1. Introduction
There are myriad environmental parameters, such as light, pressure, temperature and humidity, that have a significant importance in different applications. Of these, humidity plays a pivotal role, especially in medical, agricultural, food, and other industrial applications. Controlling and monitoring humidity has been attracting increasing interest in various fields, including pharmaceutical processing, food storage, medicine processing, and weather forecasting [
1,
2,
3,
4]. The humidity-sensing mechanism is based on changes in the electrical properties of the sensing film in response to water molecules. The change due to water adsorption can be resistive [
5], capacitive [
6], optical [
7], impeditive [
8], or acoustic [
9]. Generally, humidity sensors are characterized by various essential parameters, including high sensitivity, quick response time, wide detection range, low fabrication cost, simple fabrication methodology, and linearity. A wide range of materials, techniques, and mechanisms have been investigated with respect to their humidity-sensing capabilities. Active sensing layers may include polymers [
10], ceramics [
11], metal oxides [
12], and carbon nanotubes [
13,
14]. Recently, 2D materials with excellent sensing properties have also been explored for humidity-sensing applications [
15,
16].
Different functional materials have been proposed for sensing humidity in the environment. For instance, polymer moisture sensors exhibit excellent sensing qualities. In addition, polymeric sensors possess the advantages of simple fabrication, light weight, and low cost [
17,
18]. Similarly, chitosan, a biocompatible polymer, is considered an ideal humidity-sensing material that can interact with water molecules via hydrogen bonding. Chitosan is a polysaccharide composed of B-(1,4)-linked D-glucosamine residues with a varying number of randomly positioned N-acetyl-glucosamine groups, depending on the mode of preparation [
19,
20]. It has two types of reactive functional groups: an amino group and a hydroxyl group [
21]. Chitosan-based devices have been widely investigated in nanoelectronics as humidity and gas sensors, because they are biocompatible and naturally biodegradable [
22,
23]. In addition, they have excellent film-forming properties and the ability to swell when exposed to water molecules [
24,
25]. This change in the physical structure of chitosan affects both its absorption spectrum and electrical conductivity, which ensures its application as a sensor of relative humidity. Moreover, PVA is a non-toxic and inexpensive hydrophilic semicrystalline polymer with strong film-forming properties. It can boost the humidity-sensing effectiveness of chitosan owing to the additional water adsorption sites. Although an enormous amount of research has been performed on the development of cost-effective and inexpensive materials for robust sensing, most of these sensors rely on external power sources, which limits their application in the case of power failure. Simultaneously, conventional batteries fail to address permanent energy demands because of their heavy weight, limited life span, and potential to cause environmental damage. Therefore, these shortcomings necessitate the establishment of highly desirable self-powered humidity sensors that combine humidity sensing and power generation.
To achieve the self-powered feature in humidity sensors, piezoelectric energy harvesters are promising candidates in a variety of applications [
26,
27,
28,
29]. They harvest different types of mechanical energy, which is the most abundant, eco-friendly, and readily available energy source. To date, several studies have reported self-powered sensors with piezoelectric-based nanogenerators as their power source. For example, Xu et al. reported a self-powered UV sensor with a piezoelectric-based nanogenerator fabricated from ZnO nanowires [
30]. When UV irradiation changes across the sensor, the output voltage of the piezoelectric generator changes with the sensor resistance. Lee et al. investigated the practicability of a self-powered piezoelectric-based sensor for detecting mercury ions in aqueous solutions [
31]. The piezoelectric generator lights up a light emission diode when mercury ions are released in the water solution. In a recent study, Vivekana et. al. then reported a self-powered piezoelectric biopolymer device with average sensitivity in the range of 50–90% RH [
32]. The collagen-nanofibril biopolymer was coated onto a cotton fabric, which simultaneously works as an energy harvester and HS. The reported studies suggest that both the power unit and sensing properties are crucial for the development of high-performance self-powered sensors. Therefore, this suggests that there is a need for the exploration of biocompatible materials that can not only harvest energy for the operation of device, but can also sense the wide range of humidity in the environment.
