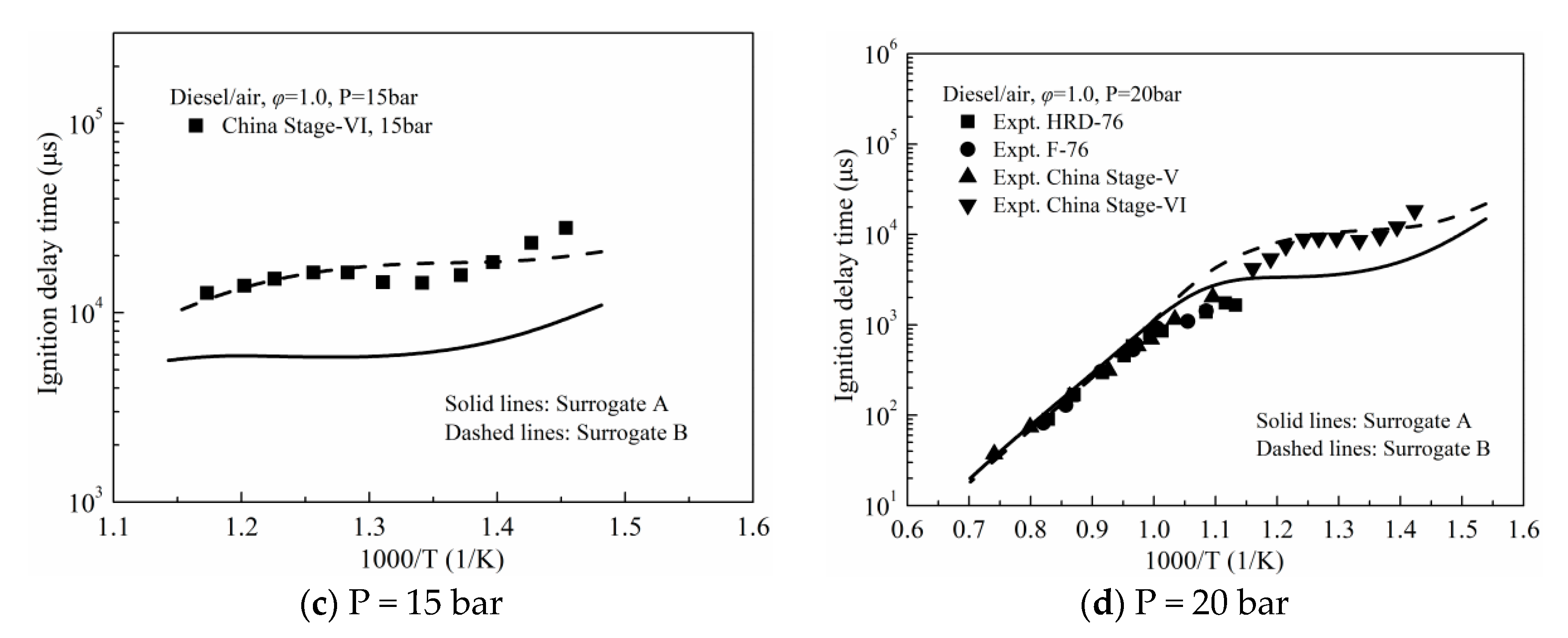
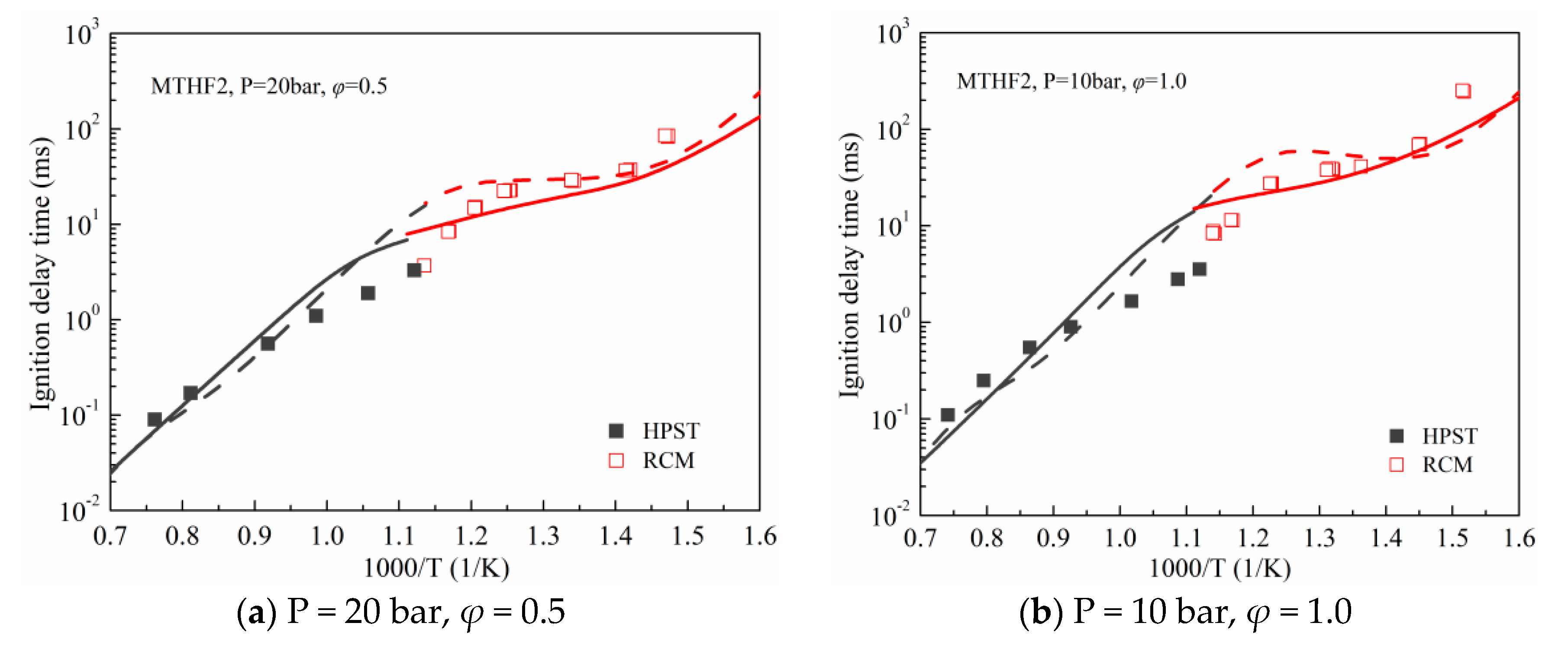
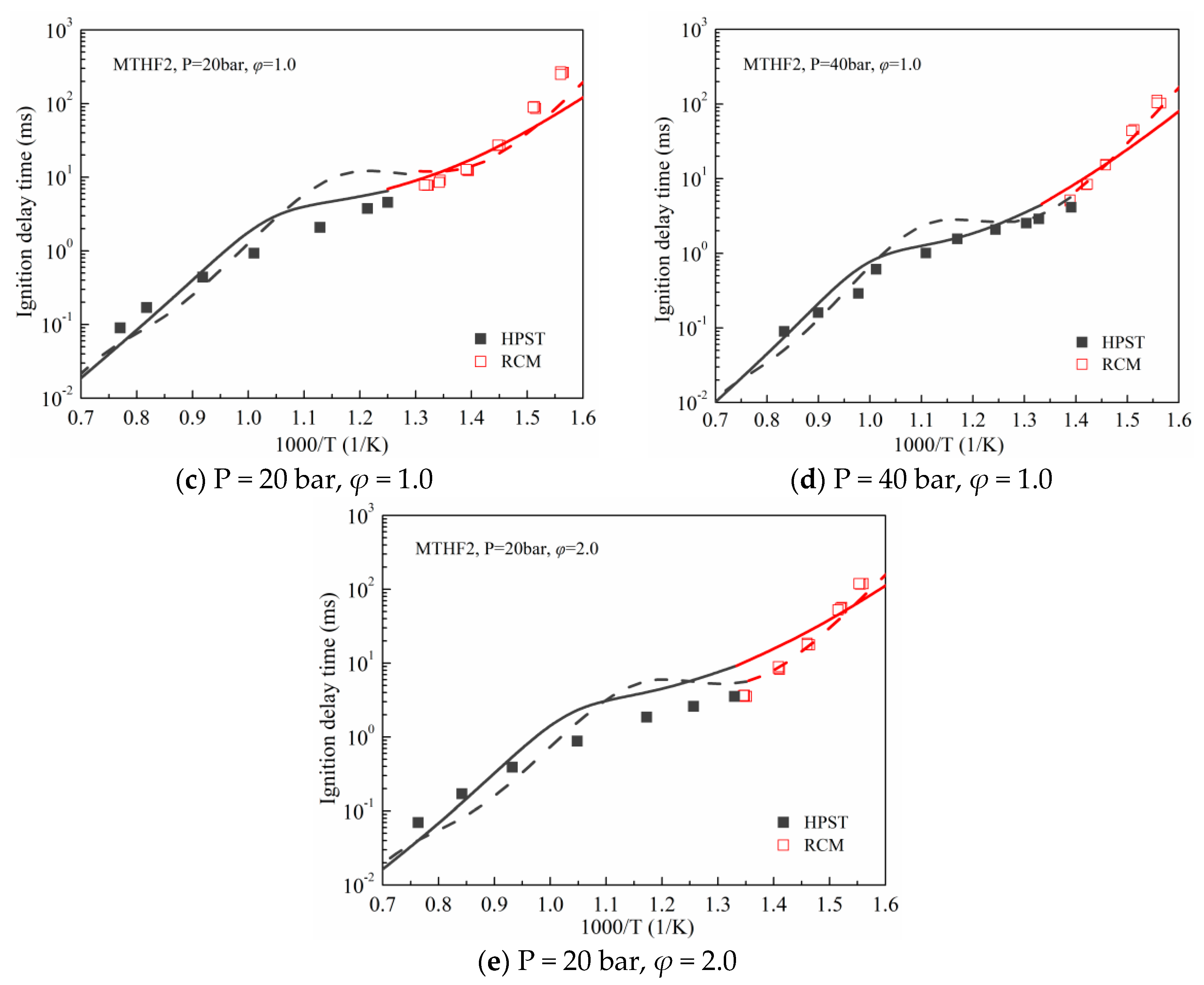
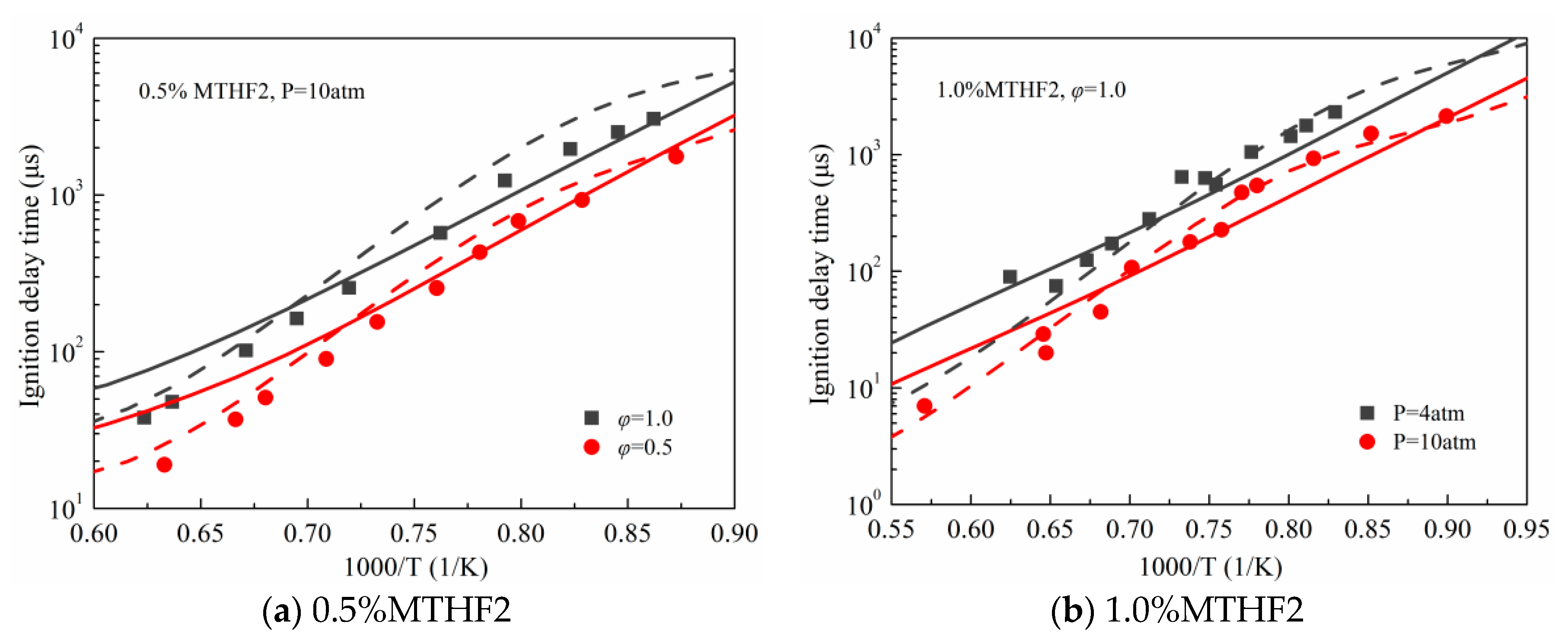
3.1. Validation against Ignition Delay Times
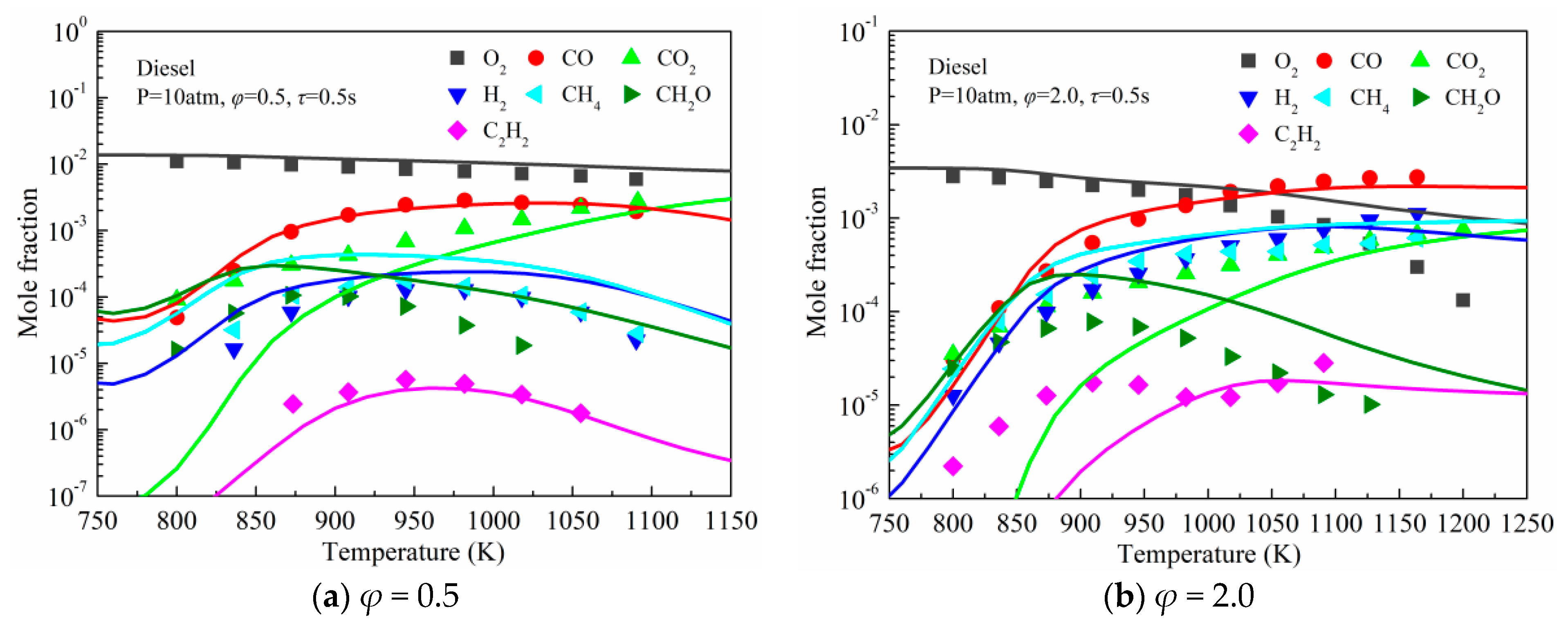
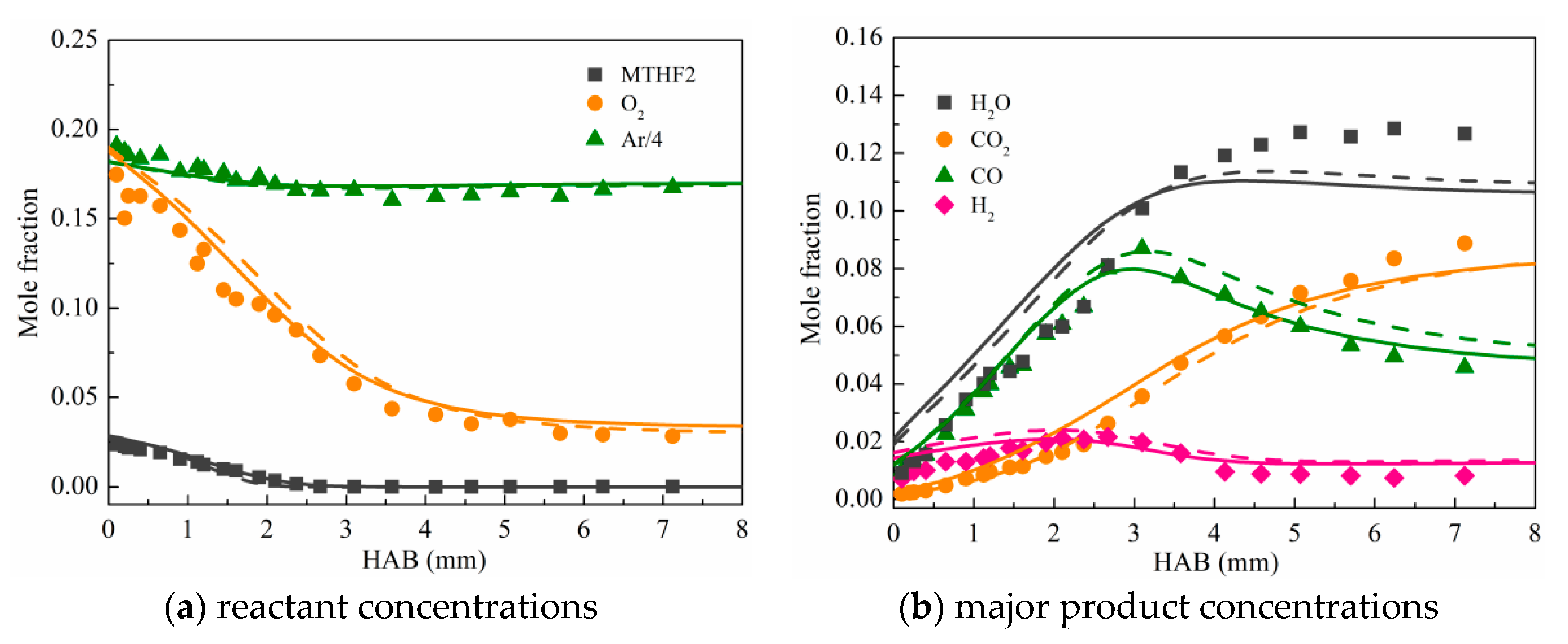
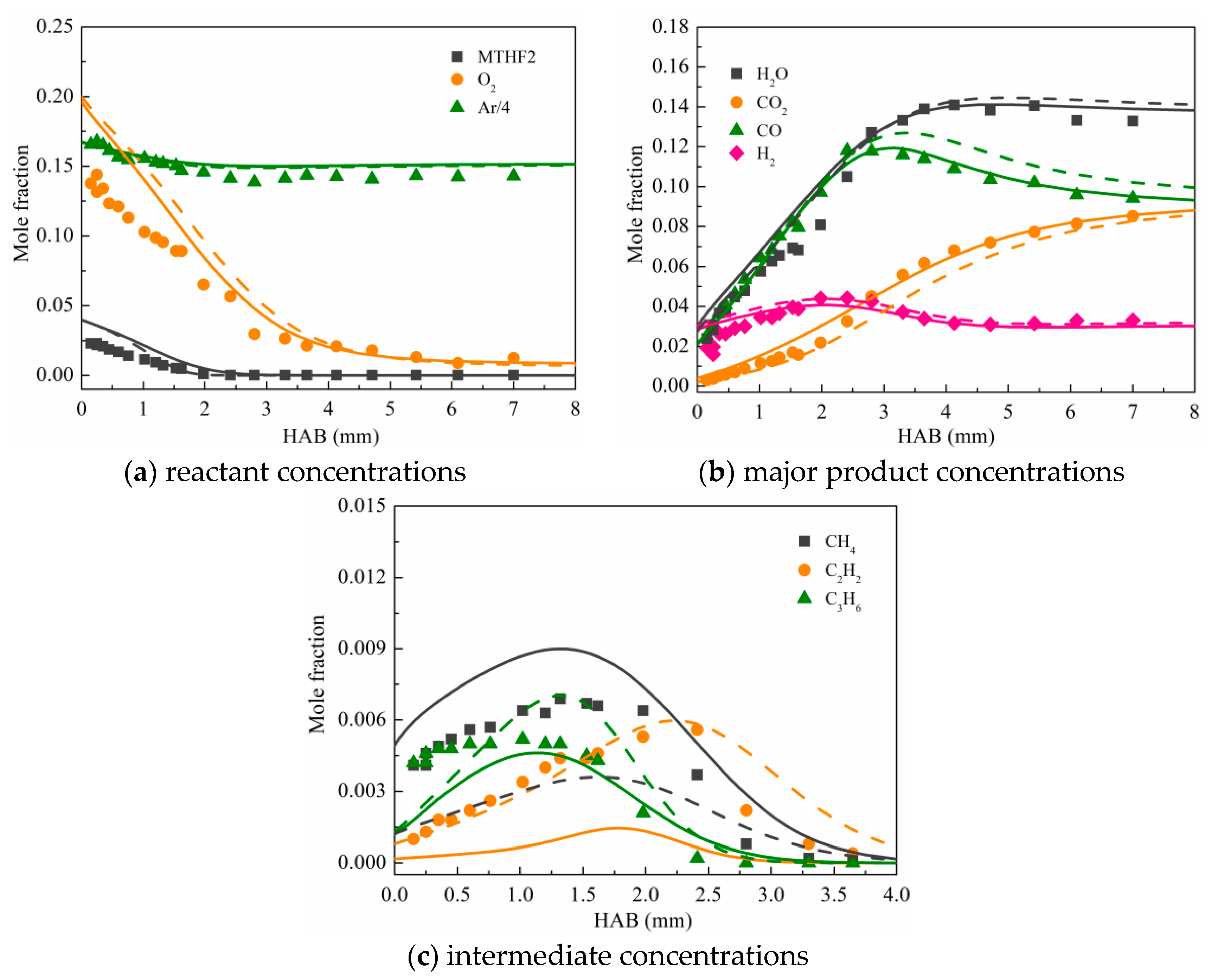
3.2. Validation against Species Concentration Profiles
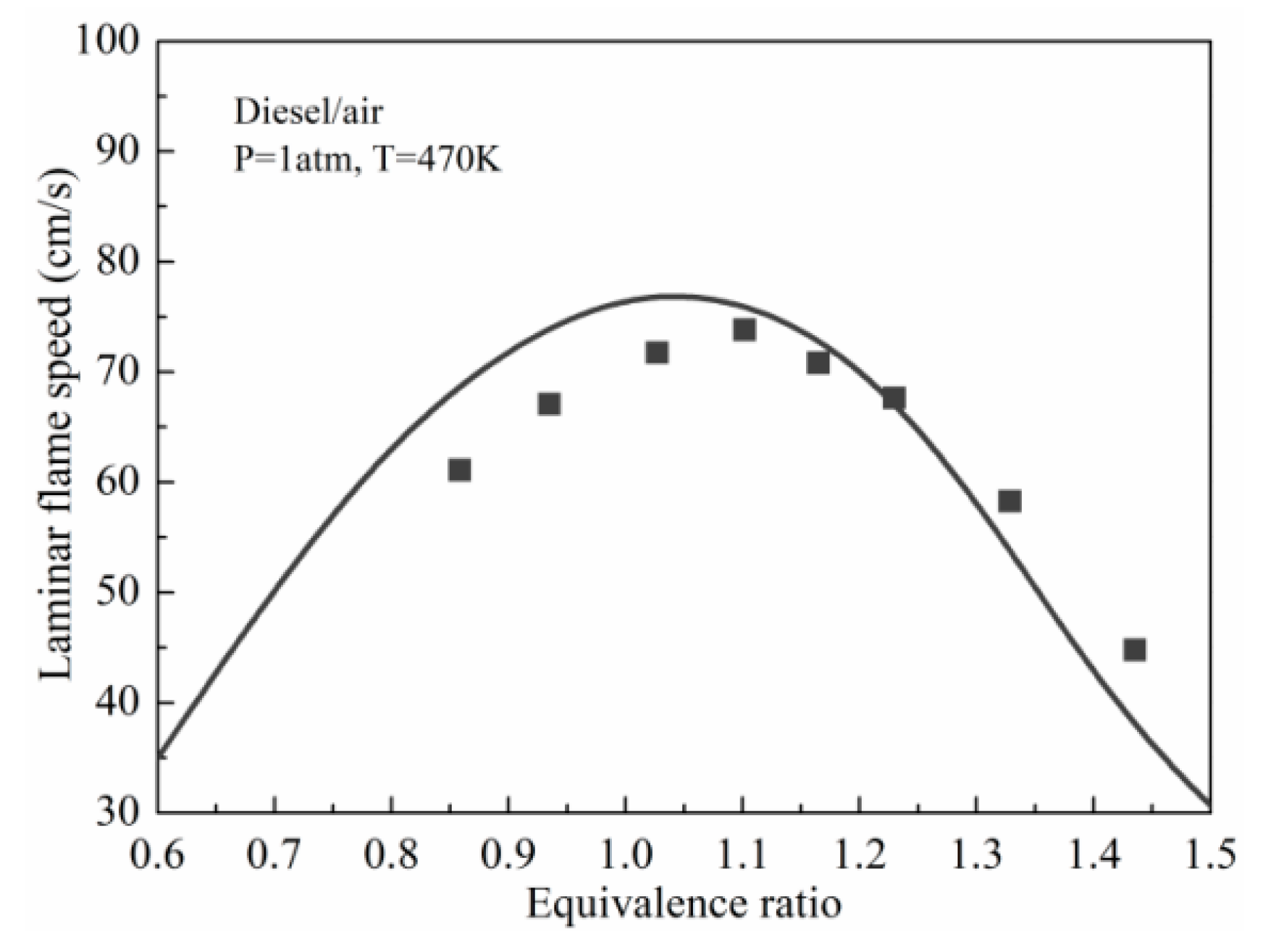
3.3. Validation against Laminar Flame Speeds
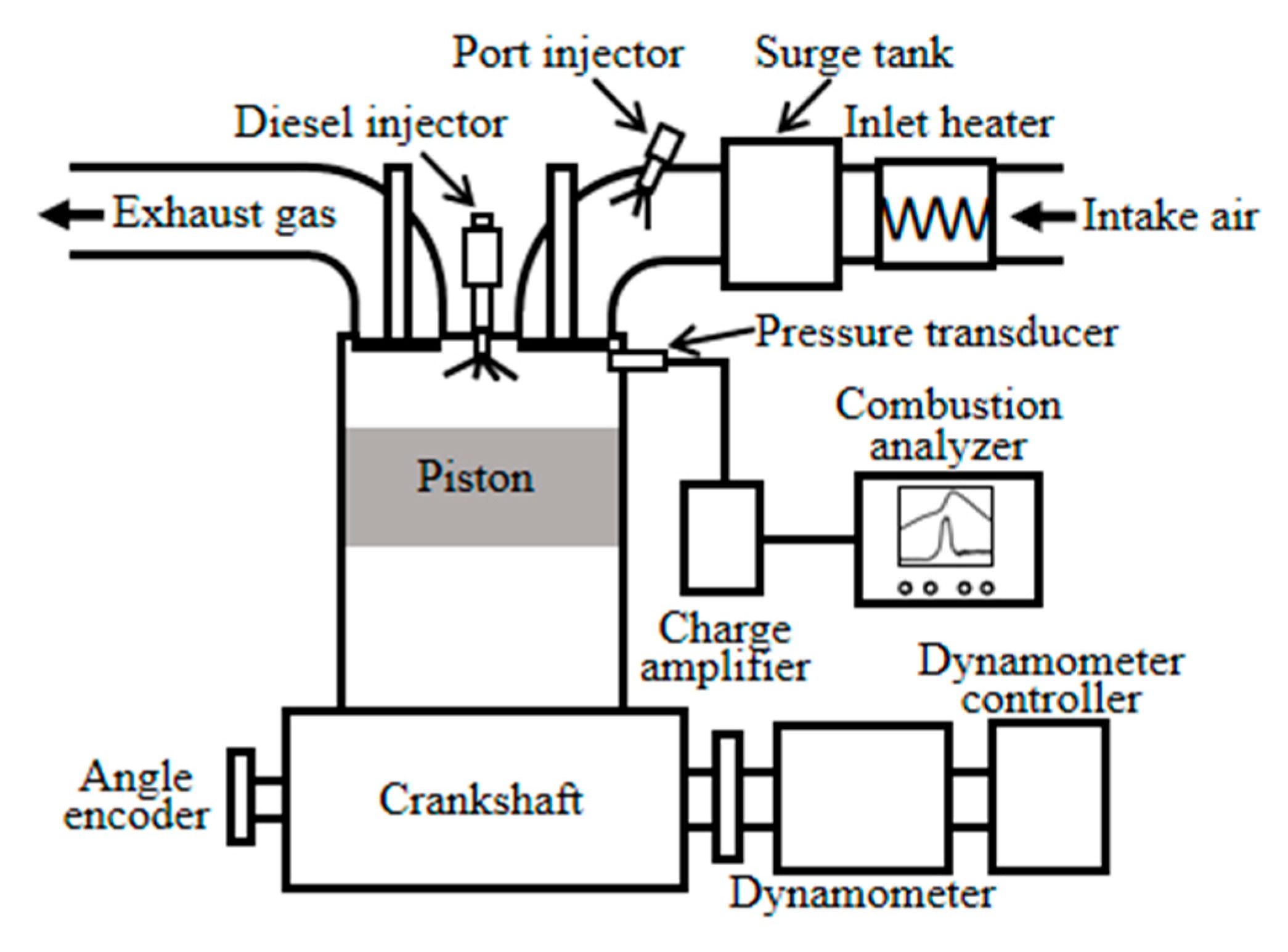
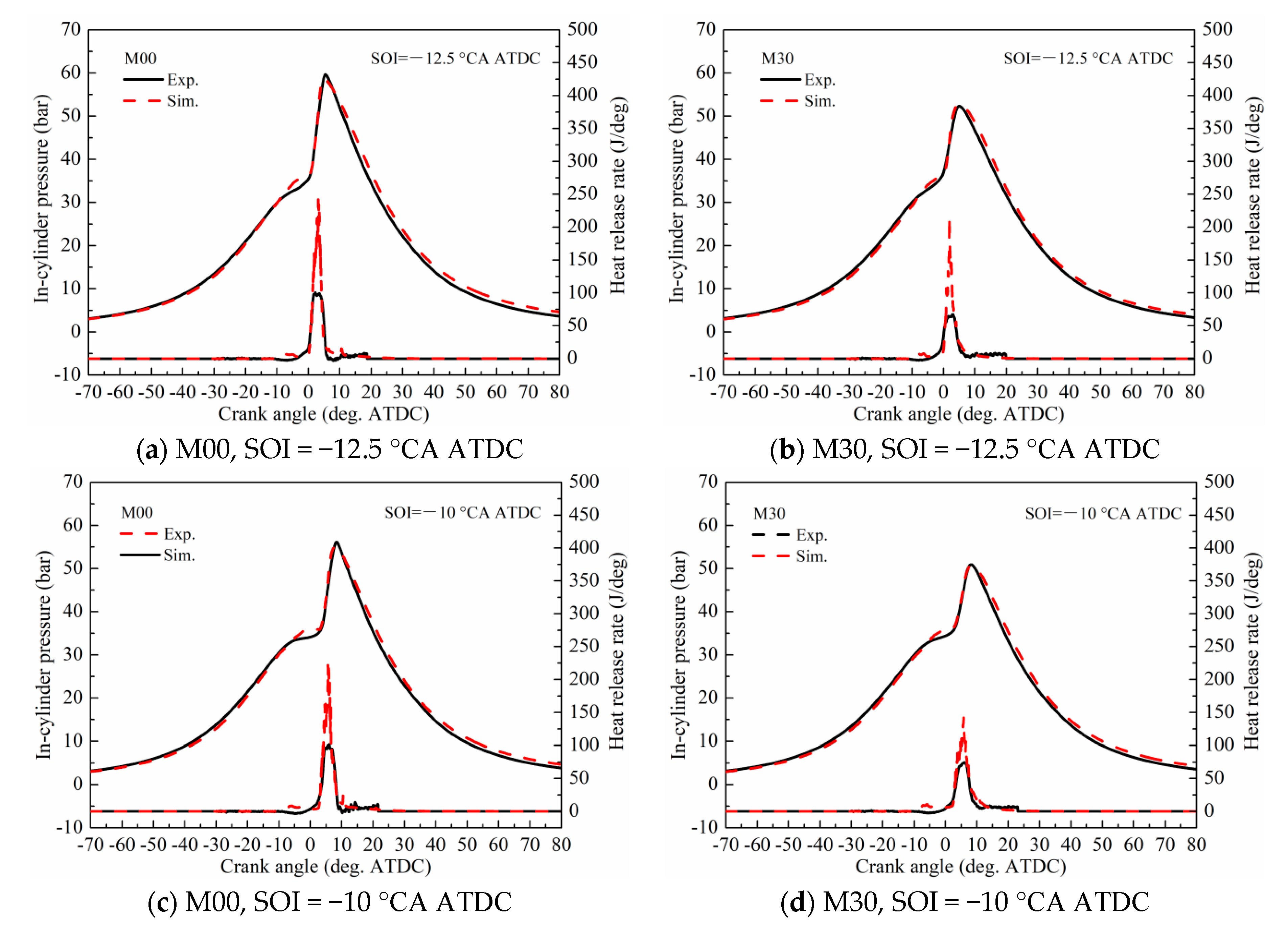
3.4. Validation against the Dual Fuel Combustion in the Engine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daniel, R.; Wei, L.X.; Xu, H.M.; Wang, C.M.; Wyszynski, M.L.; Shuai, S.J. Speciation of hydrocarbon and carbonyl emissions of 2,5-dimethylfuran combustion in a DISI engine. Energy Fuels 2012, 26, 6661–6668. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Zhang, Z.; Wang, X.; Geng, Z.; Jin, C.; Liu, H.F.; Yao, M.F. Effects of diesel-ethanol-THF blend fuel on the performance and exhaust emissions on a heavy-duty diesel engine. Fuel 2020, 271, 117633. [Google Scholar] [CrossRef]
- Xiao, H.L.; Yang, X.L.; Hou, B.B.; Wang, R.; Xue, Q.; Ju, H.L. Combustion performance and pollutant emissions analysis of a diesel engine fueled with biodiesel and its blend with 2-methylfuran. Fuel 2019, 237, 1050–1056. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wei, L.; Liu, J.; Reitz, R.D.; Yao, M. Development of a reduced toluene reference fuel (TRF)-2,5-dimethylfuran-polycyclic aromatic hydrocarbon (PAH) mechanism for engine applications. Combust. Flame 2016, 165, 453–465. [Google Scholar] [CrossRef]
