Possibilities of Biogas Upgrading on a Bio-Waste Sorbent Derived from Anaerobic Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
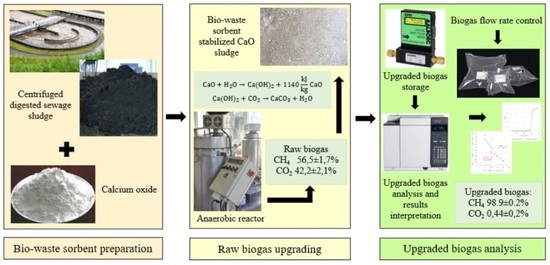
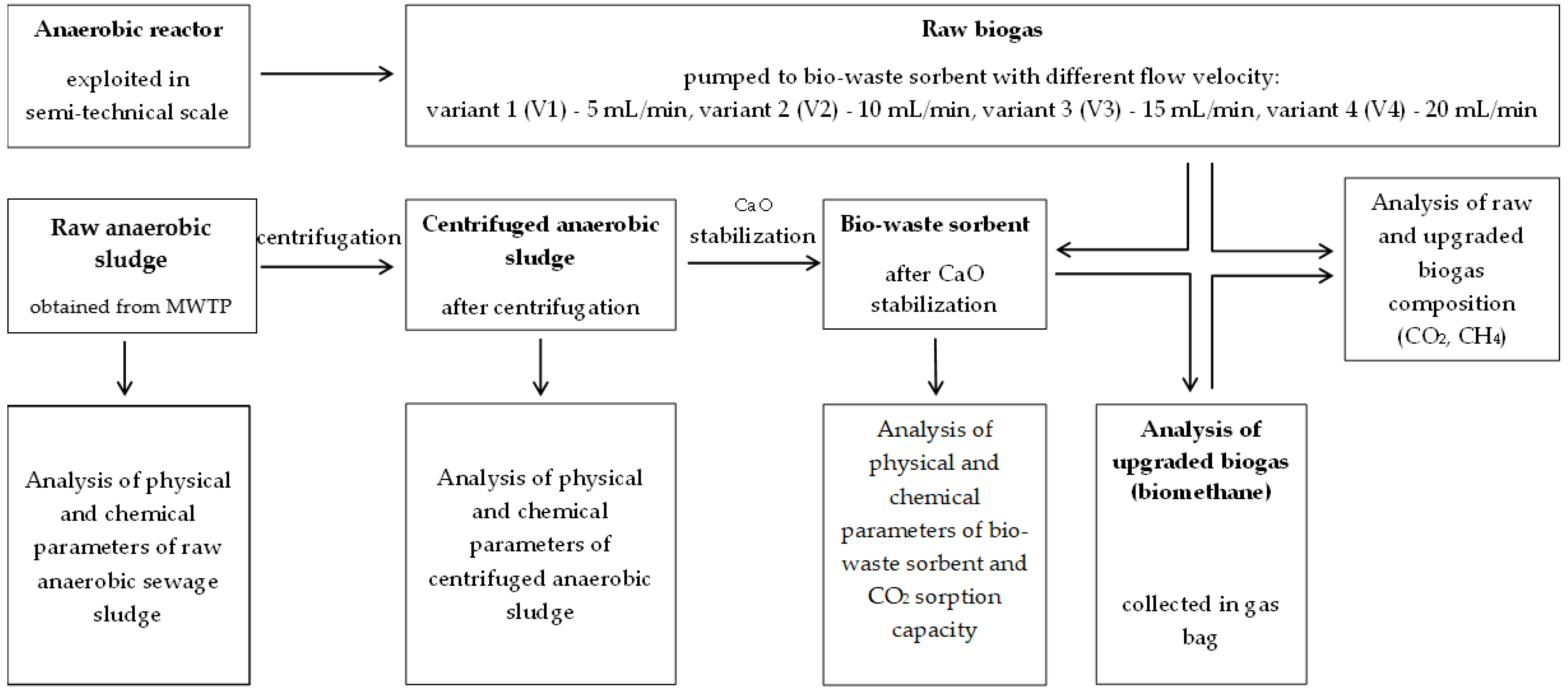
2.1. Study Organization