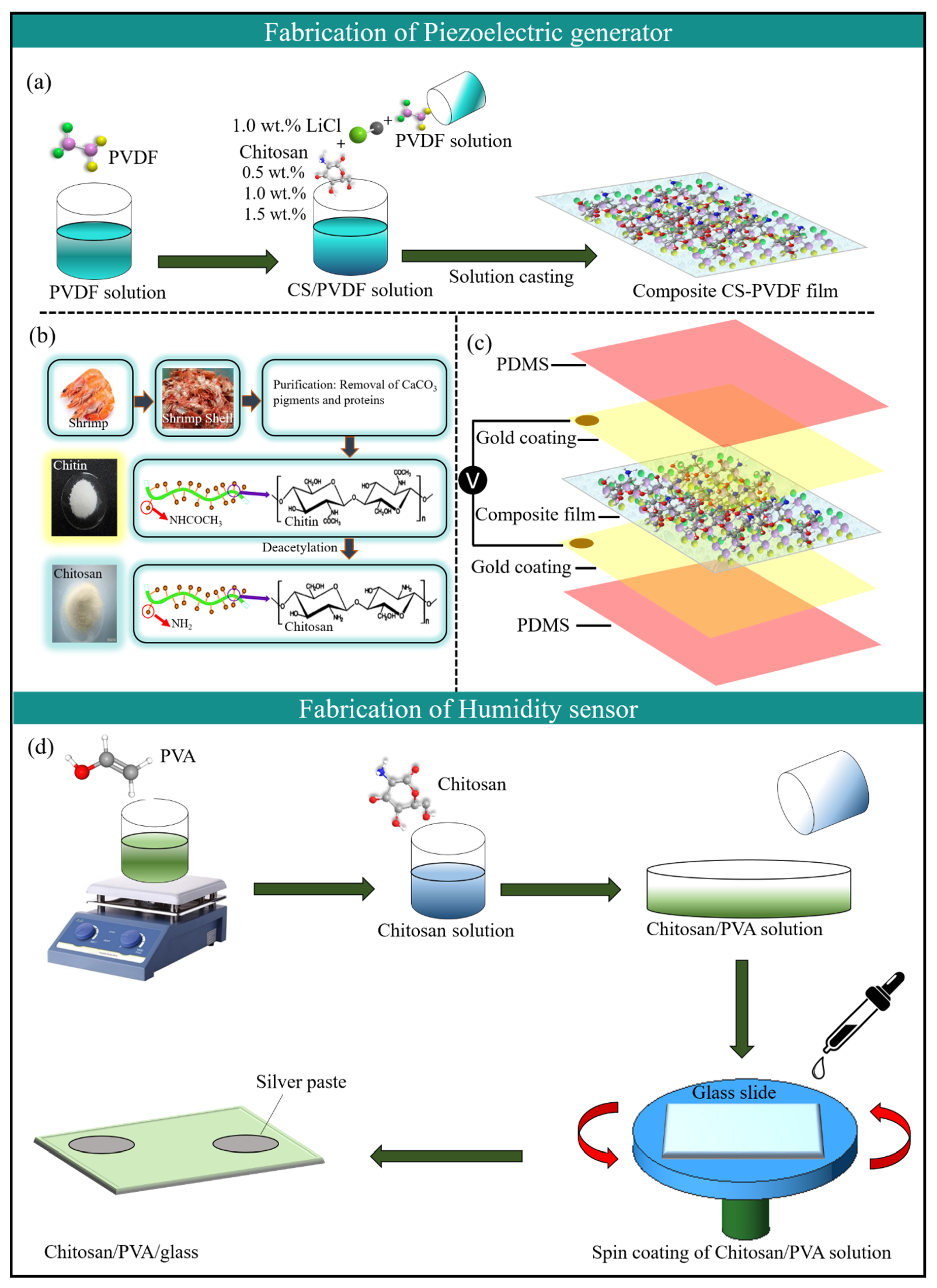
In this study, we propose a self-powered humidity sensor enabled by the piezoelectric effect from biocompatible materials such as chitosan (CS), polyvinylidene difluoride (PVDF), and polyvinyl alcohol (PVA). Chitosan was conjugated with PVDF at different concentrations to develop a piezoelectric nanogenerator as a power source for HSs. Meanwhile, the humidity senor was developed using a composite of CS-PVA. Moreover, the structure of the materials and their concentration were studied in detail while making the composite materials for both the piezoelectric generator and humidity sensor. Both devices were carefully optimized on the basis of the output performance and thoroughly investigated for various parameters. The voltage applied to the sensing film by the PNG changes with the sensor resistance when the humidity in the environment changes. The sensing performance of the humidity sensor was studied by measuring the piezoelectric potential against different percentages of RH under a periodically changing force with an amplitude of 10 N applied to the piezoelectric device. The CS-PVA-based HS exhibited a linear response (R2 = 0.981) and good sensitivity (0.23 V/% RH) in the range of 21–89% RH.
3. Characterization
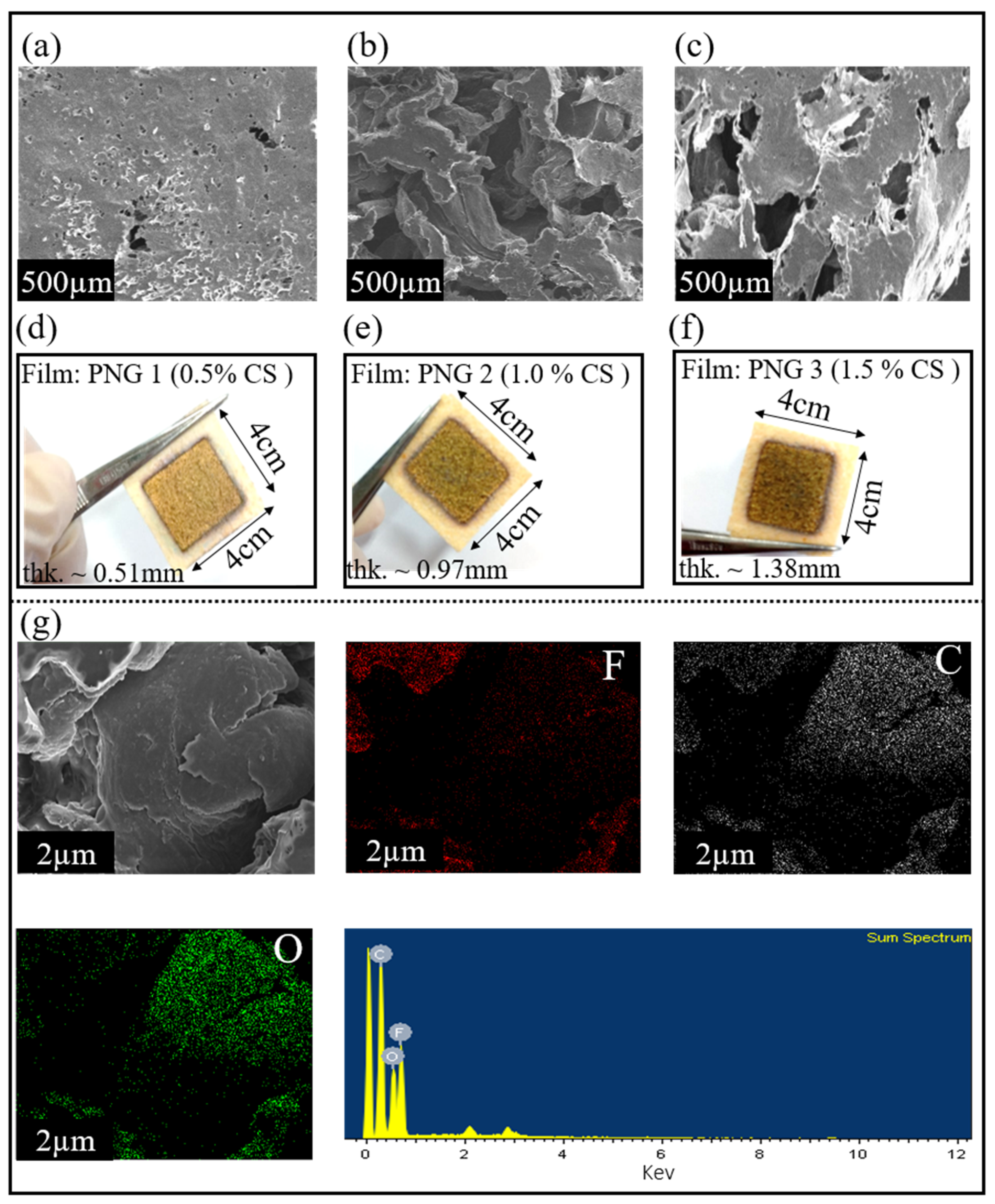
The surface morphologies of the pure PVDF and composite (PVDF-CS) films were analyzed using scanning electron microscopy (SEM).
Figure 2a reveals that the composite (PVDF-CS) film containing 0.5 wt.% of chitosan has a smooth morphology.
Figure 2b shows that the membrane pore size and surface roughness increased with increasing concentration of chitosan, ensuring a large surface area. However, increasing the chitosan concentration beyond 1 wt.% decreases the surface area, as can be seen in
