Alkaline Direct Ethanol Fuel Cell: Effect of the Anode Flow Field Design and the Setup Parameters on Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
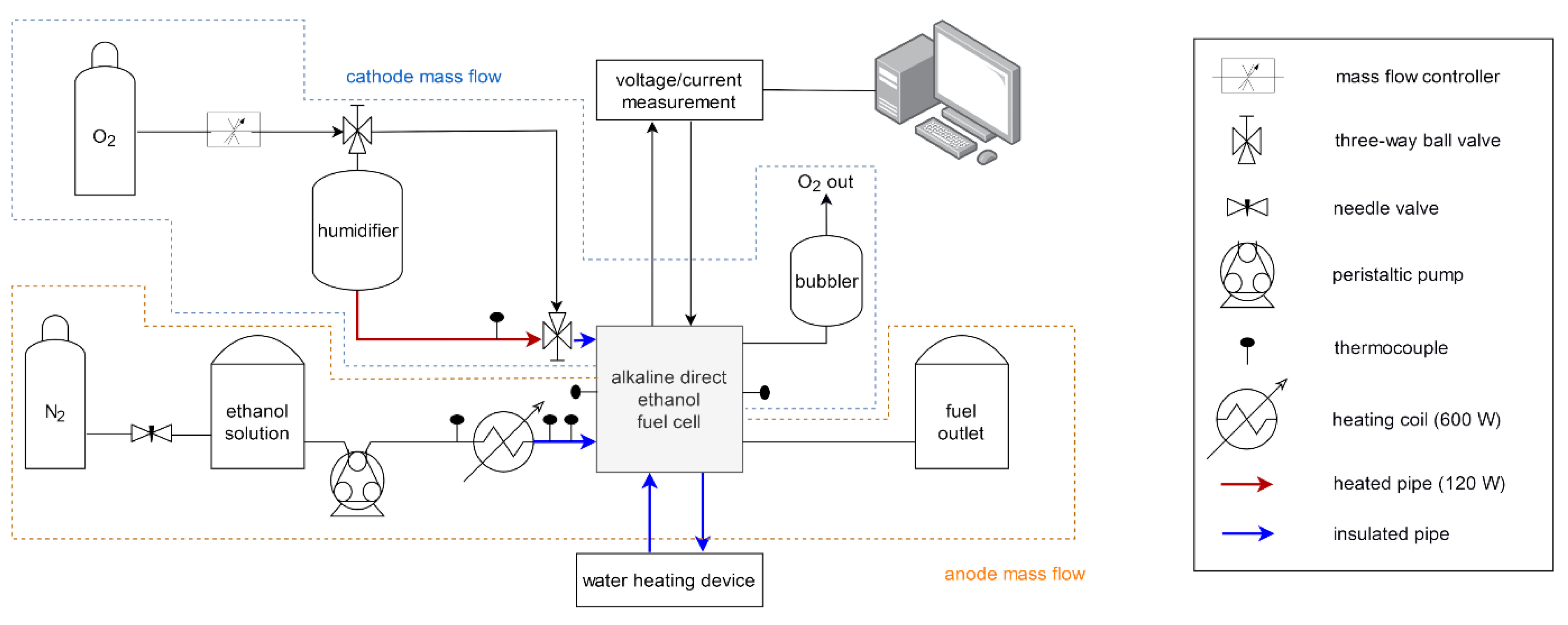
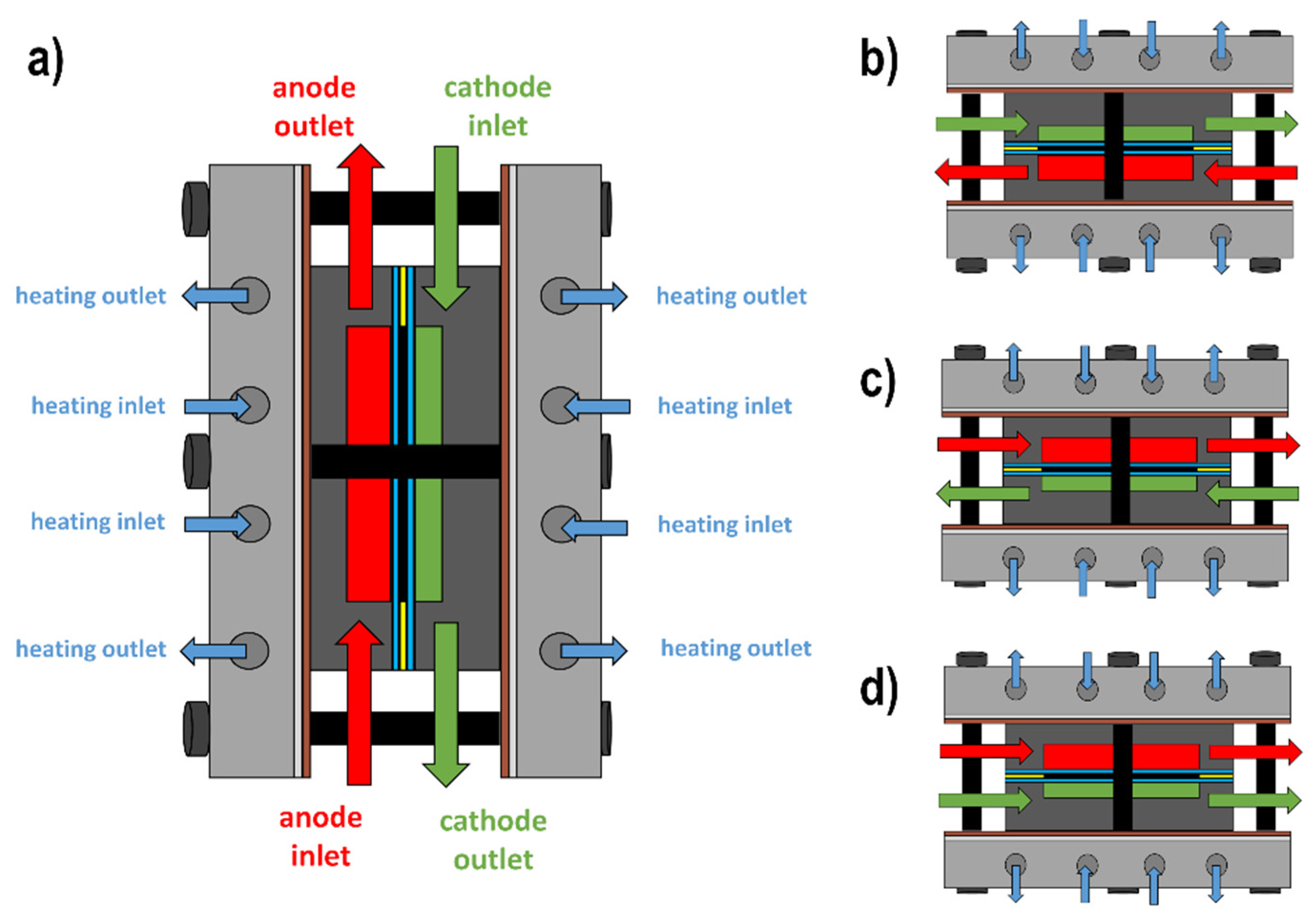
2.2. Testrig and Fuel Cell
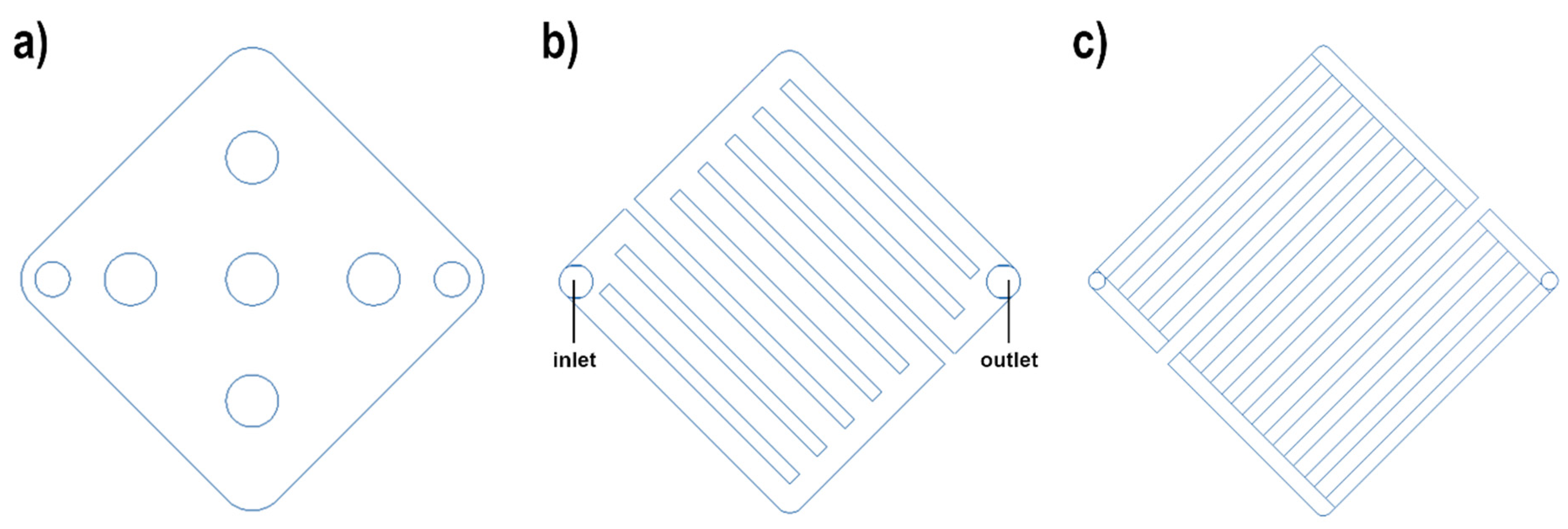
2.3. Flow Field
2.4. Computational Fluid Dynamics Simulation
2.5. Single Cell Tests
2.5.1. Membrane Electrode Assembly Production
2.5.2. Polarization Measurements
2.5.3. Electrochemical Impedance Spectra
3. Results and Discussion
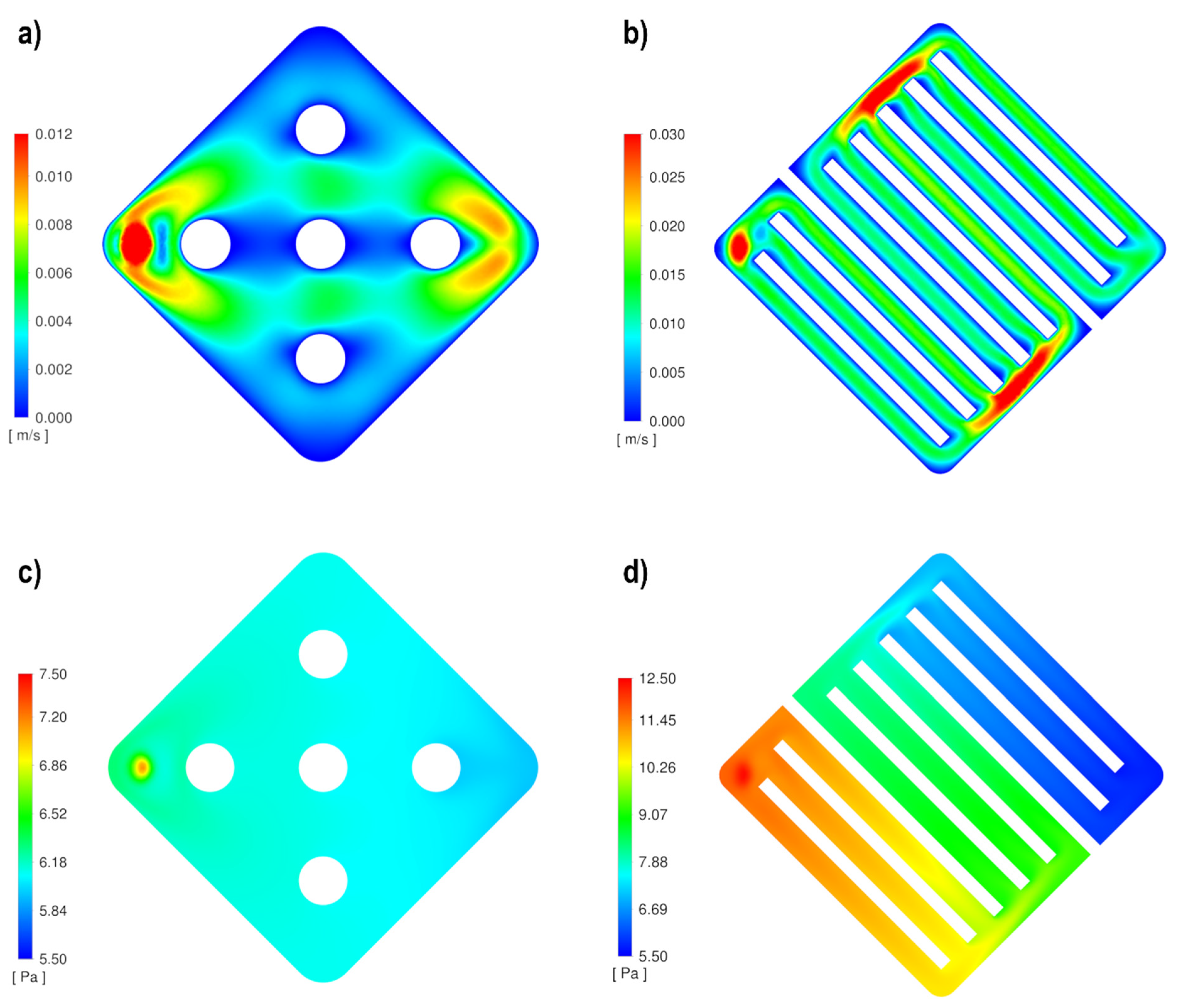
3.1. Computational Fluid Dynamics Simulation
3.2. Single Cell Tests
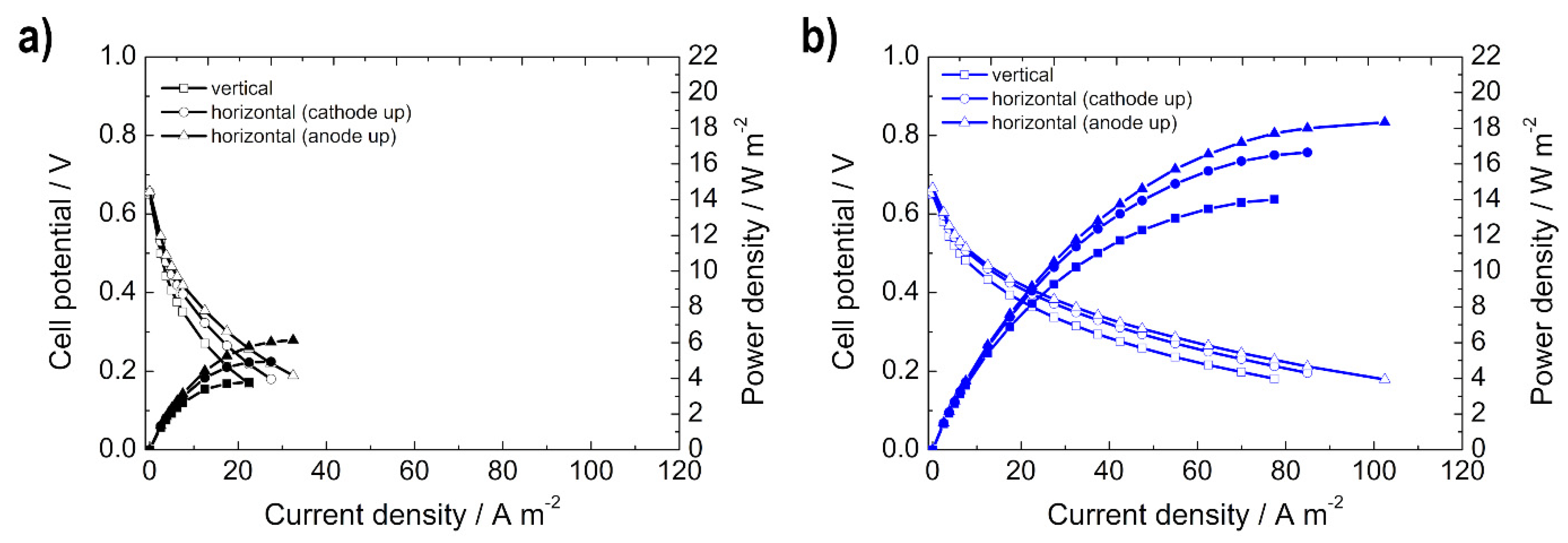
3.2.1. Influence of Orientation on Alkaline DEFC Performance
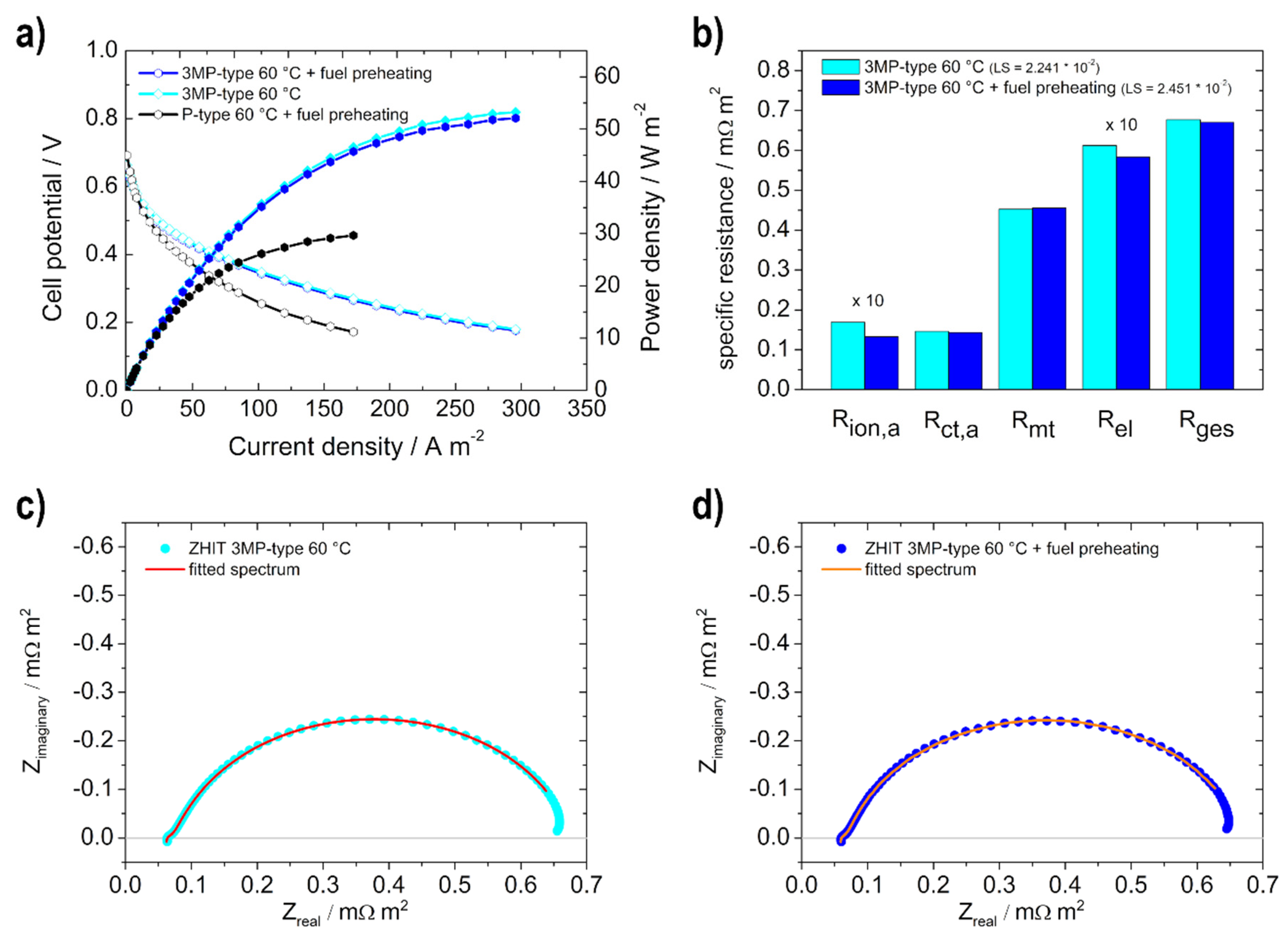
3.2.2. Effect of the Fuel Temperature on Performance
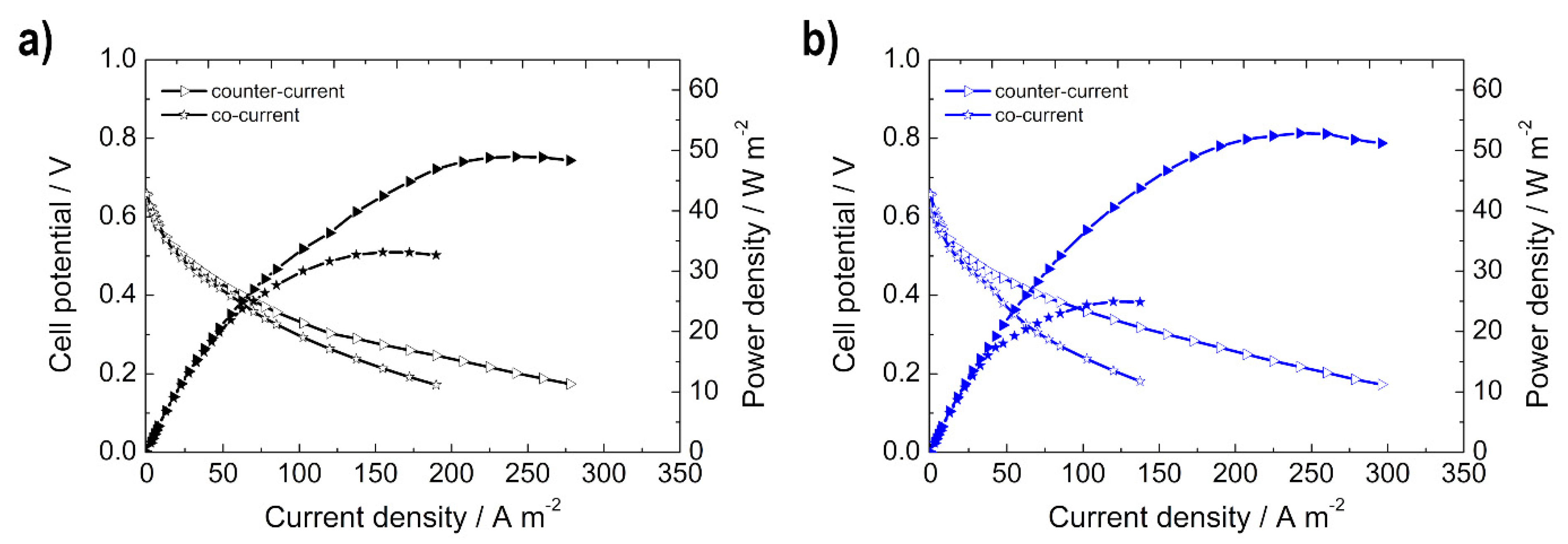
3.2.3. Influence of Co-Current vs. Counter-Current
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, E.H.; Wang, X.; Krewer, U.; Li, L.; Scott, K. Direct Oxidation Alkaline Fuel Cells: From Materials to Systems. Energy Environ. Sci. 2012, 5, 5668–5680. [Google Scholar] [CrossRef]
- Kamarudin, M.Z.F.; Kamarudin, S.K.; Masdar, M.S.; Daud, W.R.W. Review: Direct Ethanol Fuel Cells. Int. J. Hydrogen Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- Huo, S.; Deng, H.; Chang, Y.; Jiao, K. Water Management in Alkaline Anion Exchange Membrane Fuel Cell Anode. Int. J. Hydrogen Energy 2012, 37, 18389–18402. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhao, T.S.; Chen, R. Cathode Flooding Behaviour in Alkaline Direct Ethanol Fuel Cells. J. Power Sources 2011, 196, 133–139. [Google Scholar] [CrossRef]
- Carrera-Cerritos, R.; Fuentes-Ramírez, R.; Cuevas-Muñiz, F.M.; Ledesma-García, J.; Arriaga, L.G. Performance and Stability of Pd Nanostructures in an Alkaline Direct Ethanol Fuel Cell. J. Power Sources 2014, 269, 370–378. [Google Scholar] [CrossRef]
