Double Cathode Modification Improves Charge Transport and Stability of Organic Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Device Performance
3.2. Characterization of Opto-Electronic Properties
3.3. Improving Device Stability via SnO2/ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, P.; Yan, C.; Wu, Y.; Wang, Y.; Qin, M.; An, Q.; Cao, J.; Huo, L.; Zhang, F.; Ding, L.; et al. Alloy Acceptor: Superior Alternative to PCBM toward Efficient and Stable Organic Solar Cells. Adv. Mater. 2016, 28, 8021–8028. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-Junction Organic Photovoltaic Cell with 19% Efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Sun, L.; Chen, H.; Liu, Y.; Lu, X.; Wang, W.; Liu, T.; Dong, X.; Jiang, P.; Jiang, Y.; et al. 54 cm2 Large-Area Flexible Organic Solar Modules with Efficiency Above 13%. Adv. Mater. 2021, 33, 2103017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Xie, R.; Wang, Z.; Zhong, W.; Li, Y.; Huang, F.; Cao, Y. A Rational Design and Synthesis of Cross-Conjugated Small Molecule Acceptors Approaching High-Performance Fullerene-Free Polymer Solar Cells. Chem. Mater. 2018, 30, 4331–4342. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, L.; Zhang, J.; Qin, S.; Lai, W.; Qiu, B.; Yuan, J.; Wan, Y.; Huang, W.; Li, Y. A Quinoxaline-Based D-A Copolymer Donor Achieving 17.62% Efficiency of Organic Solar Cells. Adv. Mater. 2021, 33, 2100474. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.; Lau, T.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Zhan, L.; Li, S.; Xia, X.; Li, Y.; Lu, X.; Zuo, L.; Shi, M.; Chen, H. Layer-by-Layer Processed Ternary Organic Photovoltaics with Efficiency over 18%. Adv. Mater. 2021, 33, 2007231. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Yao, H.; Cui, Y.; Bi, P.; Zhang, T.; Cheng, Y.; Zu, Y.; Qin, J.; Yu, R.; Ge, Z.; et al. 18.5% Efficiency Organic Solar Cells with a Hybrid Planar/Bulk Heterojunction. Adv. Mater. 2021, 33, 2103091. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Z.; Zu, Y.; Wang, Y.; Liu, X.; Zhang, S.; Zhang, M.; Hou, J. A Tandem Organic Photovoltaic Cell with 19.6% Efficiency Enabled by Light Distribution Control. Adv. Mater. 2021, 33, 2102787. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L.; Hao, T.; Zhou, G.; Qiu, C.; Zhao, Z.; Hartmann, N.; Xiao, B.; Zou, Y.; Feng, W.; et al. High-Efficiency Organic Photovoltaics using Eutectic Acceptor Fibrils to Achieve Current Amplification. Adv. Mater. 2021, 33, 2007177. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.J.; Szarko, J.M.; Xu, T.; Yu, L.; Marks, T.J.; Chen, L.X. Effects of additives on the morphology of solution phase aggregates formed by active layer components of high-efficiency organic solar cells. J. Am. Chem. Soc. 2011, 133, 20661–20663. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Li, X.; Zhang, Y.; Xu, D.; Geng, A.; Zhao, J.; Chen, X. Performance improvement of polymer solar cells with binary additives induced morphology optimization and interface modification simultaneously. Sol. Energy 2020, 201, 330–338. [Google Scholar] [CrossRef]
- Cai, C.; Yao, J.; Chen, L.; Yuan, Z.; Zhang, Z.; Hu, Y.; Zhao, X.; Zhang, Y.; Chen, Y.; Li, Y. Silicon Naphthalocyanine Tetraimides: Cathode Interlayer Materials for Highly Efficient Organic Solar Cells. Angew. Chem. Int. Ed. 2021, 60, 19053–19057. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Liao, C.; Deng, M.; Xu, X.; Yu, L.; Peng, Q. 18.77% Efficiency Organic Solar Cells Promoted by Aqueous Solution Processed Cobalt(II) Acetate Hole Transporting Layer. Angew. Chem. Int. Ed. 2021, 60, 22554–22561. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, S.; Qu, Y.; Gao, X.; Han, L.; Lin, Z.; Yang, L.; Wang, W.; Zheng, N.; Liang, Y.; et al. Nanographene-Osmapentalyne Complexes as a Cathode Interlayer in Organic Solar Cells Enhance Efficiency over 18%. Adv. Mater. 2021, 33, 2101279. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, Y.; Du, D.; Ouyang, J. PEDOT:PSS Films with Metallic Conductivity through a Treatment with Common Organic Solutions of Organic Salts and Their Application as a Transparent Electrode of Polymer Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 11629–11638. [Google Scholar] [CrossRef]
- Girotto, C.; Voroshazi, E.; Cheyns, D.; Heremans, P.; Rand, B.P. Solution-processed MoO3 thin films as a hole-injection layer for organic solar cells. ACS Appl. Mater. Interfaces 2011, 3, 3244–3247. [Google Scholar] [CrossRef]
