Microorganisms as New Sources of Energy
Abstract
1. Introduction
2. Microbial Technologies for Biofuel Production
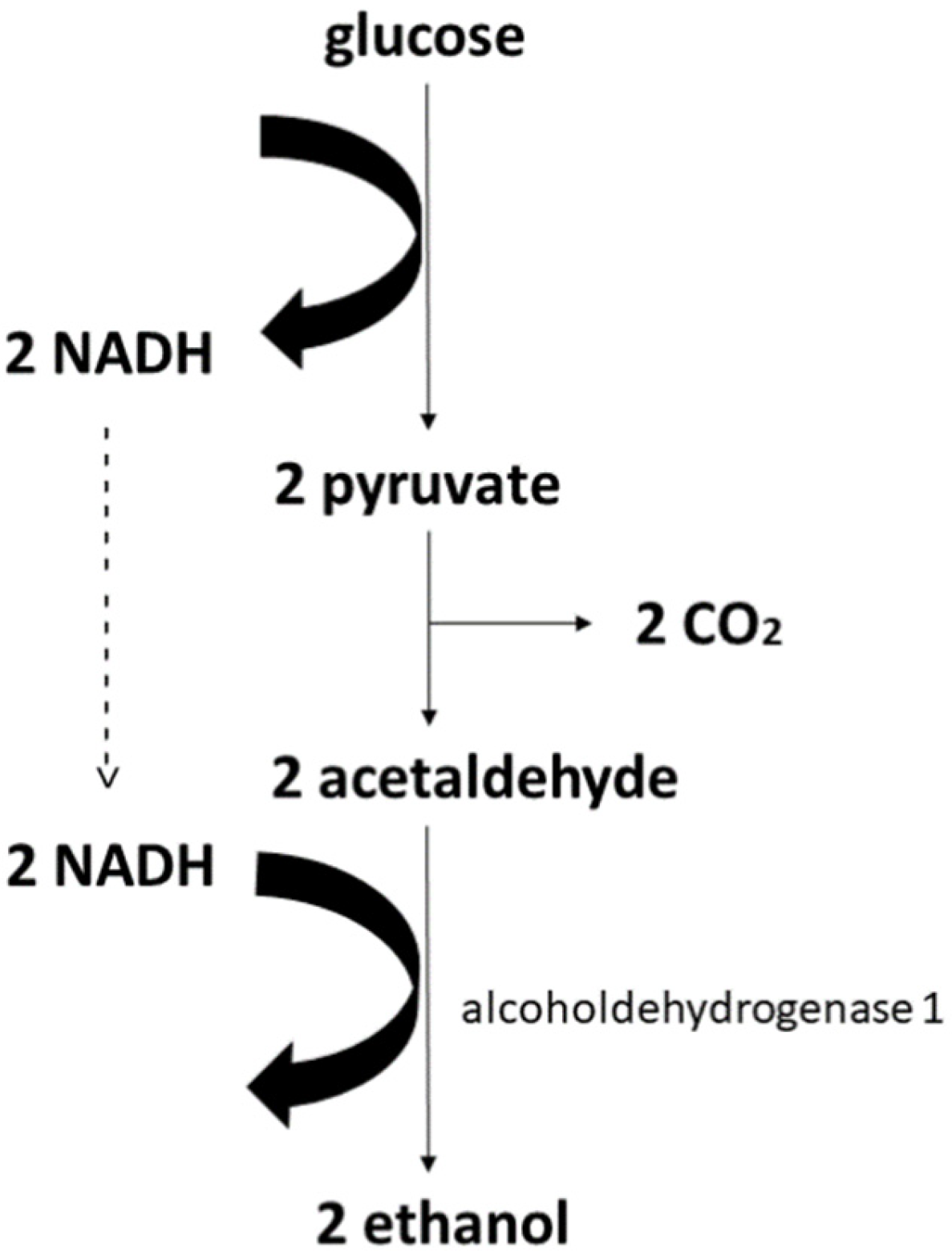
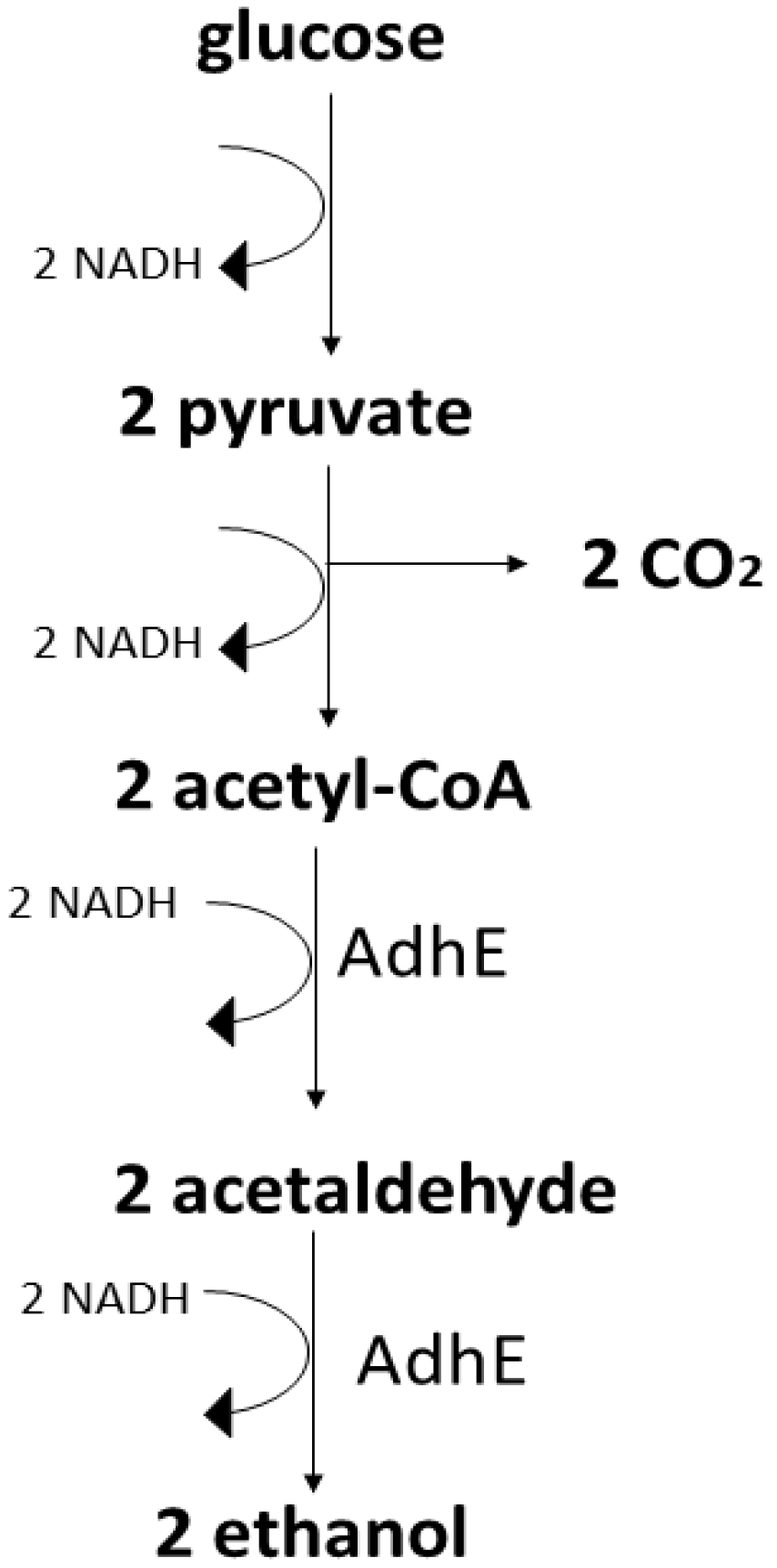
3. Production of Ethanol and Butanol
Main Metabolic Pathways for Ethanol in the Most Prominent Microorganisms
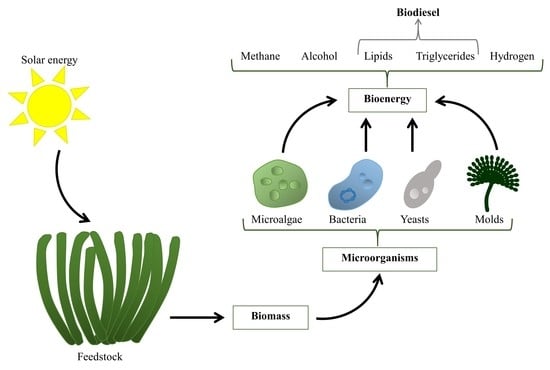
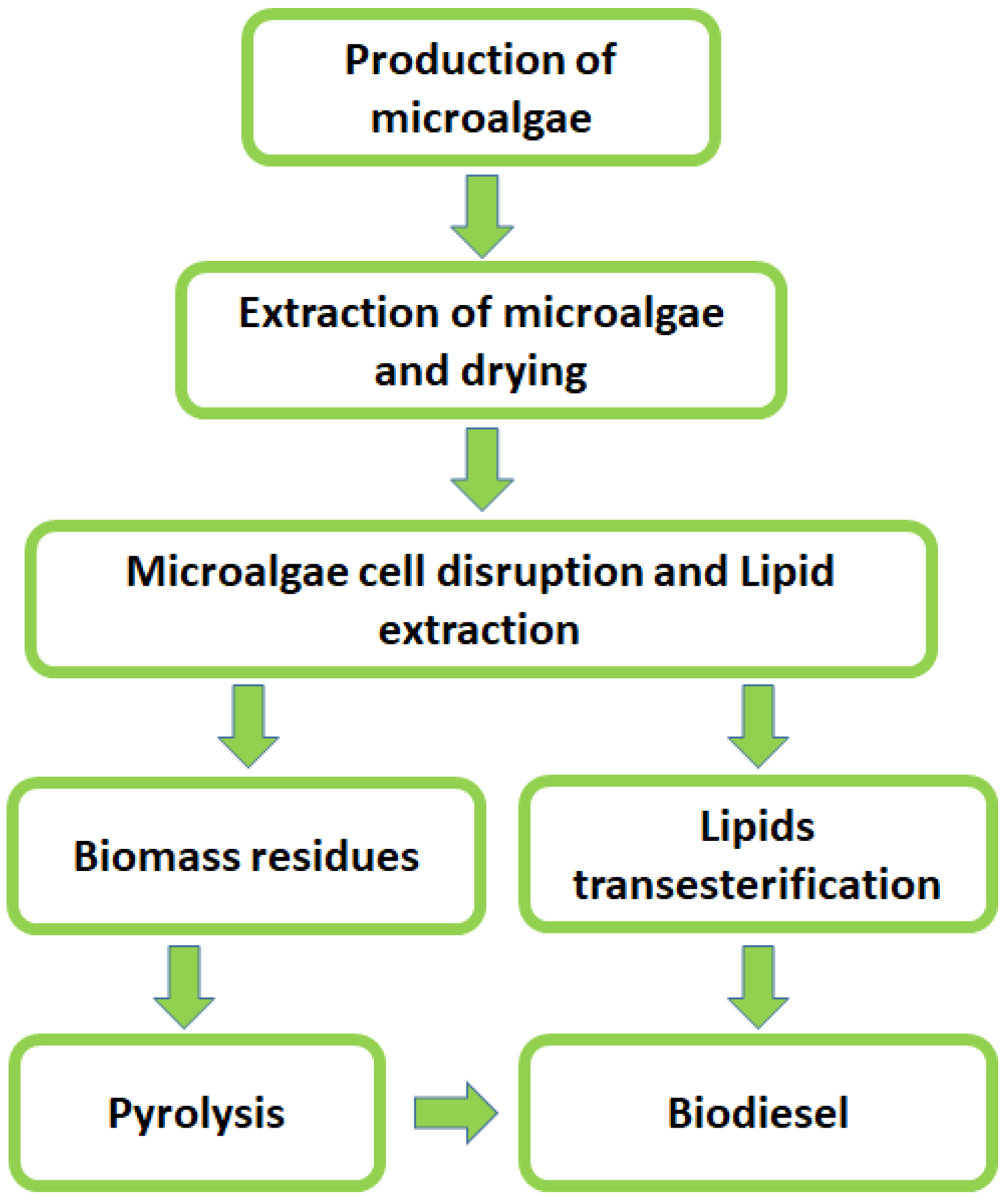
4. Biodiesel Production
Main Metabolic Pathways for Biodiesel in the Most Prominent Microorganisms
| Microorganism | Substrate | Mass Proportion of Oil (%) | Ref. | |
|---|---|---|---|---|
| Acinetobacter calcoaceticus | dry matter | 27–38 | [95] | |
| Bacteria | Arthrobacter sp. | dry matter | >40 | [95] |
| Bacillus alcalophilus | dry matter | 18–24 | [95] | |
| Rhodococcus opacus | dry matter | 24–24 | [95] | |
| Candida curvata | dry matter | 58 | [95] | |
| Cryptococcus albidus | dry matter | 65 | [95] | |
| Yeasts | Lipomyces starkeyi | dry matter | 64 | [95] |
| Rhodotorula glutinis | dry matter | 72 | [95] | |
