Effect of WLTP CLASS 3B Driving Cycle on Lithium-Ion Battery for Electric Vehicles
Abstract
:1. Introduction
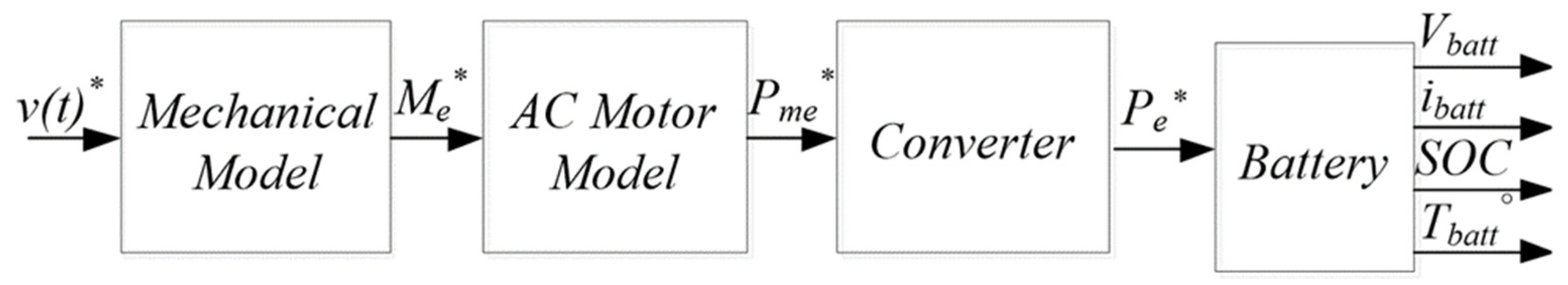
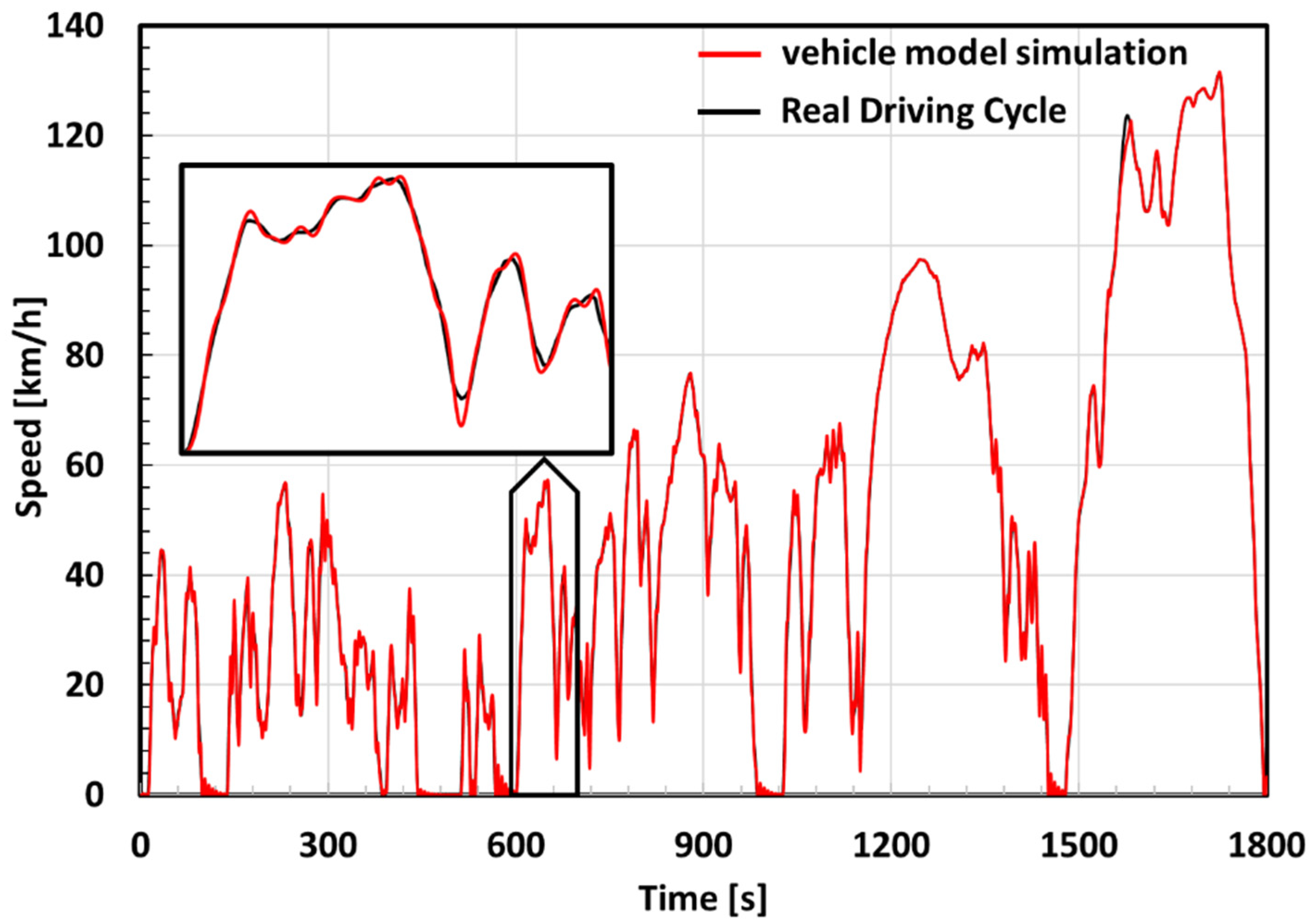
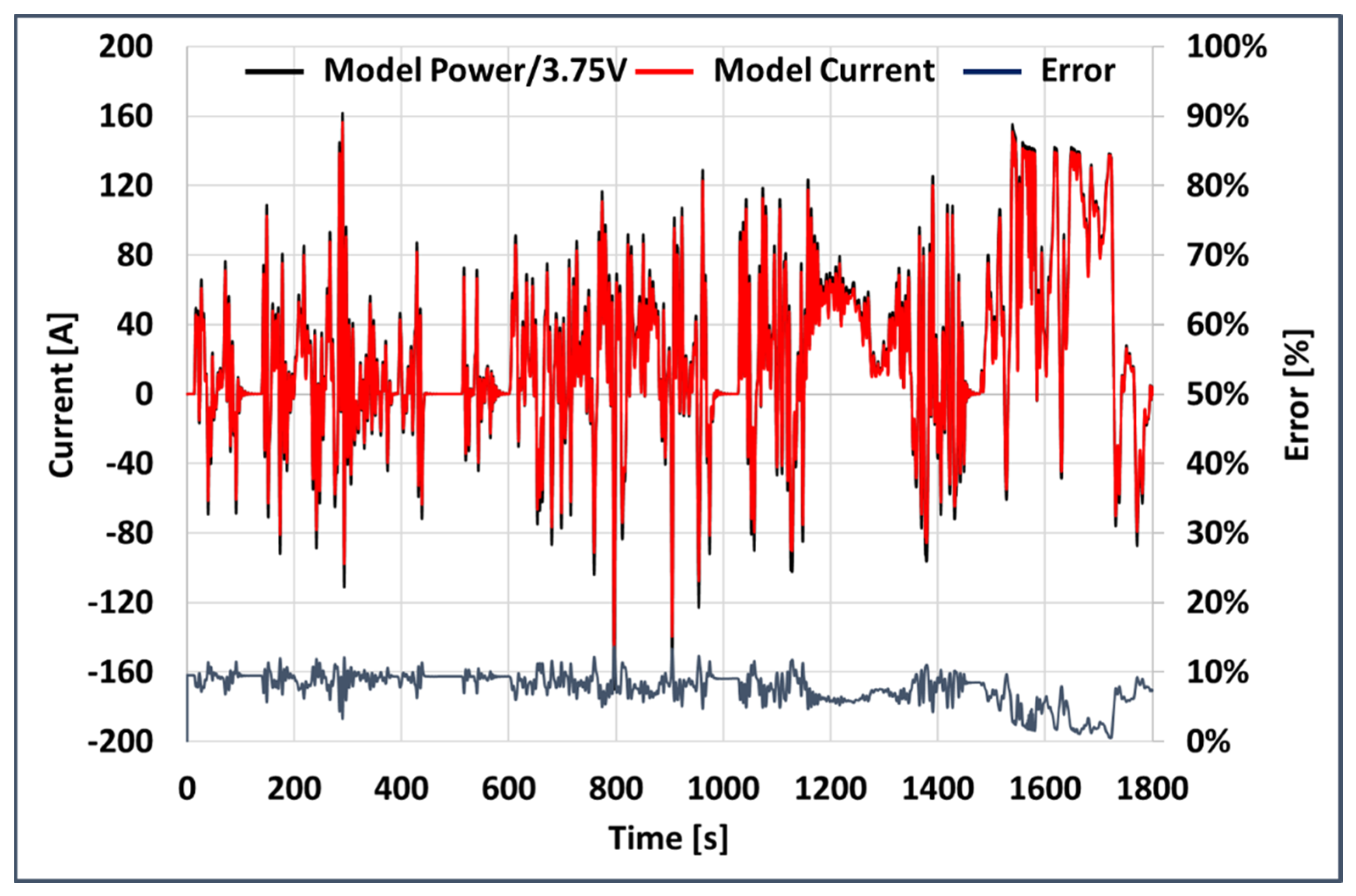
- A dynamic model [22] of an Electric Vehicle was used for the estimation of a current profile delivery by, or supplied to, the battery pack.