Figure 2c. This change in the structure could be attributed to the high concentration of chitosan, leading to poor penetration of CS particles into the membrane pores, resulting in the formation of a thicker film. Moreover, the elemental composition of the materials was identified using EDS analysis.
Figure 2g shows the EDS mapping of fluoride (F), oxygen (O), and carbon (C) in the composite (CS-PVDF) film. PVDF ((C
2H
2F
2)
n) does not contain oxygen; however, chitosan does, because of the presence of the hydroxyl (-OH) group. The EDS maps show the distribution of oxygen, confirming the presence of chitosan in the composite film.
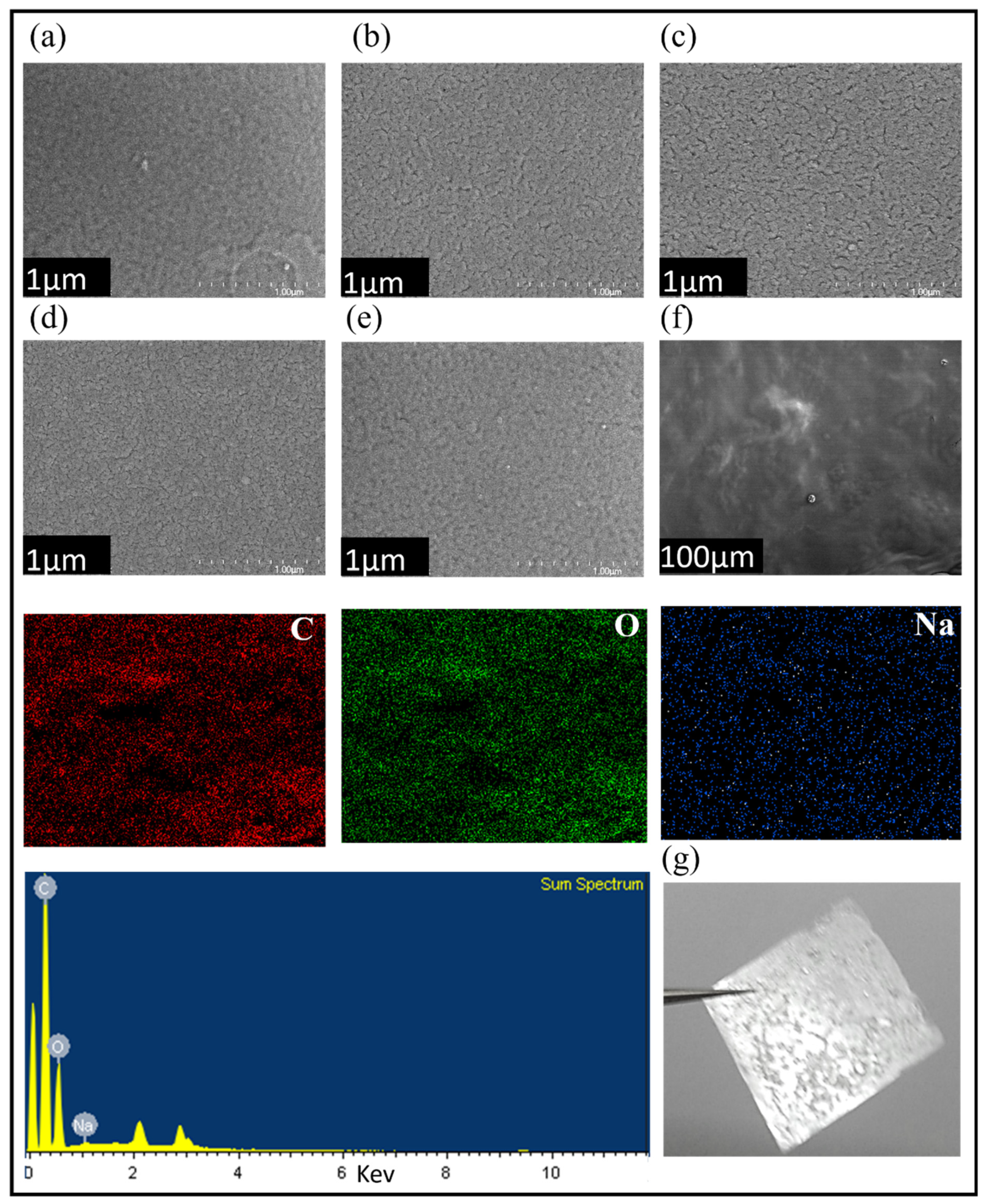
Similarly, the surface morphologies of the pure PVA and composite (CS-PVA) films were investigated using scanning electron microscopy (SEM). From the SEM results shown in
Figure 3, it can be observed that CS-PVA composite with 70/30 of volume ratio (
Figure 3c) have good microstructure compared to the rest of the CS-PVA composites with volume ratio of 90/10, 50/50 and 40/60. This means that there is an optimum CS-PVA volume ratio that improves the mixing of chitosan and PVA with good microstructure. The EDS analysis shows the elemental composition, which is uniformly distributed over the entire surface of the CS-PVA blend. The characteristic peaks of carbon (C), oxygen (O) and sodium (Na) elements owing to the CS-PVA polymeric blend are shown in
Figure 3f.
FTIR analysis was performed to study the chemical interactions between PVDF and chitosan in all of the CS-PVDF composite films, as shown in
Figure 4a. The functional groups from the pure chitosan spectra are recognizable, as the strong band between 3600 and 3000 cm
−1 corresponds to O-H and N-H stretching, while the band ranging from 2800 to 3000 cm
−1 is related to C-H stretching. The characteristic bands at 1508 and 1634 cm
−1 indicate NH vibrations in the amino group and C=O stretching of the N-acetyl group, respectively. The composite CS-PVDF films showed absorption peaks for chitosan at approximately 3230 cm