- Li, S.; Huang, C.; Liu, J.P.; Zhang, T.T. Development of a reduced n-heptane/toluene/tetrahydrofuran mechanism for dual-fuel engine combustion prediction. Fuel 2022, 326, 124914. [Google Scholar] [CrossRef]
- Grochowski, M.R.; Yang, W.; Sen, A. Mechanistic study of a one-step catalytic conversion of fructose to 2,5-dimethyltetrahydrofuran. Chem. A Eur. J. 2012, 18, 12363–12371. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sen, A. One-step catalytic transformation of carbohydrates and cellulosic biomass to 2,5-dimethyltetrahydrofuran for liquid fuels. ChemSusChem 2010, 3, 597–603. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Jiang, Y.; Liew, K.M.; Qiu, R. Laminar burning velocities of 2-methyltetrahydrofuran at elevated pressures. Proc. Combust. Inst. 2021, 38, 2175–2183. [Google Scholar] [CrossRef]
- Rudolph, T.W.; Thomas, J.J. NOx, NMHC and CO emissions from biomass derived gasoline extenders. Biomass 1988, 16, 33–49. [Google Scholar] [CrossRef]
- Lucas, S.V.; Loehr, D.A.; Meyer, M.E.; Thomas, J.J.; Gordon, E.E. Exhaust emissions and field trial results of a new, oxygenated, non-petroleum-based, waste-derived gasoline blending component: 2-methyltetrahydrofuran. In Proceedings of the Fuels and Lubricants Meeting and Exposition, Philadelphia, PA, USA, 18–21 October 1993. [Google Scholar]
- Thewes, M.; Muther, M.; Brassat, A.; Pischinger, S.; Sehr, A. Analysis of the effect of biofuels on the combustion in a fownsized DI SI Engine. SAE Int. J. Fuels Lubr. 2011, 5, 274–288. [Google Scholar] [CrossRef]
- Janssen, A.J.; Kremer, F.W.; Baron, J.H.; Muether, M.; Pischinger, S.; Klankermayer, J. Tailor-Made fuels from biomass for homogeneous low-temperature diesel combustion. Energy Fuels 2011, 25, 4734–4744. [Google Scholar] [CrossRef]