- The current profile extracted from the WLTP CLASS 3B driving cycle simulation was used on a commercial electrochemical cell used for automotive applications.
- Test data were analyzed in order to understand degradation effects to identify capacity loss, resistance increase, and ageing phenomena using IC and DV curves.
- A mathematical model for capacitance loss from cycle ageing and calendar ageing was extracted.
- The model was used to estimate battery ageing over possible vehicle operating scenarios.
2. Materials and Methods
2.1. Lithium Ion Cell Characteristics
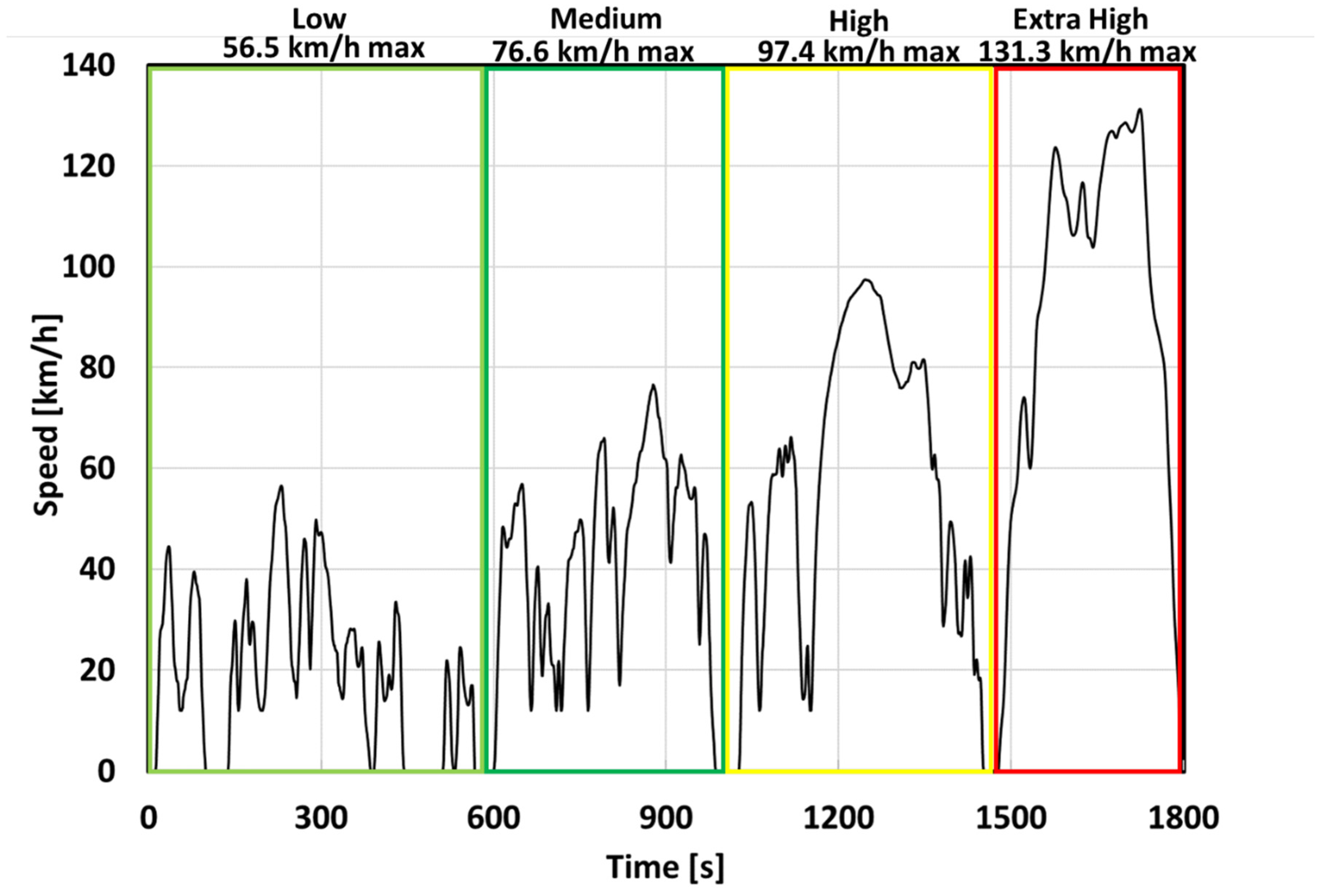
2.2. EV Mission Profile
- Class 1—low power vehicles with PMR ≤ 22;
- Class 2—vehicles with 22 < PMR ≤ 34;
- Class 3—high-power vehicles with PMR > 34;
2.3. Test Procedures
2.4. Test Facilities
2.5. Test Schedule
3. Results and Discussion
3.1. Cells Inspection and Beginning of Life (BOL) Conditions
3.2. Cells Cycling under Current Driving Cycle
3.3. Lifetime Modeling
4. Conclusions
- A reduction in available cell capacity of 11% after 8 test years from BOL condition is found and it does not cause a large increase in the internal resistance.
- IC curve shows three different peaks mainly related to LLI and LAM of the negative electrode. Important variations are observed on the first peak, which dropped almost 36% and shifting of 5 mV to higher potential in comparison to the fresh cell after ageing.
- DV analysis shows an alteration on the lithium storage capability of the graphite anode, then it is representative of anode degradation due to LAM. Q2 changing is indicative of possible LLI side reaction. An increment of peaks is noted in the first year; this evolution of peak magnitude has been related to an initial enhancement of the HLD of the cell and then to a change in graphite material or microstructure.
