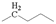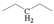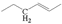1. Introduction
In recent years, with increasingly stringent environmental regulations and the increasing shortage of petroleum resources, researchers around the world are actively looking for a new sustainable energy sources [
1]. Compared with petroleum, vegetable oil—a renewable energy source—contains almost no S, N, or metal substances. Vegetable oil is a clean raw material and is considered to be one of the important sources of biomass fuel production. In addition, vegetable oil is also receiving wide attention due to its lower net CO
2 emissions [
2].
The annual production rate of waste cooking oil in China is up to 5 million tons; therefore, the use of waste cooking oil with high added value is an important research subject. The main methods of producing biomass fuel from vegetable oil are transesterification, catalytic hydrogenation and FCC. The transesterification of vegetable oils is the main production process used to produce biodiesel, which is a mixture of fatty acid methyl esters (FAMEs). However, this reaction also produces glycerin as a byproduct, which will not only lead to a decline in the quality of oil products but also reduce the market price of biodiesel [
3,
4]. Otherwise, it has the drawbacks of byproduct generation and a higher cost. Catalytic hydrogenation is performed at a moderate temperature (300–600 °C) and high hydrogen pressure (10–20 MPa), over a variety of catalysts, with the removal of oxygen as H
2O, CO
2 or CO. Nonetheless, due to the use of high pressure and hydrogen, its investment and operation cost are relatively high. Therefore, more researchers are paying attention to the study of biodiesel production from FCC vegetable oil [
5].
The catalysts used for the FCC of animal and vegetable oils are mainly include molecular sieve catalysts (HZSM-5 [
6,
7], USY [
8], MCM-41 [
9]), amorphous catalysts (activated alumina gamma-Al
2O
3 [
10], and alkaline catalysts (CaO, Na
2CO
3 [
11]), etc. Thanh-An [
12] used waste cooking oil as a raw material and carried out FCC experiments in a fixed-bed microreactor. The results showed that by using HZSM-5 as a catalyst, the aromatics contents—such as benzene, toluene and xylene—were higher in the liquid products. Atsushi Ishihara [
13] used ZSM-5 zeolite-containing silica-aluminas as a catalyst; by comparison, ZSM-5 zeolite showed the highest yield of aromatics among the three types of zeolite.
In the aspect of reactor selection, most researchers choose a fixed-bed microreactor as the FCC reactor [
14,
15,
16]. However, the fixed-fluidized-bed (FFB) reactor will have better commercial characteristics. FCC, the most important technology in petrochemical industry, can transform high-molecular-weight hydrocarbons to more gasoline products [
17,
18]. Its units are usually designed for the treatment of complex and heavy feeds [
19]. According to the characteristics of FCC, it is possible for it to be used for the upgrading of waste cooking oil. The direct feeding of bio-oil in a FCC unit has been considered to be infeasible. Attempts to directly upgrade bio-oil have reported significant amounts of char, coke and water as the main products [
20,
21,
22,
23]. Bio-oil’s immiscibility with hydrocarbons has also been singled out as an impediment to its direct introduction into the FCC process [
24].
LDO-75 is a kind of industrial catalyst. It has been widely used in heavy-oil FCC. The experimental results show that a high liquid yield can be obtained by using LDO-75 as the catalyst in heavy-oil FCC. Thus, in this paper, LDO-75 was selected as a catalyst, waste cooking oil was selected as a raw material, and a fixed fluidized bed was selected as a reactor to study waste cooking oil FCC characterization. The effect of the temperature, catalyst–oil ratio, and weight hourly space velocity (WHSV) on the yield of product was investigated. The formation and distribution of products in the FCC of waste cooking oil were analyzed by gas chromatography (GC), gas chromatography-mass spectrometry (GC-MS), and nuclear magnetic resonance (NMR).
2. Material and Methods
2.1. Materials
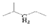
The crude waste cooking oil was purchased from a local supermarket, and was used without any further treatment. The crude waste cooking oil was mainly composed of C16–C20 unsaturated fatty acids, and a small quantity of saturated fatty acid was detected using gas chromatography–mass spectrometry (GC–MS): hexadecanoic acid (7.53%), 9-octadecenoic acid (32.94%), octadecanoic acid (3.13%), 9,12-octadecadienoic acid (52.46%), arachidic acid (0.57%), 12-octadecenoic acid (0.82%), and α-linolenic acid (2.55%).
The industrial catalyst LDO-75 produced by petroChina Lanzhou Petrochemical company (Lanzhou, China) catalyst plant was used in this research. The catalyst characterization was as follows: pore volume 0.38 mL·g−1, specific surface area 267 m2·g−1, and mean pore size 3.82 nm. The catalyst was calcinated for 6 h at 600 °C before experiment.
2.2. Cracking Reaction
The experiment was carried out in a fluidized bed reactor system. The device is shown in
Figure 1. Firstly, the water was pumped into the preheater and heated to 350 °C. Then, the steam formed in the reactor and contacted with the catalyst at a high temperature in the reactor to form fluidization. Secondly, the waste cooking oil was extracted by the oil pump and injected into the preheater; then, the preheated waste cooking oil entered the reactor and reacted with the catalyst in the fluidized state. After the reaction, the oil gas was condensed and cooled from the reactor; the heavy oil entered the heavy oil receiving bottle, the light oil entered the light oil receiving bottle, and the remaining oil and gas are absorbed into the liquefied gas by the liquefied gas absorption bottle with industrial ethanol, and then enters the wet flow meter to measure the dry gas. The effects of the reaction temperature (400–550 °C), catalyst–oil ratio (3–6), and weight hourly space velocity (WHSV) (6–22/h) on the yield of products were investigated. However, in this experiment, steam was used as a carrier, such that the water content produced by the FCC of waste cooking oil cannot be accurately measured during the experiment.
2.3. Product Collection, Calculation and Characterization
The pyrolysis gases included dry gas (H2, CO, CO2 and C1–C2 hydrocarbons) and liquefied gas (C3–C5 hydrocarbons). The dry gas was calculated using a wet-gas flowmeter; the calculation of the dry-gas yield was performed according to the ideal gas equation of state. The liquefied gas was absorbed by industrial alcohol (95 wt%).
After the removal of the water, the liquid products collected from the separation system were subjected to a reduced-pressure Engler distillation experiment. The liquid products were divided into gasoline (<205 °C), diesel (205–350 °C) and heavy oil (>350 °C) fractions according to the distillation range. The coke deposited on the catalyst surface was calculated by the charred difference method.