- Aqoma, H.; Park, S.; Park, H.; Hadmojo, W.T.; Oh, S.; Nho, S.; Kim, D.; Seo, J.; Park, S.; Ryu, D.Y.; et al. 11% Organic Photovoltaic Devices Based on PTB7-Th: PC71BM Photoactive Layers and Irradiation-Assisted ZnO Electron Transport Layers. Adv. Sci. 2018, 5, 1700858. [Google Scholar] [CrossRef]
- Wen, X.; Fang, S.; Xu, Y.; Zheng, N.; Liu, L.; Xie, Z.; Würthner, F. Enhanced Electron Transportation by Dye Doping in Very Low-Temperature (<130 °C)-Processed Sol-Gel ZnO toward Flexible Organic Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 34151–34157. [Google Scholar] [CrossRef]
- You, J.; Chen, C.; Dou, L.; Murase, S.; Duan, H.; Hawks, S.A.; Xu, T.; Son, H.; Yu, L.; Li, G.; et al. Metal oxide nanoparticles as an electron-transport layer in high-performance and stable inverted polymer solar cells. Adv. Mater. 2012, 24, 5267–5272. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J.; Shaheen, S.E.; Winder, C.; Sariciftci, N.S. Effect of LiF/metal electrodes on the performance of plastic solar cells. Appl. Phys. Lett. 2002, 80, 1288–1290. [Google Scholar] [CrossRef]
- Yu, S.; Yang, W.; Li, L.; Zhang, W. Improved Chemical Stability of ITO Transparent Anodes with a SnO2 Buffer Layer for Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 144, 652–656. [Google Scholar] [CrossRef]
- Lee, E.; Heo, S.; Han, Y.; Moon, D. An organic-inorganic hybrid interlayer for improved electron extraction in inverted polymer solar cells. J. Mater. Chem. C 2016, 4, 2463–2469. [Google Scholar] [CrossRef]
- He, R.; Yu, L.; Cai, P.; Peng, F.; Xu, J.; Ying, L.; Chen, J.; Yang, W.; Cao, Y. Narrow-Band-Gap Conjugated Polymers Based on 2,7-Dioctyl-Substituted Dibenzo[a,c]phenazine Derivatives for Polymer Solar Cells. Macromolecules 2014, 47, 2921–2928. [Google Scholar] [CrossRef]
- Xiong, S.; Hu, L.; Hu, L.; Sun, L.; Qin, F.; Liu, X.; Fahlman, M.; Zhou, Y. 12.5% Flexible Nonfullerene Solar Cells by Passivating the Chemical Interaction Between the Active Layer and Polymer Interfacial Layer. Adv. Mater. 2019, 31, 1806616. [Google Scholar] [CrossRef]
- Pan, F.; Sun, C.; Li, Y.; Tang, D.; Zou, Y.; Li, X.; Bai, S.; Wei, X.; Lv, M.; Chen, X.; et al. Solution-Processable n-Doped Graphene-Containing Cathode Interfacial Materials for High-Performance Organic Solar Cells. Energy Environ. Sci. 2019, 12, 3400–3411. [Google Scholar] [CrossRef]
