Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production
Abstract
1. Introduction
2. Microalgae as a Feedstock for Biodiesel Production
3. Microalgae Cell Disruption
4. In Situ Transesterification of Microalgae
4.1. Conventional Techniques for In Situ Transesterification
4.2. Advanced Methods for In Situ Transesterification
4.2.1. Use of Co-Solvents
4.2.2. Use of Microwave Assistance
4.2.3. Use of Ultrasound Assistance
4.2.4. Synergistic Microwave and Ultrasound Assistance
4.2.5. Supercritical Fluid Conditions
4.2.6. Hydrothermal Liquefaction
5. Perspectives and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- IEA. World Energy Outlook 2017; IEA: Paris, France, 2017. [Google Scholar]
- Zhou, Z.; Slaný, M.; Kuzielová, E.; Zhang, W.; Ma, L.; Dong, S.; Zhang, J.; Chen, G. Influence of Reservoir Minerals and Ethanol on Catalytic Aquathermolysis of Heavy Oil. Fuel 2022, 307, 121871. [Google Scholar] [CrossRef]
- Chiari, L.; Zecca, A. Constraints of Fossil Fuels Depletion on Global Warming Projections. Energy Policy 2011, 39, 5026–5034. [Google Scholar] [CrossRef]
- Ideris, F.; Shamsuddin, A.H.; Nomanbhay, S.; Kusumo, F.; Silitonga, A.S.; Ong, M.Y.; Ong, H.C.; Mahlia, T.M.I. Optimization of Ultrasound-Assisted Oil Extraction from Canarium Odontophyllum Kernel as a Novel Biodiesel Feedstock. J. Clean. Prod. 2021, 288, 125563. [Google Scholar] [CrossRef]
- Khairudin, N.F.; Bidin, R.; Shamsuddin, A.H.; Akhiar, A.; Ideris, F.; Abd Rahman, A. Renewable Energy Development in Malaysia: Communication Barriers towards Achieving the National Renewable Energy Target. In Proceedings of the 2nd International Conference on Civil & Environmental Engineering, Langkawi, Malaysia, 20–21 November 2019; IOP Conference Series: Earth and Environmental Science. Volume 476. [Google Scholar] [CrossRef]
- Masjuki, H.H.; Kalam, M.A.; Mofijur, M.; Shahabuddin, M. Biofuel: Policy, Standardization and Recommendation for Sustainable Future Energy Supply. Energy Procedia 2013, 42, 577–586. [Google Scholar] [CrossRef]
- Kusumo, F.; Shamsuddin, A.H.; Ahmad, A.R.; Dharma, S.; Milano, J.; Silitonga, A.S.; Fazril, I.; Marzuki, H.; Akhiar, A.; Sebayang, R.; et al. Production of Biodiesel from Jatropha Curcas Mixed with Waste Cooking Oil Assisted by Ultrasound. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 012082. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the Engine Performance and Exhaust Emissions of Biodiesel-Bioethanol-Diesel Blends Using Kernel-Based Extreme Learning Machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Ong, H. Non-Edible Vegetable Oils: A Critical Evaluation of Oil Extraction, Fatty Acid Compositions, Biodiesel Production, Characteristics, Engine Performance and Emissions. Sustain. Energy 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Imtenan, S.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Sajjad, H.; Arbab, M.I.; Rizwanul Fattah, I.M. Impact of Low Temperature Combustion Attaining Strategies on Diesel Engine Emissions for Diesel and Biodiesels: A Review. Energy Convers. Manag. 2014, 80, 329–356. [Google Scholar] [CrossRef]
- Marzuki, H.; Shamsuddin, A.H.; Saifuddin, N.M.; Ideris, F. Energy Saving Potential Using Elite Jatropha Curcas Hybrid for Biodiesel Production in Malaysia. Int. J. Recent Technol. Eng. 2019, 8, 6281–6287. [Google Scholar] [CrossRef]
- Hafriz, R.S.R.M.; Arifin, N.A.; Salmiaton, A.; Yunus, R.; Taufiq-Yap, Y.H.; Saifuddin, N.M.; Shamsuddin, A.H. Multiple-Objective Optimization in Green Fuel Production via Catalytic Deoxygenation Reaction with NiO-Dolomite Catalyst. Fuel 2022, 308, 122041. [Google Scholar] [CrossRef]
- Lane, J. Biofuels Digest. Available online: https://www.biofuelsdigest.com/bdigest/2015/09/10/methanols-moment-with-low-prices-and-proven-process-is-meth-your-new-best-friend/ (accessed on 5 June 2021).
- Phongamwong, T.; Chantaprasertporn, U.; Witoon, T.; Numpilai, T.; Poo-arporn, Y.; Limphirat, W.; Donphai, W.; Dittanet, P.; Chareonpanich, M.; Limtrakul, J. CO2 Hydrogenation to Methanol over CuO–ZnO–ZrO2–SiO2 Catalysts: Effects of SiO2 Contents. Chem. Eng. J. 2017, 316, 692–703. [Google Scholar] [CrossRef]
- Greenhalgh, K. Methanol Production Capacity May Quintuple on Decarbonized Industry Transformation: Study|IHS Markit. Available online: https://cleanenergynews.ihsmarkit.com/research-analysis/methanol-production-capacity-may-quintuple-on-decarbonized-ind.html (accessed on 22 September 2022).
- Babarit, A.; Body, E.; Gilloteaux, J.C.; Hetet, J.F. Energy and Economic Performance of the FARWIND Energy System for Sustainable Fuel Production from the Far-Offshore Wind Energy Resource. In Proceedings of the Fourteenth International Conference on Ecological Vehicles and Renewable Energies (EVER), Monte-Carlo, Monaco, 8–10 May 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Borisut, P.; Nuchitprasittichai, A. Methanol Production via CO2 Hydrogenation: Sensitivity Analysis and Simulation—Based Optimization. Front. Energy Res. 2019, 7, 81. [Google Scholar] [CrossRef]
- Bos, M.J.; Kersten, S.R.A.; Brilman, D.W.F. Wind Power to Methanol: Renewable Methanol Production Using Electricity, Electrolysis of Water and CO2 Air Capture. Appl. Energy 2020, 264, 114672. [Google Scholar] [CrossRef]
- von der Assen, N.; Jung, J.; Bardow, A. Life-Cycle Assessment of Carbon Dioxide Capture and Utilization: Avoiding the Pitfalls. Energy Environ. Sci. 2013, 6, 2721–2734. [Google Scholar] [CrossRef]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel Properties and Emission Characteristics of Biodiesel Produced from Unused Algae Grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef]
- Sugözü, İ.; Öner, C.; Altun, Ş. The Performance and Emissions Characteristics of a Diesel Engine Fueled with Biodiesel and Diesel Fuel. Int. J. Eng. Res. 2010, 2, 50–53. [Google Scholar]
- James, G.; Speight, K.S. Fuels From Biomass. In Environmental Management of Energy from Biofuels and Biofeedstocks; Wiley: Hoboken, NY, USA, 2014; Web; pp. 1–51. ISBN 9781118915141. [Google Scholar]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Im, H.; Kim, B.; Lee, J.W. Bioresource Technology Concurrent Production of Biodiesel and Chemicals through Wet in Situ Transesterification of Microalgae. Bioresour. Technol. 2015, 193, 386–392. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Leong, K.Y. Optimization of Biodiesel Production and Engine Performance from High Free Fatty Acid Calophyllum Inophyllum Oil in CI Diesel Engine. Energy Convers. Manag. 2014, 81, 30–40. [Google Scholar] [CrossRef]
- Bagchi, S.K.; Rao, P.S.; Mallick, N. Bioresource Technology Development of an Oven Drying Protocol to Improve Biodiesel Production for an Indigenous Chlorophycean Microalga scenedesmus sp. Bioresour. Technol. 2015, 180, 207–213. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, Z.; Wang, Q.; Liu, Y. Bioresource Technology Biodiesels from Microbial Oils: Opportunity and Challenges. Bioresour. Technol. 2018, 263, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Salam, K.A.; Velasquez-orta, S.B.; Harvey, A.P. A Sustainable Integrated in Situ Transesteri Fi Cation of Microalgae for Biodiesel Production and Associated Co-Product-a Review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Kim, B.; Young, H.; Son, J.; Yang, J.; Chang, Y.; Lee, J.H.; Lee, J.W. Simplifying Biodiesel Production from Microalgae via Wet in Situ Transesteri Fi Cation: A Review in Current Research and Future Prospects. Algal Res. 2019, 41, 101557. [Google Scholar] [CrossRef]
- Park, J.; Park, M.S.; Lee, Y.; Yang, J. Bioresource Technology Advances in Direct Transesterification of Algal Oils from Wet Biomass. Bioresour. Technol. 2015, 184, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Iderisa, F.; Susan Silitonga, A.; Kusumo, F.; Halim Shamsuddina, A.; Nomanbhaya, S.M.; Sebayang, A.H.; Sutrisno, J.; Bela, N. The Effect of Ultrasound Duty Cycle in Biodiesel Production from Ceiba Pentandra. IOP Conf. Ser. Earth Environ. Sci. 2021, 753, 012031. [Google Scholar] [CrossRef]
