Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective
Abstract
1. Introduction
2. Review Methodology
3. Results
3.1. Environmental Perspective
- Battery Cell Composition, which deals with the structure of the cells.
- Ecological point of view that addresses a purely ecological point of view.
- Recycling Processes and Recovery Efficiencies, which provides a detailed summary of applied recycling techniques, their efficiencies, and findings.
- LCA (Life Cycle Assessment) study that evaluates the life cycle of the battery.
3.1.1. Battery Cell Composition
3.1.2. Ecological Point of View
- Is it materials production or battery assembly that causes more of these impacts?
- What motivates battery recycling if it is the assembly step that is the primary energy consumer?
- How do the energy and environmental performance of EVs and internal combustion engine vehicles (ICVs) compare?
3.1.3. Recycling Processes and Recovery Efficiencies
| Section | Recovered Material | Process | Description | Ref. |
|---|---|---|---|---|
| Cobalt (Co) | Co | Synthesis of Co3S4 | Extraction of Co and Ni from the pregnant leach solution (PLS) by a xanthate complex; ammonia solution wash; heat treatment (250 °C, 1 h). | [54] |
| Co(II) | Co(II) extraction from chloride using toluene diluted Cyphos IL 102 | Co(II) transferred into the organic phase; stripped by 0.05 mol L−1 HCl with 99.9% efficiency in a single stage at O/A 1/1. | [55] | |
| Co, Cu | Electrodeposition | The instantaneous nucleation mechanism occurs at pH 2.7 and progresses at pH 5.4 for Co electrodeposited multilayer on platinum, vitreous carbon, and Al; the same for Cu electrodeposited on Co. | [56] | |
| Co, Ni | Solvent extraction | Efficient H2SO4 + H2O2 leaching assisted by diluent heptane and ammonium thiocyanate; reaching factor 372. | [57] | |
| Co | Carbothermal reduction, magnetic separation | Separation to 53 µm fraction; carbothermal reduction (5–45 min, 500–900 °C); distilled water leaching; magnetic separation of 90% Co. | [58] | |
| Co | Electrochemical reduction | Molten salt fluidized cathode technique; characterization: voltammograms, chronoamperometry; efficiency 70–80% for the commercial LiCoO2 and upwards of 80% for the spent Li-ion battery. | [59] | |
| Co, Ni | Leaching | Acid leaching using H2SO4, HNO3, HCl, 1–4 mol L−1, 3–18 h, 25–90 °C, with a solid to liquid ratio fixed at 5% (w/v); the recovery yields of Co and Ni are 100% and 99.99%. | [60] | |
| Co | Precipitation | The Co(II) hydroxide precipitation; optimal at pH = 9; Co recovery is close to 100% and the filtration flow rate is high. | [61] | |
| β-Co(OH)2, Co3O4 | Precipitation, calcination | Chemical (CP) and electrochemical precipitation (EP); Co3O4 formation by heat-treating β-Co(OH)2 at 450 °C for 3 h. | [62] | |
| Co(OH)2, Co3O4 | Leaching | The Co(OH)2 is electrodeposited onto conductive glass using−0.85 V, 20 C cm−2, with an efficiency of 66.67%; Co3O4 is obtained by heat treatment at 450 °C after 3 h, with an efficiency of 64.29%. | [63] | |
| Co | Leaching | Acidic dissolution of LiCoO2; next electrodeposition on steel to Co3O4. | [64] | |
| Co | Synthesis | Recovery using 3D sea-urchin-like cobalt nitride composite material (CoN-Gr-2) used as a bi-functional catalyst for water splitting; potentials of 128.9 mV and 280 mV for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively. | [65] | |
| Lithium (Li)/Lithium Cobalt Oxide (LiCoO2)/ Cobalt (Co) | Li | Separation from solution | The maximum uptake range 20–25 mg Lig−1 reached for Amberlite IR 120 resin and molecular sieve 13X. | [66] |
| Li2CO3, Li3PO4 | Precipitation | Two-stage precipitation process using Na2CO3 and Na3PO4, with recovery rates of 74.72% and 92.21%, respectively. | [67] | |
| LiCoO2 | Metal-based leaching | Leaching by Co2+ or Mn2+; 95% Li recovery rate and no metal-ions in the leachate. | [68] | |
| LiCoO2 | Eco-friendly leaching | Resynthesis of cathode materials using oxalic acid; 90.13% purity of Li. | [69] | |
| LiCoO2 | Leaching | Using H2SO4 and HCl as leaching agents; optimal 2 M HCl, 60–80 °C, for 90 min. | [70] | |
| Li2CO3 | Low Li high-salt solution | Li precipitation by P; Li3PO4 anolyte dissolution, electrodialysis with cation-exchange membranes used for Li, and P separation with P/Li mass ratio 0.23; Li2CO3 precipitation rate reached 88.3%. | [71] | |
| LiCoO2 | Structure restoration | LiCoO2 powder and Li salts sintering to layered structure; Li2CO3 addition; calcination at a temperature of 800 °C; coating with nanosized Al2O3 particles for performance improvement. | [72] | |
| Li, Co | Ultrasonic-assisted leaching | Recovery of 96% Co and nearly 100% Li by using 0.5 M citric acid with 0.55 M H2O2, a solid-to-liquid ratio of 25 g L−1, a temperature of 60 °C, 5 h, and ultrasonic power of 90 W. | [73] | |
| Li, Co | Leaching | Leaching using oxalic acid (H2C2O4) at 0.46 M at 100 °C; addition of hydrogen peroxide (H2O2) resulted in a 33% of activation energy, and 50% of energy consumption reduction. | [74] | |
| Li, Co | Leaching | Leaching using biodegradable organic methane sulfonic acid (MSA); recovery efficiencies ~100% for Li, Co. | [75] | |
| Li, Co | Hydrometallurgical-electro dialytic method | Batch extraction of 30% (Co), 69% (Li) using 0.1 M HCl by LiCoO2 dissolution; extraction by compartment electro dialytic cells, and cation-exchange membranes; it yielded a recovery of 62% (Li) and 33% (Co), whereas 80% of Co was electrodeposited at the cathode. | [76] | |
| Li, Co | Leaching | Cathode leaching using a mixture of citric acid (CA), tartaric acid (TA) and ascorbic acid (AA), to recover the metals; almost complete dissolution of Li, nearly 90% dissolution of Co (80 °C, 6 h). | [77] | |
| Li, Co | Combination of crushing, ultrasonic washing, acid leaching, and precipitation | Crushing with a 12 mm aperture screen; undersize products ultrasonic washing; filtration through a 2 mm aperture; 4.0 M HCl for 2.0 h, at 80 °C leaching; 97% of Li and 99% of Co recovery. | [78] | |
| Co, Li2CO3, graphite | Thermodynamical | Thermogravimetry analysis, oxygen-free roasting, and wet magnetic separation used to transfer LiCoO2 and graphite powders to Co, Li2CO3, and graphite. | [79] | |
| LiCoO2 | Leaching, calcination | Separation of Al foil using dimethyl acetamide (DMAC), next the PVDF and carbon elimination by calcining; well-crystallized single phase LiCoO2 without Co3O4 synthesized at 850 °C,12 h. | [80] | |
| LiCoO2 | Suspension analysis | System based on NH4HCO3, (NH4)2SO3, and NaF, where NH4+ represents a complexing agent of NH3. | [81] | |
| Li, Co | Ammonia leaching | Leaching rate of 91.16% (Co) and 97.57% (Li), using NH3·H2O 120 g/L, NH4HCO3 75 g/L, n (Na2SO3), 80 °C, 240 min. | [82] | |
| Valuable metals (Co, Ni, Mn, Li) | Co, Ni, Mn, Li | Smelting reduction | Li was concentrated and recovered in the flue dust as Li2CO3 and LiF. The absence of a slag allows a nearly 100% recovery of Co, Ni, and Mn (alloy) and a nearly 100% recovery of Li (in flue dust). | [83] |
| Co, Ni, Mn, Li | Smelting reduction | Smelting reduction in a pilot-scale Electric Arc Furnace in two trials; Co, Ni, Mn and Li’s yields are 98.2%, 98.4%, 91.5%, and 68.3%, respectively, in Trial I, and 97.9%, 97.7%, 85.3%, and 60,9%, respectively, in Trial II; carbonated water leaching reaches up the purity of Li2CO3 to 95.8%. | [84] | |
| Co, Ni, Mn, Li | Thermal treatment-ammoniacal leaching | Based on the TG-DSC analysis, cathode material calcined at 300 °C and 550 °C in air atmosphere; Ni, Co, Mn, and Li leached out with efficiencies of 98%, 81%, 92% and 98%, respectively. | [85] | |
| Co, Ni, Mn, Li, Cu, Al | Wet crushing, screening, and a ternary leaching system | Selective leaching under conditions: leaching time (0–300 min), temperature (40–90 °C), solid-to-liquid ratio (10–50 g/L), and agitation speed (300–700 rpm); almost completely leaching Ni and Cu, hard recovery of Al, leaching for Li (60.53%) and Co (80.99%). | [86] | |
| Co, Ni, Mn, Li | Leaching and sol-gel method resynthesis | Leaching using 0.4 mol/L DL-malic acid and 0.1 mol/L ascorbic acid; under conditions (70 °C, 30 min, slurry density: 20 g/L), yields: 99.06% (Li), 97.11% (Ni), 96.46% (Co), and 97.22% (Mn). | [87] | |
| Co, Ni, Mn, Li | Dissolution−chelation mechanism | Leaching by solution (malonic acid, hydrogen peroxide); procedure efficiency reaches 95% (Li), over 98% (Ni, Co, Mn). | [88] | |
| Co, Ni, Mn, Li | Leaching | Leaching by sulfuric acid leaching liquor (ammonium oxalate, sodium carbonate solution); precipitation using dimethylglyoxime reagent; solvent extraction using D2EHPA; recovery efficiencies as follows: 98.7% (Ni), 97.1% (Mn), 98.2% (Co), and 81.0% (Li). | [60] | |
| Co, Ni, Mn, Li | Leaching | Recovery from spent LiNi1/3Mn1/3Co1/3O2; the efficiencies for Li, Ni, Co, and Mn reached 99.7% under the optimized conditions of 1 M H2SO4, 1 vol% H2O2, 400 rpm stirring speed, 40 g/L pulp density, and 60 min leaching at 40 °C. | [89] | |
| Co, Ni, Mn, Li | Leaching | Replacing the oxalates/carbonates of the precipitation process with sulfides; reducing the solubility of Li2CO3 in the Li precipitation step with ethanol; reaching the recycling ratio of 94.9% (Li), 94.5% (Co), 94.4% (Ni), and 95.5% (Mn). | [90] | |
| Co, Ni, Mn, Li | Leaching | Selective leaching system of NH3–(NH4)2CO3-Na2SO3; for multistage leaching, high recovery of 98.4% (Li), 99.4% (Co), 97.3% (Ni), and a high-purity (>99%) MnCO3 products. | [91] | |
| Co, Ni, Mn, Li | Hydrothermal | Using (NH4)2SO3 as a reductant in a one-step leaching process; recovery of 100% (Co), 98.3% (Ni), and 90.3% (Li). | [92] | |
| Li, Fe, P, Al | Physical separation | Discharging of spent LFP batteries in 5 wt% sodium chloride solution for approx. 3 h; extended heat treatment time within the temperature range of 240–300 °C; corona electrostatic separation for metallic particles from the nonmetallic particles. | [93] | |
| Co, Ni, Mn, Li | Hydrometallurgical | Recycling LiNi0.5Co0.2Mn0.3O2 materials using 0.2 M phosphoric acid and 0.4 M citric acid with a solid to liquid (S/L) ratio of 20 g/L at 90 °C, 30 min; leaching efficiency of ~100% (Li), 93.38% (Ni), 91.63% (Co), and 92.00% (Mn). | [94] | |
| Co, Ni, Mn, Li | High-temperature calcination, and coprecipitation | Regeneration of a ternary cathode material (LiNi0.6Co0.2Mn0.2O2) using leaching, high-temperature calcination, and coprecipitation procedure; above 97.9% transition-metal elements and 81.2% Li element in the LIBs reused. | [95] | |
| Co, Ni, Mn, Li | Leaching | Recycling LiNi0.33Mn0.33Co0.33O2 (NMC) using water under strong agitation, and pH-adjusted solutions. | [96] | |
| Co, Ni, Mn, Li | Extraction and co-precipitation | Extraction by D2EHPA in kerosene—100% (Mn), 99% (Co), and 85% (Ni); Li recovery (purity of 99.2%) from the raffinate as Li2CO3 by precipitation; organic load phase stripped with 0.5 M H2SO4; cathode material directly regenerated from stripping liquor. | [97] | |
| Co, Ni, Mn, Li | Leaching | Leaching with 1.0 M H2SO4 mixed with 0.62 wt% H2O2 at a liquid-to-solid ratio of 25.8 mL g−1, 51 °C, 60 min results in ∼100% recovery of Li, Ni, Co, and Mn; after leaching precipitation into Ni0.15Mn0.15Co0.70(OH)2, and Li2CO3. | [98] | |
| Co, Ni, Mn, Li | Leaching | Using of D, L-malic acid for leaching, and as a chelating agent; synthesis of LiNi1/3Co1/3Mn1/3O2 through a sol-gel process (no other chelating reagents). | [99] | |
| Co, Ni, Mn, Li | Leaching, oxalate co-precipitation, and solid-phase reaction | Preparation of precursor: 50 °C, pH of 1.98, the aging time of 24 h; for calcination 850 °C, 12 h. | [100] | |
| Co, Ni, Mn, Li | Froth flotation | For multiple stages above 95% of NMC111 in the froth product and 95% of LMO in the tailing product separated. | [101] | |
| LiNi1/3Co1/3Mn1/3O2 | Hydrometallurgy | Dismantling, crushing, leaching and impurity removing; LiNi1/3Co1/3Mn1/3O2 prepared from the leaching solution via co-precipitation followed by solid-state synthesis. | [102] | |
