Source Identification, Toxicity, and Persistence of PAHs in Sediment Core from a Natural Protected Area in Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sediment Core Extraction
2.3. Determination of PAHs
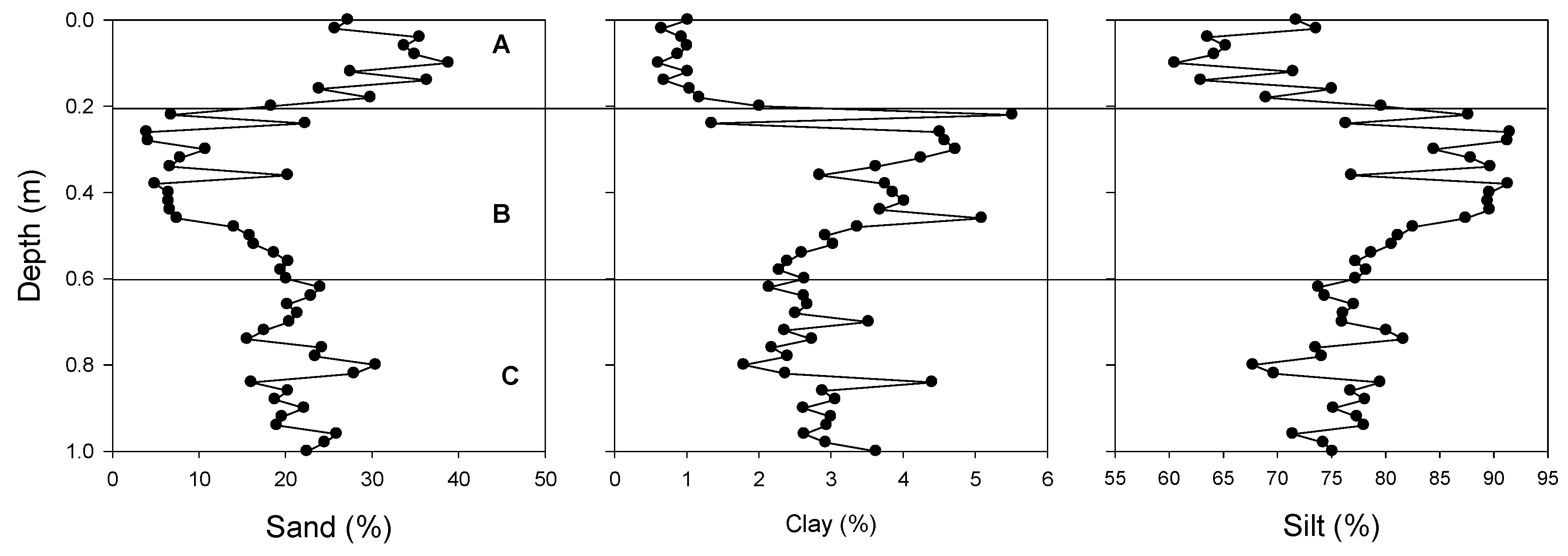
2.4. Granulometric Analysis
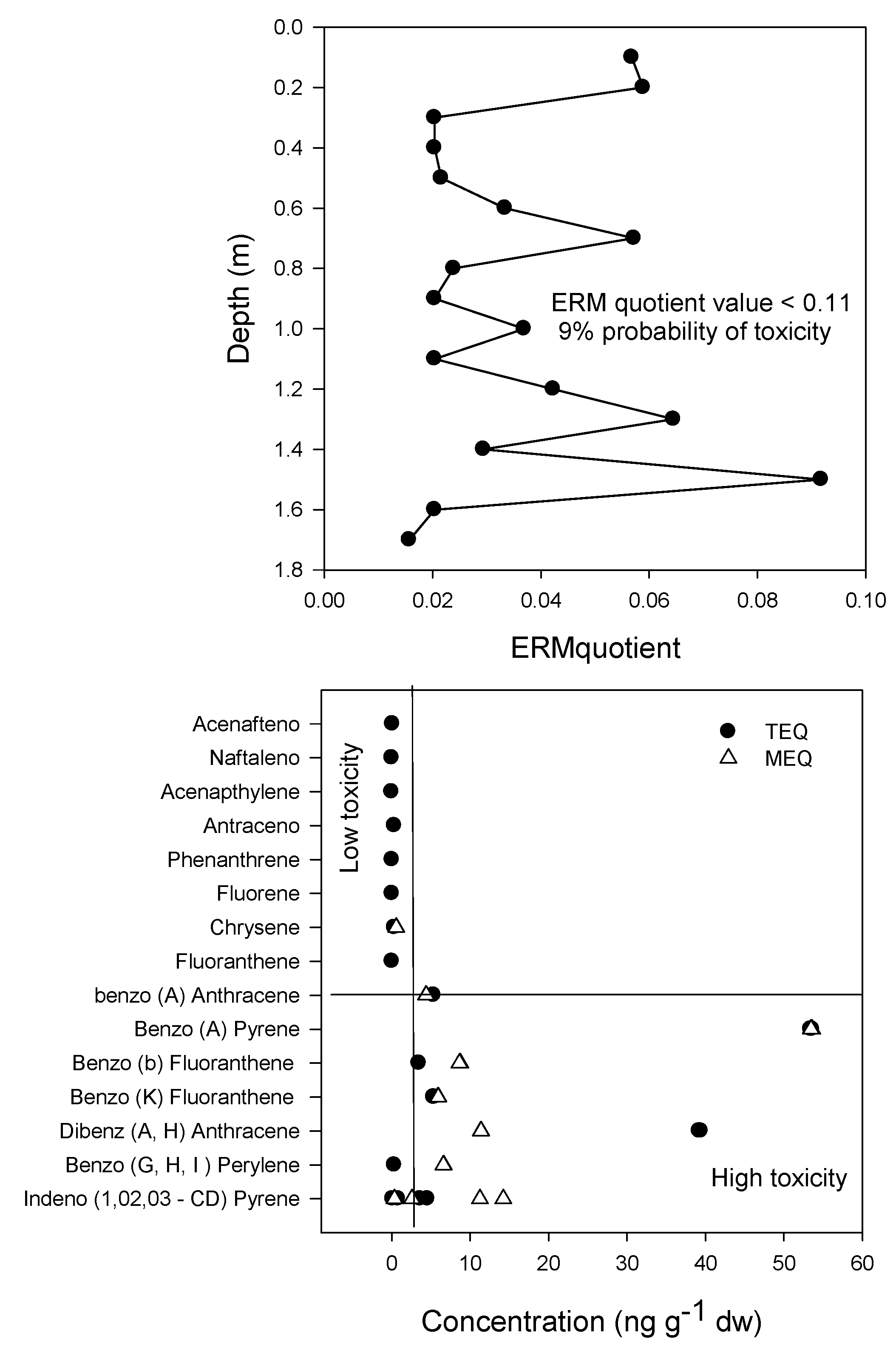
2.5. Sediment Quality Evaluation
3. Results and Discussion
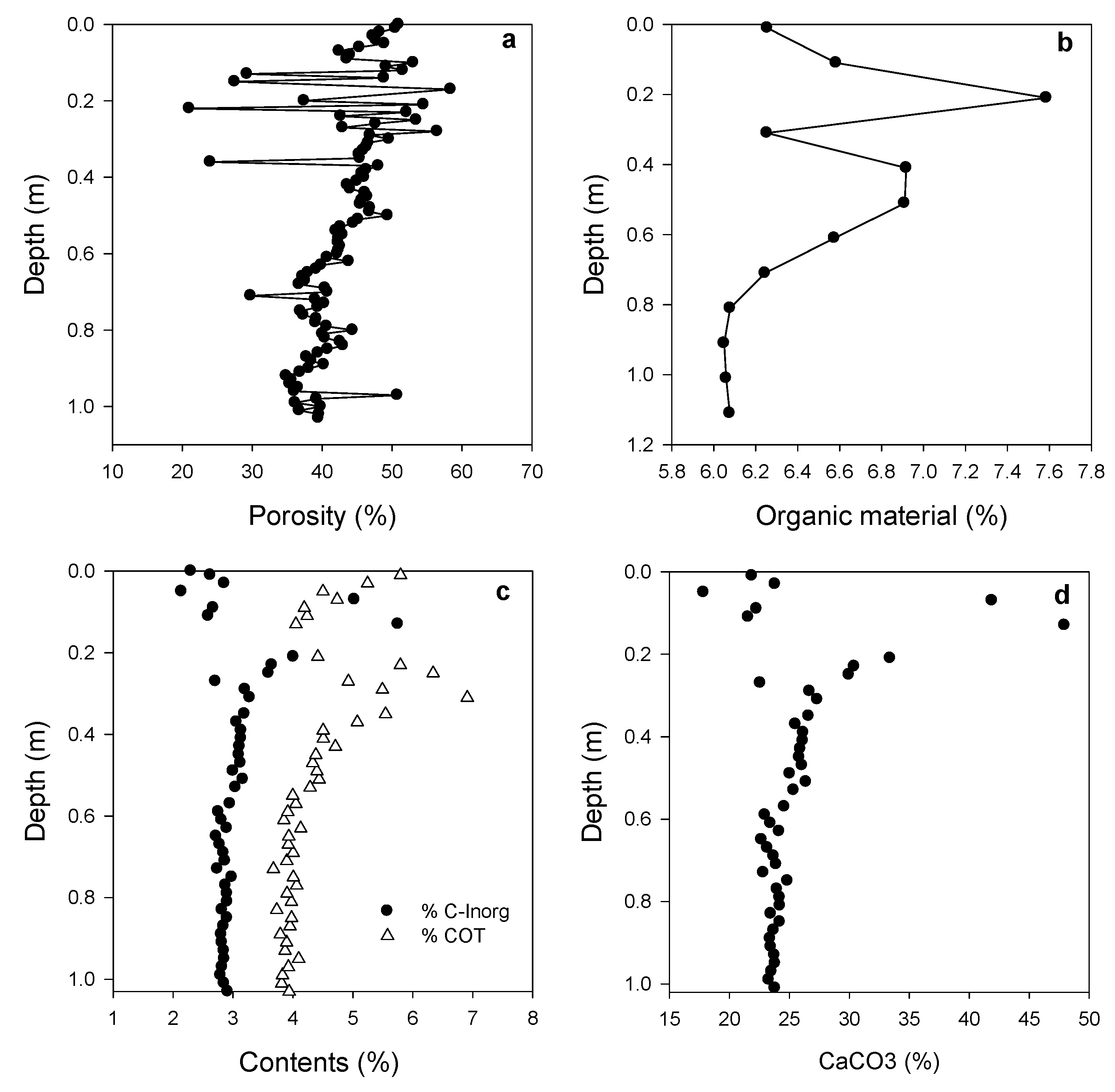
3.1. Sediment Characteristics
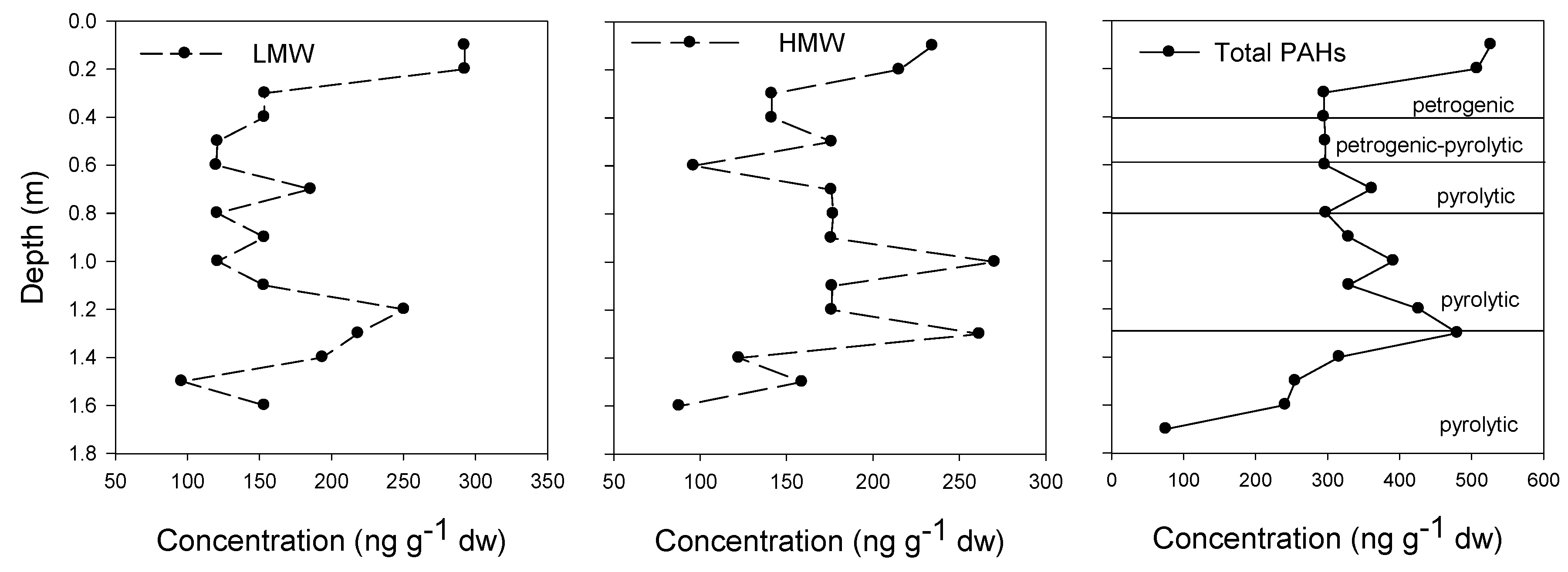
3.2. PAH Source and Toxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oleszczu, P.; Baran, S. Leaching of individual PAHs in soil varies with the amounts of sewage sludge applied and total organic carbon content. Pol. J. Environ. Stud. 2005, 14, 491–500. [Google Scholar]
- Reddy, M.S.; Basha, J.H.V.; Ramachandraiah, G. Seasonal distribution and contamination levels of total PHCs, PAHs and heavy metals in coastal water of the Alang-Sosiya ship scrapping yard, Gulf Cambay India. Chemosphere 2005, 61, 1587–1593. [Google Scholar] [CrossRef]
- Quilliam, R.A.; Rangecroft, S.; Emmett, B.A.; Deluca, T.H.; Jones, D.I. Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural solis? GCB Bioenergy 2013, 5, 96–103. [Google Scholar] [CrossRef]
- Kuśmierz, M.; Oleszczuk, P.; Kraska, P.; Pałys, E.; Andruszczak, S. Persistence of polycyclic aromatic hydrocarbons (PAHs) in biochar-amended soil. Chemosphere 2016, 146, 272–279. [Google Scholar] [CrossRef]
- Doick, K.J.; Klingelmann, E.; Burauel, P.; Jones, K.C.; Semple, K.T. Long-Term Fate of Polychlorinated Biphenyls and Polycyclic Aromatic Hydrocarbons in an Agricultural Soil. Environ. Sci. Technol. 2005, 39, 3663–3670. [Google Scholar] [CrossRef]
