Isolation and Purification of Actinides Using N,O-Hybrid Donor Ligands for Closing the Nuclear Fuel Cycle
Abstract
1. Introduction
2. Amides of 2-Pyridinecarboxylic (Picolinic) Acid (PA)
3. Diamides of 2,6-Pyridinedicarboxylic (Dipicolinic) Acid (DPA)
4. Amides of 1,10-Phenanthroline-2-carboxylic Acid (PTA)
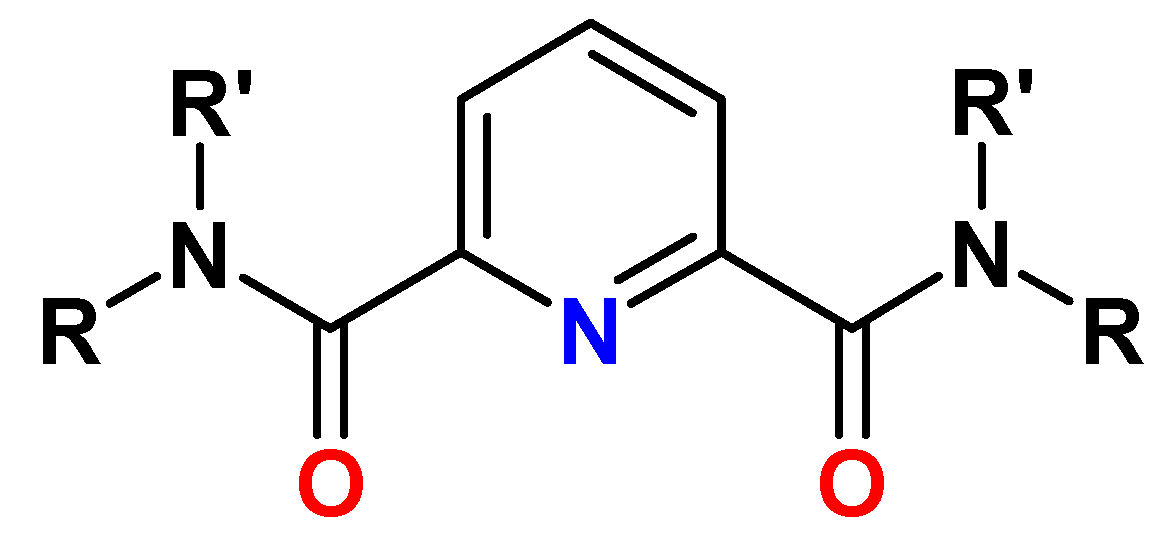
5. Diamides of 2,2′-Dipyridyl-6,6′-dicarboxylic Acid (BPyDA)
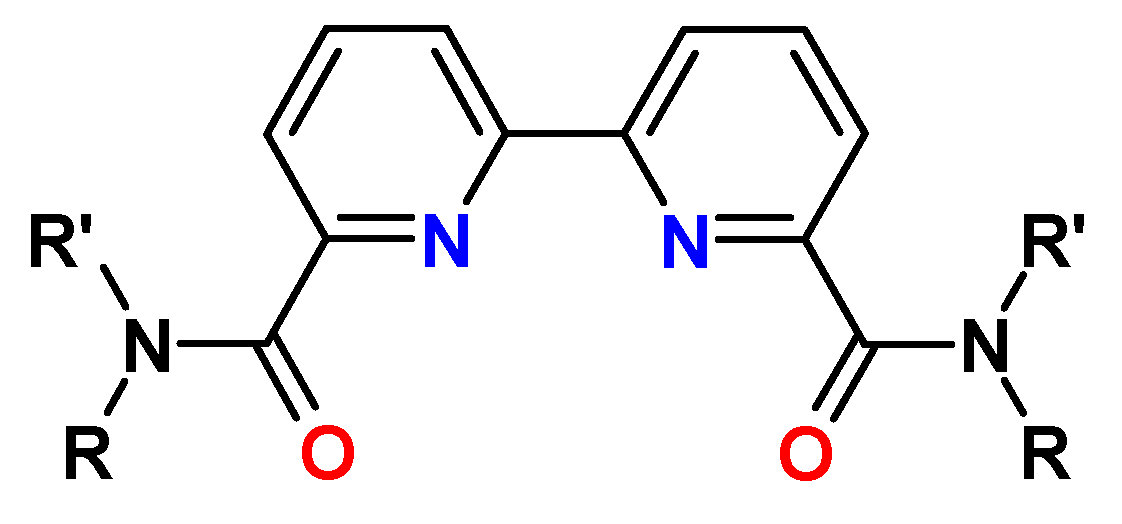
6. Diamides of 6,6″(2,2′:6′,2″-Tripyridine)-dicarboxylic Acid (TPyDA)
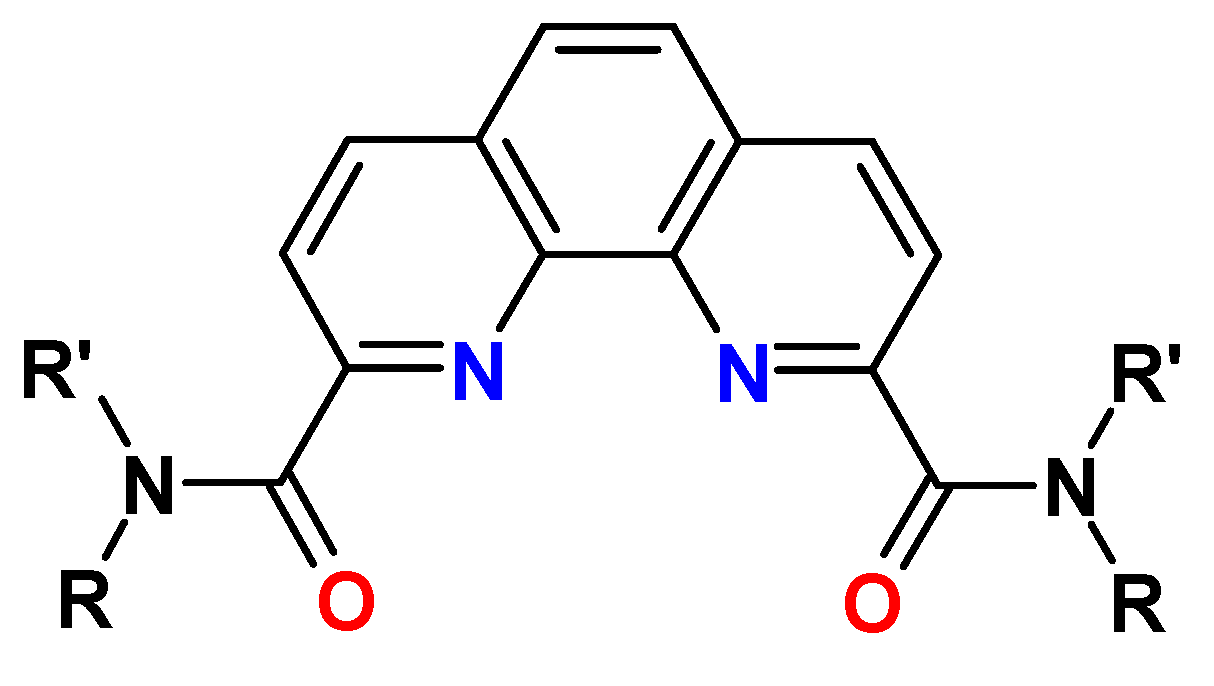
7. Diamides of 1,10-Phenanthroline-2,9-dicarboxylic Acid (PHENDA)
8. Py-Lactams
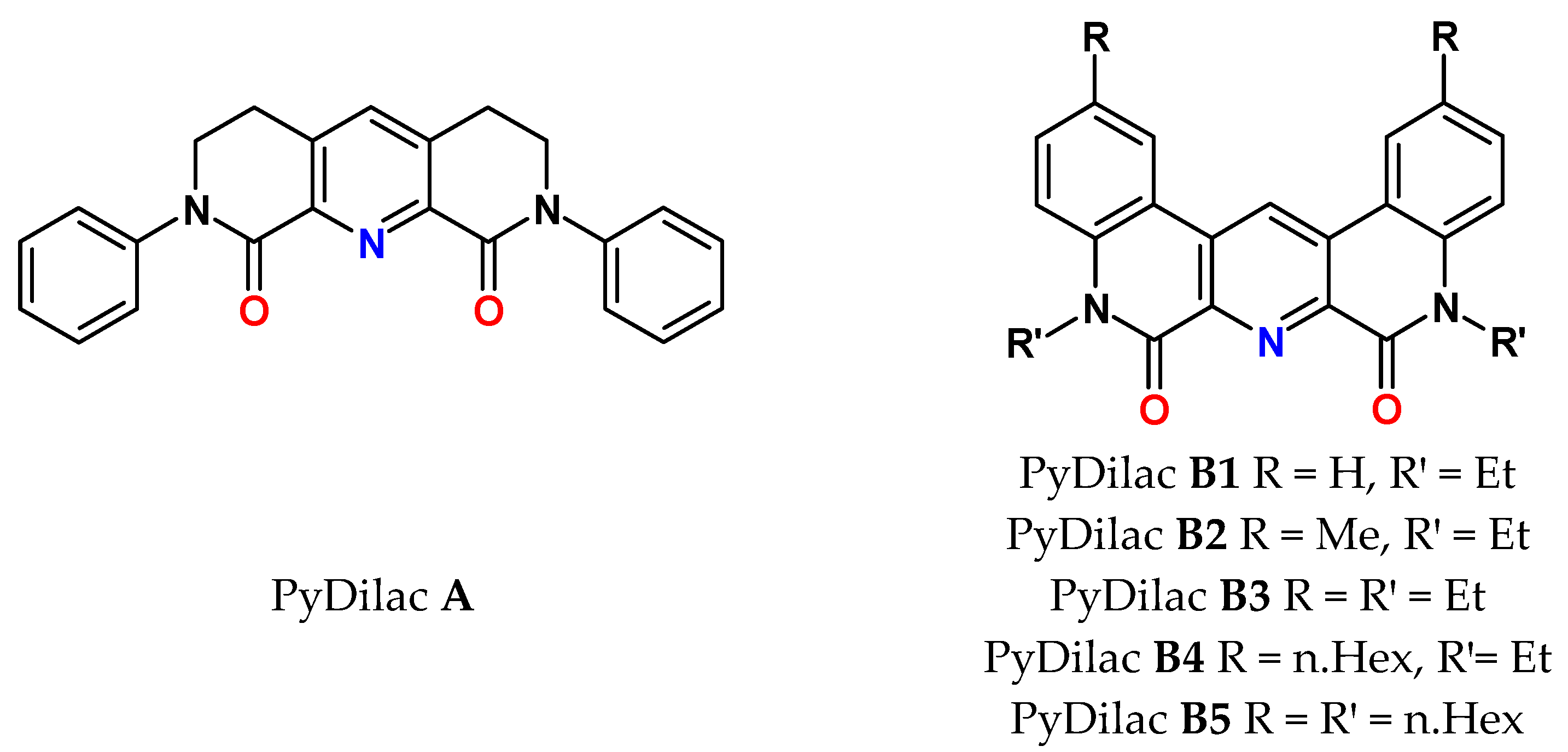
9. Py-Dilactams (PyDilac)
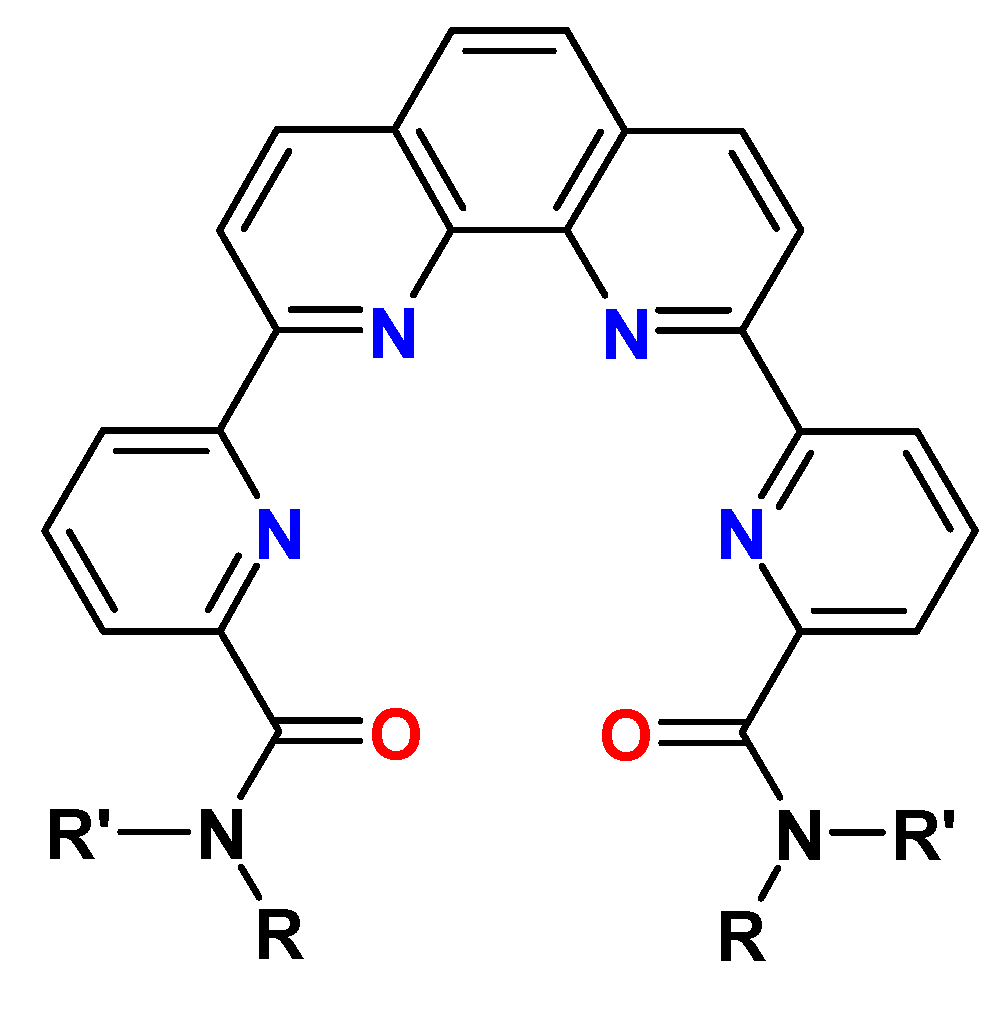
10. Phen-Dilactams (PhenDilac)
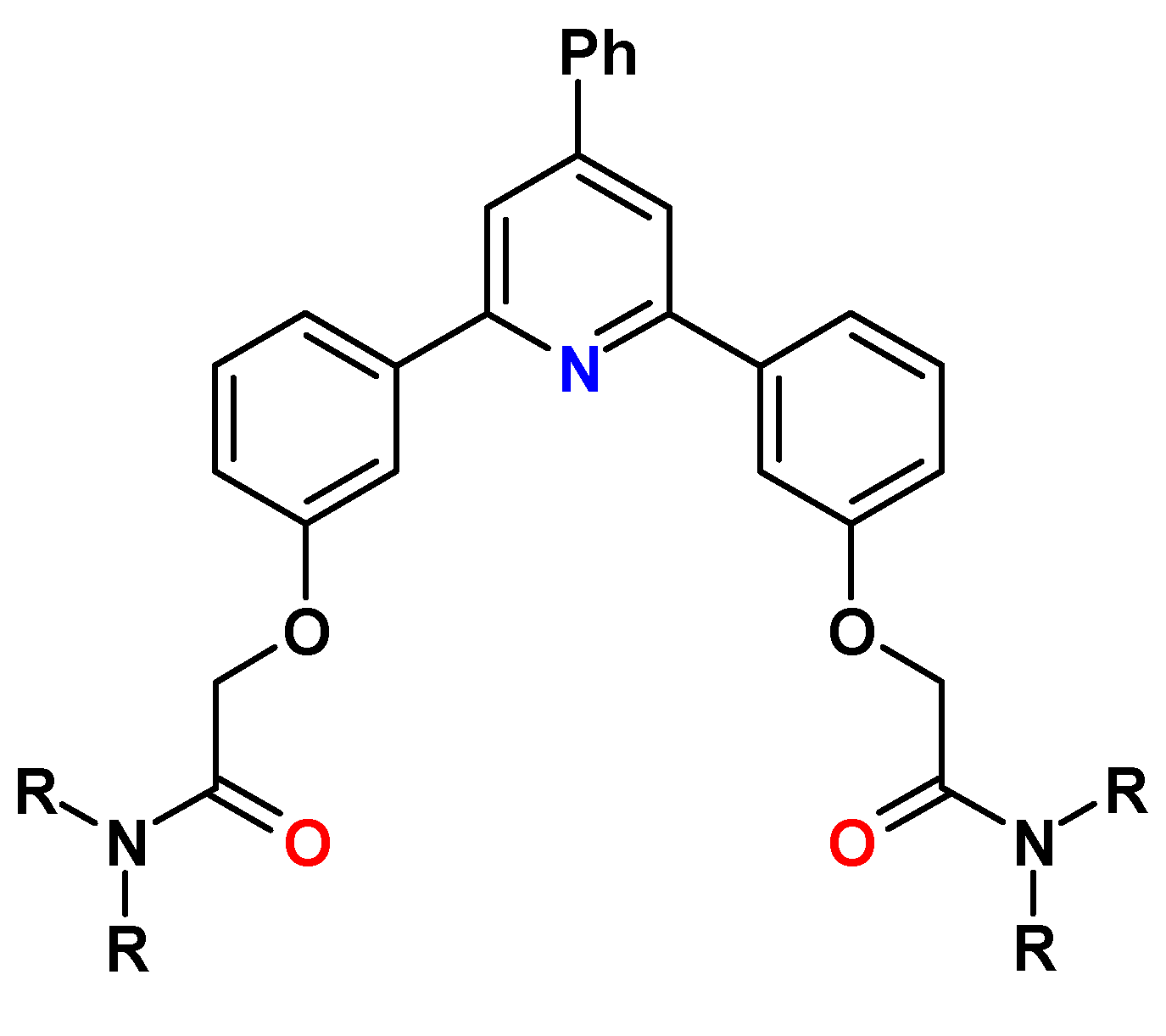
11. 1,10-Phenanthroline-2,9-(2-dipyridyl)-6,6′-dicarboxylic Acid Diamides (Py-Phen)
12. Other N,O Hybrid Amide Extractants
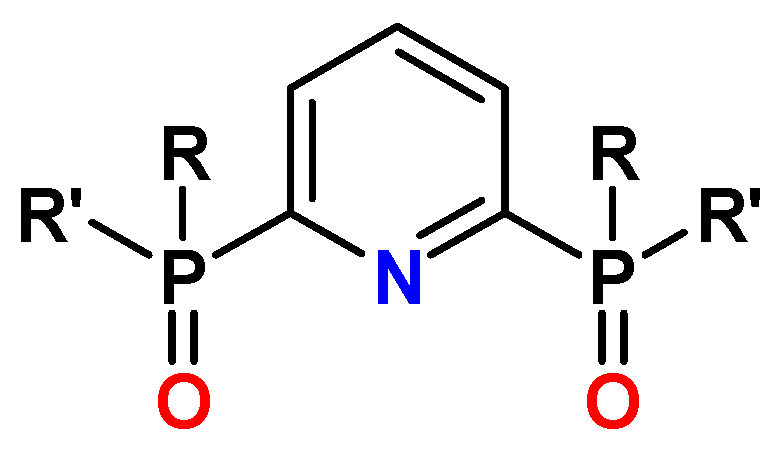
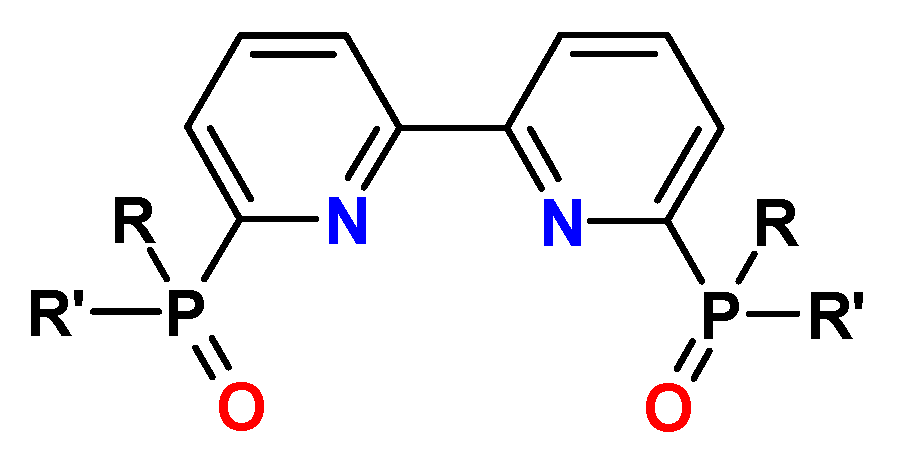
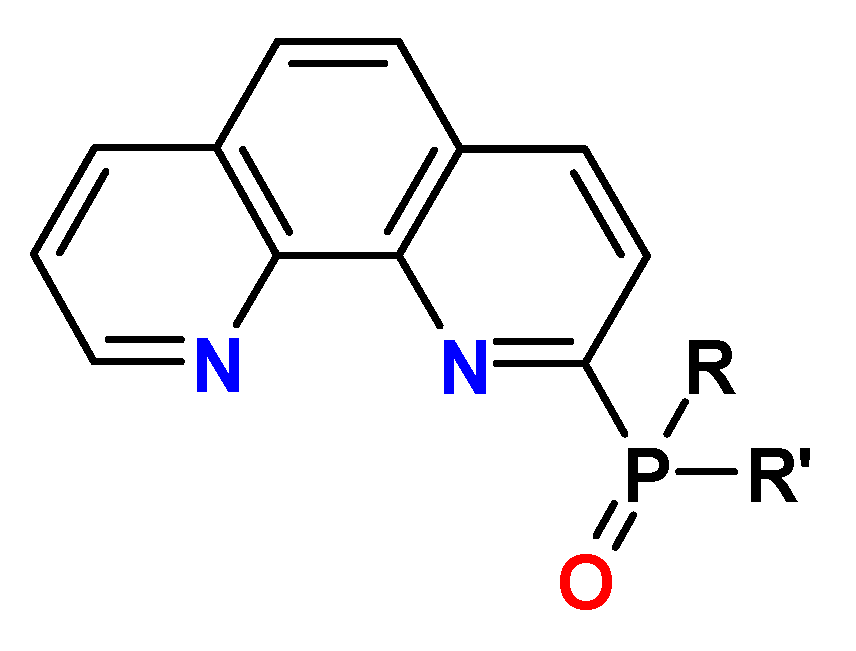
13. N-Heterocyclic Diphosphine Oxides (PyPO, BPyPO, PhenPO)
14. N,O-Hybrid Donor Ligands for Sensors in SNF Reprocessing
15. Conclusions
- -
- All amides of N-heterocyclic dicarboxylic acids are characterized by an “anomalous aryl hardening” effect. The extraction capacity of diamides substituted with both alkyl and aryl substituents is higher than that of diamides having only alkyl substituents. The type of substituent at the amide nitrogen atoms has a significant influence on the properties of diamides of N-heterocyclic dicarboxylic acids. The maximum extraction capacity is achieved for diethyl diaryl derivatives with donor substituents in the phenyl rings. The introduction of additional acceptor substituents in the aryl and pyridine rings, as one would expect, leads to a decrease in the extraction ability of the diamides.
- -
- In the case of diamides of N-heterocyclic dicarboxylic acids, an increase in structural rigidity leads to an increase in the extraction capacity and selectivity of actinide extraction. When comparing two pairs of ligands, DPA–DilacDPA and PhenDA–DilacPhen, it is clearly seen that the ligands with greater structural rigidity—dilactams and diamides of 1,10-phenanthroline-2,9-dicarboxylic acid, whose “pre-organization” energy is minimal—have the greatest extraction ability.