The gas components and their percentages were determined by GC analysis (GC 950), equipped with a Porapak-Q column (3 m × 3 mm) and a TDX-01 column (3 m × 3 mm). The C1∼C5 hydrocarbons were detected by a FID using nitrogen as a carrier gas, and the H2 and COx were detected by a TCD using helium as a carrier gas, respectively. The qualitative and quantitative analysis of the pyrolysis gas were determined by the external standard method with a standard gas.
The characterization parameters of the diesel and heavy oil fractions were identified by NMR. The 13C NMR curve was obtained at 100.62 MHz by a Bruker AVANCEIII−400 MHz with a 5-mm probe insert and TMS as the internal standard. In order to guarantee the total solution organic matter, CDCl3 was used as the solvent. The acquisition time of the 13C spectra was 3 s. MestReNova 6.1.1 was used to analyze the NMR spectra and quantify the relative proportion of different carbon and hydrogen types in the liquid product samples.
GC-MS was performed with ACION TQ (Bruker) in order to determine the composition of the gasoline fraction. The type of chromatographic column was an HP-5MS (30 m × 0.25 mm × 0.25 μm) fused silica capillary column. The oven temperature was maintained at 20 °C for 5 min and then slowly increased to 280 °C at a rate of 10 °C min
−1, followed by holding the pyrolysis temperature for 20 min. The analysis was performed using a Qual Browser and Technology mass spectra library search. In order to quantitatively analyze the peaks in the total ion chromatogram, the curves were compared with the spectra in the National Institute of Standards and Technology (NIST) [
25,
26].
3. Results and Discussion
3.1. The Yield of the Catalytic Cracking Products
In this section, the yields of FCC products—as affected by the catalyst–oil ratio, WHSV and temperature—were investigated.
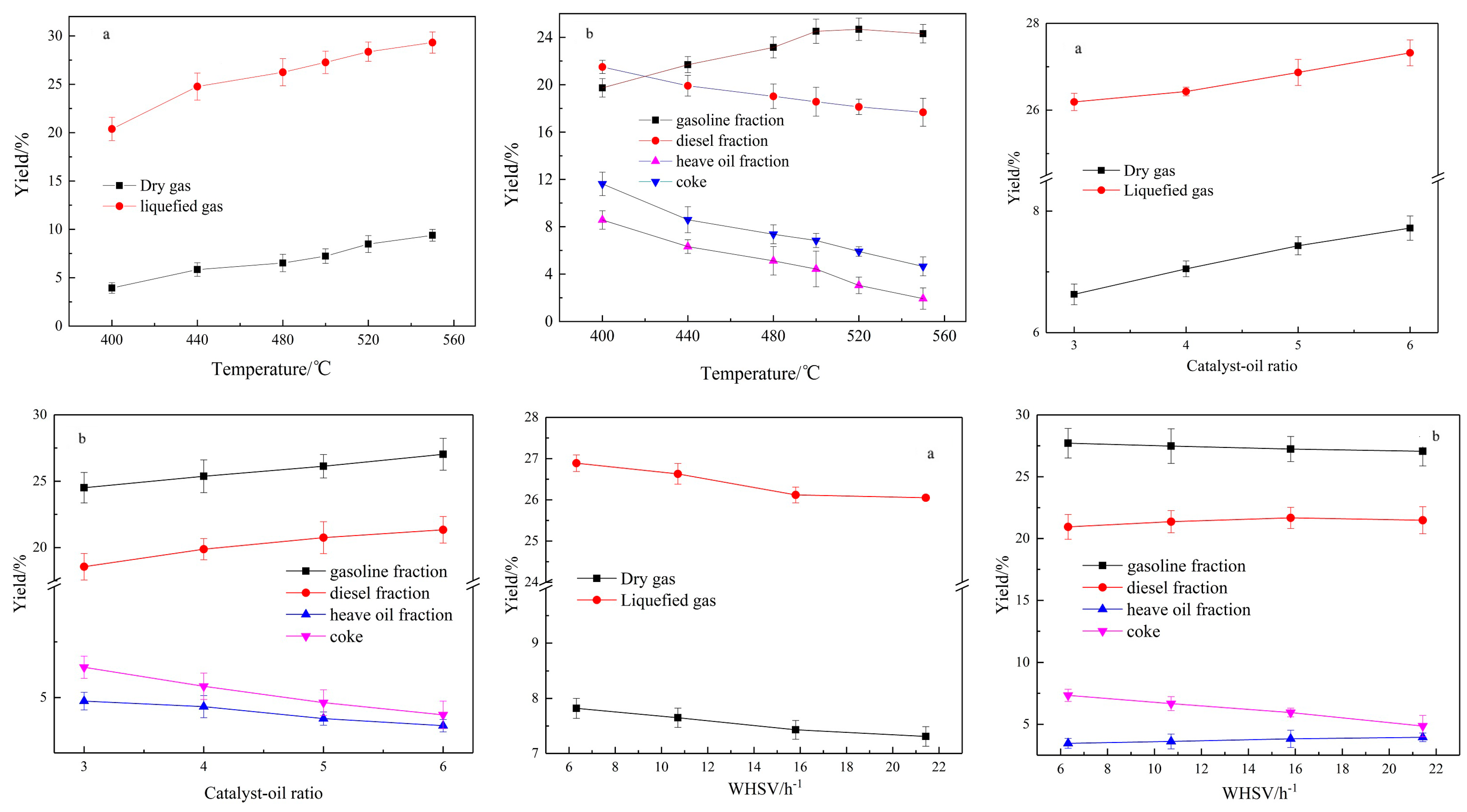
3.1.1. Products Distribution
As shown in
Figure 2, the temperature, catalyst–oil ratio and WHSV will all affect the product yield, of which temperature has the greatest impact. The yield of dry gas and liquefied gas increases with the increasing temperature, and the yield of liquefied gas is more obvious. Katikaneni [
16] indicated that the fatty acid ester FCC has two steps: firstly, the fatty acid esters initial crack and deoxidate on the surface of the catalyst; secondly, the intermediates, such as oxygen-containing derivatives, spread into the catalyst channel for the secondary cracking, and most of the oxygen-containing derivatives will be removed in the form of H
2O, CO and CO
2.
