Abstract
This work aimed to synthesize biodiesel from Ricinus communis L., using calcium oxide (CaO) nanoparticles as a catalyst. The CaO nanoparticles were examined by scanning electron microscopy (SEM) and X-Ray Diffraction (XRD). The physico-chemical properties of biodiesel were studied through H and C-NMR, GC-MS, FT-IR, and fuel properties were studied according to ASTM and EN standard methods. The oil content of the feedstock was 53.7% with a free fatty acid (FFA) content of 0.89 mg KOH/g. The suitable condition for the optimum yield (89%) of biodiesel was 1:15 of oil to methanol using 20 mg of catalyst at a temperature of 60 °C for 80 to 100 min of reaction time. The H and C-NMR confirm the biodiesel synthesis by showing important peaks at 3.661, 2.015–2.788, 24.83–34.16 and 174.26 and 130.15 ppm. Similarly, GC-MS spectroscopy confirmed 18 different types of fatty acid methyl esters (FAME) in the biodiesel sample. FT-IR spectroscopy confirmed the synthesis of biodiesel by showing characteristic peaks of biodiesel formation in the range of 1725–1750 cm−1 and 1000–1300 cm−1. The fuel properties were compared with the international ASTM and EN standards. The physico-chemical properties confirm that RCB is both an engine and environmentally friendly fuel.
1. Introduction
Sustainability in the steady and continuous supply of energy sources and energy security are the key parameters of sustainable economic development. The different sectors of agriculture, industrial, and transportation require many sources of energy to sustain their routine activities. The utilization of conventional and non-renewable energy sources are dominant and found to be costly [1]. Furthermore, several environmental impacts such as decrease in the quality of life, increase in pollution, and climate change are gradually rising with the increase in the demand for petro-fuels for the production of energy. Petro-fuel being utilized overwhelmingly as a nonrenewable power source will prompt energy deficiency in the coming years [2]. Greenhouse gases are released into the environment as a result of the combustion of fossil fuels for energy purposes, resulting in an increase in the temperature of the atmosphere of the Earth and ultimately putting the survival of organisms at risk. Furthermore, drilling for fossil fuels leads to contamination of water by the discharge of heavy metals, radioactive materials, and toxins into the water, which are toxic to plants and animals. Because of these impacts of the combustion of fossil fuels for energy generation, researchers throughout the world are now focusing their attention on the sustainable development of renewable and clean energy sources to replace these non-renewable and conventional fossil fuels and to meet the energy demands [1,3,4].
Beside their use as a fuel for automobiles and energy production, biodiesels are also used as an additive for the improvement of the lubricity property of fossil diesel. Saturated fatty acids (SFAs), monounsaturated (MUFAs), and polyunsaturated (PUFAs) fatty acids are the three types of fatty acids (FAs) found in biodiesel. The unsaturated fatty acids (USFAs) are classified into two types: MUFAs with only one (C=C) double bond and PUFAs with more than two (C=C) double bonds. As the number of double bonds increases, the melting temperature decreases, hence USFAs have a lower melting point than SFAs. Chemical reactivity increases as the amount of double bonds increases; thus, USFAs are more chemically reactive than SFAs [5].
In the presence of either a base or an acid catalyst, the biodiesel synthesis process is carried out efficiently. There are two main disadvantages of acid catalysts, i.e., equipment corrosion and slower reaction rate, which makes acid catalysts not suitable for commercial purposes. However, base catalysts are much more efficient due to the higher rate of reaction (4000 times more) compared to acidic catalysts [6]. Although base catalysts are widely available and cheap, base catalysts still have one main drawback, these catalysts work best when the oil has a free fatty acid (FFA) content below 0.5%, at a higher concentration of FFA their catalytic activity is reduced [3].
Based on the chemical nature, the catalysts used to synthesize biodiesel are of three types, i.e., enzyme catalysts, nanocatalysts, and homogeneous acid/base catalysts [3]. Biodiesel synthesis is commonly carried out on the industrial scale in the presence of homogeneous catalysts (sodium hydroxides or alkoxides). However, homogenous catalysts have some limitations; for example, homogenous catalysts are nonrecyclable and non-environmentally friendly by polluting biodiesel with potassium or sodium ions [6]. Furthermore, these catalysts also favor more soap formation during biodiesel synthesis. Moreover, homogeneous catalysts become less active when the feedstock has more humidity than 0.3 wt.% [7,8]. Thus, these catalysts need pure raw materials from feedstocks to synthesize biodiesel.
The feedstocks such as animal fats, cooking oils, non-edible plant oils, etc., are used to synthesize biodiesel because these feedstocks are less expensive [5,7,9]. However, the main problems with these feedstocks are their high humidity (∼3% by weight) and the content of FFAs (up to 12%) which are suitable for the synthesis of biodiesel using homogeneous catalysts. To solve this problem, researchers have synthesized nanocatalysts, which have many advantages over homogeneous catalysts such as their good performance as a catalyst and recyclability at the end of the reaction. Furthermore, these catalysts maintain their activity even with high FFA content and humidity of the feedstock [7].
Nearly 21 million tonnes of biodiesel are produced annually all over the world. The most commonly used non-edible plants as feedstock for biodiesel are Jatropha curcus, Pongamia pinnata, Madhuca indica, and Ricinus communis. The oil contents of these plants vary from 21–73%. The Ricinus communis plant has the property of growing easily in almost every environment, which is why about 30 countries cultivate it on a commercial scale. The seed of this plant has 45–55% oil content [10]. Due to its high oil content, we selected the Ricinus communis as feedstock for biodiesel synthesis using calcium oxide (CaO) nanoparticles as a catalyst.