- Gonul, L.T.; Kucuksezgin, F. Aliphatic and polycyclic aromatic hydrocarbons in the surface sediments from the Eastern Aegean: Assessment and source recognition of petroleum hydrocarbons. Environ. Sci. Pollut. Res. 2012, 19, 31–41. [Google Scholar] [CrossRef]
- Gschwend, P.M.; Hites, R.A. Fluxes of polycyclic aromatic hydrocarbons to marine and lacustrine sediments in the north-eastern United States. Geochim. Cosmochim. Acta 1981, 45, 2359–2367. [Google Scholar] [CrossRef]
- Sicre, M.; Marty, J.; Saliot, A. Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: Occurrence and origin. Atmos. Environ. (1967) 1987, 21, 2247–2259. [Google Scholar] [CrossRef]
- Kavouras, I.; Koutrakis, P.; Tsapakis, M.; Lagoudaki, E.; Stephanou, E.; Baer, D.; Oyola, P. Source appointment of urban aliphatic and polyaromatic hydrocarbons (PAHs) using multivariate methods. Environ. Sci. Technol. 2001, 35, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W. Alkane and PAH depositional history, source and fluxes in sediments from the Fraser River Basin and Strait of Georgia, Canada. Org. Geochem. 2003, 34, 1429–1454. [Google Scholar] [CrossRef]
- Ke, C.-L.; Gu, Y.-G.; Liu, Q.; Li, L.-D.; Huang, H.-H.; Cai, N.; Sun, Z.-W. Polycyclic aromatic hydrocarbons (PAHs) in wild marine organisms from South China Sea: Occurrence, sources, and human health implications. Mar. Pollut. Bull. 2017, 117, 507–511. [Google Scholar] [CrossRef]
- Salgado, L.D.; Marques, A.E.M.L.; Kramer, R.D.; de Oliveira, F.G.; Moretto, S.L.; de Lima, B.A.; Prodocimo, M.M.; Cestari, M.M.; de Azevedo, J.C.R.; de Assis, H.C.S. Integrated assessment of sediment contaminant levels and biological responses in sentinel fish species Atherinella brasiliensis from a sub-tropical estuary in south Atlantic. Chemosphere 2019, 219, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.F.; Castañeda, L.O. Los ecosistemas estuarinos del Estado de Sinaloa. In Atlas de los Ecosistemas de Sinaloa; Cifuentes-Lemus, J.L., Gaxiola-López, J., Eds.; Colegio de Sinaloa: Culiacan, Mexico, 2003; pp. 175–196. [Google Scholar]
- de la Lanza, G.; Sánchez, N.; Esquivel, A. Análisis temporal y espacial fisicoquímico de una laguna litoral a través del análisis multivariado. Hidrobiológica 1998, 8, 89–96. [Google Scholar]
- Mancilla, M.; Vargas, M. Los primeros estudios sobre la circulación y el flujo neto de agua a través de la Laguna de Términos, Campeche. An. Cent. Cienc. Mar Limnol. 1980, 7, 1–2. [Google Scholar]
- Yáñez-Arancibia, A.; Day, J.W. Ecological Characterization of Terminos Lagoon, a Tropical Lagoon-Estuarine System in the Southern Gulf of Mexico. Coast. Lagoons Oceanol. Acta 1982, 5, 431–440+462. [Google Scholar]
- Canedo-Lopez, Y.; Ruiz-Marin, A.; Barreto-Castro, M.D.R. Polycyclic Aromatic Hydrocarbons in Surface Sediments and Fish Tissues Collected from a Protected Lagoon Region. Bull. Environ. Contam. Toxicol. 2020, 104, 185–192. [Google Scholar] [CrossRef]
- Briggs, D. Soils: Sources and Methods in Geography; Butter-Worths: London, UK, 1977. [Google Scholar]
- Tu, Y.; Ou, J.; Tsang, D.; Dong, C.; Chen, C.; Kao, C. Source identification and ecological impact evaluation of PAHs in urban river sediments: A case study in Taiwan. Chemosphere 2018, 194, 666–674. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- NOAA. Designated Critical Habitat: Critical Habitat for 19 Evolutionarily Significant Units and Salmon and Steelhead in Washington, Oregon, Idaho and California; NOAA: Washington, DC, USA, 2000; pp. 5740–5753. [Google Scholar]