- -
- An increase in the number of “soft” donors in the ligand structure leads to an increase in the extraction selectivity of actinides, including Am(III). However, the introduction of a large number of “soft donors” into the diamide structure leads to a strong increase in the Brandsted basicity of the ligand, a decrease in its extraction ability in acidic solutions, and even a decrease in selectivity. Ligands in which each heterocycle (pyridine, phenanthroline) has two carboxamide substituents in position 2 to the heterocyclic nitrogen atom have the lowest basicity. All other compounds (monoamides of pyridine-2-carboxylic acid, amides of 1,10-phenanthroline-2-carboxylic acid and tripyridine diamides) have appreciable basicity and cannot be used to extract metals from solutions with high acid concentration.
- -
- Replacement of amide groups with phosphine oxide or phosphonate groups does not lead to an increase in actinide extraction selectivity;
- -
- As one would expect, heterocyclic phosphine oxides are more radiation resistant than phosphonate derivatives or amides;
- -
- At this point, the extraction of other fission products must be studied to decide whether N-heterocyclic phosphorus-containing ligands are suitable for the creation of process schemes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olenin, Y.A.; Ilgisonis, V.I. The actual scientific and technical problems of nuclear energy. Her. Russ. Acad. Sci. 2019, 89, 335–342. (In Russian) [Google Scholar] [CrossRef]
- Pershukov, V.; Artisyuk, V.; Kashirsky, A. Paving the way to green status for nuclear power. Sustainability 2022, 14, 9339. [Google Scholar] [CrossRef]
- Todd, T.A. Development of closed nuclear fuel cycles in the United States. In Reprocessing and Recycling of Spent Nuclear Fuel; Woodhead Publishing: Sawasto, UK, 2015; pp. 523–530. [Google Scholar]
- Tsvetkov, P.; Waltar, A.; Todd, D. Sustainable development of nuclear energy and the role of fast spectrum reactors. In Fast Spectrum Reactors; Springer: Boston, MA, USA, 2012; pp. 3–22. [Google Scholar]
- Adamov, E.O.; Mochalov, Y.S.; Rachkov, V.I.; Khomyakov, Y.S.; Shadrin, A.Y.; Kascheev, V.A.; Khaperskaya, A.V. Spent nuclear fuel reprocessing and nuclear materials recycling in two-component nuclear energy. At. Energy 2021, 130, 29–35. [Google Scholar] [CrossRef]
- Tolstoukhov, D.; Panov, S.; Presnyakov, I. Economic aspects of nuclear fuel cycle closure on the basis of fast neutron reactors in the framework of “Proryv” project direction implementation. Nucl. Eng. Des. 2021, 384, 111471. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Singh, D.; Avtar, R.; Hwang, G.H.; Setiadi, T.; Lo, W.H. Technological solutions for long-term storage of partially used nuclear waste: A critical review. Ann. Nucl. Energy 2022, 166, 108736. [Google Scholar] [CrossRef]
- Lyseid Authen, T.; Adnet, J.M.; Bourg, S.; Carrott, M.; Ekberg, C.; Galán, H.; Geist, A.; Guilbaud, P.; Miguirditchian, M.; Modolo, G.; et al. An overview of solvent extraction processes developed in Europe for advanced nuclear fuel recycling, Part 2—Homogeneous recycling. Sep. Sci. Technol. 2022, 57, 1724–1744. [Google Scholar] [CrossRef]
- Mathur, J.N.; Murali, M.S.; Nash, K.L. Actinide partitioning—A review. Solv. Extr. Ion Exch. 2001, 19, 357–390. [Google Scholar] [CrossRef]
- Tachimori, S.; Morita, Y. Overview of solvent extraction chemistry for reprocessing. In Ion Exchange and Solvent Extraction. A Series of Advances; Moyer, B., Ed.; CRC Press is an imprint of Taylor & Francis Group: New York, NY, USA, 2010; Volume 19, pp. 1–63. [Google Scholar]
- Dam, H.H.; Beijleveld, H.; Reinhoudt, D.N.; Verboom, W. In the pursuit for better actinide ligands: An efficient strategy for their discovery. J. Am. Chem. Soc. 2008, 130, 5542–5551. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Ustynyuk, Y.A. Recovery of minor actinides from high-level wastes: Modern trends. Russ. Chem. Rev. 2016, 85, 943. [Google Scholar] [CrossRef]
- Leoncini, A.; Huskens, J.; Verboom, W. Ligands for f-element extraction used in the nuclear fuel cycle. Chem. Soc. Rev. 2017, 46, 7229–7273. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Nash, K.L. A review of the development and operational characteristics of the TALSPEAK process. Solv. Extr. Ion Exch. 2007, 25, 665–701. [Google Scholar] [CrossRef]
- Braley, J.C.; Carter, J.C.; Sinkov, S.I.; Nash, K.L.; Lumetta, G.J. The role of carboxylic acids in TALSQuEAK separations. J. Coord. Chem. 2012, 65, 2862–2876. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Gelis, A.V.; Braley, J.C.; Carter, J.C.; Pittman, J.W.; Warner, M.G.; Vandegrift, G.F. The TRUSPEAK concept: Combining CMPO and HDEHP for separating trivalent lanthanides from the transuranic elements. Solv. Extr. Ion Exch. 2013, 31, 223–236. [Google Scholar] [CrossRef]
- Gelis, A.V.; Lumetta, G.J. Actinide lanthanide separation process ALSEP. Ind. Eng. Chem. Res. 2014, 53, 1624–1631. [Google Scholar] [CrossRef]
- Suneesh, A.S.; Kumaresan, R.; Rajeswari, S.; Nayak, P.K.; Syamala, K.V.; Venkatesan, K.A.; Antony, M.P.; Rao, P.V. Development and demonstration of americium (III)-europium (III) separation using diglycolamic acid. Sep. Sci. Technol. 2013, 48, 1998–2006. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Kenf, E.V.; Tkachenko, L.I.; Logunov, M.V.; Voroshilov, Y.A.; Vidanov, V.L. Method for Isolating Americium from Liquid Radioactive Waste and for Separating Americium from Rare Earth Elements. WIPO WO2016182472A1, 17 March 2020. RU Pat. 2603405, 27 November 2016; Bull. No. 33. [Google Scholar]
- Tkachenko, L.; Kenf, E.; Babain, V.; Alyapyshev, M.; Logunov, M.; Voroshilov, Y.; Vidanov, V.; Shadrin, A.; Zverev, D. Dynamic Test of Extraction Process for Americium Partitioning from the PUREX Raffinate. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/49/085/49085971.pdf (accessed on 22 September 2022).
- Kolarik, Z. Complexation and separation of lanthanides(III) and actinides(III) by heterocyclic N-donors in solutions. Chem. Rev. 2008, 108, 4208–4252. [Google Scholar] [CrossRef]
- Ekberg, C.; Fermvik, A.; Retegan, T.; Skarnemark, G.; Foreman, M.R.S.; Hudson, M.J.; Englund, S.; Nilsson, M. An overview and historical look back at the solvent extraction using nitrogen donor ligands to extract and separate An(III) from Ln(III). Radiochim. Acta 2008, 96, 225–233. [Google Scholar] [CrossRef]
- Panak, P.J.; Geist, A. Complexation and extraction of trivalent actinides and lanthanides by triazinylpyridine N-donor ligands. Chem. Rev. 2013, 113, 1199–1236. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.J.; Harwood, L.M.; Laventine, D.M.; Lewis, F.W. Use of soft heterocyclic N-donor ligands to separate actinides and lanthanides. Inorg. Chem. 2013, 52, 3414–3428. [Google Scholar] [CrossRef]
- Zalupski, P.R.; Ensor, D.D.; Riddle, C.L.; Peterman, D.R. Complete recovery of actinides from UREX-like raffinates using a combination of hard and soft donor ligands. Solv. Extr. Ion Exch. 2013, 31, 430–441. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kitatsuji, Y.; Tsubata, Y.; Sugo, Y.; Morita, Y. Separation of Am, Cm and lanthanides by solvent extraction with hydrophilic and lipophilic organic ligands. Solv. Extr. Res. Dev. Jpn. 2011, 18, 93–101. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tsubata, Y.; Kitatsuji, Y.; Sugo, Y.; Shirasu, N.; Morita, Y. Novel extractant, NTAamide, and its combination with TEDGA for mutual separation of Am/Cm/Ln. Solv. Extr. Ion Exch. 2014, 32, 179–188. [Google Scholar] [CrossRef]
- Suzuki, H.; Tsubata, Y.; Kurosawa, T.; Sagawa, H.; Matsumura, T. Continuous extraction and separation of Am(III) and Cm(III) using a highly practical diamide amine extractant. J. Nucl. Sci. Technol. 2017, 54, 1163–1167. [Google Scholar] [CrossRef]
- Ban, Y.; Suzuki, H.; Hotoku, S.; Tsutsui, N.; Tsubata, Y.; Matsumura, T. Minor actinides separation by N, N, N’, N’, N’’, N’’-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell. Solv. Extr. Ion Exch. 2019, 37, 489–499. [Google Scholar] [CrossRef]
- Kaneko, M.; Suzuki, H.; Matsumura, T. Theoretical elucidation of Am (III)/Cm (III) separation mechanism with diamide-type ligands using relativistic density functional theory calculation. Inorg. Chem. 2018, 57, 14513–14523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Ding, S.; Liu, Y.; Zhang, L.; Song, L.; Chen, Z.; Yang, X.; Wang, X. Non-heterocyclic N-donor ligands of nitrilotriacetamide for Am3+/Eu3+ separation. Sep. Purif. Technol. 2019, 210, 107–116. [Google Scholar] [CrossRef]
- Matveev, P.; Mohapatra, P.K.; Kalmykov, S.N.; Petrov, V. Solvent extraction systems for mutual separation of Am (III) and Cm (III) from nitric acid solutions. A review of recent state-of-the-art. Solvent Extr. Ion Exch. 2021, 39, 679–713. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Tkachenko, L.I. Amides of heterocyclic carboxylic acids as novel extractants for high-level waste treatment. Radiochemistry 2014, 56, 565–574. [Google Scholar] [CrossRef]
- Kwon, S.G.; Lee, E.H.; Yoo, J.H.; Park, H.S.; Kim, J.S. Extraction of Eu-152, Nd and Am-241 from the simulated liquid wastes by picolinamide(C8H17). J. Korean Soc. 1999, 31, 498–505. [Google Scholar]
- Nigond, L.; Condamines, N.; Cordier, P.-Y.; Livet, J.; Madic, C.; Cuillerdier, C.; Musikas, C. Recent advances in the treatment of nuclear wastes by the use of diamide and picolinamide extractants. Sep. Scie. Technol. 1995, 30, 2075–2099. [Google Scholar] [CrossRef]
- Casnati, A.; Della Ca’, N.; Fontanella, M.; Sansone, F.; Ugozzoli, F.; Ungaro, R.; Liger, K.; Dozol, J.F. Calixarene-Based Picolinamide Extractants for Selective An/Ln Separation from Radioactive Waste. Eur. J. Org. Chem. 2005, 2005, 2338–2348. [Google Scholar] [CrossRef]