- Hülser, T.; Grünefeld, G.; Brands, T.; Günther, M.; Pischinger, S. Optical investigation on the origin of pre ignition in a highly boosted SI engine using bio-fuels. In Proceedings of the SAE 2013 World Congress & Exhibition, Detroit, MI, USA, 16–18 April 2013. [Google Scholar]
- Moshammer, K.; Vranckx, S.; Chakravarty, H.K.; Parab, P.; Fernandes, R.X.; Kohse-Höinghaus, K. An experimental and kinetic modeling study of 2-methyltetrahydrofuran flames. Combust. Flame 2013, 160, 2729–2743. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Fan, X.; Yang, K. Shock tube experimental and modeling study of MTHF ignition characteristics at high temperatures. In Proceedings of the JSAE/SAE 2015 International Powertrains, Fuels & Lubricants Meeting, Kyoto, Japan, 1–4 September 2015. [Google Scholar]
- De Bruycker, R.; Tran, L.S.; Carstensen, H.H.; Glaude, P.A.; Monge, F.; Alzueta, M.U.; Battin-Leclerc, F.; van Geem, K.M. Experimental and modeling study of the pyrolysis and combustion of 2-methyltetrahydrofuran. Combust. Flame 2017, 176, 409–428. [Google Scholar] [CrossRef]
- Tripathi, R.; Lee, C.; Fernandes, R.X.; Olivier, H.; Curran, H.J.; Sarathy, S.M.; Pitsch, H. Ignition characteristics of 2-methyltetrahydrofuran: An experimantal and kinetic study. Proc. Combust. Inst. 2017, 36, 587–595. [Google Scholar] [CrossRef]
- Fenard, Y.; Boumehdi, M.A.; Vanhove, G. Experimental and kinetic modeling study of 2-methyltetrahydrofuran oxidation under engine-relevant conditions. Combust. Flame 2017, 178, 168–181. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Fan, X.; Yang, K.; Zhang, Y. An ignition delay time and kinetic study of 2-methyltetrahydrofuran at high temperatures. Fuel 2016, 186, 758–769. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Yang, K.; Wang, J.; Jiao, X.; Guo, Z. Laminar flame characteristics and chemical kinetics of 2-methyltetrahydrofuran and the effect of blending with iso-octane. Combust. Flame 2018, 191, 213–225. [Google Scholar] [CrossRef]
- Wu, S.H.; Tay, K.L.; Yu, W.B.; Lin, Q.J.; Li, H.; Zhao, F.Y.; Yang, W.M. Development of a highly compact and robust chemical reaction mechanism for the oxidation of tetrahydrofurans under engine relevant conditions. Fuel 2020, 276, 118034. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Li, Y.; Liu, Y.; Xie, M.; Wang, H.; Reltz, R.D. Development of a skeletal mechanism for diesel surrogate fuel by using a decoupling methodology. Combust. Flame 2015, 162, 3785–3802. [Google Scholar] [CrossRef]
- Liu, Y.D.; Jia, M.; Xie, M.Z.; Pang, B. Improvement on a skeletal chemical kinetic model of iso-octane for internal combustion engine by using a practical methodology. Fuel 2013, 103, 884–891. [Google Scholar] [CrossRef]
- Pitz, W.J.; Mueller, C.J. Recent progress in the development of diesel surrogate fuels. Prog. Energy Combust. Sci. 2011, 37, 330–350. [Google Scholar] [CrossRef]
- Pepiot-Desjardins, P.; Pisch, H. An efficient error-propagation-based reduction method for large chemical kinetic mechanisms. Combust. Flame 2008, 154, 67–81. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, J.P.; Meng, W.; Wang, M.Y.; Cao, Y.S.; Cao, S.; Yao, L.B.; Zhang, K.P. Development of a phenomenological soot model integrated with a reduced TRF-PAH mechanism for diesel engine application. Fuel 2021, 283, 118810. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, H.; Zheng, Z.Q.; Liu, J.L.; Reitz, R.D.; Yao, M.F. Development of a combined reduced primary reference fuel-alcohols (methanol/ethanol/propanols/butanols/n-pentanol) mechanism for engine applications. Energy 2016, 114, 542–558. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.F.; Yue, Z.Y.; Jia, M.; Reitz, R.D. A reduced toluene reference fuel chemical kinetic mechanism for combustion and polycyclic-aromatic hydrocarbon predictions. Combust. Flame 2015, 162, 2390–2404. [Google Scholar] [CrossRef]
- Kee, R.J.; Rupley, F.M.; Miller, J.A. CHEMKIN II: A Fortran Chemical Kinetics Package for the Analysis of Gas-Phase Chemical Kinetics; Technical Report; Sandia National Laboratories: Livermore, CA, USA, September 1989. [Google Scholar]
- Amsden, A.A. Kiva-3v: A Block-Structured Kiva Program for Engines with Vertical or Canted Valves; Technical Report; Los Alamos National Lab: Los Alamos, NM, USA, July 1997. [Google Scholar]
- Gowdagiri, S.; Wang, W.; Oehlschlaeger, M.A. A shock tube ignition delay study of conventional diesel fuel and hydroprocessed renewable diesel fuel from algal oil. Fuel 2014, 128, 21–29. [Google Scholar] [CrossRef]
- Kukkadapu, G.; Sung, C.J. Autoignition study of ULSD#2 and FD9A diesel blends. Combust. Flame 2016, 166, 45–54. [Google Scholar]
- Wang, S.X.; Mao, Y.B.; Raza, M.; Yu, L.; Lu, X.C. Autoignition of diesel/oxygen/nitrogen mixture under elevated temperature in a heated shock tube. Fuel 2019, 254, 115635. [Google Scholar] [CrossRef]