- Monyoncho, E.A.; Woo, T.K.; Baranova, E.A. Ethanol Electrooxidation Reaction in Alkaline Media for Direct Ethanol Fuel Cells. In Electrochemistry; SPR Electrochemistry; Royal Society of Chemistry: Cambridge, UK, 2019; Volume 15, pp. 1–57. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.S. Transport Phenomena in Alkaline Direct Ethanol Fuel Cells for Sustainable Energy Production. J. Power Sources 2017, 341, 199–211. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active Direct Methanol Fuel Cell: An Overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Abdullah, S.; Kamarudin, S.K.; Hasran, U.A.; Masdar, M.S.; Daud, W.R.W. Modeling and Simulation of a Direct Ethanol Fuel Cell: An Overview. J. Power Sources 2014, 262, 401–406. [Google Scholar] [CrossRef]
- Fadzillah, D.M.; Kamarudin, S.K.; Zainoodin, M.A.; Masdar, M.S. Critical Challenges in the System Development of Direct Alcohol Fuel Cells as Portable Power Supplies: An Overview. Int. J. Hydrogen Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, J.; Zhang, Y. Design and Simulation of Air-Breathing Micro Direct Methanol Fuel Cells with Different Anode Flow Fields. Micromachines 2021, 12, 253. [Google Scholar] [CrossRef]
- Gamea, O.E.; Ookawara, S.; Mori, S.; Ahmed, M. Performance Enhancement of Direct Methanol Fuel Cell Using Multi-Zone Narrow Flow Fields. Int. J. Energy Res. 2019, 43, 8257–8274. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Lim, T.H.; Oh, I.H.; Ha, H.Y. Operational Characteristics of a 50 W DMFC Stack. J. Power Sources 2006, 155, 203–212. [Google Scholar] [CrossRef]
- Azam, A.M.I.N.; Lee, S.H.; Masdar, M.S.; Zainoodin, A.M.; Kamarudin, S.K. Parametric Study on Direct Ethanol Fuel Cell (DEFC) Performance and Fuel Crossover. Int. J. Hydrogen Energy 2019, 44, 8566–8574. [Google Scholar] [CrossRef]
- Alzate, V.; Fatih, K.; Wang, H. Effect of Operating Parameters and Anode Diffusion Layer on the Direct Ethanol Fuel Cell Performance. J. Power Sources 2011, 196, 10625–10631. [Google Scholar] [CrossRef]
- Kamaruddin, M.Z.F.; Kamarudin, S.K.; Daud, W.R.W.; Masdar, M.S. An Overview of Fuel Management in Direct Methanol Fuel Cells. Renew. Sustain. Energy Rev. 2013, 24, 557–565. [Google Scholar] [CrossRef]
- Sundar Pethaiah, S.; Arunkumar, J.; Ramos, M.; Al-Jumaily, A.; Manivannan, N. The Impact of Anode Design on Fuel Crossover of Direct Ethanol Fuel Cell. Bull. Mater. Sci. 2016, 39, 273–278. [Google Scholar] [CrossRef]
- Ekdharmasuit, P.; Therdthianwong, A.; Therdthianwong, S. Anode Structure Design for Generating High Stable Power Output for Direct Ethanol Fuel Cells. Fuel 2013, 113, 69–76. [Google Scholar] [CrossRef]