−1, although the peaks were not intense. Moreover, there are two notable phenomena: (1) the composite film containing 0.5% CS, which clearly shows no CS characteristics peaks, and (2) the intensity of the CS peaks also increases with increasing chitosan concentration. Notably, the LiCl concentration was maintained at 1% in all the composite films, whereas the CS concentration was allowed to vary. Similarly, the FTIR spectra of the sensing film (CS-PVA) were compared, as shown in
Figure 4b. In the case of the pure chitosan film, the characteristic peak at 3338 cm
−1 is related to O-H and N-H stretching, while the peak at 2905 cm
−1 corresponds to C-H stretching. The addition of PVA to the chitosan results in a decrease in intensity. In addition, the CS-PVA composite film displays an absorption peak at approximately 3234 cm
−1 as a result of O-H and C-H asymmetric stretching from PVA [
35].
4. Results and Discussion
This work describes in detail the fabrication process and working mechanism of a self-powered HS enabled by the piezoelectric effect. First, composite (CS-PVDF) films of different thicknesses were evaluated based on their piezoelectric capabilities. Furthermore, a parallel connection was established between the piezoelectric generator and HS (both CS and CS-PVA) to demonstrate its self-powered sensing capabilities against different percentages of RH. The electrical output generated from the piezoelectric harvester was used to drive the HS. The output results reveal that the energy-harvesting device and HS lead the path for the future in the field of self-powered sensors.
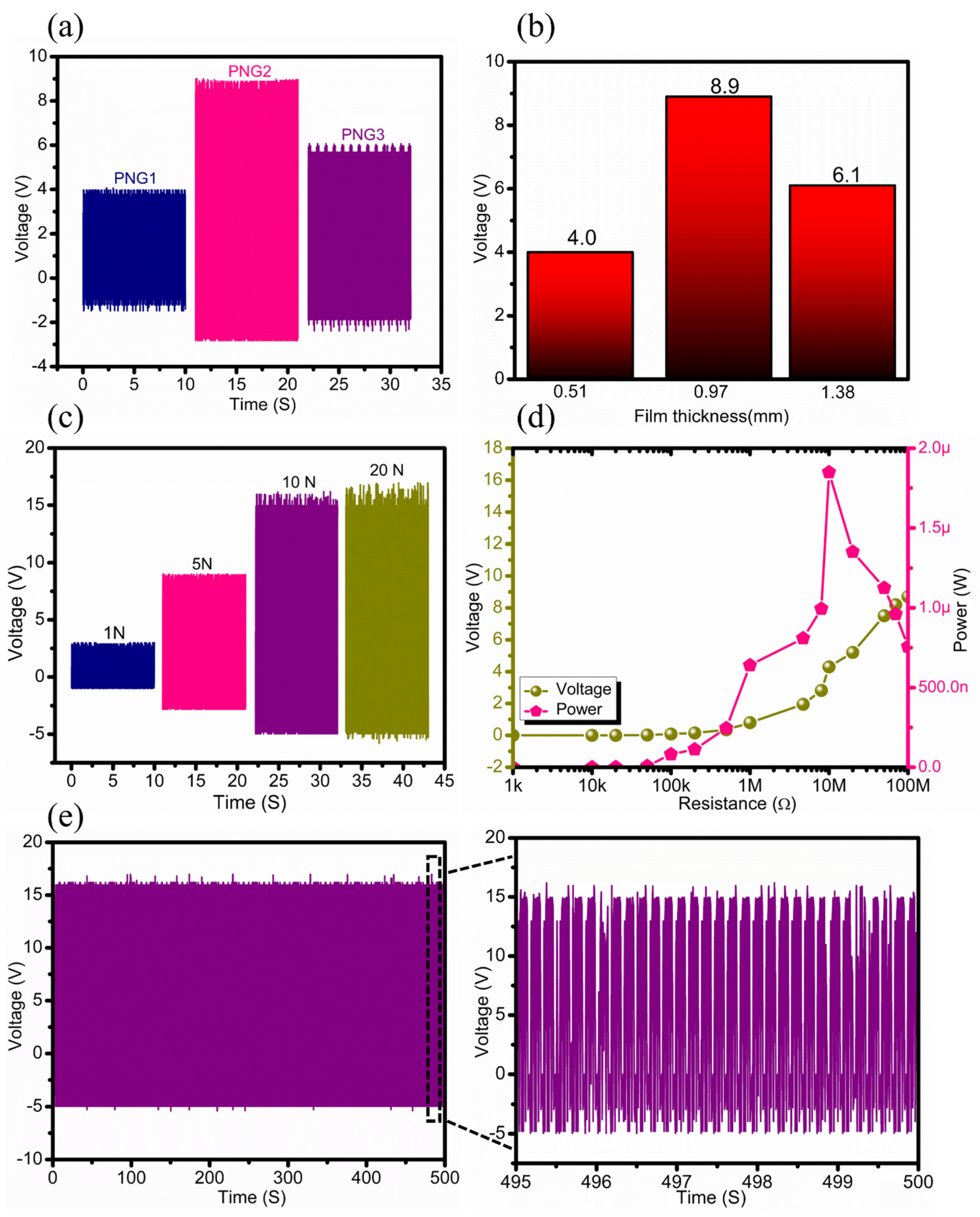
As mentioned previously, CS-PVDF-based composite films with different CS concentrations and different film thicknesses, such as 0.51 mm, 0.97 mm, and 1.38 mm were used to devise PNG1, PNG2, and PNG3 devices, respectively. The fabricated piezoelectric generators were evaluated based on their electrical output performance (electrical) under a constant mechanical force of 5 N, as shown in