- Long, E.R.; MacDonald, D.D.; Severn, C.G.; Hong, C.B. Classifying probabilities of acute toxicity in marine sediments with empirically derived sediment quality guidelines. Environ. Toxicol. Chem. 2000, 19, 2598–2601. [Google Scholar] [CrossRef]
- Durant, J.L.; Busby, W.F., Jr.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res. Genet. Toxicol. 1996, 371, 123–157. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Balgobin, A.; Ramrrop, S.N. Source apportionment and seasonal cancer risk of polycyclic aromatic hydrocarbons of sediments in a multi-use coastal environment containing a Ramsar wetland, for a Caribbean Island. Sci. Total Environ. 2019, 664, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Flemming, B. A revised textural classification of gravel-free muddy sediments on the basis of ternary diagrams. Cont. Shelf Res. 2000, 20, 1125–1137. [Google Scholar] [CrossRef]
- Araújo, M.; Jouanneau, J.-M.; Valério, P.; Barbosa, T.; Gouveia, A.; Weber, O.; Oliveira, A.; Rodrigues, A.; Dias, J. Geochemical tracers of northern Portuguese estuarine sediments on the shelf. Prog. Oceanogr. 2002, 52, 277–297. [Google Scholar] [CrossRef]
- Corredeira, C.; Araújo, M.; Gouveia, A.; Jouanneau, J. Geochemical characterization of sediment cores from the continental shelf off the western rias area (NW Iberian Peninsula). Cienc. Mar. 2005, 31, 319–325. [Google Scholar] [CrossRef]
- Partida-Gutiérrez, D.I.; Villaescusa, J.A.; Macias-Zamora, J.V.; Castillón, F.F. Persistent organic pollutants in sediment cores from the southern region of the Bight of the Californias. Cienc. Mar. 2003, 29, 521–534. [Google Scholar] [CrossRef]
- Rasheed, M.; Badran, M.I.; Huettel, M. Influence of sediment permeability and mineral composition on organic matter degradation in three sediments from the Gulf of Aqaba, Red Sea. Estuar. Coast. Shelf Sci. 2003, 57, 369–384. [Google Scholar] [CrossRef]