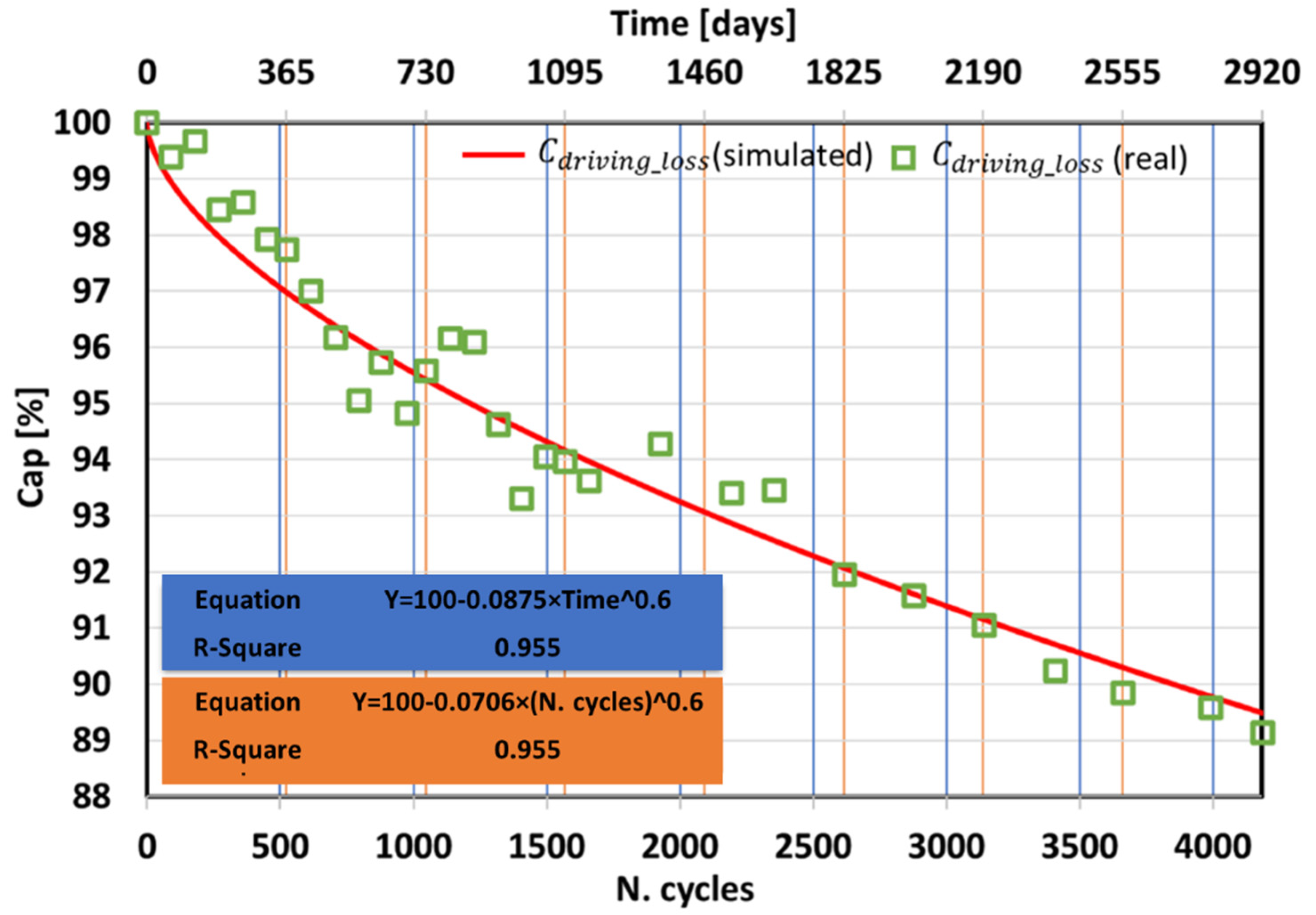
- From real data coming from the WLTP CLASS 3B driving cycle a mathematical model for capacitance loss from cycle ageing was extracted.
- Real data retrieved from a literature article [51] were analyzed and a mathematical curve dependent on temperature and SOC were extracted to estimate the calendar ageing.
- Total ageing was computed as the sum of the calendar and driving cycle contributions for 8 years, and different Scenarios were then evaluated in order to predict the capacity loss.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Parliament. New EU Regulatory Framework for Batteries Setting Sustainability Requirements. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2021/689337/EPRS_BRI(2021)689337_EN.pdf (accessed on 10 March 2022).
- Hume, N.; Terazono, E.; Wilson, T. European Gas Prices Soar and Oil Tops $105 after Russia Attacks Ukraine. Financial Times. Available online: https://www.ft.com/content/c6303127-5edf-4256-9c25-effa75766002 (accessed on 24 February 2022).
- Duh, Y.-S.; Sun, Y.; Lin, X.; Zheng, J.; Wang, M.; Wang, Y.; Lin, X.; Jiang, X.; Zheng, Z.; Zheng, S.; et al. Characterization on thermal runaway of commercial 18650 lithium-ion batteries used in electric vehicles: A review. J. Energy Storage 2021, 41, 102888. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Theng, J.-H.; Chen, C.-C.; Kao, C.-S. Comparative study on thermal runaway of commercial 14500, 18650 and 26650 LiFePO4 batteries used in electric vehicles. J. Energy Storage 2020, 31, 101580. [Google Scholar] [CrossRef]
- Verma, A.; Rakshit, D. Performance analysis of PCM-fin combination for heat abatement of Li-ion battery pack in electric vehicles at high ambient temperature. Therm. Sci. Eng. Prog. 2022, 32, 101314. [Google Scholar] [CrossRef]
- Eboli, L.; Mazzulla, G.; Pungillo, G. How drivers’ characteristics can affect driving style. Transp. Res. Procedia 2017, 27, 945–952. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Lin, C.; Tang, A.; Mu, H.; Wang, W.; Wang, C. Aging Mechanisms of Electrode Materials in Lithium-Ion Batteries for Electric Vehicles. J. Chem. 2015, 2015, 104673. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. A Mathematical Model of Stress Generation and Fracture in Lithium Manganese Oxide. J. Electrochem. Soc. 2006, 153, A1019. [Google Scholar] [CrossRef]
- Zhou, W. Effects of external mechanical loading on stress generation during lithiation in Li-ion battery electrodes. Electrochimica Acta 2015, 185, 28–33. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Gailani, A.; Mokidm, R.; El-Dalahmeh, M.; El-Dalahmeh, M.; Al-Greer, M. Analysis of Lithium-ion Battery Cells Degradation Based on Different Manufacturers. In Proceedings of the 2020 55th International Universities Power Engineering Conference (UPEC), Turin, Italy, 1–4 September 2020. [Google Scholar] [CrossRef]
- Su, L.; Wu, M.; Li, Z.; Zhang, J. Cycle life prediction of lithium-ion batteries based on data-driven methods. eTransportation 2021, 10, 100137. [Google Scholar] [CrossRef]
- Svens, P.; Smith, A.J.; Groot, J.; Lacey, M.J.; Lindbergh, G.; Lindstrom, R.W. Evaluating Performance and Cycle Life Improvements in the Latest Generations of Prismatic Lithium-Ion Batteries. IEEE Trans. Transp. Electrif. 2022, 8, 3696–3706. [Google Scholar] [CrossRef]
- Gandoman, F.H.; Jaguemont, J.; Goutam, S.; Gopalakrishnan, R.; Firouz, Y.; Kalogiannis, T.; Omar, N.; Van Mierlo, J. Concept of reliability and safety assessment of lithium-ion batteries in electric vehicles: Basics, progress, and challenges. Appl. Energy 2019, 251, 113343. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Choi, D.; Wang, D.; Viswanathan, V.V.; Bae, I.-T.; Wang, W.; Nie, Z.; Zhang, J.-G.; Graff, G.L.; Liu, J.; Yang, Z.; et al. Li-ion batteries from LiFePO4 cathode and anatase/graphene composite anode for stationary energy storage. Electrochem. Commun. 2010, 12, 378–381. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-Ion Battery Storage for the Grid—A Review of Stationary Battery Storage System Design Tailored for Applications in Modern Power Grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- Micari, S.; Foti, S.; Testa, A.; De Caro, S.; Sergi, F.; Andaloro, L.; Aloisio, D.; Napoli, G. Reliability assessment and lifetime prediction of Li-ion batteries for electric vehicles. Electr Eng. 2022, 104, 165–177. [Google Scholar] [CrossRef]
- Stroe, D.-I.; Schaltz, E. Lithium-Ion Battery State-of-Health Estimation Using the Incremental Capacity Analysis Technique. IEEE Trans. Ind. Appl. 2020, 56, 678–685. [Google Scholar] [CrossRef]
- Orecchini, F.; Santiangeli, A.; Zuccari, F. Real Drive Well-to-Wheel Energy Analysis of Conventional and Electrified Car Powertrains. Energies 2020, 13, 4788. [Google Scholar] [CrossRef]
- Luigi, F.; Tarsitano, D. Modeling of Full Electric and Hybrid Electric Vehicles. In New Generation of Electric Vehicles; Chapter 7; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Technical Specifications. BMW i3 (120 Ah). Available online: https://www.press.bmwgroup.com/global/article/attachment/T0284828EN/415571 (accessed on 12 December 2020).
- Ahern, A.; Weyman, G.; Redelbach, M. Analysis of National Travel Statistics in Europe. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC83304/tch-d2.1_final.pdf (accessed on 3 July 2022).