| Co, Ni, Mn, Li | Leaching | Two-step leaching of the LiNixCoyMnzO2; in the first step, Li and Co selectively leached into oxalic acid at the optimal condition of C2H2O4, 0.25 M, pulp density, 10%, H2O2 dosage, 0.5%, 80 °C, 90 min.; next H2SO4, 3.0 M, pulp density, 6%, H2O2 dosage, 2%, 60 ◦C, 120 min. performed; approximately 99% of all remaining metals leached. | [103] | |
| Co, Ni, Mn, Li | Leaching | Leaching NMC 811 by using hydrochloric acid (37% w., Sigma Aldrich). | [104] | |
| Co, Ni, Mn, Li | Leaching, thermal treatment | Solvent method for detaching the current collectors; thermal treatment for removing the polymer binders (PVDF using dissolution with N-methyl pyrrolidone); the polymer solution for carbon separation. | [105] | |
| Co, Ni, Mn, Li | Leaching | Two-step leaching of the exhausted LiNixCoyMnzO2 with 99% recovery rate; C2H2O4, 0.25 M, pulp density, 10%, H2O2 dosage, 0.5%, 80 °C, 90 min for Li, Co; H2SO4, 3.0 M, pulp density, 6%, H2O2 dosage, 2%, 60 °C, 120 min for other. | [106] | |
| Co, Ni, Mn, Li Co, Ni, Mn, Li | Leaching | The current collector of Al preforms used as the in-situ reductant of thermite reduction transforming valuable metals of Li, Ni, Co, and Mn were effectively leached into H2SO4 solution with efficiencies of 99.78%, 98.62%, 99.29%, and 99.91%, respectively. | [107] | |
| Li, Fe, P | Sintering | A direct regeneration from spent LiFePO4 batteries using a solid phase sintering; after dismantling, the cathode plate is soaked in DMAC (30 min, 30 °C, and solid-liquid ratio of 1:20 g mL−1); next regeneration at 600–700 °C. | [108] | |
| Co, Ni, Mn, Li | Calcination, Dissolution | Scraps regeneration suing solvent dissolution and heating at 800 °C. | [109] | |
| Co, Ni, Mn, Li | Leaching | Method including sol−gel method for resynthesis, and lactic acid (leaching and chelating agent); under 1.5 mol L−1, solid/liquid ratio of 20 g L−1, 70 °C, H2O2 content of 0.5 vol%, reaction for 20 min, the results: 97.7% (Li), 98.2% (Ni), 98.9% (Co), and 98.4% (Mn). | [110] | |
| Co, Ni, Mn, Li | Leaching | The recovery process is based on ammonia, ammonium carbonate, and ammonium sulfite. Co and Cu are completely leached out (~100%), whereas Mn and Al are hardly leached (<10%), and Ni with moderate leaching efficiency (30–50%). | [111] | |
| Co, Ni, Mn, Li | Hydrometallurgy | Process for recovery LiNixMnyCozO2 by applying closed loop recycling; recovery of active cathode material states for over 70% of the battery value. | [112] | |
| Co, Ni, Mn, Li | Hydrometallurgy | Selectively precipitation using dimethylglyoxime reagent, D2EHPA, ammonium oxalate solution, and saturated sodium carbonate solution. Recovery efficiencies as follows: 98.7% for Ni, 97.1% for Mn, 98.2% for Co, and 81.0% for Li under optimized conditions. | [113] | |
| Co, Ni, Mn, Li | Mechanical treatment, Chemical leaching | Mechanical treatment for recovering fractions: ferrous metals, non-ferrous metals, and electronic powders; leaching using Cyanex 272 for Ni and Co, D2EHPA for Mn. | [114] | |
| Co, Ni, Mn, Li | Leaching | The sulfuric acid was combined with H2O2 as a reducing agent; above 99% of valuable metals at 2 M H2SO4, 10 vol.% H2O2, 75 °C, 300 rpm agitation speed, 250 g/5 L solid/liquid ratio, and after 75 min, was recovered. More than 99% of Li and less than 1% of Co were dissolved at 3 M oxalic acid, at 80 °C, 300 rpm agitation speed, 50 g/L initial solid/liquid ratio, and in 90 min. | [115] | |
| Co, Ni, Mn, Li | Calcination, solvent extraction, fusion | The 5 h long calcination at 500 °C, solvent extraction aimed at 90 wt% recovery yield for Li salts; the H2SO4 and H2O2 evaporation resulted in the high purity Co and Mn sulfates; fusion with KHSO4 at 500 °C for 5 h. | [116] | |
| Graphite (C) | C | Leaching and calcination | Sulfuric acid curing-acid leaching; sequential calcination at 1500 °C, where the XRD, Raman spectroscopy, and SAEM analysis were used; final purity of regenerated graphite around 99.6%. | [117] |
| Electrolyte | PF6, PO2F2, P, F | Transcritical extraction | Combination of extraction and separation; products of hexafluorophosphate (PF6−), fluoride (F−), and difluorophosphate (PO2F2−) were detected by 19F and 31P. | [118] |
Active Anode Materials
Manganese Recovery
Lithium Iron Phosphate (LFP) Batteries
Organic Binders
Aluminum (Al) Foils
Valuable Metals
Others (Not Specified)
3.1.4. Life Cycle Analysis (LCA) Study
- ISO 14,040—that provides the ‘principles and framework’ of the Standard, and in simple terms, is written for a managerial audience,
- ISO 14,044—that offers concepts of the ‘requirements and guidelines’ typically used by practitioners [159].
- Attributional LCA—attempts to answer ‘how and which impacts are flowing within the chosen temporal window?’,
- Consequential LCA—tries to answer ‘how will they flow beyond the immediate system change in response to our decisions?’ [160].
- Two basic kinds of recycling treatments, the pyrometallurgical and hydrometallurgical one, based on secondary inventory data from the current state-of-the-art LCA models,
- One advanced hydrometallurgical technique modeled on the first-hand data obtained from industry.
3.2. Economic Perspective
4. Discussion and Conclusions
- Only a few publications are devoted to the recycling of the electrolyte issue. Although the organic electrolyte is the least profitable item of the whole battery (compared to the recovery of high-in-cost metals), it is required to complete studies characterizing its safe waste treatment, toxicity analysis, and environmental impacts, including wastewater treatment procedures or proper disposal.
- Several studies have been conducted addressing the effect of reclaimed materials on a new production of raw materials, either on a small scale or based on laboratory techniques. Nevertheless, it would be beneficial to study this issue further, especially on the data of implemented lines, which could represent a real scenario and outline future possibilities.
- According to Table 5, considering the available recycling processes, more than 99% of all valuable metals (Ni, Co, Mn, and Li), Al coverings, or organic binders can be recovered. Currently, these processes are being optimized to reduce necessary costs or environmental impacts.
- Several comprehensive LCA studies have been conducted, characterizing the issue of LIBs from EVs during their active and waste life. Although most works deal with GHG topics, there are extensions evaluating the effects of TETP (terrestrial ecotoxicity potential) or CED (cumulative energy demand). Including other environmental indicators would be beneficial, such as global warming potential (GWP) or abiotic resource depletion potential (ADP).
- So far, few publications have addressed the economic aspect of recycling LIBs from EVs. Although some complex works can be found, it is necessary to devote further research in this direction to achieve a high-quality evaluation of financial impacts with full use of EVs LIBs.
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Battery Cell Composition | |||
|---|---|---|---|
| Reference | Type | Publication Year | Summary Content |
| [11] | Review | 2020 | The recycling process of anode materials and electrolyte; recently used techniques; recovery materials application. |
| [12] | Proceedings Paper | 2012 | Summary of recycling techniques used for LIBs. |
| [13] | Proceedings Paper | 2005 | Discussion of recycling techniques used for LIBs. |
| [15] | Proceedings Paper | 2018 | Direct recycling of bare and coated NMC 622 cells. |
| [14] | Article | 2021 | Strategy for recycling LFP batteries; cathode composition based on recycled materials, design of new-type dual-ion battery. |
| [16] | Article | 2021 | Physical separation/recycling process, implemented via thermal and mechanical treatments on LFP cathode materials and current collectors (including Al fragments). |
| Environmental and Ecological Point of View | |||
|---|---|---|---|
| Reference | Type | Publication Year | Summary Content |
| [23] | Review | 2020 | Current-state developments of recycling active cathode materials by using various leaching techniques (organic acids). |
| [25] | Proceedings Paper | 2019 | Three-stage diafiltration process for recycling LIBs (Co, Li). |
| [27] | Proceedings Paper | 2016 | Summarization of recent recycling techniques; LCA; environmental impacts (GWP, TETP, HTP). |
| [18] | Article | 2009 | Critical analysis of natural resource savings. |
| [19] | Article | 2017 | Environmental and economic impacts of reusing recovery material during new LIB production. |
| [20] | Article | 2014 | Comprehensive analysis of energy and environmental impacts devoted to material production and battery assembly; motivation for recycling LIBs if considering high energy consumption of assembly; comparison EVs vs. ICVs. |
| [21] | Article | 2021 | The dis/advantages of currently used recycling techniques for LIBs from EVs; environmental issues of EVs’ LIBs’ production, use, and EOL procedures. |
| [22] | Article | 2021 | Current scenario and future perspectives for LIBs recycling; correlation between the vehicles in use and GDP. |
| [24] | Article | 2018 | Ecological recycling cathode materials (especially Co, Li); organic acid leaching (NA, AA, ascorbic acid). |
| [26] | Article | 2012 | Cradle-to-Gate energy consumption and GHG emissions of LIBs from EVs. |
| [28] | Article | 2020 | Summary description of current recycling techniques;concept of Battery Identity Global Passport (BIGP). |
| [29] | Article | 2019 | The social-economic-environmental impacts of spent LIBs from EVs; three scenarios for Stackelberg game theoretical model. |
| [30] | Article | 2020 | Determination of the future lithium availability in China. |
| Life Cycle Assessment (LCA) | |||
|---|---|---|---|
| Reference | Type | Publication Year | Summary Content |
| [162] | Proceedings Paper | 2016 | LCA (cradle-to-cradle) for lead acid, LIBs, and vanadium redox flow based on ReCiPe2008 method. |
| [164] | Proceedings Paper | 2016 | LCA of cathode materials (NMC, LFP, three types: LMR-NMC), and a lithium anode; GHG comparison EVs and CVs. |
| [161] | Article | 2020 | LCA for NCA, NMC, LFP, and SIB; comparison of basic pyrometallurgy, hydrometallurgy, and advanced hydrometallurgy; evaluation using GWP, ADP. |
| [163] | Article | 2011 | Life cycle energy performance analysis—PHEV-20. |
| [166] | Article | 2019 | LCA of LCP battery, focused on CED and GHG. |
| Recovery of Materials | |||
|---|---|---|---|
| Reference | Type | Publication Year | Summary Content |
| [43] | Review | 2019 | Summary of Li recovery procedures and their evaluation. |
| [44] | Review | 2018 | A brief review of Li recovery from aqueous resources. |
| [45] | Review | 2019 | A comprehensive review of Li recovery processes. |
| [47] | Editorial Material | 2019 | Discussion of profitable recycling of low Co LIBs considering new process developments. |
| [48] | Editorial Material | 2018 | Study of currently used recycling strategies for LIBs. |
| [51] | Proceedings Paper | 2019 | The LCA of energy consumption and GHG from critical minerals recycling of LIBs. |
| [144] | Proceedings Paper | 2015 | The liquid membrane permeation with supported flat sheet membranes. |
| [31] | Article | 2021 | Up-to-date review on the methods for the recovery of Co. |
| [54] | Article | 2021 | Selective recovery of Co from nano-Co3S4 using PSL. |
| [55] | Article | 2019 | Co(II) extraction using toluene diluted Cyphos IL 102 and chemical precipitation; recovery Co, Li, Mn, Ni, Al, Fe, Cu. |
| [56] | Article | 2010 | Electrodeposition of Co, Cu multilayers. |
| [57] | Article | 2020 | Complexation-assisted solvent extraction of Co, Ni with factor 372; study of leaching kinetics Li, Ni, Co, Mn. |
| [58] | Article | 2019 | Carbothermal reduction in a muffle furnace, magnetic separation with fraction containing 90% of Co. |