- Kusumo, F.; Mahlia, T.M.I.; Shamsuddin, A.H.; Ahmad, A.R.; Silitonga, A.S.; Dharma, S.; Mofijur, M.; Ideris, F.; Ong, H.C.; Sebayang, R.; et al. Optimisation of Biodiesel Production from Mixed Sterculia Foetida and Rice Bran Oil. Int. J. Ambient Energy 2021, 1–11. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Veiga-del-Baño, J.M.; Chica, A.; Quesada-Medina, J. Waste Animal Fats as Feedstock for Biodiesel Production Using Non-Catalytic Supercritical Alcohol Transesterification: A Perspective by the PRISMA Methodology. Energy Sustain. Dev. 2022, 69, 150–163. [Google Scholar] [CrossRef]
- Godwin John, J.; Hariram, V.; Kavuru Rakesh, V.S.S.; Harsha Vardhan, T.; Vamsi Manikanta, T.Y.; Shafi, S. Waste Cooking Oil Biodiesel with FeO Nanoparticle–A Viable Alternative Fuel Source. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Yunus Khan, T.M.; Bashir, M.N.; Akram, N.; Farade, R.; Afzal, A. The Effects of Graphene Oxide Nanoparticle Additive Stably Dispersed in Dairy Scum Oil Biodiesel-Diesel Fuel Blend on CI Engine: Performance, Emission and Combustion Characteristics. Fuel 2019, 257, 116015. [Google Scholar] [CrossRef]
- Fazril, I.; Shamsuddin, A.H.; Nomanbhay, S.; Kusomo, F.; Hanif, M.; Ahmad Zamri, M.F.M.; Akhiar, A.; Ismail, M.F. Microwave-Assisted in Situ Transesterification of Wet Microalgae for the Production of Biodiesel: Progress Review. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 12078. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent Landscape Review on Biodiesel Production: Technology Updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel Synthesis from Ceiba Pentandra Oil by Microwave Irradiation-Assisted Transesterification: ELM Modeling and Optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks. Vitr. Cell. Dev. Biol.-Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Mofijur, M.; Atabani, A.E.; Masjuki, H.H.; Kalam, M.A.; Masum, B.M. A Study on the Effects of Promising Edible and Non-Edible Biodiesel Feedstocks on Engine Performance and Emissions Production: A Comparative Evaluation. Renew. Sustain. Energy Rev. 2013, 23, 391–404. [Google Scholar] [CrossRef]
- Han, B.; Goh, H.; Chyuan, H.; Yee, M.; Chen, W.; Ling, K. Sustainability of Direct Biodiesel Synthesis from Microalgae Biomass: A Critical Review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. Review Article A Review on the Extraction of Lipid from Microalgae for Biodiesel Production. Algal 2015, 7, 117–123. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biofuels from Algae for Sustainable Development. Appl. Energy 2011, 88, 3473–3480. [Google Scholar] [CrossRef]
- Martins, A.; Caetano, N.S.; Mata, T.M. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Silveira Junior, E.; Simionatto, E.; Perez, V.; Justo, O.; Zárate, N.; Vieira, M. Potential of Virginia-Type Peanut (Arachis hypogaea L.) as Feedstock for Biodiesel Production. Ind. Crops Prod. 2016, 89, 448–454. [Google Scholar] [CrossRef]
- Moser, B. Preparation of Fatty Acid Methyl Esters from Hazelnut, High-Oleic Peanut and Walnut Oils and Evaluation as Biodiesel. Fuel 2012, 92, 231–238. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hanifzadeh, M.; Sarrafzadeh, M.-H.; Nabati, Z.; Tavakoli, O.; Feyzizarnagh, H. Technical, Economic and Energy Assessment of an Alternative Strategy for Mass Production of Biomass and Lipid from Microalgae. J. Environ. Chem. Eng. 2018, 6, 866–873. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biore Fi Nery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Nadiah, W.; Kadir, A.; Kee, M.; Uemura, Y.; Wei, J.; Teong, K. Harvesting and Pre-Treatment of Microalgae Cultivated in Wastewater for Biodiesel Production: A Review. Energy Convers. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Hanif, M.; Hesam, N.M.; Akhiar, A.; Fazril, I.; Zamri, M.F.M.A.; Shamsuddin, A.H. Economic Feasibility of Smart City Power Generation from Biogas Produced by Food Waste in Malaysia via Techno-Economic Analysis. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 012076. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Bioresource Technology Wastewater Treatment High Rate Algal Ponds for Biofuel Production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Otero-Gonzalez, L.; Michiels, J.; Lens, P.N.L.; Du Laing, G.; Ferrer, I. Production of Selenium-Enriched Microalgae as Potential Feed Supplement in High-Rate Algae Ponds Treating Domestic Wastewater. Bioresour. Technol. 2021, 333, 125239. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Microalgae Conversion to Biogas: Thermal Pretreatment Contribution on Net Energy Production. Environ. Sci. Technol. 2014, 48, 7171–7178. [Google Scholar] [CrossRef] [PubMed]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel Production by Simultaneous Extraction and Conversion of Total Lipids from Microalgae, Cyanobacteria, and Wild Mixed-Cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef]
- Dasgupta, C.N.; Suseela, M.R.; Mandotra, S.K.; Kumar, P.; Pandey, M.K.; Toppo, K.; Lone, J.A. Dual Uses of Microalgal Biomass: An Integrative Approach for Biohydrogen and Biodiesel Production. Appl. Energy 2015, 146, 202–208. [Google Scholar] [CrossRef]
- Wan, C.; Bai, F.-W.; Zhao, X.-Q. Effects of Nitrogen Concentration and Media Replacement on Cell Growth and Lipid Production of Oleaginous Marine Microalga Nannochloropsis Oceanica DUT01. Biochem. Eng. J. 2013, 78, 32–38. [Google Scholar] [CrossRef]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal Species Selection for Biodiesel Production Based on Fuel Properties Derived from Fatty Acid Profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef]
- Ho, S.; Lai, Y.; Chiang, C.; Chen, C.N.; Chang, J. Bioresource Technology Selection of Elite Microalgae for Biodiesel Production in Tropical Conditions Using a Standardized Platform. Bioresour. Technol. 2013, 147, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Cristina, A. Microalgae as a Raw Material for Biofuels Production. J. Ind. Microbiol. Biotechnol. 2009, 142, 269–274. [Google Scholar] [CrossRef]
- Khan, S.; Siddique, R.; Sajjad, W.; Nabi, G.; Hayat, K.M.; Duan, P.; Yao, L. Biodiesel Production From Algae to Overcome the Energy Crisis. Hayati J. Biosci. 2017, 24, 163–167. [Google Scholar] [CrossRef]
- Zhu, L.; Nugroho, Y.K.; Shakeel, S.R.; Li, Z.; Martinkauppi, B.; Hiltunen, E. Using Microalgae to Produce Liquid Transportation Biodiesel: What Is Next? Renew. Sustain. Energy Rev. 2017, 78, 391–400. [Google Scholar] [CrossRef]
- Hidalgo, P.; Ciudad, G.; Schober, S.; Mittelbach, M.; Navia, R. Improving the FAME Yield of in Situ Transesterification from Microalgal Biomass through Particle Size Reduction and Cosolvent Incorporation. Energy Fuels 2015, 29, 823–832. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhang, X.; Tan, T. Bioresource Technology Biodiesel Production by Direct Transesterification of Microalgal Biomass with Co-Solvent. Bioresour. Technol. 2015, 196, 712–715. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. The Potential of Microalgae in Biodiesel Production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Shwetharani, R.; Balakrishna, R.G. Efficient Algal Lipid Extraction via Photocatalysis and Its Conversion to Biofuel. Appl. Energy 2016, 168, 364–374. [Google Scholar] [CrossRef]
- Ehimen, E.A.; Sun, Z.F.; Carrington, C.G. Variables Affecting the in Situ Transesterification of Microalgae Lipids. Fuel 2010, 89, 677–684. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of Oil from Microalgae for Biodiesel Production: A Review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Naghdi, F.; González González, L.M.; Chan, W.; Schenk, P.M. Progress on Lipid Extraction from Wet Algal Biomass for Biodiesel Production. Microb. Biotechnol. 2016, 9, 718–726. [Google Scholar] [CrossRef]
- Ríos, S.D.; Castañeda, J.; Torras, C.; Farriol, X.; Salvadó, J. Bioresource Technology Lipid Extraction Methods from Microalgal Biomass Harvested by Two Different Paths: Screening Studies toward Biodiesel Production. Bioresour. Technol. 2013, 133, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Cohen, Z. Microbial and Algal Oils: Do They Have a Future for Biodiesel or as Commodity Oils? Lipid Technol. Wiley 2008, 20, 155–160. [Google Scholar] [CrossRef]