- de la Lanza-Espino, G.; Flores-Verdugo, F.J.; Hernandez-Pulido, S.; Penié-Rodriguez, I. Concentration of nutrients and C:N:P rations in Surface sediments of a tropical coastal lagoon complex affected by agricultural runoff. Univ. Cienc. Trop. Humed. 2011, 27, 145–155. [Google Scholar]
- Pereira, W.E.; Hostettler, F.D.; Luoma, S.N.; van Geen, A.; Fuller, C.C.; Anima, R.J. Sedimentary record of anthropogenic and biogenic polycyclic aromatic hydrocarbons in San Francisco Bay, California. Mar. Chem. 1999, 64, 99–113. [Google Scholar] [CrossRef]
- Magni, P.; De Falco, G.; Como, S.; Casu, D.; Floris, A.; Petrov, A.; Castelli, A.; Perilli, A. Distribution and ecological relevance of fine sediments in organic-enriched lagoons: The case study of the Cabras lagoon (Sardinia, Italy). Mar. Pollut. Bull. 2008, 56, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Kuc, A.G.; Posada-Vanegas, G.; Vega-Serratos, B.E. Evaluación hidrodinámica de la Laguna de Términos. In Aspectos Socioambientales de la Región de la Laguna de Términos; Miranda, J.R., Zapata, G.J.V., Eds.; Instituto EPOMEX, Universidad Autónoma de Campeche: Campeche, Mexico, 2015; pp. 145–166. [Google Scholar]
- Villéger, S.; Ramos, M.J.; Flores, H.D.; Moullinot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Hites, R.A.; Laflamme, R.E.; Windsor, J.G.; Farrington, J.W.; Deuser, W.G. Polycyclic aromatic hydrocarbons in an anoxic sediment core from the Pettaquamscutt River (Rhode Island, USA). Geochim. Cosmochim. Acta 1980, 44, 873–878. [Google Scholar] [CrossRef]