2.2. Materials
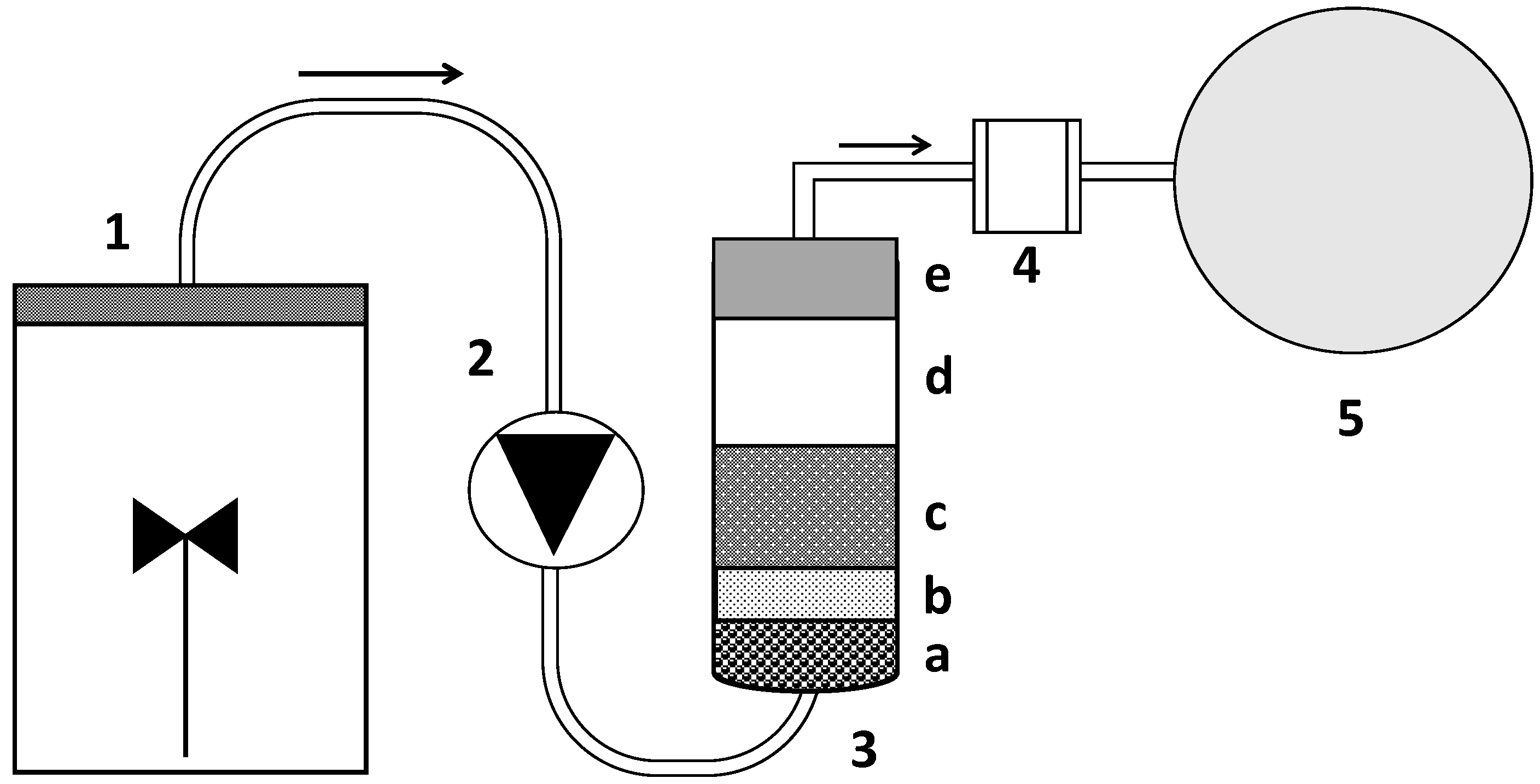
2.3. Fixed-Bed Column Construction and Exploitation
2.4. Analytical Methods
2.5. Calculation Methods
2.6. Statistical Methods
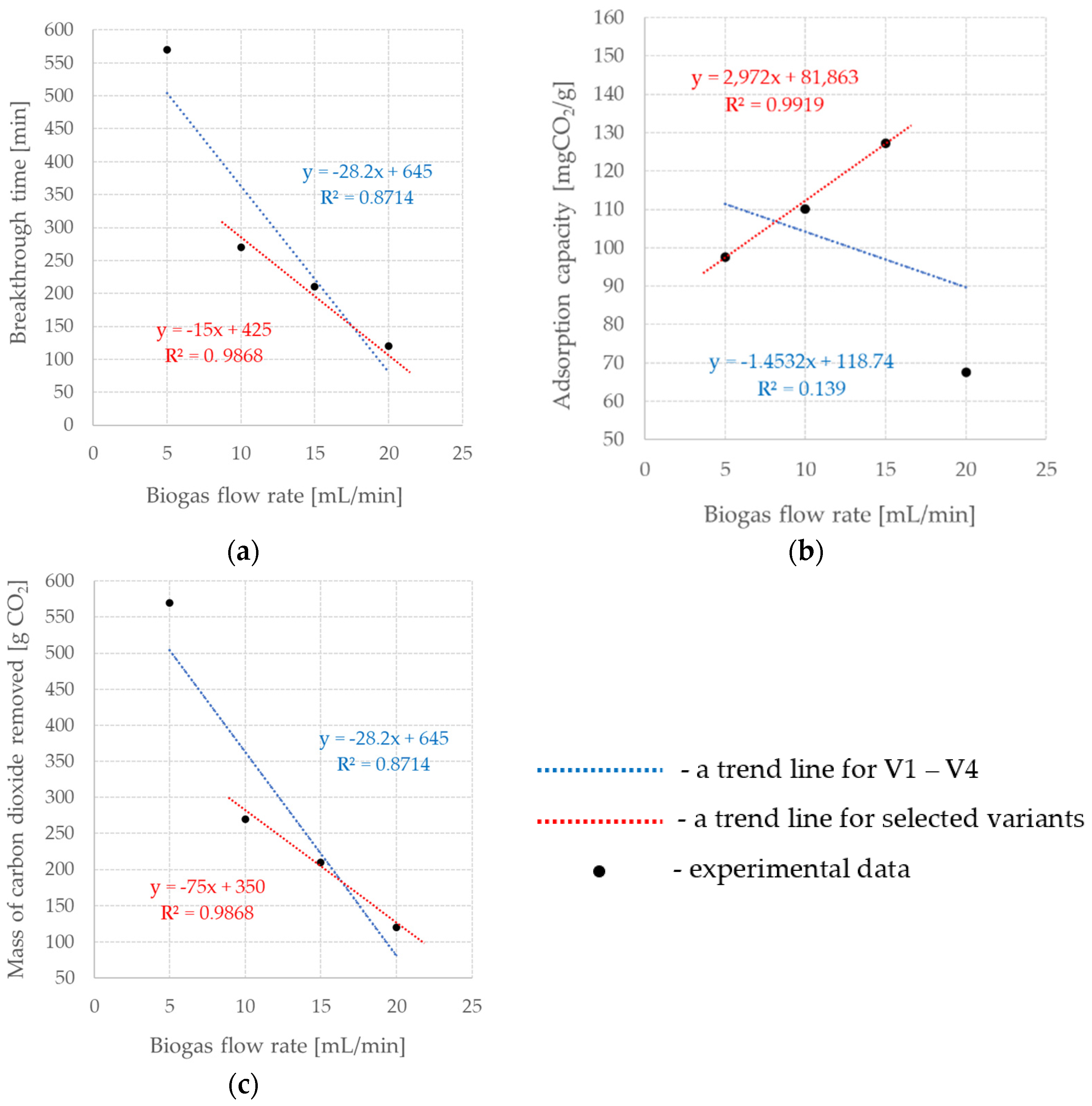
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. 2030 Climate and Energy Framework. Available online: https://ec.europa.eu/clima/eu-action/climate-strategies-targets/2030-climate-energy-framework_en (accessed on 9 June 2022).