2. Materials and Methods
2.1. Calcium Oxide Nanoparticle Preparation
The sol-gel technique described by Tahvildari et al. [11] was adopted for the synthesis of calcium oxide (CaO) nanoparticles. Calcium nitrate tetrahydrate was taken as a precursor substance and dissolved in distilled water with constant stirring. An amount of 25 mL of ethylene glycol was also added slowly to the solution. Furthermore, 2 gm of NaOH pellets was dissolved in 25 mL of distilled water and added drop-wise to it. After that, it was stirred for two hours. The Whatman filter paper (grade 3) was used for filtration. Then, the gel was kept in the oven at 105 °C for two hours to evaporate the aqueous layer. Further, to reduce alkalinity, the gel was washed 4–5 times with distilled water. After washing with distilled water, the gel was kept again in the oven for four hours at 105 °C for drying. The dried gel was finally powdered in pestle mortar and kept in a furnace for calcination at 850 °C for one hour. Then, it was stored in the desiccator [12].
2.2. XRD and SEM Analysis of CaO Nanoparticles
The XRD spectroscopic analysis of the already prepared CaO nanoparticles was carried out using a SHIMADZU 6000, Japan diffractometer equipped with a Cukα (K = 1.54 A°) source, maintaining an applied voltage of 40 kV and a current at 30 mA, and data were acquired at 2θ within the range of (10°–90°) to safeguard the establishment of a desired crystal-like assembly of all nanoparticles. With the help of the Scherrer equation, the calculation was completed, which provided a heterogeneous ordinary diameter of the nanoparticles. All dimensions were achieved between (10–60 °C). The scanning electron microscopy was performed through SEM, Model JEOL JSM-5910, and HT7800 Ruli. (Made in Japan) Scanned images were obtained through operating-field emissions of a SEM microscope with (20 kV) accelerating voltage. It added in the interpretation of the phenomena that occurred during calcination and pretreatment and permits qualitative characterization of the surface of catalysts.
2.3. Oil Extraction from Feedstock
To synthesize biodiesel, the first step is oil extraction; in the current study, we have used two methods for oil extraction, Chemical (through Soxhlet) and Mechanical. Before oil extraction, the seedlings of the feedstock were washed with mildly diluted distilled water to eradicate the dust [13].
2.3.1. Chemical Extraction through Soxhlet
Chemical extraction was carried out to determine the amount of oil in the seeds of Ricinus communis L. The chemical extraction was performed with the help of Soxhlet. About 10 gm of dried seeds was finely powdered in pastle mortar. This fine powder was then subjected to the Soxhlet apparatus for the chemical extraction of oil using ether as the solvent [14]. The quantity of oil in the feedstock seeds was calculated using the equation [13];
where
- W1 = empty flask weight
- W2 = fine powder sample weight
- W3 = flask + weight of extracted oil
- W4 = extracted oil weight
2.3.2. Mechanical Extraction of Oil
Through mechanical extraction, the oil was extracted in bulk amount for the experimental work. For this purpose, the oil expeller Model YZS-130A/C was used. The extracted oil was collected and stored in a glass jar.
2.4. Oil Filtration
The technique adopted by Seffati et al. [15] was used for the filtration and storage of crude oil.
2.5. Free Fatty Acid (FFA) Content Determination
The amount of FFA in the oil of feedstock was determined using the acid-based titration method [13]. The FFA percentage is calculated using the given formula.
where
- A = potassium hydroxide (KOH) volume used in the sample titration.
- B = potassium hydroxide (KOH) volume used in blank titration.
- C = potassium hydroxide (KOH) concentration (g/L).
- V = volume of oil sample.
2.6. Biodiesel Synthesis
The transesterification technique was followed to synthesize biodiesel using CaO as a nanocatalyst. The following formula was used to calculate the percentage yield of biodiesel after the experiments [10].
2.7. Analysis of Fuel Properties
Various fuel characteristics of synthesized biodiesel such as Flash Point °C (PMDC), Density at 15 °C Kg/L, K Viscosity at 40 °C CST, Pour Point °C, Sulphur % wt, Calorific value KJ/Kg, and Cetane no. were analyzed according to the ASTM standard methods (Table 1) [14,15].

Table 1.
Fuel properties of the biodiesel prepared from Ricinus communis.
2.8. Chemical Assessment of Biodiesel
To confirm the synthesis and check the chemical properties of RCB FT-IR, GC-MS, and NMR, analyses were performed.
2.8.1. Fourier Transform Infrared (FT-IR) Analysis of RCB
To confirm the synthesis of various chemical species present in the Ricinus communis Biodiesel (RCB), Fourier transform infrared (FT-IR) spectroscopy was performed. FT-IR analysis was performed using a VARIAN AA280Z (made in Canada) atomic absorption spectrometer with a GTA-120 graphite tube atomizer (made in Canada) in the range of 400–4000 cm−1.
2.8.2. NMR Spectroscopy of the Ricinus communis Biodiesel (RCB)
Both the 1H and 13C NMR spectroscopy were performed at 20 °C on an 11.75 T Avance NEO Bunker 600 MHz spectrometer (Made in Japan) equipped with a 5 mm BBF smart probe. As an internal standard for authentication, chloroform-d and Si(CH3)4 solvents were used. The 1H NMR spectrum (300 MHz) were recorded at a recycle delay of 1.0 s and 8 scans and pulse duration of 30°.
