1. Introduction
Packaging, storage, and transport of fertilizers that are based on ammonium nitrate (AN) as their main component pose much more potential hazards than it seems to many producers and prospective users. Our previous publication [
1] showed that the activity of a mixture of molten AN and polyolefins (polyethylene (PE) and polypropylene (PP)) should influence the revision of views on the safety of packing AN into polyethylene bags or polypropylene flexible intermediate bulk containers. These, of course, facilitate AN transport and storage and protect this highly hygroscopic substance from moisture. However, the circumstances change dramatically in the presence of heat, when the temperature rises above 170 °C (PE, PP, AN). Such an impact and the resulting threats also apply to flameless fire zones. Due to the heat transfer—occurring through the walls of containers, silos, rooms, etc.—metal doors, walls, and similar partitions cannot be considered entirely safe.
It should be remembered that, in the case of AN, an explosion occurs much more easily in confined spaces, as soon as at around 190 °C. Such a situation is likely to take place as a result of heat radiation arising from a fire. Combining these molten substances (optimal ratio 1:5 for the mentioned polyolefins with AN) gives a very effective explosive mixture with properties similar to ANFO. In the conducted research, a homogeneous mixture with a specified ratio of ammonium nitrate (V) and selected polymers—granulation of 0.29–0.1 mm—was used. On the basis of the differential scanning calorimeter (DSC) analysis, transformations occurring in <1 min with a significant heat flux effect, at the standard scanning speed of 10 °C/min, were defined as an explosion–like reaction. Of course, the explosion phenomenon on the macroscopic scale includes the thermal effect, mechanical effect (pressure increase), chemical transformations, etc. In the DSC measurement, the reaction can be observed in the form of narrow and very sharp peaks, also observed during the DSC analysis of typical explosives, e.g., ANFO. Explosive AN mixtures include, i.e., oils, greases, rubbers, metal dust, or acids [
2,
3,
4,
5,
6]. Relatively sharp peaks are observed for several phenomena, such as the melting of crystalline bodies. However, the energy effect, unlike an explosion, is at least 1–2 orders of magnitude smaller.
Therefore, it was decided to closely examine one of the potential causes underlying the uncontrolled progress of events. The research was carried out in accordance with the findings based on the analyses of numerous disasters, as well as on tests and results carried out and published by many experts previously dealing with AN-related issues. Plastics have long been ubiquitous in our surroundings. AN is the cheapest and most widely used agricultural fertilizer in the world, and, at the same time, a popular base for the production of explosives used in various mixtures applied in mining. The fact that the materials in question can melt in a fairly similar temperature range prompted us to conduct such studies. They pass into the liquid phase almost simultaneously, which greatly facilitates the formation of an explosive mixture.
From among numerous plastics, we selected those that have a melting point within the appropriate range and of which items, used and potentially used where AN is stored or applied, are produced. Potential reduction properties of the molten material or of its thermal decomposition products were the second selection factor. The results of the research clearly showed that the choice was right.
The first of the selected polymers was the aforementioned polyvinyl chloride (PVC); its chemical formula is (-[CH2CHCl]-)n. PVC is a popular synthetic thermoplastic polymer from the group of vinyl polymers. Due to different properties, two forms of this material can be distinguished: unplasticized PVC (uPVC), also referred to as hard PVC, and plasticized PVC (PVC-P), also referred to as soft PVC. From the chemical point of view, they differ mainly in terms of the number of added plasticizers. Due to high chemical and mechanical resistance, PVC is widely used in various industries. As it comes to the agricultural industry, PVC is most frequently applied in the production of irrigation pipes and pipes in sewage and water supply systems inside farm buildings. Concerning the safety of AN storage, the first of these applications is crucial because elements of water supply networks on farms may be situated near other materials.
Additionally, PVC is used in the construction of large-area tents. It may also be applied in construction materials as well as in equipment of utility rooms, such as door frames, window frames, and gutters. Furthermore, PVC can be used in electrical wiring insulation, packaging, or as a cover for coated fabrics.
In comparison to most polymers, PVC has a relatively high flash point and auto-ignition temperature. Moreover, the high limiting oxygen index (LOI) value, 50% for uPVC and 21–36% for PVC-P (depending on composition), suggests that this polymer is a fire-resistant material and requires a strong external heat source for ignition. PVC, while burning, produces much less heat than most organic materials. Therefore, it is believed that the heat released by combustion may be insufficient to initiate ignition of nearby materials (26 MJ/kg). Moreover, during the combustion, the behavior of PVC also differs from that of most popular polymers. No dripping occurs while melting; instead, the surface becomes charred, which, together with the hydrogen chloride released during decomposition, may limit the spread of fire. From the chemical point of view, the fact that carbon monoxide, carbon dioxide, hydrogen chloride, and water vapor are main products of PVC combustion is also very important. During the laboratory combustion of the material, no observable amounts of gaseous chlorine and phosgene are released.
Polystyrene (PS) is another of the selected polymers; its chemical formula is (-[-CH2CH(C6H5)-]-)n and it belongs to the group of polyolefins. Polystyrene also comes in various forms: solid polystyrene, expanded polystyrene (EPS), and extruded polystyrene (XPS). Due to different properties, various types of the mentioned forms can be distinguished. For example, among the solid material, it is possible to distinguish, i.e., a general-purpose polystyrene (GPPS) or a high-impact polystyrene (HIPS). Numerous everyday objects are made of solid polystyrene; concerning the analyzed case, this material does not, however, pose a significant threat compared to the other two forms. Expanded PS, in turn, is one of the most widely used insulation materials. The walls of storage rooms are often covered with EPS, which greatly contributes to the protection of rooms against moisture as well as to reduction of thermal losses of buildings.
Like the vast majority of polymers, polystyrene is combustible. Due to its commonness, additives are used to retard the flammability of the material. Typically, hexabromocyclododecane (HBCD) serves as such an additive, in an amount of up to 0.5% by mass. This type of polystyrene is marked as self-extinguishing (SE) (EPS SE). It is not only much more difficult to ignite, but also has a lower fire spread rate. In addition, the method of assembly and special preparation and insulation of the outer surface of the panels are aimed at preventing the polystyrene from direct contact with the heat source in the event of fire. When exposed to high temperatures, the material softens and melts, with a possibility of dripping. Due to the low density of the material, the amount of heat released during its combustion is relatively small. Thermal decomposition products of PS do not support combustion, so the material should self-extinguish after the removal of heat source.
Another group of polymers that may potentially come into direct contact with AN during its life cycle are polyamides (PA). Polyamides are a group of polymers that, in their main chains, contain a unit with amide bonds: -C(O)-NH-. Due to different chemical composition and production methods, ready-made materials from the polyamides’ group may have different functional properties. Due to the widespread use, in further analysis, the research was carried out on nylon (PA66). The synthesis of PA66 is based on the condensation of adipic acid and hexamethylenediamine. Polyamides show a very strong tendency to crystallize (35–70%), additionally reinforced by the formation of hydrogen bonds between the oxygen and nitrogen atoms from two different amide groups. As a result, polyamides are harder and more difficult to melt than, for example, polyesters, being far superior to vinyl polymers (e.g., PVC) in this respect. They are most often available on the market in the form of fibrous materials. Polyamides are used to produce nylons (aliphatic polyamides) and aramid fibers (aromatic polyamides). They are widely used in the textile industry, in the production of both clothing and large-area textiles. Polyamide fibers are harder and have a higher mechanical resistance than polyesters and vinyl polymers, thanks to which they are also used in very niche applications such as, e.g., the production of chew-resistant fabrics (resistant to being chewed through by rodents) or the production of materials for noise and vibration suppression (ropes, nets, mesh, etc.). Due to the possibility of direct contact with AN, it is also worth mentioning the use of PA in the automotive industry where many components, such as engine covers or radiator covers, can be made of this material due to the reduction of costs and weight compared to such elements made of steel. In addition, PA are used for the production of braiding for electrical wires, parts of automated devices, fishing nets, or as packaging. Due to good mechanical properties, high tensile strength, abrasion resistance, low friction coefficient, and shape stability at elevated temperatures, as well as resistance to gasoline and solvents, polyamides are currently the most important construction material used in many industries, incl. the automotive, aerospace, electronics, and electrotechnical industries, as well as in textiles and medicine. PA are also used for the production of gears and other hardware. Glass fiber-reinforced elements show an increase in specific weight (up to 1.60 g/cm3) and very high stiffness, increased creep resistance, and high dimensional stability.