- Zhang, Z.; Qi, B.; Jin, Z.; Chi, D.; Qi, Z.; Li, Y.; Wang, J. Perylene Diimides: A Thickness-Insensitive Cathode Interlayer for High Performance Polymer Solar Cells. Energy Environ. Sci. 2014, 7, 1966–1973. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, Y.; Chen, X.; Zhang, Z.; Li, Y. Design of a Thiophene-fused Benzotriazole Unit as an Electron Acceptor to Build D-A Copolymers for Polymer Solar Cells. J. Mater. Chem. C 2017, 5, 2951–2957. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Tao, Y.; Fan, C.; Xue, L.; Zhang, Z.; Li, H.; Li, Y.; Huang, W. Random Terpolymer with a Cost-effective Monomer and Comparable Efficiency to PTB7-Th for Bulk-heterojunction Polymer Solar Cells. Polym. Chem. 2016, 7, 926–932. [Google Scholar] [CrossRef]
- Uddin, A.; Upama, M.B.; Yi, H.; Duan, L. Encapsulation of Organic and Perovskite Solar Cells: A Review. Coatings 2019, 9, 65. [Google Scholar] [CrossRef]
- Burlingame, Q.; Ball, M.; Loo, Y. It’s time to focus on organic solar cell stability. Nat. Energy 2020, 5, 947–949. [Google Scholar] [CrossRef]
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. [Google Scholar] [CrossRef]
- Hains, A.W.; Liu, J.; Martinson, A.B.F.; Irwin, M.D.; Marks, T.J. Anode Interfacial Tuning via Electron-Blocking/Hole-Transport Layers and Indium Tin Oxide Surface Treatment in Bulk-Heterojunction Organic Photovoltaic Cells. Adv. Funct. Mater. 2010, 20, 595–606. [Google Scholar] [CrossRef]
- Son, H.; Kim, S.; Kim, D. Critical Impact of Hole Transporting Layers and Back Electrode on the Stability of Flexible Organic Photovoltaic Module. Adv. Energy Mater. 2017, 7, 1601289. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Kwon, H.; Yang, W.; Hwang, H.; Lee, H.; Lee, E.; Ma, S.; Moon, J. La2O3 interface modification of mesoporous TiO2 nanostructures enabling highly efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 15478–15485. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Kwon, H.; Yang, W.; Mane, R.S.; Moon, J. Performance enhancement of mesoporous TiO2-based perovskite solar cells by ZnS ultrathin-interfacial modification layer. J. Alloys Compd. 2018, 738, 405–414. [Google Scholar] [CrossRef]