- Abdullah, S.; Kamarudin, S.K.; Hasran, U.A.; Masdar, M.S.; Daud, W.R.W. Electrochemical Kinetic and Mass Transfer Model for Direct Ethanol Alkaline Fuel Cell (DEAFC). J. Power Sources 2016, 320, 111–119. [Google Scholar] [CrossRef]
- Heysiattalab, S.; Shakeri, M.; Safari, M.; Keikha, M.M. Investigation of Key Parameters Influence on Performance of Direct Ethanol Fuel Cell (DEFC). J. Ind. Eng. Chem. 2011, 17, 727–729. [Google Scholar] [CrossRef]
- Sarris, I.; Tsiakaras, P.; Song, S.; Vlachos, N. A Three-Dimensional CFD Model of Direct Ethanol Fuel Cells: Anode Flow Bed Analysis. Solid State Ion. 2006, 177, 2133–2138. [Google Scholar] [CrossRef]
- Maia, L.K.K.; Sousa, R. Three-Dimensional CFD Modeling of Direct Ethanol Fuel Cells: Evaluation of Anodic Flow Field Structures. J. Appl. Electrochem. 2017, 47, 25–37. [Google Scholar] [CrossRef]
- Sokmez, E.; Taymaz, I.; Eker Kahveci, E. Performance Evaluation of Direct Ethanol Fuel Cell Using a Three-Dimensional CFD Model. Fuel 2022, 313, 123022. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Pinar, F.J.; Mena, E.; Úbeda, D. Three-Dimensional Model of a 50 cm2 High Temperature PEM Fuel Cell. Study of the Flow Channel Geometry Influence. Int. J. Hydrogen Energy 2010, 35, 5510–5520. [Google Scholar] [CrossRef]
- Wang, J. Theory and Practice of Flow Field Designs for Fuel Cell Scaling-up: A Critical Review. Appl. Energy 2015, 157, 640–663. [Google Scholar] [CrossRef]
- Li, X.; Sabir, I. Review of Bipolar Plates in PEM Fuel Cells: Flow-Field Designs. Int. J. Hydrogen Energy 2005, 30, 359–371. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, T.S. A New Flow Field Design for Polymer Electrolyte-Based Fuel Cells. Electrochem. Commun. 2007, 9, 497–503. [Google Scholar] [CrossRef]
- Gomes, R.S.; De Bortoli, A.L. A Three-Dimensional Mathematical Model for the Anode of a Direct Ethanol Fuel Cell. Appl. Energy 2016, 183, 1292–1301. [Google Scholar] [CrossRef]
- Gomes, R.S.; De Souza, M.M.; De Bortoli, A.L. Modeling and Simulation of a Direct Ethanol Fuel Cell Considering Overpotential Losses and Variation of Principal Species Concentration. Chem. Eng. Res. Des. 2018, 136, 371–384. [Google Scholar] [CrossRef]