- Reijnders, L. Do Biofuels from Microalgae Beat Biofuels from Terrestrial Plants? Trends Biotechnol. 2008, 1, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from Algae: Challenges and Potential Biofuels from Algae: Challenges and Potential. Biofuels 2014, 1, 37–41. [Google Scholar] [CrossRef]
- Chen, C.; Huang, C.; Ho, K.; Hsiao, P.; Wu, M. Bioresource Technology Biodiesel Production from Wet Microalgae Feedstock Using Sequential Wet Extraction/Transesterification and Direct Transesterification Processes. Bioresour. Technol. 2015, 194, 179–186. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.-P.; Chang, J.-S. Mild Cell Disruption Methods for Bio-Functional Proteins Recovery from Microalgae—Recent Developments and Future Perspectives. Algal Res. 2018, 31, 506–516. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell Disruption and Lipid Extraction for Microalgal Biorefineries: A Review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic Cultivation of a Chlorella Sorokiniana Strain for Enhanced Biomass and Lipid Production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Rashidi, B.; Trindade, L.M. Detailed Biochemical and Morphologic Characteristics of the Green Microalga Neochloris Oleoabundans Cell Wall. Algal Res. 2018, 35, 152–159. [Google Scholar] [CrossRef]
- Guiry, M.D. P Erspective How Many Species of Algae Are There? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Gerken, H.G.; Donohoe, B.; Knoshaug, E.P. Enzymatic Cell Wall Degradation of Chlorella Vulgaris and Other Microalgae for Biofuels Production. Planta 2013, 237, 239–253. [Google Scholar] [CrossRef]
- Adair, W.S.; Steinmetz, S.A.; Mattson, D.M.; Goodenough, U.W.; Heuser, J.E. Chlamydomonas Volvox. J. Cell Biol. 1987, 105, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhteseb, S.; Emeish, S. Producing Natural Mixed Carotenoids from Dunaliella Salina. J. Nat. Sci. Res. 2015, 5, 53–59. [Google Scholar]
- Praveenkumar, R.; Lee, K.; Lee, J.; Oh, Y.-K. Breaking Dormancy: An Energy-Efficient Means of Recovering Astaxanthin from Microalgae. Green Chem. 2015, 17, 1226–1234. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Posewitz, M.C.; Gerken, H.G.; Biology, C.; State, A. Ultrastructure and Composition of the Nannochloropsis Gaditana Cell Wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Alam, A.; Pan, Y.; Wu, J.; Wang, Z.; Yuan, Z. Bioresource Technology A New Approach of Microalgal Biomass Pretreatment Using Deep Eutectic Solvents for Enhanced Lipid Recovery for Biodiesel Production. Bioresour. Technol. 2016, 218, 123–128. [Google Scholar] [CrossRef]
- Halim, R.; Rupasinghe, T.W.T.; Tull, D.L.; Webley, P.A. Bioresource Technology Mechanical Cell Disruption for Lipid Extraction from Microalgal Biomass. Bioresour. Technol. 2013, 140, 53–63. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Raehanah, S.; Shaleh, M. Rapid Biodiesel Production Using Wet Microalgae via Microwave Irradiation. Energy Convers. Manag. 2014, 84, 227–233. [Google Scholar] [CrossRef]
- Günerken, E.; Hondt, E.D.; Eppink, M.H.M.; Garcia-gonzalez, L.; Elst, K.; Wijffels, R.H. Cell Disruption for Microalgae Biore Fi Neries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Show, K.; Lee, D.; Tay, J.; Lee, T.; Chang, J. Bioresource Technology Microalgal Drying and Cell Disruption–Recent Advances. Bioresour. Technol. 2015, 184, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing Microalgae on the Biofuels Priority List: A Review of the Technological Challenges Placing Microalgae on the Biofuels Priority List: A Review of the Technological Challenges. J. R. Soc. Interface 2010, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Shirgaonkar, I.Z.; Lothe, R.R.; Pandit, A.B. Comments on the Mechanism of Microbial Cell Disruption in High-Pressure and High-Speed Devices. Biotechnol. Prog. 1998, 14, 657–660. [Google Scholar] [CrossRef]
- Joannes, C.; Sipaut, C.S.; Dayou, J.; Yasir, S.; Mansa, R.F. The Potential of Using Pulsed Electric Field (PEF) Technology as the Cell Disruption Method to Extract Lipid from Microalgae for Biodiesel Production. Int. J. Renew. Energy Res. 2015, 5, 598–621. [Google Scholar]
- Zhang, Y.; Soldatov, S.; Papachristou, I.; Nazarova, N.; Link, G.; Frey, W.; Silve, A. Pulsed Microwave Pretreatment of Fresh Microalgae for Enhanced Lipid Extraction. Energy 2022, 248, 123555. [Google Scholar] [CrossRef]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H. Disruption of Chlorella Vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 167, 1215–1224. [Google Scholar] [CrossRef]
- Luengo, E.; Martínez, J.M.; Bordetas, A.; Álvarez, I.; Raso, J. Influence of the Treatment Medium Temperature on Lutein Extraction Assisted by Pulsed Electric Fields from Chlorella Vulgaris. Innov. Food Sci. Emerg. Technol. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Jazrawi, C.; Biller, P.; He, Y.; Montoya, A.; Ross, A.B.; Maschmeyer, T.; Haynes, B.S. Two-Stage Hydrothermal Liquefaction of a High-Protein Microalga. Algal 2015, 8, 15–22. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, A.; Chen, H.; Nizami, A.-S.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. Biobased Carbon Dots Production via Hydrothermal Conversion of Microalgae Chlorella Pyrenoidosa. Sci. Total Environ. 2022, 839, 156144. [Google Scholar] [CrossRef]
- Grossmann, L.; Ebert, S.; Hinrichs, J.; Weiss, J. Effect of Precipitation, Lyophilization, and Organic Solvent Extraction on Preparation of Protein-Rich Powders from the Microalgae Chlorella Protothecoides. Algal Res. 2018, 29, 266–276. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Bioresource Technology Oil Extraction from Microalgae for Biodiesel Production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ho Row, K. Evaluation of CO2-Induced Azole-Based Switchable Ionic Liquid with Hydrophobic/Hydrophilic Reversible Transition as Single Solvent System for Coupling Lipid Extraction and Separation from Wet Microalgae. Bioresour. Technol. 2020, 296, 122309. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable Green Solvents and Techniques for Lipid Extraction from Microalgae: A Review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Seo, J.Y.; Praveenkumar, R.; Kim, B.; Seo, J.-C.; Park, J.-Y.; Na, J.-G.; Jeon, S.G.; Park, S.B.; Lee, K.; Oh, Y.-K. Downstream Integration of Microalgae Harvesting and Cell Disruption by Means of Cationic Surfactant-Decorated Fe3O4 Nanoparticles. Green Chem. 2016, 18, 3981–3989. [Google Scholar] [CrossRef]
- Taylor, P.; Huang, Y.; Hong, A.; Zhang, D.; Li, L. Comparison of Cell Rupturing by Ozonation and Ultrasonication for Algal Lipid Extraction from Chlorella Vulgaris. Environ. Technol. 2014, 35, 931–937. [Google Scholar] [CrossRef]
- Krishna Koyande, A.; Tanzil, V.; Murraly Dharan, H.; Subramaniam, M.; Robert, R.N.; Lau, P.-L.; Khoiroh, I.; Show, P.-L. Integration of Osmotic Shock Assisted Liquid Biphasic System for Protein Extraction from Microalgae Chlorella Vulgaris. Biochem. Eng. J. 2020, 157, 107532. [Google Scholar] [CrossRef]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercritical Fluid Extraction from Microalgae with High Content of LC-PUFAs. A Case of Study: Sc-CO2 Oil Extraction from Schizochytrium sp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Sierra, L.S.; Dixon, C.K.; Wilken, L.R. Enzymatic Cell Disruption of the Microalgae Chlamydomonas Reinhardtii for Lipid and Protein Extraction. Algal Res. 2017, 25, 149–159. [Google Scholar] [CrossRef]
- Martinez-guerra, E.; Gnaneswar, V.; Mondala, A.; Holmes, W.; Hernandez, R. Bioresource Technology Extractive-Transesterification of Algal Lipids under Microwave Irradiation with Hexane as Solvent. Bioresour. Technol. 2014, 156, 240–247. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Mohd, N.; Hisham, N.; Kamis, H. Optimization of the Ionic Liquid-Microwave Assisted One-Step Biodiesel Production Process from Wet Microalgal Biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae Biofuel: Current Status and Future Applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Boey, P.-L.; Ganesan, S.; Maniam, G.P.; Ali, D.M.H. Ultrasound Aided in Situ Transesterification of Crude Palm Oil Adsorbed on Spent Bleaching Clay. Energy Convers. Manag. 2011, 52, 2081–2084. [Google Scholar] [CrossRef]