- Khan, U.; Iqbal, T.; Khan, M.; Wu, R. SnO2/ZnO as double electron transport layer for halide perovskite solar cells. Sol. Energy 2021, 223, 346–350. [Google Scholar] [CrossRef]
- Fan, Q.; Zhu, Q.; Xu, Z.; Su, W.; Chen, J.; Wu, J.; Guo, X.; Ma, W.; Zhang, M.; Li, Y. Chlorine substituted 2D-conjugated polymer for high-performance polymer solar cells with 13.1% efficiency via toluene processing. Nano Energy 2018, 48, 413–420. [Google Scholar] [CrossRef]
- Pan, M.; Lau, T.; Tang, Y.; Wu, Y.; Liu, T.; Li, K.; Chen, M.; Lu, X.; Ma, W.; Zhan, C. 16.7%-efficiency ternary blended organic photovoltaic cells with PCBM as the acceptor additive to increase the open-circuit voltage and phase purity. J. Mater. Chem. A 2019, 7, 20713–20722. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Yu, J.; Wang, H.; Zhang, M.; Yang, L.; Geng, R.; Cao, J.; Du, F.; Liu, F.; et al. Enhancing phase separation with a conformation-locked nonfullerene acceptor for over 14.4% efficiency solar cells. J. Mater. Chem. C 2019, 7, 13279–13286. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, B.; Lu, J.; Cheng, M.; Jeng, R.; Lu, Y.; Chen, C.; Wong, K. A Near-Infrared Absorption Small Molecule Acceptor for High-Performance Semitransparent and Colorful Binary and Ternary Organic Photovoltaics. ChemSusChem 2020, 13, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, L.; Jiang, H.; Yuan, D.; Wang, Q.; Cao, Y.; Chen, J. Investigation of halogen-free solvents towards high-performance additive-free non-fullerene organic solar cells. Org. Electron. 2020, 85, 105871. [Google Scholar] [CrossRef]
- Dai, T.; Li, X.; Zhang, Y.; Zhou, X.; Zhu, Y.; Zhou, J.; Xu, D.; Lin, T. Electric-Induced Degradation of Cathode Interface Layer in PM7:IT-4F Polymer Solar Cells. Sol. RRL 2021, 5, 2100151. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, T.; Li, X.; Yan, Y.; Xiong, W.; Lin, T.; Zhou, J.; Xu, D.; Zhu, Y.; Zhao, J.; et al. Improved Charge Transport and Reduced Carrier Recombination of Nonfullerene Organic Solar Cells with the Binary Solvent. ACS Appl. Energy Mater. 2021, 4, 8175–8182. [Google Scholar] [CrossRef]