- Zehtabiyan-Rezaie, N.; Arefian, A.; Kermani, M.J.; Noughabi, A.K.; Abdollahzadeh, M. Effect of Flow Field with Converging and Diverging Channels on Proton Exchange Membrane Fuel Cell Performance. Energy Convers. Manag. 2017, 152, 31–44. [Google Scholar] [CrossRef]
- Yen, T.J.; Fang, N.; Zhang, X.; Lu, G.Q.; Wang, C.Y. A Micro Methanol Fuel Cell Operating at near Room Temperature. Appl. Phys. Lett. 2003, 83, 4056–4058. [Google Scholar] [CrossRef]
- Cavalca, C.; Homeyer, S.T.; Walsworth, E. Flow Field Plate for Use in a Proton Exchange Membrane Fuel Cell. U.S. Patent US5686199, 11 November 1997. [Google Scholar]
- Yuan, W.; Tang, Y.; Yang, X.; Liu, B.; Wan, Z. Structural Diversity and Orientation Dependence of a Liquid-Fed Passive Air-Breathing Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2012, 37, 9298–9313. [Google Scholar] [CrossRef]
- Hutzenlaub, T.; Paust, N.; Zengerle, R.; Ziegler, C. The Effect of Wetting Properties on Bubble Dynamics and Fuel Distribution in the Flow Field of Direct Methanol Fuel Cells. J. Power Sources 2011, 196, 8048–8056. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhao, Y.; An, Z.; Zhou, Z.; Liu, X. Design, Fabrication and Testing of an Air-Breathing Micro Direct Methanol Fuel Cell with Compound Anode Flow Field. J. Micromech. Microeng. 2011, 21, 104012. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Wu, H.C.; Her, B.S. A Novel Design for a Flow Field Configuration, of a Direct Methanol Fuel Cell. J. Power Sources 2010, 195, 3224–3230. [Google Scholar] [CrossRef]
- Jung, G.B.; Tu, C.H.; Chi, P.H.; Su, A.; Weng, F.B.; Lin, Y.T.; Chiang, Y.C.; Lee, C.Y.; Yan, W.M. Investigations of Flow Field Designs in Direct Methanol Fuel Cell. J. Solid State Electrochem. 2009, 13, 1455–1465. [Google Scholar] [CrossRef]
- Krewer, U.; Pfafferodt, M.; Kamat, A.; Menendez, D.F.; Sundmacher, K. Hydrodynamic Characterisation and Modelling of Anode Flow Fields of Direct Methanol Fuel Cells. Chem. Eng. J. 2007, 126, 87–102. [Google Scholar] [CrossRef]
- Ouellette, D.; Gencalp, U.; Colpan, C.O. Effect of Cathode Flow Field Configuration on the Performance of Flowing Electrolyte-Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2017, 42, 2680–2690. [Google Scholar] [CrossRef]