The yield of the gasoline fraction increases gradually from 19.73% at 400 °C to 24.30% at 550 °C. To the contrary, the yields of diesel, the heavy oil fraction and coke decrease with the increasing temperature. The results show that the higher the temperature is, the higher the yield of light components is. In the cracking process, the thermal and FCC reactions occur at the catalyst’s surface to produce light compounds, then the light compounds will produce heavier alkane and aromatic hydrocarbons through oligomerization and aromatization, respectively [
27]. The degree of thermal cracking will deepen with the increase of the reaction temperature. Not only the coke but also some tar components will attach to the catalyst. When the reaction temperature rises, the relatively heavy molecular weight of the tar components will volatilize into the product collection device.
With the increase of the catalyst–oil ratio, the yields of dry gas and liquefied gas also gradually increase; at the same time, the yields of the gasoline and diesel fractions gradually increase from 24.51% and 18.56% of the catalyst–oil ratio 3 to 27.03% and 21.34% of catalyst–oil ratio 6, respectively. On the contrary, the yields of heavy oil fractions and coke decrease with the increase of the catalyst–oil ratio. The results showed that with the increase of the catalyst–oil ratio, the number of active catalyst bits per unit of feed molecule was increased, which promoted the secondary cracking reaction, deepened the reaction, and made more products move to small molecules.
The WHSV reflects the material flow rate. Within the operational range of the reactor, the WHSV has little effect on the yield of the products. As a result, the yields of dry gas, liquefied gas, gasoline and coke gradually decrease with the increased WHSV. This was because the WHSV decreased and the secondary reaction intensified, leading to the deepening of aromatization, and the olefin polymerized to form coke. When the WHSV was 15.80 h−1, the total yield of gasoline and diesel was the highest, reaching 48.91%.
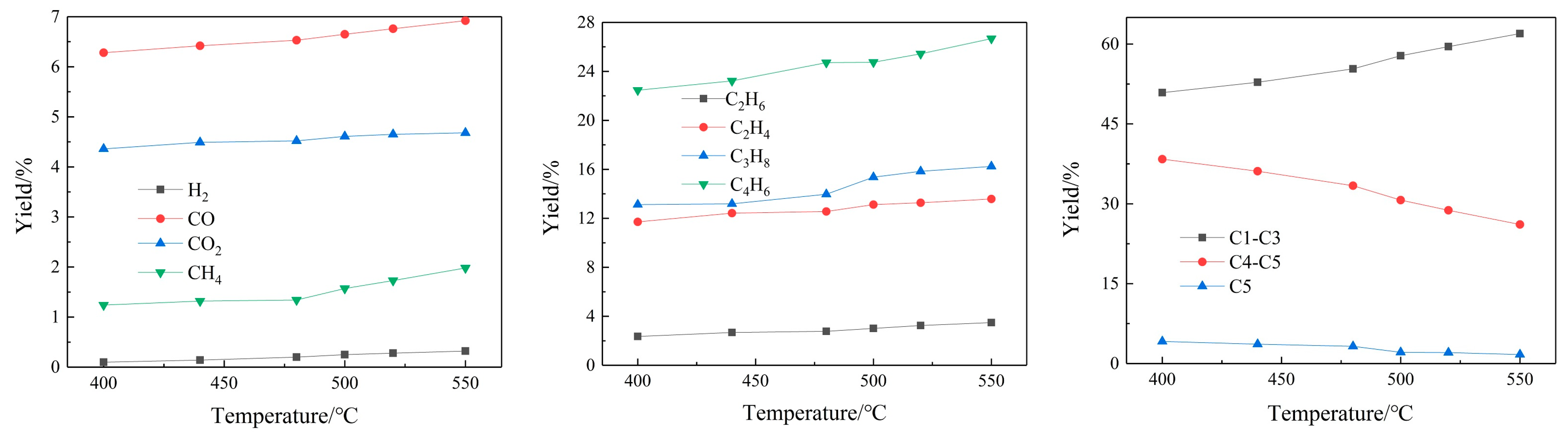
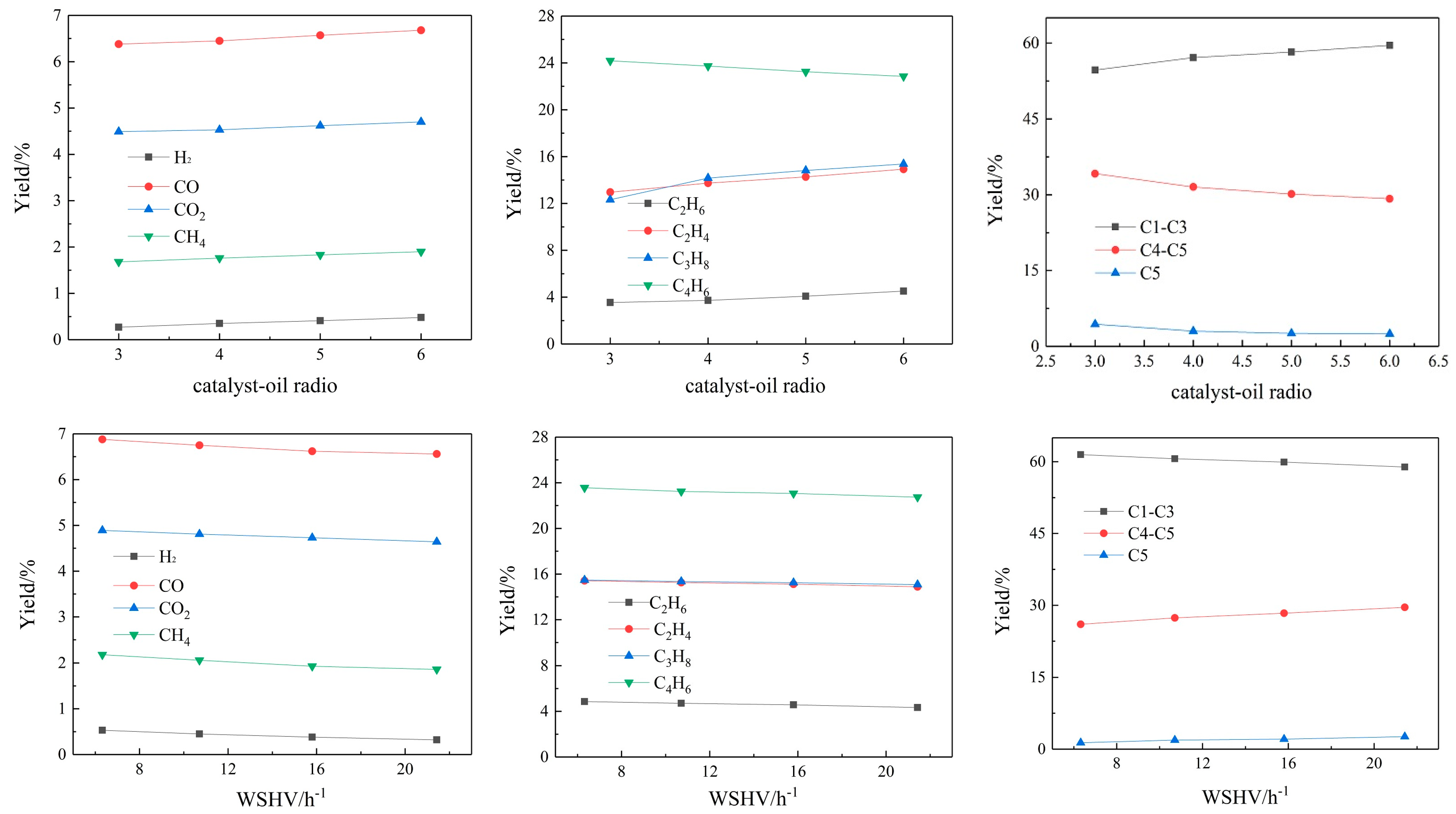
3.1.2. Gas Yields
The content of the FCC gas yield is listed in
Figure 3. The FCC gases mainly consist of CO, CO
2, CH4 and C2–C5 alkanes and alkenes. Compared with other catalysts, the LDO-75 catalyst has a higher propylene yield [
5,