These plastics, however, have a disadvantage, which is significant for some specialized applications: They are highly absorbent to moisture (2.5–2.8%). PA electrical properties are strongly dependent on the moisture content. In any case of practical application, it is recommended to dry the polyamide to a minimum value of 0.2% before processing. The drying temperature, depending on the technology, remains in the range of 80–110 °C. The material shows relatively high thermal stability. However, after exceeding the temperature of 310 °C, thermal decomposition of the polymer occurs, with the separation of decomposition products in the form of carbon monoxide, ammonia, and caprolactam. This has a negative impact on human safety nearby and may cause undesirable reactions. Polyamides are inherently flammable, as evidenced, i.e., by the low value of the LOI coefficient, which, for a pure polymer, is about 22%. The appropriate number of additives (flame retardants) allows improving thermal properties, increasing the LOI level to a safer value (above 30), and changing the material behavior under high temperatures and under combustion.
Another polymer for which the tests were performed is poly(methyl methacrylate) (PMMA). It is the main component of acrylic glass, also known as organic glass (plexiglass). For certain applications, it may contain admixtures of other materials, polymers, and copolymers. This material has been widely used due to its properties, such as high transparency in the field of visible light, ease of processing, resistance to UV radiation, or high resistance to mechanical stress. Moreover, it has good insulating properties (thermal and acoustic). Organic glass is used for the production of protective screens, covers, sound barriers, and electrical insulation spacers between materials and serves as an alternative to ordinary glass in windows and sight glasses. Greenhouses and garden tunnels are its typical application in agriculture. Plexiglass is more durable and its light transmission is more effective, which has a positive effect on plant growth. On the other hand, it is more sensitive to tarnishing as a result of superficial scratches.
Another disadvantage of organic glass is its flammability, unlike in the case of polycarbonate, which has a similar application to PMMA. During its combustion, the molten material drips. When PMMA is burned, relatively little smoke is produced. In an oxygen-rich atmosphere, pure PMMA decomposes into carbon dioxide and water vapor. Irritating and toxic gases may also be emitted (partial depolymerization effect). In addition, PMMA can actively contribute to the fire propagation.
Selected polymer materials can potentially accompany production, as well as transport, storage, or application of ammonium nitrate. Mixing of molten materials is likely during emergency events (fires). Previous studies showed that, in the case of popular polyolefins, such a randomly produced blend may have properties that can lead to the initiation of pure AN explosion due to the generated heat and shock wave.
2. Materials and Methods
In order to obtain representative results of the analysis, the selection of polymer samples was accompanied by the inspection of the sites related to the production and use of ammonium nitrate and obtaining information from people working there.
Previous experience in the preparation of test samples was also used. For the results concerning different samples to be easily comparable, the same measurement conditions were applied. Similar phenomena may be observed while testing typical explosives.
For selected types of plastics, samples were taken for their most common forms applied in utility items. For example, for polyvinyl chloride, uPVC, used in construction joinery (doors, windows, façade panels, covers of electric wiring harnesses, and all types of pipes) and PVC-P (insulation of electric cables, pipes for transferring aggressive liquids, bags for such liquids, specially sealed protective clothing) were chosen. The difference between these varieties is based upon the syntactic or atactic arrangement of the polymer substituents and significant content of the plasticizer, e.g., phthalates.
The test mixtures were prepared with the polymer content of 8%, 20%, 50%, and 75%. Explosion was most often observed for the content of 20% of the polymer in AN mixture. The following samples were selected:
- -
PVC: A fragment of a typical water main pipe was used in the analysis. The processing was done by hand in order not to lead to undesirable changes in the structure of the material. A sample of PVC-P was also taken in a similar manner, coming from a piece of colorless tubing for transportation of liquids and gases [
7].
- -
PA: A forklift element made of PA66 was used for the analysis. The sampling and sample preparation procedure was analogous to that of uPVC. Due to the surface sorption of moisture, the sample was kept in the desiccator the entire time [
8].
- -
PMMA: The sample used in the analysis was taken from a colorless general purpose plexiglass board [
9].
- -
PS: The analysis used expanded polystyrene (EPS). White EPS, without dyes and modifying additives, foamed with CO
2/H
2O was selected [
10].
Mixtures of AN with selected polymers were analyzed with the use of differential scanning calorimetry (DSC). It is one of the methods of thermal analysis of materials that allows measuring the heat flux received or supplied to the environment by a sample during its heating. This allows determining the temperatures of chemical reactions and physical changes of the material, as well as determining the thermal effects of these changes. The analysis of the course of these reactions in a function of temperature changes allows determining whether, i.e., a given reaction may take place in an explosive manner.
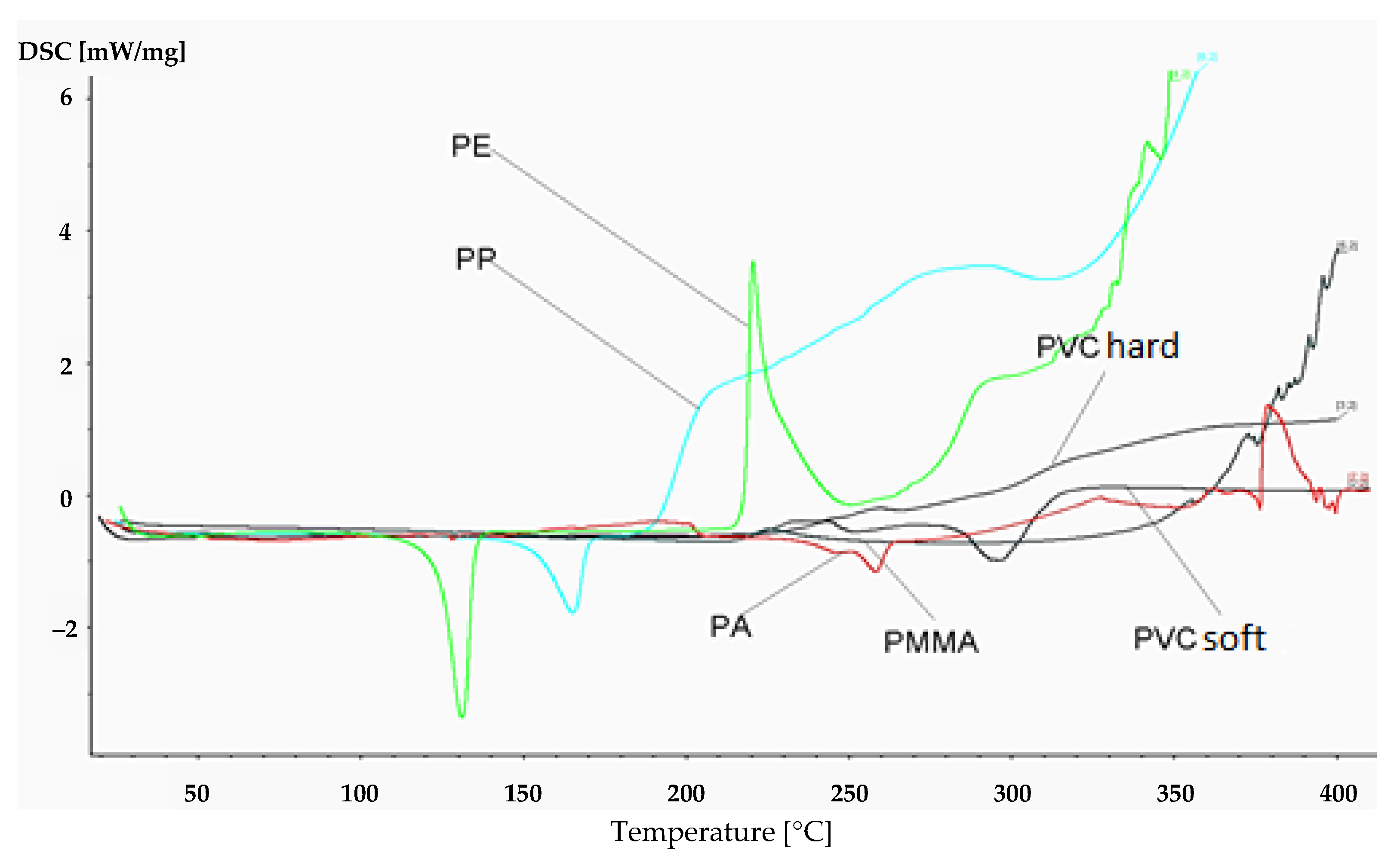
Figure 1 presents the thermal analysis of the polymer samples used in the research in the range of 20–400 °C. A thermal effect was observed for each of the plastics, indicating thermal decomposition. The effect of the energy release rate was, however, several times less significant than during changes taking place in the presence of AN.