The 13C NMR spectrum (75 MHz) was recorded with a recycle delay of 1.89 and 160 scans and a pulse duration of 30°. The conversion percentage was mathematically determined with the help of the formula given below [16].
Percentage of Biofuel,
where
C = 100 × 2AMe/3ACH2
- C = oil to biodiesel conversion percentage
- AMe = integration value of methoxy protons in biodiesel
- ACH2 = α-methylene protons’ integration value of methylene protons in biodiesel
2.8.3. FAMEs Determination through GC-MS
GC-MS was used for the determination of the chemical composition of the fatty acid methyl esters (FAMEs) of Ricinus communis Biodiesel (RCB). Approximately 1 mL of RCB was injected into GC-MS (Model QP 2010 Plus; Shimadzu, Japan) using hexane as the solvent. The vector gas was helium. The column used was a DB-5MS capillary column with 30 mm length and 0.25 mm diameter, 0.25 µm of film thickness. The column temperature was set at 50–300 °C. Both the injector and detector temperature were set at 250 °C.
3. Results and Discussion
3.1. X-ray Diffraction of CaO Nanocatalysts
The XRD analysis of the already prepared CaO nanoparticles was carried out using a SHIMADZU 6000 diffractometer equipped with a Cukα (K = 1.54 A°) source, maintaining an applied voltage of 40 kV and a current at 30 mA and data were acquired at 2θ with a range of (10°–90°). The XRD design of the CaO nanocatalyst (Figure 1) indicates strong deflection heights at 2 theta angles of 32.48, 37.58, 53.94, 64.38, and 67.54 corresponding to (111), (200), (220), (311), and (222), respectively, of cubic phase CaO and are consistent with the standard XRD data for cubic phase CaO.

Figure 1.
XRD patterns confirming the CaO nanoparticles synthesis.
The magnitude of the sample’s XRD points reveals that the designed nanoparticles stay crystalline and wide-ranging diffraction peaks specify the very small extent of crystallite [17]. The size of the nanoparticles was determined with the help of the Scherrer equation.
where k is the shape factor = 0.9, λ is the radiation wavelength (1.54 Å), β is the full width of half of the maximum intensity (FWHM) in radians.
3.2. Scanning Electron Microscopic Study of CaO
The surface and morphological characterizations of the prepared catalyst CaO were performed using SEM, Jeol model appliance (JSM-6390LV-Japan). The synthesized nanoparticles were spherical and densely packed (Figure 2).
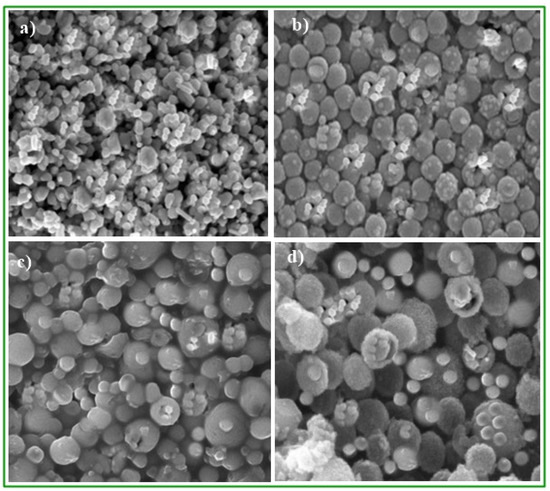
Figure 2.
SEM analysis of CaO nanoparticles to confirm the synthesis of nanoparticles and size, (a) magnification power 1 µm × 5000, (b) magnification power 1 µm × 10,000 (c) magnification power 1 µm × 20,000 (d) magnification power 1 µm × 30,000.
The images of the synthesized nanoparticles were taken at a magnification of 1 µm × 5000, 1 µm × 10,000, 1 µm × 20,000, and 1 µm × 30,000, which confirms that the nanoparticles are spherical and densely packed (Figure 2). Furthermore, the surface of nanoparticles shows roughness, and their sizes are uneven. The surface of the particles is porous, and nature is amorphous. CaO-CaO nanoparticles are bound to each other due to the presence of nodule formation [18,19,20]. The ordered and regular shape of particles is the sign of a better interconnected regular pore distribution system [21]. All the nanoparticles have a uniform size ranging from 5.68 to 8.33 nm, although some scattered particles have sizes up to 100 nm in diameter [12].
3.3. Oil Extraction and Free Fatty Acids Content Determination
We used two approaches (chemical through Soxhlet and mechanical) of oil extraction to compare our result with the international standard techniques stated by various researchers in their works. The Soxhlet extraction technique is specified by the European Union for oil extraction from the feedstock. Furthermore, the use of these techniques is cost-effective and easy to handle [13].
The oil content in the feedstock was 53.7%. Other researchers have also reported almost the same results [22]. Furthermore, the FFA content was also determined by the acid titration method before the biodiesel synthesis process. The concentration was reported as 0.89 mg KOH/g; this is below the limit specified internationally. If the feedstock has a FFA content exceeding 3%, the oil-to-biodiesel conversion efficacy gradually decreased [13].
3.4. Biodiesel Synthesis through Transesterification Technique
The transesterification technique was followed to synthesize biodiesel. To determine conditions suitable for maximum biodiesel yield through the transesterification reaction, a series of experiments were intended to consider four main reaction variables, i.e., the oil-to-methanol ratio, catalyst concentration, reaction time, and temperature. The optimum yield (89%) of biodiesel was 1:15 of oil to methanol using 20 mg of catalyst at a temperature of 60 °C for 80 to 100 min of reaction time.