- Liang, J.; Luo, Y.; Zheng, S.; Wang, D. Enhance Performance of Micro Direct Methanol Fuel Cell by in Situ CO2 Removal Using Novel Anode Flow Field with Superhydrophobic Degassing Channels. J. Power Sources 2017, 351, 86–95. [Google Scholar] [CrossRef]
- Wang, S.J.; Huo, W.W.; Zou, Z.Q.; Qiao, Y.J.; Yang, H. Computational Simulation and Experimental Evaluation on Anodic Flow Field Structures of Micro Direct Methanol Fuel Cells. Appl. Therm. Eng. 2011, 31, 2877–2884. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Yuan, Z.; He, H.; Zhao, Y.; Liu, X. A Tapered Serpentine Flow Field for the Anode of Micro Direct Methanol Fuel Cells. J. Power Sources 2011, 196, 3255–3259. [Google Scholar] [CrossRef]
- El-Zoheiry, R.M.; Mori, S.; Ahmed, M. Using Multi-Path Spiral Flow Fields to Enhance under-rib Mass Transport in Direct Methanol Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 30663–30681. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, T.S.; Ye, Q. Pressure Drop Behavior in the Anode Flow Field of Liquid Feed Direct Methanol Fuel Cells. J. Power Sources 2005, 142, 117–124. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, A.; Ye, G.; Pan, B.; Tang, K.; Chen, H. Dynamic Relationship between the CO2 Gas Bubble Behavior and the Pressure Drop Characteristics in the Anode Flow Field of an Active Liquid-Feed Direct Methanol Fuel Cell. Appl. Energy 2017, 188, 431–443. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, T.S. Effect of Anode Flow Field Design on the Performance of Liquid Feed Direct Methanol Fuel Cells. Electrochim. Acta 2005, 50, 3243–3252. [Google Scholar] [CrossRef]
- Park, Y.C.; Chippar, P.; Kim, S.K.; Lim, S.; Jung, D.H.; Ju, H.; Peck, D.H. Effects of Serpentine Flow-Field Designs with Different Channel and Rib Widths on the Performance of a Direct Methanol Fuel Cell. J. Power Sources 2012, 205, 32–47. [Google Scholar] [CrossRef]
- Wong, C.W.; Zhao, T.S.; Ye, Q.; Liu, J.G. Experimental Investigations of the Anode Flow Field of a Micro Direct Methanol Fuel Cell. J. Power Sources 2006, 155, 291–296. [Google Scholar] [CrossRef]
- Tüber, K.; Oedegaard, A.; Hermann, M.; Hebling, C. Investigation of Fractal Flow-Fields in Portable Proton Exchange Membrane and Direct Methanol Fuel Cells. J. Power Sources 2004, 131, 175–181. [Google Scholar] [CrossRef]