| Trichosporon oleaginosus | Lignocellulosic substrate | 80 | [101] | |
| Aspergillus oryzae | dry matter | 57 | [95] | |
| Molds | Humicola lanuginosa | dry matter | 75 | [95] |
| Mortierella isabellina | dry matter | 86 | [95] | |
| Mortierella vinacea | dry matter | 66 | [95] |
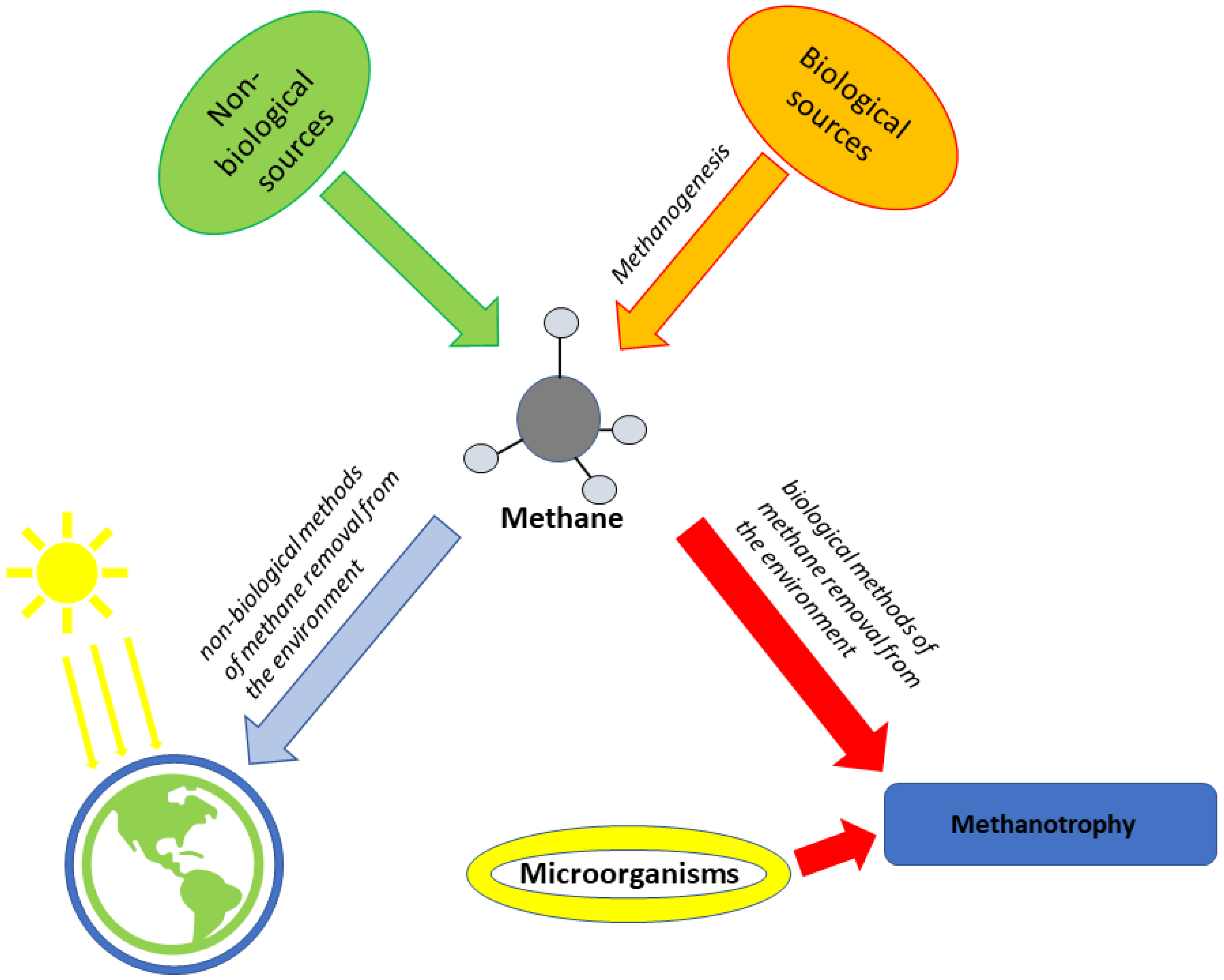
5. Hydrogen
5.1. Main Metabolic Pathways for Hydrogen in the Most Prominent Microorganisms
5.2. Hydrogen Production via Photofermentation with Photofermenting Bacteria
6. Cyanobacteria
7. Future Perspective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Gupta, R.; Singh, R.L. Microbes and Environment. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Singapore, 2017; pp. 43–84. [Google Scholar] [CrossRef]
- Hoppe, T.; Butenko, A.; Heldeweg, M. Innovation in the European Energy Sector and Regulatory Responses to It: Guest Editorial Note. Sustainability 2018, 10, 416. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, Y.; Wang, X.; Christie, P. Function of Biohydrogen Metabolism and Related Microbial Communities in Environmental Bioremediation. Front. Microbiol. 