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, D.P.; Ok, S.Y.; Tong, W.Y.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Kazimierowicz, J. Organic waste used in agricultural biogas plants. J. Ecol. Eng. 2014, 15, 88–92. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. The Effect of Electromagnetic Microwave Radiation on Methane Fermentation of Selected Energy Crop Species. Processes 2022, 10, 45. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of microwave thermohydrolysis and liquid hot water pretreatment of energy crop Sida hermaphrodita for enhanced methane production. Biomass Bioenergy 2019, 128, 105324. [Google Scholar] [CrossRef]
- Ravindran, R.; Donkor, K.; Gottumukkala, L.; Menon, A.; Guneratnam, A.J.; McMahon, H.; Koopmans, S.; Sanders, J.P.M.; Gaffey, J. Biogas, Biomethane and Digestate Potential of By-Products from Green Biorefinery Systems. Clean Technol. 2022, 4, 35–50. [Google Scholar] [CrossRef]
- European Biogas Association (EBA). Available online: https://www.argusmedia.com/en/news/2294127-european-biomethane-production-hits-record-high-in-2021 (accessed on 9 June 2022).
- Hosseini, S.E.; Wahid, M.A. Development of Biogas Combustion in Combined Heat and Power Generation. Renew. Sustain. Energy Rev. 2014, 40, 868–875. [Google Scholar] [CrossRef]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for Biogas Upgrading to Biomethane: A Review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef]
- Pfau, S.F.; Hagens, J.E.; Dankbaar, B. Biogas between renewable energy and bio-economy policies—Opportunities and constraints resulting from a dual role. Energy Sustain. Soc. 2017, 7, 17. [Google Scholar] [CrossRef]
- Brudermann, T.; Mitterhuber, C.; Posch, A. Agricultural biogas plants—A systematic analysis of strengths, weaknesses, opportunities and threats. Energy Policy 2015, 76, 107–111. [Google Scholar] [CrossRef]
- Lóránt, B.; Tardy, G.M. Current Status of Biological Biogas Upgrading Technologies. Period. Polytech. Chem. Eng. 2022, 66, 465–481. [Google Scholar] [CrossRef]
- European Biogas. Market State and Trends in Renewable and Low-Carbon Gases in Europe, A Gas Climate Report. 2020. Available online: https://www.europeanbiogas.eu/market-state-and-trends-in-renewable-and-low-carbon-gases-in-europe/ (accessed on 6 September 2021).
- Brunetti, A.; Barbieri, G. Membrane Engineering for Biogas Valorization. Front. Chem. Eng. 2021, 26, 59. [Google Scholar] [CrossRef]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R. Biogas upgrading to fuel grade methane using pressure swing adsorption: Parametric sensitivity analysis on an industrial scale. Fuel 2022, 308, 121986. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through Physical Adsorption using Carbonaceous and non-Carbonaceous Adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar] [CrossRef]
- Mulu, E.; M’Arimi, M.M.; Ramkat, R.C. A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sustain. Energy Rev. 2021, 145, 111081. [Google Scholar] [CrossRef]
- Chomiak, K.; Gryglewicz, S.; Kierzek, K.; Machnikowski, J. Optimizing the properties of granular walnut-shell based KOH activated carbons for carbon dioxide adsorption. J. CO2 Util. 2017, 21, 436–443. [Google Scholar] [CrossRef]
- Correia, L.B.; Fiuza, R.A., Jr.; de Andrade, R.C.; Andrade, H.M.C. CO2 capture on activated carbons derived from mango fruit (Mangifera indica L.) seed shells. J. Therm. Anal. Calorim. 2018, 131, 579–586. [Google Scholar] [CrossRef]
- Rattanaphan, S.; Rungrotmongkol, T.; Kongsune, P. Biogas improving by adsorption of CO2 on modified waste tea activated carbon. Renew. Energy 2020, 145, 622–631. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Alobiad, F. CO2 capture from biogas by biomass-based adsorbents: A review. Fuel 2022, 328, 125276. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2021, 14, 150. [Google Scholar] [CrossRef]
- Council Directive 91/271/EEC: Council Directive 91/271/EEC of 21 March 1991 Concerning Urban Waste-Water Treatment (Amended by the 98/15/EC of 27 February 1998). Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31991L0271) (accessed on 11 June 2022).
- Council Directive 86/278/EEC: Council Directive 86/278/EEC of 4 July 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge is Used in Agriculture. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31986L0278) (accessed on 11 June 2022).