3.4.1. Oil-to-Methanol Ratio
According to Hanif et al. [23], the oil-to-methanol ratio greatly affects the biodiesel yield; therefore, the oil-to-methanol ratio was taken as 1:03, 1:06, 1:09, 1:12, and 1:15. The reported results clearly show that at 1:15 of oil to methanol the optimum yield was achieved (Figure 3).
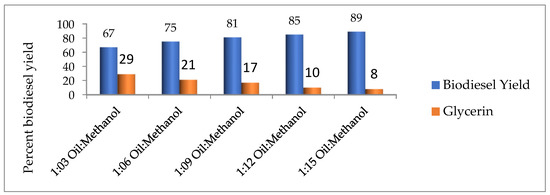
Figure 3.
Effect of variation in oil to methanol ratio on biodiesel yield.
Furthermore, Sharma et al. [11] reported the maximum yield of biodiesel at a 1:16 oil to methanol ratio, while Ullah et al. [13] and Worapun et al. [24] reported almost similar results in their studies.
As reported in the literature for transesterification, the stoichiometric molar ratio for alcohol to oil is 3:1, and this reaction is reversible. Therefore, a higher molar ratio enhances the miscibility and interaction between the triglycerides and alcohol molecules. According to the literature for the completion of the reaction or the maximum biodiesel yield, the molar ratio would be kept higher than the stoichiometric ratio [1].
According to the literature for the breakdown of the linkages between glycerin and fatty acids in the transesterification reaction, the availability of adequate amounts of methanol is desirable [13,25]. However, a very high amount of methanol must be avoided because higher oil-to-methanol ratios cannot optimize the biodiesel yield and esters content; however, it complicates the ester recovery process and increases the cost [13]. Furthermore, in the biodiesel synthesis process, a high amount of methanol of more than 1:70 molar ratio slows the separation of glycerol and ester [26].
3.4.2. Catalyst Concentration
The catalyst plays an important role in biodiesel production [24]. In this work, we study the influence of catalyst concentration by keeping the concentrations at 10, 15, 20, 25, and 30 mg to obtain the maximum yield of biodiesel. The result clearly shows that the optimum yield was obtained at 20 mg of catalyst concentration (Figure 4).
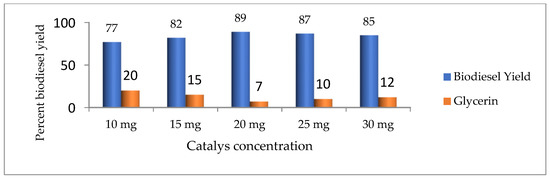
Figure 4.
Effect of catalyst concentration on biodiesel yield.
According to Bojan and Durairaj [27], a high concentration of catalyst causes the formation of soap due to emulsification. According to Ullah et al. [13], a high amount of catalyst increases the viscosity of the reactants, which results in the reduction of the biodiesel yield.
3.4.3. Reaction Temperature
Another substantial factor that markedly influenced the biodiesel yield is the reaction temperature, since a high reaction temperature increases the reaction speed and minimizes the duration of the reaction due to a reduction in oil viscosity [13]. Therefore, to determine the ideal temperature to obtain the maximum biodiesel yield, the temperature was kept at 45, 50, 55, 60, and 65 °C, respectively. The research findings express the influence of temperature on biodiesel yield. It was observed that when the temperature increased from 55 °C to 60 °C, the biodiesel yield increased to 89%, but when the temperature was increased above 60 °C, the biodiesel yields gradually decreased (Figure 5).
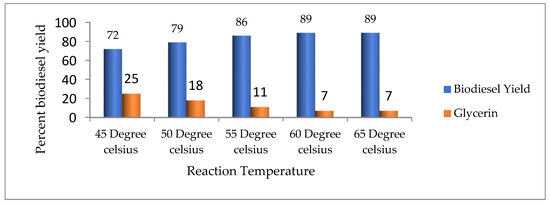
Figure 5.
Effect of reaction temperature on biodiesel yield.
Our result is almost similar to the results of Ullah et al. [13], Phan et al. [28], and Zhang et al. [29]. The decrease in the amount of biodiesel at high temperatures (more than 65 °C) may be due to the high miscibility that results in reduced phase separation, as well as yield [13].
3.4.4. Reaction Time
According to Mathiyazhagan and Ganapathi [30], the yield of biodiesel increases when the reaction time is increased. We also varied the duration of the reaction to 20, 40, 60, 80, and 100 min to determine the ideal time for optimum biodiesel yield while keeping other conditions constant. The result of this study clearly shows that an optimal yield (89%) of biodiesel was obtained at 80 and 100 min (Figure 6).
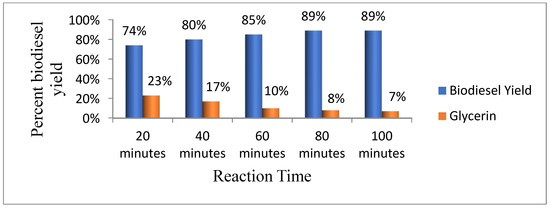
Figure 6.
Effect of reaction time on biodiesel yield.
Sharma et al. [10] reported that for the same feedstock the maximum yield of biodiesel was obtained at 90 min of reaction time and Ullah et al. [13] reported that the maximum yield of biodiesel was obtained at 45 min of reaction time.