- Aricò, A.S.; Cretì, P.; Baglio, V.; Modica, E.; Antonucci, V. Influence of Flow Field Design on the Performance of a Direct Methanol Fuel Cell. J. Power Sources 2000, 91, 202–209. [Google Scholar] [CrossRef]
- Gholami, O.; Imen, S.J.; Shakeri, M. Effect of Anode and Cathode Flow Field Geometry on Passive Direct Methanol Fuel Cell Performance. Electrochim. Acta 2015, 158, 410–417. [Google Scholar] [CrossRef]
- Ozden, A.; Ercelik, M.; Ouellette, D.; Colpan, C.O.; Ganjehsarabi, H.; Hamdullahpur, F. Designing, Modeling and Performance Investigation of Bio-Inspired Flow Field Based DMFCs. Int. J. Hydrogen Energy 2017, 42, 21546–21558. [Google Scholar] [CrossRef]
- Ouellette, D.; Ozden, A.; Ercelik, M.; Colpan, C.O.; Ganjehsarabi, H.; Li, X.; Hamdullahpur, F. Assessment of Different Bio-Inspired Flow Fields for Direct Methanol Fuel Cells through 3D Modeling and Experimental Studies. Int. J. Hydrogen Energy 2018, 43, 1152–1170. [Google Scholar] [CrossRef]
- Sudaroli, B.M.; Kolar, A.K. Experimental and Numerical Study of Serpentine Flow Fields for Improving Direct Methanol Fuel Cell Performance. Fuel Cells 2015, 15, 826–838. [Google Scholar] [CrossRef]
- El-Zoheiry, R.M.; Ookawara, S.; Ahmed, M. Efficient Fuel Utilization by Enhancing the Under-Rib Mass Transport Using New Serpentine Flow Field Designs of Direct Methanol Fuel Cells. Energy Convers. Manag. 2017, 144, 88–103. [Google Scholar] [CrossRef]
- Lu, Y.; Reddy, R.G. Effect of Flow Fields on the Performance of Micro-Direct Methanol Fuel Cells. Int. J. Hydrogen Energy 2011, 36, 822–829. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Rangel, C.M.; Pinto, A.M.F.R. Effect of Anode and Cathode Flow Field Design on the Performance of a Direct Methanol Fuel Cell. Chem. Eng. J. 2010, 157, 174–180. [Google Scholar] [CrossRef]
- Vasile, N.S.; Monteverde Videla, A.H.A.; Simari, C.; Nicotera, I.; Specchia, S. Influence of Membrane-Type and Flow Field Design on Methanol Crossover on a Single-Cell DMFC: An Experimental and Multi-Physics Modeling Study. Int. J. Hydrogen Energy 2017, 42, 27995–28010. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, T.S.; Ye, Q. In Situ Visualization Study of CO2 Gas Bubble Behavior in DMFC Anode Flow Fields. J. Power Sources 2005, 139, 79–90. [Google Scholar] [CrossRef]