- Hemavathi, S.; Shinisha, A. A study on trends and developments in electric vehicle charging technologies. J. Energy Storage 2022, 52, 105013. [Google Scholar] [CrossRef]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; He, R.; Gao, X.; Li, X.; Wang, H.; Liu, X.; Yan, X.; Yang, S. Degradation identification of LiNi0.8Co0.1Mn0.1O2/graphite lithium-ion batteries under fast charging conditions. Electrochim. Acta 2021, 392, 138979. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Yu, T.F.; Widanage, W.D.; Marco, J. Critical review of non-invasive diagnosis techniques for quantification of degradation modes in lithium-ion batteries. Renew. Sustain. Energy Rev. 2019, 109, 138–159. [Google Scholar] [CrossRef]
- Li, Y.; Abdel-Monem, M.; Gopalakrishnan, R.; Berecibar, M.; Nanini-Maury, E.; Omar, N.; van den Bossche, P.; Van Mierlo, J. A quick on-line state of health estimation method for Li-ion battery with incremental capacity curves processed by Gaussian filter. J. Power Sources 2018, 373, 40–53. [Google Scholar] [CrossRef]
- Leonardi, S.G.; Aloisio, D.; Brunaccini, G.; Stassi, A.; Ferraro, M.; Antonucci, V.; Sergi, F. Investigation on the ageing mechanism for a lithium-ion cell under accelerated tests: The case of primary frequency regulation service. J. Energy Storage 2021, 41, 102904. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Cugnet, M.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part I: Initial characterizations. J. Power Sources 2011, 196, 10328–10335. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Synthesize battery degradation modes via a diagnostic and prognostic model. J. Power Sources 2012, 219, 204–216. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; Marco, J. A Comparison between Electrochemical Impedance Spectroscopy and Incremental Capacity-Differential Voltage as Li-ion Diagnostic Techniques to Identify and Quantify the Effects of Degradation Modes within Battery Management Systems. J. Power Sources 2017, 360, 301–318. [Google Scholar] [CrossRef]
- Krupp, A.; Ferg, E.; Schuldt, F.; Derendorf, K.; Agert, C. Incremental Capacity Analysis as a State of Health Estimation Method for Lithium-Ion Battery Modules with Series-Connected Cells. Batteries 2021, 7, 2. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of commercial lithium-ion cells based on composite positive electrode for plug-in hybrid electric vehicle applications. Part II. Degradation mechanism under 2C cycle aging. J. Power Sources 2011, 196, 10336–10343. [Google Scholar] [CrossRef]
- Fath, J.P.; Dragicevic, D.; Bittel, L.; Nuhic, A.; Sieg, J.; Hahn, S.; Alsheimer, L.; Spier, B.; Wetzel, T. Quantification of aging mechanisms and inhomogeneity in cycled lithium-ion cells by differential voltage analysis. J. Energy Storage 2019, 25, 100813. [Google Scholar] [CrossRef]
- Gyenes, B.; Stevens, D.A.; Chevrier, V.; Dahn, J.R. Understanding Anomalous Behavior in Coulombic Efficiency Measurements on Li-Ion Batteries. J. Electrochem. Soc. 2014, 162, A278–A283. [Google Scholar] [CrossRef]
- Keil, P.; Schuster, S.F.; Wilhelm, J.; Travi, J.; Hauser, A.; Karl, R.C.; Jossen, A. Calendar Aging of Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1872–A1880. [Google Scholar] [CrossRef]
- O’Kane, S.E.J.; Ai, W.; Madabattula, G.; Alonso-Alvarez, D.; Timms, R.; Sulzer, V.; Edge, J.S.; Wu, B.; Offer, G.J.; Marinescu, M. Lithium-ion battery degradation: How to model it. Phys. Chem. Chem. Phys. 2022, 24, 7909–7922. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Deshpande, R.D.; Pan, J.; Cheng, Y.-T.; Battaglia, V.S. Electrode Side Reactions, Capacity Loss and Mechanical Degradation in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2026–A2035. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4–Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium ion batteries. Part I: Cycle aging. J. Power Sources 2014, 251, 439–450. [Google Scholar] [CrossRef]
- Smith, A.J.; Svens, P.; Varini, M.; Lindbergh, G.; Lindström, R.W. Expanded In Situ Aging Indicators for Lithium-Ion Batteries with a Blended NMC-LMO Electrode Cycled at Sub-Ambient Temperature. J. Electrochem. Soc. 2021, 168, 110530. [Google Scholar] [CrossRef]
- Lewerenz, M.; Sauer, D.U. Evaluation of cyclic aging tests of prismatic automotive LiNiMnCoO2-Graphite cells considering influence of homogeneity and anode overhang. J. Energy Storage 2018, 18, 421–434. [Google Scholar] [CrossRef]
- Lewerenz, M.; Dechent, P.; Sauer, D.U. Investigation of capacity recovery during rest period at different states-of-charge after cycle life test for prismatic Li(Ni1/3Mn1/3Co1/3)O2-graphite cells. J. Energy Storage 2019, 21, 680–690. [Google Scholar] [CrossRef]
- Sieg, J.; Storch, M.; Fath, J.; Nuhic, A.; Bandlow, J.; Spier, B.; Sauer, D.U. Local degradation and differential voltage analysis of aged lithium-ion pouch cells. J. Energy Storage 2020, 30, 101582. [Google Scholar] [CrossRef]
- Lewerenz, M.; Marongiu, A.; Warnecke, A.; Sauer, D.U. Differential voltage analysis as a tool for analyzing inhomogeneous aging: A case study for LiFePO4|Graphite cylindrical cells. J. Power Sources 2017, 368, 57–67. [Google Scholar] [CrossRef]
- Lewerenz, M.; Münnix, J.; Schmalstieg, J.; Käbitz, S.; Knips, M.; Sauer, D.U. Systematic aging of commercial LiFePO 4 |Graphite cylindrical cells including a theory explaining rise of capacity during aging. J. Power Sources 2017, 345, 254–263. [Google Scholar] [CrossRef]
- Montaru, M.; Fiette, S.; Koné, J.-L.; Bultel, Y. Calendar ageing model of Li-ion battery combining physics-based and empirical approaches. J. Energy Storage 2022, 51, 104544. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Woirgard, E.; Vinassa, J. Remaining useful life prediction of lithium batteries in calendar ageing for automotive applications. Microelectron. Reliab. 2012, 52, 2438–2442. [Google Scholar] [CrossRef]
- Kebede, A.A.; Hosen, S.; Messagie, M.; Behabtu, H.A.; Jemal, T.; Van Mierlo, J.; Coosemans, T.; Berecibar, M. Development of a lifetime model for large format nickel-manganese-cobalt oxide-based lithium-ion cell validated using a real-life profile. J. Energy Storage 2022, 50, 104289. [Google Scholar] [CrossRef]
- Belt, J.; Utgikar, V.; Bloom, I. Calendar and PHEV cycle life aging of high-energy, lithium-ion cells containing blended spinel and layered-oxide cathodes. J. Power Sources 2011, 196, 10213–10221. [Google Scholar] [CrossRef]
- Schmalstieg, J.; Käbitz, S.; Ecker, M.; Sauer, D.U. A holistic aging model for Li(NiMnCo)O2 based 18650 lithium-ion batteries. J. Power Sources 2014, 257, 325–334. [Google Scholar] [CrossRef]
- National Centers for Environmental Information. Past-Weather in Messina, Italy. 2022. Available online: https://www.ncei.noaa.gov/access/past-weather/messina (accessed on 20 July 2022).