- Bratina, B.; Sorgo, A.; Kramberger, J.; Ajdnik, U. From municipal/industrial wastewater sludge and FOG to fertilizer: A proposal for economic sustainable sludge management. J. Environ. Manag. 2016, 183 Pt 3, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, F.; Marcinkowski, T.A. Sewage Sludge Stabilisation with Calcium Hydroxide: Effect on Physicochemical Properties and Molecular Composition. Water Res. 2006, 40, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Wolny-Koładka, K.; Malinowski, M.; Żukowski, W. Impact of Calcium Oxide on Hygienization and Self-Heating Prevention of Biologically Contaminated Polymer Materials. Materials 2020, 13, 4012. [Google Scholar] [CrossRef] [PubMed]
- Famielec, S.; Gliniak, M.; Kapjor, A.; Łukasiewicz, M.; Malinowski, M. Thermographic evaluation of CaO additive on the process of waste hygienization. Stow. Infrastrukt. I Ekol. Teren. Wiej. 2016, IV, 1857–1865. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Sudlitz, M. Zero energy electron beam technology for sludge hygienization. Nukleonika 2019, 64, 55–63. [Google Scholar] [CrossRef]
- Rusanowska, P.; Zieliński, M.; Dudek, M.R.; Dębowski, M. Mechanical Pretreatment of Lignocellulosic Biomass for Methane Fermentation in Innovative Reactor with Cage Mixing System. J. Ecol. Eng. 2018, 19, 219–224. [Google Scholar] [CrossRef]
- Górka, J.Ł.; Cimochowicz-Rybicka, M. Water and sewage sludge co-digestion: Characteristic of the process and its possible applications. Arch. Environ. Prot. 2019, 45, 84–91. [Google Scholar] [CrossRef]
- Kuglarz, M.; Grübel, K.; Bohdziewicz, J. Post-digestion liquor treatment in the method combining chemical precipitation with reverse osmosis. Arch. Environ. Prot. 2014, 40, 29–42. [Google Scholar] [CrossRef]
- Monsalvo, A.; Mohedano, A.F.; Rodriguez, J.J. Adsorption of 4-chlorophenol by inexpensive sewage sludge-based adsorbents. Chem. Eng. Res. Design 2012, 90, 1807–1814. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Płonka, I. Application of excess activated sludge as waste sorbent for dyes removal from their aqueous solutions. Ecol. Chem. Eng. 2019, 26, 773–784. [Google Scholar] [CrossRef]
- Zaker, A.; Chen, Z.; Lee, K.; Hammouda, S. Development of sludge-based activated char sorbent with enhanced hydrophobicity for oil spill cleanup. Environ. Technol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yadaw, A.; Singh, S.; Garg, A. Optimization for the conditions to prepare sewage sludge derived adsorbent and ciprofloxacin adsorption. Water Environ. Res. 2021, 93, 2754–2768. [Google Scholar] [CrossRef]