2019, 10, 106. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiumparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Biocatalysis and biomass conversion: Enabling a circular economy. Philos. Trans. R. Soc. A 2020, 378, 20190274. [Google Scholar] [CrossRef] [PubMed]
- Óhaiseadha, C.; Quinn, G.; Connolly, R.; Connolly, M.; Soon, W. Energy and Climate Policy—An Evaluation of Global Climate Change Expenditure 2011–2018. Energies 2020, 13, 4839. [Google Scholar] [CrossRef]
- Alzate, C.A.C.; Serna-Loaiza, S.; Ortiz-Sanchez, M. Sustainable Biorefineries: What was Learned from the Design, Analysis and Implementation. J. Sustain. Dev. Energy Water Environ. Syst. 2020, 8, 88–117. [Google Scholar] [CrossRef]
- Waldrop, M.M. News Feature: Microbes for better sewage treatment. Proc. Natl. Acad. Sci. USA 2021, 118, e2112863118. [Google Scholar] [CrossRef]
- Zetterholm, J.; Bryngemark, E.; Ahlström, J.; Söderholm, P.; Harvey, S.; Wetterlund, E. Economic Evaluation of Large-Scale Biorefinery Deployment: A Framework Integrating Dynamic Biomass Market and Techno-Economic Models. Sustainability 2020, 12, 7126. [Google Scholar] [CrossRef]
- Janssen, P.; Lambreva, M.D.; Plumeré, N.; Bartolucci, C.; Antonacci, A.; Buonasera, K.; Frese, R.; Scognamiglio, V.; Rea, G. Photosynthesis at the forefront of a sustainable life. Front. Chem. 2014, 2, 36. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Li, H.; Hu, R.; Yao, X.; Liu, Y.; Zhou, Y.; Lyu, T. Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere 2021, 273, 128578. [Google Scholar] [CrossRef]
- Rather, R.A.; Wani, A.W.; Mumtaz, S.; Padder, S.A.; Khan, A.H.; Almohana, A.I.; Almojil, S.F.; Alam, S.S.; Baba, T.R. Bioenergy: A foundation to environmental sustainability in a changing global climate scenario. J. King Saud. Univ. Sci. 2022, 34, 101734. [Google Scholar] [CrossRef]
- Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 22 August 2022).