- Pevec, D.; Babic, J.; Carvalho, A.; Ghiassi-Farrokhfal, Y.; Ketter, W.; Podobnik, V. Electric Vehicle Range Anxiety: An Obstacle for the Personal Transportation (R)evolution? In Proceedings of the 2019 4th International Conference on Smart and Sustainable Technologies (SpliTech), Split, Croatia, 18–21 June 2019. [Google Scholar] [CrossRef]
- Napoli, G.; Micari, S.; Dispenza, G.; Andaloro, L.; Antonucci, V.; Polimeni, A. Freight distribution with electric vehicles: A case study in Sicily. RES, infrastructures and vehicle routing. Transp. Eng. 2021, 3, 100047. [Google Scholar] [CrossRef]

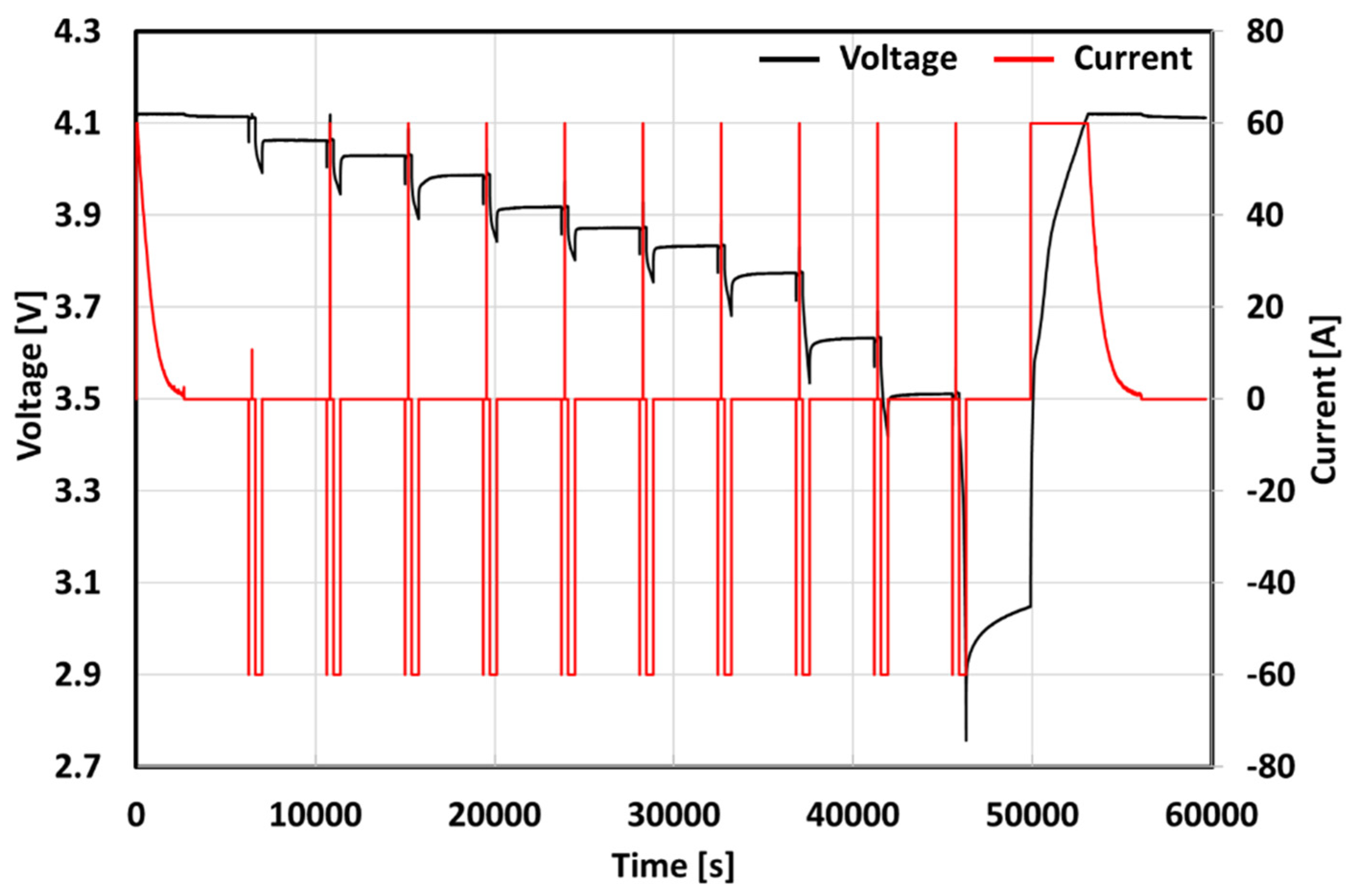

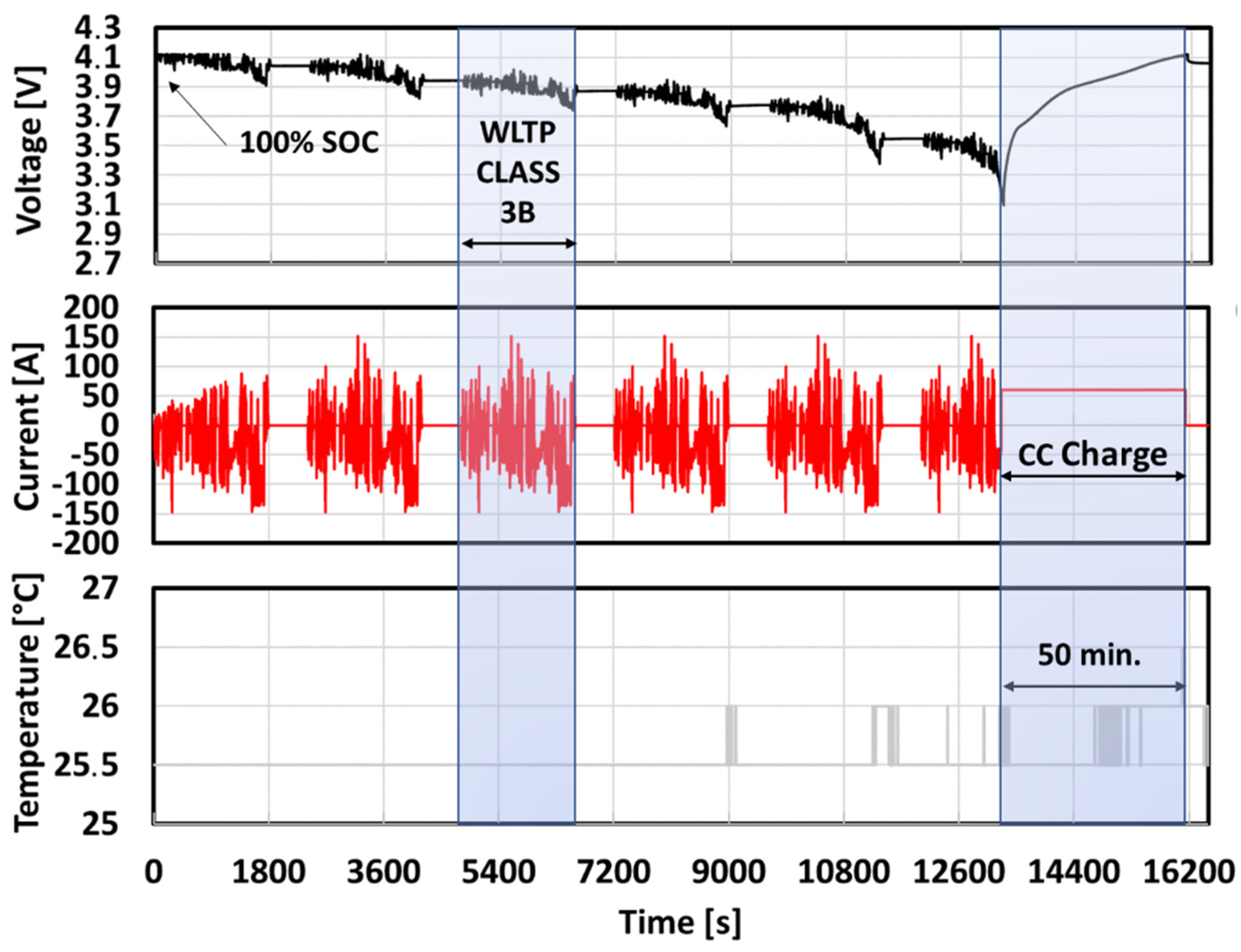





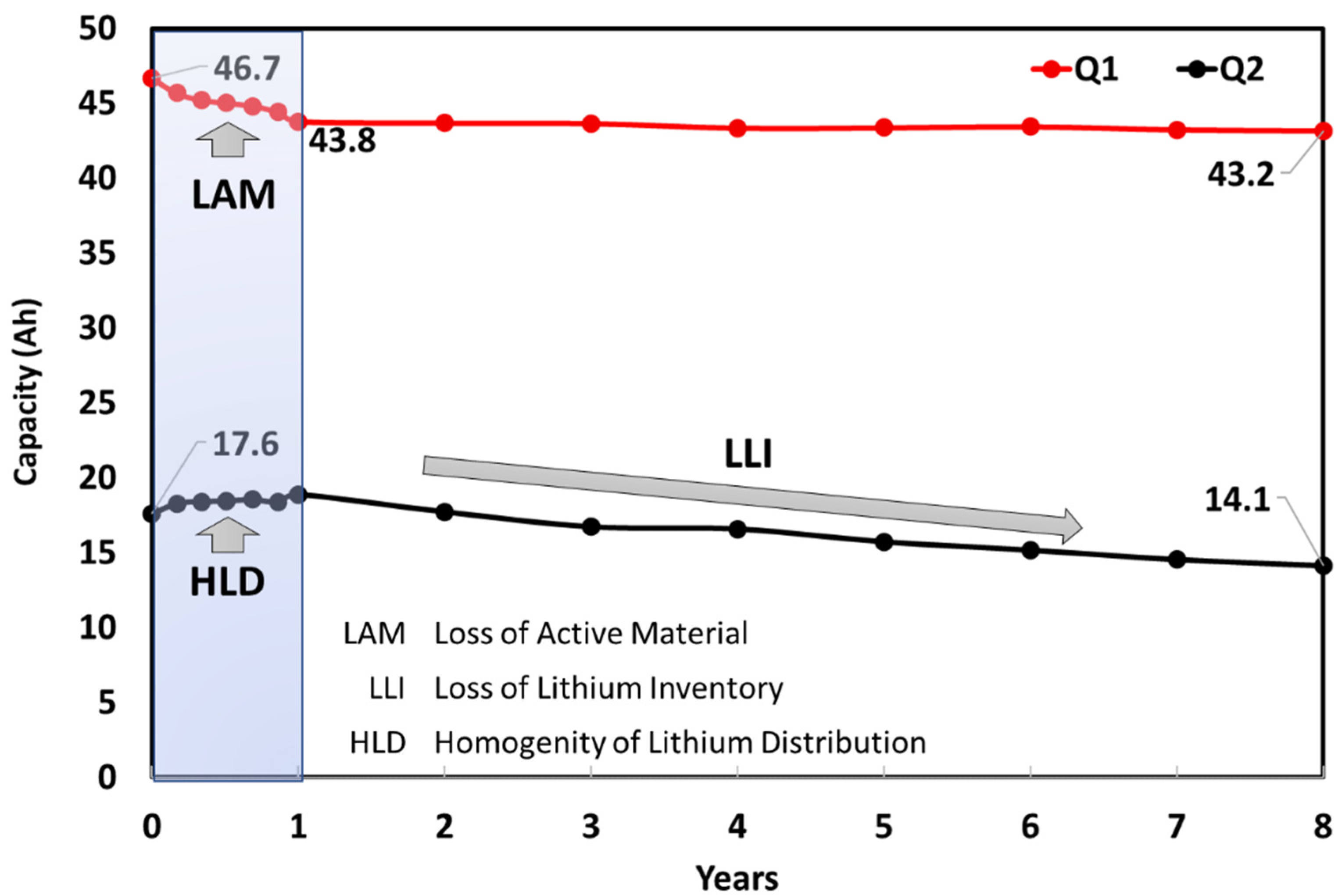

| Cause | Degradation Mechanism | Degradation Mode | Effect |
|---|---|---|---|
| Time High Temperature High Voltage Current Load Load Temperature Stoichiometry Mechanical Stress Low Voltage | SEI growth SEI decomposition Electrolyte decomposition Binder decomposition Graphite Exfoliation Structural Disordering Lithium Plating Dendrite formation Loss of electric contact Electrode particle cracking Transition metal dissolution Corrosion of current collector | Loss of Lithium Inventory Loss of active anode material Loss of active cathode material | Capacity Fade Power Fade |
| Cell Type | Lithium-Ion |
|---|---|
| Chemistry Cathode | LMO-NMC |
| Chemistry Anode | Graphite |
| Capacity | 63 Ah |
| Nominal voltage | 3.75 V |
| Operating voltage | 2.7 V–4.12 V |
| Battery temperature | −40–65 °C |
| Vehicle Type | Battery Electric Vehicle (BEV) |
|---|---|
| Vehicle mass | 1195 kg |
| Frontal area | 2.8 m2 |
| Drag coefficient | 0.29 |
| Rolling friction coefficient | 0.012 |
| Transmission gear ratio | 9.7 |
| Hub diameter | 48 cm |
| Tire width | 15.5 cm |
| Tire aspect ratio | 70% |
| Wheel radius | 25.4 cm |
| Motor type | AC-PMSM |
| Motor rated power | 75 kW |
| Motor rated speed | 4800 rpm |
| Motor pole pairs | 4 |
| Battery pack | 85 cells in series |
| Step | Description | Parameter | Stop Condition |
|---|---|---|---|
| 1 | Temperature conditioning | T = 25 °C | t = 1 h |
| 2 | CC Discharge | I = 63 A | V = 2.7 V |
| 3 | CC-CV Full Charge | I = 63 A CV = 4.2 V | I = 0.2 A |