Due to the high sensitivity of the research equipment and the risk of damaging the sensors, it was decided to use a relatively low mass of samples, about 5 mg. In order to map the conditions that occur on a larger scale, the prefrozen samples of AN and polymer products, cooled with liquid nitrogen (−196 °C), were crushed by hand using an agate mortar. Thanks to this, changes in the structure of the material were avoided. Test samples with a fraction of 0.1–0.29 mm were then placed in a chamber dryer at the temperature of 28 °C and at 25 kPa below atmospheric pressure for 24 h. The dried material was stored in a desiccator under nitrogen atmosphere (conditioning for at least 48 h). Such measures were necessary due to the hygroscopicity of some of the analyzed substances, in particular ammonium nitrate. DSC measurements showed such high sensitivity that even a slight humidity of samples noticeably affected the final measurement results. Ammonium nitrate, min. 99.0% pure, was used in the analysis. This choice was made to ensure the repeatability of measurements. The influence of additives used with AN for agricultural purposes (content <28% N), such as calcium salts, magnesium, dolomite flour, or chlorides, was avoided. The mixture was prepared just before performing the analysis, thanks to which the risk of a chemical reaction between its components and the vessel walls was minimized. An analytical balance with an accuracy of ±0.01 mg was used for its preparation. Measured samples weighing 5.00 ± 0.15 mg were placed in a designated aluminum vessel with a volume of 25 µL with a lid with a hole of 0.3 mm in diameter. The presence of an opening in the upper part of the vessel prevented a gradual increase in pressure in the analyzed sample due to the released gases. This is important due to the fact that the decomposition parameters of AN change with the change of pressure: In the case of a significant increase, an explosion may occur. The sample (mixture) was tamped using a PTFE rod to remove air and improve the thermal conductivity of the material. The prepared sample was then placed in the DSC 3500 Sirius NETZSCH apparatus and the analysis began in the range of 25–400 °C under nitrogen atmosphere with a constant flow of 50 mL/min. The sample was heated at a standard rate of 10 °C/min.
3. Results