3.5. Physical and Fuel Properties of Biodiesel
In the current study, Ricinus communis biodiesel had a 0.89 mg KOH/g acid value that was below the standard range defined by EN-14214. The acid value of the Ricinus communis biodiesel was more or less similar to the values reported by Avila Vazquez et al. [31]. The acid value of biodiesel is also influenced by its purification quality. The kinetic viscosity of Ricinus communis biodiesel was 3.98 mm2/s at 40 °C. It is within the limit of the ASTM D-445 standard. According to literature, higher viscosity creates resistance to fluid flow. Furthermore, the high viscosity of fuel causes poor injection performance, which then leads to poor combustion inside the engine cylinder [10]. The kinematic viscosity of RCB is also close to that of fossil diesel [13]. Moreover, the density of RCB was obtained as 869 kg/L, which is within the D-4052 standards range [32]. According to Kaisan et al. [33], due to the high oxygen content, biodiesel has a lower calorific value than fossil diesel. In this study, RCB also has a little lower calorific value than fossil diesel (Table 1).
The flash point of RCB in this study was calculated as 84 °C (Table 1). This is within the EN 14,214 and ASTM D 6751 standards range. The EN 14,214 standard identified that biodiesel must have a flash point of more than 120 °C, while the ASTM D-6751 allows the range to be below 130 °C. The methanol content of any biodiesel has a pronounced effect on the flash point of biodiesel [13]. According to Ullah et al. [13], when the methanol content of biodiesel increases by 0.5%, its flash point decreases by 50 °C. According to previous studies, the range of flash points for biodiesel is 160–202 °C [34,35].
In this study, the cetane number of RCB was determined to be 51 (Table 1), which was below the limits mentioned in the EN 14,214 standard, while Sharma et al. [10] reported that the CN value of RCB is 42. If the cetane number is above the limit, we can adjust it by adding a small amount of nitric acid, iso-octyl, to it for the quality conformation [36]. The statement above indicates that there is an inverse proportionality between the degree of unsaturation of fatty acids and the cetane number [13].
In the current study, CP and PP for RCB were documented as −4 °C and −9 °C. However, there is no limit for CP and PP according to the ASTM D 6751 standard; however, Mofijur et al. [37] have specified values for CP and PP. In the present study, the sulfur content was determined at 0.00038 ppm, which is in the range limit of ASTM D 6751 (0.05 ppm). Therefore, Ricinus communis biodiesel is considered an eco-friendly fuel [25].
3.6. H NMR of Ricinus communis Biodiesel
The characteristic singlet peak obtained at 3.661 ppm confirmed the presence of a methoxy proton (-OCH3). Furthermore, for the alpha-methylene proton (α-CH2), the peaks were obtained in triplicate from 2.015 to 2.788 ppm. These two peaks confirmed the formation of FAMEs from triglycerides. The other important peaks observed were for terminal methyl protons (−CH3) at 0.885–0.910 ppm. The next strong indication was the peak obtained from 1.253 to 1.641 ppm for beta-carbonyl methylene protons (Figure 7).
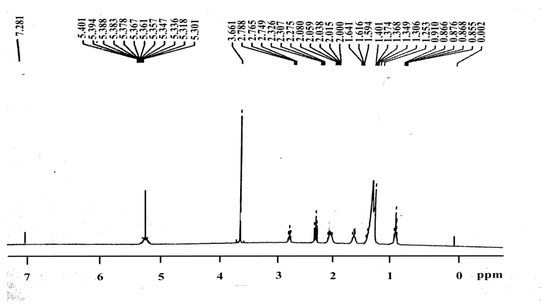
Figure 7.
H-NMR spectroscopy for the confirmation of the synthesized biodiesel.
Furthermore, the peaks obtained at 5.301–5.401 ppm confirmed the olefinic hydrogen [38,39]. The above mentioned peaks are the indications for the transformation of triglycerides into FAMEs [40].
3.7. 13. C-NMR of Ricinus communis Biodiesel
In the present study, the peaks obtained at 24.81–34.26 ppm confirmed the presence of long-chain ethylene carbons (-CH2-). The peaks at 174.18 and 130.18 ppm confirmed the carbonyl carbon (-C=O) and also the unsaturation position in the Ricinus communis biodiesel. Furthermore, the peak at 128.12 ppm is an indication of vinylic group (C=H). The absence of signal lines at “172.75–173.16 ppm” approved the occurrence of C=O (Figure 8).
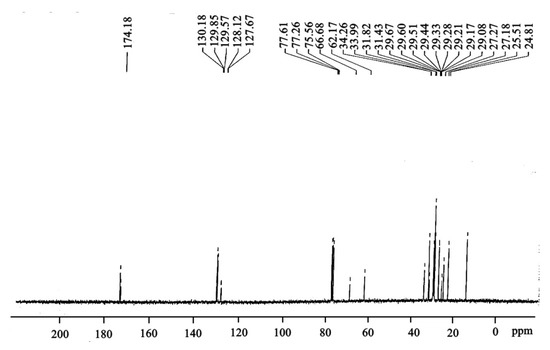
Figure 8.
C-NMR spectroscopy confirms the synthesis of biodiesel.
Peaks for the same functional groups in the specified range were also reported in other studies [40,41].
3.8. GC-MS Analysis of Ricinus communis Biodiesel
The GC-MS results showed 18 peaks of different types of FAMEs. Every single peak represents a specific FAME and was confirmed through No. NIST 02 library match software. The retention time data identify each FAME after mass spectrometric analysis (Table 2).

Table 2.
FAMEs composition of biodiesel Ricinus communis determined by GC-MS.