9]. With the rising temperature, C1–C3 components increase from 50.88% at 400 °C to 61.97% at 550 °C, while the C4–C5 components decrease obviously from 38.38% to 26.11%. This phenomenon also indicates that a high temperature can promote a cracking reaction and generate small-molecule components. Besides this, the content of alkenes in cracking gas is much higher than that in alkanes; this is because the waste cooking oil is mainly constituted of unsaturated acid. Moreover, H
2 almost cannot be detected in the cracked gas; the possible reason is H
2 generated by dehydrogenation during the reaction, and may also involve the hydrogenation reaction through catalytic means.
As the catalyst–oil ratio increases, the content of C1–C3 increases from 54.68% when the catalyst oil ratio is 3 to 59.57% when the catalyst–oil ratio is 6, while the relative content of C4–C5 components decreases from 34.18% to 29.20%. This is because as the catalyst–oil ratio increases, the number of active catalyst bits per unit of raw material molecules increases, leading to the deepening of the secondary cracking. On the one hand, the dehydrogenation reaction of macromolecular hydrocarbons produces more small molecular hydrocarbons. On the other hand, propylene will generate aromatic hydrocarbons through condensation and polymerization, resulting in a gradual decrease in the propylene content. With the deepening of the secondary cracking, isobutane, n-butane and other C4-and-above substances will gradually undergo a hydrogen transfer reaction to produce light olefins, and olefins will continue to carry out the above reaction.
As the WHSV decreases, the content of methane, ethane, ethylene and other cracked final products gradually increases, and the contents of butane and butene gradually decrease. The smaller WHSV can deepen the degree of the cracking reaction and promote secondary cracking. Accordingly, larger molecular hydrocarbons will be cracked into small molecules. The hydrocarbons, such as ethane, ethylene, butene, etc., as intermediate products, will be more retained when the residence time decreases. It can be seen from
Figure 3 that as the WHSV decreases, the contents of CO and CO
2 gradually increase, because as the degree of secondary cracking deepens, the deoxidation reaction becomes intense. The oxygen-containing derivatives of macromolecules will undergo more cracking deoxygenation reactions, and more of the oxygen will exist in the form of CO, CO
2 and H
2O.
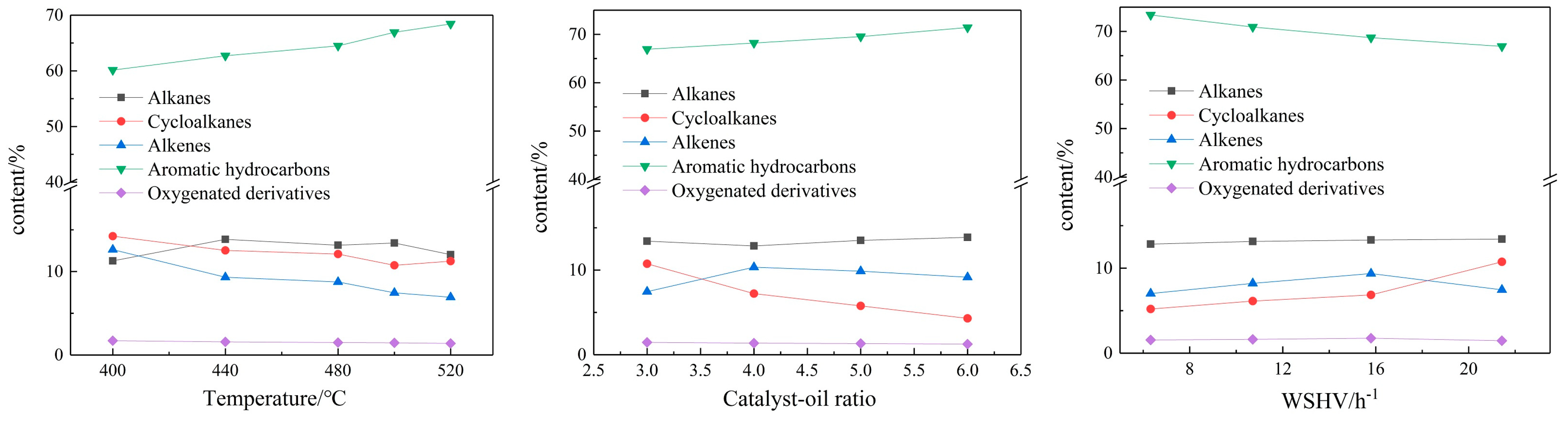
3.1.3. GC-MS Analysis
GC-MS was used to qualitatively and quantitatively analyze the FCC gasoline fraction of waste cooking oil. According to the composition classification, the composition of the gasoline fraction was divided into alkanes, cycloalkanes, alkenes, aromatics and oxidized derivatives; the results are shown in
Figure 4.