- Yu, G.-W.; Nie, J.; Lu, L.-G.; Wang, S.-P.; Li, Z.-G.; Lee, M.-R. Transesterification of Soybean Oil by Using the Synergistic Microwave-Ultrasonic Irradiation. Ultrason. Sonochem. 2017, 39, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Mijangos, G.E.; Romero-Ibarra, I.C.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y. In-Situ Transesterification of Jatropha Curcas L. Seeds Using Homogeneous and Heterogeneous Basic Catalysts. Fuel 2019, 235, 277–287. [Google Scholar] [CrossRef]
- Dasari, S.R.; Chaudhary, A.J.; Goud, V.V.; Sahoo, N.; Kulkarni, V.N. In-Situ Alkaline Transesterification of Castor Seeds: Optimization and Engine Performance, Combustion and Emission Characteristics of Blends. Energy Convers. Manag. 2017, 142, 200–214. [Google Scholar] [CrossRef]
- Koutsouki, A.A.; Tegou, E.; Badeka, A.; Kontakos, S.; Pomonis, P.J.; Kontominas, M.G. In Situ and Conventional Transesterification of Rapeseeds for Biodiesel Production: The Effect of Direct Sonication. Ind. Crops Prod. 2016, 84, 399–407. [Google Scholar] [CrossRef]
- Taherkhani, M.; Sadrameli, S.M. An Improvement and Optimization Study of Biodiesel Production from Linseed via In-Situ Transesterification Using a Co-Solvent. Renew. Energy 2018, 119, 787–794. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Kaur, R.; Tyagi, R.D. Detergent Assisted Ultrasonication Aided in Situ Transesterification for Biodiesel Production from Oleaginous Yeast Wet Biomass. Bioresour. Technol. 2017, 224, 365–372. [Google Scholar] [CrossRef]
- Woo, A.; Sutanto, S.; Ki, L.; Tran-nguyen, P.L.; Ismadji, S.; Ju, Y. Developments in In-Situ (Trans) Esteri Fi Cation for Biodiesel Production: A Critical Review. Renew. Sustain. Energy Rev. 2016, 60, 284–305. [Google Scholar] [CrossRef]
- Eguchi, S.; Kagawa, S.; Okamoto, S. Environmental and Economic Performance of a Biodiesel Plant Using Waste Cooking Oil. J. Clean. Prod. 2015, 101, 245–250. [Google Scholar] [CrossRef]
- Fulton, L.; Mejia, A.; Arioli, M.; Dematera, K.; Lah, O. Climate Change Mitigation Pathways for Southeast Asia: CO2 Emissions Reduction Policies for the Energy and Transport Sectors. Sustainability 2017, 9, 1160. [Google Scholar] [CrossRef]
- Vandyck, T.; Keramidas, K.; Saveyn, B.; Kitous, A.; Vrontisi, Z. A Global Stocktake of the Paris Pledges: Implications for Energy Systems and Economy. Glob. Environ. Chang. 2016, 41, 46–63. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Keun, Y.; Lee, J.W. Bioresource Technology Wet in Situ Transesterification of Microalgae Using Ethyl Acetate as a Co-Solvent and Reactant. Bioresour. Technol. 2017, 230, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pradana, Y.S.; Sudibyo, H.; Suyono, E.A.; Indarto; Budiman, A. Oil Algae Extraction of Selected Microalgae Species Grown in Monoculture and Mixed Cultures for Biodiesel Production. Energy Procedia 2017, 105, 277–282. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of Biodiesel Fuel from the Microalga Schizochytrium Limacinum by Direct Transesterification of Algal Biomass. Energy Fuels 2009, 48, 5179–5183. [Google Scholar] [CrossRef]
- Harrington, K.J.; D’Arcy-Evans, C. Transesterification in Situ of Sunflower Seed Oil. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 314–318. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Bioresource Technology Microalgae as Feedstock for Biodiesel Production under Ultrasound Treatment–A Review. Bioresour. Technol. 2018, 250, 877–887. [Google Scholar] [CrossRef]
- Hidalgo, P.; Ciudad, G.; Mittelbach, M.; Navia, R. Biodiesel Production by Direct Conversion of Botryococcus Braunii Lipids: Reaction Kinetics Modelling and Optimization. Fuel 2015, 153, 544–551. [Google Scholar] [CrossRef]
- Kim, B.; Im, H.; Lee, J.W. Bioresource Technology In Situ Transesterification of Highly Wet Microalgae Using Hydrochloric Acid. Bioresour. Technol. 2015, 185, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Lemões, J.S.; Alves, R.C.M.; Farias, S.P.; De Moura, R.R.; Primel, E.G.; Abreu, P.C.; Martins, A.F.; Montes, M.G.; Oca, D. Sustainable Production of Biodiesel from Microalgae by Direct Transesteri Fi Cation. Sustain. Chem. Pharm. 2016, 3, 33–38. [Google Scholar] [CrossRef]
- Vonortas, A.; Papayannakos, N. Comparative Analysis of Biodiesel versus Green Diesel. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 3–23. [Google Scholar] [CrossRef]
- Im, H.; Lee, H.; Park, M.S.; Yang, J.; Lee, J.W. Bioresource Technology Concurrent Extraction and Reaction for the Production of Biodiesel from Wet Microalgae. Bioresour. Technol. 2014, 152, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.-T.; Yeh, K.-L.; Chen, C.-L.; Chang, J.-S. Enzymatic Transesterification of Microalgal Oil from Chlorella Vulgaris ESP-31 for Biodiesel Synthesis Using Immobilized Burkholderia Lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.; Callejas, N.; Morais, E.G.; Vieira Costa, J.A.; Jachmanián, I.; Vieitez, I. Obtaining Biodiesel from Microalgae Oil Using Ultrasound-Assisted in-Situ Alkaline Transesterification. Fuel 2017, 202, 512–519. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-orta, S.B.; Harvey, A.P. Kinetics of Fast Alkali Reactive Extraction/in Situ Transesteri Fi Cation of Chlorella Vulgaris That Identi Fi Es Process Conditions for a Signi Fi Cant Enhanced Rate and Water Tolerance. Fuel Process. Technol. 2016, 144, 212–219. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A Review on Biodiesel Production Using Catalyzed Transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A. Alkaline in Situ Transesterification of Chlorella Vulgaris. Fuel 2012, 94, 544–550. [Google Scholar] [CrossRef]
- Thliveros, P.; Uçkun Kiran, E.; Webb, C. Microbial Biodiesel Production by Direct Methanolysis of Oleaginous Biomass. Bioresour. Technol. 2014, 157, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sani, Y.M.; Daud, W.M.A.W.; Abdul Aziz, A.R. Solid Acid-Catalyzed Biodiesel Production from Microalgal Oil—The Dual Advantage. J. Environ. Chem. Eng. 2013, 1, 113–121. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Biodiesel Production from Algae by Using Heterogeneous Catalysts: A Critical Review. Energy 2014, 78, 72–83. [Google Scholar] [CrossRef]
- Vicente, G.; Carrero, A.; Rodríguez, R.; Peso, G.L. Heterogeneous-Catalysed Direct Transformation of Microalga Biomass into Biodiesel-Grade FAMEs. Fuel 2017, 200, 590–598. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Hydrothermal Liquefaction of Algae and Bio-Oil Upgrading into Liquid Fuels: Role of Heterogeneous Catalysts. Renew. Sustain. Energy Rev. 2018, 81, 1037–1048. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, Y.; Huang, R.; Yang, W.; Zhou, J.; Cen, K. Bioresource Technology Biodiesel Production from Wet Microalgae by Using Graphene Oxide as Solid Acid Catalyst. Bioresour. Technol. 2016, 221, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of Heterogeneous Solid Acid Catalyst Performance on Low Grade Feedstocks for Biodiesel Production: A Review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Skorupskaite, V.; Makareviciene, V.; Gumbyte, M. Opportunities for Simultaneous Oil Extraction and Transesteri Fi Cation during Biodiesel Fuel Production from Microalgae: A Review. Fuel Process. Technol. 2016, 150, 78–87. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Rawat, I.; Bux, F. Synthesis of Biodiesel from Scenedesmus Sp. by Microwave and Ultrasound Assisted in Situ Transesterification Using Tungstated Zirconia as a Solid Acid Catalyst. Chem. Eng. Res. Des. 2014, 92, 1503–1511. [Google Scholar] [CrossRef]
- Choo, M.; Eng, L.; Loke, P.; Chang, J.; Chuan, T. Journal of the Taiwan Institute of Chemical Engineers Recent Progress in Catalytic Conversion of Microalgae Oil to Green Hydrocarbon: A Review. J. Taiwan Inst. Chem. Eng. 2017, 79, 116–124. [Google Scholar] [CrossRef]