| 4 | CC Discharge | I = 63 A | V = 2.7 V |
| 5 | Repeat from 3 to 4 | Counter = 3 | |
| 6 | CC-CV Full Charge | I = 63 A CV = 4.2 V | I = 0.2 A |
| Step | Description | Parameter | Stop Condition |
|---|---|---|---|
| 1 | Temperature conditioning | T = 25 °C | t = 1 h |
| 2 | CC-CV Full Charge | I = 63 A CV = 4.2 V | I = 0.2 A |
| 3 | Rest | t = 1 h | |
| 4 | CC Discharge | I = 63 A | t = 10 s V = 2.7 V |
| 5 | Rest | t = 3 min | |
| 6 | CC Charge | I = 63 A | t = 10 sV = 4.12 V |
| 7 | Rest | t = 3 min | |
| 8 | CC Discharge | I = 63 A | Discharge Capacity = 10% Capacity test V = 2.7 V |
| 9 | Rest | t = 1 h | |
| 10 | Repeat from 4 to 9 | Counter = 10 | |
| 11 | Rest | t = 1 h | |
| 12 | CC-CV Full Charge | I = 63 A CV = 4.2 V | I = 0.2 A |
| Description | Characteristics |
|---|---|
| Model | Bitrode cycler FTV-1 1000/100/10–20 |
| DC Voltage Range | 0–20 V (Res. 0.001 V) |
| Maximum DC Current | 1000 A |
| Maximum Data Acquisition Rate | 0.1 s/Data Sample |
| Current Ranges Max | 500 A (single channel) 1000 A (2 channels in parallel) (Res. 1 A) 100 A (Acc. 0.1 A) 10 A (Acc. 0.01 A) |
| Assignable Data Channel Inputs | 2 |
| Thermocouples Type | J |
| Temperature Range | −40 to 200 °C (Res. 0.5 °C) |
| Temperature Tolerance | ±2 °C (for all measured temperatures) |
| Description | Characteristics |
|---|---|
| Model | Angelantoni Discovery 340 L |
| Useful Volume | 337 l |
| External Measurement (L × D × W) | 919 × 1786 × 1765 mm |
| Useful Internal Measurement (L × D × W) | 601 × 810 × 694 mm |
| Temperature Range | −40 to +180 °C |
| Temperature Precision | 0.1 ÷ 0.3 ± K |
| Humidity Range | 10–98% |
| Maximum Internal Thermal Load | 2300 W (+25 °C) |
| Cooling Gas | R404 |
| Additional Temperature sensors | N°5 PT100 |
| Step | Parametric Checks | Driving Cycles | Total Driving Cycle | Total Ageing Test Years |
|---|---|---|---|---|
| 1 | Capacity + Pulse test | 261 | 261 | ½ year |
| 2 | Capacity + Pulse test | 262 | 523 | 1 year |
| 3 | Capacity + Pulse test | 261 | 784 | 1 ½ year |
| 4 | Capacity + Pulse test | 262 | 1046 | 2 year |
| 5 | Capacity + Pulse test | 261 | 1307 | 2 ½ year |
| 6 | Capacity + Pulse test | 262 | 1569 | 3 year |
| 7 | Capacity + Pulse test | 261 | 1830 | 3 ½ year |