The analysis showed that Ricinoleic acid methyl ester (C18:1-OH), Linoleic acid methyl ester (C18:2c), Nervonic acid methyl ester (C24:1), and Stearic acid methyl ester (C18:0) were the major FAMEs identified in the RCB. The mass fragmentation patterns and the retention time of the eluted components were used for the confirmation of various FAMEs. It is obvious from the GC-MS data that the RCB is primarily composed of various FAMEs (Figure 9).
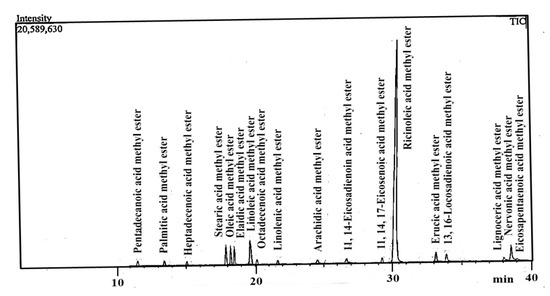
Figure 9.
FAMEs composition of the Ricinus communis biodiesel, determined by GC-MS.
In the present study, 18 different types of FAME in Ricinus communis biodiesel were reported for the first time (Table 2). Other researchers using the same feedstock (Ricinus communis L.) reported fewer FAMEs such as Berman et al. [42], who reported nine FAMEs, Ola et al. [43] reported five FAMEs, Kundu et al. [44] reported seven FAMEs, Keera et al. [45] reported six FAMEs, Palconite et al. [46] reported ten FAMEs, and Gebrehiwot and Zelelew [47] reported ten FAMEs.
3.9. FT-IR Analysis of the Ricinus communis Biodiesel
According to Soon et al. [48], the position of the carbonyl group is sensitive to the molecular structure and the effects of the substituent. In FT-IR spectroscopy of the Ricinus communis biodiesel, the dimeric OH stretch was observed at 3447.2 cm−1 and the normal polymeric OH stretch was observed at 3308 cm−1. The stretching bands for methyl and methylene were observed at 2839.67 cm−1 and 2934.2 cm−1, respectively. The medium stretching for allene was obtained at 1961.34 cm−1. The methoxycarbonyl group (methyl ester) was observed at 1748.35 cm−1. Stretching for alkenyl was obtained at 1678.43 cm−1. The stretching for the aryl group (C=C-C) was obtained at 1512.67 cm−1. The peak for the vinyl C-H in-plane bend was obtained at 1409.54 cm−1. The peak for isomethyl was obtained at 1388.71 cm−1. The peak of aromatic ether (the aryl–O stretching) was obtained at 1227 cm−1. The stretch for secondary amine (CN) was obtained at 1169.22 cm−1. The peak for the cyclohexane ring was obtained at 1002.63 cm−1. The peak obtained at 831.51 cm−1 confirmed the presence of the C-O-O stretch (Figure 10).
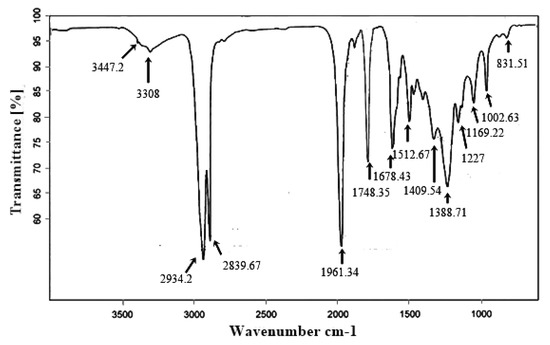
Figure 10.
FT-IR spectroscopy confirmation of various constituents found in synthesized biodiesel.
FTIR spectroscopic study was conducted to confirm biodiesel synthesis and various functional groups formed during the transesterification process. There are two main peaks for ester formation; one is carbonyl, for which the maximum range is 1725–1750 cm−1, and the other is C-O, for which the maximum range is 1000–1300 cm−1 [13].
4. Conclusions
Biodiesel is with no doubt a less pollutant-causing fuel with a soft chemical nature of FAMEs. Currently, various techniques are being applied for biodiesel production. In this study, the transesterification technique was followed to synthesize biodiesel using Ricinus communis as feedstock and CaO as the catalyst. The FFA content of 0.89 mg KOH/g was observed and was considered feasible for biodiesel synthesis. The optimized protocol for the maximum conversion of Ricinus communis biodiesel in the transesterification reaction was found to be 1:15 (oil-methanol molar ratio), 20 mg (CaO catalyst), 60 °C (reaction temperature), and 100 min (reaction time). The physicochemical properties of the biodiesel synthesized in this study revealed that it meets the fundamental prerequisites for petro-diesel. Subsequently, it may be a potential substitute for fossil fuels. The chemical and physical properties of Ricinus communis L. clearly showed that the oil of Ricinus communis L. could be a potential non-edible feedstock in the biodiesel industry. Furthermore, this feedstock is economical, indigenously available, and can be easily grown in various environments. However, further research and development on additional fuel property measures and long term run and wear analysis of biodiesel-fueled engines are also necessary along with engine hardware modification.