- Venkatesan, M.; Brenner, S.; Ruth, E.; Bonilla, J.; Kaplan, I. Hydrocarbons in age-dated sediment cores from two basins in the Southern California Bight. Geochim. Cosmochim. Acta 1980, 44, 789–802. [Google Scholar] [CrossRef]
- Barakat, A.O.; Mostafa, A.; Wade, T.L.; Sweet, S.T.; El Sayed, N.B. Distribution and characteristics of PAHs in sediments from the Mediterranean coastal environment of Egypt. Mar. Pollut. Bull. 2011, 62, 1969–1978. [Google Scholar] [CrossRef]
- Reddy, C.M.; Eglinton, T.I.; Hounshell, A.; White, H.K.; Xu, L.; Gaines, R.B.; Frysinger, G.S. The West Falmouth Oil Spill after Thirty Years: The Persistence of Petroleum Hydrocarbons in Marsh Sediments. Environ. Sci. Technol. 2002, 36, 4754–4760. [Google Scholar] [CrossRef]
- Hassan, H.M.; Castillo, A.B.; Yigiterhan, O.; Elobaid, E.A.; Al-Obaidly, A.; Al-Ansari, E.; Obbard, J.P. Baseline concentrations and distributions of Polycyclic Aromatic Hydrocarbons in surface sediments from the Qatar marine environment. Mar. Pollut. Bull. 2018, 126, 58–62. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No.835/2011 of 19 August 2011 amending Regulation (EC) No.1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons foodstuffs. Off. J. Eur. Union 2011, 215, 4–8. [Google Scholar]

| PAH | Abbreviation | Rings | TEFi | MEFi | NOAA-ERL | NOAA-ERM | Literature Data * T1/2 |
|---|---|---|---|---|---|---|---|
| Naphthalene | Nap | 2 | 0.001 | 260 | 2100 | - | |
| Acenaphthylene | Acy | 3 | 0.001 | 44 | 640 | - | |
| Acenaphthene | Ace | 3 | 0.001 | 16 | 500 | - | |
| Fluorene | Fl | 3 | 19 | 540 | 340 | ||
| Anthracene | Ant | 3 | 0.01 | 85 | 1100 | 160 | |
| Phenanthrene | Phen | 3 | 0.001 | 240 | 1500 | 310 | |
| Fluoranthene | Flu | 0.001 | 290 | 600 | 110–4745 | ||
| Pyrene | Pyr | 4 | 0.001 | 670 | 2600 | 250 | |