- Matheri, A.N.; Eloko, N.S.; Freeman, N.; Ngila, J.C. Influence Of Pyrolyzed Sludge Use As An Adsorbent In Removal Of Selected Trace Metals From Wastewater Treatment. Chem. Environ. Eng. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Ali, Y.A.H.; Ahrouch, M.; Lahcen, A.A.; Demba, N.A. Dried sewage sludge as an efficient adsorbent for pollutants: Cationic methylene blue removal case study. Nanotechnol. Environ. Eng. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Militaru, B.A.; Pode, R.; Lupa, L.; Schmidt, W.; Tekle-Röttering, A.; Kazamer, N. Using Sewage Sludge Ash as an Efficient Adsorbent for Pb (II) and Cu (II) in Single and Binary Systems. Molecules 2020, 25, 2559. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ghanegaonkar, P.M. Methane enrichment of biogas produced from floral waste: A potential energy source for rural India. Energy Sources Part A 2019, 41, 2757–2768. [Google Scholar] [CrossRef]
- Tippayawong, I.N.; Thanompongchart, P. Biogas quality upgrade by simultaneous removal of CO2 and H2S in a packed column reactor. Energy 2010, 35, 4531–4535. [Google Scholar] [CrossRef]
- Srichat, A.; Suntivarakorn, R.; Kamwilaisak, K. Development of Biogas Purification System Using Calcium Hydroxide and Amine Solution. Energy Proc. 2017, 138, 441–445. [Google Scholar] [CrossRef]
- Verougstraete, B.; Schuddinck, D.; Lefevere, J.; Baron, G.V.; Denayer, J.F.M. A 3D-Printed Zeolitic Imidazolate Framework-8 Monolith For Flue- and Biogas Separations by Adsorption: Influence of Flow Distribution and Process Parameters. Front. Chem. Eng. 2020, 2, 589686. [Google Scholar] [CrossRef]
- Kapur, M.; Mondal, M.K. Design and model parameters estimation for fixed–bed column adsorption of Cu(II) and Ni(II) ions using magnetized saw dust. Desalination Water Treat. 2016, 57, 12192–12203. [Google Scholar] [CrossRef]
- Karimi, M.; de Tuesta, J.L.D.; Gonçalves, C.N.d.P.; Gomes, H.T.; Rodrigues, A.E.; Silva, J.A.C. Compost from Municipal Solid Wastes as a Source of Biochar for CO2 Capture. Chem. Eng. Technol. 2020, 43, 1336–1349. [Google Scholar] [CrossRef]
- Shahkarami, S.; Dalai, A.K.; Soltan, J.; Hu, Y.; Wang, D. Selective CO2 Capture by Activated Carbons: Evaluation of the Effects of Precursors and Pyrolysis Process. Energy Fuels 2015, 29, 7433–7440. [Google Scholar] [CrossRef]