Author Contributions
Conceptualization: H.A.J., A.S.A.-F. and F.R.; Methodology: H.A.J., I.Š. and A.Z.; Formal analysis: R.L.A.-O.; Investigation: H.A.J., A.S.A.-F. and F.R.; Resources: H.A.J.; Writing—original draft preparation: H.A.J.; Writing—review and editing: H.A.J. and I.Š.; Funding acquisition: A.S.A.-F. and I.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Researchers Supporting Project number (RSP-2021/368), King Saud University, Riyadh, Saudi Arabia, and Slovak Research and Development Agency under the contracts APVV-14-0393 and APVV-16-0088, and by the Slovak Scientific Grant Agency VEGA by the contract VEGA 1/0012/19.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the Researchers Supporting Project number (RSP-2021/368), King Saud University, Riyadh, Saudi Arabia, and Slovak Research and Development Agency under the contracts APVV-14-0393 and APVV-16-0088, and by the Slovak Scientific Grant Agency VEGA by the contract VEGA 1/0012/19.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASTM | American Society for Testing and Materials |
| CP | Cloud Point |
| FAME | Fatty Acid Methyl Esters |
| FFA | Free Fatty Acid |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| FT-IR spectroscopy | Fourier Transform Infrared Spectroscopy |
| H and C-NMR | Nuclear Magnetic Resonance |
| HHV | Higher Heating Value |
| PMCC | Flash Point (Pensky-Martens Closed Cup) |
| PP | Pour Point |
| SEM | Scanning Electron Microscopy |
| SMB | Silybum Marianum Biodiesel |
| XRD | X-ray Diffraction |
| ZnO | Zinc Oxide |
References
- Qadeer, M.U.; Ayoub, M.; Komiyama, M.; Daulatzai, M.U.K.; Mukhtar, A.; Saqib, S.; Bokhari, A. Review of biodiesel synthesis technologies, current trends, yield influencing factors and economical analysis of supercritical process. J. Clean. Prod. 2021, 309, 127388. [Google Scholar] [CrossRef]
- Bagher, A.M.; Vahid, A.; Mohsen, M.; Reza, B.M. Effect of Using Renewable Energy in Public Health. Am. J. En. Sci. 2016, 3, 1–9. [Google Scholar]
- Narasimhan, M.; Chandrasekaran, M.; Govindasamy, S.; Aravamudhan, A. Heterogeneous nanocatalysts for sustainable biodiesel production: A review. J. Environ. Chem. Eng. 2021, 9, 104876. [Google Scholar] [CrossRef]
- Sharma, V.; Duraisamy, G. Production and characterization of bio-mix fuel produced from a ternary and quaternary mixture of raw oil feedstock. J. Clean. Prod. 2019, 221, 271–285. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Duraisamy, G. Experimental Investigation of Neat Biodiesels’ Saturation Level on Combustion and Emission Characteristics in a CI Engine. Energies 2021, 14, 5203. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, J.; Martinez, M.; Flores, H. Metakaolinite as a catalyst for biodiesel production from waste cooking oil. Front. Chem. Sci. Eng. 2012, 6, 403–409. [Google Scholar] [CrossRef]
- Toledo Arana, J.; Torres, J.J.; Acevedo, D.F.; Illanes, C.O.; Ochoa, N.A.; Pagliero, C.L. One-step synthesis of CaO-ZnO efficient catalyst for biodiesel production. Int. J. Chem. Eng. 2019, 2019, 1806017. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Gogate, P.R.; Pandit, A.B. Ultrasound-assisted synthesis of biodiesel from palm fatty acid distillate. Ind. Eng. Chem. Res. 2009, 48, 7923–7927. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of synthesis of biodiesel from nonedible oils using sonochemical reactors. Ind. Eng. Chem. Res. 2012, 51, 11866–11874. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Duraisamy, G.; Vijay, M. Transesterification of Pyrolysed Castor Seed Oil in the Presence of CaCu(OCH3)2 Catalyst. Energies 2021, 14, 6064. [Google Scholar] [CrossRef]
- Tahvildari, K.; Anaraki, Y.N.; Fazaeli, R.; Mirpanji, S.; Delrish, E. The study of CaO and MgO heterogenic nano-catalyst coupling on transesterification reaction efficacy in the production of biodiesel from recycled cooking oil. J. Environ. Health Sci. Eng. 2015, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Bharti, P.; Bhaskar, S.; Dey, R.K. Process optimization of biodiesel production catalyzed by CaO nanocatalyst using response surface methodology. J. Nanostructure Chem. 2019, 9, 269–280. [Google Scholar] [CrossRef]
- Ullah, K.; Jan, H.A.; Ahmad, M.; Ullah, A. Synthesis and structural characterization of biofuel from Cocklebur sp., using zinc oxide nano-particle: A novel energy crop for energy industry. Front. Bioeng. Biotech. 2020, 8, 756. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Antolın, G.; Tinaut, F.V.; Briceno, Y.; Castano, V.; Perez, C.; Ramırez, A.I. Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef]
- Holser, R.A.; O’Kuru, R.H. Transesterified milkweed (Asclepias) seed oil as a biodiesel fuel. Fuel 2006, 85, 2106–2110. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Nano sized copper particles by electrolytic synthesis and characterizations. Int. J. Phy. Sci. 2011, 6, 3662–3671. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Y.; Zhang, P.; Jiang, P. Blocky shapes Ca-Mg mixed oxides as a water-resistant catalyst for effective synthesis of biodiesel by transesterification. Fuel Process. Technol. 2016, 149, 163–168. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Shiwaku, H. Design a novel optical adsorbent for simultaneous ultra-trace cerium (III) detection, sorption and recovery. Chem. Eng. J. 2013, 228, 327–335. [Google Scholar] [CrossRef]