| [59] | Article | 2021 | Electrochemical reduction of Co from LiCoO2 that uses a molten salt fluidized cathode technique. |
| [60] | Article | 2014 | Hydrometallurgical process for recovery valuable metals from NCA, LIBS cathodes by using acids (H2SO4, HNO3 and HCl); the recovery efficiency of Co (100%), Ni (99.99%). |
| [61] | Article | 2021 | The thermodynamic simulations for cobalt (II) hydroxide recovery; experimental; the precipitation under different pH conditions; optimal: pH 9, efficiency close to 100%. |
| [63] | Article | 2014 | Leaching and calcination of Co(OH)2 and Co3O4 films with efficiency of 66.67% and 64.29%, respectively. |
| [64] | Article | 2011 | Electrodeposition of Co onto 430 steel in order to obtain Co3O4 film; recovery of pure Co using acidic dissolution of LiCoO2. |
| [67] | Article | 2017 | Li recovery by ion sieve, two-step precipitation using Na2CO3 and Na3PO4; recovery rates 74.72% and 92.21%, respectively. |
| [68] | Article | 2021 | Metal-based strategy for selective Li leaching from NCM, LCO, and LMO by Co2+ or Mn2+; 95% leaching rate, without metal ions left in the leachate; study of electrochemical performance of LiCoO2 particles. |
| [69] | Article | 2020 | Oxalic acid-based recycling process; 90.13% purity of LiCoO2. |
| [70] | Article | 2016 | Experimental study for LiCoO2 recovery by using H2SO4 and HCl as leaching agents; optimal 2 M HCl, 60–80 °C, for 90 min; explanation of temperature influence. |
| [71] | Article | 2018 | Li recovery from low Li high-salt solution; precipitation of Li by P; the P/Li mass ratio of the catholyte reduced to 0.23 (the feed of 1.48), Li2CO3 precipitation rate reached 88.3% at 80 °C. |
| [72] | Article | 2020 | Recovery of LiCoO2 via structure restoration; sintering of LiCoO2 powder and Li salts mixture, improvement of regeneration using nanosized Al2O3 particles. |
| [73] | Article | 2014 | Ultrasonic-assisted leaching of Li, Co; testing H2SO4, HCL, and citric acid; optimal conditions for using 0.5 M citric acid with 0.55 M H2O2, a solid-to-liquid ratio of 25 g L−1, a temperature of 60 °C, 5 h, and ultrasonic power of 90 W; recovery of 96% of Co and nearly 100% of Li. |
| [74] | Article | 2021 | Kinetic investigation of an oxalate-based process for recovery of Li and Co from LiCoO2; a combined shrinking core model (cSCM) was used for LiCoO2 digestions; description of importance of cost-effective, environmentally friendly, and energy-effective recycling processes. |
| [75] | Article | 2020 | Leaching valuable metals from LiCoO2 powders using biodegradable organic MSA; leaching efficiencies of Li and Co are achieved at nearly ~100% and ~100%, respectively. |
| [76] | Article | 2020 | Hydrometallurgical-electro dialytic method for Li, and Co recovery from LiCoO2; recovery rate 62% for Li and 33% for Co, whereas 80% of Co was electrodeposited at the cathode. |
| [77] | Article | 2018 | Recycling LiCoO2 cathode powders using organic acids (CA, TA, AA) for metal recovery; almost complete dissolution of Li, nearly 90% dissolution of Co occurred at temperature of 80 °C after 6 h. |
| [78] | Article | 2009 | Recovering Co and Li using a combination of crushing, ultrasonic washing, acid leaching and precipitation; results: 97% of Li and 99% of Co was dissolved. |
| [79] | Article | 2015 | Thermodynamic (including thermogravimetry) analysis of possible reaction between LiCoO2 and graphite; obtaining products of Co, Li2Co3 and graphite. |
| [80] | Article | 2006 | Recovery of LiCoO2 including separation of Al foil using DMAC, and the PVDF and carbon powders elimination by calcining; elements morphology and structure analysis. |
| [81] | Article | 2020 | Suspension electrolysis system for directly recycling LiCoO2 at atmospheric condition without any usage of acid and alkalis. |
| [83] | Article | 2021 | Laboratory-scale study of smelting reduction and recovery of nearly 100% recovery of Co, Ni, and Mn in the formed alloy and a nearly 100% recovery of Li in the flue dust. |
| [84] | Article | 2021 | Smelting reduction of Co, Ni, Mn (alloy), and Li (in the flue dust) in a pilot-scale Electric Arc Furnace demonstrated for two trials; recovery material yields over 90%. |
| [85] | Article | 2018 | A thermal treatment-ammoniacal leaching process; based on TG-DSC analysis calcination at 300 °C and 550 °C in air atmosphere. Complete leaching of Ni, Co, Mn, and Li with efficiencies of 98%, 81%, 92% and 98%, respectively. |
| [86] | Article | 2019 | A complex procedure of wet crushing, screening, and ternary leaching system composed of ammonia, ammonium sulfite, and ammonium bicarbonate for monitoring behavior of Li, Ni, Co, Cu, and Al. Almost fully leaching out of Ni and Cu, while Al is hardly leached, and Li (60.53%) and Co (80.99%) exhibit a moderate leaching efficiency. |
| [87] | Article | 2020 | A closed-loop recycling system of mixed organic acid leaching and sol-gel method resynthesis for LiNi0.5Co0.2Mn0.3O2 cathode material. Under optimal conditions (temp.: 70 °C, time: 30 min, slurry density: 20 g/L), the leaching efficiency of Li, Ni, Co, and Mn is 99.06%, 97.11%, 96.46%, and 97.22%, respectively. |
| [88] | Article | 2020 | Dissolution−chelation mechanism of the LiNi1/3Mn1/3Co1/3O2 cathode materials in acidic solution (malonic acid, hydrogen peroxide); under the optimal conditions the leaching efficiency of the Li is 95%, for Ni, Co, and Mn it reaches over 98%. |
| [89] | Article | 2017 | Leaching process for the recovery of Li, Ni, Co, and Mn from spent LiNi1/3Mn1/3Co1/3O2-based LIBs and cathode scraps;the efficiencies for Li, Ni, Co, and Mn reached 99.7% under the optimized conditions. |
| [90] | Article | 2020 | A higher recycling ratio of valuable metals, that is, 94.9% of Li, 94.5% of Co, 94.4% of Ni, and 95.5% of Mn, was achieved by using sulfides and reducing the solubility of lithium carbonate in the lithium precipitation step at room temperature with ethanol. |
| [91] | Article | 2020 | Selective leaching system of NH3-(NH4)2CO3-Na2SO3 for NMC; single and multistage leaching; high recovery of 98.4% (Li), 99.4% (Co), 97.3% (Ni), and high-purity (>99%) MnCO3 products were obtained. |
| [92] | Article | 2019 | Reduction-ammoniacal method (hydrothermal) devoted to the effects of various species of ammonia, ammonium salts, and reductants on the leaching of Li, Co, Ni, Mn, and Al from spent LIBs. |
| [93] | Article | 2020 | Process of physical separation of materials from spent LFP batteries; heat treatment, and corona electrostatic separation for metallic from the nonmetallic particles. |
| [94] | Article | 2018 | A hydrometallurgical process for recycling cathode materials dissolved in a mixed acid containing phosphoric and citric acid; leaching efficiency of ca. 100% for Li, 93.38% for Ni, 91.63% for Co, and 92.00% for Mn, respectively. |
| [95] | Article | 2021 | Regeneration process for a ternary cathode material using high-temperature calcination, and coprecipitation procedures; results: above 97.9% transition-metal elements and 81.2% Li element in the spent LIBs could be reused; NCM-r characterization using physical and electrochemical measurements compared to commercial NCM-c. |
| [96] | Article | 2019 | Approach for recovery of NMC particles while preserving their chemical and morphological properties, with a minimal use of chemicals. |
| [97] | Article | 2017 | Extraction and co-precipitation processes for 100% of Mn, 99% of Co and 85% of Ni recovery by D2EHPA in kerosene; Li recovery using the raffinate as Li2CO3 with the purity of 99.2% by precipitation method. |
| [98] | Article | 2021 | Recovery of valuable metals from LiNi0.15Mn0.15Co0.70O2 using the best leachant between HCl and H2SO4 + H2O2 resulting in almost 100% recovery. |
| [99] | Article | 2016 | Recovery by using D,L-malic acid; synthesis of LiNi1/3Co1/3Mn1/3O2 through a sol-gel process. |
| [100] | Article | 2020 | Regeneration process for LiNi0.5Co0.2Mn0.3O2 using mixed acid leaching, oxalate co-precipitation and solid-phase reaction. |
| [101] | Article | 2021 | Froth flotation process for NMC111, and LMO materials separation; for multiple stages 95% grade or above of NMC111 in the froth product and 95% grade of LMO in the tailing product was separated. |
| [103] | Article | 2022 | Two-step leaching of the LiNixCoyMnzO2 by sequential application of organic and mineral acids; ~99% of metals could be leached. |
| [102] | Article | 2016 | Regeneration of a LiNi1/3Co1/3Mn1/3O2 cathode material directly from the purified leaching solution via co-precipitation followed by solid-state synthesis. |
| [104] | Article | 2019 | Material recovery from NMC 811 using leaching by hydrochloric acid. |
| [105] | Article | 2021 | Three-step treatment for the separation of cathode components for sustainable LIBs recycling. |
| [106] | Article | 2019 | Two-step leaching of the exhausted LiNixCoyMnzO2 by sequential application of both organic and mineral acids; achieving more than 99% efficiency for Li and Co recovery. |
| [107] | Article | 2020 | Eco-friendly recycling; the current collector of Al preforms as the in situ reductant of thermite reduction transforming valuable metals in LiNixCoyMnzO2 cathode into LiAlO2, Li2O, NiO, CoO, and MnO. |
| [108] | Article | 2016 | A direct regeneration of cathode materials from spent LiFePO4 batteries using a solid phase sintering. |
| [110] | Article | 2017 | A leaching process for recycling valuable metals using sol−gel method and lactic acid as a leaching and chelating agent; the leaching efficiency of Li, Ni, Co, and Mn reached 97.7, 98.2, 98.9, and 98.4%, respectively. |
| [109] | Article | 2016 | Three different separation processes, including direct calcination, solvent dissolution, and basic solution dissolution, were applied to obtain the active materials from LIBs scraps. |
| [111] | Article | 2016 | The leaching behavior of Ni, Mn, Co, Al, and Cu from treated cathode active materials; study of ammonium sulfite as a reductant, and ammonium carbonate as a pH buffer. |
| [112] | Article | 2016 | Process for recovery LiNixMnyCozO2 recovery. |
| [113] | Article | 2015 | Hydrometallurgical process using sulfuric acid leaching liquor (ammonium oxalate, saturated sodium carbonate solution) for treating waste cathode materials; recovery efficiencies attained as follows: 98.7% for Ni; 97.1% for Mn, 98.2% for Co and 81.0% for Li under optimized experimental conditions. |
| [114] | Article | 2012 | Recycling NiMH and LIBs using three mechanical treatment routes for each type followed by chemical leaching. |
| [119] | Article | 2020 | Spent carbon cathode recycled (kind of roasting technologies) and used applied as the anode of Li-ion batteries (LIBs). |
| [120] | Article | 2019 | Strategy devoted to preparation of Si/CNF/C composite for LIBs by using self-prepared micron-sized silicon and waste high-density polyethylene (HDPE) as raw materials. |
| [178] | Article | 2019 | Preparation of carbon paper that is coated with recycled silicon powder (CP-RSP); it acts as both the current collector and the active material for the anodes of LIBs. |
| [121] | Article | 2020 | Analysis of reusing spent graphite as anode material for LIBs and SIBs after reconstruction process. |
| [122] | Article | 2021 | Preparation of ORR electrocatalyst applied in fuel cells based on recycled anode graphite of spent LIBs. |
| [125] | Article | 2021 | Lab-scale DN50 pulsed disc and doughnut column solvent extraction of Mn from LIBs resulting in a 94% extraction yield. |
| [127] | Article | 2021 | Extensive characterization analysis (including XRD, EPMA, XANES, etc.) of slags of the system Li2O-CaO-SiO2-Al2O3-MgO-MnOx with up to 17 mol% MnO2 content for recycling. |
| [128] | Article | 2020 | Direct recycling of cathode scrap from spent LIBs based on sulfate radical-based advance oxidation processes (SR-AOPs) that entail a complex synthesis process. |