- Yu, L.; Mao, Y.B.; Qiu, Y.; Wang, S.X.; Li, H.; Tao, W.C.; Qian, Y.; Lu, X.C. Experimental and modeling study of the autoignition characteristics of commercial diesel under engine-relevant conditions. Proc. Combust. Inst. 2019, 37, 4805–4812. [Google Scholar] [CrossRef]
- Villano, S.M.; Huynh, L.K.; Carstensen, H.H.; Dean, A.M. High-pressure rate rules for Alkyl+O2 reactions. 1. the dissociation, concerted elimination, and isomerization channels of the alkyl peroxy radical. J. Phys. Chem. A 2015, 115, 13425–13442. [Google Scholar] [CrossRef]
- Mati, K.; Ristori, A.; Gaiel, S.; Pengloan, G.; Dagaut, P. The oxidation of a diesel fuel at 1–10 atm: Experimental study in a JSR and detailed chemical kinetic modeling. Proc. Combust. Inst. 2007, 31, 2939–2946. [Google Scholar] [CrossRef]
- Bai, Y.Q.; Wang, Y.; Wang, X.C.; Wang, P. Development of a skeletal mechanism for tri-component diesel surrogate fuel: N-hexadecane/iso-cetane/1-methylnaphthalene. Fuel 2020, 259, 116217. [Google Scholar] [CrossRef]
- Chong, C.T.; Hochgreb, S. Measurements of laminar flame speeds of liquid fuels: JetA1, diesel, palm methyl esters and blends using particle imaging velocimetry (PIV). Proc. Combust. Inst. 2011, 33, 979–986. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Li, Y.; Zhang, Y.; Xie, M.; Wang, H.; Reitz, R.D. Development of a skeletal oxidation mechanism for biodiesel surrogate. Proc. Combust. Inst. 2015, 35, 3037–3044. [Google Scholar] [CrossRef]
- Wang, P.; Jia, M.; Zhang, Y.; Xu, G.; Chang, Y.; Xu, Z. Development of a decoupling physical-chemical surrogate (DPCS) model for simulation of the spray and combustion of multi-component biodiesel fuels. Fuel 2019, 240, 16–30. [Google Scholar] [CrossRef]
- Han, Z.; Reitz, R.D. Turbulence modeling of internal combustion engines using RNG k-ε; models. Combust. Sci. Technol. 1995, 106, 267–295. [Google Scholar] [CrossRef]