- Tran, V.; Eom, S.; Yoon, S.; Kim, S.; Lee, S. Enhancing device performance of inverted organic solar cells with SnO2/Cs2CO3 as dual electron transport layers. Org. Electron. 2019, 68, 85–95. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, W.; Shou, C.; Yan, J.; Li, N.; Yang, Z.; Song, W.; Yan, B.; Sheng, J.; Ye, J. SnO2 surface defects tuned by (NH4)2S for high-efficiency perovskite solar cells. Sol. Energy 2019, 194, 541–547. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, Y.; Wang, X.; Zhao, Y.; Tian, J. Defect engineering on SnO2 nanomaterials for enhanced gas sensing performances. Adv. Powder Mater. 2022, 1, 100033. [Google Scholar] [CrossRef]
- An, Q.; Ma, X.; Gao, J.; Zhang, F. Solvent additive-free ternary polymer solar cells with 16.27% efficiency. Sci. Bull. 2019, 64, 504–506. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Jiang, X.; Gao, K.; Liu, F.; Gong, X.; Chen, J.; Cao, Y. Using o-Chlorobenzaldehyde as a Fast Removable Solvent Additive during Spin-Coating PTB7-Based Active Layers: High Efficiency Thick-Film Polymer Solar Cells. Adv. Energy Mater. 2017, 7, 1601344. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, Z.; Zhang, G.; Li, Q.; Yin, Q.; Jiang, X.; Huang, F.; Xu, Y.; Yip, H.; Cao, Y. Efficient device engineering for inverted non-fullerene organic solar cells with low energy loss. J. Mater. Chem. C 2018, 6, 4457–4463. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device Physics of Polymer:Fullerene Bulk Heterojunction Solar Cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Li, Z.; Gao, F.; Greenham, N.C.; McNeill, C.R. Comparison of the Operation of Polymer/Fullerene, Polymer/Polymer, and Polymer/Nanocrystal Solar Cells: A Transient Photocurrent and Photovoltage Study. Adv. Funct. Mater. 2011, 21, 1419–1431. [Google Scholar] [CrossRef]
- Chatri, A.R.; Torabi, S.; Corre, V.M.L.; Koster, L.J.A. Impact of Electrodes on Recombination in Bulk Heterojunction Organic Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 12013–12020. [Google Scholar] [CrossRef]
- Soci, C.; Moses, D.; Xu, Q.; Heeger, A.J. Charge-carrier Relaxation Dynamics in Highly Ordered Poly(p-phenylene vinylene): Effects of Carrier Bimolecular Recombination and Trapping. Phys. Rev. B 2005, 72, 245204. [Google Scholar] [CrossRef]