| [129] | Article | 2016 | An environmentally friendly Mn-based material, where binder-free self-supporting (BFSS) electrodes are prepared using a fibrous, high aspect ratio MnO2 active material. |
| [130] | Article | 2015 | A green process route for recycling LiFePO4/C materials using a crystalline FePO4·2H2O phase (metastrengite I). |
| [131] | Article | 2015 | An approach for reuse, recycle, and regeneration of a spent LFP cathode for rechargeable lithium- and sodium-ion batteries. |
| [132] | Article | 2017 | Direct regeneration for scrapped LFP; high yield of high-purity products of cathode material mixture (LiFePO4 + acetylene black), anode material mixture (graphite + acetylene black) and other outputs (shell, Al foil, Cu foil, and electrolyte solvent, etc.). |
| [147] | Article | 2016 | Conversion method for the recycled LCO from spent LIBs into an efficient electrocatalyst for oxygen evolution reaction (OER); after 500 cycles a current density of 9.68 mA cm−2 at 1.65 V. |
| [138] | Article | 2019 | A high voltage/energy and long life LiFe0.6Mn0.4PO4/C (LFMP/C) composite prepared by reusing the whole LiMn2O4 cathode. |
| [139] | Article | 2019 | The soft-chemical treatment non-destructively recycles cathodes: NCM 523 and NCM 622; the reproduction of electrodes with performance equivalent to the original. |
| [140] | Article | 2018 | Four representative recycling streams were produced by a hydroxide co-precipitation to demonstrate the flexibility of the recycling process and generation of consistentquality cathode materials (NMC111). |
| Recycling of Materials | |||
|---|---|---|---|
| [32] | Review | 2020 | Recycling strategies for valuable metals in mixed-metal LIB cathodes and scrap for different types of chemistries. |
| [33] | Review | 2020 | Advances in the anode and cathode materials for the next-generation LIBs. |
| [133] | Article | 2019 | Mechanical separation and high-temperature pyrolysis of used LFP cathode active materials from retired EVs. |
| [134] | Article | 2021 | The SC CO2 extraction of organic binders from spent LIBs to facilitate the liberation of the cathode material from Al foil. |
| [135] | Article | 2015 | Thermal decomposition of the PVDF binder used between coating and foil; ANVIIL separation process. |
| [136] | Article | 2021 | Complete separation of positive active materials from Al foil, without foil destruction. |
| [137] | Article | 2020 | In situ separation and recycling procedure of coating materials and Al foils from spent LIBs using ultrasonic-assisted acid scrubbing method. |
| [141] | Article | 2020 | High-capacity Si/C anode LIBs materials that are based on Si and lignin waste from PV and the traditional paper industry. |
| [142] | Article | 2017 | Recycling procedure for waste Cu scraps in the form of CuCl powders via the facile hydrothermal route. |
| [143] | Article | 2020 | Adsorption performance of spent LFP and LMO cathodes as adsorbents toward heavy metals in water. |
| [145] | Article | 2020 | The pyrolysis kinetics of active cathode material using various methods, including Flynn–Wall–Ozawa (FWO), Friedman, Kissinger–Akahira–Sunose, Starink, Tang, and Boswell. |
| [146] | Article | 2019 | A study focused on using collected silicon oxides (SiOx) particles that are condensed from Si vapors exhausted from the ingot-growing furnace as an anode material. |
| [8] | Article | 2016 | Summary of procedures for the recycling and recovery of spent LIBs. |
| [7] | Article | 2018 | Review of the state-of-the-art techniques for metal recycling from spent LIBs. |
| [46] | Article | 2020 | Summary of technologies and issues in the disposal of spent LIBs from EVs. |
| [124] | Article | 2015 | Separation of the active electrode materials from the Co and Al foils in case of post-vehicle-application LIBs. |
| [123] | Article | 2020 | Separation of electrode materials from the current collectors using ethylene glycol. |
| [52] | Article | 2020 | Importance of recovering critical materials and improving battery designs from the cell to module level to facilitate recyclability. |
| [53] | Article | 2013 | Insulation of minerals processing operations and their effects in the case of LIBs and NiMH scraps. |
| [34] | Article | 2020 | The perspectives of hydrometallurgy and pyrometallurgy, including the process of optimization and novel recycling. |
| [50] | Article | 2021 | Global warming potential of a new waterjet-based recycling process for cathode materials of LIBs. |
| [49] | Article | 2018 | Overview of challenges in the material supply chain for automotive LIBs. |
| Economic Point of View | |||
|---|---|---|---|
| [10] | Review | 2019 | An analysis of recycling technologies from a CE perspective; overview of currently-in-service recycling facilities. |
| [167] | Review | 2019 | A circular economy insight devoted to energy storage systems on the world-wide scale. |
| [168] | Review | 2020 | Study of a circular economy for LIBs recycling techniques; a literature review of opportunities and challenges for recycling LIBs; recycling patents. |
| [169] | Review | 2021 | Environmental and economic aspects of recycling by hydrometallurgical processes. |
| [170] | Proceedings Paper | 2012 | Review of the state-of-the-art of recycling processes of spent LIBs based on the LiCoO2 system. |
| [171] | Proceedings Paper | 2015 | Different scenarios for the return rates of LIBs from EVs considering their first and second life application. |
| [172] | Proceedings Paper | 2019 | Insights for future development options for recycling EOL LIBs based on the techno-economic analyses. |
| [173] | Proceedings Paper | 2018 | The economic profits estimation of recycling spent LFP and NMC batteries in conditions of China. |
| [174] | Article | 2019 | An AI approach for evaluating the residual energy of the LIBs embedded in battery packs used in EVs. |
| [4] | Article | 2021 | The techno-economic model for comparing recycling locations and techniques; six different locations, five LIB types. |
| [175] | Article | 2013 | Optimization model for the profitability analysis for recycling facilities of LIB technologies. |
| [176] | Article | 2017 | Leaching of NMC LIBs using acetic and maleic acid with 98% recovery of valuable metals; the economic analysis of performed hydrometallurgical technique. |
| [177] | Article | 2020 | An environmental economic model based on a real case study from a Chinese EV manufacturer. |
| Recycling of EV LIBs | |||
|---|---|---|---|
| [36] | Proceedings Paper | 2016 | Hazards and consequences of incorrect recycling processing and procedures. |
| [37] | Proceedings Paper | 2013 | Overview of the framework of EV LIBs recycling. |
| [35] | Article | 2020 | Solutions for the screening and regrouping of retired LIBs considering the secondary application and future recycling. |
| [179] | Correction | 2020 | Correction to review article by Harper et al. [180]. |
| Recycling of LIBs | |||
|---|---|---|---|
| [38] | Review | 2020 | Critical overview devoted to sustainability of LIBs recycling processes. |
| [39] | Review | 2020 | A guide for suited recycling methods for metal recovery and future repurposing of spent LIBs. |
| [40] | Review | 2020 | Categorization according to state-of-the-art schemes of waste treatment technology in terms of LIBs recycling. |
| [41] | Review | 2018 | Review devoted to recent advancements in recycling technologies of spent LIBs. |
| [42] | Review | 2020 | A comparison of recycling challenges, processes, and impacts in the case of ASSBs and LIBs. |
| [115] | Proceedings Paper | 2010 | LCO batteries’ recycling based on sulfuric and oxalic acid. |
| [148] | Proceedings Paper | 2017 | Secondary Al production by spent LIBs’ recycling. |
| [149] | Proceedings Paper | 2014 | Lithium recovery from seawater by electrodialysis. |
| [82] | Article | 2019 | Ammonia leaching recycling procedure for LIBs. |
| [116] | Article | 2007 | Two recycling processes for spent LIBs: calcination and fusion. |
| [150] | Article | 2016 | Waste-to-Li system based on electrochemical reaction with water and Li precursors. |
| [151] | Article | 2019 | Critical discussion of Polish waste treatment management systems and disposal solutions. |
| [152] | Article | 2019 | Recycling procedure for spent LIBs; comparison of recycled materials with LNCM-R and LNCM-N. |
| [153] | Article | 2014 | Eco-friendly process for Li recycling from organic electrode materials for secondary used LIBs. |
| [154] | Article | 2013 | Chemical analysis, questionnaire survey, and flow analysis for Co recovery from LIBs recycling in conditions of Japan. |
| [155] | Article | 2019 | Direct physical and combined recycling procedure for LIBs. |
| [156] | Article | 2021 | LIBs discharging in aqueous salt solutions; performance and optimalization. |
References
- Dyatkin, B.; Meng, Y.S. COVID-19 disrupts battery materials and manufacture supply chains, but outlook remains strong. MRS Bull. 2020, 45, 700–702. [Google Scholar] [CrossRef] [PubMed]
- European Parliament Council of the European Union Regulation (EU) 2019/631 of the European Parliament and of the Council of 17 April 2019 Setting CO2 Emission Performance Standards for New Passenger Cars and for New Light Commercial Vehicles, and Repealing Regulations (EC) No 443/2009 and (EU) No 510/2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32019R0631 (accessed on 19 July 2022).
- Duarte Castro, F.; Mehner, E.; Cutaia, L.; Vaccari, M. Life cycle assessment of an innovative lithium-ion battery recycling route: A feasibility study. J. Clean. Prod. 2022, 368, 133130. [Google Scholar] [CrossRef]
- Lander, L.; Cleaver, T.; Rajaeifar, M.A.; Nguyen-Tien, V.; Elliott, R.J.R.; Heidrich, O.; Kendrick, E.; Edge, J.S.; Offer, G. Financial viability of electric vehicle lithium-ion battery recycling. iScience 2021, 24, 102787. [Google Scholar] [CrossRef]
- Pražanová, A.; Knap, V.; Stroe, D.-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part I: Recycling Technology. Energies 2022, 15, 1086. [Google Scholar] [CrossRef]
- Global EV Sales by Scenario, 2020–2030—Charts—Data & Statistics—IEA. Available online: https://www.iea.org/data-and-statistics/charts/global-ev-sales-by-scenario-2020-2030 (accessed on 19 July 2022).
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Arshad, F.; Li, L.; Amin, K.; Fan, E.; Manurkar, N.; Ahmad, A.; Yang, J.; Wu, F.; Chen, R. A Comprehensive Review of the Advancement in Recycling the Anode and Electrolyte from Spent Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13527–13554. [Google Scholar] [CrossRef]
- Wen, R.M.; Qi, F.P.; Hu, Y.J.; Liu, C.H.; You, P.Q. Progress of recycling and seperation of the electrode materials from spent lithium-ion batteries. Adv. Mater. Res. 2012, 550–553, 2319–2324. [Google Scholar] [CrossRef]
- Zhou, E.B.; Shi, P.X.; Li, J.H.; Lu, M.X. Recycling of Electrode Materials from Spent Lithiumion Batteries. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000397260207173 (accessed on 1 October 2021).
- Meng, Y.F.; Liang, H.J.; De Zhao, C.; Li, W.H.; Gu, Z.Y.; Yu, M.X.; Zhao, B.; Hou, X.K.; Wu, X.L. Concurrent recycling chemistry for cathode/anode in spent graphite/LiFePO4 batteries: Designing a unique cation/anion-co-workable dual-ion battery. J. Energy Chem. 2022, 64, 166–171. [Google Scholar] [CrossRef]
- Sloop, S.E.; Trevey, J.E.; Gaines, L.; Lerner, M.M.; Xu, W. Advances in Direct Recycling of Lithium-Ion Electrode Materials. ECS Trans. 2018, 85, 397–403. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, H.; Bi, H.; He, P.; Gao, S. Recycling of electrode materials from spent lithium-ion power batteries via thermal and mechanical treatments. Waste Manag. Res. 2021, 39, 607–619. [Google Scholar] [CrossRef]
- Council of the EU Waste Management and Recycling: Council Adopts New Rules—Consilium. Available online: https://www.consilium.europa.eu/en/press/press-releases/2018/05/22/waste-management-and-recycling-council-adopts-new-rules/ (accessed on 18 July 2022).