As shown in
Figure 4, the reaction temperature and WHSV have a great influence on the group composition of the gasoline fraction. The greatest content of the gasoline fraction in the FCC products of the waste cooking oil is aromatic hydrocarbons; this is because there are many oxygen-containing compounds in waste cooking oil, which are easy to aromatize to aromatic compounds. With the increasing temperature, the content of aromatics increased and the contents of the other compounds decreased gradually. The fluid catalytic cracking of unsaturated aliphatic acid would form more alkenes, which can form more aromatics through a condensation reaction. It is generally considered that alkenes are the most active type of hydrocarbon in FCC. More alkenes may be produced due to secondary cracking at a higher temperature. Moreover, branched alkenes can react with naphthenic hydrocarbons to form aromatics by hydrogen transfer and condensation reactions. In addition, cyclic alkenes would be produced by side chain breaks of alkanes and alkenes with side chains or conjugated dienes occurring in Diels–Alder reactions; then, aromatic hydrocarbons may be generated by the hydrogen transfer reaction of cyclic alkenes. Besides this, only a small amount of oxygen compounds are detected in the gasoline fraction, mainly composed of ethers and alcohols, which indicate that in the fluid catalytic cracking reaction of waste cooking oil, the oxygen compounds are decomposed to CO
2, CO and H
2O.
As the WHSV became smaller, the content of aromatics gradually increased, and the content of alkanes, cycloalkanes, alkenes and oxygen-containing derivatives gradually decreased. The smaller the WHSV, the longer the contact time between the raw material molecules and the catalyst, and the deeper the cracking reaction. The degree of reaction and secondary cracking deepened. The rings of cycloalkanes will be broken to produce olefins, and olefins will produce more aromatics through polymerization.
3.1.4. NMR Analysis
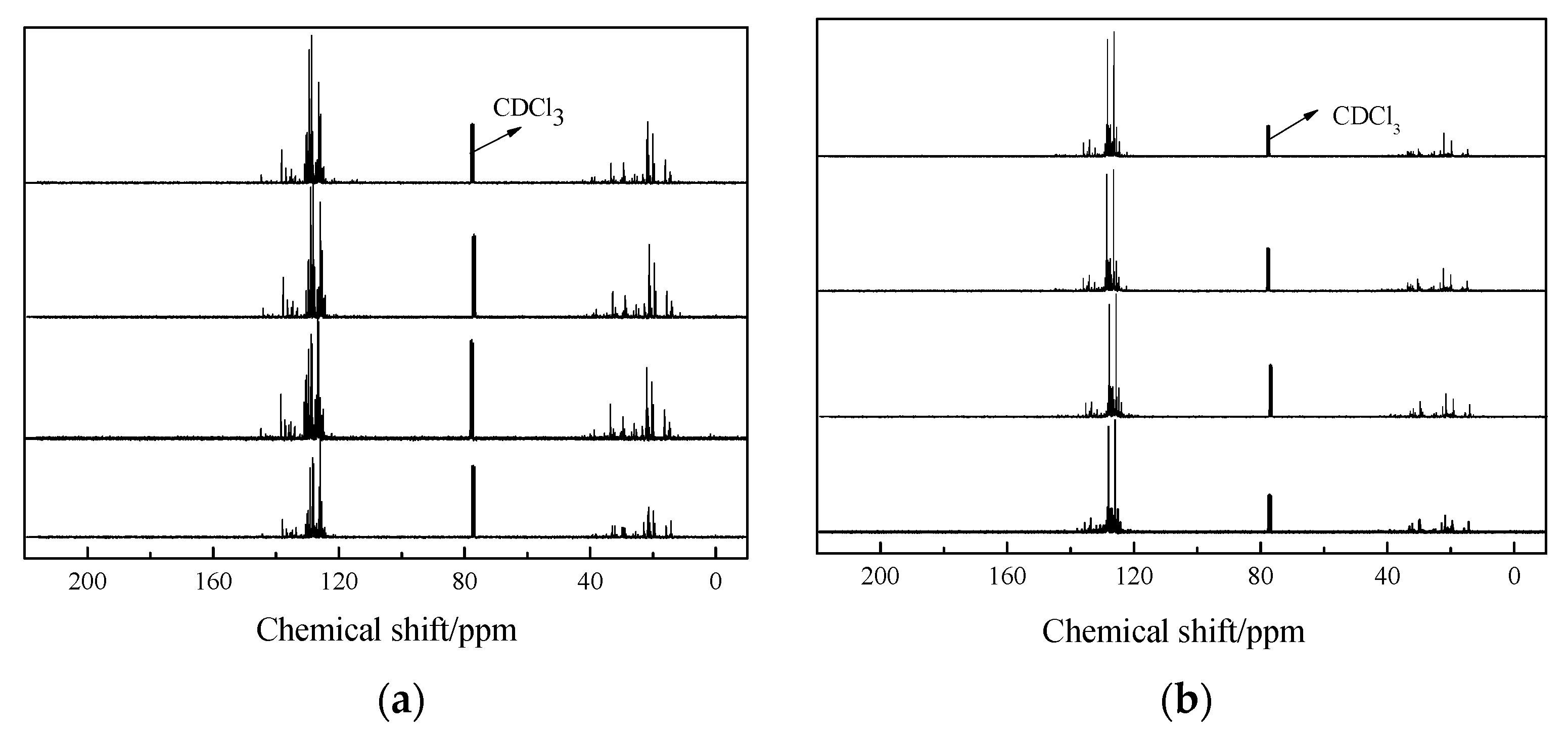
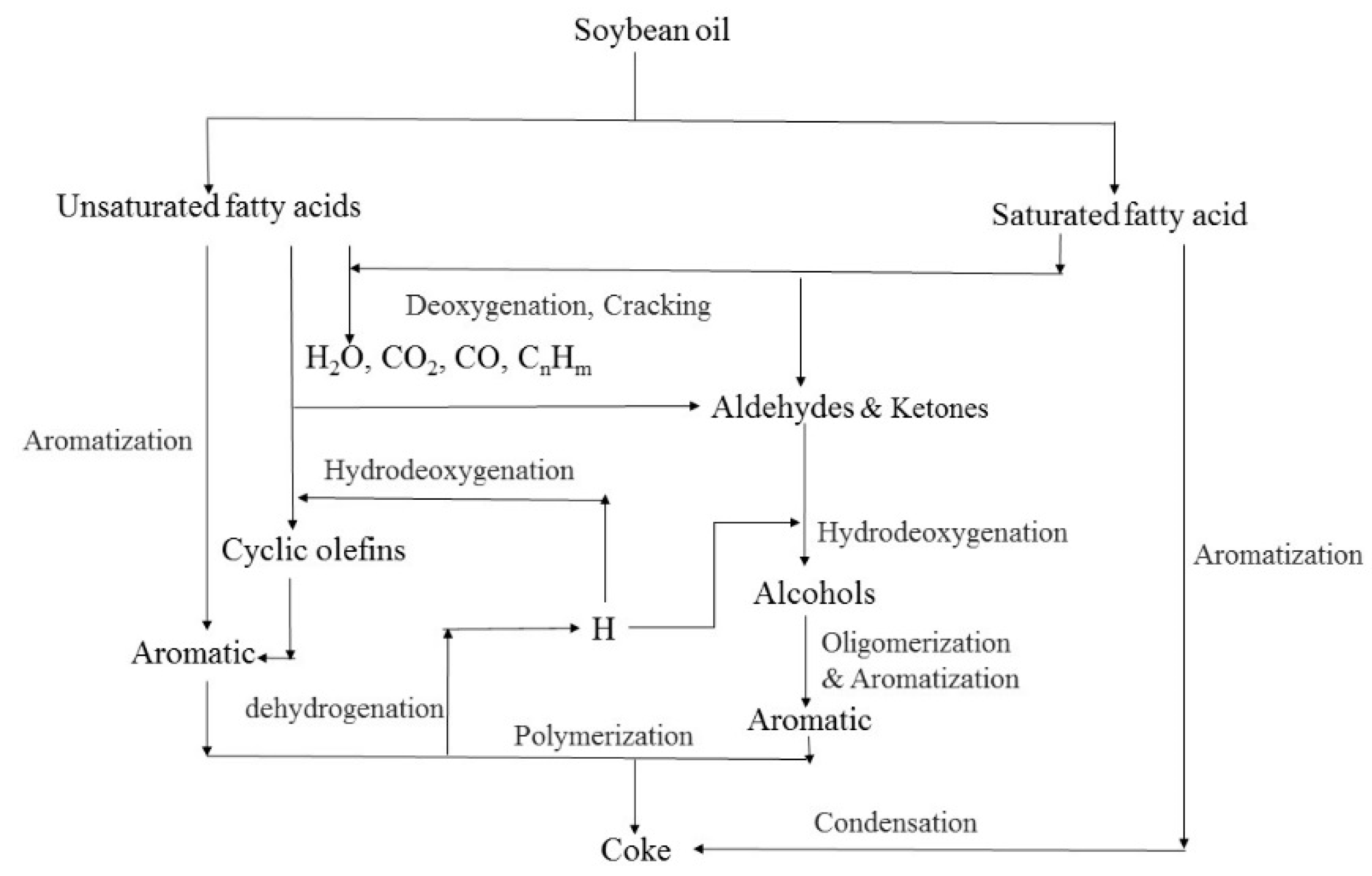
In order to obtain the composition and structure information of the diesel fraction and heavy oil fraction, the samples at different temperatures were analyzed by NMR. The
13C NMR curves of the diesel and heavy oil fractions obtained at different temperatures were similar, indicating that the compounds of the samples demonstrate an overall similar distribution. Thus, the
13C NMR technique is only employed for the samples at 400 °C, 440 °C, 500 °C and 550 °C. The
13C NMR curves are shown in
Figure 5.
The
13C NMR spectra exhibit signals at 15–40 ppm, which are assigned to the carbons of alkane and alkene, whereas the signals at 120–160 ppm correspond to aromatic carbons (
Figure 3; the signal at 77 ppm is assigned to the solvent of the NMR measurement). The carbon skeleton structure information corresponding to different chemical shifts is listed in
Table 1.
As shown in
Figure 5 and