- Acevedo, S.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of CO2 on Activated Carbons Prepared by Chemical Activation with Cupric Nitrate. ACS Omega 2020, 5, 10423–10432. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.S. Efficient carbon dioxide capture over a nitrogen-rich carbon having a hierarchical micro-mesopore structure. Fuel 2012, 95, 360–364. [Google Scholar] [CrossRef]
- Murge, P.; Dinda, S.; Roy, S. Zeolite-Based Sorbent for CO2 Capture: Preparation and Performance Evaluation. Langmuir 2019, 35, 14751–14760. [Google Scholar] [CrossRef]
- Liu, S.H.; Hsiao, W.C.; Sie, W.H. Tetraethylene-pentamine-modified mesoporous adsorbents for CO2 capture: Effects of preparation methods. Adsorption 2012, 18, 431–437. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Behaein, S. Intensified biogas upgrading via various wastewater using microchannel. Chem. Eng. Process.-Process Intensif. 2022, 175, 108927. [Google Scholar] [CrossRef]
- Bałys, M.; Brodawka, E.; Jodłowski, G.S.; Szczurowski, J. Alternative materials for the enrichment of biogas with methane. Materials 2021, 14, 7759. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Behaien, S.; Vaferi, B. Experimental and modeling analyzing the biogas upgrading in the microchannel: Carbon dioxide capture by seawater enriched with low-cost waste materials. Environ. Technol. Innov. 2022, 27, 102770. [Google Scholar] [CrossRef]
- Mininni, G.; Blanch, A.R.; Lucena, F.; Berselli, S. EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environ. Sci. Pollut. Res. 2015, 22, 7361–7374. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Raw Anaerobic Sludge | Centrifuged Anaerobic Sludge | Bio-Waste Sorbent |
|---|---|---|---|
| Total solids (%) | 4.9 ± 0.7 | 22.4 ± 0.5 | 35.1 ± 0.7 |
| Volatile solids (% TS) | 72.7 ± 0.6 | 71.9 ± 1.3 | 39.4 ± 0.9 |
| Total nitrogen (mg/g TS) | 32.9 ± 2.7 | 33.8 ± 2.9 | 17.1 ± 2.0 |
| Total phosphorus (mg/g TS) | 1.7 ± 0.2 | 1.9 ± 0.4 | 0.88 ± 0.13 |
| Total carbon (mg/g TS) | 311 ± 21 | 317 ± 19 | 165 ± 17 |
| Total organic carbon (mg/g TS) | 201 ± 37 | 204 ± 21 | 107 ± 19 |
| Calcium (mg/g TS) | 17.2 ± 3.7 | 17.1 ± 1.9 | 351 ± 13 |
| Magnesium (mg/g TS) | 5.8 ± 0.9 | 5.7 ± 0.2 | 3.1 ± 0.3 |
| C/N ratio | 9.4 ± 0.1 | 9.4 ± 0.2 | 9.6 ± 0.2 |
| pH | 7.2 ± 0.2 | 7.3 ± 0.2 | 12.2 ± 0.3 |
| Proteins (% TS) | 20.9 ± 1.8 | 20.1 ± 1.9 | 10.1 ± 0.4 |