- Malins, K.; Kampars, V.; Brinks, J.; Neibolte, I.; Murnieks, R. Synthesis of Activated Carbon Based Heterogenous Acid Catalyst for Biodiesel Preparation. Appl. Catal. B Environ. 2015, 176–177, 553–558. [Google Scholar] [CrossRef]
- Jeon, J.L.T. A Review of the Terahertz Conductivity of Bulk and Nano-Materials. J. Infrared Millim. Waves 2012, 33, 871–925. [Google Scholar] [CrossRef]
- Konwar, L.J.; Wärnå, J.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.-P. Reaction Kinetics with Catalyst Deactivation in Simultaneous Esterification and Transesterification of Acid Oils to Biodiesel (FAME) over a Mesoporous Sulphonated Carbon Catalyst. Fuel 2016, 166, 1–11. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on Latest Developments in Biodiesel Production Using Carbon-Based Catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, Y.; Zhang, J.; Huang, R.; Yang, W.; Fan, Z. Bioresource Technology Conversion of Lipids from Wet Microalgae into Biodiesel Using Sulfonated Graphene Oxide Catalysts. Bioresour. Technol. 2017, 244, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Mota, G.; Germano de Sousa, I.; Luiz Barros de Oliveira, A.; Luthierre Gama Cavalcante, A.; da Silva Moreira, K.; Thálysson Tavares Cavalcante, F.; Erick da Silva Souza, J.; Rafael de Aguiar Falcão, Í.; Guimarães Rocha, T.; Bussons Rodrigues Valério, R.; et al. Biodiesel Production from Microalgae Using Lipase-Based Catalysts: Current Challenges and Prospects. Algal Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Hama, S.; Kondo, A. Enzymatic Biodiesel Production: An Overview of Potential Feedstocks and Process Development. Bioresour. Technol. 2013, 135, 386–395. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Kumari, S.; Rawat, I.; Permaul, K.; Bux, F. Biodiesel Synthesis from Microalgae Using Immobilized Aspergillus Niger Whole Cell Lipase Biocatalyst. Renew. Energy 2016, 85, 1002–1010. [Google Scholar] [CrossRef]
- Tian, X.; Dai, L.; Liu, M.; Liu, D.; Du, W.; Wu, H. Lipase-Catalyzed Methanolysis of Microalgae Oil for Biodiesel Production and PUFAs Concentration. Catal. Commun. 2016, 84, 44–47. [Google Scholar] [CrossRef]
- Teo, C.L.; Jamaluddin, H.; Zain, N.A.M.; Idris, A. Biodiesel Production via Lipase Catalysed Transesterification of Microalgae Lipids from Tetraselmis Sp. Renew. Energy 2014, 68, 1–5. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Muthukumar, K. Direct Transesterification of Oedogonium Sp. Oil Be Using Immobilized Isolated Novel Bacillus Sp. Lipase. J. Biosci. Bioeng. 2014, 117, 86–91. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, T.; Wang, X.; Chen, B.; Chen, F. Bioresource Technology Cost-e Ff Ective Biodiesel Production from Wet Microalgal Biomass by a Novel Two-Step Enzymatic Process. Bioresour. Technol. 2018, 268, 583–591. [Google Scholar] [CrossRef]
- Raou, Z.; Latif, S.; Gargari, M. Biodiesel Production from Microalgae Oil by Lipase from Pseudomonas Aeruginosa Displayed on Yeast Cell Surface. Biochem. Eng. J. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Ozalp, V.C.; Arica, M.Y. Immobilized Lipase on Micro-Porous Biosilica for Enzymatic Transesterification of Algal Oil. Chem. Eng. Res. Des. 2015, 95, 12–21. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Direct Transesterification of Botryococcus sp. Catalysed by Immobilized Lipase: Ultrasound Treatment Can Reduce Reaction Time with High Yield of Methyl Ester. Fuel 2017, 191, 363–370. [Google Scholar] [CrossRef]
- Huang, J.; Xia, J.; Jiang, W.; Li, Y.; Li, J. Biodiesel Production from Microalgae Oil Catalyzed by a Recombinant Lipase. Bioresour. Technol. 2015, 180, 47–53. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Seo, J.-W.; Oh, B.-R.; Lee, E.Y. Lipase-Catalyzed in-Situ Biosynthesis of Glycerol-Free Biodiesel from Heterotrophic Microalgae, Aurantiochytrium Sp. KRS101 Biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef]
- Wu, S.; Song, L.; Sommerfeld, M.; Hu, Q.; Chen, W. Optimization of an Effective Method for the Conversion of Crude Algal Lipids into Biodiesel. Fuel 2017, 197, 467–473. [Google Scholar] [CrossRef]
- Bautista, L.F.; Vicente, G.; Mendoza, A.; Gonza, S.; Morales, V.; Rey, U.; Carlos, J.; Tulipa, C. Enzymatic Production of Biodiesel from Nannochloropsis Gaditana Microalgae Using Immobilized Lipases in Mesoporous Materials. Energy Fuels 2015, 29, 4981–8989. [Google Scholar] [CrossRef]
- Navarro López, E.; Robles Medina, A.; Esteban Cerdán, L.; González Moreno, P.A.; Macías Sánchez, M.D.; Molina Grima, E. Fatty Acid Methyl Ester Production from Wet Microalgal Biomass by Lipase-Catalyzed Direct Transesterification. Biomass Bioenergy 2016, 93, 6–12. [Google Scholar] [CrossRef]
- Amoah, J.; Ho, S.-H.; Hama, S.; Yoshida, A.; Nakanishi, A.; Hasunuma, T.; Ogino, C.; Kondo, A. Lipase Cocktail for Efficient Conversion of Oils Containing Phospholipids to Biodiesel. Bioresour. Technol. 2016, 211, 224–230. [Google Scholar] [CrossRef]
- Jin, Z.; Han, S.; Zhang, L.; Zheng, S.; Wang, Y.; Lin, Y. Bioresource Technology Combined Utilization of Lipase-Displaying Pichia Pastoris Whole-Cell Biocatalysts to Improve Biodiesel Production in Co-Solvent Media. Bioresour. Technol. 2013, 130, 102–109. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Purification and Characterization of Solvent Tolerant Lipase from Bacillus Sp. for Methyl Ester Production from Algal Oil. J. Biosci. Bioeng. 2016, 121, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Nordblad, M.; Martel, H.H.; Chrabas, B.; Wang, H.; Nielsen, P.M.; Woodley, J.M.; Llc, V.F. Scale-Up of Industrial Biodiesel Production to 40 m 3 Using a Liquid Lipase Formulation. Biotechnol. Bioeng. 2016, 113, 1719–1728. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Ahmad, F.; Singh, B.; Bux, F. Biodiesel Synthesis from Microalgal Lipids Using Tungstated Zirconia as a Heterogeneous Acid Catalyst and Its Comparison with Homogeneous Acid and Enzyme Catalysts. Fuel 2017, 187, 180–188. [Google Scholar] [CrossRef]
- Environ, E.; Gu, H.; Jiang, Y.; Zhou, L.; Gao, J. Energy & Environmental Science Reactive Extraction and in Situ Self-Catalyzed Methanolysis of Germinated Oilseed for Biodiesel Production. Energy Environ. Sci. 2011, 4, 1337–1344. [Google Scholar] [CrossRef]
- Prommuak, C.; Pavasant, P.; Quitain, A.T.; Goto, M. Microalgal Lipid Extraction and Evaluation of Single-Step Biodiesel Production. Eng. J. 2012, 16, 157–166. [Google Scholar] [CrossRef]
- Abedini Najafabadi, H.; Vossoughi, M.; Pazuki, G. The Role of Co-Solvents in Improving the Direct Transesterification of Wet Microalgal Biomass under Supercritical Condition. Bioresour. Technol. 2015, 193, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, B.; Lee, J.W. In-Situ Transesterification of Wet Spent Coffee Grounds for Sustainable Biodiesel Production. Bioresour. Technol. 2016, 221, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Z.; Sada, A.; Muhammad, N.; Ullah, R.; Alqahtani, A.S.; Nasir, S.; Ben, O.; Azmi, M.; Man, Z. A Review on Ionic Liquids as Perspective Catalysts in Transesteri Fi Cation of Different Feedstock Oil into Biodiesel. J. Mol. Liq. 2018, 266, 673–686. [Google Scholar] [CrossRef]
- Lee, Y.R.; Row, K.H. Comparison of Ionic Liquids and Deep Eutectic Solvents as Additives for the Ultrasonic Extraction of Astaxanthin from Marine Plants. J. Ind. Eng. Chem. 2016, 39, 87–92. [Google Scholar] [CrossRef]
- Suarez Garcia, E.; Suarez Ruiz, C.A.; Tilaye, T.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Fractionation of Proteins and Carbohydrates from Crude Microalgae Extracts Using an Ionic Liquid Based-Aqueous Two Phase System. Sep. Purif. Technol. 2018, 204, 56–65. [Google Scholar] [CrossRef]
- Young, G.; Nippen, F.; Titterbrandt, S.; Cooney, M.J.; Young, G.; Nippen, F.; Titterbrandt, S.; Cooney, M.J. Direct Transesterification of Biomass Using an Ionic Liquid Co-Solvent System Direct Transesterification of Biomass Using an Ionic Liquid Co-Solvent System. Biofuels 2011, 2, 261–266. [Google Scholar] [CrossRef]