Table 1, the main components of the diesel fraction and heavy oil fraction are aromatic compounds, which account for more than 68% of the diesel fraction and more than 75% of the heavy fraction. It should be noted that there are no aromatic hydrocarbons in waste cooking oil; the aromatic compounds detected in the products were formed by the aromatization of the unsaturated fatty acids in waste cooking oil. Besides this, with the increase of the fluid catalytic cracking temperature, the content of polycyclic aromatic hydrocarbons in the diesel and heavy oil fractions increases, which indicates that the degree of reaction will deepen with the increase of the fluid catalytic cracking temperature.
Waste cooking oil is mainly composed of C
18 unsaturated fatty acids; with the fluid catalytic cracking reaction, aromatic compounds with hydrocarbyl substituted are mainly produced in the products. Among them, alkenyl groups are the main substitution. Because there is also a small amount of saturated fatty acids in waste cooking oil, there is a small quantity of alkyl-substituted aromatic compounds in the liquid products. Carboxyl groups were not detected in the liquid products at different temperatures; it has been reported that the oxygen in the feedstock would be removed through the formation of water, CO
2 and CO though deoxygenation [
28].
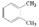
3.2. Reaction Pathway
Waste cooking oil contains a variety of fatty acids, including saturated fatty acids and unsaturated fatty acids, which are mainly unsaturated fatty acids. Undergoing a series of reactions, various hydrocarbons, gases and water are generated. The possible reaction pathway for the FCC of waste cooking oil over a LDO-75 catalyst is proposed in
Figure 6.
The process of the fluid catalytic cracking of waste cooking oil is mainly composed of two simultaneous reactions: deoxygenation and cracking [
29,
30]. In the deoxygenation process, H
2O, CO, and CO
2 are generated, and then the oxygenated compounds produce saturated or unsaturated hydrocarbons, which are reacted with hydrogen generated from aromatization to form light alcohols [
31]. The light alcohols will produce heavier aromatic hydrocarbons through oligomerization and aromatization. Unsaturated fatty acids will form cyclic olefin compounds through hydrodeoxygenation, and then undergo further dehydrogenation and hydrodeoxygenation to form aromatic compounds. At the same time, thermal and fluid catalytic cracking reactions occurred at the catalysts’ surface to produce light olefins, light paraffins, light aldehydes, and ketones, which will further produce heavier alkane and aromatic hydrocarbons through oligomerization and aromatization [
32,
33]. The coke will be generated through the further polymerization of aromatic compounds. During this polymerization process, hydrogen was produced by dehydrogenation reactions. It can not only be used to stabilize unsaturated hydrocarbon compounds via a hydrogen transfer reaction but also participate in the deoxygenation of unsaturated oxygenated compounds. Besides this, due to the high temperature of the catalyst surface, some components will rapidly coke when it contacts the catalyst surface [
34,
35,
36].