Ammonium nitrate (AN) has quite specific properties that cause its reduced stability in contact with other substances, especially at elevated temperatures. It shows polymorphism, i.e., the existence of various crystalline forms and their mutually influenced changes depending on pressure and temperature (in the range <200 °C there may be five crystalline varieties). They differ not only in terms of their structure but also their properties. Under atmospheric pressure, the changes between various crystalline structures are endothermic, and so are the melting reaction (~169 °C) and thermal decomposition into gaseous products (~281 °C). The decomposition of PVC, PA, PS, and PMMA is very gentle: Slow decomposition with a relatively slow release of heat occurs. In the analyzed temperature range (<400 °C), the tested material did not decompose entirely. The process of pyrolysis with a temperature increase led to carbonization. Summing up, the analyzed samples did not show any sudden changes during heating, and their decomposition was relatively safe. They did not ignite until reaching significantly higher temperatures. Completely different conclusions could be drawn after mixing AN with the tested polymers. For each of the mixtures, slight changes in temperature and thermal effects of crystalline changes and AN melting were observed, but these changes were not significant from the point of view of safety. Therefore, the remaining part of the analysis will be focused solely on changes related to thermal decomposition (
Figure 2).
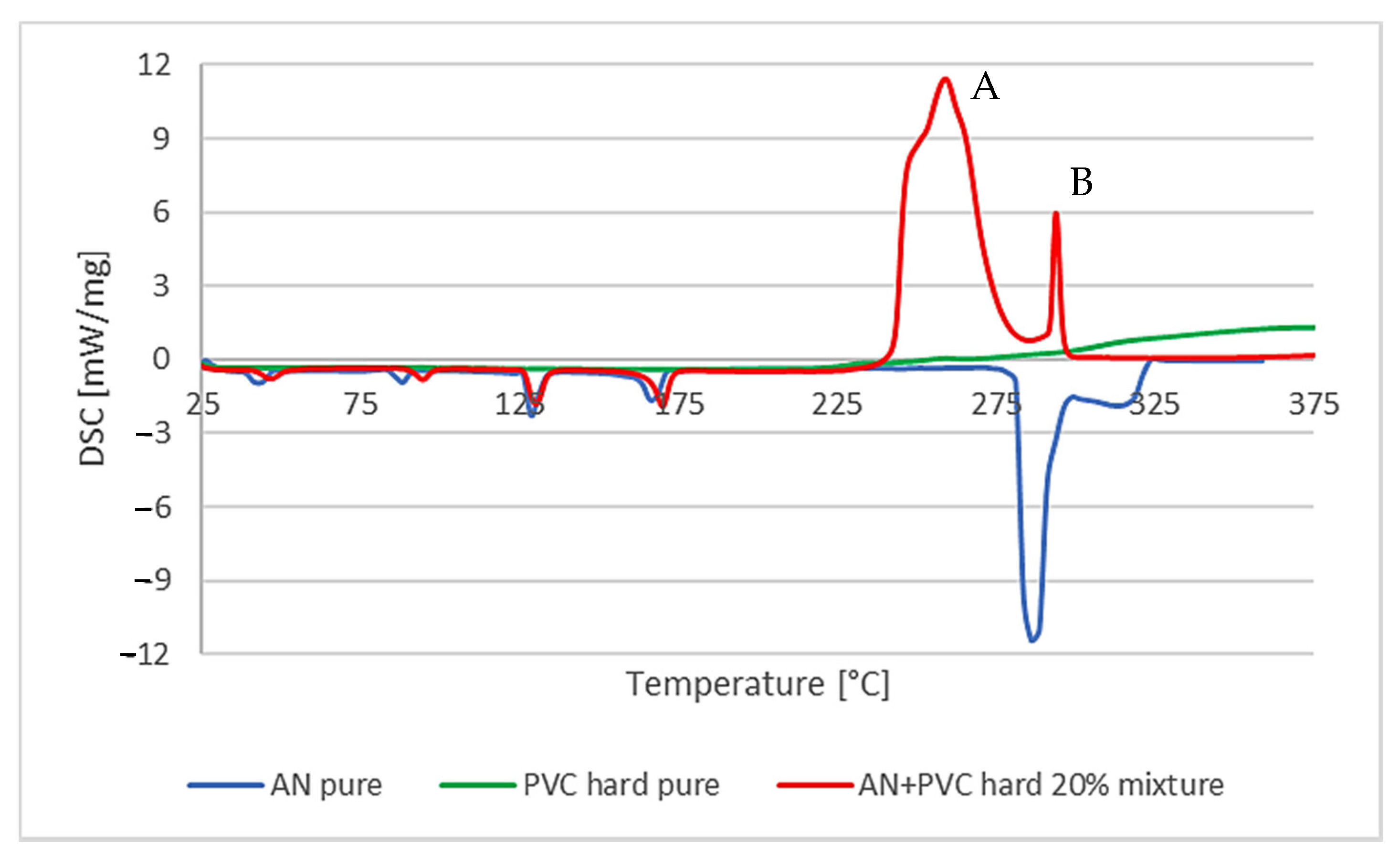
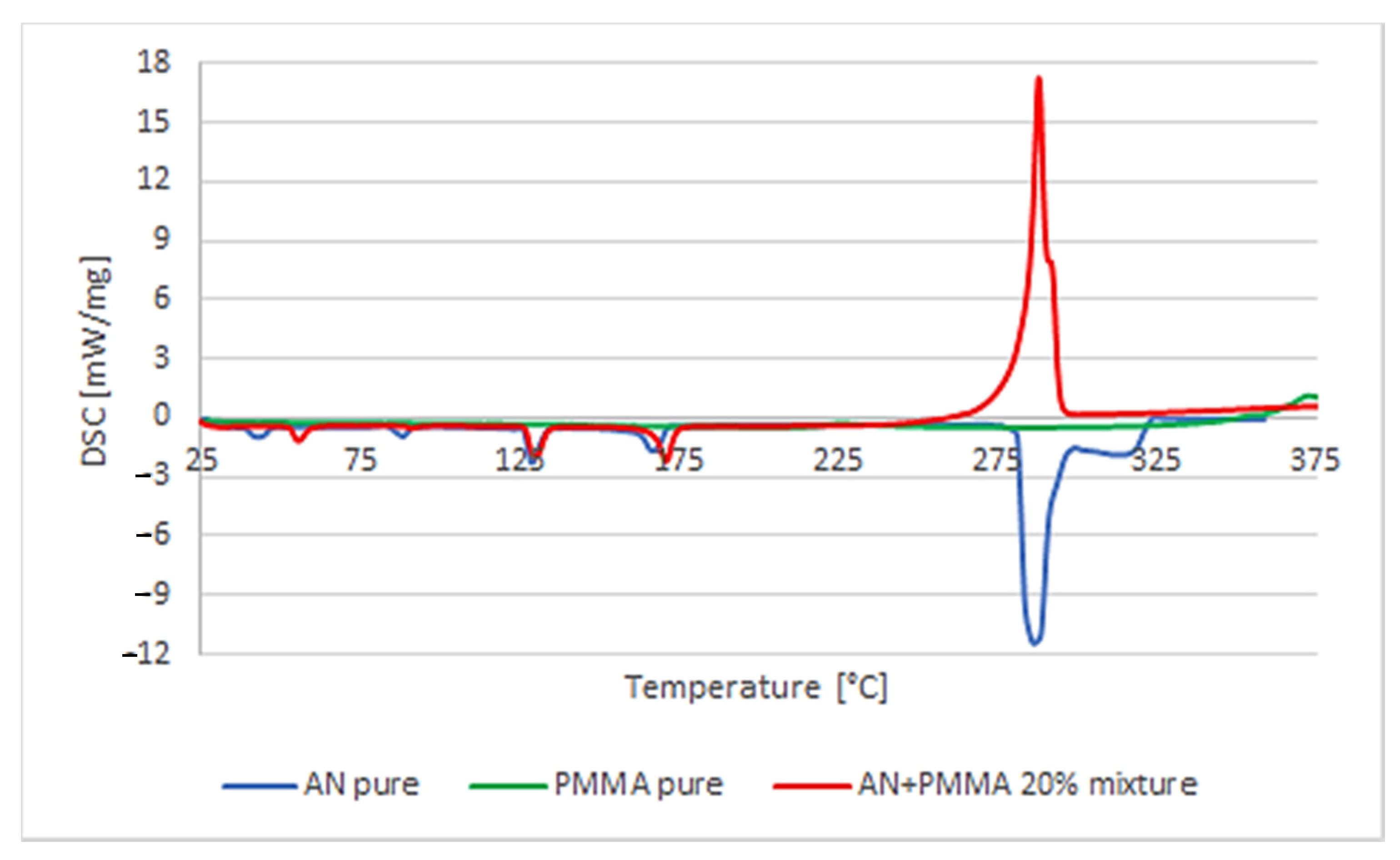
In the case of the mixture of AN with uPVC, the characteristic multistep course of the mixture decomposition reaction was noticeable. It was assumed that it was related to the earlier transformation of PVC, i.e., dehydrohalogenation. It is a characteristic chemical process for this material, in which gaseous HCl is detached from the structure of polymer chains. For a pure polymer, this process usually takes place at a temperature of about 180 °C. It is followed by an explosive reaction of the resulting unsaturated polypropylene chain with the decomposition products of AN. Moreover, a significant reduction in the temperature of the decomposition reaction was observed: from 281.6 to 243.8 °C for pure AN. The enthalpy of the decomposition process increased from −877 to −1384 J/g for the first reaction and −66 J/g for the second one. The amount of heat released during the main decomposition process, despite the relatively quick course of the reaction, did not indicate an explosive course of the reaction; however, the reaction proceeding this way may initiate other reactions inside the sample. The course of the second chemical reaction was explosive, but the amount of released heat was relatively small (
Figure 3).
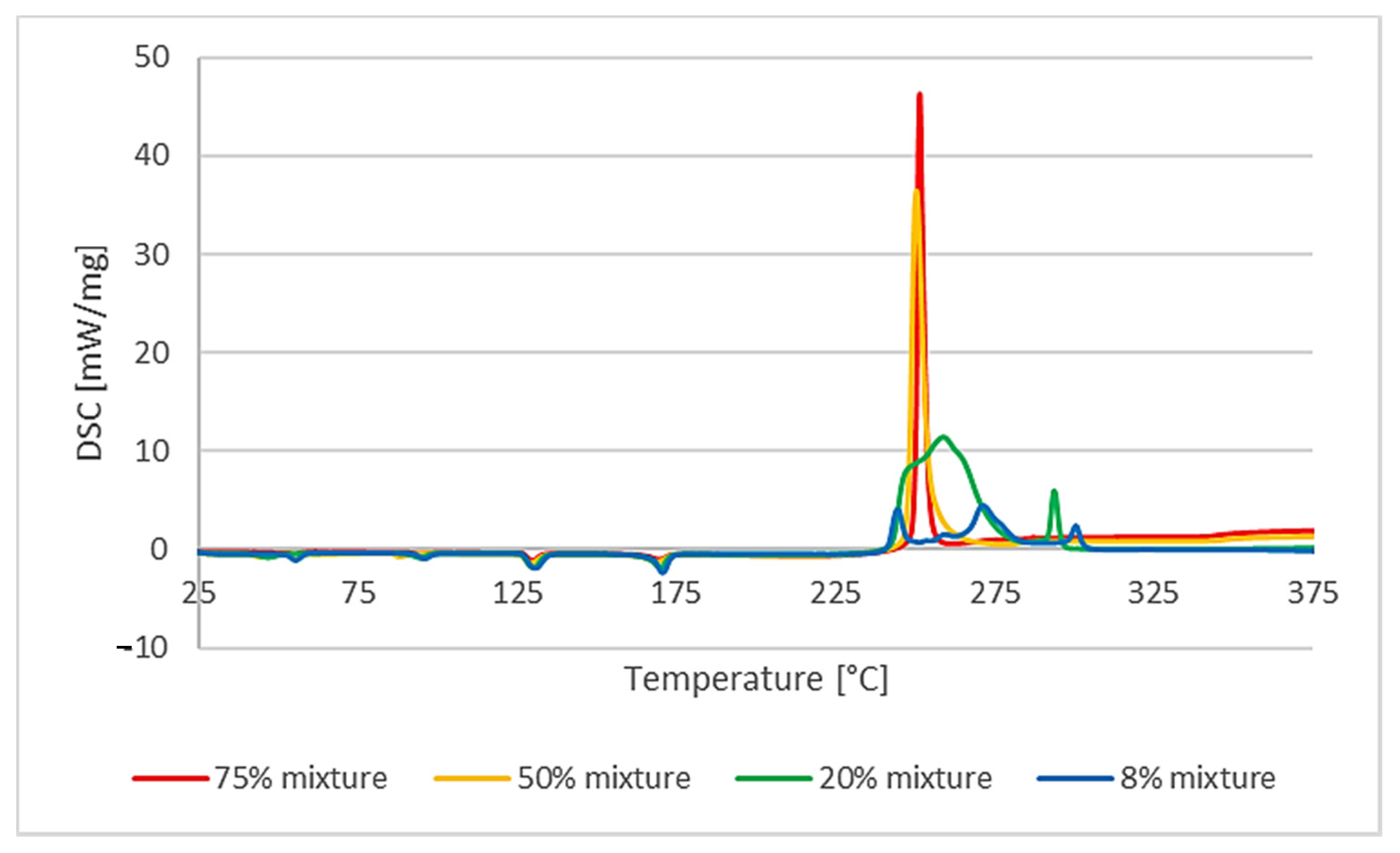
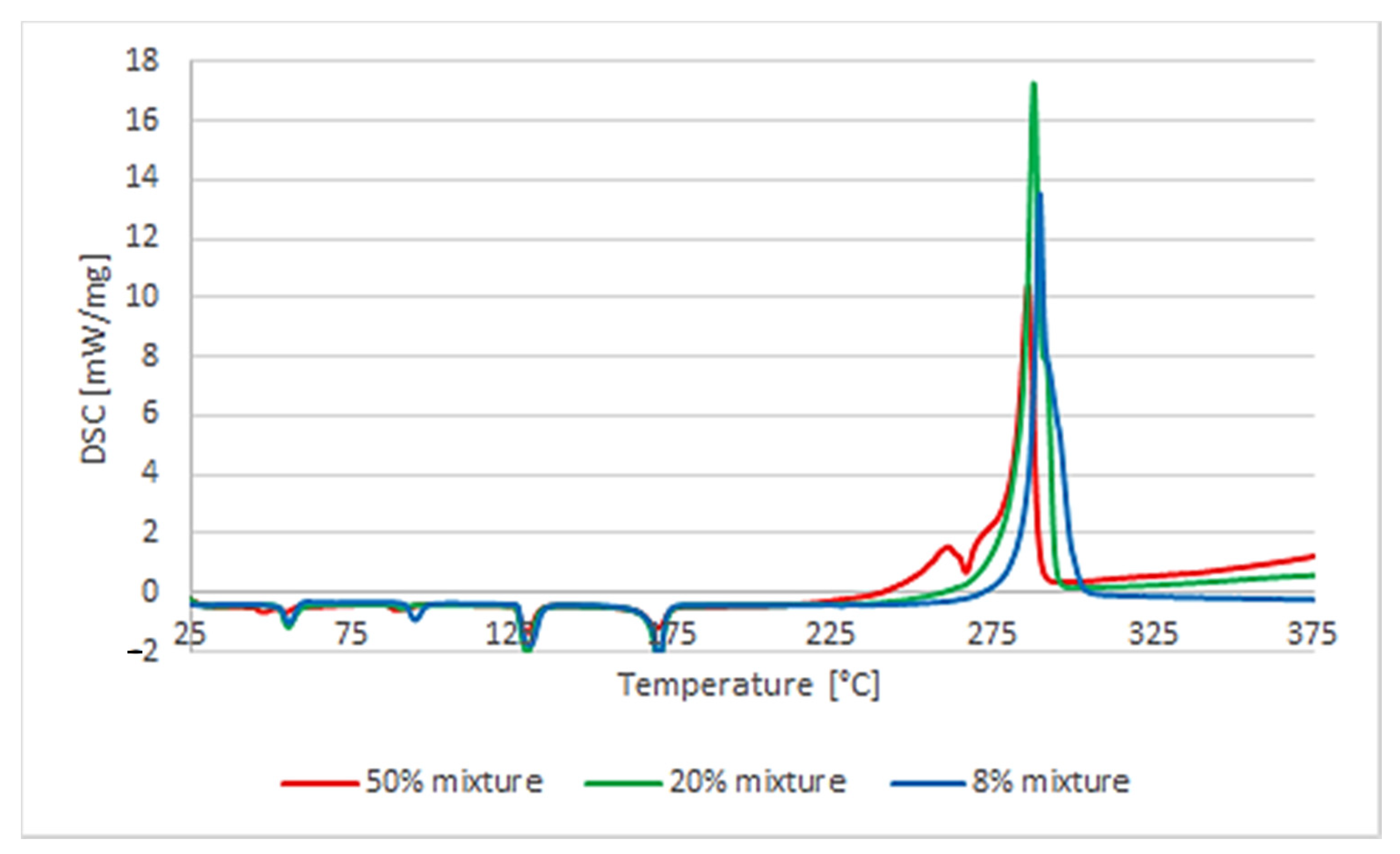
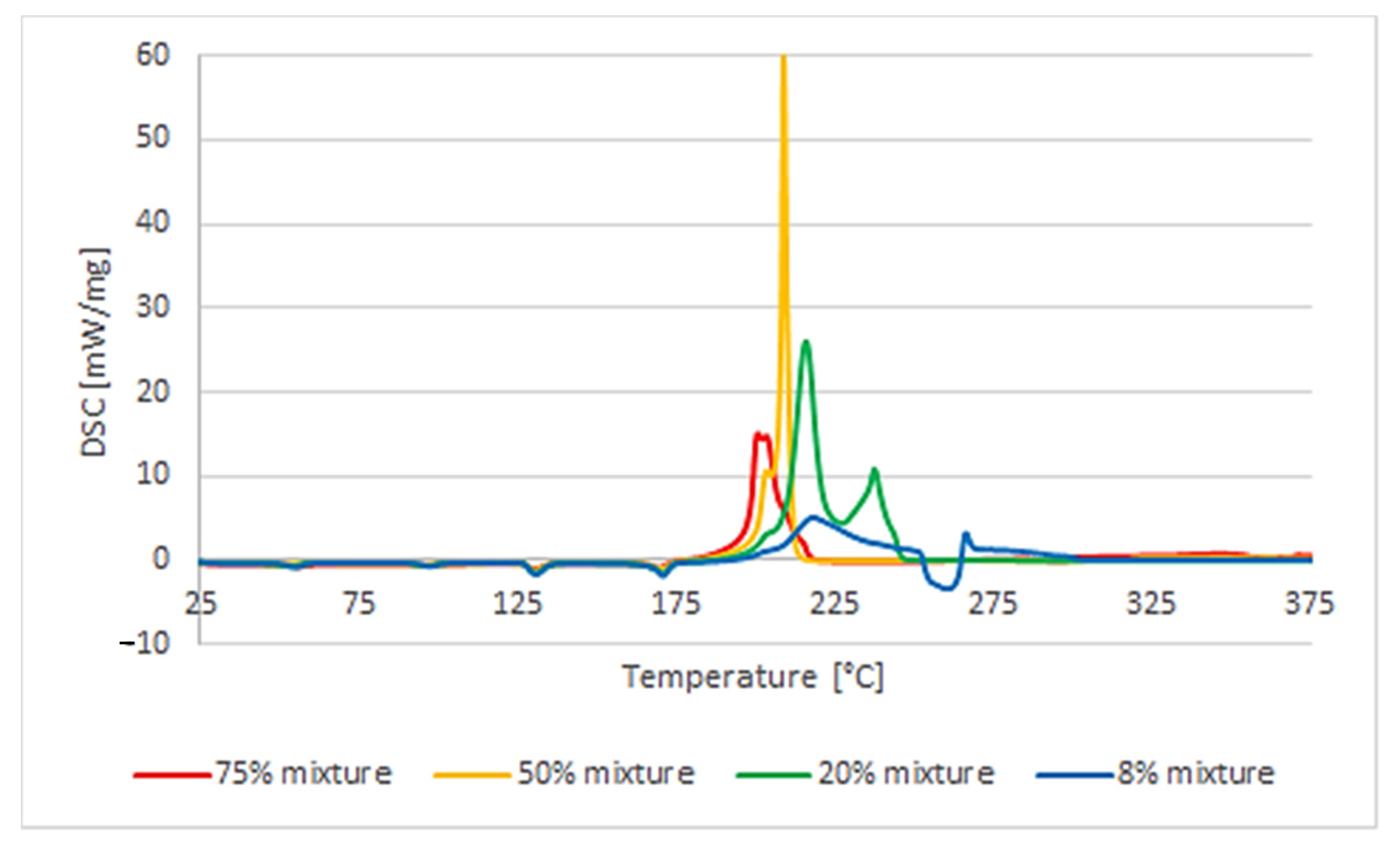
The influence of the percentage of polymer in the mixture with AN on the course of its decomposition was also analyzed. It turned out to be different for PVC than for other polymers. As it comes to the mixture of AN with uPVC, mixtures containing 8%, 20%, 50%, and 75% by mass of the polymer were analyzed. Reducing the polymer content from 20% to 8% resulted in a behavior change of a similar nature. The heat effect was much lower: −396 J/g compared to −1384 J/g for the higher polymer content. Moreover, the splitting of the decomposition reaction was clearly observed. The decomposition process was a multistep one. On the other hand, increasing the amount of PVC to 50% of the mixture mass caused a change in the course of the process observed with the DSC analysis. The decomposition process became explosive and single-step. A more-than-three-times-higher heat release rate was observed compared to the 20% AN/PVC mixture. The measured enthalpy of the reaction was lower than that of the 20% mixture and amounted to −880 J/g. The difference between the temperature of the reaction’s termination and its beginning was 5.7 °C, while in the case of the 8% and 20% mixtures the difference was over 30 °C. Due to the explosive nature of the decomposition of the mixture containing 50% polymer, it was decided to also analyze the mixture containing 75% polymer. The nature of the decomposition process was not changed in relation to the 50% mixture (
Table 1). The initiation temperature of the reaction rose slightly to 250.3 °C. The enthalpy of the reaction decreased to −537 J/g, and the rate of heat released increased more than four times (to 46.3 W/g). The course of the reaction itself was very similar and also indicated the explosive nature of the decomposition reaction (
Figure 4).