- Flemmer, A.C.; Franchini, M.C.; Lindström, L.I. Description of safflower (Carthamus tinctorius) phenological growth stages according to the extended BBCH scale. Ann. App. Biol. 2015, 166, 331–339. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized production and advanced assessment of biodiesel: A review. Int. J. Energy Res. 2018, 42, 2070–2083. [Google Scholar] [CrossRef]
- Worapun, I.; Pianthong, K.; Thaiyasuit, P. Two-step biodiesel production from crude Jatropha curcas L. oil using ultrasonic irradiation assisted. J. Oleo Sci. 2012, 61, 165–172. [Google Scholar] [CrossRef]
- Kumar, K. Standardization of non-edible Pongamia pinnata oil methyl ester conversion using hydroxyl content and GC–MS analysis. J. Taiwan Inst. Chem. Eng. 2013, 45, 1485–1489. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Bojan, S.G.; Durairaj, S.K. Producing Biodiesel from High Free Fatty Acid Jatropha Curcas Oil by a Two Step Method—An Indian Case Study. J. Sustain. Energy Environ. 2012, 3, 63–66. [Google Scholar]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Mathiyazhagan, M.; Ganapathi, A. Factors affecting biodiesel production. Res. Plant Biol. 2011, 1, 1–5. [Google Scholar] [CrossRef]
- Ávila Vázquez, V.; Díaz Estrada, R.A.; Aguilera Flores, M.M.; Escamilla Alvarado, C.; Correa Aguado, H.C. Transesterification of non-edible castor oil (Ricinus communis L.) from Mexico for biodiesel production: A physicochemical characterization. Biofuels 2020, 11, 753–762. [Google Scholar] [CrossRef]
- Wang, R.; Hanna, M.A.; Zhou, W.W.; Bhadury, P.S.; Chen, Q.; Song, B.A.; Yang, S. Production and selected fuel properties of biodiesel from promising non-edible oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Kaisan, M.U.; Anafi, F.O.; Nuszkowski, J.; Kulla, D.M.; Umaru, S. Calorific value, flash point and cetane number of biodiesel from cotton, jatropha and neem binary and multi-blends with diesel. Biofuels 2017, 11, 321–327. [Google Scholar] [CrossRef]
- Refaat, A.A.; Attia, N.K.; Sibak, H.A.; El Sheltawy, S.T.; El Diwani, G.I. Production optimization and quality assessment of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2008, 5, 75–82. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.; Almeida, M.F. Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel 2008, 87, 3572–3578. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Rasul, M.G.; Atabani, A.E.; Hazrat, M.A.; Mahmudul, H.M. Effect of biodiesel-diesel blending on physico-chemical properties of biodiesel produced from Moringa oleifera. Procedia Eng. 2015, 105, 665–669. [Google Scholar] [CrossRef]
- Killner, M.H.M.; Linck, Y.G.; Danieli, E.; Rohwedder, J.J.R.; Blümich, B. Compact NMR spectroscopy for real-time monitoring of a biodiesel production. Fuel 2015, 139, 240–247. [Google Scholar] [CrossRef]
- Dutra, E.D.; de Lima, T.A.; de Oliveira Souza, J.L.; Silva, J.G.V.; da Silva Aquino, K.A.; da Silva Aquino, F.; Menezes, R.S.C. Characterization of fat and biodiesel from mango seeds using 1HNMR spectroscopy. Biomass Convers. Biorefinery 2018, 8, 135–141. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rokhum, L.; Gupta, R.; Chatterjee, S. Zinc oxide supported silver nanoparticles as a heterogeneous catalyst for production of biodiesel from palm oil. Environ. Prog. Sustain. Energy 2020, 39, e13369. [Google Scholar] [CrossRef]
- Kalanakoppal Venkatesh, Y.; Mahadevaiah, R.; Haraluru Shankaraiah, L.; Ramappa, S.; Basanagouda, A.S. Preparation of a CaO nanocatalyst and its application for biodiesel production using butea monosperma oil: An optimization study. J. Am. Oil Chem. Soc. 2018, 95, 635–649. [Google Scholar] [CrossRef]
- Berman, P.; Nizri, S.; Wiesman, Z. Castor oil biodiesel and its blends as alternative fuel. Biomass Bioenergy 2011, 35, 2861–2866. [Google Scholar] [CrossRef]
- Ola, P.D.; Karim, R.A.; Suherdin, M.F. The optimum condition for synthesis of biodiesel from castor (Ricinus communis) oil through transesterification reaction. J. Appl. Chem. Sci. 2013, 2, 267–272. [Google Scholar]
- Kundu, A.; Mukherjee, A.; Halder, G.; Datta, D. A kinetic study on acid catalyzed esterification of free fatty acids in Ricinus Communis oil for the production of biodiesel. Int. J. Res. Eng. Technol. 2016, 5, 31–44. [Google Scholar]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Palconite, C.L.; Edrolin, A.C.; Lustre, S.N.B.; Manto, A.A.; Caballero, J.R.L.; Tizo, M.S.; Arazo, R.O. Optimization and characterization of bio-oil produced from Ricinus communis seeds via ultrasonic-assisted solvent extraction through response surface methodology. Sustain. Environ. Res. 2018, 28, 444–453. [Google Scholar] [CrossRef]
- Gebrehiwot, H.; Zelelew, D. Ricinus communis Seed Oils as a Source of Biodiesel; A Renewable Form of Future Energy. J. Turk. Chem. Soc. Sect. Chem. 2022, 9, 339–354. [Google Scholar] [CrossRef]
- Soon, L.B.; AZ, M.R.; Hasan, S. Continuous biodiesel production using ultrasound clamp on tubular reactor. Int. J. Automot. Mech. Eng. 2013, 8, 1396–1405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).