| Chrysene | Chr | 0.01 | 0.017 | - | - | 390 | |
| Benzo(a)anthracene | BaA | 0.1 | 0.082 | 261 | 1600 | 240–730 | |
| Benzo(b)fluoranthrene | BbF | 0.1 | 0.25 | - | - | 87–5183 | |
| Benzo(k)fluoranthrene | BkF | 5 | 0.1 | 0.11 | - | - | 139–4015 |
| Benzo(a)pyrene | BaP | 1 | 1 | 430 | 1600 | 151–5329 | |
| Dibenzo(a,h)anthracene | DhA | 1 | 0.29 | 63 | 260 | 240–730 | |
| Benzo(ghi)perylene | BgP | 6 | 0.01 | 0.19 | - | - | 173–657 |
| Indeno(1,2,3-cd)pyrene | InP | 0.1 | 0.31 | - | - | 58–730 |
| Depth (m) | Zone | Clay | Sand | Silt |
|---|---|---|---|---|
| 0–0.2 | A | 0.9 ± 0.04 a | 31.36 ± 26.5 a | 67.73 ± 25.6 a |
| 0.2–0.4 | B | 3.71 ± 1.69 b | 10.61 ± 49.6 b | 85.66 ± 36.1 b |
| 0.4–0.6 | 3.32 ± 0.75 b | 13.20 ± 33.6 b | 83.47 ± 25.5 b | |
| 0.6–0.9 | C | 2.58 ± 0.15 c | 21.01 ± 8.1 c | 76.41 ± 7.4 ab |
| 0.9–1.1 | 2.86 ± 0.44 c | 22.48 ± 20.9 c | 75.31 ± 17.5 ab |
| Nap | Acy | Ace | Fl | Ant | Phen | Flu | Chr | BaA | BbF | BkF | BaP | DhA | BgP | InP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nap | 1.00 | ||||||||||||||
| Acy | 0.21 | 1.00 | |||||||||||||
| Ace | −0.14 | −0.18 | 1.00 | LWM | |||||||||||
| Fl | 0.39 | −0.12 | 0.30 | 1.00 | |||||||||||
| Ant | 0.25 | −0.39 | −0.06 | −0.04 | 1.00 | ||||||||||
| Phen | 0.21 | −0.06 | 0.33 | 0.54 | −0.39 | 1.00 | |||||||||
| Flu | 0.55 | 0.11 | 0.03 | 0.22 | 0.72 | 0.11 | 1.00 | ||||||||
| Chr | 0.67 | 0.14 | −0.17 | 0.27 | 0.56 | 0.14 | 0.84 | 1.00 | |||||||
| BaA | 0.08 | 0.54 | −0.02 | 0.19 | −0.04 | −0.12 | 0.22 | 0.27 | 1.00 | ||||||
| BbF | 0.30 | 0.07 | 0.19 | 0.12 | 0.38 | 0.06 | 0.54 | 0.45 | 0.13 | 1.00 | |||||
| BkF | 0.38 | 0.19 | 0.29 | 0.02 | 0.33 | 0.18 | 0.63 | 0.47 | 0.02 | 0.34 | 1.00 | HMW | |||
| BaP | 0.30 | 0.06 | 0.19 | 0.12 | 0.39 | 0.06 | 0.54 | 0.45 | 0.12 | 1.00 | 0.34 | 1.00 | |||