- Dewulf, J.; Van der Vorst, G.; Denturck, K.; Van Langenhove, H.; Ghyoot, W.; Tytgat, J.; Vandeputte, K. Recycling rechargeable lithium ion batteries: Critical analysis of natural resource savings. Resour. Conserv. Recycl. 2010, 54, 229–234. [Google Scholar] [CrossRef]
- Rahman, A.; Afroz, R.; Safrin, M. Recycling and disposal of lithium batteries: An economical and environmental approach. IIUM Eng. J. 2017, 18, 238–252. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; James, C.; Gallagher, K.G. The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ. Sci. 2015, 8, 158–168. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Campo, F.J.D.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric car battery: An overview on global demand, recycling and future approaches towards sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef]
- Meshram, P.; Mishra, A.; Abhilash; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Zhou, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.V.; et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J. Environ. Chem. Eng. 2019, 7, 102854. [Google Scholar] [CrossRef]
- Eugene, E.A.; Phillip, W.A.; Dowling, A.W. Material Property Goals to Enable Continuous Diafiltration Membrane Cascades for Lithium-ion Battery Recycling. Comput. Aided Chem. Eng. 2019, 47, 469–474. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef]
- Boyden, A.; Soo, V.K.; Doolan, M. The Environmental Impacts of Recycling Portable Lithium-Ion Batteries. Procedia CIRP 2016, 48, 188–193. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Q.; Li, Y.; Li, H.; Pan, X.; Mclellan, B. The social-economic-environmental impacts of recycling retired EV batteries under reward-penalty mechanism. Appl. Energy 2019, 251, 113313. [Google Scholar] [CrossRef]
- Qiao, D.; Wang, G.; Gao, T.; Wen, B.; Dai, T. Potential impact of the end-of-life batteries recycling of electric vehicles on lithium demand in China: 2010–2050. Sci. Total Environ. 2021, 764, 142835. [Google Scholar] [CrossRef]
- Mansur, M.B.; Guimarães, A.S.; Petraniková, M. An Overview on the Recovery of Cobalt from End-of-life Lithium Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 43, 489–509. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Kim, H.J.; Krishna, T.N.V.; Zeb, K.; Rajangam, V.; Muralee Gopi, C.V.V.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A Comprehensive Review of Li-Ion Battery Materials and Their Recycling Techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Wu, T.; Liu, B.; Huang, Q.; Su, Y. Recycling and Regeneration of Spent Lithium-Ion Battery Cathode Materials. Prog. Chem. 2020, 32, 2064. [Google Scholar] [CrossRef]
- Garg, A.; Yun, L.; Gao, L.; Putungan, D.B. Development of recycling strategy for large stacked systems: Experimental and machine learning approach to form reuse battery packs for secondary applications. J. Clean. Prod. 2020, 275, 124152. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, H.; Li, C.-D. Research on Systems Engineering of Recycling EV Battery. In Proceedings of the 2nd International Conference on Advances in Mechanical Engineering and Industrial Informatics (AMEII 2016), Hangzhou, China, 9–10 April 2016. [Google Scholar] [CrossRef][Green Version]
- Yu, H.; Zhang, T.; Yuan, J.; Li, C.; Li, J. Trial study on EV battery recycling standardization development. Adv. Mater. Res. 2013, 610–613, 2170–2173. [Google Scholar] [CrossRef]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnyk, S.; Sipponen, M.H.; Slabon, A. Sustainable Li-Ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2020, 11, 2003456. [Google Scholar] [CrossRef]
- Garole, D.J.; Hossain, R.; Garole, V.J.; Sahajwalla, V.; Nerkar, J.; Dubal, D.P. Recycle, Recover and Repurpose Strategy of Spent Li-ion Batteries and Catalysts: Current Status and Future Opportunities. ChemSusChem 2020, 13, 3079–3100. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling chain for spent lithium-ion batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Azhari, L.; Bong, S.; Ma, X.; Wang, Y. Recycling for All Solid-State Lithium-Ion Batteries. Matter 2020, 3, 1845–1861. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Li, L.; Deshmane, V.G.; Paranthaman, M.P.; Bhave, R.; Moyer, B.A.; Harrison, S. Lithium recovery from aqueous resources and batteries: A brief review. Johnson Matthey Technol. Rev. 2018, 62, 161–176. [Google Scholar] [CrossRef]
- Meng, F.; McNeice, J.; Zadeh, S.S.; Ghahreman, A. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, L.; Li, W.; Liu, C.; Jiang, M.; Shi, J. Recovery and Regeneration of Spent Lithium-Ion Batteries From New Energy Vehicles. Front. Chem. 2020, 8, 807. [Google Scholar] [CrossRef]
- Gaines, L. Profitable Recycling of Low-Cobalt Lithium-Ion Batteries Will Depend on New Process Developments. One Earth 2019, 1, 413–415. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. Recycling Strategies for Spent Li-Ion Battery Mixed Cathodes. ACS Energy Lett. 2018, 3, 2101–2103. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2018, 19, e00087. [Google Scholar] [CrossRef]
- Kurz, L.; Faryadras, M.; Klugius, I.; Reichert, F.; Scheibe, A.; Schmidt, M.; Wörner, R. Global warming potential of a new waterjet-based recycling process for cathode materials of lithium-ion batteries. Batteries 2021, 7, 29. [Google Scholar] [CrossRef]
- Golroudbary, S.R.; Calisaya-Azpilcueta, D.; Kraslawski, A. The Life Cycle of Energy Consumption and Greenhouse Gas Emissions from Critical Minerals Recycling: Case of Lithium-ion Batteries. Procedia CIRP 2019, 80, 316–321. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Xu, P.; Chen, Z. Enabling sustainable critical materials for battery storage through efficient recycling and improved design: A perspective. MRS Energy Sustain. 2020, 7, 27. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Behrad Vakylabad, A.; Darezereshki, E.; Hassanzadeh, A. Selective Recovery of Cobalt and Fabrication of Nano-Co3S4 from Pregnant Leach Solution of Spent Lithium-Ion Batteries. J. Sustain. Metall. 2021, 7, 1027–1044. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. J. Clean. Prod. 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Celante, V.G.; Pietre, M.K. Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits. J. Power Sources 2010, 195, 3309–3315. [Google Scholar] [CrossRef]
- Wang, W.Y.; Yang, H.C.; Xu, R. Bin High-performance recovery of cobalt and nickel from the cathode materials of nmc type li-ion battery by complexation-assisted solvent extraction. Minerals 2020, 10, 662. [Google Scholar] [CrossRef]
- Vishvakarma, S.; Dhawan, N. Recovery of Cobalt and Lithium Values from Discarded Li-Ion Batteries. J. Sustain. Metall. 2019, 5, 204–209. [Google Scholar] [CrossRef]
- Mirza, M.; Abdulaziz, R.; Maskell, W.C.; Tan, C.; Shearing, P.R.; Brett, D.J.L. Recovery of cobalt from lithium-ion batteries using fluidised cathode molten salt electrolysis. Electrochim. Acta 2021, 391, 138846. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Djoudi, N.; Le Page Mostefa, M.; Muhr, H. Hydrometallurgical process to recover cobalt from spent li-ion batteries. Resources 2021, 10, 58. [Google Scholar] [CrossRef]
- Barbieri, E.M.S.; Lima, E.P.C.; Lelis, M.F.F.; Freitas, M.B.J.G. Recycling of cobalt from spent Li-ion batteries as β-Co(OH)2 and the application of Co3O4 as a pseudocapacitor. J. Power Sources 2014, 270, 158–165. [Google Scholar] [CrossRef]
- Barbieri, E.M.S.; Lima, E.P.C.; Cantarino, S.J.; Lelis, M.F.F.; Freitas, M.B.J.G. Recycling of spent ion-lithium batteries as cobalt hydroxide, and cobalt oxide films formed under a conductive glass substrate, and their electrochemical properties. J. Power Sources 2014, 269, 158–163. [Google Scholar] [CrossRef]
- Garcia, E.M.; Tarôco, H.A.; Matencio, T.; Domingues, R.Z.; Dos Santos, J.A.F.; De Freitas, M.B.J.G. Electrochemical recycling of cobalt from spent cathodes of lithium-ion batteries: Its application as coating on SOFC interconnects. J. Appl. Electrochem. 2011, 41, 1373–1379. [Google Scholar] [CrossRef]
- Liu, T.; Cai, S.; Zhao, G.; Gao, Z.; Liu, S.; Li, H.; Chen, L.; Li, M.; Yang, X.; Guo, H. Recycling valuable cobalt from spent lithium ion batteries for controllably designing a novel sea-urchin-like cobalt nitride-graphene hybrid catalyst: Towards efficient overall water splitting. J. Energy Chem. 2021, 62, 440–450. [Google Scholar] [CrossRef]
- Lemaire, J.; Svecova, L.; Lagallarde, F.; Laucournet, R.; Thivel, P.X. Lithium recovery from aqueous solution by sorption/desorption. Hydrometallurgy 2014, 143, 1–11. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Huang, G.; Tian, Q.; Sun, H. Recovery of lithium from the effluent obtained in the process of spent lithium-ion batteries recycling. J. Environ. Manage. 2017, 198, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, B.; Hu, X.; Sun, C.F.; Hu, Y.S.; Yang, C.; Liu, H.; Zhao, J. Recycling Cathodes from Spent Lithium-Ion Batteries Based on the Selective Extraction of Lithium. ACS Sustain. Chem. Eng. 2021, 9, 10196–10204. [Google Scholar] [CrossRef]
- Anwani, S.; Methekar, R.; Ramadesigan, V. Resynthesizing of lithium cobalt oxide from spent lithium-ion batteries using an environmentally benign and economically viable recycling process. Hydrometallurgy 2020, 197, 105430. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Li, J.; Xie, H.; Chen, Y. Direct recovery of LiCoO2 from the recycled lithium-ion batteries via structure restoration. J. Alloys Compd. 2020, 845, 156234. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Verma, A.; Corbin, D.R.; Shiflett, M.B. Lithium and cobalt recovery for lithium-ion battery recycle using an improved oxalate process with hydrogen peroxide. Hydrometallurgy 2021, 203, 105694. [Google Scholar] [CrossRef]
- Wang, B.; Lin, X.Y.; Tang, Y.; Wang, Q.; Leung, M.K.H.; Lu, X.Y. Recycling LiCoO2 with methanesulfonic acid for regeneration of lithium-ion battery electrode materials. J. Power Sources 2019, 436, 226828. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Recovery of Li and Co from LiCoO2 via hydrometallurgical-electrodialytic treatment. Appl. Sci. 2020, 10, 2367. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Pai, K.V.; Manjanna, J.; Santhosh, G.; Duan, J.; Zhou, Z.; Xiao, J. Effective and environmentally friendly recycling process designed for LiCoO2 cathode powders of spent Li-ion batteries using mixture of mild organic acids. Waste Manag. 2018, 78, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.C. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Hu, Q.Y.; Li, X.H.; Wang, Z.X.; Guo, H.J. Recycle and synthesis of LiCoO2 from incisors bound of Li-ion batteries. Trans. Nonferrous Met. Soc. China 2006, 16, 956–959. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Zhang, M.; Tang, M.; Lu, Q.; Qin, Y.; Lu, Y.; Yu, B. Recycling lithium cobalt oxide from its spent batteries: An electrochemical approach combining extraction and synthesis. J. Hazard. Mater. 2021, 405, 124211. [Google Scholar] [CrossRef]
- Qi, Y.; Meng, F.; Yi, X.; Shu, J.; Chen, M.; Sun, Z.; Sun, S.; Xiu, F.R. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries. J. Clean. Prod. 2020, 251, 119665. [Google Scholar] [CrossRef]
- Hu, X.; Mousa, E.; Tian, Y.; Ye, G. Recovery of Co, Ni, Mn, and Li from Li-ion batteries by smelting reduction—Part I: A laboratory-scale study. J. Power Sources 2021, 483, 228936. [Google Scholar] [CrossRef]
- Hu, X.; Mousa, E.; Ye, G. Recovery of Co, Ni, Mn, and Li from Li-ion batteries by smelting reduction—Part II: A pilot-scale demonstration. J. Power Sources 2021, 483, 229089. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Hu, F.; Ye, L.; Xi, Y.; Yang, S. Thermal treatment and ammoniacal leaching for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2018, 75, 469–476. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Sun, C.; Zhou, T.; Zhuang, L.; Xie, H. Recycling of LiNi0.5Co0.2Mn0.3O2 Material from Spent Lithium-ion Batteries Using Mixed Organic Acid Leaching and Sol-gel Method. ChemistrySelect 2020, 5, 6482–6490. [Google Scholar] [CrossRef]
- Fan, E.; Yang, J.; Huang, Y.; Lin, J.; Arshad, F.; Wu, F.; Li, L.; Chen, R. Leaching Mechanisms of Recycling Valuable Metals from Spent Lithium-Ion Batteries by a Malonic Acid-Based Leaching System. ACS Appl. Energy Mater. 2020, 3, 8532–8542. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Song, X.F.; Yu, J.G. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag. 2017, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhang, L.; Fu, B.; Ahn, J.W.; Wang, X. Recycling of mixed lithium-ion battery cathode materials with spent lead-acid battery electrolyte with the assistance of thermodynamic simulations. J. Clean. Prod. 2020, 266, 121827. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Yan, F.; Zhang, Z.; Shen, X.; Zhang, Z. Recycling of spent lithium-ion batteries: Selective ammonia leaching of valuable metals and simultaneous synthesis of high-purity manganese carbonate. Waste Manag. 2020, 114, 253–262. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Lai, F.; Yan, F.; Zhang, Z. Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: Effect of reductants and ammonium salts. Waste Manag. 2020, 102, 122–130. [Google Scholar] [CrossRef]
- Bi, H.; Zhu, H.; Zu, L.; Gao, Y.; Gao, S.; Bai, Y. Environment-friendly technology for recovering cathode materials from spent lithium iron phosphate batteries. Waste Manag. Res. 2020, 38, 911–920. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef]
- Xing, L.; Lin, S.; Yu, J. Novel Recycling Approach to Regenerate a LiNi0.6Co0.2Mn0.2O2 Cathode Material from Spent Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2021, 60, 10303–10311. [Google Scholar] [CrossRef]
- Sieber, T.; Ducke, J.; Rietig, A.; Langner, T.; Acker, J. Recovery of Li(Ni0.33Mn0.33Co0.33)O2 from lithium-ion battery cathodes: Aspects of degradation. Nanomaterials 2019, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Anawati, J.; Malik, M.; Azimi, G. Closed-Loop Recycling of Lithium, Cobalt, Nickel, and Manganese from Waste Lithium-Ion Batteries of Electric Vehicles. ACS Sustain. Chem. Eng. 2021, 9, 4398–4410. [Google Scholar] [CrossRef]
- Yao, L.; Yao, H.; Xi, G.; Feng, Y. Recycling and synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries using d,l-malic acid. RSC Adv. 2016, 6, 17947–17954. [Google Scholar] [CrossRef]
- Gao, R.; Sun, C.; Xu, L.; Zhou, T.; Zhuang, L.; Xie, H. Recycling LiNi0.5Co0.2Mn0.3O2 material from spent lithium-ion batteries by oxalate co-precipitation. Vacuum 2020, 173, 109181. [Google Scholar] [CrossRef]
- Folayan, T.O.; Lipson, A.L.; Durham, J.L.; Pinegar, H.; Liu, D.; Pan, L. Direct Recycling of Blended Cathode Materials by Froth Flotation. Energy Technol. 2021, 9, 2100468. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, W.; Dai, Y.; Ma, Q.; Liu, Y.; Mu, D.; Li, R.; Ren, J.; Dai, C. A closed-loop process for recycling LiNixCoyMn(1−x−y)O2 from mixed cathode materials of lithium-ion batteries. Green Energy Environ. 2017, 2, 42–50. [Google Scholar] [CrossRef]
- Chabhadiya, K.; Srivastava, R.R.; Pathak, P. Two-step leaching process and kinetics for an eco-friendly recycling of critical metals from spent Li-ion batteries. J. Environ. Chem. Eng. 2021, 9, 105232. [Google Scholar] [CrossRef]
- Xuan, W.; Otsuki, A.; Chagnes, A. Investigation of the leaching mechanism of NMC 811 (LiNi0.8Mn0.1Co0.1O2) by hydrochloric acid for recycling lithium ion battery cathodes. RSC Adv. 2019, 9, 38612–38618. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, L.Z.; Kang, Y.Q.; Wang, L.; Zhou, Y.N.; Liu, X.Y.; Li, T.; Li, Y.X.; Liang, Z.; Zhang, Z.X.; et al. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Met. 2021, 40, 1431–1436. [Google Scholar] [CrossRef]
- Ning, P.; Meng, Q.; Dong, P.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system. Waste Manag. 2020, 103, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Zhang, L.; Xu, S. Cleaner recycling of cathode material by in-situ thermite reduction. J. Clean. Prod. 2020, 249, 119340. [Google Scholar] [CrossRef]
- Song, X.; Hu, T.; Liang, C.; Long, H.L.; Zhou, L.; Song, W.; You, L.; Wu, Z.S.; Liu, J.W. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017, 7, 4783–4790. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, Q.; Li, L.; Fan, E.; Wu, F.; Chen, R. Sustainable Recycling and Regeneration of Cathode Scraps from Industrial Production of Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 7041–7049. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Granata, G.; Pagnanelli, F.; Moscardini, E.; Takacova, Z.; Havlik, T.; Toro, L. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: Accomplishment of European Guidelines by optimizing mechanical pre-treatment and solvent extraction operations. J. Power Sources 2012, 212, 205–211. [Google Scholar] [CrossRef]
- Sohn, J.S.; Shin, S.M.; Yang, D.H.; Kim, S.K.; Lee, C.K. Comparison of Two Acidic Leaching Processes for Selecting the Effective Recycle Process of Spent Lithium Ion Battery-Web of Science Core Collection. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000235216700094 (accessed on 1 October 2021).
- Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, C.; Zhang, J.; Jing, Q.; Ma, B.; Chen, Y.; Zhang, W. Graphite Recycling from the Spent Lithium-Ion Batteries by Sulfuric Acid Curing-Leaching Combined with High-Temperature Calcination. ACS Sustain. Chem. Eng. 2020, 8, 9447–9455. [Google Scholar] [CrossRef]
- Mu, D.; Liu, Y.; Li, R.; Ma, Q.; Dai, C. Transcritical CO2 extraction of electrolytes for lithium-ion batteries: Optimization of the recycling process and quality-quantity variation. New J. Chem. 2017, 41, 7177–7185. [Google Scholar] [CrossRef]
- Yang, K.; Gong, P.; Tian, Z.; Lai, Y.; Li, J. Recycling spent carbon cathode by a roasting method and its application in Li-ion batteries anodes. J. Clean. Prod. 2020, 261, 121090. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, Z.; Zhu, Z.; Zhou, X.; Wang, Y.; Wang, Y.; Zhuang, Q. Recycling of waste plastics and scalable preparation of Si/CNF/C composite as anode material for lithium-ion batteries. Ionics 2019, 25, 1523–1529. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Luo, L.; Pan, Q.; Zhang, P.; Huang, Y.; Zheng, F.; Wang, H.; Li, Q. From spent graphite to recycle graphite anode for high-performance lithium ion batteries and sodium ion batteries. Electrochim. Acta 2020, 356, 136856. [Google Scholar] [CrossRef]
- Ruan, D.; Zou, K.; Du, K.; Wang, F.; Wu, L.; Zhang, Z.; Wu, X.; Hu, G. Recycling of Graphite Anode from Spent Lithium-ion Batteries for Preparing Fe-N-doped Carbon ORR Catalyst. ChemCatChem 2021, 13, 2025–2033. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Li, J.; Essehli, R.; Belharouak, I. Sustainable Direct Recycling of Lithium-Ion Batteries via Solvent Recovery of Electrode Materials. ChemSusChem 2020, 13, 5664–5670. [Google Scholar] [CrossRef]
- Li, H.; Corneal, L.M.; Standridge, C.R. Effects of acid concentration, temperature, and time on recycling of post-vehicle-application lithium-ion batteries of varying chemistries. Mater. Renew. Sustain. Energy 2015, 4, 7. [Google Scholar] [CrossRef]
- Keller, A.; Hlawitschka, M.W.; Bart, H.J. Manganese recycling of spent lithium ion batteries via Solvent extraction. Sep. Purif. Technol. 2021, 275, 119166. [Google Scholar] [CrossRef]
- Elwert, T.; Strauß, K.; Schirmer, T.; Goldmann, D. Phase composition of high lithium slags from the recycling of lithium ion batteries. World Met. 2012, 65, 163–171. [Google Scholar]
- Wittkowski, A.; Schirmer, T.; Qiu, H.; Goldmann, D.; Fittschen, U.E.A. Speciation of manganese in a synthetic recycling slag relevant for lithium recycling from lithium-ion batteries. Metals 2021, 11, 188. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dai, L.; Guo, H.; Shi, P.; Min, Y.; Xu, Q. Recycling the Cathode Scrap of Spent Lithium-Ion Batteries as an Easily Recoverable Peroxymonosulfate Catalyst with Enhanced Catalytic Performance. ACS Sustain. Chem. Eng. 2020, 8, 11337–11347. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Huang, J.; Cheng, S.; Bock, D.C.; Wu, L.; Zhu, Y.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Effective recycling of manganese oxide cathodes for lithium based batteries. Green Chem. 2016, 18, 3414–3421. [Google Scholar] [CrossRef]
- Shin, E.J.; Kim, S.; Noh, J.K.; Byun, D.; Chung, K.Y.; Kim, H.S.; Cho, B.W. A green recycling process designed for LiFePO4 cathode materials for Li-ion batteries. J. Mater. Chem. A 2015, 3, 11493–11502. [Google Scholar] [CrossRef]
- Gangaja, B.; Nair, S.; Santhanagopalan, D. Reuse, Recycle, and Regeneration of LiFePO4Cathode from Spent Lithium-Ion Batteries for Rechargeable Lithium- And Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 4711–4721. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Song, D.; Song, J.; Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 2017, 345, 78–84. [Google Scholar] [CrossRef]
- Liang, Q.; Yue, H.; Wang, S.; Yang, S.; Lam, K.-H.; Hou, X. Recycling and crystal regeneration of commercial used LiFePO4 cathode materials. Electrochim. Acta 2020, 330, 135323. [Google Scholar] [CrossRef]
- Fu, Y.; Schuster, J.; Petranikova, M.; Ebin, B. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Resour. Conserv. Recycl. 2021, 172, 105666. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Chu, W.; Zhang, Y.; Chen, L.; Wu, K.; Huang, Y.; Jia, Y. Comprehensive recycling of Al foil and active materials from the spent lithium-ion battery. Sep. Purif. Technol. 2021, 269, 118704. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Wu, X.; Zhou, T.; Ma, H. In-situ recycling of coating materials and Al foils from spent lithium ion batteries by ultrasonic-assisted acid scrubbing. J. Clean. Prod. 2020, 258, 120943. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.Z.; Gu, Z.Y.; Sun, Z.H.; Hou, B.H.; Yang, A.B.; Ning, Q.L.; Li, W.H.; Wu, X.L. Effective recycling of the whole cathode in spent lithium ion batteries: From the widely used oxides to high-energy/stable phosphates. ACS Sustain. Chem. Eng. 2019, 7, 12014–12022. [Google Scholar] [CrossRef]
- Sloop, S.E.; Crandon, L.; Allen, M.; Lerner, M.M.; Zhang, H.; Sirisaksoontorn, W.; Gaines, L.; Kim, J.; Lee, M. Cathode healing methods for recycling of lithium-ion batteries. Sustain. Mater. Technol. 2019, 22, e00113. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, M.; Wang, Q.; Zhang, Y.; Ma, X.; Shen, C.; Xu, D.; Liu, J.; Liu, Y.; Gionet, P.; et al. High Performance Cathode Recovery from Different Electric Vehicle Recycling Streams. ACS Sustain. Chem. Eng. 2018, 6, 13977–13982. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Zhu, M.; Wang, W.; Wang, L.; Xie, S.; Wang, L.; Yang, X.; He, X.; Sun, Y. Recycling of Lignin and Si Waste for Advanced Si/C Battery Anodes. ACS Appl. Mater. Interfaces 2020, 12, 57055–57063. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Yao, Y.; Liu, S.; Duan, J.; Liao, Q.; Yu, C.; Li, D.; Dai, Z. Recycled tetrahedron-like CuCl from waste Cu scraps for lithium ion battery anode. Waste Manag. 2017, 65, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhang, H.; Li, Y.; Zhang, Z.; Zhang, W. Recycling spent lithium-ion battery as adsorbents to remove aqueous heavy metals: Adsorption kinetics, isotherms, and regeneration assessment. Resour. Conserv. Recycl. 2020, 156, 104688. [Google Scholar] [CrossRef]
- Siebenhofer, M.; Noll, H.; Fritz, M. Liquid Membrane Permeation with Supported Liquid Membranes and their Application in Li-ion Battery Recycling. Sep. Sci. Technol. 2015, 50, 2937–2947. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, B.; Xiong, J.; Yao, Z.; Wu, D.; Liu, J.; Xu, S.; Tang, J. Pyrolysis kinetic study of cathode material derived from spent lithium ion batteries (LIBs): Comparison of different models. J. Air Waste Manag. Assoc. 2021, 71, 844–850. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.Y.; Yang, C.M.; Lee, G.W. Possibility of Recycling SiOx Particles Collected at Silicon Ingot Production Process as an Anode Material for Lithium Ion Batteries. Sci. Rep. 2019, 9, 13313. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Qi, J.; Du, X.; Wang, Y.; Zhang, W.; Wang, Y.; Lu, Y.; Wang, S. Recycled LiCoO2 in spent lithium-ion battery as an oxygen evolution electrocatalyst. RSC Adv. 2016, 6, 103541–103545. [Google Scholar] [CrossRef]
- Beheshti, R.; Tabeshian, A.; Aune, R.E. Lithium-ion battery recycling through secondary aluminum production. In The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2017; pp. 267–274. [Google Scholar] [CrossRef]
- Hoshino, T. Lithium Recovery from Seawater by Electrodialysis Using Ionic Liquid-based Membrane Technology. ECS Trans. 2014, 58, 173–177. [Google Scholar] [CrossRef]
- Bae, H.; Hwang, S.M.; Seo, I.; Kim, Y. Electrochemical Lithium Recycling System toward Renewable and Sustainable Energy Technologies. J. Electrochem. Soc. 2016, 163, E199–E205. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Urbańska, W. Future portable li-ion cells’ recycling challenges in Poland. Batteries 2019, 5, 75. [Google Scholar] [CrossRef]
- Peng, F.; Mu, D.; Li, R.; Liu, Y.; Ji, Y.; Dai, C.; Ding, F. Impurity removal with highly selective and efficient methods and the recycling of transition metals from spent lithium-ion batteries. RSC Adv. 2019, 9, 21922–21930. [Google Scholar] [CrossRef]
- Renault, S.; Brandell, D.; Edström, K. Environmentally-Friendly Lithium Recycling from a Spent Organic Li-Ion Battery. ChemSusChem 2014, 7, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Asari, M.; Sakai, S.I. Li-ion battery recycling and cobalt flow analysis in Japan. Resour. Conserv. Recycl. 2013, 81, 52–59. [Google Scholar] [CrossRef]
- Jo, C.H.; Myung, S.T. Efficient recycling of valuable resources from discarded lithium-ion batteries. J. Power Sources 2019, 426, 259–265. [Google Scholar] [CrossRef]
- Rouhi, H.; Karola, E.; Serna-Guerrero, R.; Santasalo-Aarnio, A. Voltage behavior in lithium-ion batteries after electrochemical discharge and its implications on the safety of recycling processes. J. Energy Storage 2021, 35, 102323. [Google Scholar] [CrossRef]
- Ilgin, M.A.; Gupta, S.M. Environmentally conscious manufacturing and product recovery (ECMPRO): A review of the state of the art. J. Environ. Manag. 2010, 91, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, M.Z.; Rosenbaum, R.K.; Olsen, S.I. Life Cycle Assessment; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Klöpffer, W.; Grahl, B. Life Cycle Assessment (LCA): A Guide to Best Practice; Wiley-VCH: Weinheim, Germany, 2014; ISBN 9783527329861. [Google Scholar] [CrossRef]
- Scientific Applications International Corporation; EPA/600/R-06/060 Life Cycle Assessment: Principles And Practice. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NRMRL&dirEntryId=155087 (accessed on 27 May 2022).