- Orr, V.C.A.; Plechkova, N.V.; Seddon, K.R.; Rehmann, L. Disruption and Wet Extraction of the Microalgae Chlorella Vulgaris Using Room-Temperature Ionic Liquids. ACS Sustain. Chem. Eng. 2016, 4, 591–600. [Google Scholar] [CrossRef]
- Lee, H.; Jung, W.S.J.; Woong, C.; Jae, K.; Kwon, W.L.J. Optimization of Variables Affecting the Direct Transesterification of Wet Biomass from Nannochloropsis Oceanica Using Ionic Liquid as a Co-Solvent. Bioprocess Biosyst. Eng. 2015, 38, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Q.; Hu, Z.-L.; Wang, P.-W.; Yang, Z. Enzymatic Production of Microalgal Biodiesel in Ionic Liquid [BMIm][PF6]. Fuel 2012, 95, 329–333. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. Deep Eutectic Solvents: Synthesis, Application, and Focus on Lipase-Catalyzed Reactions. Eur. J. Lipid Sci. Technol. 2013, 115, 379–385. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of Deep Eutectic Solvents in Analytical Chemistry. A Review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M.A.; Wang, Z.; Wu, J.; Zhang, Y.; Yuan, Z. Enhanced Esterification of Oleic Acid and Methanol by Deep Eutectic Solvent Assisted Amberlyst Heterogeneous Catalyst. Bioresour. Technol. 2016, 220, 543–548. [Google Scholar] [CrossRef]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Radojčić Redovniković, I. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of Ionic Liquids and Deep Eutectic Solvents in Biodiesel Production: A Review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M.A.; Wang, Z.; Huang, D.; Hu, K.; Chen, H.; Yuan, Z. One-Step Production of Biodiesel from Wet and Unbroken Microalgae Biomass Using Deep Eutectic Solvent. Bioresour. Technol. 2017, 238, 157–163. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Hashemi, M.M. Polyethylene Glycol (PEG) as a Green Solvent for Carbon–Carbon Bond Formation Reactions. J. Mol. Liq. 2015, 207, 73–79. [Google Scholar] [CrossRef]
- Liu, J.; Cao, X.; Chu, Y.; Zhao, Y.; Wu, P.; Xue, S. Novel Approach for the Direct Transesterification of Fresh Microalgal Cells via Micro-Reactor. Algal Res. 2018, 32, 38–43. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, Y. Direct Transesterification of Wet Cryptococcus Curvatus Cells to Biodiesel through Use of Microwave Irradiation. Appl. Energy 2014, 119, 438–444. [Google Scholar] [CrossRef]
- Patil, P.D.; Gnaneswar, V.; Mannarswamy, A.; Cooke, P.; Munson-mcgee, S.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Bioresource Technology Optimization of Microwave-Assisted Transesterification of Dry Algal Biomass Using Response Surface Methodology. Bioresour. Technol. 2011, 102, 1399–1405. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Z.K. Biodiesel Production by Direct Methanolysis of Oleaginous Microbial Biomass. J. Chem. Technol. Biotechnol. 2007, 780, 775–780. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Microwave-Assisted Conversion of Biomass and Waste Materials to Biofuels. Renew. Sustain. Energy Rev. 2018, 82, 1149–1177. [Google Scholar] [CrossRef]
- Latorre, M.E.; de Escalada Plá, M.F.; Rojas, A.M.; Gerschenson, L.N. Blanching of Red Beet (Beta vulgaris L. Var. conditiva) Root. Effect of Hot Water or Microwave Radiation on Cell Wall Characteristics. LWT Food Sci. Technol. 2013, 50, 193–203. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, J.; Huang, Y.; Feng, J.; Zhou, J.; Cen, K. Dynamic Microstructures and Fractal Characterization of Cell Wall Disruption for Microwave Irradiation-Assisted Lipid Extraction from Wet Microalgae. Bioresour. Technol. 2013, 150, 67–72. [Google Scholar] [CrossRef]
- Loong, T.C.; Idris, A. One Step Transesteri Fi Cation of Biodiesel Production Using Simultaneous Cooling and Microwave Heating. J. Clean. Prod. 2017, 146, 57–62. [Google Scholar] [CrossRef]
- Idris, A.; Bukhari, A.; Mohd Yusof, N.; Tan, K. A Simultaneous Cooling and Dielectric Heating: An Advanced Technology to Improve the Yield of Lactides. Prog. Electromagn. Res. Symp. Proc. 2012, 1559–9450, 141–145. [Google Scholar]
- Gude, V.G.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave Energy Potential for Biodiesel Production. Sustain. Chem. Process. 2013, 1, 5. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from Microwave Pyrolysis of Biomass: A Review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Fattah, I.M.; Noraini, M.Y.; Mofijur, M.; Silitonga, A.S.; Badruddin, I.A.; Khan, T.M.; Mahlia TM, I. Lipid Extraction Maximization and Enzymatic Synthesis of Biodiesel from Microalgae. Appl. Sci. 2020, 10, 6103. [Google Scholar] [CrossRef]
- Ido, A.L.; Daniel, M.; De Luna, G.; Capareda, S.C.; Maglinao, A.L.; Nam, H. Application of Central Composite Design in the Optimization of Lipid Yield from Scenedesmus Obliquus Microalgae by Ultrasound-Assisted Solvent Extraction. Energy 2018, 157, 949–956. [Google Scholar] [CrossRef]
- Mofijur, M.; Kusumo, F.; Fattah, I.M.R.; Mahmudul, H.M.; Rasul, M.G.; Shamsuddin, A.H.; Mahlia, T.M.I. Resource Recovery from Waste Coffee Grounds Using Ultrasonic-Assisted Technology for Bioenergy Production. Energies 2020, 13, 1770. [Google Scholar] [CrossRef]
- Toro, P.H.C.; Navia, G.C.R. Advances in Direct Transesterification of Microalgal Biomass for Biodiesel Production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 179–199. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gude, V.G.; Mondala, A.; Holmes, W.; Hernandez, R. Microwave and Ultrasound Enhanced Extractive-Transesterification of Algal Lipids. Appl. Energy 2014, 129, 354–363. [Google Scholar] [CrossRef]
- Cercado, A.P.; Ballesteros, F.; Capareda, S. Ultrasound Assisted Transesterification of Microalgae Using Synthesized Novel Catalyst. Sustain. Environ. Res. 2018, 28, 234–239. [Google Scholar] [CrossRef]
- Gude, V.G. Synergism of Microwaves and Ultrasound for Advanced Biorefineries. Resour. Technol. 2015, 1, 116–125. [Google Scholar] [CrossRef]
- Ma, G.; Hu, W.; Pei, H.; Jiang, L.; Song, M.; Mu, R. In Situ Heterogeneous Transesterification of Microalgae Using Combined Ultrasound and Microwave Irradiation. Energy Convers. Manag. 2015, 90, 41–46. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Howlader, M.S.; Shields-Menard, S.; French, W.T.; Gude, V.G. Optimization of Wet Microalgal FAME Production from Nannochloropsis Sp. under the Synergistic Microwave and Ultrasound Effect. Int. J. Energy Res. 2018, 42, 1934–1949. [Google Scholar] [CrossRef]
- Duarte, S.H.; dos Santos, P.; Michelon, M.; de Pinho Oliveira, S.M.; Martínez, J.; Maugeri, F. Recovery of Yeast Lipids Using Different Cell Disruption Techniques and Supercritical CO2 Extraction. Biochem. Eng. J. 2017, 125, 230–237. [Google Scholar] [CrossRef]
- Jafari, A.; Esmaeilzadeh, F.; Mowla, D.; Sadatshojaei, E.; Heidari, S.; Wood, D.A. New Insights to Direct Conversion of Wet Microalgae Impregnated with Ethanol to Biodiesel Exploiting Extraction with Supercritical Carbon Dioxide. Fuel 2021, 285, 119199. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.-M.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Lin, K.-Y.A.; Choi, Y.-E.; Kwon, E.E. Biodiesel from Microalgae: Recent Progress and Key Challenges. Prog. Energy Combust. Sci. 2022, 93, 101020. [Google Scholar] [CrossRef]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of Carotenoids and Lipids from Algae by Supercritical CO2 and Subcritical Dimethyl Ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Tobar, M.; Núñez, G.A. The Journal of Supercritical Fluids Supercritical Transesteri Fi Cation of Microalgae Triglycerides for Biodiesel Production: E Ff Ect of Alcohol Type and Co-Solvent. J. Supercrit. Fluids 2018, 137, 50–56. [Google Scholar] [CrossRef]
- Reddy, H.K.; Muppaneni, T.; Patil, P.D.; Ponnusamy, S.; Cooke, P.; Schaub, T.; Deng, S. Direct Conversion of Wet Algae to Crude Biodiesel under Supercritical Ethanol Conditions. Fuel 2014, 115, 720–726. [Google Scholar] [CrossRef]
- Mohamadzadeh, H.; Karimi-sabet, J.; Ghotbi, C. Bioresource Technology Biodiesel Production from Spirulina Microalgae Feedstock Using Direct Transesterification near Supercritical Methanol Condition. Bioresour. Technol. 2017, 239, 378–386. [Google Scholar] [CrossRef]