- Macerata, E.; Sansone, F.; Baldini, L.; Ugozzoli, F.; Brisach, F.; Haddaoui, J.; Hubscher-Bruder, V.; Arnaud-Neu, F.; Mariani, M.; Ungaro, R.; et al. Calix[6]arene-Picolinamide Extractants for Radioactive Waste Treatment: Effect of Additional Carboxy Binding Sites in the Pyridine 6-Positions on Complexation, Extraction Efficiency and An/Ln Separation. Eur. J. Org. Chem. 2010, 2010, 2675–2686. [Google Scholar] [CrossRef]
- Galletta, M.; Baldini, L.; Sansone, F.; Ugozzoli, F.; Ungaro, R.; Casnati, A.; Mariani, M. Calix[6]arene-picolinamide extractants for radioactive waste: Effect of modification of the basicity of the pyridine N atom on the extraction efficiency and An/Ln separation. Dalton Trans. 2010, 39, 2546–2553. [Google Scholar] [CrossRef]
- Cai, Y.; Ansari, S.A.; Fu, K.; Zhu, B.; Ma, H.; Chen, L.; Conradson, S.D.; Qin, S.; Fu, H.; Mohapatra, P.K.; et al. Highly efficient actinide (III)/lanthanide (III) separation by novel pillar[5]arene-based picolinamide ligands: A study on synthesis, solvent extraction and complexation. J. Hazard. Mater. 2021, 405, 124214. [Google Scholar] [CrossRef] [PubMed]
- Mowafy, E.A.; El-Nagar, I.M.; Shalash, A.M. Development of novel organic extractants for applications in various radioactive waste remediation. Arab J. Nucl. Scie. Appl. 2004, 37, 43–56. [Google Scholar]
- Mowafy, E.A.; El-Nagar, I.M.; Shalash, A.M. Extraction and separation of U(VI), Th(IV) and Fe(III) with N, N-di-alkyl-4-pyridine carboxamides and N, N’-tetra-alkyl-2, 6-pyridine dicarboxamides as new organic extractants from chloride medium. Arab J. Nucl. Scie. Appl. 2004, 37, 69–82. [Google Scholar]
- Mowafy, E.A.; Shalash, A.M.; El-Nagar, I.M. Extraction of certain radionuclides by bipicolinamides as new extractants from nitric acid medium. Ind. J. Chem. A 2003, 42, 3012–3016. [Google Scholar]
- Babain, V.A.; Alyapyshev, M.Y.; Smirnov, I.V.; Shadrin, A.Y. Extraction of Am and Eu with N, N′-substituted pyridine-2, 6-dicarboxamides in fluorinated diluents. Radiochemistry 2006, 48, 369–373. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Smirnov, I.V. Extractive properties of synergistic mixtures of dipicolinic acid diamides and chlorinated cobalt dicarbollide. Radiochemistry 2004, 46, 270–271. [Google Scholar] [CrossRef]
- Shimada, A.; Yaita, T.; Narita, H.; Tachimori, S.; Kimura, T.; Okuno, K.; Nakano, Y. Extraction of Am (III) and lanthanide (III) ions from HNO3 solutions N, N’-dimethyl-N, N’-diphenylpyridine-2, 6-dicarboxyamide. Solv. Extr. Res. Dev. Jpn. 2004, 11, 1–10. [Google Scholar]
- Alyapyshev, M.Y.; Babain, V.A.; Tkachenko, L.I.; Eliseev, I.I.; Didenko, A.V.; Petrov, M.L. Dependence of extraction properties of 2, 6-dicarboxypyridine diamides on extractant structure. Solv. Extr. Ion Exch. 2011, 29, 619–636. [Google Scholar] [CrossRef]
- Babain, V.A.; Alyapyshev, M.Y.; Kiseleva, R.N. Metal extraction by N, N’-dialkyl-N, N’-diaryl-dipicolinamides from nitric acid solutions. Radiochim. Acta 2007, 95, 217–223. [Google Scholar] [CrossRef]
- Lapka, J.L.; Paulenova, A.; Alyapyshev, M.Y.; Babain, V.A.; Law, J.D.; Herbst, R.S. The extraction of actinides from nitric acid solutions with diamides of dipicolinic acid. IOP Conf. Series: Mater. Sci. Eng. 2010, 9, 012068. [Google Scholar] [CrossRef]
- Shimada, A.; Yaita, T.; Narita, H.; Tachimori, S.; Kimura, T.; Okuno, K. Extraction studies of lanthanide(III) ions with N, N’-dimethyl-N, N’-diphenylpyridine-2, 6-dicarboxyamide (DMDPhPDA) from nitric acid solutions. Solv. Extr. Ion Exch. 2004, 22, 147–161. [Google Scholar] [CrossRef]
- Paulenova, A.; Alyapyshev, M.Y.; Babain, V.A.; Herbst, R.S.; Law, J.D. Extraction of lanthanides with diamides of dipicolinic acid from nitric acid solutions. I. Sep. Sci. Technol. 2008, 43, 2606–2618. [Google Scholar] [CrossRef]
- Ren, Y.; Polukeev, V.A.; Kenf, E.V.; Tkachenko, L.I.; Alyapyshev, M.Y.; Babain, V.A.; Nechaev, A.V.; Legin, A.V.; Kirsanov, D.O. Substituted diamides of dipicolinic acid as extractants and ionophores for scandium, yttrium and lanthanides. J. Rare Earths 2022, in press. [CrossRef]
- Alyapyshev, M.; Babain, V.; Tkachenko, L.; Kenf, E.; Voronaev, I.; Dar’In, D.; Matveev, P.; Petrov, V.; Kalmykov, S.; Ustynyuk, Y. Extraction of actinides with heterocyclic dicarboxamides. J. Radioanal. Nucl. Chem. 2018, 316, 419–428. [Google Scholar] [CrossRef]
- Hu, P.; Qian, L.; He, Y.; Wang, H.; Wu, W. Solvent extraction of uranium (VI) and thorium (IV) by N, N′-di-p-tolylpyridine-2, 6-dicarboxamide from nitric acid solution. J. Radioanal. Nucl. Chem. 2013, 297, 133–137. [Google Scholar] [CrossRef]
- Ustynyuk, Y.A.; Gloriozov, I.P.; Kalmykov, S.N.; Mitrofanov, A.A.; Babain, V.A.; Alyapyshev, M.Y.; Ustynyuk, N.A. Pyridinedicarboxylic acid diamides as selective ligands for extraction and separation of trivalent lanthanides and actinides: DFT study. Solv. Extr. Ion Exch. 2014, 32, 508–528. [Google Scholar] [CrossRef]
- Lapka, J.L.; Paulenova, A.; Herbst, R.S.; Law, J.D. The radiolytic and thermal stability of diamides of dipicolinic acid. Sep Sci Technol. 2010, 45, 1706–1710. [Google Scholar] [CrossRef]
- Bubeníková, M.; Rais, J.; Selucký, P.; Kvíčala, J. Am (III) separation from acidic solutions by diamides of dipicolinic acid. Radiochim. Acta 2013, 101, 753–759. [Google Scholar] [CrossRef]
- Patil, A.B.; Pathak, P.N.; Shinde, V.S.; Alyapyshev, M.Y.; Babain, V.A.; Mohapatra, P.K. A novel solvent system containing a dipicolinamide in room temperature ionic liquids for actinide ion extraction. J. Radioanal. Nucl. Chem. 2015, 305, 521–528. [Google Scholar] [CrossRef]
- Lapka, J.; Paulenova, A.; Alyapyshev, M.; Babain, V.; Herbst, R.; Law, J. Extraction of molybdenum and technetium with diamides of dipicolinic acid from nitric acid solutions. J. Radioanal. Nucl. Chem. 2009, 280, 307–313. [Google Scholar] [CrossRef]
- Alyapyshev, M.; Babain, V.; Tkachenko, L. Various flowsheets of actinides recovery with diamides of heterocyclic dicarboxylic acids. J. Radioanal. Nucl. Chem. 2017, 312, 47–58. [Google Scholar] [CrossRef]
- Makrlík, E.; Vaňura, P.; Selucký, P.; Babain, V.A.; Smirnov, I.V. Extractive properties of synergistic mixture of hydrogen dicarbollylcobaltate and N, N, N′, N′-tetraisobutyl-2, 6-dipicolinamide in the water–nitrobenzene system with regard to Eu3+ and Am3+. Acta Chim. Slov. 2009, 56, 718–722. [Google Scholar]
- Makrlík, E.; Vaňura, P.; Selucký, P.; Babain, V.; Smirnov, I. Solvent extraction of europium and americium into phenyltrifluoromethyl sulfone by using synergistic mixture of hydrogen dicarbollylcobaltate and N, N, N′, N′-tetraethyl-2, 6-dipicolinamide. J. Radioanal. Nucl. Chem. 2010, 284, 629–633. [Google Scholar] [CrossRef]
- Romanovskiy, V.N.; Babain, V.A.; Alyapyshev, M.Y.; Smirnov, I.V.; Herbst, R.S.; Law, J.D.; Todd, T.A. Radionuclide Extraction by 2, 6-Pyridinedicarboxylamide Derivatives and Chlorinated Cobalt Dicarbollide. Sep. Sci. Technol. 2006, 41, 2111–2127. [Google Scholar] [CrossRef]
- Makrlík, E.; Vaňura, P.; Selucký, P.; Babain, V.; Smirnov, I. Extraction of europium and americium into phenyltrifluoromethyl sulfone by using hydrogen dicarbollylcobaltate in the presence of N, N′-diethyl-N, N′-diphenyl-2, 6-dipicolinamide. J. Radioanal. Nucl. Chem. 2010, 283, 839–844. [Google Scholar] [CrossRef]
- Makrlík, E.; Vaňura, P.; Selucký, P.; Babain, V.A.; Alyapyshev, M.Y. Extraction of microamounts of Eu and Am into nitrobenzene using a synergistic mixture of chlorinated hydrogen dicarbollylcobaltate and N, N′-diethyl-N, N′-diphenyl-2, 6-dipicolinamide. Radiochemistry 2009, 51, 479–483. [Google Scholar] [CrossRef]
- Paulenova, A.; Alyapyshev, M.Y.; Babain, V.A.; Herbst, R.S.; Law, J.D. Extraction of lanthanoids with diamides of dipcolinic acid from nitric acid solutions. II. Synergistic effect of ethyl-tolyl derivates and dicarbollide cobalt. Solvent Extr. Ion Exch. 2013, 31, 184–197. [Google Scholar] [CrossRef]
- Babain, V.; Smirnov, I.; Alyapyshev, M.; Pokrovskaya, E.; Logunov, M.; Skobtsov, A.; Voroshilov, Y. Using the UNEX process to process waste with high rare-earth element content. I. Vopr. Radiatsionnoi Bezop. (Вoпрoсы Радиациoннoй Безoпаснoсти) 2006, 3–12. (In Russian) [Google Scholar]
- Paulenova, A.; Lapka, J.L.; Alyapyshev, M.Y.; Babain, V.A.; Eliseev, I.I.; Law, J.D.; Herbst, R.S.; Todd, T.A. Extraction of Actinides and Fission Products with a Modified UNEX Group Separation. In Proceedings of the GLOBAL 2009 Congress: The Nuclear Fuel Cycle: Sustainable Options and Industrial Perspectives, Paris, France, 6–11 September 2009. [Google Scholar]
- Lapka, J.L.; Paulenova, A.; Zakharov, L.N.; Alyapyshev, M.Y.; Babain, V.A. Coordination of uranium(VI) with N, N’-diethyl-N, N’-ditolyldipicolinamide. IOP Conf. Ser. Mater. Sci. Eng. 2010, 9, 012029. [Google Scholar] [CrossRef]
- Alyapyshev, M.; Babain, V.; Tkachenko, L.; Gurzhiy, V.; Zolotarev, A.; Ustynyuk, Y.; Gloriozov, I.; Lumpov, A.; Dar’in, D.; Paulenova, A. Complexes of uranyl nitrate with 2, 6-pyridinedicarboxamides: Synthesis, crystal structure, and DFT study. Z. Anorg. Allg. Chem. 2017, 643, 585–592. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; Gong, Y. Coordination structures of the uranyl (VI)–diamide complexes: A combined mass spectrometric, EXAFS spectroscopic, and theoretical study. Inorg. Chem. 2019, 58, 5695–5702. [Google Scholar] [CrossRef]
- Renaud, F.; Piguet, C.; Bernardinelli, G.; Biinzli, J.-C.G.; Hopfgartner, G. In search for mononuclear helical lanthanide building blocks with predetermined properties: Triple-stranded helical complexes with N, N, N’, N’-tetraethylpyridine-2, 6-dicarboxamide. Chem. Eur. J. 1997, 3, 1646–1659. [Google Scholar] [CrossRef]