- Han, Z.; Reitz, R.D. A temperature wall function formulation for variable-density turbulent flows with application to engine convective heat transfer modeling. Int. J. Heat Mass Transf. 1997, 40, 613–625. [Google Scholar] [CrossRef]
- Ricart, L.M.; Reltz, R.D.; Dec, J.E. Comparisons of diesel spray liquid penetration and vapor fuel distributions with in-cylinder optical measurements. J. Eng. Gas Turbines Power 2000, 122, 588–595. [Google Scholar] [CrossRef]
- Nordin, N. Complex Chemistry Modeling of Diesel Spray Combustion. Ph.D. Thesis, Chalmers University of Technology, Goteborg, Sweden, 2001. [Google Scholar]
- Han, Z.Y.; Xu, Z.; Trigui, N. Spray/wall interaction models for multidimensional engine simulation. Int. J. Engine Res. 2000, 1, 127–146. [Google Scholar] [CrossRef]






| Reactions | A (cm, mol, s) | β | E (cal/mol) | Ref. | |
|---|---|---|---|---|---|
| R1 | MTHF2 + OH = MTHF25J + H2O | 2.400 × 106 | 2.00 | −2630 | [17] |
| R2 | MTHF2 + H = MTHF25J + H2 | 5.800 × 104 | 2.49 | 2330 | This work |
| R3 | MTHF2 + HO2 = MTHF25J + H2O2 | 2.800 × 105 | 2.69 | 15,348 | This work |
| R4 | MTHF2 + O2 = MTHF25J + HO2 | 2.865 × 1013 | 0.00 | 47,640 | [17] |
| R5 | MTHF25J + O2 = MTHF25OOJ | 6.046 × 1014 | −0.92 | −130 | [17] |
| R6 | MTHF25OOJ = MTHF25OOH2J | 3.080 × 106 | 1.53 | 14,622 | [17] |
| R7 | MTHF25OOH2J + O2 = MTHF25OOH2O2 | 2.464 × 1010 | 0.40 | −800 | [17] |
| R8 | MTHF25OOH2O2 = MTHF22OOH5KET + OH | 3.616 × 1010 | 0.13 | 14,470 | [17] |
| R9 | MTHF22OOH5KET => CH3CO + C2H4 + CO2 + OH | 4.494 × 1013 | 0.00 | 41,600 | This work |
| R10 | MTHF25OOH2J => MTHF2O2-5 + OH | 6.520 × 109 | 0.10 | 9330 | [17] |