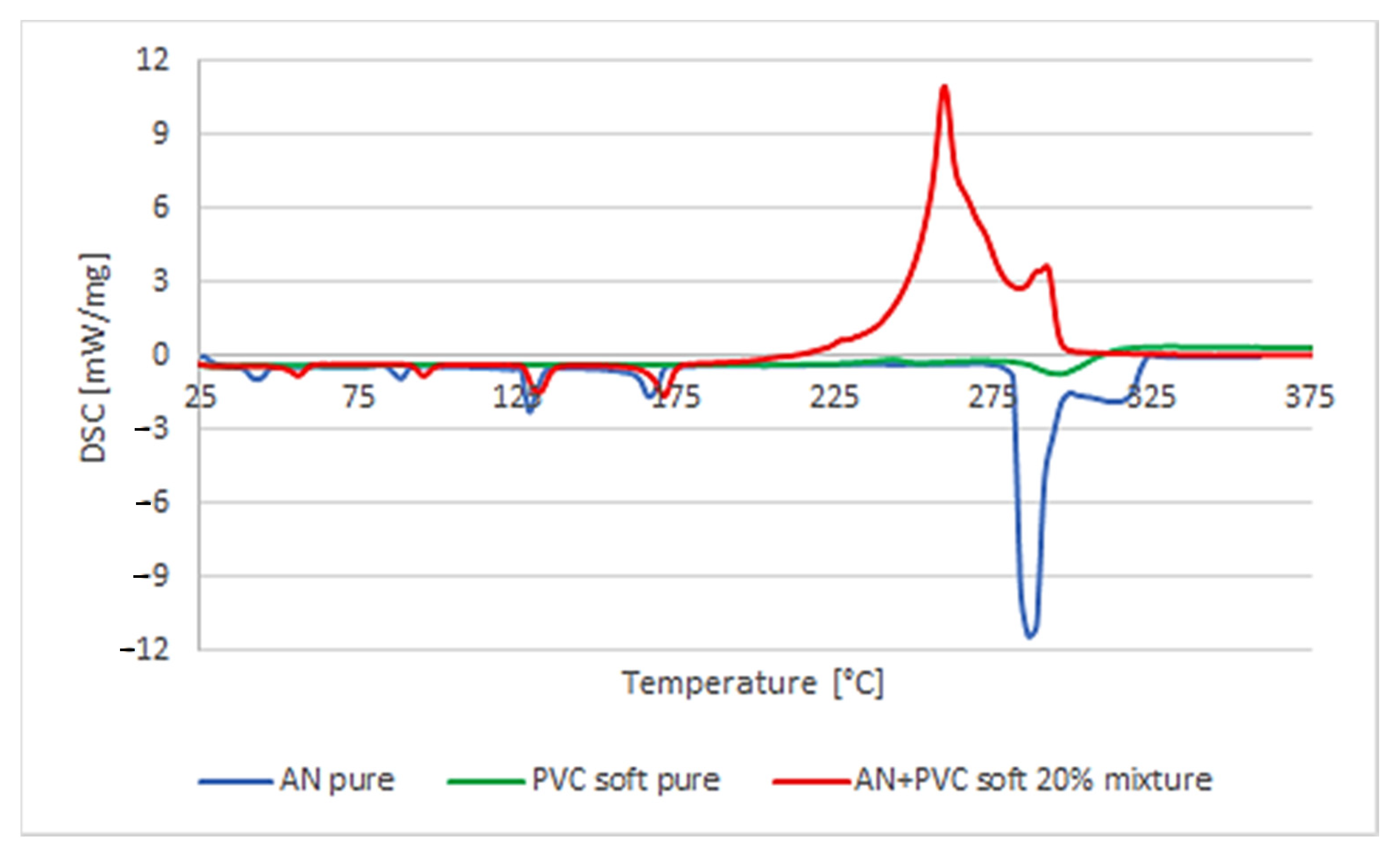
In the case of products made of PVC-P, the observations were similar to those of uPVC-made products. Their AN mixture containing 20% polymer also decomposed in several steps. Some differences were due to the different chemical composition compared to uPVC. In contrast to uPVC, the pure polymer content of PVC-P in the AN mixture can be up to about 50% lower due to the increased plasticizer content. For this reason, it was decided to choose a colorless material, since the addition of dyes, for example, ZnO or TiO
2, in colored uPVC and PVC-P products is typically 20–30%. In the case of a 20% PVC-P mixture with AN, the process was much gentler than in the case of uPVC. At temperatures above 190 °C, we observed the decomposition of the mixture, which accelerated significantly only at above 250 °C. However, in this case, in the measurement conditions we did not register the explosive nature of either the first or the second process. Stagnation enthalpy of the decomposition reaction was −1560 J/g (
Figure 5).
As in the case of uPVC, reducing the amount of material to 8% by mass reduced both the reaction initiation temperature (248.1 °C) and the reduction of its heat effect (−1265 J/g). Moreover, similarly to the previous case, a splitting of the decomposition reaction was observed. In contrast to uPVC, increasing the amount of polymer in the mixture to 50% also resulted in a reduction of the heat effect to an even greater extent than in the case of the 8% mixture (−813.2 J/g). In addition, a significant reduction in the reaction initiation temperature was observed, which, in this case, was 244 °C (
Table 2).
Summing up, for neither of the analyzed cases (range of tested mass concentrations) were typical explosive reactions observed. However, attention should be paid to the test conditions, as the tests were carried out in a way that minimized the pressure increase. Moreover, it should be kept in mind that the products formed as a result of thermal carbonization of PVC-P and uPVC (pyrolytic carbon or chlorine compounds) should enhance the reactivity of the mixture (
Figure 6). Additional hazards may arise from phosgene formation. It has been known since World War I as a chemical warfare agent.
The tests performed for polystyrene (PS) also provided interesting observations concerning the potential explosive properties of the mixture of this polymer with AN. This is another example that the components of a mixture may have explosive properties in certain proportions. These observations are particularly important because EPS is a common material in our vicinity, used for packing mechanically sensitive goods and all kinds of thermal insulation. Observations regarding the course of the reaction for the 20% mixture of AN with EPS were quite similar to those observed for PVC. The decomposition reaction was exothermic. A decrease in the temperature of its initiation was observed (242.1 °C) in relation to pure AN. Despite the relatively high reaction enthalpy value, which amounted to −762 J/g, the reaction corresponding to the explosion was not observed in the measurement conditions. The heat was released quite evenly in the range from 225 to 325 °C. The shape of the DSC graph also indicated that EPS decomposition is a composite process (
Figure 7).
Reducing the PS polymer content in the mixture to 8% resulted in a significant reduction of the decomposition reaction enthalpy (−450 J/g). The decomposition onset temperature dropped slightly, to 242.2 °C. Moreover, attention was drawn to the partial splitting of the course of the reaction on the thermal decomposition diagram. A relatively small, but relatively severe, course of the reaction appeared at a temperature of about 300 °C (
Table 3). However, it did not have a course of an explosive reaction.