4. Conclusions
Bio-oil is an important sustainable energy source. In this paper, waste cooking oil was used as a raw material, and FCC treatment was carried out on LDO-75 through an FFB reactor. The effects of the reaction temperature, catalyst–oil ratio and WHSV on the yield of the product were studied. In addition, the GC, GC-MS and NMR analysis products were characterized. The results were as follows.
(1) As the reaction temperature increased, the yield of dry gas and liquefied gas gradually increased. In addition, the yield of the gasoline fractions gradually increased from 19.73% at 400 °C to 24.30% at 550 °C. The GC-MS and NMR analysis results showed that the liquid product was mainly composed of aromatic compounds, accounting for more than 60%.
(2) As the catalyst–oil ratio increased, the yields of dry gas, liquefied gas, gasoline and diesel gradually increased, while the yields of the heavy oil fractions and coke gradually decreased. Similarly, the GC-MS and NMR analysis results showed that the liquid product is mainly composed of aromatic compounds, accounting for more than 65%.
(3) As the WHSV increases, the yield of dry gas, liquefied gas, gasoline, diesel, and coke slightly decreases, while the yield of heavy oil gradually increases. In general, the change of the WHSV has little effect on the distribution of the catalytic cracking products of waste cooking oil.
In addition, based on the characterization results, the reaction pathway of waste cooking oil FCC in the FFB reactor was proposed. This will provide encouraging research to further optimize the waste cooking oil FCC process and target product yield.
Author Contributions
J.S. analyzed and interpreted the experiment data regarding the yields of the products, GCMS, and NMR, and was a major contributor in writing the manuscript; H.A. performed the data analysis of GC and FTIR; C.W. and Y.C. provided technical support for the experiment. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (NO. 51301028), General Program Education Department of Jiangsu Province (19KJB480004), Prospective Joint Research Project of Jiangsu Province (NO. BY2012091), and Sinopec Joint Research and Development Project (NO. 411024).
Institutional Review Board Statement
The author agrees to publication in the energies and also to the publication of the article by in MDPI’s corresponding English-language journal.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and materials generated or analysed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lovás, P.; Hudec, P.; Hadvinová, M.; Ház, A. Use of ZSM-5 catalyst in deoxygenation of waste cooking oil. Chem. Pap. 2015, 69, 1454–1464. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Dian, C.; Schenkel, J.V.D.; de Castilhos, Z. Biodiesel production from soybean oil and dimethyl carbonate catalyzed by potassium methoxide. Fuel 2018, 212, 101–107. [Google Scholar]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Emori, E.Y.; Hirashima, F.H.; Zandonai, C.H.; Ortiz-Bravo, C.A. Fluid catalytic cracking of soybean oil using ZSM5 zeolite. Olsen-Scaliante. Catal. Today 2017, 279, 168–176. [Google Scholar] [CrossRef]
- Katikaneni, S.P.R.; Adjaye, J.D.; Idem, R.O.; Bakhshi, N.N. Catalytic Conversion of Canola Oil over Potassium-Impregnated HZSM-5 Catalysts: C2−C4 Olefin Production and Model Reaction Studies. Ind. Eng. Chem. 1996, 35, 3332–3346. [Google Scholar] [CrossRef]
- Naranov, E.R.; Dement’Ev, K.I.; Gerzeliev, I.M.; Kolesnichenko, N.V.; Maksimov, A.L. The role of zeolite catalysis in modern petroleum refining: Contribution from domestic technologies. Petrol Chem. 2019, 59, 247–261. [Google Scholar] [CrossRef]
- Li, L.; Quan, K.; Xu, J.; Liu, F.; Liu, S.; Yu, S.; Xie, C.; Zhang, B.; Ge, X. Liquid hydrocarbon fuels from fluid catalytic cracking of rubber seed oil using USY as catalyst. Fuel 2014, 123, 189–193. [Google Scholar] [CrossRef]
- Li, L.; Quan, K.; Xu, J.; Liu, F.; Ge, X. Liquid Hydrocarbon Fuels from Fluid catalytic cracking of Waste Cooking Oils Using Basic Mesoporous Molecular Sieves K2O/Ba-MCM-41 as Catalysts. ACS Sustain. Chem. Eng. 2013, 1, 412–1416. [Google Scholar] [CrossRef]
- Kuchonthara, P.; Puttasawat, B.; Piumsomboon, P.; Mekasut, L.; Vitidsant, T. Catalytic steam reforming of biomass-derived tar for hydrogen production with K2CO3/NiO/γ-Al2O3, catalyst. Korean J. Chem. Eng. 2012, 29, 1525–1530. [Google Scholar]
- Dandik, L.; Aksoy, H.A. Pyrolysis of used sunflower oil in the presence of sodium carbonate by using fractionating pyrolysis reactor. Fuel Process. Technol. 1998, 57, 81–92. [Google Scholar] [CrossRef]
- Ngo, T.A.; Kim, R.; Kim, R.K.; Kim, R.S. Pyrolysis of soybean oil with H-ZSM5 (Proton-exchange of Zeolite Socony Mobil #5) and MCM41 (Mobil Composition of Matter No. 41) catalysts in a fixed-bed reactor. Energy 2010, 35, 2723–2728. [Google Scholar]
- Ishihara, A.; Tsukamoto, T.; Hashimoto, T.; Nasu, H. Fluid catalytic cracking of soybean oil by ZSM-5 zeolite-containing silica-aluminas with three layered micro-meso-meso-structure. Catal. Today 2017, 303, 123–129. [Google Scholar] [CrossRef]
- Ishihara, A.; Ishida, R.; Ogiyama, T.; Nasu, H.; Hashimoto, T. Dehydrocyclization-cracking reaction of soybean oil using zeolite-metal oxide composite-supported PtNiMo sulfided catalysts. Fuel Process. Technol. 2017, 161, 17–22. [Google Scholar] [CrossRef]