Figure 5a. The generated output voltage reached a maximum value of V
oc ~ 8.9 V for the PNG2 device. The PNG1 and PNG3 devices showed a lower output voltage than the PNG2 device, as shown in
Figure 5a. This change in the output performance of the PNG3 device can be attributed to the high concentration of chitosan (1.5 wt.%), which leads to poor penetration of CS particles into the PVDF membrane, and results in the formation of a relatively thicker film with less surface area and in a lower output performance. In addition, lower concentrations of chitosan (0.5 wt.%) cause the improper distribution of CS particles into the PVDF membrane, which limits the output performance of PNG1. Therefore, PNG2 was preferred for further studies because it has the highest output performance among the other two piezoelectric devices (PNG1 and PNG3).
Furthermore, the electrical output response of the PNG2 device was tested under different mechanical forces ranging from 1 N to 20 N, as shown in
Figure 5c. The figure shows that increasing the compressive force (from 1 to 10 N) causes an increase in the output response of PNG2. However, the output response of the PNG was stabilized with a further increase in the mechanical force, as there was no significant change in the output performance, as shown in
Figure 5c. This means that PNG2 exhibits better performance at a compressive force of 10 N, which is favorable for further studies. The load resistance analysis was carried out by deploying external resistances to monitor the power output of the PNG2 device, as shown in
Figure 5d. A stability test of the device was performed, as shown in
Figure 5e. Moreover, the PNG device was operated at three different frequencies (0.5 Hz, 1 Hz and 2 Hz). The relationship between the generated voltage and the frequency of changes of the force applied to the PNG device is shown in
Figure S1 in the Supplementary Materials.