- Tanase, S.; Marques Gallego, P.; de Gelder, R.; Wen, T.F. Synthesis, crystal structure and photophysical properties of europium(III) and terbium(III) complexes with pyridine-2, 6-dicarboxamide. Inorg. Chim. Acta 2007, 360, 102–108. [Google Scholar] [CrossRef]
- Fujiwara, A.; Nakano, Y.; Yaita, T.; Okuno, K. Structural studies of lanthanide nitrate—N, N’-dimethyl-N, N’-diphenylpyridine-2, 6-dicarboxyamide complexes. J. Alloy. Comp. 2008, 456, 429–435. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, Z.; Gong, Y. Complexation of Ln3+ with Pyridine-2, 6-dicarboxamide: Formation of the 1:2 Complexes in Solution and Gas Phase. Inorg. Chem. 2020, 59, 14486–14492. [Google Scholar] [CrossRef] [PubMed]
- Sittel, T.; Weßling, P.; Großmann, D.; Engels, E.; Geist, A.; Panak, P.J. Spectroscopic investigation of the covalence of An (III) complexes with tetraethylcarboxamidopyridine. Dalton Trans. 2022, 51, 8028–8035. [Google Scholar] [CrossRef]
- Lehman-Andino, I.; Su, J.; Papathanasiou, K.E.; Eaton, T.M.; Jian, J.; Dan, D.; Albrecht-Schmitt, T.E.; Dares, C.J.; Batista, E.R.; Yang, P.; et al. Soft-donor dipicolinamide derivatives for selective actinide (III)/lanthanide (III) separation: The role of S-vs. O-donor sites. Chem. Commun. 2019, 55, 2441–2444. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mohapatra, P.K. Separation of trivalent actinides and lanthanides using various ‘N’, ‘S’and mixed ‘N, O’donor ligands: A review. Radiochim. Acta 2019, 107, 931–949. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Wang, Z.; Li, X.; Feng, W.; Deng, P.; Yuan, L. An insight into the extraction of transition metal ions by picolinamides associated with intramolecular hydrogen bonding and rotational isomerization. RSC Adv. 2014, 4, 29702–29714. [Google Scholar] [CrossRef]
- Molochnikova, N.P.; Myasoedova, G.V.; Eliseev, I.I.; Alyapyshev, M.Y. Solid-phase extractant with dipicolinic acid N, N’-diethyl-N, N’-di(p-tolyl)diamide for preconcentration and separation of actinides and lanthanides. Radiochemistry 2010, 52, 65–67. [Google Scholar] [CrossRef]
- Arisaka, M.; Watanabe, M.; Kimura, T. New alkyl-pyridinedicarboxyamides as extractants for separation of trivalent minor actinides from fission products by extraction chromatography. Radiochim. Acta 2013, 101, 711–717. [Google Scholar] [CrossRef]
- Arisaka, M.; Watanabe, M.; Sugo, Y.; Kobayashi, K.; Kanao, O.; Kimura, T. Development of extraction chromatographic adsorbent using alkylpyridinedicarboxyamides as extractant for separation of trivalent minor actinides from lanthanides—Stability and separation ability against nitric acid exposure and gamma-ray irradiation. J. Nucl. Sci. Technol. 2014, 51, 457–464. [Google Scholar] [CrossRef][Green Version]
- Black, C.D.; Paulenova, A.; Lapka, J.L.; Babain, V.A.; Alyapyshev, M.Y. Separation of f-elements by resins impregnated with amides of pyridine-2, 6-dicarboxylic acid. J. Radioanal. Nucl. Chem. 2019, 320, 299–307. [Google Scholar] [CrossRef]
- Kobayashi, T.; Suzuki, S.; Shiwaku, H.; Yaita, T. Crystal Structure of N-Methyl-N-phenyl-1, 10-phenanthroline- 2-carboxamide. X-ray Struct. Anal. Online 2012, 28, 77–78. [Google Scholar] [CrossRef][Green Version]
- Shiwaku, H.; Suzuki, S.; Okamoto, Y. Method for Selective Separation of Trivalent Actinoids from Trivalent Lanthanoide Using Hybrid Donor-Type Extraction Agent Having Functional Group Carrying Active Oxygen and Nitrogen Atoms. European Patent 2128871, 2 December 2009. [Google Scholar]
- Kobayashi, T.; Yaita, T.; Suzuki, S.; Shiwaku, H.; Okamoto, Y.; Akutsu, K.; Nakano, Y.; Fujii, Y. Effect of the introduction of amide oxygen into 1, 10-phenanthroline on the extraction and complexation of trivalent lanthanide in acidic condition. Sep. Sci. Technol. 2010, 45, 2431–2436. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.; Tan, C.; Zhang, X.; Li, S.; Tian, W.; Guo, H.; Wang, L.; Qin, Z. Solvent extraction of americium (III) and europium (III) with tridentate N, N-dialkyl-1, 10-phenanthroline-2-amide-derived ligands: Extraction, complexation and theoretical study. New J. Chem. 2016, 40, 10560–10568. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Liu, Y.; Ma, J.; Li, X.; Yang, Y. Selective extraction of Am (III) from Cm (III) and Eu (III) using a novel phenanthrolinamide ligand: Thermodynamics, species, and structure. Sep. Purif. Technol. 2021, 274, 119119. [Google Scholar] [CrossRef]
- Simonnet, M.; Suzuki, S.; Miyazaki, Y.; Kobayashi, T.; Yokoyama, K.; Yaita, T. Lanthanide intra-series separation by a 1, 10-phenanthroline derivative: Counterion effect. Solv. Extr. Ion Exch. 2020, 38, 430–440. [Google Scholar] [CrossRef]
- Sun, M.; Xu, L.; Yang, X.; Wang, S.; Lei, L.; Xiao, C. Complexation Behaviors of a Tridentate Phenanthroline Carboxamide Ligand with Trivalent f-Block Elements in Different Anion Systems: A Thermodynamic and Crystallographic Perspective. Inorg. Chem. 2022, 61, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Tamaki, S.; Yajima, H.; Hashimoto, B.; Yaita, T. Selective separation of samarium (III) by synergistic extraction with β-diketone and methylphenylphenanthroline carboxamide. Talanta 2011, 85, 1543–1548. [Google Scholar] [CrossRef]
- Awual, M.R.; Kobayashi, T.; Shiwaku, H.; Miyazaki, Y.; Motokawa, R.; Suzuki, S.; Okamoto, Y.; Yaita, T. Evaluation of lanthanide sorption and their coordination mechanism by EXAFS measurement using novel hybrid adsorbent. Chem. Eng. J. 2013, 225, 558–566. [Google Scholar] [CrossRef]
- Awual, M.R.; Kobayashi, T.; Miyazaki, Y.; Motokawa, R.; Shiwaku, H.; Suzuki, S.; Okamoto, Y.; Yaita, T. Selective lanthanide sorption and mechanism using novel hybrid Lewis base (N-methyl-N-phenyl-1, 10-phenanthroline-2-carboxamide) ligand modified adsorbent. J. Hazard. Mat. 2013, 252–253, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Alharthi, N.H.; Okamoto, Y.; Karim, M.R.; Halim, M.E.; Hasan, M.M.; Rahman, M.M.; Islam, M.M.; Khaleque, M.A.; Sheikh, M.C. Ligand field effect for dysprosium (III) and lutetium (III) adsorption and EXAFS coordination with novel composite nanomaterials. Chem. Eng. J. 2017, 320, 427–435. [Google Scholar] [CrossRef]
- Kobayashi, T.; Suzuki, S.; Shiwaku, H.; Yaita, T. Lanthanides complexation properties of O, N-hetero donor ligand PTA. Prog. Nucl. Energy 2018, 5, 74–77. [Google Scholar] [CrossRef]
- Nakase, M.; Kobayashi, T.; Shiwaku, H.; Suzuki, S.; Grimes, T.S.; Mincher, B.J.; Yaita, T. Relationship between structure and coordination strength of N and N, O-hybrid donor ligands with trivalent lanthanides. Solvent Extr. Ion Exch. 2018, 36, 633–646. [Google Scholar] [CrossRef]
- Borisova, N.E.; Eroshkina, E.A.; Korotkov, L.A.; Ustynyuk, Y.A.; Alyapyshev, M.Y.; Eliseev, I.I.; Babain, V.A. Actinide-lanthanide separation by bipyridyl-based ligands. DFT calculations and experimental results. In Proceedings of the GLOBAL 2011, Chiba, Japan, 11–16 December 2011; p. 392539. [Google Scholar]
- Alyapyshev, M.Y.; Babain, V.A.; Borisova, N.E.; Kiseleva, R.N.; Safronov, D.V.; Reshetova, M.D. New systems based on 2, 2′-dipyridyl-6, 6′-dicarboxylic acid diamides for Am–Eu separation. Mendeleev Commun. 2008, 6, 336–337. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Eliseev, I.I., Jr.; Kiseleva, R.N.; Borisova, N.E.; Kostin, A.A.; Reshetova, M.D. Am-lanthanides separation using new solvents based on 2, 2′-dipyridyl-6, 6′-dicarboxylic acid diamides. In Proceedings of the International Conference Global-2009, Paris, France, 6–11 September 2009; p. 9415. [Google Scholar]
- Alyapyshev, M.; Babain, V.; Borisova, N.; Eliseev, I.; Kirsanov, D.; Kostin, A.; Legin, A.; Reshetova, M.; Smirnova, Z. 2, 2′-Dipyridyl-6, 6′-dicarboxylic acid diamides: Synthesis, complexation and extraction properties. Polyhedron 2010, 29, 1998–2005. [Google Scholar] [CrossRef]
- Borisova, N.E.; Korotkov, L.A.; Ivanov, A.V.; Lapka, J.; Paulenova, A.; Belova, E.V.; Stefanovsky, S.V.; Myasoedov, B.F. New potentialities of the UNEX process using polyheterocyclic diamides. Radiochemistry 2016, 58, 606–616. [Google Scholar] [CrossRef]
- Kirsanov, D.O.; Borisova, N.E.; Reshetova, M.D.; Ivanov, A.V.; Korotkov, L.A.; Eliseev, I.I.; Alyapyshev, M.Y.; Spiridonov, I.G.; Legin, A.V. Vlasov Yu. G.; Babain, V.A. Novel diamides of 2, 2′-dipyridyl-6, 6′-dicarboxylic acid: Synthesis, coordination properties, and possibilities of use in electrochemical sensors and liquid extraction. Russ. Chem. Bull. 2012, 61, 881–890. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Tkachenko, L.I.; Paulenova, A.; Popova, A.A.; Borisova, N.E. New diamides of 2, 2′-dipyridyl-6, 6′-dicarboxylic acid for actinide-lanthanide separation. Solvent Extr. Ion Exch 2014, 32, 138–152. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ivanov, A.V.; Matveev, P.I.; Smirnova, A.A.; Belova, E.V.; Kalmykov, S.N.; Myasoedov, B.F. Screening of the structure of americium extractants based on a 2, 2′-bipyridyl scaffold: A simple way to a N2, O2-tetradentate ligands library for rational design of An/Ln extractants. ChemistrySelect 2018, 3, 1983–1989. [Google Scholar] [CrossRef]
- Alyapyshev, M.Y.; Babain, V.A.; Eliseev, I.I.; Tkachenko, L.I.; Ustynjuk, Y.A.; Reshetova, M.D.; Borisova, N.E.; Ivanov, A.V.; Logunov, M.V. Extraction Mixture for Separating Actinides from Liquid Radioactive Wastes. Patent RU 2499308, 20 November 2013. [Google Scholar]
- Vidanov, V.L.; Shadrin, A.Y.; Tkachenko, L.I.; Kenf, E.V.; Parabin, P.V.; Shirokov, S.S. Separation of americium and curium for transmutation in the fast neutron reactor. Nucl. Eng. Des. 2021, 385, 111434. [Google Scholar] [CrossRef]
- Borisova, N.E.; Kostin, A.A.; Eroshkina, E.A.; Reshetova, M.D.; Lyssenko, K.A.; Spodine, E.N.; Puntus, L.N. Lanthanide complexes with tetradentate N, N′, O, O′-dipyridyl-based ligands: Structure, stability, and photophysical properties. Eur. J. Inorg. Chem. 2014, 2014, 2219–2229. [Google Scholar] [CrossRef]