- Bewer, T.; Beckmann, T.; Dohle, H.; Mergel, J.; Stolten, D. Novel Method for Investigation of Two-Phase Flow in Liquid Feed Direct Methanol Fuel Cells Using an Aqueous H2O2 Solution. J. Power Sources 2004, 125, 1–9. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, A.; Yan, Z.; Tan, Z.; Tang, Y.; Xia, H. Visualization of Two-Phase Flow and Temperature Characteristics of an Active Liquid-Feed Direct Methanol Fuel Cell with Diverse Flow Fields. Appl. Energy 2016, 179, 85–98. [Google Scholar] [CrossRef]
- Jung, G.B.; Su, A.; Tu, C.H.; Weng, F.B.; Chan, S.H. Innovative Flow-Field Combination Design on Direct Methanol Fuel Cell Performance. J. Fuel Cell Sci. Technol. 2007, 4, 365–368. [Google Scholar] [CrossRef]
- Grimmer, C.; Grandi, M.; Zacharias, R.; Weinberger, S.; Schenk, A.; Aksamija, E.; Mautner, F.-A.; Bitschnau, B.; Hacker, V. Carbon Supported Nanocrystalline Manganese Oxide: Surpassing Platinum as Oxygen Reduction Catalyst in Direct Borohydride Fuel Cells. J. Electrochem. Soc. 2016, 163, F885–F890. [Google Scholar] [CrossRef]
- Gorgieva, S.; Osmić, A.; Hribernik, S.; Božič, M.; Svete, J.; Hacker, V.; Wolf, S.; Genorio, B. Efficient Chitosan/Nitrogen-Doped Reduced Graphene Oxide Composite Membranes for Direct Alkaline Ethanol Fuel Cells. Int. J. Mol. Sci. 2021, 22, 1740. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, A.M.; Wolf, S.; Roschger, M.; Hacker, V. Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Direct Ethanol Fuel Cells. Int. J. Renew. Energy Dev. 2021, 10, 435–443. [Google Scholar] [CrossRef]
- Cermenek, B.; Genorio, B.; Winter, T.; Wolf, S.; Connell, J.G.; Roschger, M.; Letofsky-Papst, I.; Kienzl, N.; Bitschnau, B.; Hacker, V. Alkaline Ethanol Oxidation Reaction on Carbon Supported Ternary PdNiBi Nanocatalyst Using Modified Instant Reduction Synthesis Method. Electrocatalysis 2020, 11, 203–214. [Google Scholar] [CrossRef]
- Wnuk, P.; Jurczakowski, R.; Lewera, A. Electrochemical Characterization of Low-Temperature Direct Ethanol Fuel Cells Using Direct and Alternate Current Methods. Electrocatalysis 2020, 11, 121–132. [Google Scholar] [CrossRef]


| Flow Field Structures | Flow Field Parameters (Width and Depth of Channel) | Results | Ref. |
|---|---|---|---|
| Different Geometries | |||
| trapezoidal, parallel, perforated |