| Lipids (% TS) | 3.2 ± 0.7 | 3.3 ± 0.8 | 1.6 ± 0.4 |
| Sugars (% TS) | 1.6 ± 0.4 | 1.4 ± 0.2 | 0.75 ± 0.3 |
| Parameter | Variant | |||
|---|---|---|---|---|
| V1 | V2 | V3 | V4 | |
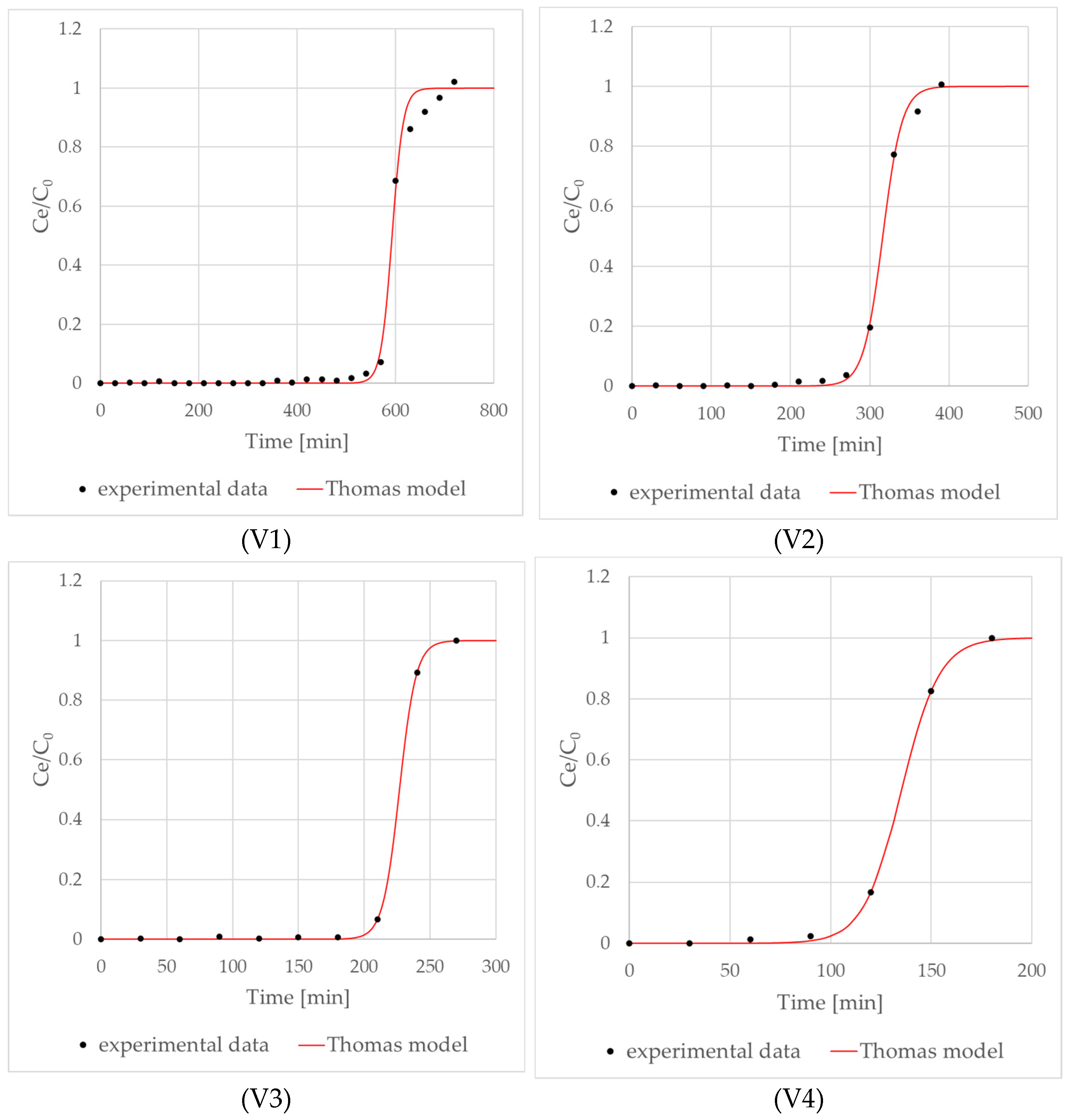
| KTh (mL/mg·min) | 0.491 | 0.0391 | 0.0730 | 0.0625 |
| Sorption capacity (mg CO2/g TS) | 106.73 ± 3.5 | 132.85 ± 3.2 | 147.20 ± 2.8 | 91.19 ± 4.4 |
| R2 | 0.998 | 0.998 | 0.999 | 0.999 |
| Breakthrough time (min) | 570 ± 10 | 270 ± 5 | 210 ± 7 | 120 ± 12 |
| Ce/C0 | 0.07 ± 0.01 | 0.04 ± 0.02 | 0.07 ± 0.01 | 0.17 ± 0.01 |
| Parameter | Variant | |||
|---|---|---|---|---|
| V1 | V2 | V3 | V4 | |
| Inlet carbon dioxide (g/L) | 1.84 ± 0.08 | 2.11 ± 0.07 | 2.16 ± 0.09 | 1.69 ± 6.5 |
| Outlet carbon dioxide (g/L) | 0.130 ± 0.009 | 0.076 ± 0.008 | 0.145 ± 0.007 | 0.281 ± 0.008 |
| Mass of carbon dioxide removed (g CO2) | 4.87 ± 0.06 | 5.50 ± 0.05 | 6.36 ± 0.04 | 3.38 ± 0.05 |
| Sorption capacity (mg CO2/g TS) | 97.50 ± 3.7 | 110.03 ± 2.1 | 127.22 ± 1.5 | 67.55 ± 4.8 |
| Parameter | % by Volume | ||||
|---|---|---|---|---|---|
| Raw Biogas | Upgraded Biogas V1 | Upgraded Biogas V2 | Upgraded Biogas V3 | Upgraded Biogas V4 | |
| Carbon dioxide (v% CO2) | 42.2 ± 2.1 | 0.53 ± 0.2 | 0.50 ± 0.2 | 0.44 ± 0.2 | 2.14 ± 0.3 |
| Methane (v% CH4) | 56.5 ± 1.7 | 97.7 ± 0.3 | 98.6 ± 0.2 | 98.9 ± 0.2 | 96.9 ± 0.2 |
| Other gases (v%) | 1.3 ± 0.8 | 1.77 ± 0.06 | 0.9 ± 0.05 | 0.66 ± 0.07 | 0.96 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, M.; Karczmarczyk, A.; Kisielewska, M.; Dębowski, M. Possibilities of Biogas Upgrading on a Bio-Waste Sorbent Derived from Anaerobic Sewage Sludge. Energies 2022, 15, 6461. https://doi.org/10.3390/en15176461
Zieliński M, Karczmarczyk A, Kisielewska M, Dębowski M. Possibilities of Biogas Upgrading on a Bio-Waste Sorbent Derived from Anaerobic Sewage Sludge. Energies. 2022; 15(17):6461. https://doi.org/10.3390/en15176461
Chicago/Turabian StyleZieliński, Marcin, Aleksandra Karczmarczyk, Marta Kisielewska, and Marcin Dębowski. 2022. "Possibilities of Biogas Upgrading on a Bio-Waste Sorbent Derived from Anaerobic Sewage Sludge" Energies 15, no. 17: 6461. https://doi.org/10.3390/en15176461
APA StyleZieliński, M., Karczmarczyk, A., Kisielewska, M., & Dębowski, M. (2022). Possibilities of Biogas Upgrading on a Bio-Waste Sorbent Derived from Anaerobic Sewage Sludge. Energies, 15(17), 6461. https://doi.org/10.3390/en15176461