- Kharcheva, A.V.; Borisova, N.E.; Ivanov, A.V.; Reshetova, M.D.; Kaminskaya, T.P.; Popov, V.V.; Yuzhakov, V.I.; Patsaeva, S.V. Effect of aliphatic chain length in the ligand on photophysical properties and thin films morphology of the europium complexes. Russ. J. Inorg. Chem. 2018, 63, 219–228. [Google Scholar] [CrossRef]
- Farat, O.K.; Kharcheva, A.V.; Ioutsi, V.A.; Borisova, N.E.; Reshetova, M.D.; Patsaeva, S.V. Europium complex of 2, 2′-bipyridine-6, 6′-dicarboxylic acid bis [di (phosphonomethyl) amide] as a new efficient water-soluble luminescent dye. Mendeleev Comm. 2019, 29, 282–284. [Google Scholar] [CrossRef]
- Borisova, N.E.; Ivanov, A.V.; Kharcheva, A.V.; Sumyanova, T.B.; Surkova, U.V.; Matveev, P.I.; Patsaeva, S.V. Effect of heterocyclic ring on LnIII coordination, luminescence and extraction of diamides of 2, 2′-bipyridyl-6, 6′-dicarboxylic acid. Molecules 2019, 25, 62. [Google Scholar] [CrossRef]
- Borisova, N.E.; Reshetova, M.D. Quantum chemical modeling of 2, 2’-bipyridine-6, 6´-dicarboxylic acid diamide structures: A relationship between the extraction ability and conformational behavior of the ligands. Russ. Chem. Bull. 2015, 64, 1882–1890. [Google Scholar] [CrossRef]
- Skvortsov, I.V.; Belova, E.V.; Rodin, A.V.; Borisova, N.E.; Ivanov, A.V.; Myasoedov, B.F. Thermal stability of irradiated solutions of 2, 2′-bipyridine-6, 6′-dicarboxylic acid bis (N-ethyl-4-hexylanilide) in fluorinated sulfones. Radiochemistry 2017, 59, 607–611. [Google Scholar] [CrossRef]
- Skvortsov, I.V.; Kalistratova, V.V.; Belova, E.V.; Rodin, A.V.; Sokolov, I.P.; Myasoedov, B.F. Thermal properties of 2, 2′-bipyridine-6, 6′-dicarboxylic acid bis (N-ethyl-4-hexylanilide), an extractant for radioactive waste components. Radiochemistry 2017, 59, 612–617. [Google Scholar] [CrossRef]
- Skvortsov, I.V.; Kalistratova, V.V.; Rodin, A.V.; Belova, E.V.; Myasoedov, B.F.; Borisova, N.E.; Tsarev, D.A. Thermal stability of extractants based on diamides of heterocyclic carboxylic acids. Radiochemistry 2018, 60, 601–606. [Google Scholar] [CrossRef]
- Belova, E.V.; Skvortsov, I.V.; Kadyko, M.I.; Yudintsev, S.V. The effect of irradiation on hydrodynamic properties of extraction mixtures based on diamides of N-heterocyclic dicarboxylic acids in heavy fluorinated diluents. Nucl. Eng. Technol. 2019, 51, 1163–1168. [Google Scholar] [CrossRef]
- Kadyko, M.I.; Skvortsov, I.V.; Ivanov, A.V.; Belova, E.V.; Myasoedov, B.F. Effect of Irradiation on Hydrodynamic Properties of Extraction Mixtures Based on Carboxylic Acid Diamides in FS-13 Diluent. Radiochemistry 2019, 61, 579–584. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, L.; Liu, Y.; Chen, B.; Liu, J.; Li, X.; Luo, S. Complexes of Th(IV) with neutral O–N–N–O hybrid ligands: A thermodynamic and crystallographic study. Dalton Trans. 2021, 50, 705–714. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Chen, B.; Lv, L.; Li, Q.; Li, X.; Ding, S.; Yang, Y. Oxygen and peroxide bridged uranyl (VI) dimers bearing tetradentate hybrid ligands: Supramolecular self-assembly and generation pathway. Inorg. Chem. Front. 2020, 7, 3412–3423. [Google Scholar] [CrossRef]
- Kostikova, G.V.; Fedoseev, A.M.; Zhilov, V.I. Method for Selective Extraction of Scandium from Rare Earth Concentrates. RU Patent 2767924, 22 March 2022. [Google Scholar]
- Marie, C.; Miguirditchian, M.; Bisson, J.; Guillaneux, D.; Dubreuil, D. Compounds Useful as Ligands of Actinides, Their Synthesis and Their Uses. U.S. Patent 2012/0186396 A1, 26 July 2012. [Google Scholar]
- Marie, C.; Bisson, J.; Miguirditchian, M.; Charbonnel, M.-C. New bitopic ligands for separation os actinides: Influence of the structure on complexation and extraction properties. In Proceedings of the ATALANTE 2012 International Conference, Montpellier, France, 3–7 September 2012, CEA-R-6298. ISSN 0429-3460. [Google Scholar]
- Marie, C.; Miguirditchian, M.; Guillaumont, D.; Bisson, J.; Pipelier, M.; Dubreuil, D. New bitopic ligands for the group actinide separation by solvent extraction. Solvent Extr. Ion Exch. 2011, 29, 292–315. [Google Scholar] [CrossRef]
- Marie, C.; Miguirditchian, M.; Guillaumont, D.; Tosseng, A.; Berthon, C.; Guilbaud, P.; Duvail, M.; Bisson, J.; Guillaneux, D.; Pipelier, M.; et al. Complexation of lanthanides(III), americium(III), and uranium(VI) with bitopic N, O ligands: An experimental and theoretical study. Inorg. Chem. 2011, 50, 6557–6566. [Google Scholar] [CrossRef] [PubMed]
- Marie, C. Synthèse et Évaluation de Nouvelles Molecules Polyfonctionnelles Pour la Séparation Groupée des Actinides. Ph.D. Thesis, Université de Nantes, Nantes, France, 2009. [Google Scholar]
- Merrill, D.; Hancock, R.D. Metal ion selectivities of the highly preorganized tetradentate ligand 1, 10-phenanthroline-2, 9-dicarboxamide with lanthanide (III) ions and some actinide ions. Radiochim. Acta 2011, 99, 161–166. [Google Scholar] [CrossRef]
- Merrill, D.; Harrington, J.M.; Lee, H.S.; Hancock, R.D. Unusual metal ion selectivities of the highly preorganized tetradentrate ligand 1, 10-phenanthroline-2, 9-dicarboxamide: A thermodynamic and fluorescence study. Inorg. Chem. 2011, 50, 8348–8355. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Song, Y.T.; Ji, L.; Wang, C.Z.; Chai, Z.F.; Shi, W.Q. Theoretical unraveling the separation of Am(III)/Eu(III): Insights from mixed N, O-donor ligands with variations of central heterocyclic moieties. Phys. Chem. Chem. Phys. 2017, 19, 26969–26979. [Google Scholar] [CrossRef]
- Alyapyshev, M.; Ashina, J.; Dar’in, D.; Kenf, E.; Kirsanov, D.; Tkachenko, L.; Legin, A.; Starova, G.; Babain, V. 1, 10-Phenanthroline-2, 9-dicarboxamides as ligands for separation and sensing of hazardous metals. RSC Adv. 2016, 73, 68642–68652. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, L.; Chai, Z.; Shi, W. Towards understanding the correlation between UO22+ extraction and substitute groups in 2, 9-diamide-1, 10-phenanthroline. Sci. China Chem. 2018, 61, 1285–1292. [Google Scholar] [CrossRef]
- Lemport, P.S.; Matveev, P.I.; Yatsenko, A.V.; Evsiunina, M.V.; Petrov, V.S.; Tarasevich, B.N.; Roznyatovsky, V.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Zhokhov, S.S.; et al. The impact of alicyclic substituents on the extraction ability of new family of 1, 10-phenanthroline-2, 9-diamides. RSC Adv. 2020, 10, 26022–26033. [Google Scholar] [CrossRef]
- Wang, H.; Cui, T.; Sui, J.; Mocilac, P.; Wang, Y.; Guo, Z. Efficient UO22+ extraction by DAPhens with asymmetric terminal groups: The molecular design, spectral titration, liquid-liquid extraction and mechanism study. Sep. Purif. Technol. 2022, 282, 120046. [Google Scholar] [CrossRef]
- Xiao, C.L.; Wang, C.Z.; Yuan, L.Y.; Li, B.; He, H.; Wang, S.; Zhao, Y.L.; Chai, Z.F.; Shi, W.Q. Excellent selectivity for actinides with a tetradentate 2, 9-diamide-1, 10-phenanthroline ligand in highly acidic solution: A hard-soft donor combined strategy. Inorg.Chem. 2014, 53, 1712–1720. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Lan, J.; Yuan, L.; Xu, C.; Chai, Z.; Shi, W. Highly selective extraction of Pu (IV) and Am (III) by N, N′-diethyl-N, N′-ditolyl-2, 9-diamide-1, 10-phenanthroline ligand: An experimental and theoretical study. Sep. Purif. Technol. 2019, 223, 274–281. [Google Scholar] [CrossRef]
- Tsutsui, N.; Ban, Y.; Suzuki, H.; Nakase, M.; Ito, S.; Inaba, Y.; Matsumura, T.; Takeshita, K. Effects of diluents on the separation of minor actinides from lanthanides with tetradodecyl-1, 10-phenanthroline-2, 9-diamide (TDdPTDA) from nitric acid medium. Anal. Sci. 2019, 36, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Li, F.F.; Ren, P.; Yuan, L.Y.; Liu, K.; Geng, J.S.; Tang, H.B.; Chai, Z.F.; Shi, W.Q. Selective separation between UO22+ and Pu4+ by novel tetradentate chelate phenanthroline diamide ligand in 1-octanol. Sep. Purif. Technol. 2021, 277, 119521. [Google Scholar] [CrossRef]
- Xiao, C.L.; Wang, C.Z.; Mei, L.; Zhang, X.R.; Wall, N.; Zhao, Y.L.; Chai, Z.F.; Shi, W.Q. Europium, uranyl, and thorium-phenanthroline amide complexes in acetonitrile solution: An ESI-MS and DFT combined investigation. Dalton Trans. 2015, 44, 14376–14387. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.H.; Wu, Q.Y.; Zhang, X.R.; Wang, C.; Hu, K.Q.; Chai, Z.F.; Nie, C.M.; Shi, W.Q. Coordination behavior of uranyl with PDAM derivatives in solution: Combined study with ESI-MS and DFT. J. Mol. Liq. 2020, 300, 112287. [Google Scholar] [CrossRef]
- Xiao, C.L.; Wu, Q.Y.; Wang, C.; Chai, Z.F.; Shi, W.Q. Quantum chemistry study of uranium(VI), neptunium(V), and plutonium(IV, VI) complexes with preorganized tetradentate phenanthrolineamide ligands. Inorg.Chem. 2014, 53, 10846–10853. [Google Scholar] [CrossRef] [PubMed]
- Lipin, R.; Ebenezer, C.; Solomon, R.V. Theoretical evaluation of mixed N-, O-donor based TMPhenDA ligand in selective complexation with actinide (III) ions over lanthanide (III) ions. J. Mol. Liq. 2021, 332, 115819. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Q.Y.; Wang, C.Z.; Lan, J.H.; Nie, C.M.; Chai, Z.F.; Shi, W.Q. Theoretical insights into selective separation of trivalent actinide and lanthanide by ester and amide ligands based on phenanthroline skeleton. Dalton Trans. 2020, 49, 4093–4099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.Z.; Wu, Q.Y.; Lan, J.H.; Chai, Z.F.; Liu, Q.; Shi, W.Q. Theoretical prediction of the potential applications of phenanthroline derivatives in separation of transplutonium elements. Inorg.Chem. 2020, 59, 11469–11480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, L.; Chai, Z.; Shi, W. A new solvent system containing N, N′-diethyl-N, N′-ditolyl-2, 9-diamide-1, 10-phenanthroline in 1-(trifluoromethyl)-3-nitrobenzene for highly selective UO22+ extraction. Sep. Purif. Technol. 2016, 168, 232–237. [Google Scholar] [CrossRef]