- Vučurović, D.; Bajić, B.; Vučurović, V.; Jevtić-Mučibabić, R.; Dodić, S. Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis. Fermentation 2022, 8, 114. [Google Scholar] [CrossRef]
- Nordahl, S.L.; Devkota, J.P.; Amirebrahimi, J.; Smith, S.J.; Breunig, H.M.; Preble, C.V.; Satchwell, A.J.; Jin, L.; Brown, N.J.; Kirchstetter, T.W.; et al. Life-Cycle Greenhouse Gas Emissions and Human Health Trade-Offs of Organic Waste Management Strategies. Environ. Sci. Technol. 2020, 54, 9200–9209. [Google Scholar] [CrossRef]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef]
- Buan, N.R. Methanogens: Pushing the boundaries of biology. Emerg. Top. Life Sci. 2018, 2, 629–646. [Google Scholar] [CrossRef]
- Hook, S.E.; Wright, A.-D.G.; McBride, B.W. Methanogens: Methane Producers of the Rumen and Mitigation Strategies. Archaea 2010, 2010, 945785. [Google Scholar] [CrossRef]
- Srivastava, P.; Marjo, C.; Gerami, A.; Jones, Z.; Rahman, S. Surface Analysis of Coal Indicating Neutral Red Enhances the Precursor Steps of Methanogenesis. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Ney, B.; Ahmed, F.H.; Carere, C.R.; Biswas, A.; Warden, A.C.; E Morales, S.; Pandey, G.; Watt, S.J.; Oakeshott, J.G.; Taylor, M.C.; et al. The methanogenic redox cofactor F420 is widely synthesized by aerobic soil bacteria. ISME J. 2017, 11, 125–137. [Google Scholar] [CrossRef]
- Grinter, R.; Greening, C. Cofactor F420: An expanded view of its distribution, biosynthesis and roles in bacteria and archaea. FEMS Microbiol. Rev. 2021, 45, fuab021. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, P.; Yang, J.; Zhu, Y.-A.; Chen, D. C–H bond activation in light alkanes: A theoretical perspective. Chem. Soc. Rev. 2021, 50, 4299–4358. [Google Scholar] [CrossRef] [PubMed]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Wang, S.; Dames, E.E.; Davidson, D.F.; Hanson, R.K. Reaction Rate Constant of CH2O + H = HCO + H2 Revisited: A Combined Study of Direct Shock Tube Measurement and Transition State Theory Calculation. J. Phys. Chem. A 2014, 118, 10201–10209. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Meng, X.; Wang, S.; Zhou, W.; Wang, X.; Kako, T.; Ye, J. Direct and Selective Photocatalytic Oxidation of CH4 to Oxygenates with O2 on Cocatalysts/ZnO at Room Temperature in Water. J. Am. Chem. Soc. 2019, 141, 20507–20515. [Google Scholar] [CrossRef]
- Nisbet-Jones, P.B.R.; Fernandez, J.M.; Fisher, R.E.; France, J.L.; Lowry, D.; Waltham, D.A.; Maisch, C.A.W.; Nisbet, E.G. Is the destruction or removal of atmospheric methane a worthwhile option? Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2022, 380, 20210108. [Google Scholar] [CrossRef]
- Islam, J.; Shrestha, N.; Bathi, J.R.; Sani, R.K.; Gadhamshetty, V. Surface Modification Approaches for Methane Oxidation in Bioelectrochemical Systems. In Bioelectrochemical Systems; Springer: Singapore, 2020; pp. 343–374. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Dlugokencky, E.J.; Nisbet, E.G.; Fisher, R.; Lowry, D. Global atmospheric methane: Budget, changes and dangers. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2011, 369, 2058–2072. [Google Scholar] [CrossRef]
- De Souza, R.F.B.; Florio, D.Z.; Antolini, E.; Neto, A.O. Partial Methane Oxidation in Fuel Cell-Type Reactors for Co-Generation of Energy and Chemicals: A Short Review. Catalysts 2022, 12, 217. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Renewable Fuels Association. 2022 Ethanol Industry Outlook; Renewable Fuels Association: Washington, DC, USA, 2020. [Google Scholar]
- Lawford, H.G.; Rousseau, J.D. Corn steep liquor as a cost-effective nutrition adjunct in high-performance zymomonas ethanol fermentations. Appl. Biochem. Biotechnol. 1997, 63–65, 287–304. [Google Scholar] [CrossRef]
- Linde, M.; Galbe, M.; Zacchi, G. Simultaneous saccharification and fermentation of steam-pretreated barley straw at low enzyme loadings and low yeast concentration. Enzym. Microb. Technol. 2007, 40, 1100–1107. [Google Scholar] [CrossRef]
- Choi, I.S.; Wi, S.G.; Kim, S.-B.; Bae, H.-J. Conversion of coffee residue waste into bioethanol with using popping pretreatment. Bioresour. Technol. 2012, 125, 132–137. [Google Scholar] [CrossRef]