- Patil, P.D.; Gnaneswar, V.; Mannarswamy, A.; Cooke, P.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Comparison of Direct Transesterification of Algal Biomass under Supercritical Methanol and Microwave Irradiation Conditions. Fuel 2012, 97, 822–831. [Google Scholar] [CrossRef]
- Crampon, C.; Nikitine, C.; Zaier, M.; Lépine, O.; Tanzi, C.D.; Vian, M.A.; Chemat, F.; Badens, E. Oil Extraction from Enriched Spirulina Platensis Microalgae Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 119, 289–296. [Google Scholar] [CrossRef]
- Li, J.; Si, B.; Liao, Q.; Fu, Q.; Liu, Z. Insights into Hydrothermal Process of Microalgae via Novel Modified Kinetic Model and Thermodynamic Analysis. Fuel 2022, 317, 123540. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Maria, A.; Pari, L. Review and Experimental Study on Pyrolysis and Hydrothermal Liquefaction of Microalgae for Biofuel Production. Appl. Energy 2017, 185, 963–972. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.; Son, J.; Lee, J.W. Bioresource Technology Catalyst-Free Production of Alkyl Esters from Microalgae via Combined Wet in Situ Transesteri Fi Cation and Hydrothermal Liquefaction (ITHL). Bioresour. Technol. 2017, 244, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Marangon, B.B.; Castro, J.S.; Assemany, P.P.; Couto, E.A.; Calijuri, M.L. Environmental Performance of Microalgae Hydrothermal Liquefaction: Life Cycle Assessment and Improvement Insights for a Sustainable Renewable Diesel. Renew. Sustain. Energy Rev. 2022, 155, 111910. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Techno-Economic and Life Cycle Analysis of Biofuel Production via Hydrothermal Liquefaction of Microalgae in a Methanol-Water System and Catalytic Hydrotreatment Using Hydrochar as a Catalyst Support. Biomass Bioenergy 2021, 151, 106168. [Google Scholar] [CrossRef]
- Chen, P.H.; Quinn, J.C. Microalgae to Biofuels through Hydrothermal Liquefaction: Open-Source Techno-Economic Analysis and Life Cycle Assessment. Appl. Energy 2021, 289, 116613. [Google Scholar] [CrossRef]
- Zhou, Y.; Schideman, L.; Yu, G.; Zhang, Y. A Synergistic Combination of Algal Wastewater Treatment and Hydrothermal Biofuel Production Maximized by Nutrient and Carbon Recycling. Energy Environ. Sci. 2013, 6, 3765–3779. [Google Scholar] [CrossRef]
- Kumar, L.; Anand, R.; Shah, M.P.; Bharadvaja, N. Microalgae Biodiesel: A Sustainable Source of Energy, Unit Operations, Technological Challenges, and Solutions. J. Hazard. Mater. Adv. 2022, 8, 100145. [Google Scholar] [CrossRef]
- Kim, T.; Oh, Y.; Lee, J.W.; Keun, Y. Levulinate Production from Algal Cell Hydrolysis Using in Situ Transesteri Fi Cation. Algal Res. 2017, 26, 431–435. [Google Scholar] [CrossRef]
- White, R.; Ryan, R. Long-Term Cultivation of Algae in Open-Raceway Ponds: Lessons from the Field. Ind. Biotechnol. 2015, 11, 213–220. [Google Scholar] [CrossRef]
- Nishshanka, G.K.S.H.; Anthonio, R.A.D.P.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Chang, J.-S. Marine Microalgae as Sustainable Feedstock for Multi-Product Biorefineries. Biochem. Eng. J. 2022, 187, 108593. [Google Scholar] [CrossRef]
- Lim, J.Y.; Teng, S.Y.; How, B.S.; Nam, K.; Heo, S.; Máša, V.; Stehlík, P.; Yoo, C.K. From Microalgae to Bioenergy: Identifying Optimally Integrated Biorefinery Pathways and Harvest Scheduling under Uncertainties in Predicted Climate. Renew. Sustain. Energy Rev. 2022, 168, 112865. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae. Natl. Renew. Energy Lab. 1998, 328, 1–294. [Google Scholar]
- Mourya, M.; Khan, M.J.; Ahirwar, A.; Schoefs, B.; Marchand, J.; Rai, A.; Varjani, S.; Rajendran, K.; Banu, J.R.; Vinayak, V. Latest Trends and Developments in Microalgae as Potential Source for Biofuels: The Case of Diatoms. Fuel 2022, 314, 122738. [Google Scholar] [CrossRef]
- Martín, L.A.; Popovich, C.A.; Martínez, A.M.; Scodelaro, P.G.; Damiani, M.C.; Leonardi, P.I. Hybrid Two-Stage Culture of Halamphora Coffeaeformis for Biodiesel Production: Growth Phases, Nutritional Stages and Biore Fi Nery Approach. Renew. Energy 2018, 118, 984–992. [Google Scholar] [CrossRef]
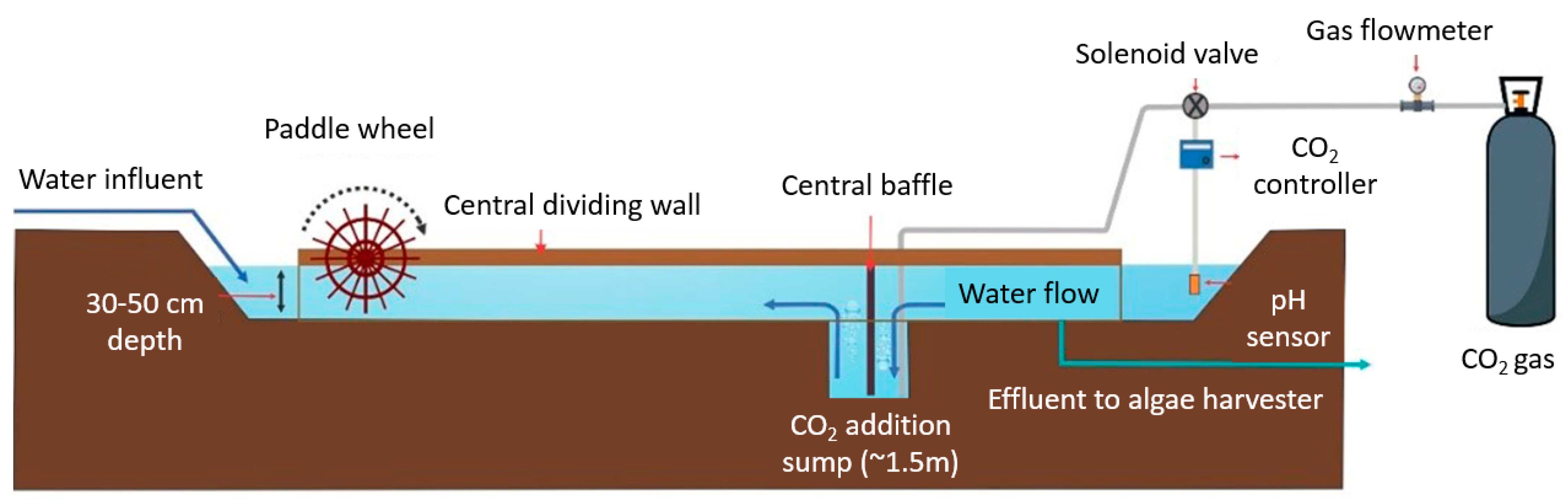


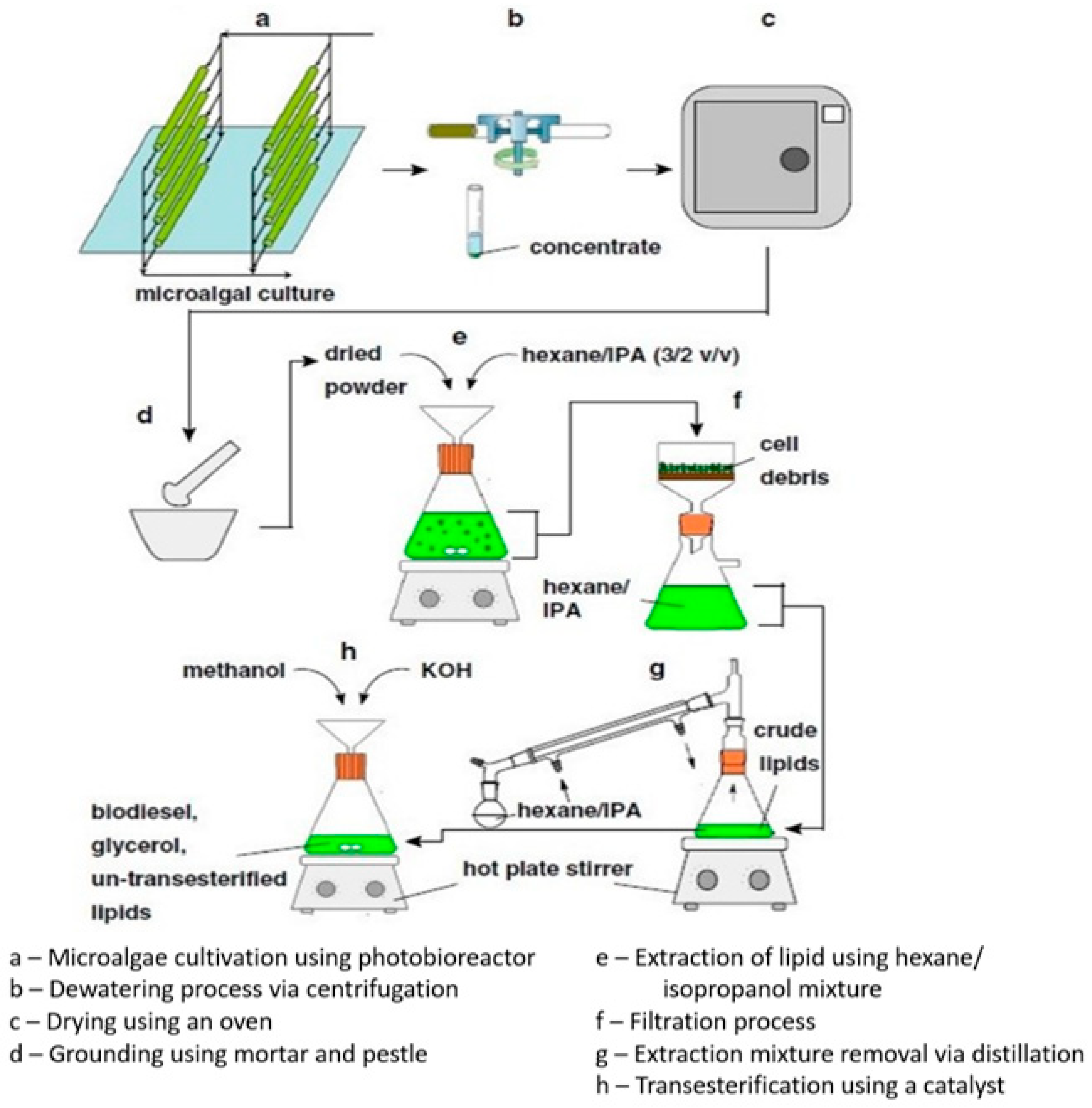

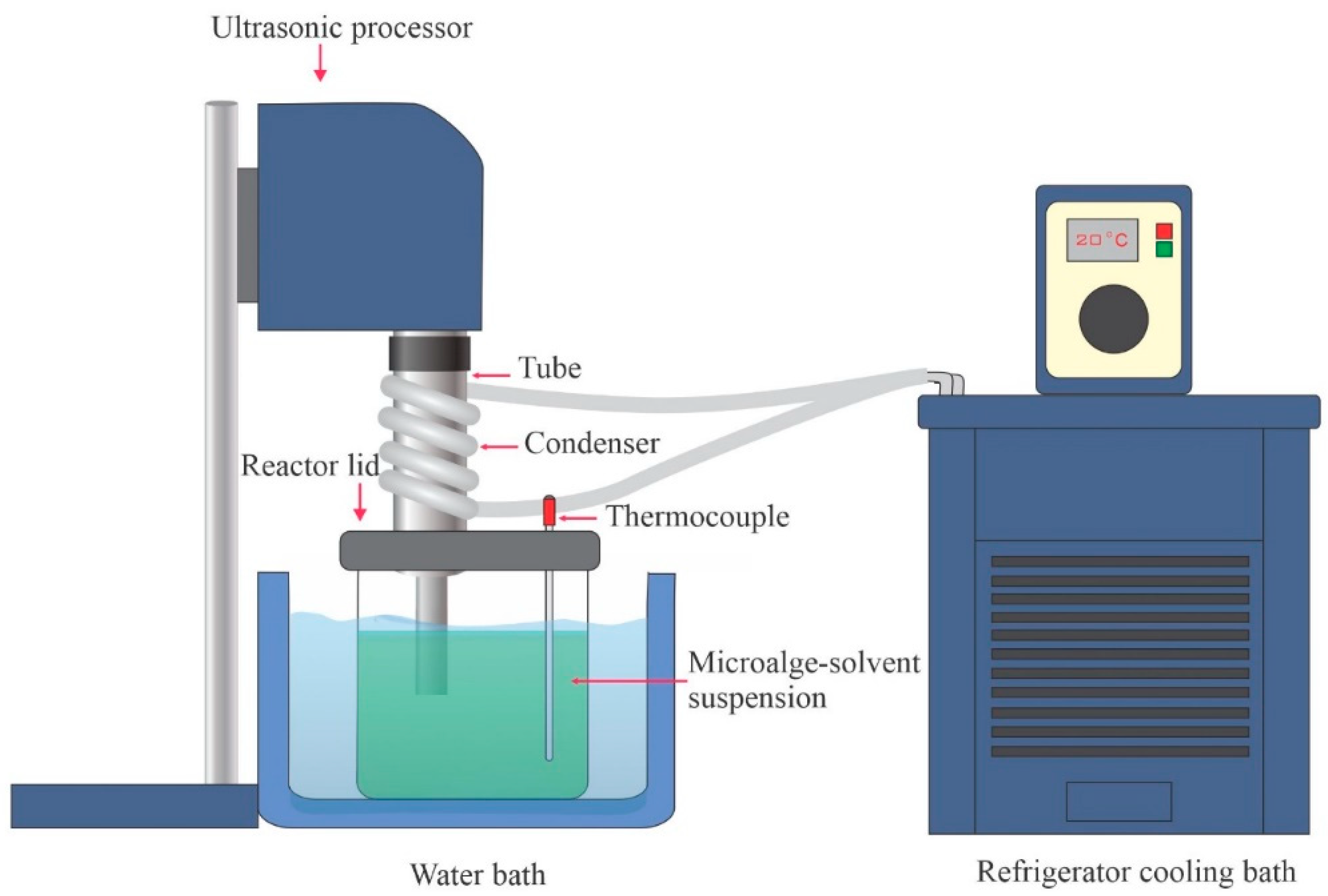
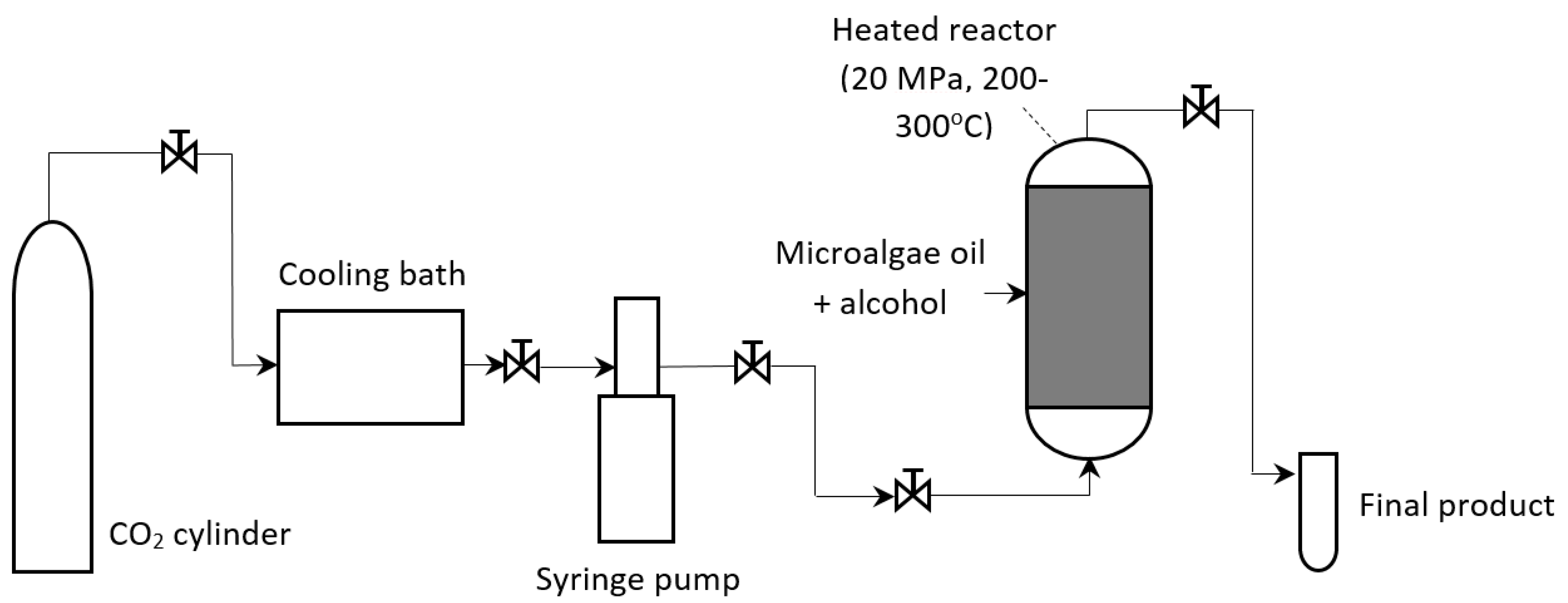
| Feedstock | Oil Content (% of Dry Weight) | Oil Yield (L Oil/ha/Year) | Land Area Requirement (m2/Year/kg Biodiesel) | Biodiesel Productivity (kg Biodiesel/ha/Year) | References |
|---|---|---|---|---|---|
| Castor (Ricinus communis) | 48 | 1307 | 9 | 1156 | [44] |
| Corn/maize (Zea mays L.) | 44 | 172 | 66 | 152 | [44] |
| Hemp (Cannabis sativa L.) | 33 | 363 | 31 | 321 | [44] |
| Soybean (Glycine max L.) | 18 | 636 | 18 | 562 | [44] |
| Jatropha (Jatropha curcas L.) | 28 | 741 | 15 | 656 | [44] |
| Camelina (Camelina sativa L.) | 42 | 915 | 12 | 809 | [44] |
| Canola/rapeseed (Brassica napus L.) | 41 | 974 | 12 | 862 | [44] |
| Sunflower (Helianthus annuus L.) | 40 | 1070 | 11 | 946 | [44] |
| Peanut oil (Arachis hypogaea L.) | 50 | 1059 | - | 1425–1782 | [45,46] |
| Hazelnut (Corylus avellana) | 51–75 | - | - | 1000 | [46] |
| Palm oil (Elaeis guineensis) | 36 | 5366 | 2 | 4747 | [44] |
| Walnut (Juglans regia) | 51–72 | - | - | 780–1750 | [46] |
| Microalgae (low oil content) | 30 | 58,700 | 0.2 | 51,927 | [44] |
| Microalgae (medium oil content) | 50 | 97,800 | 0.1 | 86,515 | [44] |
| Microalgae (high oil content) | 70 | 136,900 | 0.1 | 121,104 | [44] |
| Microalgae Species | Lipid Content (% Dry Weight Biomass) | Lipid Productivity (mg/L/Day) | Volumetric Productivity of Biomass (g/L/Day) | Areal Productivity of Biomass (g/m3/Day) |
|---|---|---|---|---|
| Ankistrodesmus sp. | 24.0–31.0 | - | - | 11.5–17.4 |
| Botryococcus braunii | 25.0–75.0 | – | 0.02 | 3.0 |
| Chaetoceros muelleri | 33.6 | 21.8 | 0.007 | – |
| Chaetoceros calcitrans | 14.6–16.4/39.8 | 17.6 | 0.04 | – |
| Chlorella emersonii | 25.0–63.0 | 10.3–50.0 | 0.036–0.041 | 0.91–0.97 |
| Chlorella protothecoides | 14.6–57.8 | 1214 | 2.00–7.70 | – |
| Chlorella sorokiniana | 19.0–22.0 | 44.7 | 0.23–1.47 | – |
| Chlorella vulgaris | 5.0–58.0 | 11.2–40.0 | 0.02–0.20 | 0.57–0.95 |
| Chlorella pyrenoidosa | 2.0 | – | 2.90–3.64 | 72.5/130 |
| Chlorococcum sp. | 19.3 | 53.7 | 0.28 | – |
| Crypthecodinium cohnii | 20.0–51.1 | – | 10 | – |
| Dunaliella salina | 6.0–25.0 | 116.0 | 0.22–0.34 | 1.6–3.5/20–38 |
| Dunaliella primolecta | 23.1 | – | 0.09 | 14 |
| Dunaliella tertiolecta | 16.7–71.0 | – | 0.12 | – |
| Dunaliella sp. | 17.5–67.0 | 33.5 | – | – |
| Ellipsoidion sp. | 27.4 | 47.3 | 0.17 | – |
| Euglena gracilis | 14.0–20.0 | – | 7.70 | – |
| Haematococcus pluvialis | 25.0 | – | 0.05–0.06 | 10.2–36.4 |
| Isochrysis galbana | 7.0–40.0 | – | 0.32–1.60 | – |
| Monodus subterraneus | 16.0 | 30.4 | 0.19 | – |
| Monallanthus salina | 20.0–22.0 | – | 0.08 | 12 |
| Nannochloris sp | 20.0–56.0 | 60.9–76.5 | 0.17–0.51 | – |
| Nannochloropsis oculata. | 22.7–29.7 | 84.0–142.0 | 0.37–0.48 | – |
| Nannochloropsis sp. | 12.0–53.0 | 37.6–90.0 | 0.17–1.43 | 1.9–5.3 |
| Neochloris oleoabundans | 29.0–65.0 | 90.0–134.0 | – | – |
| Nitzschia sp. | 16.0–47.0 | 8.8–21.6 | ||
| Oocystis pusilla | 10.5 | – | – | 40.6–45.8 |
| Pavlova salina | 30.9 | 49.4 | 0.16 | – |
| Pavlova lutheri | 35.5 | 40.2 | 0.14 | – |
| Phaeodactylum tricornutum | 18.0–57.0 | 44.8 | 0.003–1.9 | 2.4–21 |
| Porphyridium cruentum | 9.0–18.8/60.7 | 34.8 | 0.36–1.50 | 25 |
| Scenedesmus obliquus | 11.0–55.0 | – | 0.004–0.74 | – |
| Scenedesmus quadricauda | 1.9–18.4 | 35.1 | 0.19 | – |
| Scenedesmus sp. | 19.6–21.1 | 40.8–53.9 | 0.03–0.26 | 2.43–13.52 |
| Skeletonema sp. | 13.3–31.8 | 27.3 | 0.09 | – |
| Skeletonema costatum | 13.5–51.3 | 17.4 | 0.08 | – |
| Spirulina platensis | 4.0–16.6 | – | 0.06–4.3 | 1.5–14.5/24–51 |
| Spirulina maxima | 4.0–9.0 | – | 0.21–0.25 | 25 |
| Thalassiosira pseudonana | 20.6 | 17.4 | 0.08 | – |
| Tetraselmis suecica | 8.5–23.0 | 27.0–36.4 | 0.12–0.32 | 19 |
| Tetraselmis sp. | 12.6–14.7 | 43.4 | 0.30 | – |