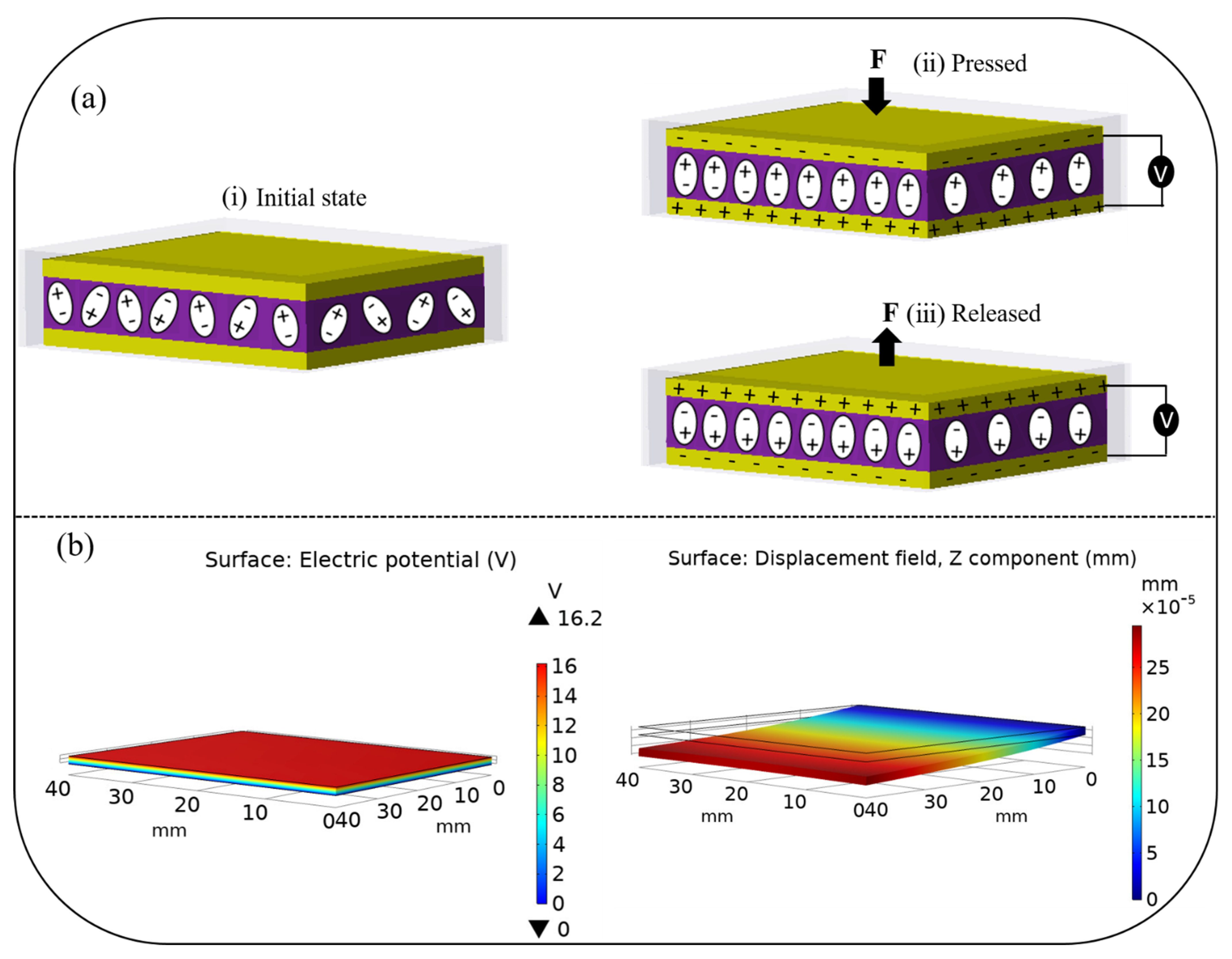
The working mechanism of the PNG device under compressive force is schematically shown in
Figure 6a. Initially, there is no piezoelectric potential, and the surface charges are exactly balanced in the absence of an external force. However, deforming the structure of piezoelectric crystals by employing an external force results in the alignment of the dipoles. The surface charges become unbalanced, which produces a net electric charge on the crystal surface. Hence, the charge starts flowing from one electrode to another through an external circuit. To maintain the charge balance after the external force is removed, the electric dipoles are aligned in the opposite direction, during which a reverse signal is generated. Furthermore, the reverse piezoelectric effect was simulated in COMSOL to investigate the mechanical energy produced by the PNG in response to applied electrical energy, as shown in
Figure 6b.
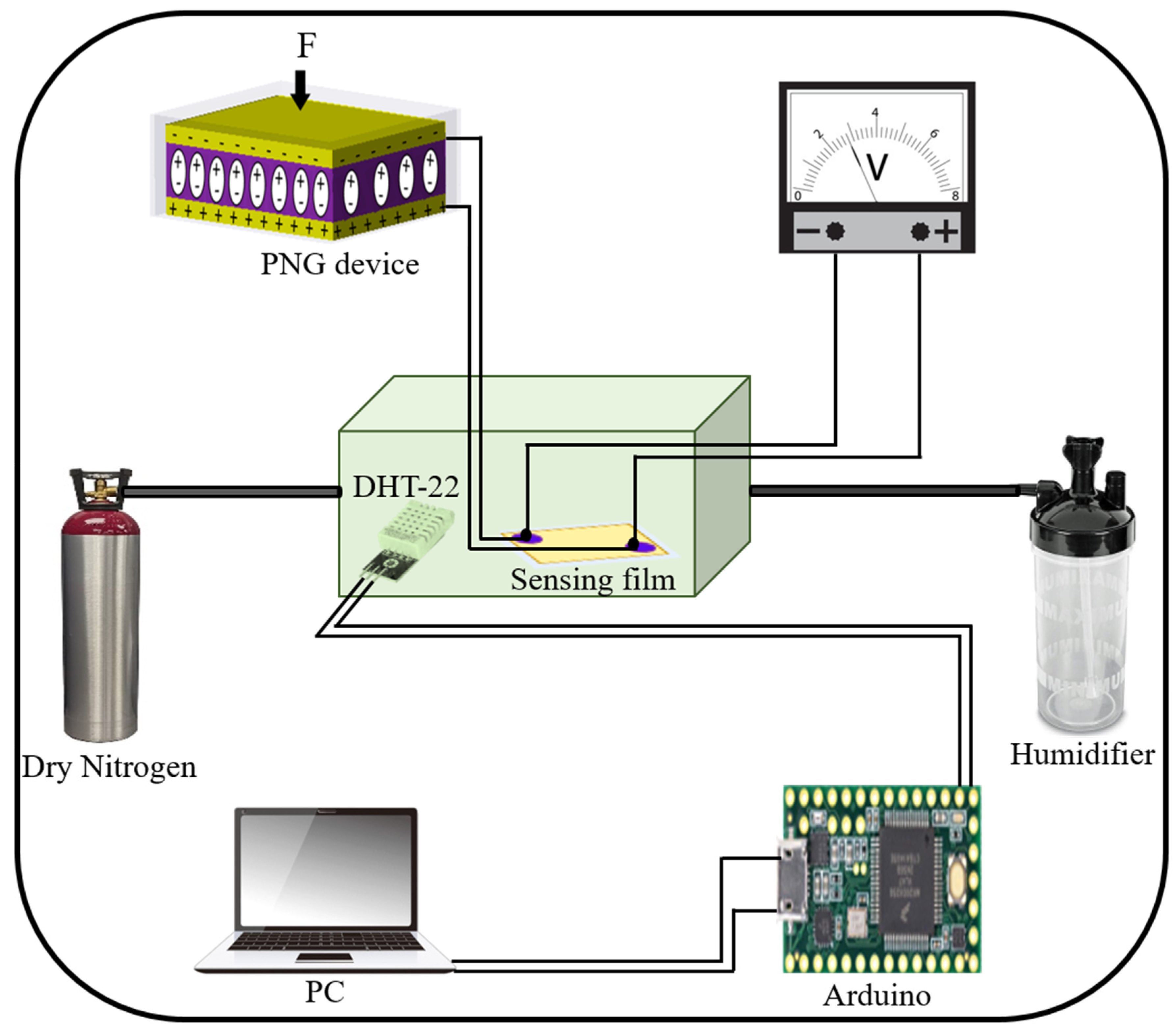
After studying the structural and piezoelectric properties of the CS-PVDF-based PNG device, humidity analysis was performed to examine the affinity of both the CS and CS-PVA films for water molecules. The force was applied perpendicular to the planer surface of the PNG device. Accordingly, a parallel connection was individually established between the PNG device and HSs (CS and CS-PVA) to determine the sensing behavior on the basis of the IV characteristics, as well as its potential to operate as a self-powered system. The humidity-sensing experiment was performed in a customized humidity chamber, and the humidity within the chamber was controlled using a humidifier and nitrogen. A commercially available humidifier was used to regulate the humidification level between 0% RH and 100% inside the chamber. Dry nitrogen gas (N
2) was used to adjust the dehumidification level inside the chamber. Furthermore, to monitor the humidity level inside the chamber, a commercially available DHT-22 sensor was employed as a reference HS with a measuring range of 0 to 100% with a 2–5% accuracy. The reference sensor was connected to an Arduino and PC for data acquisition. The detailed humidity-sensing mechanism is illustrated in
Figure 7. Meanwhile, an optical image of the operating humidity-sensing mechanism is given in
Figure S2 in the Supplementary Materials.
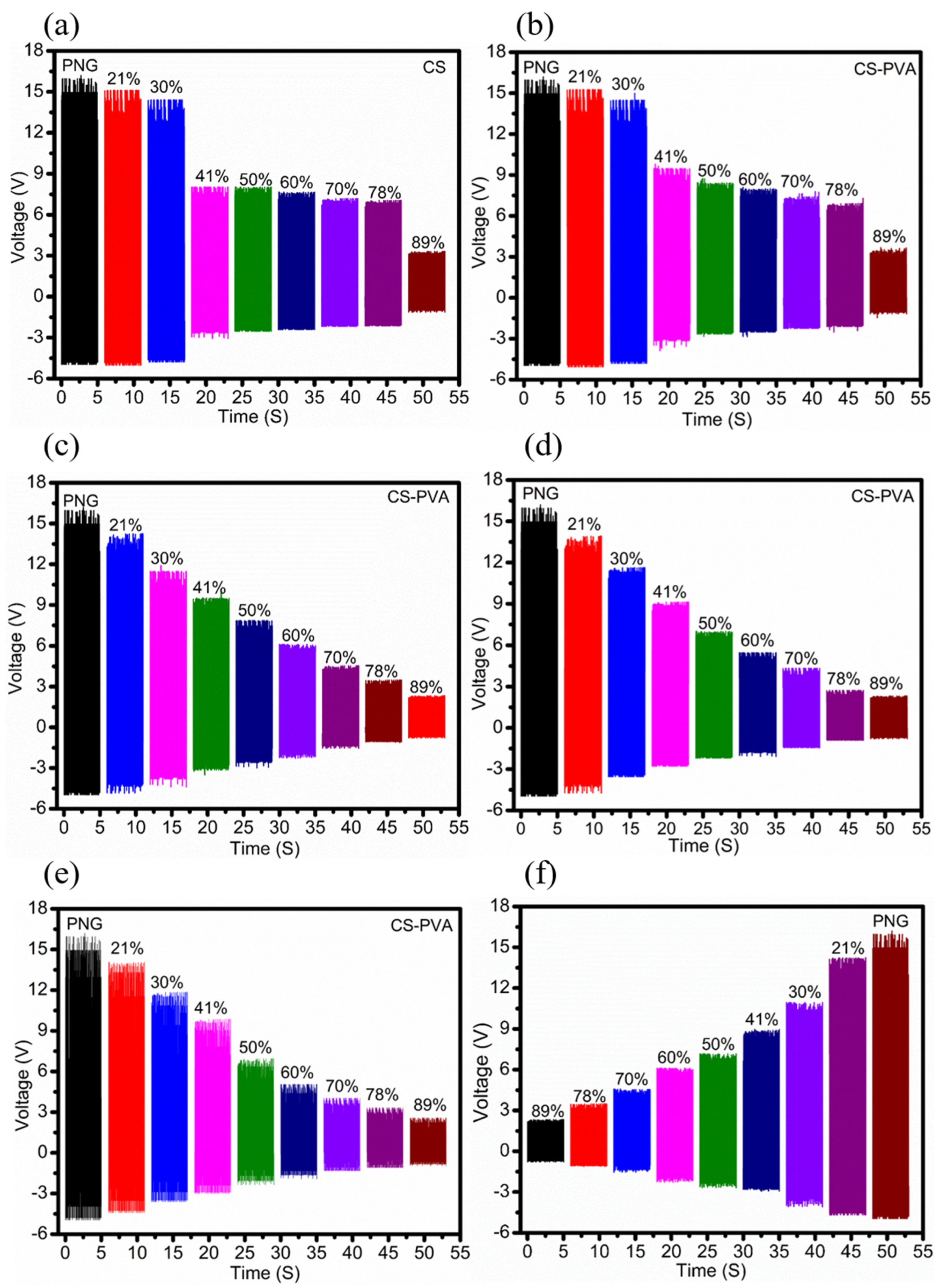
The PNG device was given a constant force of 10 N using a linear motor, and the derived electrical output from the PNG was fed as an input to both the CS- and CS-PVA-based humidity sensors in succession to determine the humidity-sensing response on the basis of the I–V measurement. The humidity-sensing performance of the CS-based humidity sensor is shown in