- Stephen, M.; Genevičius, K.; Juška, G.; Arlauskas, K.; Hiorns, R.C. Charge transport and its characterization using photo-CELIV in bulk heterojunction solar cells. Polym. Int. 2017, 66, 13–25. [Google Scholar] [CrossRef]
- Cowan, S.R.; Roy, A.; Heeger, A.J. Recombination in polymer-fullerene bulk heterojunction solar cells. Phys. Rev. B 2010, 82, 245207. [Google Scholar] [CrossRef]
- Huang, S.; Tang, Y.; Yu, A.; Wang, Y.; Shen, S.; Kang, B.; Silva, S.R.P.; Lu, G. Solution-processed SnO2 Nanoparticle Interfacial Layers for Efficient Electron Transport in ZnO-based Polymer Solar Cells. Org. Electron. 2018, 62, 373–381. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q.; Chen, S.; Cai, D. Interface Control of Semiconducting Metal Oxide Layers for Efficient and Stable Inverted Polymer Solar Cells with Open-Circuit Voltages over 1.0 Volt. ACS Appl. Mater. Interfaces 2013, 5, 9015–9025. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, O.J.; Nyman, M. Charge extraction by a linearly increasing voltage of photo-generated carriers: The influence of two mobile carrier types, bimolecular recombination, and series resistance. Org. Electron. 2019, 64, 97–103. [Google Scholar] [CrossRef]
- Neukom, M.; Züfle, S.; Jenatsch, S.; Ruhstaller, B. Opto-electronic characterization of third-generation solar cells. Sci. Technol. Adv. Mater. 2018, 19, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Singh, R.; Lee, J.; Sin, D.; Lee, H.; Cho, K. Propeller-shaped small molecule acceptors containing a 9,9′-spirobifluorene core with imide-linked perylene diimides for non-fullerene organic solar cells. J. Mater. Chem. C 2016, 4, 10610–10615. [Google Scholar] [CrossRef]
- Dkhil, S.B.; Pfannmöller, M.; Schröder, R.R.; Alkarsifi, R.; Gaceur, M.; Köntges, W.; Heidari, H.; Bals, S.; Margeat, O.; Ackermann, J.; et al. Interplay of Interfacial Layers and Blend Composition To Reduce Thermal Degradation of Polymer Solar Cells at High Temperature. ACS Appl. Mater. Interfaces 2018, 10, 3874–3884. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, L.; Jiang, F.; Xie, C.; Hu, L.; Dong, X.; Qin, F.; Liu, T.; Hu, L.; Jiang, X.; et al. Photocatalytic effect of ZnO on the stability of nonfullerene acceptors and its mitigation by SnO2 for nonfullerene organic solar cells. Mater. Horiz. 2019, 6, 1438–1443. [Google Scholar] [CrossRef]