- Ballesteros, M.; Oliva, J.; Negro, M.; Manzanares, P. Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem. 2004, 39, 1843–1848. [Google Scholar] [CrossRef]
- Pessani, N.K.; Atiyeh, H.K.; Wilkins, M.; Bellmer, D.D.; Banat, I. Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: The effect of enzyme loading, temperature and higher solid loadings. Bioresour. Technol. 2011, 102, 10618–10624. [Google Scholar] [CrossRef]
- Tanimura, A.; Nakamura, T.; Watanabe, I.; Ogawa, J.; Shima, J. Isolation of a novel strain of Candida shehatae for ethanol production at elevated temperature. SpringerPlus 2012, 1, 27. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Enhanced ethanol production from brewer’s spent grain by a Fusarium oxysporum consolidated system. Biotechnol. Biofuels 2009, 2, 4. [Google Scholar] [CrossRef]
- Van Aalst, A.C.; de Valk, S.C.; van Gulik, W.M.; Jansen, M.L.; Pronk, J.T.; Mans, R. Pathway engineering strategies for improved product yield in yeast-based industrial ethanol production. Synth. Syst. Biotechnol. 2022, 7, 554–566. [Google Scholar] [CrossRef]
- Lin, P.P.; Jaeger, A.J.; Wu, T.-Y.; Xu, S.C.; Lee, A.S.; Gao, F.; Chen, P.-W.; Liao, J.C. Construction and evolution of an Escherichia coli strain relying on nonoxidative glycolysis for sugar catabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3538–3546. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Unaegbunam, E.; Stuart, D.T. Co-Production of Isobutanol and Ethanol from Prairie Grain Starch Using Engineered Saccharomyces cerevisiae. Fermentation 2021, 7, 150. [Google Scholar] [CrossRef]
- Kolesinska, B.; Fraczyk, J.; Binczarski, M.; Modelska, M.; Berlowska, J.; Dziugan, P.; Antolak, H.; Kaminski, Z.J.; Witonska, I.A.; Kregiel, D. Butanol Synthesis Routes for Biofuel Production: Trends and Perspectives. Materials 2019, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-Del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G.; Energia, D.; Ferraris, G. Biofuels from Micro-Organisms: Thermodynamic Considerations on the Role of Electrochemical Potential on Micro-Organisms Growth. Appl. Sci. 2021, 11, 2591. [Google Scholar] [CrossRef]
- Poudel, J.; Choi, J.H.; Oh, S.C. Process Design Characteristics of Syngas (CO/H2) Separation Using Composite Membrane. Sustainability 2019, 11, 703. [Google Scholar] [CrossRef]
- Ciliberti, C.; Biundo, A.; Albergo, R.; Agrimi, G.; Braccio, G.; De Bari, I.; Pisano, I. Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses. Processes 2020, 8, 1567. [Google Scholar] [CrossRef]
- Znad, H.; Awual, R.; Martini, S. The Utilization of Algae and Seaweed Biomass for Bioremediation of Heavy Metal-Contaminated Wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Fact. 2021, 20, 1–31. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Jiang, C.; Peces, M.; Andersen, M.H.; Kucheryavskiy, S.; Nierychlo, M.; Yashiro, E.; Andersen, K.S.; Kirkegaard, R.H.; Hao, L.; Høgh, J.; et al. Characterizing the growing microorganisms at species level in 46 anaerobic digesters at Danish wastewater treatment plants: A six-year survey on microbial community structure and key drivers. Water Res. 2021, 193, 116871. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Ojuederie, O.B.; Talia, P.M.; Babalola, O.O. Bioprospecting of microbial strains for biofuel production: Metabolic engineering, applications, and challenges. Biotechnol. Biofuels 2021, 14, 5. [Google Scholar] [CrossRef]
- Eram, M.S.; Ma, K. Decarboxylation of Pyruvate to Acetaldehyde for Ethanol Production by Hyperthermophiles. Biomolecules 2013, 3, 578–596. [Google Scholar] [CrossRef]
- Gambacorta, F.V.; Dietrich, J.J.; Yan, Q.; Pfleger, B.F. Rewiring yeast metabolism to synthesize products beyond ethanol. Curr. Opin. Chem. Biol. 2020, 59, 182–192. [Google Scholar] [CrossRef]
- Koppolu, V.; Vasigala, V.K. Role of Escherichia coli in Biofuel Production. Microbiol. Insights 2016, 9, 29–35. [Google Scholar] [CrossRef]