- Grecco, S.D.T.F.; Carvalho, D.R.D.; Zandonai, C.H.; Machado, N.R.C.F.; Lião, L.M.; González, E.A.U. Fluid catalytic cracking of crude soybean oil on Beta nanozeolites. J. Mol. Catal. A-Chem. 2016, 422, 89–102. [Google Scholar] [CrossRef]
- Katikaneni, S.P.R.; Adjaye, J.D.; Idem, R.O.; Bakhshi, N.N. Performance studies of various cracking catalysts in the conversion of canola oil to fuels and chemicals in a fluidized-bed reactor. J. Am. Oil Chem. Soc. 1998, 75, 381–391. [Google Scholar] [CrossRef]
- Graça, I.; Ribeiro, F.R.; Cerqueira, H.S.; Lam, Y.L.; Almeida, M.B.B.D. Fluid catalytic cracking of mixtures of model bio-oil compounds and gasoil. Appl. Catal. B Environ. 2009, 90, 556–563. [Google Scholar] [CrossRef]
- Pinho, A.D.R.; Almeida, M.B.B.D.; Mendes, F.L.; Casavechia, L.C.; Talmadge, M.S.; Kinchin, C.M.; Chum, H.L. Fast pyrolysis oil from pinewood chips co-processing with vacuum gas oil in an FCC unit for second generation fuel production. Fuel 2017, 188, 462–473. [Google Scholar] [CrossRef]
- Ibarra, Á.; Veloso, A.; Bilbao, J.; Arandes, J.M.; Castano, P. Dual coke deactivation pathways during the fluid catalytic cracking of raw bio-oil and vacuum gasoil in FCC conditions. Appl. Catal. B Environ. 2016, 182, 336–346. [Google Scholar] [CrossRef]
- Vitolo, S.; Bresci, B.; Seggiani, M. Catalytic upgrading of pyrolytic oils over HZSM-5 zeolite: Behaviour of the catalyst when used in repeated upgrading–regenerating cycles. Fuel 2001, 80, 17–26. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Andrés, T.A.; Atutxa, A.; Valle, B.; Bilbao, J. Undesired components in the transformation of biomass pyrolysis oil into hydrocarbons on an HZSM-5 zeolite catalyst. J. Chem. Technol. Biothnol. 2010, 80, 1244–1251. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Katikaneni, S.P.R.; Bakhshi, N.N. Catalytic conversion of a biofuel to hydrocarbons: Effect of mixtures of HZSM-5 and silica-alumina catalysts on product distribution. Fuel Process. Technol. 1996, 48, 115–143. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Bakhshi, N.N. Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil. Part II: Comparative catalyst performance and reaction pathways. Fuel Process. Technol. 1995, 45, 185–202. [Google Scholar] [CrossRef]
- Graça, I.; Lopes, J.M.; Cerqueira, H.S.; Ribeiro, M.F. Bio-oils Upgrading for Second Generation Biofuels. Ind. Eng. Chem. Res. 2013, 52, 275–287. [Google Scholar] [CrossRef]
- Shi, J.; Ma, Y.; Li, S.Y.; Wu, J.X.; Zhu, Y.K.; Teng, J.S. Characteristics of Estonian oil shale kerogen and its pyrolysates with thermal bitumen as a pyrolytic intermediate. Energy Fuels 2017, 31, 4808–4816. [Google Scholar] [CrossRef]
- Shi, J.; Ma, Y.; Li, S.Y.; Zhang, L. Characteristics of Thermal Bitumen Structure as the Pyrolysis Intermediate of Longkou Oil Shale. Energy Fuels 2017, 31, 10535–10544. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Barta-Rajnai, E.; Bozi, J.; Blazsó, M.; Jakab, E.; Miskolczib, N.; Sójab, J.; Czégénya, Z. Thermo-catalytic pyrolysis of biomass and plastic mixtures using HZSM-5. Appl. Energy 2017, 207, 114–122. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Liu, C.; Ma, W.; Yan, B.; Zhang, J. Hydrodeoxygenation of lignin-derived bio-oil using molecular sieves supported metal catalysts: A critical review. Renew. Sustain. Energy Rev. 2017, 71, 296–308. [Google Scholar] [CrossRef]
- Leng, T.Y.; Mohamed, A.R.; Bhatia, S. Catalytic conversion of palm oil to fuels and chemicals. Can. J. Chem. Eng. 2010, 77, 156–162. [Google Scholar] [CrossRef]
- Roldugina, E.A.; Naranov, E.R.; Maximov, A.L.; Karakhanov, E.A. Hydrodeoxygenation of guaiacol as a model compound of bio-oil in methanol over mesoporous noble metal catalysts. Appl. Catal. A Gen. 2018, 553, 24–35. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Shanks, B.H.; Brown, R.C. Catalytic conversion of carbohydrate-derived oxygenates over HZSM-5 in a tandem micro-reactor system. Green Chem. 2014, 17, 557–564. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Ding, K.; Zhang, B.; Deng, A.; Min, M. Successive desilication and dealumination of HZSM-5 in catalytic conversion of waste cooking oil to produce aromatics. Energy Convers. Manag. 2017, 147, 100–107. [Google Scholar] [CrossRef]
- Chao, W.; Liu, B.; Zhang, R.; Gu, T.; Ji, X.; Zhong, L.; Chen, G.; Ma, L.; Chen, Z.; Li, X. Co-upgrading of raw bio-oil with kitchen waste oil through fluid catalytic cracking (FCC). Appl. Energy 2018, 217, 233–240. [Google Scholar]
- Ong, Y.K.; Bhatia, S. The current status and perspectives of biofuel production via catalytic cracking of edible and non-edible oils. Energy 2010, 35, 111–119. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, Y.; Li, X.; Yu, N.; Yin, H. Catalytic upgrading of pyrolytic vapors from the vacuum pyrolysis of rape straw over nanocrystalline HZSM-5 zeolite in a two-stage fixed-bed reactor. J. Anal. Appl. Pyrol. 2014, 108, 185–195. [Google Scholar] [CrossRef]
- Trabelsi, A.B.H.; Kraiem, T.; Naoui, S.; Belayouni, H. Pyrolysis of waste animal fats in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. Waste Manag. 2014, 34, 210–218. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).