| DhA | 0.01 | 0.38 | 0.32 | 0.37 | −0.44 | 0.38 | −0.04 | 0.05 | 0.37 | −0.40 | 0.20 | −0.40 | 1.00 | ||
| BgP | −0.28 | 0.17 | 0.21 | −0.04 | −0.14 | 0.16 | 0.04 | −0.06 | −0.04 | −0.17 | 0.07 | −0.17 | 0.14 | 1.00 | |
| InP | −0.49 | −0.10 | 0.46 | −0.19 | −0.17 | −0.10 | −0.31 | −0.24 | −0.17 | 0.10 | −0.12 | 0.10 | 0.18 | 0.18 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queb-Suarez, J.E.; Ruiz-Marin, A.; Canedo-Lopez, Y.; Aguilar-Ucan, C.A.; Montalvo-Romero, C.; Flores-Trujillo, J.G.; Perez-Morga, N. Source Identification, Toxicity, and Persistence of PAHs in Sediment Core from a Natural Protected Area in Mexico. Energies 2022, 15, 7116. https://doi.org/10.3390/en15197116
Queb-Suarez JE, Ruiz-Marin A, Canedo-Lopez Y, Aguilar-Ucan CA, Montalvo-Romero C, Flores-Trujillo JG, Perez-Morga N. Source Identification, Toxicity, and Persistence of PAHs in Sediment Core from a Natural Protected Area in Mexico. Energies. 2022; 15(19):7116. https://doi.org/10.3390/en15197116
Chicago/Turabian StyleQueb-Suarez, Jose Emilio, Alejandro Ruiz-Marin, Yunuen Canedo-Lopez, Claudia Alejandra Aguilar-Ucan, Carlos Montalvo-Romero, Juan Gabriel Flores-Trujillo, and Nancy Perez-Morga. 2022. "Source Identification, Toxicity, and Persistence of PAHs in Sediment Core from a Natural Protected Area in Mexico" Energies 15, no. 19: 7116. https://doi.org/10.3390/en15197116
APA StyleQueb-Suarez, J. E., Ruiz-Marin, A., Canedo-Lopez, Y., Aguilar-Ucan, C. A., Montalvo-Romero, C., Flores-Trujillo, J. G., & Perez-Morga, N. (2022). Source Identification, Toxicity, and Persistence of PAHs in Sediment Core from a Natural Protected Area in Mexico. Energies, 15(19), 7116. https://doi.org/10.3390/en15197116