Figure 8a. When the sensor was exposed to water molecules, the V
OC decreased with increasing %RH, as can be seen in
Figure 8a. At low RH, between 0% and 30%, a small change in voltage could be attributed to the exposure of the chitosan sensor to very few hydrophilic functional groups for chemisorption of water molecules. However, a dramatic decrease in voltage values was observed in the range of 30% RH to 41% RH. This is due to the physisorption of water molecules onto the surface of the previously chemisorbed water molecules. With a further increase in relative humidity (41% RH to 78% RH), excessive water molecules were chemisorbed by the internal hydrophilic group, leading to a slight reduction in the voltage values. Again, sharp decrements in the voltage values were recorded in the high relative humidity environment (78% RH to 89%RH). This was caused by the physisorption of additional water molecules in the chitosan sensing film.
The sensing mechanism of the CS-PVA-based humidity sensors with different volume ratios (90/10, 70/30, 50/50 and 40/60) of CS-PVA were tested with different ranges of relative humidity as shown in
Figure 8. Meanwhile,
Figure 9 demonstrates the linearity between the voltage and percentage of RH on the basis of the correlation coefficient and sensitivity of both the CS and CS-PVA-based sensors. According to the results, the CS-PVA (70/30) HS showed better sensitivity and linearity compared to others. This means that the CS-PVA-based humidity sensor with a volume ratio of 70/30 facilitates a good microstructure, resulting in better sensitivity (0.23 V/% RH) and linearity (R
2 = 0.981), as shown in
Figure 9c. It should also be noted here that all the CS-PVA-based humidity sensors exhibit a better humidity-sensing response than the CS-based HS. This can be attributed to the greater number of hydrophilic functional groups in the CS-PVA. To validate the experiment, the same tests were repeated for other devices that exhibited high repeatability, and a 5% error was observed. Therefore, the CS-PVA-based HS with volume ratio of (70/30) was chosen for further studies with respect to its humidity-sensing properties.
Figure 8f demonstrates the desorption response of the 70/30 (CS-PVA)-based HS. Meanwhile,