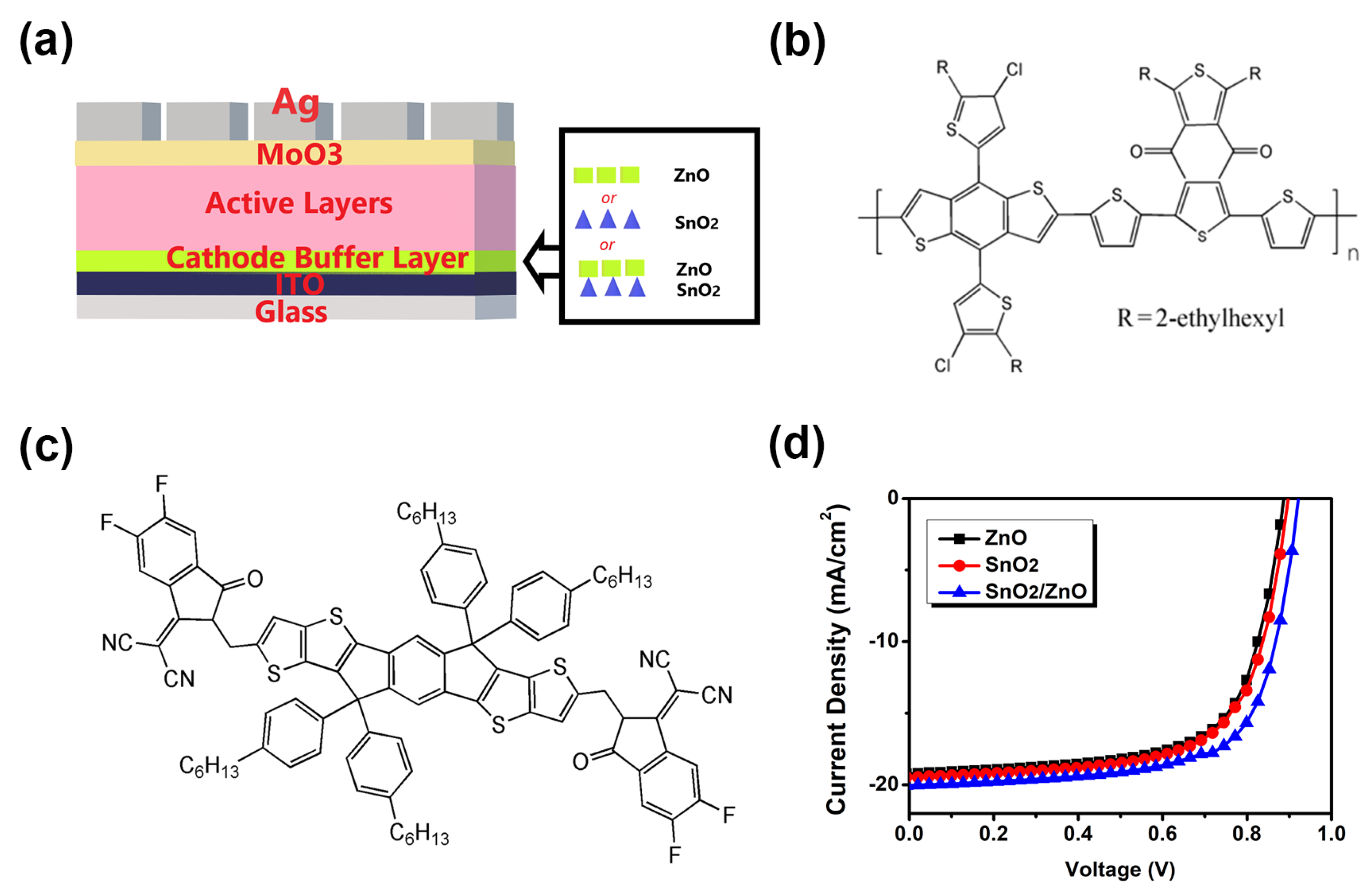
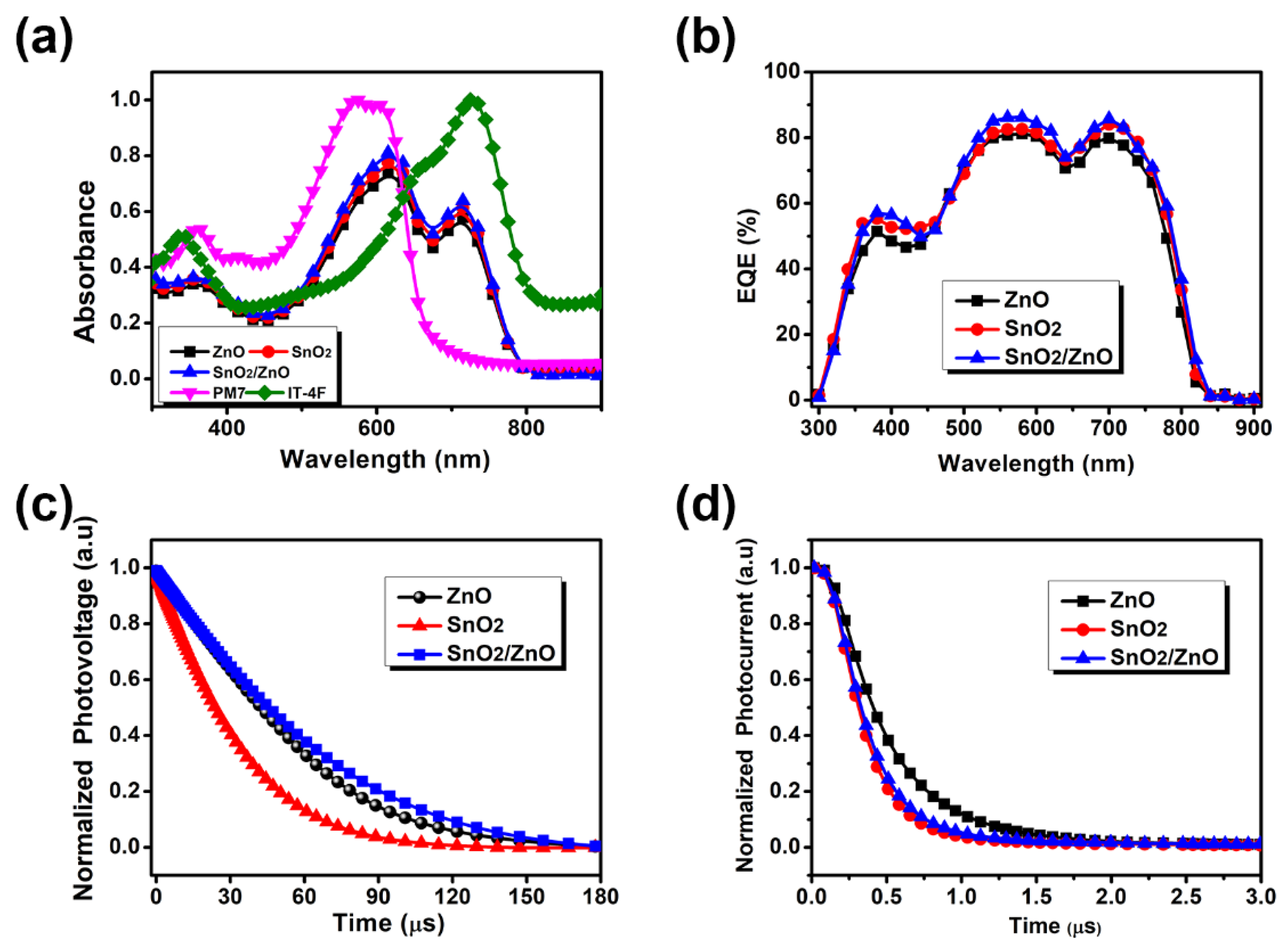

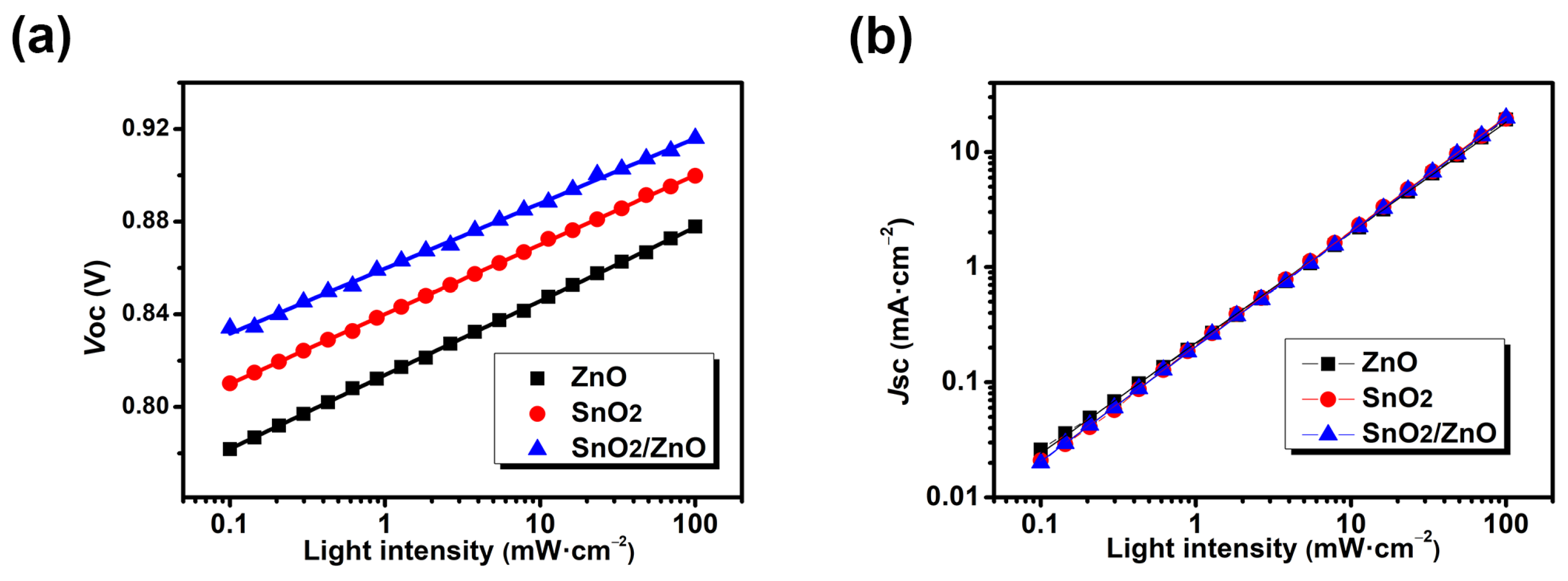
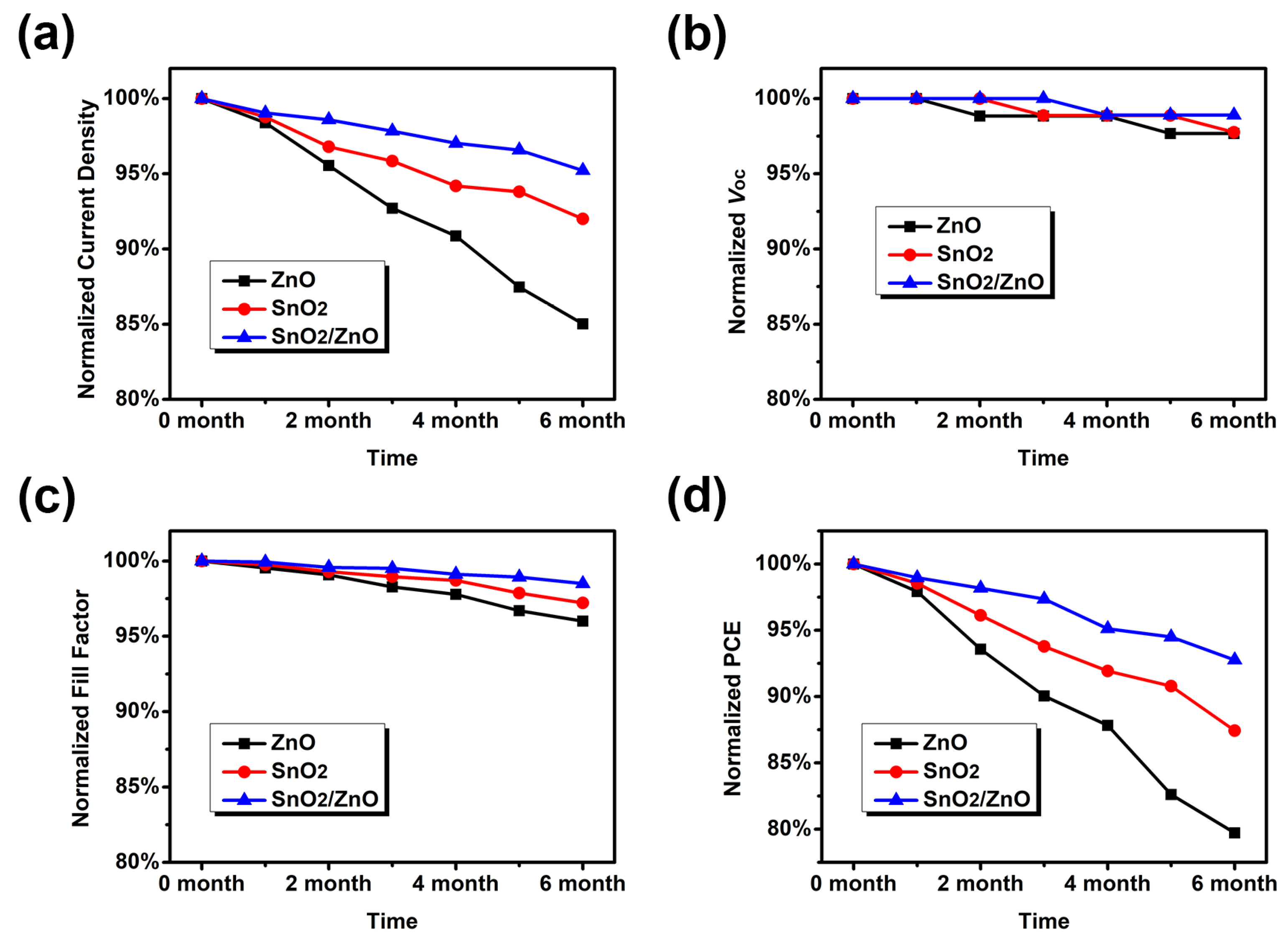

| Jsc (mA/cm2) | Voc (V) | FF | PCE (%) | |
|---|---|---|---|---|
| ZnO | 19.22 a (19.06 b ± 0.15) | 0.88 a (0.88 b ± 0.01) | 0.67 a (0.66 b ± 0.01) | 11.45 a (11.42 b ± 0.04) |
| SnO2 | 19.45 a (19.28 b ± 0.12) | 0.90 a (0.90 b ± 0.01) | 0.68 a (0.68 b ± 0.01) | 12.03 a (11.92 b ± 0.12) |
| SnO2/ZnO | 20.04 a (19.72 b ± 0.29) | 0.92 a (0.91 b ± 0.01) | 0.70 a (0.69 b ± 0.01) | 12.91 a (12.66 b ± 0.24) |
| Device Structure | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| ITO/NP-ZnO/PM7:IT-4F/MoO3/Al [39] | 20.9 | 0.88 | 0.71 | 13.1 |
| ITO/PEDOT:PSS/PM7:IT-4F/PDINO/Al [40] | 20.5 | 0.85 | 0.77 | 13.4 |
| ITO/ZnO/PM7:IT-4F/MoO3/Ag [41] | 20.21 | 0.86 | 0.72 | 12.48 |
| ITO/ZnO/PM7:IT-4F/MoO3/Ag [42] | 15.8 | 0.90 | 0.64 | 9.1 |
| ITO/PEDOT:PSS/PM7:IT-4F/PFN-Br/Al [43] | 17.14 | 0.93 | 0.73 | 11.72 |
| ITO/PEDOT:PSS/PM7:IT-4F/PFN-Br/Al [44] | 20.48 | 0.87 | 0.55 | 9.77 |
| ITO/ZnO/PM7:IT-4F/MoO3/Ag [45] | 20.47 | 0.90 | 0.69 | 12.74 |
| This work | 20.04 | 0.92 | 0.70 | 12.91 |
| τTPV (μs) | τTPC (μs) | Carrier Mobility (cm2·V−1·s−1) | |
|---|---|---|---|
| ZnO | 69.18 | 0.45 | 8.10 × 10−5 |
| SnO2 | 34.27 | 0.31 | 9.89 × 10−5 |
| SnO2/ZnO | 77.42 | 0.33 | 1.54 × 10−4 |
| α | β (κT/q) | |
|---|---|---|
| ZnO | 0.9594 | 1.22 |
| SnO2 | 0.9977 | 1.16 |
| SnO2/ZnO | 0.9949 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Dai, T. Double Cathode Modification Improves Charge Transport and Stability of Organic Solar Cells. Energies 2022, 15, 7643. https://doi.org/10.3390/en15207643
Lin T, Dai T. Double Cathode Modification Improves Charge Transport and Stability of Organic Solar Cells. Energies. 2022; 15(20):7643. https://doi.org/10.3390/en15207643
Chicago/Turabian StyleLin, Tao, and Tingting Dai. 2022. "Double Cathode Modification Improves Charge Transport and Stability of Organic Solar Cells" Energies 15, no. 20: 7643. https://doi.org/10.3390/en15207643
APA StyleLin, T., & Dai, T. (2022). Double Cathode Modification Improves Charge Transport and Stability of Organic Solar Cells. Energies, 15(20), 7643. https://doi.org/10.3390/en15207643