- Li, F.; Yang, Z.; Weng, H.; Chen, G.; Lin, M.; Zhao, C. High efficient separation of U(VI) and Th(IV) from rare earth elements in strong acidic solution by selective sorption on phenanthroline diamide functionalized graphene oxide. Chem. Eng. J. 2018, 332, 340–350. [Google Scholar] [CrossRef]
- Ustynyuk, Y.A.; Borisova, N.E.; Babain, V.A.; Gloriozov, I.P.; Manuilov, A.Y.; Kalmykov, S.N.; Alyapyshev, M.Y.; Tkachenko, L.I.; Kenf, E.V.; Ustynyuk, N.A. N, N′-Dialkyl-N, N′-diaryl-1, 10-phenanthroline-2, 9-dicarboxamides as donor ligands for separation of rare earth elements with a high and unusual selectivity. DFT computational and experimental studies. Chem. Comm. 2015, 51, 7466–7469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, X.; Yuan, L.; Chai, Z.; Shi, W. Coordination of Eu(III) with 1, 10-phenanthroline-2, 9-dicarboxamide derivatives: A Combined study by MS, TRLIF, and DFT. Inorg.Chem. 2019, 58, 10239–10247. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Ren, P.; Yang, Q.; Geng, J.S.; Zhang, J.Y.; Yuan, L.Y.; Tang, H.B.; Chai, Z.F.; Shi, W.Q. Strong periodic tendency of trivalent lanthanides coordinated with a phenanthroline-based ligand: Cascade countercurrent extraction, spectroscopy, and crystallography. Inorg. Chem. 2021, 60, 9745–9756. [Google Scholar] [CrossRef]
- Simonnet, M.; Kobayashi, T.; Shimojo, K.; Yokoyama, K.; Yaita, T. Study on Phenanthroline Carboxamide for Lanthanide Separation: Influence of Amide Substituents. Inorg.Chem. 2021, 60, 13409–13418. [Google Scholar] [CrossRef] [PubMed]
- Ustynyuk, Y.A.; Lemport, P.S.; Roznyatovsky, V.A.; Lyssenko, K.A.; Gudovannyy, A.O.; Matveev, P.I.; Khult, E.K.; Evsiunina, M.V.; Petrov, V.G.; Gloriozov, I.P.; et al. Synthesis, structure, stereodynamics and complexation with Ln(III). Molecules 2022, 27, 3114. [Google Scholar] [CrossRef]
- Borisova, N.E.; Kostin, A.A.; Reshetova, M.D.; Lyssenko, K.A.; Belova, E.V.; Myasoedov, B.F. The structurally rigid tetradentate N, N′, O, O′-ligands based on phenanthroline for binding of f-elements: The substituents vs. structures of the complexes. Inorg. Chim. Acta 2018, 478, 148–154. [Google Scholar] [CrossRef]
- Zarubin, D.N.; Bushkov, N.S.; Lavrov, H.V.; Dolgushin, F.M.; Ustynyuk, N.A.; Ustynyuk, Y.A. 4, 7-Di-n-butoxy-1, 10-phenanthroline-2, 9-dicarboxamide: A tetradentate ligand featuring excellent solubility in nonpolar media. INEOS OPEN 2019, 2, 130–133. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Lv, L.; Yang, L.; Luo, S.; Yang, Y.; Peng, S. Complexation of lanthanides with N, N, N′, N′-tetramethylamide derivatives of bipyridinedicarboxylic acid and phenanthrolinedicarboxylic acid: Thermodynamics and coordination modes. Inorg.Chem. 2019, 58, 7416–7425. [Google Scholar] [CrossRef]
- Dehaudt, J.; Williams, N.J.; Shkrob, I.A.; Luo, H.; Dai, S. Selective separation of trivalent f-ions using 1, 10-phenanthroline-2, 9-dicarboxamide ligands in ionic liquids. Dalton Trans. 2016, 45, 11624–11627. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, X.; Yuan, J.; Pu, N.; Wei, P.; Sun, T.; Shi, W.; Chen, J.; Wang, J.; Xu, C. Performance and mechanism for the selective separation of trivalent americium from lanthanides by a tetradentate phenanthroline ligand in ionic liquid. Inorg.Chem. 2020, 59, 3905–3911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Ren, P.; Sun, T.; Shi, W.; Wang, J.; Chen, J.; Xu, C. Substituent Effect on the selective separation and complexation of trivalent americium and lanthanides by N, O-hybrid 2, 9-diamide-1, 10-phenanthroline ligands in ionic liquid. Inorg.Chem. 2021, 60, 5131–5139. [Google Scholar] [CrossRef]
- Yang, X.F.; Liu, Y.; Tao, W.Q.; Wang, S.; Ren, P.; Yang, S.L.; Yuan, L.Y.; Tang, H.B.; Chai, Z.F.; Shi, W.Q. Lipophilic phenanthroline diamide ligands in 1-octanol for separation of Am (III) from Eu (III). J. Environ. Chem. Eng. 2022, 10, 108401. [Google Scholar] [CrossRef]
- Jia, L.; Li, Z.; Shi, W.; Shen, X. A novel CPE procedure by oil-in-water microemulsion for preconcentrating and analyzing thorium and uranium. Radiochim. Acta 2022, 110, 239–249. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Hay, B.P. Theoretical prediction of Am(III)/Eu(III) selectivity to aid the design of actinide-lanthanide separation agents. Dalton Trans. 2015, 44, 7935–7942. [Google Scholar] [CrossRef] [PubMed]
- Alyapyshev, M.Y. Diamides of N-heterocyclic dicarboxylic acids—New class of ligands for extraction and separation of f-elements. Ph.D. Thesis, Moscow State University, Moscow, Russia, 2022. (In Russian). [Google Scholar]
- Ustynyuk, Y.A.; Alyapyshev, M.Y.; Babain, V.A.; Ustynyuk, N.A. Quantum chemical modelling of extraction separation of minor actinides and lanthanides: The state of the art. Russ. Chem. Rev. 2016, 85, 917–942. [Google Scholar] [CrossRef]
- Lavrov, H.V.; Ustynyuk, N.A.; Matveev, P.I.; Gloriozov, I.P.; Zhokhov, S.S.; Alyapyshev, M.Y.; Tkachenko, L.I.; Voronaev, I.G.; Babain, V.A.; Kalmykov, S.N.; et al. A novel highly selective ligand for separation of actinides and lanthanides in the nuclear fuel cycle. Experimental verification of the theoretical prediction. Dalton Trans. 2017, 46, 10926–10934. [Google Scholar] [CrossRef] [PubMed]
- Matveev, P.I. Extraction Systems on the Base of 4.7-Substituted Phenanthroline Dicarboxamides for Extraction and Separation of Americium(III) and Curium(III). Ph.D. Thesis, Moscow State University, Moscow, Russia, 2019. (In Russian). [Google Scholar]
- Karslyan, Y.; Sloop, F.V.; Delmau, L.H.; Moyer, B.A.; Popovs, I.; Paulenova, A.; Jansone-Popova, S. Sequestration of trivalent americium and lanthanide nitrates with bis-lactam-1, 10-phenanthroline ligand in a hydrocarbon solvent. RSC Adv. 2019, 9, 26537–26541. [Google Scholar] [CrossRef] [PubMed]
- Matveev, P.I.; Mitrofanov, A.A.; Petrov, V.G.; Zhokhov, S.S.; Smirnova, A.A.; Ustynyuk, Y.A.; Kalmykov, S.N. Testing a simple approach for theoretical evaluation of radiolysis products in extraction systems. A case of N, O-donor ligands for Am/Eu separation. RSC Adv. 2017, 7, 55441–55449. [Google Scholar] [CrossRef]
- Ustynyuk, N.A.; Lavrov, H.V.; Zarubin, D.N.; Dolgushin, F.M.; Ezernitskaya, M.G.; Gloriozov, I.P.; Zhokhov, S.S.; Zhokhova, N.I.; .Ustynyuk, Y.A. New benzo[f]quinolino [3, 4-b][1, 7]naphthyridine-6, 8(5H, 9H)-diones: Synthesis, electronic, molecular, and crystal structures. Protonation and complexation with lanthanum and europium salts. Russ. Chem. Bull. Int. Ed. 2018, 67, 1878–1890. [Google Scholar] [CrossRef]
- Jansone-Popova, S.; Ivanov, A.S.; Bryantsev, V.S.; Sloop, F.V., Jr.; Custelcean, R.; Popovs, I.; Dekarske, M.M.; Moyer, B.A. Bis-lactam-1, 10-phenanthroline (BLPhen), a new type of preorganized mixed N, O-donor ligand that separates Am (III) over Eu (III) with exceptionally high efficiency. Inorg.Chem. 2017, 56, 5911–5917. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.; Berthon, C.; Berthon, L.; Boubals, N.; Dubreuil, D.; Charbonnel, M.C. Effect of the structure of Amido-polynitrogen Molecules on the complexation of Actinides. Procedia Chem. 2012, 7, 13–19. [Google Scholar] [CrossRef]
- Healy, M.R.; Ivanov, A.S.; Karslyan, Y.; Bryantsev, V.S.; Moyer, B.A.; Jansone-Popova, S. Efficient separation of light lanthanides (III) by using bis-lactam phenanthroline ligands. Chem. Eur. J 2019, 25, 6326–6331. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.D.N.; Ivanov, A.S.; Driscoll, D.M.; Jansone-Popova, S.; Jiang, D.E. Atomistic Insights into Structure and Dynamics of Neodymium (III) Complexation with a Bis-lactam Phenanthroline Ligand in the Organic Phase. ACS Omega 2022, 7, 21317–21324. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.; Dehaudt, J.; Charbonnel, M.C.; Guillaneux, D.; Miguirditchian, M.; Marie, C.; Boubals, N.; Dutech, G.; Pipelier, M.; Blot, V.; et al. 1, 10-phenantroline and non-symmetrical 1, 3, 5-triazine dipicolinamide-based ligands for group Actinide extraction. Chem. Eur. J. 2014, 20, 7819–7829. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Q.Y.; Wang, C.Z.; Lan, J.H.; Liu, Z.R.; Chaia, Z.F.; Shi, W.Q. Theoretical insights into the separation of Am(III) over Eu(III) with PhenBHPPA. Dalton Trans. 2015, 44, 16737–16745. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, P.; Nair, D.; Panja, S.; Ali, S.M.; Banerjee, D.; Sugilal, G.; Kaushik, C.P. Pyridine diglycolamide: A novel ligand for plutonium extraction from nitric acid medium. Sep. Purif. Technol. 2022, 282, 120026. [Google Scholar] [CrossRef]
- Sivaramakrishna, M.; Raut, D.R.; Nayak, S.K.; Mohapatra, P.K. Extraction of plutonium (IV) from acidic feeds using several diamides with a tri-phenyl pyridine centre. J. Radioanal. Nucl. Chem. 2019, 320, 245–253. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Li, Q.; Wu, Y.; Shang, X.; Yang, P.; Feng, W. Novel phenanthroline-derived pyrrolidone ligands for efficient uranium separation: Liquid-liquid extraction, spectroscopy, and molecular simulations. J. Mol. Liq. 2022, 364, 119909. [Google Scholar] [CrossRef]
- Matveev, P.I.; Borisova, N.E.; Andreadi, N.G.; Zakirova, G.G.; Petrov, V.G.; Belova, E.V.; Myasoedov, B.F. A first phosphine oxide-based extractant with high Am/Cm selectivity. Dalton Trans. 2019, 48, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Konopkina, E.A.; Matveev, P.I.; Huang, P.W.; Kirsanova, A.A.; Chernysheva, M.G.; Sumyanova, T.B.; Borisova, N.E. Pyridine-di-phosphonates as chelators for trivalent f-elements: Kinetics, thermodynamic and interfacial study of Am (III)/Eu (III) solvent extraction. Dalton Trans. 2022, 51, 11180–11192. [Google Scholar] [CrossRef]