Figure 9f shows the adsorption and desorption responses, which exhibited a small humidity hysteresis of ~3.8% RH in the humidity range of 21% to 89% RH. The larger response value and wider detection range of the CS-PVA-based HS operated by a piezoelectric generator confirms that the fabricated HS is a favorable candidate for humidity detection and possesses remarkable advantages in the field of self-powered humidity detection. The proposed self-powered humidity-sensing device can target various applications, such as in remote areas where power delivery is not frequently available. The Internet of Things (IOT)-based nodes that must deliver continuous power to sensors for operation can be qualified by just pressing once on the sensor to obtain an instantaneous relative humidity reading of the environment. Similarly, this can also be a robust candidate for green roads or paths on which humans might normally walk, as the walking force can easily enable our proposed sensor to generate voltage proportional to the humidity at that point of time. Moreover, this study provides the prototype with all required characterizations.
5. Conclusions
In this study, chitosan (CS) and chitosan-polyvinyl alcohol (CS-PVA)-based sensing films and a piezoelectric nanogenerator, which acted as humidity sensors and a power generator, respectively, were successfully fabricated. The piezoelectric generator performance was tested on different concentrations of chitosan (0.5,1.0, and 1.5 wt.%) in the CS-PVDF composite material. The enhanced initial output voltage of CS-PVDF composite piezoelectric material, which contains 1.0 wt.% of chitosan, was attributed to its rough surface structure and large surface area. Similarly, the performance of the humidity sensor was investigated based on the volume ratio of CS/PVA in the composite polymeric blend. According to the results, the CS-PVA HS with a volume ratio of 70/30 showed better performance compared to CS-PVA HSs with different volume ratios (90/10, 50/50 and 40/60). This is because of the good microstructure, which was facilitated by an optimized amount of chitosan and PVA. Moreover, the affinity of CS- and CS-PVA-based humidity sensors toward water molecules was investigated at different humidity levels in a closed electronically controlled chamber. The as-developed self-powered device provided an output relation inverse to the humidity in the environment. Moreover, the sensor showed excellent performance, including high linearity (R2 = 0.981) and sensitivity (0.23 V/% RH), with a wide detection capability in the range of 21% to 89% RH. This work is intended to consolidate self-powered technology in humidity-sensing mechanisms, proposing its candidature for a variety of applications.