Significant changes in the course of the reaction were observed for the mixture containing 50% EPS. The value of the enthalpy of the decomposition reaction in this case was −547 J/g. A relatively low value may be related to the preceding endothermic effect in the range corresponding to the decomposition of AN. Probably both effects partially compensated each other. A significant increase of the temperature of rapid mixture decomposition, which, in this case, was over 280 °C, was noteworthy. At a temperature of around 242 °C, however, slow decomposition of the mixture was observed. Contrary to the measurements of mixtures with lower EPS content, in the case of the 50% polymer mixture there was potentially a real danger of an explosive reaction while heating. For this reason, it was decided to perform the analysis of a mixture with a larger amount of polymer. For the mixture containing 75% EPS, however, a similar reaction course was observed to that of the mixtures containing 8% and 20% polymer. The measured decomposition initiation temperature was almost identical, and the enthalpy of the decomposition process was the lowest among the analyzed samples (−332 J/g). Unlike a 50% mixture, this reaction was not potentially explosive (
Figure 8).
Poly(methyl methacrylate) (PMMA), also known as organic glass, is another polymer for which a risk of an explosive reaction in contact with AN can be noticed. As in the case of most of the tested polymers, the strongest explosive decomposition effect was observed for the 20% mixture. This reflected both the value of the maximum heat flux produced and the measured maximum reaction enthalpy of −799 J/g for the 20% mixture. Contrary to the previously described substances, no decrease in the temperature of the decomposition reaction was observed, but its slight increase, to 285.2 °C, occurred instead. This was largely due to the fact that PMMA began to melt in a temperature range close to the decomposition temperature. For this reason, a liquid explosive mixture was formed at a temperature close to the decomposition temperature of pure AN. This can significantly affect the behavior of the mixture during faster heating due to thermal inertia. The shape of the DSC graph also indicated a two-step mechanism for decomposition reaction of the mixture (
Figure 9).
Contrary to the previously described substances, explosive properties were observed in the case of a mixture containing 8% by weight of polymer. The enthalpy of the decomposition reaction was −681 J/g. Due to the abovementioned high melting point of PMMA, the initiation of the decomposition reaction did not change significantly and took place at a temperature of about 287 °C. The temperature was slightly reduced (280.8 °C) for a mixture containing 50% of polymer. For PMMA, the enthalpy of the decomposition reaction was the lowest among the measured polymers and amounted to −629 J/g (
Table 4). Clear splitting of chemical reactions constituting the stages of the decomposition reaction was noteworthy. The results of the measurements showed that, for each of the analyzed polymer contents in the mixture, its thermal decomposition was explosive (
Figure 10).
Another analyzed polymer is polyamide. PA66 was chosen as the model polymer. The decomposition of the 20% mixture took place in a multi-step manner. There were two main steps in this process. The first one started at 209.8 °C, the second at 233.0 °C. In the analyzed conditions, i.e., without pressure increase, these reactions were not explosive. It is also worth paying attention to the high value of the enthalpy of the decomposition reaction: For the first step of the decomposition process it was −1033 J/g, and −387 J/g for the second step (
Figure 11).
The course of the decomposition reaction of the 8% mixture was very mild. As in the case of the 20% mixture, several steps of this reaction were distinguished. The endothermic process was especially noteworthy. It coincided with the melting point of PA66, which was about 252 °C. The decomposition reaction began at 205.5 °C, and the stagnation enthalpy was −654 J/g. From the safety point of view, this was not a potentially dangerous process. The situation changed completely when the amount of polymer was increased to 50%. The course of the reaction was explosive; moreover, the process took place at low temperature (lower than 200 °C). The decomposition of the mixture was rapid and significant amounts of heat were released in the process: The instantaneous flux of released heat exceeded 60 W/g. The enthalpy of this process was −1095 J/g. Increasing the amount of polymer to 75% by mass of the mixture resulted in a much milder course of the decomposition reaction. As in the previous cases, the temperature of the decomposition process was very low, around 200 °C, and the enthalpy of the process was high (−978 J/g). The decomposition process was also multistep. Contrary to the 50% mixture, the decomposition process was not potentially explosive in the analyzed conditions. Nevertheless, all mixtures of AN with PA decomposed at relatively low temperatures (
Table 5). These processes and their products can initiate further reactions taking place in the reaction environment.
4. Discussion
The basic disadvantages of plastics include their average mechanical properties, low heat resistance, and relatively low temperature of thermal decomposition. Their combustion products additionally pose a serious threat to human life and health related to the emission of gaseous (particularly toxic) products of thermal decomposition [
11]. The conducted tests were to additionally prove whether mixing of plastics with fertilizers containing significant amounts of AN could produce explosive mixtures under fire conditions.
Particular plastics were selected and used for the research because of their enormous popularity and prevalence. Two of them (PP and PE), belonging to the so-called “big five” bulk commodity polymers dominating the market, were described in the first part of the article (a case study). Other plastics with the highest market share include polyvinyl chloride (PVC), polystyrene (solid (PS) and foamed (EPS)), and polyethylene terephthalate (PET) [
12]. In this series of tests, other popular materials, such as PA, were tested instead of PET due to the fact that they are components of building materials, which were the focus of our case study.
An introduction to the combustion processes of polymers and polymer materials along with a short thermochemical description is included in [
13]. The fact that the properties of PP and PS constitute a serious threat in fire conditions due to the high value of HRR (heat release rate), with PS appearing to be the most dangerous [
14], served as an indication for the materials’ selection.
In turn, PA is an important type of engineering material and is widely used due to its unique balance of mechanical, thermal, and electrical properties, but attention should be paid to its flammability, which limits its wider application [
15]. Research on the behavior of these materials under fire conditions was already conducted on a full scale in the early 1970s [
16], e.g., large-scale fire tests of furniture and finishing materials containing nylon components were carried out [
17,
18].
EPS, used as an insulation material in buildings, above the temperature of 200 °C emits flammable gases that ignite (with the participation of a flame) or self-ignite at a temperature of 450–500 °C [
19] (
Figure 12).
Fires in which PVC is burned are characterized by a violent and dynamic course, as a result of which significant amounts of smoke, soot, and volatile toxic substances (toxic decomposition products) are released and cause PVC-P to drip into burning droplets [
21].
The first comprehensive overview of AN explosions in transport or storage over the centuries was provided in [
22]. It was found that all of the tested explosions were caused by uncontrolled fires. Fire and explosion protection measures were identified, which, in the opinion of the author of the study, should prevent the recurrence of such disasters. The law and the implemented procedures should follow technical solutions.
Very detailed studies on the various forms of AN and the method of their classification and their impact on susceptibility to thermal and impact shock are included in [
23]. However, there are some discrepancies in the critical parameters depending on test methods, materials, and configurations (ADR, International Fire Code, HMEx) [
24].
AN is too valuable a chemical compound to disappear from the market in the foreseeable future. Therefore, having over 100 years of experience in its storage and usage, where, in the case of most catastrophes, both the causes and the course of the events are known [
25,
26,
27,
28], it is worth using these conclusions but also checking whether the reactions with other substances than those known to date can cause the AN mixture to become a self-contained explosive.
In [
29] the influence of small additions of various components, including AN, on the flammability of polyurethane foam (PUR) was examined, while in our article we deal with the addition of plastics to AN and the influence of such addition on AN’s explosiveness.