- Borisova, N.E.; Kharcheva, A.V.; Patsaeva, S.V.; Korotkov, L.A.; Bakaev, S.; Reshetova, M.D.; Myasoedov, B.F. Hard-and-soft phosphinoxide receptors for f-element binding: Structure and photophysical properties of europium (III) complexes. Dalton Trans. 2017, 46, 2238–2248. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Vologzhanina, A.V.; Turanov, A.N.; Karandashev, V.K.; Brel, V.K. Synthesis, coordination and extraction properties of 2, 3-bis (diphenylphosphoryl) pyridine toward f-block elements. Mendeleev Comm. 2021, 31, 306–308. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Li, Y.; Wei, P.; Sun, T.; Xiao, C.; Xu, C. Selective separation and complexation of trivalent actinide and lanthanide by a tetradentate soft–hard donor ligand: Solvent extraction, spectroscopy, and DFT calculations. Inorg.Chem. 2019, 58, 4420–4430. [Google Scholar] [CrossRef]
- Xu, L.; Pu, N.; Ye, G.; Xu, C.; Chen, J.; Zhang, X.; Xiao, C. Unraveling the complexation mechanism of actinide (III) and lanthanide (III) with a new tetradentate phenanthroline-derived phosphonate ligand. Inorg.Chem. Front. 2020, 7, 1726–1740. [Google Scholar] [CrossRef]
- Yang, X.; Xu, L.; Hao, Y.; Meng, R.; Zhang, X.; Lei, L.; Xiao, C. Effect of counteranions on the extraction and complexation of trivalent lanthanides with tetradentate phenanthroline-derived phosphonate ligands. Inorg.Chem. 2020, 59, 17453–17463. [Google Scholar] [CrossRef] [PubMed]
- Matveev, P.I.; Huang, P.W.; Kirsanova, A.A.; Ananyev, I.V.; Sumyanova, T.B.; Kharcheva, A.V.; Khvorostinin, E.Y.; Petrov, V.G.; Shi, W.Q.; Kalmykov, S.N.; et al. Way to enforce selectivity via steric hindrance: Improvement of Am (III)/Eu (III) solvent extraction by loaded diphosphonic acid esters. Inorg.Chem. 2021, 60, 14563–14581. [Google Scholar] [CrossRef]
- Xu, L.; Yang, X.; Wang, Z.; Wang, S.; Sun, M.; Xu, C.; Zhang, X.; Lei, L.; Xiao, C. Unfolding the Extraction and Complexation Behaviors of Trivalent f-Block Elements by a Tetradentate N, O-Hybrid Phenanthroline Derived Phosphine Oxide Ligand. Inorg.Chem. 2021, 60, 2805–2815. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Xu, L.; Yan, Q.; Xu, C.; Matveev, P.; Xiao, C. New tetradentate N, O-hybrid phenanthroline-derived organophosphorus extractants for the separation and complexation of trivalent actinides and lanthanides. Inorg. Chem. Front. 2022, 364, 119909. [Google Scholar] [CrossRef]
- Xu, L.; Hao, Y.; Yang, X.; Wang, Z.; Xu, C.; Borisova, N.E.; Sun, M.; Zhang, X.; Lei, L.; Xiao, C. Comparative investigation into the complexation and extraction properties of tridentate and tetradentate phosphine oxide-functionalized 1, 10-phenanthroline ligands toward lanthanides and actinides. Chem. Eur. J. 2021, 27, 10717–10730. [Google Scholar] [CrossRef]
- Ebenezer, C.; Solomon, R.V. Does the length of the alkyl chain affect the complexation and selectivity of phenanthroline-derived phosphonate ligands?–Answers from DFT calculations. Polyhedron 2021, 210, 115533. [Google Scholar] [CrossRef]
- Mitrofanov, A.; Andreadi, N.; Matveev, P.; Zakirova, G.; Borisova, N.; Kalmykov, S.; Petrov, V. An (III)/Ln (III) solvent extraction: Theoretical and experimental investigation of the role of ligand conformational mobility. J. Mol. Liq. 2021, 325, 115098. [Google Scholar] [CrossRef]
- Ebenezer, C.; Solomon, R.V. Preorganization of N, O-hybrid phosphine oxide chelators for effective extraction of trivalent Am/Eu ions–a computational study. New J. Chem. 2022, 46, 5761–5770. [Google Scholar] [CrossRef]
- Huang, P.W. Theoretical unraveling of the separation of trivalent Am and Eu ions by phosphine oxide ligands with different central heterocyclic moieties. Dalton Trans. 2022, 51, 7118–7126. [Google Scholar] [CrossRef] [PubMed]
- Kirsanov, D.; Rudnitskaya, A.; Legin, A.; Babain, V. UV–Vis spectroscopy with chemometric data treatment: An option for on-line control in nuclear industry. J. Radioanal. Nucl. Chem. 2017, 312, 461–470. [Google Scholar] [CrossRef]
- Tse, P.; Bryan, S.A.; Bessen, N.P.; Lines, A.M.; Shafer, J.C. Review of on-line and near real-time spectroscopic monitoring of processes relevant to nuclear material management. Anal. Chim. Acta 2020, 1107, 1–13. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- del Valle, M. Electronic Tongues Employing Electrochemical Sensors. Electroanal. 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Babain, V.A.; Legin, A.V.; Kirsanov, D.O.; Rudnitskaya, A.M.; Tatuev, Y.M.; Baulin, V.E. New chemical sensors based on extraction systems for stable fission products analysis. Radiochim. Acta 2009, 97, 479–484. [Google Scholar] [CrossRef]
- Spiridonov, I.G.; Kirsanov, D.O.; Babain, V.A.; Alyapyshev, M.Y.; Eliseev, I.I.; Vlasov, Y.G.; Legin, A.V. Polymeric sensors for determination of rare-earth metal ions, based on diamides of dipicolinic acid. Russ. J. Appl. Chem. 2011, 84, 1354–1361. [Google Scholar] [CrossRef]
- Kirsanov, D.; Khaydukova, M.; Tkachenko, L.; Legin, A.; Babain, V. Potentiometric sensor array for analysis of complex rare earth mixtures. Electroanalysis 2012, 24, 121–130. [Google Scholar] [CrossRef]
- Oleneva, E.; Savosina, J.; Agafonova-Moroz, M.; Lumpov, A.; Babain, V.; Jahatspanian, I.; Legin, A.; Kirsanov, D. Potentiometric multisensor system for tetra-and hexavalent actinide quantification in complex rare earth metal mixtures related to spent nuclear fuel reprocessing. Sens. Actuators B Chem. 2019, 288, 155–162. [Google Scholar] [CrossRef]
- Savosina, J.; Agafonova-Moroz, M.; Yaroshenko, I.; Ashina, J.; Babain, V.; Lumpov, A.; Legin, A.; Kirsanov, D. Plutonium (IV) quantification in technologically relevant media using potentiometric sensor array. Sensors 2020, 20, 1604. [Google Scholar] [CrossRef]
- Agafonova-Moroz, M.; Savosina, J.; Voroshilov, Y.; Lukin, S.; Lumpov, A.; Babain, V.; Legin, A.; Kirsanov, D. Quantification of thorium and uranium in real process streams of Mayak radiochemical plant using potentiometric multisensor array. J. Radioanal. Nucl. Chem. 2020, 323, 605–612. [Google Scholar] [CrossRef]
- Carrott, M.; Bell, K.; Brown, J.; Geist, A.; Gregson, C.; Hères, X.; Maher, C.; Malmbeck, R.; Mason, C.; Modolo, G.; et al. Development of a new flowsheet for co-separating the transuranic actinides: The “EURO-GANEX” process. Solvent Extr. Ion Exch. 2014, 32, 447–467. [Google Scholar] [CrossRef]
- Taylor, R.; Carrott, M.; Galan, H.; Geist, A.; Hères, X.; Maher, C.; Mason, C.; Malmbeck, R.; Miguirditchian, M.; Modolo, G.; et al. The EURO-GANEX process: Current status of flowsheet development and process safety studies. Procedia Chem. 2016, 21, 524–529. [Google Scholar] [CrossRef]









| Complex M(L)2(NO3)3 | ΔGext, kcal·mol−1 | ΔΔGext(Am/Eu) | |
|---|---|---|---|
| Am | Eu | ||
| 1 (R = Me) | −23.66 | −21.77 | −1.89 |
| 2 | −16.60 | −14.47 | −2.14 |
| 3 | −18.39 | −16.84 | −1.55 |
| 4 | −19.62 | −18.10 | −1.53 |
| 5 | −21.36 | −18.92 | −2.44 |
| Complex | ΔΔGext(Am/Eu) | SFAm/Eu | Reference |
|---|---|---|---|
| Me(PyDilac A)(NO3)3 | −1.9 | 24.8 | [54] |
| Me(PyDilac B1)(NO3)3 | −2.2 | 40.1 | [158] |
| Me(PyDilac B1)2(NO3)3 | −2.0 | 29.1 | [158] |
| Ligand | DAm | DEu | SFAm/Eu |
|---|---|---|---|
| PhenDilac A2 | >1000 | >1000 | n.a. |
| PhenDilac B1 | 3525 ± 292 | 17 ± 5 | 211 ± 47 |
| TOctPhenDA | 0.0031 ± 0.0002 | 0.0005 ± 0.0001 | 6.5 ± 1.5 |
| Ligand | Abbreviation | Substituents | Working Area | SF(Am/Eu) | SF(Am/Ln) |
|---|---|---|---|---|---|
 | PA | R = R′ = alkyl | 0.1–0.5 M HNO3 + 5 M LiNO3 | 4–13 | no data |
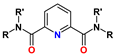 | DPA | R = alkyl R′ = aryl | 1–6 M HNO3 | 4–6 | >3–4 |
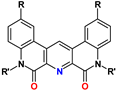 | PyDilac | R = R′ = alkyl | 1–6 M HNO3 | 15–30 | >10 |
 | BPyDA | R = alkyl R′ = aryl | 1–6 M HNO3 | 6–18 | >10 |
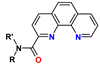 | PTA | R = alkyl R′ = aryl | <1 M HNO3 | 20–50 | no data |
 | PhenDA | R = alkyl R′ = aryl | 1–6 M HNO3 | 30–70 | >0.1–1 |
| R = R′ = alkyl | 9–10 | >3–5 | |||
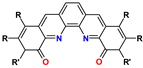 | PhenDilac | R = aryl R′= alkyl | 1–6 M HNO3 | >200 | no data |
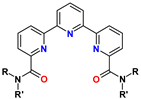 | TPyDA | R = alkyl R′ = aryl | <1 M HNO3 | 5 (almost no Am extraction) | no data |
| R = R′ = alkyl | |||||
 | Py-Phen | R = alkyl R′ = aryl | 3 M HNO3 | 19–26 | no data |
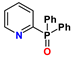 | PyPO | R = R′ = aryl | 1–3 M HNO3 | no Am extraction | no data |
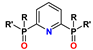 | PyDPO | R = R′ = aryl | 1–2 M HNO3 | 7–10 | no data |
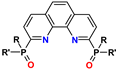 | PhenDPO | R = R′ = aryl | 0.1–4 M HNO3 | 0.5–2.1 | no data |
| R = alkyl R′ = aryl | |||||
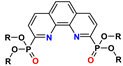 | POPhen | R = R′ = alkyl | 1–3 M HNO3 | 7–14 | no data |
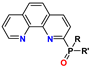 | PhenPO | R = alkyl R′ = aryl | 0.1–4 M HNO3 | no Am extraction | no data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyapyshev, M.; Babain, V.; Kirsanov, D. Isolation and Purification of Actinides Using N,O-Hybrid Donor Ligands for Closing the Nuclear Fuel Cycle. Energies 2022, 15, 7380. https://doi.org/10.3390/en15197380
Alyapyshev M, Babain V, Kirsanov D. Isolation and Purification of Actinides Using N,O-Hybrid Donor Ligands for Closing the Nuclear Fuel Cycle. Energies. 2022; 15(19):7380. https://doi.org/10.3390/en15197380
Chicago/Turabian StyleAlyapyshev, Mikhail, Vasiliy Babain, and Dmitry Kirsanov. 2022. "Isolation and Purification of Actinides Using N,O-Hybrid Donor Ligands for Closing the Nuclear Fuel Cycle" Energies 15, no. 19: 7380. https://doi.org/10.3390/en15197380
APA StyleAlyapyshev, M., Babain, V., & Kirsanov, D. (2022). Isolation and Purification of Actinides Using N,O-Hybrid Donor Ligands for Closing the Nuclear Fuel Cycle. Energies, 15(19), 7380. https://doi.org/10.3390/en15197380