- Mohr, M.; Peters, J.F.; Baumann, M.; Weil, M. Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes. J. Ind. Ecol. 2020, 24, 1310–1322. [Google Scholar] [CrossRef]
- Unterreiner, L.; Jülch, V.; Reith, S. Recycling of Battery Technologies—Ecological Impact Analysis Using Life Cycle Assessment (LCA). Energy Procedia 2016, 99, 229–234. [Google Scholar] [CrossRef]
- Gaines, L.; Sullivan, J.; Burnham, A.; Belharouak, I. Life-cycle analysis of production and recycling of lithium ion batteries. Transp. Res. Rec. 2011, 2252, 57–65. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; Gallagher, K.G. Life Cycle Analysis Summary for Automotive Lithium-Ion Battery Production and Recycling. In REWAS 2016; Springer: Cham, Switzerland, 2016; pp. 73–79. [Google Scholar] [CrossRef]
- Dunn, J.B.; James, C.; Gaines, L.; Gallagher, K.; Dai, Q.; Kelly, J.C. Material and Energy Flows in the Production of Cathode and Anode Materials for Lithium Ion Batteries; Argonne National Lab (ANL): Argonne, IL, USA, 2015. [CrossRef]
- Raugei, M.; Winfield, P. Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J. Clean. Prod. 2019, 213, 926–932. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef] [PubMed]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef] [PubMed]
- Dalini, E.A.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A Review on Environmental, Economic and Hydrometallurgical Processes of Recycling Spent Lithium-ion Batteries. Miner. Process. Extr. Met. Rev. 2020, 42, 451–472. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, W.; Qiu, W.; Liu, S.; Wang, G. Recycling opportunities for lithium-ion power batteries. Adv. Mater. Res. 2012, 518–523, 3441–3444. [Google Scholar] [CrossRef]
- Natkunarajah, N.; Scharf, M.; Scharf, P. Scenarios for the return of lithium-ion batteries out of electric cars for recycling. Procedia CIRP 2015, 29, 740–745. [Google Scholar] [CrossRef]
- Steward, D.; Mayyas, A.; Mann, M. Economics and challenges of Li-ion battery recycling from end-of-life vehicles. Procedia Manuf. 2019, 33, 272–279. [Google Scholar] [CrossRef]
- Ma, X.; Ma, Y.; Zhou, J.; Xiong, S. The Recycling of Spent Power Battery: Economic Benefits and Policy Suggestions. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 012017. [Google Scholar] [CrossRef]
- Garg, A.; Wei, L.; Goyal, A.; Cui, X.; Gao, L. Evaluation of batteries residual energy for battery pack recycling: Proposition of stack stress-coupled-AI approach. J. Energy Storage 2019, 26, 101001. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of scale for future lithium-ion battery recycling infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. Economical recycling process for spent lithium-ion batteries and macro- and micro-scale mechanistic study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Yang, W. Optimal design of electric vehicle battery recycling network—From the perspective of electric vehicle manufacturers. Appl. Energy 2020, 275, 115328. [Google Scholar] [CrossRef]
- Shen, C.W.; Ko, T.H.; Chiu, K.F.; Leu, H.J.; Liao, T.C.; Liu, C.H. Recycled silicon powder coated on carbon paper used as the anode of lithium ion batteries. Xinxing Tan Cailiao/New Carbon Mater. 2019, 34, 140–145. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Publisher Correction: Recycling lithium-ion batteries from electric vehicles. Nature 2020, 578, E20. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
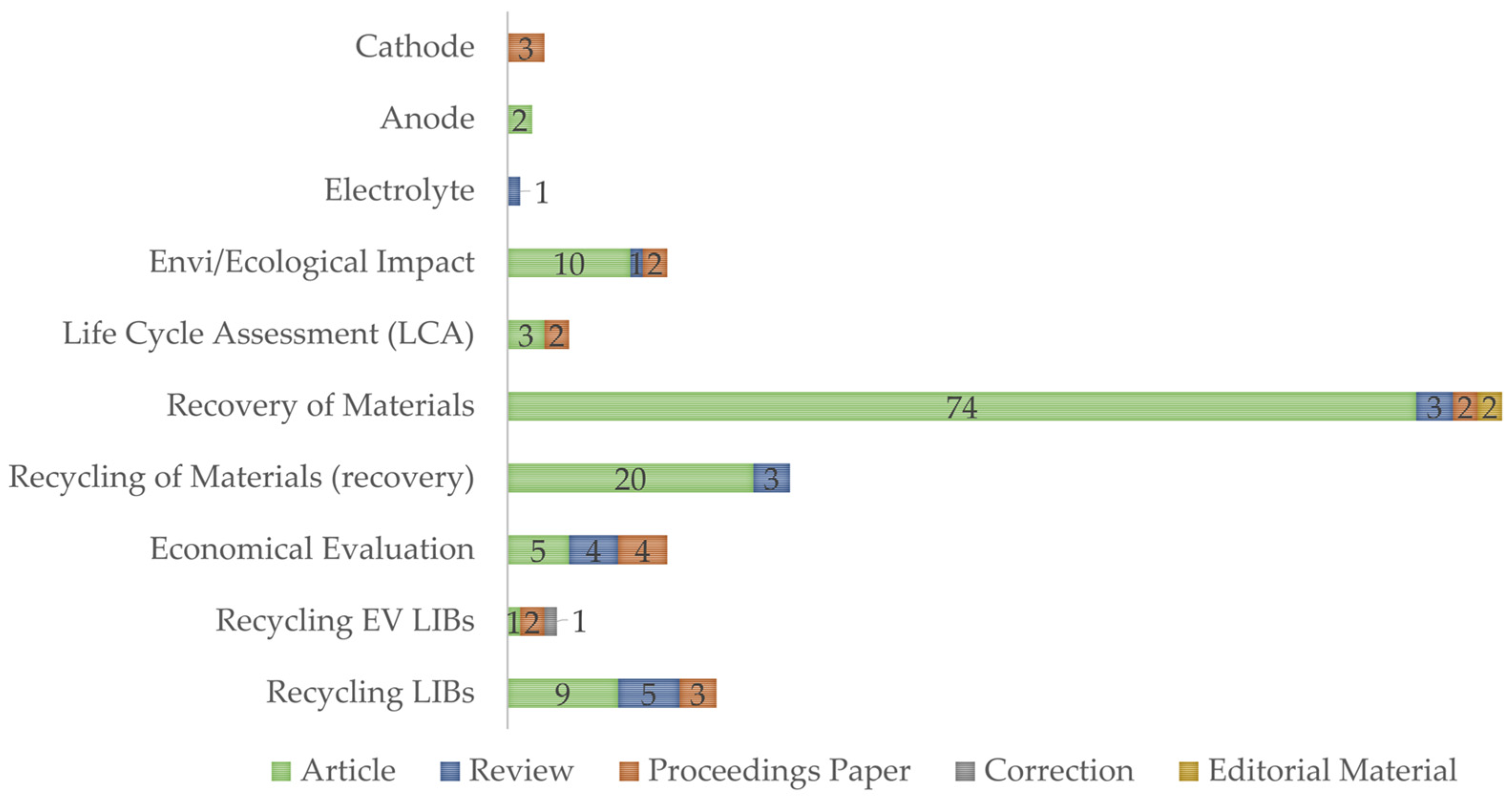
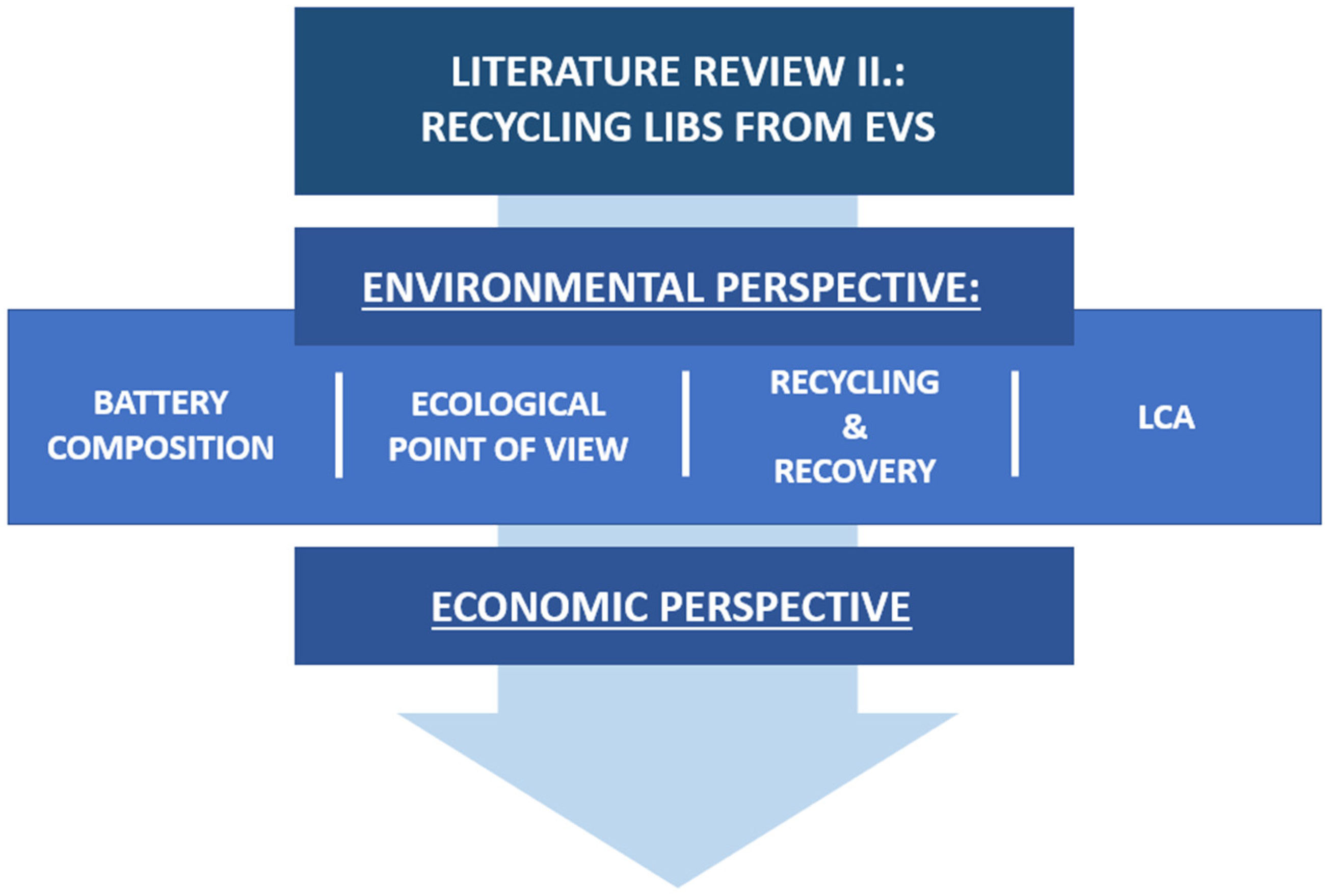
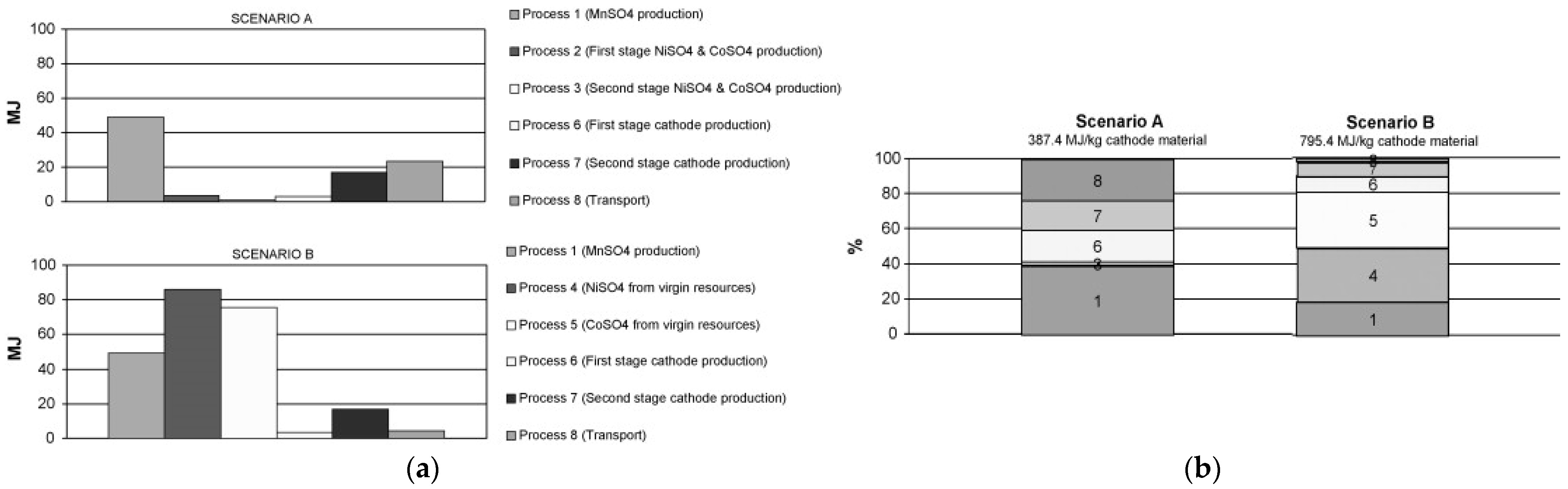

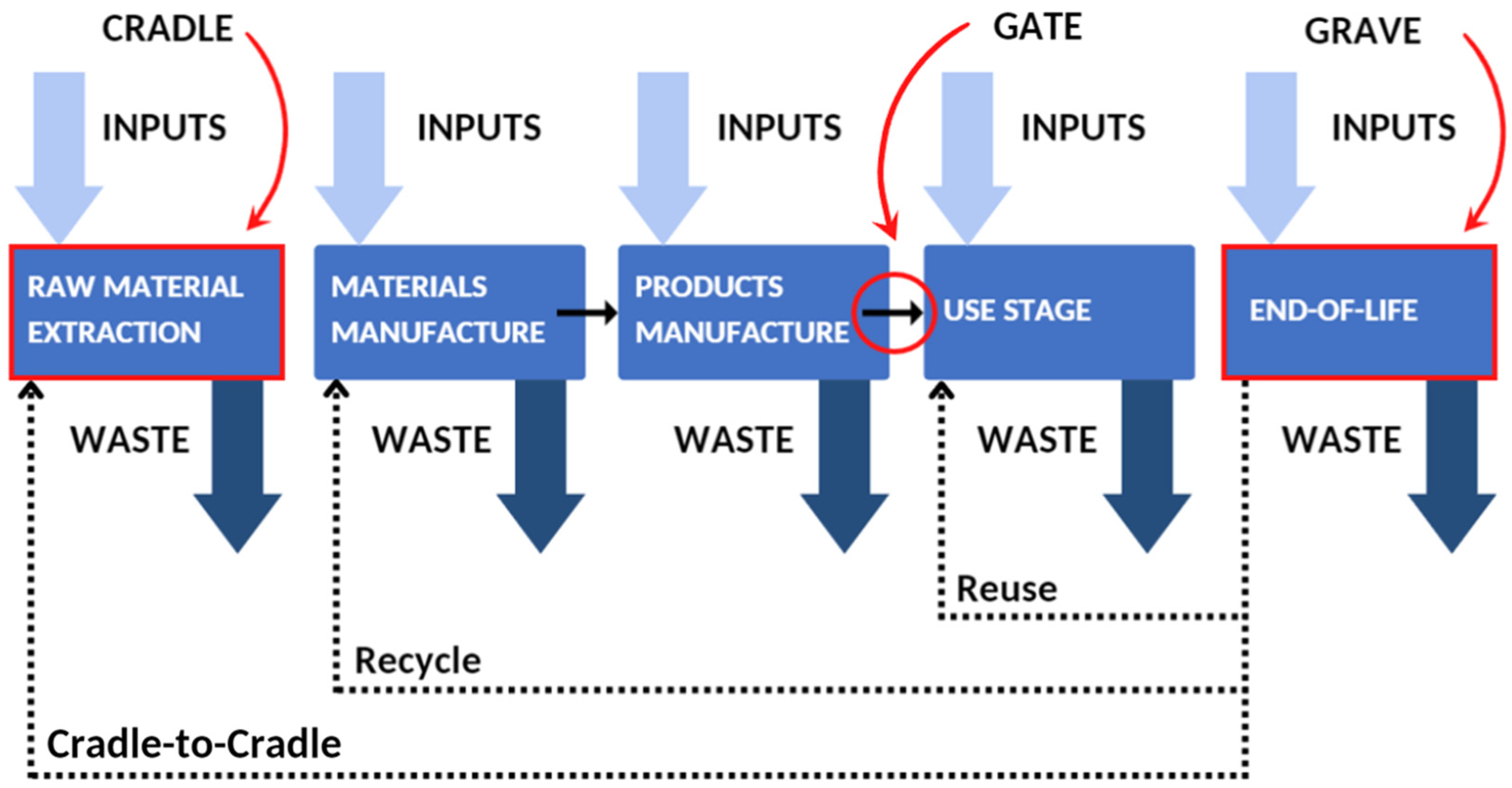
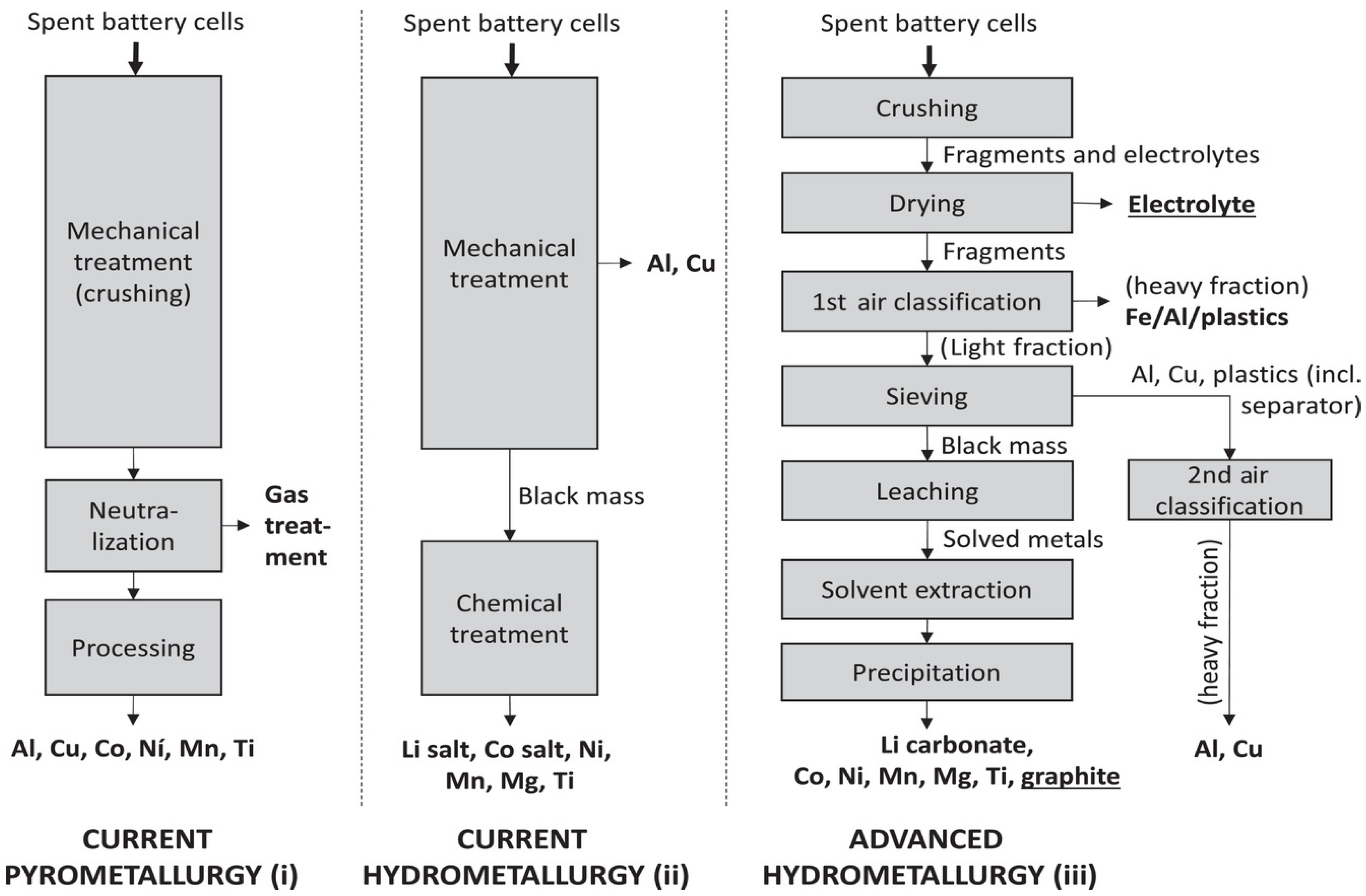
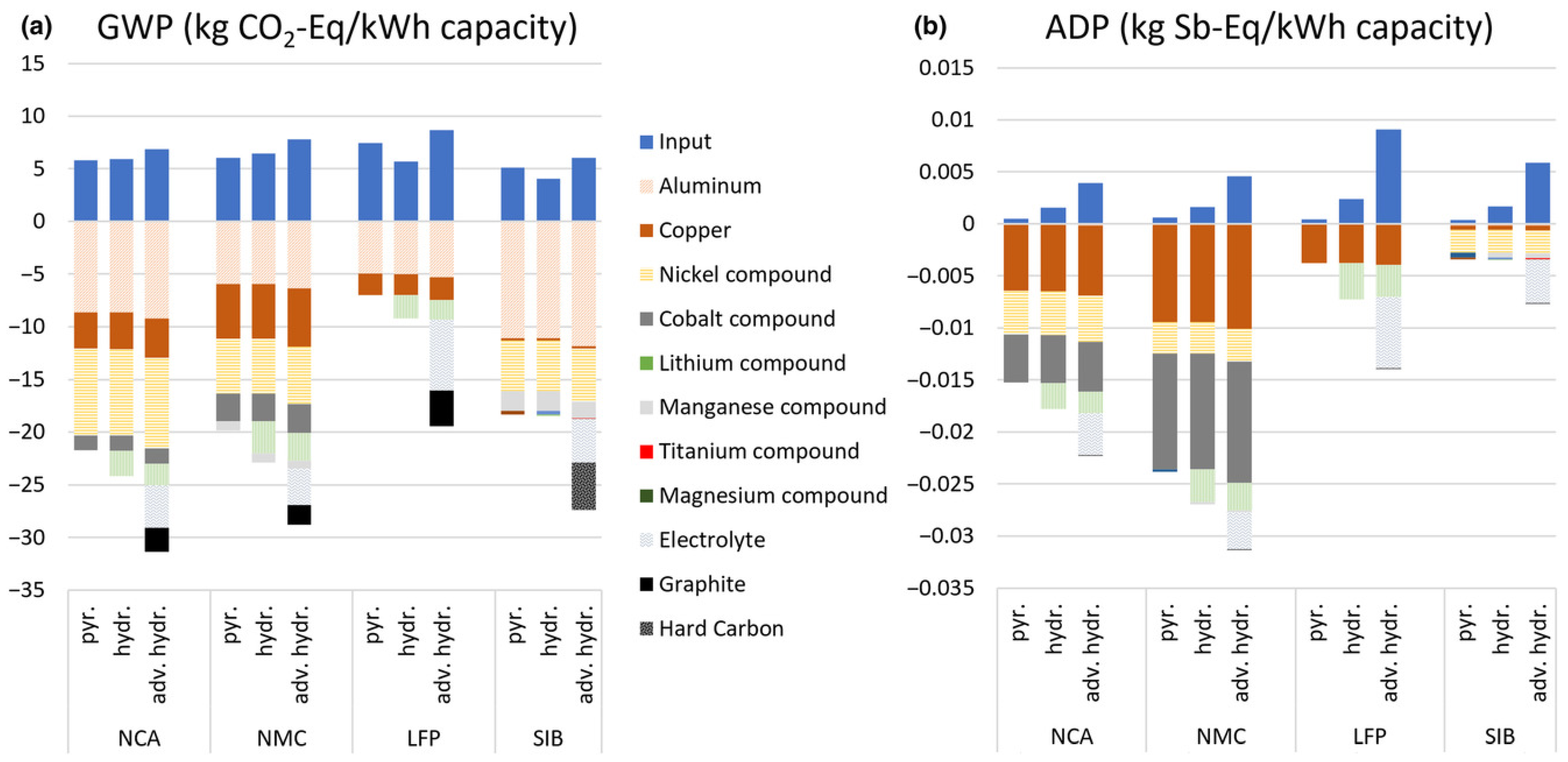

| Section | No. of Articles in Section | Category/Keyword | No. of Articles in Category | No. of Overlapped Articles |
|---|---|---|---|---|
| Recycling Processes | 61 | Pretreatment | 3 | 0 |
| Metallurgy/Mechanical | 6 | 1 | ||