| 8 | Capacity + Pulse test | 262 | 2092 | 4 year |
| 9 | Capacity + Pulse test | 261 | 2353 | 4 ½ year |
| 10 | Capacity + Pulse test | 262 | 2615 | 5 year |
| 11 | Capacity + Pulse test | 261 | 2876 | 5 ½ year |
| 12 | Capacity + Pulse test | 262 | 3138 | 6 year |
| 13 | Capacity + Pulse test | 261 | 3399 | 6 ½ year |
| 14 | Capacity + Pulse test | 262 | 3661 | 7 year |
| 15 | Capacity + Pulse test | 261 | 3922 | 7 ½ year |
| 16 | Capacity + Pulse test | 262 | 4184 | 8 year |
| Ageing Tests | Discharge Capacity (Ah) | Discharge Energy (Wh) | Energy Efficiency (%) | |
|---|---|---|---|---|
| Cell 1 | Driving cycle 25 °C | 64.26 | 241.03 | 96.01 |
| A | B | C | |
|---|---|---|---|
| Parameters | 100 | −0.0875 | 0.6 |
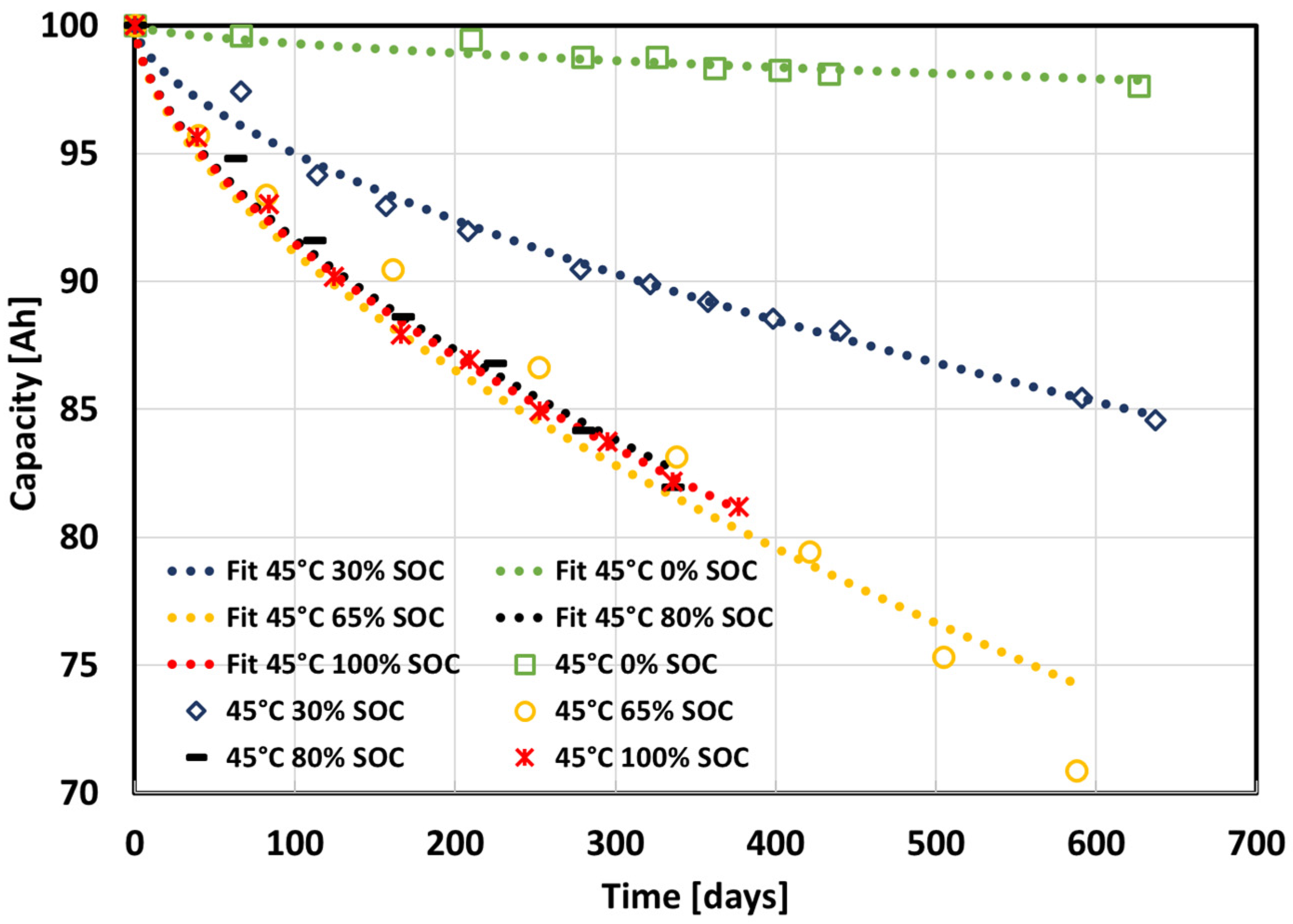
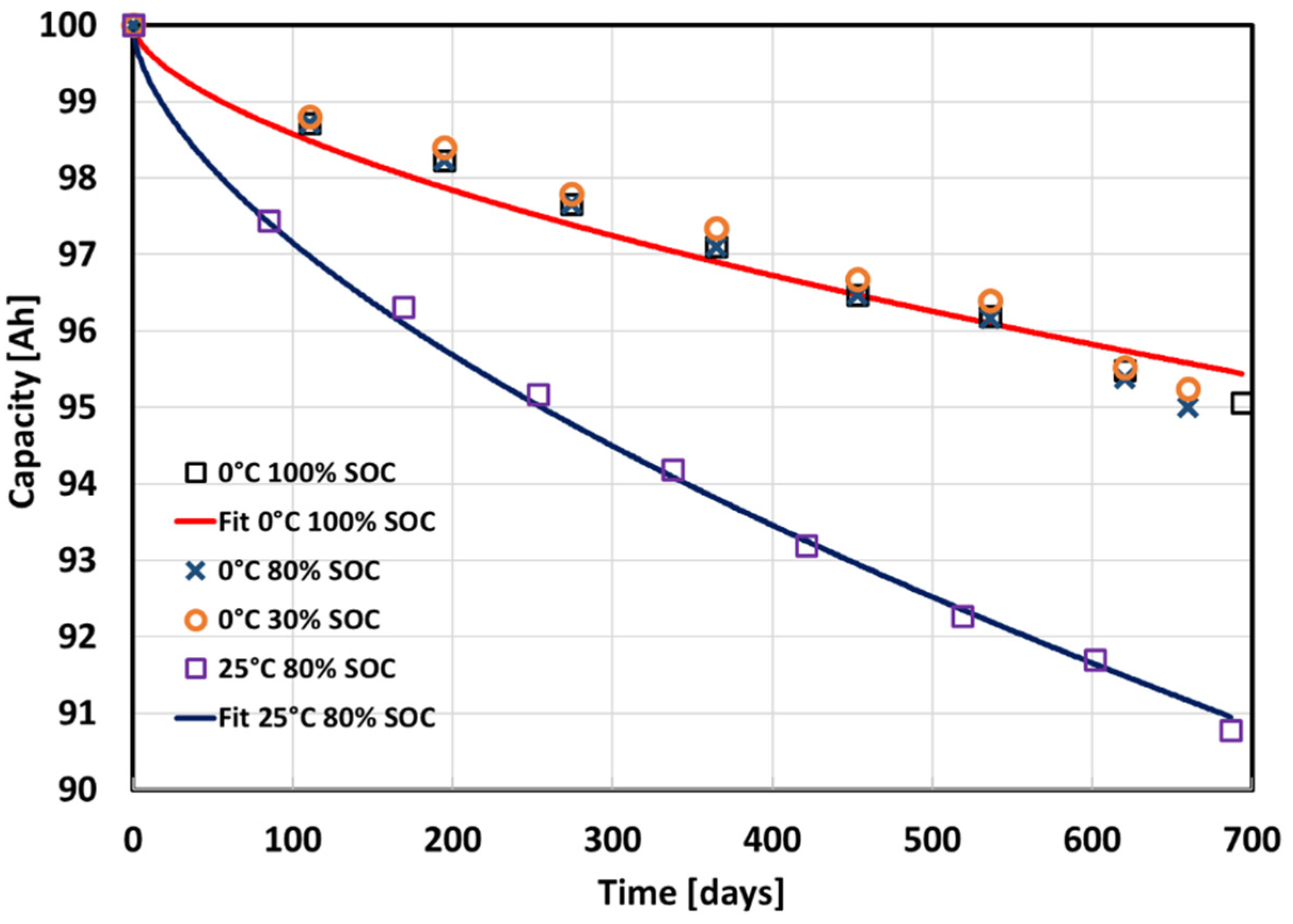
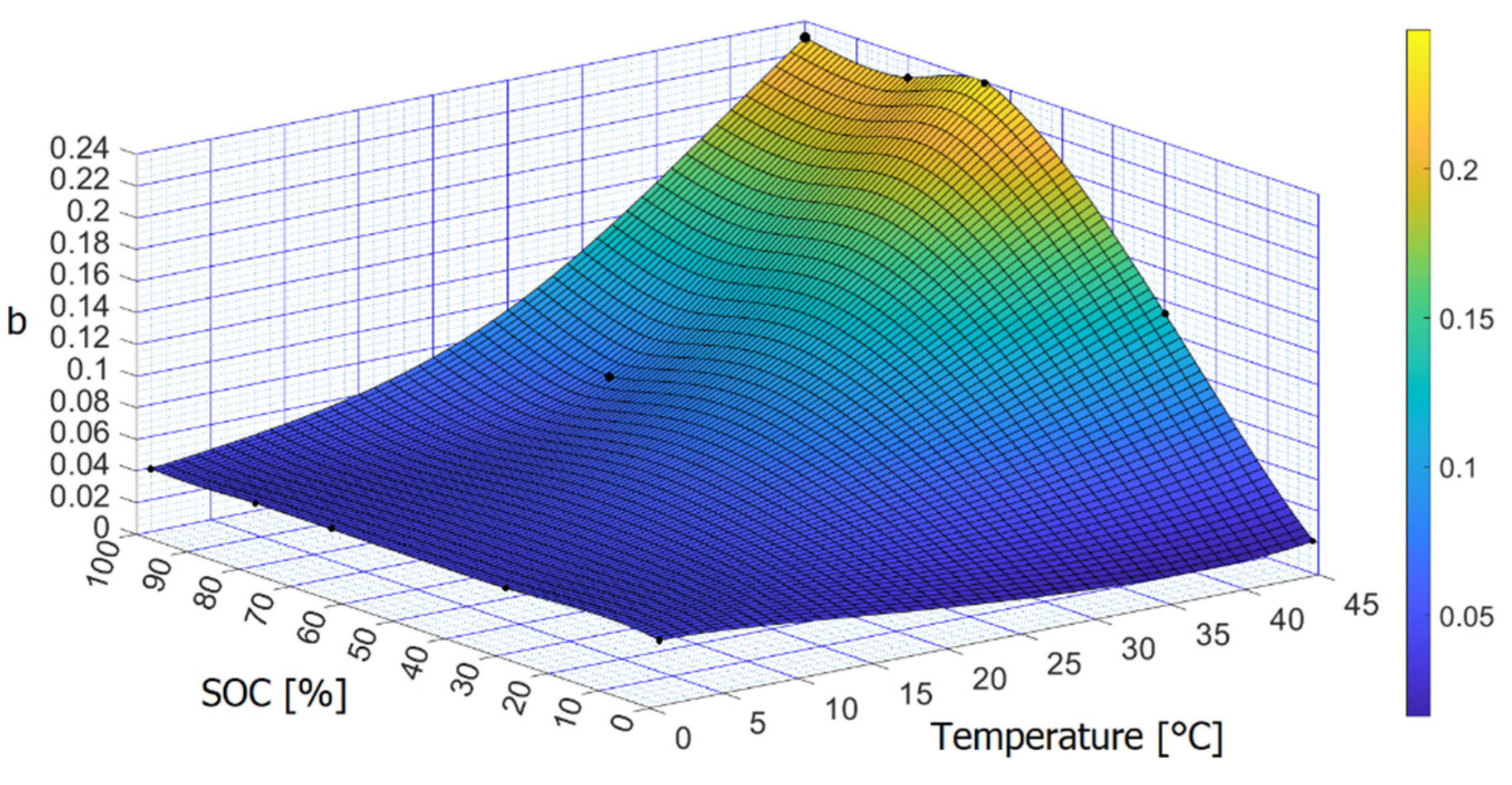
| Temperature [°C] | SOC [%] | a | b | c | R2 |
|---|---|---|---|---|---|
| 0 | 0/30/45/80/100 | 100 | −0.0899 | 0.6 | 0.9692 |
| 25 | 80 | 100 | −0.1796 | 0.6 | 0.9987 |
| 45 | 0 | 100 | −0.0446 | 0.6 | 0.8357 |
| 45 | 30 | 100 | −0.3097 | 0.6 | 0.9829 |
| 45 | 65 | 100 | −0.5609 | 0.6 | 0.9487 |
| 45 | 80 | 100 | −0.5277 | 0.6 | 0.9790 |
| 45 | 100 | 100 | −0.5380 | 0.6 | 0.9941 |
| Weekdays | Driving Cycles | Weekdays | Driving Cycles | |
|---|---|---|---|---|
| WLTP CL. 3B (23.266 km) | 154 | 1 | 53 | 1 |
| 107 | 2 | 51 | 2 | |
| Yearly Distance | 8.562 km | 3.606 km | ||
| Year | |||
|---|---|---|---|
| 0 | 0 | 0 | 100.00 |
| 1 | 5.03 | 3.02 | 91.95 |
| 2 | 2.76 | 1.56 | 87.63 |
| 3 | 2.25 | 1.26 | 84.12 |
| 4 | 1.97 | 1.10 | 81.06 |
| 5 | 1.78 | 0.99 | 78.29 |
| 6 | 1.64 | 0.92 | 75.73 |
| 7 | 1.54 | 0.86 | 73.34 |
| 8 | 1.45 | 0.81 | 71.08 |
| Scenario | Battery Working Temperature [°C] | Ambient Temperature [°C] | Yearly Distance [km] |
|---|---|---|---|
| 1 | 15 | 15 | 12,168 |
| 2 | 35 | 35 | 12,168 |
| 3 | 15 | 15 | 16,984.18 |
| 4 | 15 | 15 | 50,952.54 |
| 5 | 15 | 15 | 101,905.08 |
| 6 | 35 | 35 | 16,984.18 |
| 7 | 35 | 35 | 50,952.54 |
| 8 | 35 | 35 | 101,905.08 |
| Years | Scenario 1 | Scenario 3 | Scenario 4 | Scenario 5 |
|---|---|---|---|---|
| 0 | 100.00 | 100.00 | 100.00 | 100.00 |
| 1 | 93.78 | 93.53 | 91.51 | 89.40 |