| R11 | MTHF2O2-5 + OH => H2O + CH3 + 2CH2CO | 6.064 × 109 | 0.00 | 0 | This work |
| R12 | MTHF25J = O*CCCCJC | 5.000 × 1012 | 0.00 | 21,821 | This work |
| R13 | O*CCCCJC = C3H6 + CH2CHO | 5.000 × 1013 | 0.00 | 19,789 | [17] |
| Parameter | Value |
|---|---|
| Number of valves | 4 |
| Bore × Stroke (mm × mm) | 96 × 115 |
| Piston type | flat |
| Engine speed (rpm) | 1000 |
| Intake temperature (K) | 338 |
| Intake pressure (bar) | 1.04 |
| Diesel fuel injector | Bosch, 9 holes, 60MPa fuel injection pressure |
| Diesel injection timing (CA ATDC) | −12.5°, −10° |
| Port fuel injector | Delphi, 6 holes, 0.5MPa fuel injection pressure |
| Port fuel injection timing (CA ATDC) | −300° |
| EGR ratio (%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Huang, C.; Yang, C.; Yu, W.; Liu, J.; Zhang, T. A Reduced Reaction Mechanism for Diesel/2-Methyltetrahydrofuran Dual-Fuel Engine Application. Energies 2022, 15, 7677. https://doi.org/10.3390/en15207677
Li S, Huang C, Yang C, Yu W, Liu J, Zhang T. A Reduced Reaction Mechanism for Diesel/2-Methyltetrahydrofuran Dual-Fuel Engine Application. Energies. 2022; 15(20):7677. https://doi.org/10.3390/en15207677
Chicago/Turabian StyleLi, Song, Chen Huang, Chen Yang, Wenbin Yu, Jinping Liu, and Tingting Zhang. 2022. "A Reduced Reaction Mechanism for Diesel/2-Methyltetrahydrofuran Dual-Fuel Engine Application" Energies 15, no. 20: 7677. https://doi.org/10.3390/en15207677
APA StyleLi, S., Huang, C., Yang, C., Yu, W., Liu, J., & Zhang, T. (2022). A Reduced Reaction Mechanism for Diesel/2-Methyltetrahydrofuran Dual-Fuel Engine Application. Energies, 15(20), 7677. https://doi.org/10.3390/en15207677