- Yang, S.; Fei, Q.; Zhang, Y.; Contreras, L.M.; Utturkar, S.M.; Brown, S.D.; Himmel, M.E.; Zhang, M. Zymomonas mobilis as a model system for production of biofuels and biochemicals. Microb. Biotechnol. 2016, 9, 699–717. [Google Scholar] [CrossRef]
- Duwe, A.; Tippkötter, N.; Ulber, R. Lignocellulose-Biorefinery: Ethanol-Focused. Adv. Biochem. Eng. Biotechnol. 2019, 166, 177–215. [Google Scholar] [CrossRef]
- Lennartsson, P.R.; Erlandsson, P.; Taherzadeh, M.J. Integration of the first and second generation bioethanol processes and the importance of by-products. Bioresour. Technol. 2014, 165, 3–8. [Google Scholar] [CrossRef]
- Ruchala, J.; Kurylenko, O.O.; Dmytruk, K.V.; Sibirny, A.A. Construction of advanced producers of first- and second-generation ethanol in Saccharomyces cerevisiae and selected species of non-conventional yeasts (Scheffersomyces stipitis, Ogataea polymorpha). J. Ind. Microbiol. Biotechnol. 2020, 47, 109–132. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Raimo, N.; Malandrino, O.; Esposito, B.; Khatami, F.; Goharian, E. Beyond Profitable Shifts to Green Energies, towards Energy Sustainability. Sustainability 2022, 14, 4506. [Google Scholar] [CrossRef]
- Mizik, T.; Gyarmati, G. Economic and Sustainability of Biodiesel Production—A Systematic Literature Review. Clean Technol. 2021, 3, 19–36. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D.; Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Leach, F.; Kalghatgi, G.; Stone, R.; Miles, P. The scope for improving the efficiency and environmental impact of internal combustion engines. Transp. Eng. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Mitsis, T.; Dragoumani, K.; Efthimiadou, A.; Bacopoulou, F.; Chrousos, G.P.; Eliopoulos, E.; Vlachakis, D. Materials of biological origin and biofuels: Small environmental footprint and epigenetic impact (Review). Int. J. Epigenet. 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Popp, J.; Harangi-Rákos, M.; Gabnai, Z.; Balogh, P.; Antal, G.; Bai, A. Biofuels and Their Co-Products as Livestock Feed: Global Economic and Environmental Implications. Molecules 2016, 21, 285. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.N.; Das, P.; Gayen, K.; Mandal, M.K.; Halder, G.N. Production of biodiesel from microalgae through biological carbon capture: A review. 3 Biotech 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Hindersah, R.; Kamaluddin, N.N.; Samanta, S.; Banerjee, S.; Sarkar, S. Role and perspective of Azotobacter in crops production. SAINS Tanah J. Soil Sci. Agroclimatol. 2020, 17, 170–179. [Google Scholar] [CrossRef]
- Liu, A.; Contador, C.A.; Fan, K.; Lam, H.-M. Interaction and Regulation of Carbon, Nitrogen, and Phosphorus Metabolisms in Root Nodules of Legumes. Front. Plant Sci. 2018, 9, 1860. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N.; Nogales, S. Biodiesel by Transesterification of Rapeseed Oil Using Ultrasound: A Kinetic Study of Base-Catalysed Reactions. Energies 2018, 11, 2229. [Google Scholar] [CrossRef]
- Niehus, X.; Casas-Godoy, L.; Valadez, F.J.R.; Sandoval, G. Evaluation of Yarrowia lipolytica Oil for Biodiesel Production: Land Use Oil Yield, Carbon, and Energy Balance. J. Lipids 2018, 2018, 6393749. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- Lobo, C.B.; Tomás, M.S.J.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Zabermawi, N.M.; Alsulaimany, F.A.; El-Saadony, M.T.; El-Tarabily, K.A. New eco-friendly trends to produce biofuel and bioenergy from microorganisms: An updated review. Saudi J. Biol. Sci. 2022. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- Moradian, J.M.; Fang, Z.; Yong, Y.-C. Recent advances on biomass-fueled microbial fuel cell. Bioresour. Bioprocess. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Pandit, S.; Savla, N.; Sonawane, J.M.; Sani, A.M.; Gupta, P.K.; Mathuriya, A.S.; Rai, A.K.; Jadhav, D.A.; Jung, S.P.; Prasad, R. Agricultural Waste and Wastewater as Feedstock for Bioelectricity Generation Using Microbial Fuel Cells: Recent Advances. Fermentation 2021, 7, 169. [Google Scholar] [CrossRef]
- Taylor-Cornejo, E. Empowering Undergraduates to Fight Climate Change with Soil Microbes. DNA Cell Biol. 2022, 41, 58–63. [Google Scholar] [CrossRef]