| 2 | 90.58 | 90.18 | 87.13 | 83.93 |
| 3 | 87.99 | 87.47 | 83.59 | 79.50 |
| 4 | 85.73 | 85.11 | 80.50 | 75.64 |
| 5 | 83.69 | 82.98 | 77.71 | 72.15 |
| 6 | 81.80 | 81.01 | 75.13 | 68.93 |
| 7 | 80.04 | 79.17 | 72.72 | 65.92 |
| 8 | 78.38 | 77.43 | 70.45 | 63.08 |
| 9 | 76.80 | 75.78 | ||
| 10 | 75.28 | 74.19 | ||
| 11 | 73.83 | 72.68 | ||
| 12 | 72.43 | 71.21 | ||
| 13 | 71.07 | 69.79 | ||
| 14 | 69.76 | |||
| Overall km at 30% capacity loss | 167.189 | 218.328 | 417.392 | 576.810 |
| [%] | 20.23 | 18.56 | 13.13 | 9.51 |
| [%] | 9.77 | 11.44 | 16.87 | 20.49 |
| Years | Scenario 2 | Scenario 6 | Scenario 7 | Scenario 8 |
|---|---|---|---|---|
| 0 | 100.00 | 100.00 | 100.00 | 100.00 |
| 1 | 84.45 | 84.16 | 80.08 | 75.13 |
| 2 | 76.48 | 75.99 | 69.79 | 62.30 |
| 3 | 70.04 | 69.38 | 61.47 | 51.92 |
| 4 | 64.42 | 63.60 | 54.21 | |
| 5 | 59.34 | 58.39 | ||
| 6 | 54.66 | 53.58 | ||
| 7 | 50.28 | |||
| Overall km at 30% capacity loss | 36.550 | 49.230 | 100.788 | 139.316 |
| [%] | 21.28 | 19.59 | 14 | 10.56 |
| [%] | 8.72 | 10.41 | 16 | 19.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micari, S.; Foti, S.; Testa, A.; De Caro, S.; Sergi, F.; Andaloro, L.; Aloisio, D.; Leonardi, S.G.; Napoli, G. Effect of WLTP CLASS 3B Driving Cycle on Lithium-Ion Battery for Electric Vehicles. Energies 2022, 15, 6703. https://doi.org/10.3390/en15186703
Micari S, Foti S, Testa A, De Caro S, Sergi F, Andaloro L, Aloisio D, Leonardi SG, Napoli G. Effect of WLTP CLASS 3B Driving Cycle on Lithium-Ion Battery for Electric Vehicles. Energies. 2022; 15(18):6703. https://doi.org/10.3390/en15186703
Chicago/Turabian StyleMicari, Salvatore, Salvatore Foti, Antonio Testa, Salvatore De Caro, Francesco Sergi, Laura Andaloro, Davide Aloisio, Salvatore Gianluca Leonardi, and Giuseppe Napoli. 2022. "Effect of WLTP CLASS 3B Driving Cycle on Lithium-Ion Battery for Electric Vehicles" Energies 15, no. 18: 6703. https://doi.org/10.3390/en15186703
APA StyleMicari, S., Foti, S., Testa, A., De Caro, S., Sergi, F., Andaloro, L., Aloisio, D., Leonardi, S. G., & Napoli, G. (2022). Effect of WLTP CLASS 3B Driving Cycle on Lithium-Ion Battery for Electric Vehicles. Energies, 15(18), 6703. https://doi.org/10.3390/en15186703