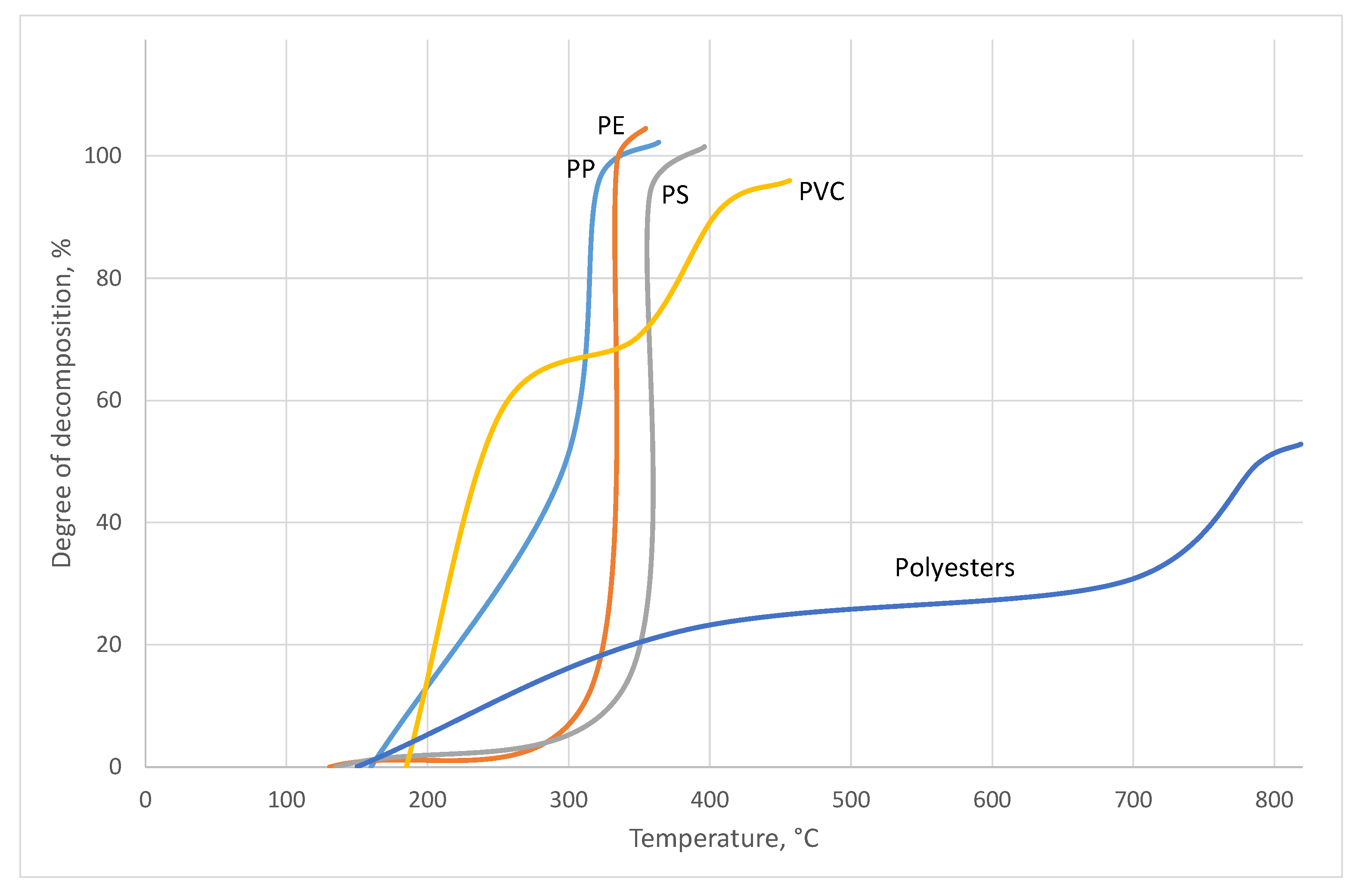
Fires of the polymers alone are violent, releasing significant amounts of toxic combustion products, but for them to occur much greater activation energy is needed. They proceed at much higher temperatures than those with a participation of AN.
Regardless of the composition of polymers, a generalized, three-stage mechanism of their combustion is taken into account [
30,
31]:
In the temperature range 100–250 °C, water is evaporated or, where present, hydrogen halides are precipitated from the polymer.
In the range of 250–500 °C, the bonds in the main chains of the polymer are broken, which leads to its depolymerization or degradation, resulting in the production of flammable monomers or elimination of small fragments of the chain that may undergo direct combustion during a fire (a recombination of chain fragments is also possible, which leads to the formation of condensed aromatic rings that are stable in this temperature range).
At temperatures above 500 °C, the aromatic compounds formed in the previous step undergo further condensation. Then a carbon char layer is formed, which limits flammability through its insulating properties: Most often at the beginning of the described step, it appears in the consistency of a thick liquid, also foamed by saturation with gaseous combustion products.
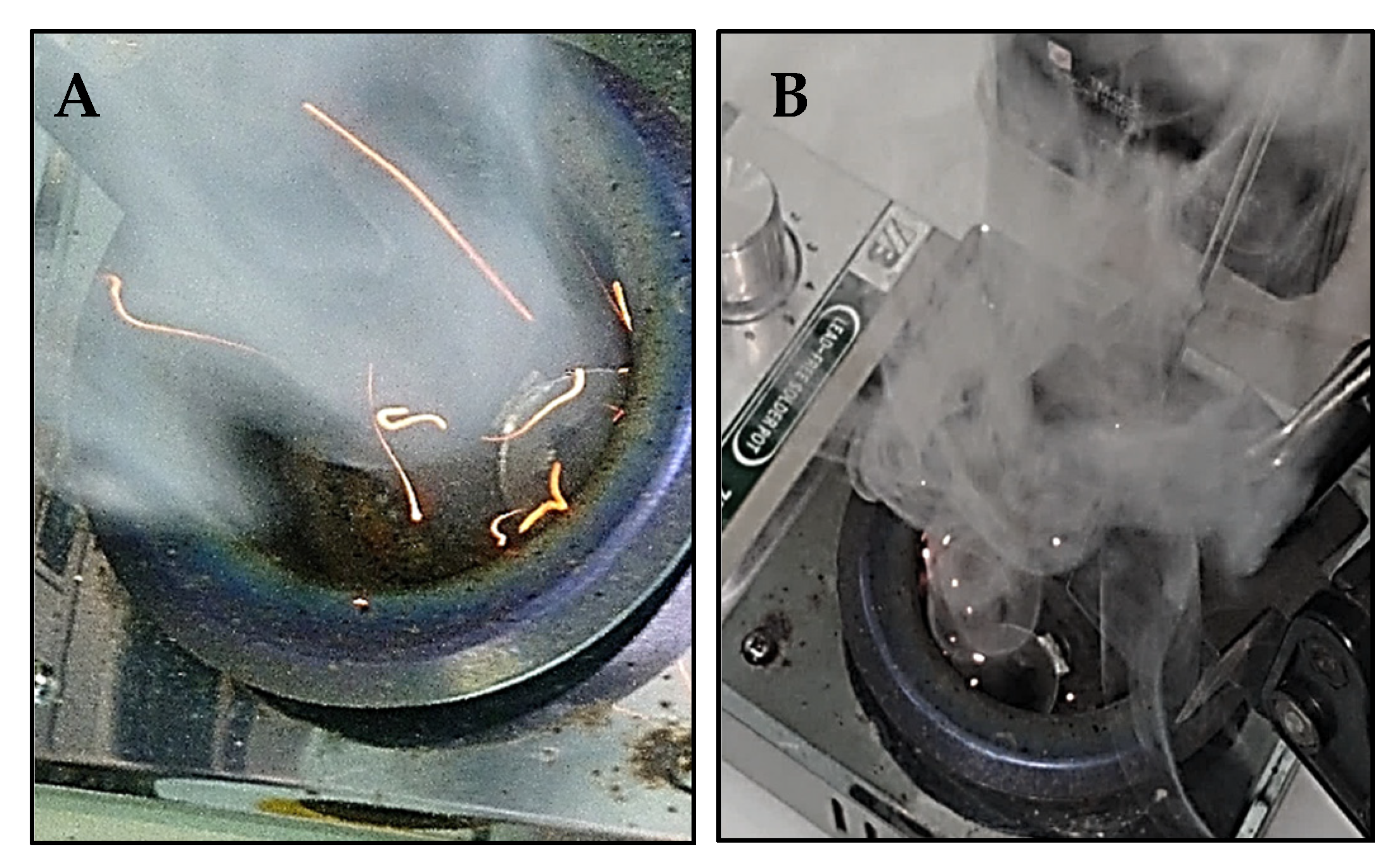
Experiments proved that the presence of AN with polymers in a confined space can lead to a mixture explosion but also to significant reduction in the temperature of the onset of polymer decomposition. The research carried out in this area, concluded on the basis of a review of literature, is innovative and unique, as it was not possible to find similar analyses of the synergy of polymers and AN under fire conditions. It should be noted that due to the microscale of DSC measurements, the explosion of the entire AN deposit should not be considered as unequivocal. For this reason, it is advisable to carry out further macroscale experiments to assess the risk of hazardous phenomena in real conditions (
Figure 13 and
Figure 14). Measurement methodology was based on [
32].
Selected examples of experiments conducted on a laboratory scale are presented above. Open crucibles made of nickel were placed in a furnace heated to 400 °C (power 200 W). The compacted samples, each of mass 0.5 g, were placed in the nickel-measuring crucibles. The experiments were filmed; time (0.1 s) corresponding to the temperature close to the sharp peak, recorded for the respective DSC measurement, was selected.