|
| Gholami et al. [51] |
| serpentine, bio-inspired interdigitated and non-interdigitated |
|
| Ouellette et al. [53] |
| grid, parallel, double serpentine, single serpentine |
|
| Deng et al. [11] |
| Different serpentine | |||
| single serpentine, multi-serpentine, mixed parallel and serpentine |
|
| Oliveira et al. [57] |
| single and double channel serpentine, mixed multichannel serpentine with wide and narrow channels |
|
| Lu et al. [56] |
| serpentine with varying number of paths, different patterning, and rib lengths |
|
| El-Zoheiry et al. [55] |
| unique serpentine, four parallel serpentine, four inlet serpentine |
|
| Vasile et al. [58] |
| Channel depth, width and rib width | |||
| single serpentine, parallel |
|
| Yang et al. [46] |
| serpentine, parallel |
|
| Wong et al. [48] |
| serpentine |
|
| Park et al. [47] |
| Flow Field | P (Anode) | 3 MP (Anode) | 4 MP (Cathode) |
|---|---|---|---|
| dimensions [cm × cm] | 2 × 2 | 2 × 2 | 2 × 2 |
| channel width [mm] | - | 1.5 | 0.8 |
| channel depth [mm] | 2.4 | 2.4 | 0.8 |
| rib width [mm] | Ø: 3 | 0.8 | 0.95 |
| open area [mm2] | 350.9 | 283.3 | 210.6 |
| rib area [mm2] | 141.4 | 109.0 | 190.5 |
| open ratio [%] | 87.7 | 70.8 | 52.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roschger, M.; Wolf, S.; Mayer, K.; Singer, M.; Hacker, V. Alkaline Direct Ethanol Fuel Cell: Effect of the Anode Flow Field Design and the Setup Parameters on Performance. Energies 2022, 15, 7234. https://doi.org/10.3390/en15197234
Roschger M, Wolf S, Mayer K, Singer M, Hacker V. Alkaline Direct Ethanol Fuel Cell: Effect of the Anode Flow Field Design and the Setup Parameters on Performance. Energies. 2022; 15(19):7234. https://doi.org/10.3390/en15197234
Chicago/Turabian StyleRoschger, Michaela, Sigrid Wolf, Kurt Mayer, Matthias Singer, and Viktor Hacker. 2022. "Alkaline Direct Ethanol Fuel Cell: Effect of the Anode Flow Field Design and the Setup Parameters on Performance" Energies 15, no. 19: 7234. https://doi.org/10.3390/en15197234
APA StyleRoschger, M., Wolf, S., Mayer, K., Singer, M., & Hacker, V. (2022). Alkaline Direct Ethanol Fuel Cell: Effect of the Anode Flow Field Design and the Setup Parameters on Performance. Energies, 15(19), 7234. https://doi.org/10.3390/en15197234