| Pyrometallurgy | 4 | 0 | ||
| Hydrometallurgy | 19 | 11 | ||
| Direct Recycling | 6 | 3 | ||
| Special Method | 23 | 9 | ||
| Battery Composition | 54 | Cathode | 43 | 43 |
| Anode | 9 | 9 | ||
| Electrolyte | 2 | 2 | ||
| Environmental Impact | 75 | Envi/Ecological Impact | 13 | 5 |
| Life Cycle Assessment (LCA) | 5 | 3 | ||
| Recovery of Materials | 35 | 1 | ||
| Recycling of Materials | 22 | 0 | ||
| Economical Assessment | 14 | Economical Evaluation | 14 | 3 |
| Recycling and Rest (R&R) | 47 | Recycling EV LIBs | 11 | 6 |
| Recycling LIBs | 36 | 19 |
| Abbreviations | Indicator |
|---|---|
| ADP | Abiotic resource depletion potential |
| CED | Cumulative energy demand |
| GHG | Greenhouse gas |
| GWP | Global warming potential |
| TETP | Terrestrial ecotoxicity potential |
| Anode | Cathode (Short Name) | Electrolyte |
|---|---|---|
| Metallic lithium | Lithium manganese oxide (LMO) | LiPF6 |
| Graphitic carbon | Lithium cobalt oxide (LCO) | LiClO4 |
| Hard carbon | Lithium nickel cobalt manganese oxide (NMC) | LiAsF6 |
| Synthetic graphite | Lithium iron phosphate (LFP) | LiCF3SO3 |
| Lithium titanate | Lithium nickel cobalt aluminum oxide (NCA) | LiBF4 |
| Tin-based alloys | FeS2 | LiSFO2 |
| Silicon-based materials | V2O5 | |
| Electronic conducting polymers |
| Method | Advantages | Disadvantages |
|---|---|---|
| Direct recycling | Environmentally friendly; High specificity; Non-destructive method. | Non-specific; Not possible processing of different cathode materials. |
| Pyrometallurgical method | High recycling rates; Solvent free. | High-temperature process; Other processes for the effective recovery of materials are necessary. |
| Hydrometallurgical method | High recycling rates; Large variety in the recovery of metals. | Complexity of the process; Application of toxic reagents; High in costs. |
| Process Company | Forms of Output Materials | ||||
|---|---|---|---|---|---|
| Co | Li | Fe | Al, Cu Foils | Loses | |
| Umicore ValéasTM | CoCl2 | x | Recovered in alloy | Recovered in alloy | Al, polymer |
| Sumitomo-Sony | CoO | x | Recovered in alloy | Recovered in alloy | Al, polymer |
| Retriev Technologies | MeO + cake | Li2CO3 | Recovered in shaking table | Not specified | Not specified |
| Recupyl Valibat | LCO/Co(OH)2/Co | Li2CO3/ Li3 PO4 | Recovered with magnetic separator | Al, Cu recovered with density separation | Not specified |
| Akkuser | Co + graphite | x | Recovered with magnetic separator | Al, Cu recovered from fin powder | Al fraction |
| Accurec | Co alloy | Li2CO3 | Recovered via air filtration | Al, Cu recovered via air separator, Al via leaching | Polymer |
| Battery Resources | NMC(OH)2 | Li2CO3 | Recovered with magnetic separator | Cu recovered via dense media separation and precipitation | Not specified |
| LithoRec | MeO | Li2CO3/ LiOH | Recovered via air filtration | Al, Cu recovered via sieves and zig-zag sifter | Not specified |
| OnTo | Cathode powder (refurbished) | Li2CO3/ cathode | Recovered, not specified | Recovered, not specified | Not specified |
| Battery Type | Profits (B) (USD/Ton) | Revenues (R) (USD/Ton) | Costs (C) (USD/Ton) |
|---|---|---|---|
| LFP NMC 523 | 505 2613 | 1.57 5.47 | 1.07 2.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pražanová, A.; Knap, V.; Stroe, D.-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies 2022, 15, 7356. https://doi.org/10.3390/en15197356
Pražanová A, Knap V, Stroe D-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies. 2022; 15(19):7356. https://doi.org/10.3390/en15197356
Chicago/Turabian StylePražanová, Anna, Vaclav Knap, and Daniel-Ioan Stroe. 2022. "Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective" Energies 15, no. 19: 7356. https://doi.org/10.3390/en15197356
APA StylePražanová, A., Knap, V., & Stroe, D.-I. (2022). Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies, 15(19), 7356. https://doi.org/10.3390/en15197356