| Species | Cell Wall Characteristics | References |
|---|---|---|
| Neochloris oleoabundans | - two distinct layers - made from 24.3% carbohydrates, 31.5% proteins - carbohydrates component consist of non-cellulosic polysaccharides | [78] |
| Chlorella vulgaris | - two distinct layers - the outer layer is an electron-dense wall, while the inner layer is low in density | [80] |
| Chlamydomonas reinhardtii | - five distinct layers - made from hydroxyproline-rich glycoproteins - made up totally from glycoproteins, with no cellulose | [81] |
| Dunaliella salina | - lack of rigid cell wall - the cell is isolated by a thin elastic plasma membrane | [82] |
| Haematococcus pluvialis | - three-layer cell wall - first layer: extracellular matrix, algaenan layer. - secondary layer: thick amorphous layer made of mannose and cellulose - tertiary layer: heterogeneous layer made of mannose and cellulose | [76,83] |
| Nannochloropsis gaditana | - two layers, with a cellulosic inner wall and an outer hydrophobic algaenan layer, which is formed by highly saturated aliphatic compounds | [84] |
| Methods | Advantages | Limitations | References |
|---|---|---|---|
| Bead milling | High-rate cell disruption; practical method of large scale mechanical cell disruption | Degree of disruption depends on characteristics of the bead; requires a large amount of energy in large-scale applications | [89,90] |
| High-speed homogenisation | High rate cell disruption, very effective; short extraction time | High energy consumption, not suitable for large scale application | [88,91] |
| High-pressure homogenisation | Effective, rapid disruption of cell; suitable for scaling up | Rapid disruption, but generally lower lipid yield compared to other methods; high level of cell debris is released, which complicates the separation process. | [86,92] |
| Ultrasonication | Short extraction time; reduced solvent consumption; greater penetration of solvent into cellular materials; improved release of intracellular contents | High power consumption; difficult to scale up | [86,89] |
| Microwave-assisted | Relatively simple, safe, rapid, economical in lab-scale | Maintenance on a large scale is a limiting factor; prone to free radicals formation | [93,94] |
| Pulse electric field | No addition of chemical; low energy consumption; rapid disruption | Prone to decreasing uniformity in the electric field due to the presence of air bubbles in chamber; the solution must be free of ions | [92,95] |
| Hydrothermal liquefaction | High-quality biocrude is obtained, environmentally friendly due to the usage of water in the extraction | High energy requirement due to very high temperature involved, more in-depth studies are needed | [96,97] |
| Organic solvent | Relatively cheap, very effective, high oil yield | Residues of solvent in extracts; most organic solvents are highly flammable and/or toxic; solvent recovery is expensive and energy-intensive, a large volume of solvent is required | [98,99] |
| Ionic liquid | Short extraction time, reusability, and high oil yield | Some ionic liquids are toxic to the environment, highly expensive | [100,101] |
| Nanoparticle | High efficiency, low energy requirement, reusability | Some are very expensive; synthesis cost needs to be evaluated for commercial purposes | [102] |
| Oxidation | An effective method; high yield, high saturated hydrocarbon products | More works need to be done for big-scale implementation | [103] |
| Osmotic shock | Simple extraction, low energy consumption | Generation of waste salt water, time-consuming | [50,104] |
| Supercritical fluid | High oil yield; non-toxic (no organic solvent residue in extracts); non-flammable | High energy consumption; expensive and difficult to scale-up | [42,105] |
| Enzymatic | Mild operating conditions; low energy requirement | Long process time; low production capacity | [88,106] |
| Lipase Origin | Microalgae Biomass | Reaction Time (h) | Biodiesel Yield (%) | References |
|---|---|---|---|---|
| Whole cell from Pseudomonas aeruginosa | Spirulina platensis | 48 | 87.6 | [158] |
| Free enzyme from Candida rugosa | Scenedesmus quadricauda | 24 | 85.7 | [159] |
| Novozyme 435 from Candida antarctica | Botryococcus sp. | 4 | 88 | [160] |
| Free enzyme from Rhizomucor miehei | Chlorella vulgaris | 25 | 90 | [161] |
| Immobilised whole cell from Aspergilous niger | Scenedesmus obliquus | 36 | 53.76 | [153] |
| Novozyme 435 from Candida antarctica | Aurantiochytrium sp. | 12 | 89.5 | [162] |
| Immobilised lipase from Candida antarctica | Chlorella vulgaris | 24 | 97 | [163] |
| Type of Catalyst | % Yield | Advantages | Disadvantages |
|---|---|---|---|
| Homogeneous alkali | 96–98 | · fast reaction time · inexpensive · reaction happens at very mild condition | · suitable for biomass with low FFA (<2%)· difficult ester purification · catalyst cannot be recycled for the next usage · a large amount of water is needed for purification phase · excessive catalyst will lead to soap formation, hence making the purification step more difficult |
| Heterogeneous alkali | <90 | · shorter reaction time than acid-catalyzed transesterification · catalysts can be separated and then reused · reaction happens at mild condition, and less energy is needed | · suitable for biomass with low FFA (<2%) · excessive catalyst will lead to soap formation, hence making the purification step more difficult · contamination of the final product might occur due to leaching · expensive synthesis method |
| Homogeneous acid | Up to 99 | · suitable for biomass with high FFA and high moisture content· very suitable for in situ transesterification process · no saponification · inexpensive | · long reaction time · corrosive to the reactor and pipelines · difficult ester purification · excessive catalyst will lead to high acidity of product; hence requiring a lot of water for purification step · difficult to recover catalyst · high ratio of alcohol to oil is needed |
| Heterogonous acid | <90 | · suitable for biomass with high FFA and high moisture content · very suitable for in situ transesterification process · no saponification · not corrosive to reactor and pipelines · catalysts can be separated and then reused | · long reaction time · expensive synthesis method in some cases · contamination of the final product might occur due to leaching · high ratio of alcohol to oil is needed |
| Biocatalyst | 99 | · suitable for biomass with high FFA and high moisture content · simple purification step · low ratio of alcohol to oil is needed · some are reusable (immobilised lipase) | · extremely long reaction time, slower than acid-catalyzed transesterification · generally very expensive · excessive methanol will lead to deactivation of biocatalyst |
| Carbon-based | <90 | · reusable and inexpensive · simple synthesis method · high thermal stability · large surface area for more effective reaction | · long reaction time · leaching of SO3H · high ratio of methanol to oil is needed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ideris, F.; Zamri, M.F.M.A.; Shamsuddin, A.H.; Nomanbhay, S.; Kusumo, F.; Fattah, I.M.R.; Mahlia, T.M.I. Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production. Energies 2022, 15, 7190. https://doi.org/10.3390/en15197190
Ideris F, Zamri MFMA, Shamsuddin AH, Nomanbhay S, Kusumo F, Fattah IMR, Mahlia TMI. Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production. Energies. 2022; 15(19):7190. https://doi.org/10.3390/en15197190
Chicago/Turabian StyleIderis, Fazril, Mohd Faiz Muaz Ahmad Zamri, Abd Halim Shamsuddin, Saifuddin Nomanbhay, Fitranto Kusumo, Islam Md Rizwanul Fattah, and Teuku Meurah Indra Mahlia. 2022. "Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production" Energies 15, no. 19: 7190. https://doi.org/10.3390/en15197190
APA StyleIderis, F., Zamri, M. F. M. A., Shamsuddin, A. H., Nomanbhay, S., Kusumo, F., Fattah, I. M. R., & Mahlia, T. M. I. (2022). Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production. Energies, 15(19), 7190. https://doi.org/10.3390/en15197190