- De_Richter, R.; Ming, T.; Davies, P.; Liu, W.; Caillol, S. Removal of non-CO2 greenhouse gases by large-scale atmospheric solar photocatalysis. Prog. Energy Combust. Sci. 2017, 60, 68–96. [Google Scholar] [CrossRef]
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Al Khourdajie, A.; House, J.; et al. A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar] [CrossRef]
- Jafarihaghighi, F.; Ardjmand, M.; Hassani, M.S.; Mirzajanzadeh, M.; Bahrami, H. Effect of Fatty Acid Profiles and Molecular Structures of Nine New Source of Biodiesel on Combustion and Emission. ACS Omega 2020, 5, 16053–16063. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Q.; Shen, Q.; Zhan, J.; Zhao, Y. Genetic engineering of microorganisms for biodiesel production. Bioengineered 2013, 4, 292–304. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E. Application of Microalgae Biomass for Biodiesel Fuel Production. Energies 2022, 15, 4178. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Li, G.; Bai, X.; Huo, S.; Huang, Z. Fast pyrolysis of LERDADEs for renewable biofuels. IET Renew. Power Gener. 2020, 14, 959–967. [Google Scholar] [CrossRef]
- Rezić, T.; Filipović, J.; Šantek, B. Microalgae–A potential source of lipids for biodiesel production. Hrvat. Časopis Prehrambenu Tehnol. Biotehnol. Nutr. 2014, 9, 26–36. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Nigam, H.; Malik, A.; Singh, V. A novel nanoemulsion-based microalgal growth medium for enhanced biomass production. Biotechnol. Biofuels 2021, 14, 111. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Subramaniam, R.; Dufreche, S.; Zappi, M.; Bajpai, R. Microbial lipids from renewable resources: Production and characterization. J. Ind. Microbiol. Biotechnol. 2010, 37, 1271–1287. [Google Scholar] [CrossRef]
- Uma, V.S.; Usmani, Z.; Sharma, M.; Diwan, D.; Sharma, M.; Guo, M.; Tuohy, M.G.; Makatsoris, C.; Zhao, X.; Thakur, V.K.; et al. Valorisation of algal biomass to value-added metabolites: Emerging trends and opportunities. Phytochem. Rev. 2022, 1–26. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from microalgae biomass: A review of conversion processes and procedures. Arab. J. Chem. 2022, 15, 103591. [Google Scholar] [CrossRef]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; da Costa Sousa, L.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production Processes, Systems and Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 45–83. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2021, 20, 153–188. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Nucleosynthesis and the origin of the elements. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1988, 325, 391–403. [CrossRef]
- Zaikowski, L.; Wilkens, R.T.; Fisher, K. Science and the Concept of Evolution: From the Big Bang to the Origin and Evolution of Life. Evol. Educ. Outreach 2007, 1, 65–73. [Google Scholar] [CrossRef][Green Version]
- Haider, Q. Nuclear Fusion: Holy Grail of Energy. In Nuclear Fusion—One Noble Goal and a Variety of Scientific and Technological Challenges; Girka, I., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-788-7. [Google Scholar]
- Croswell, K. News Feature: Tracing gold’s cosmic origin story. Proc. Natl. Acad. Sci. USA 2021, 118, e2026110118. [Google Scholar] [CrossRef]
- Chen, H.; Simoska, O.; Lim, K.; Grattieri, M.; Yuan, M.; Dong, F.; Lee, Y.S.; Beaver, K.; Weliwatte, S.; Gaffney, E.M.; et al. Fundamentals, Applications, and Future Directions of Bioelectrocatalysis. Chem. Rev. 2020, 120, 12903–12993. [Google Scholar] [CrossRef]
- Clemente, J. Energy as a Foundation of Modern Life. J. Energy Dev. 2009, 35, 33–48. [Google Scholar]
- Lambert, J.G.; Hall, C.A.S.; Balogh, S.; Gupta, A.; Arnold, M. Energy, EROI and quality of life. Energy Policy 2014, 64, 153–167. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Delucchi, M.A.; Bauer, Z.A.F.; Goodman, S.C.; Chapman, W.E.; Cameron, M.A.; Bozonnat, C.; Chobadi, L.; Clonts, H.A.; Enevoldsen, P.; et al. 100% Clean and Renewable Wind, Water, and Sunlight All-Sector Energy Roadmaps for 139 Countries of the World. Joule 2017, 1, 108–121. [Google Scholar] [CrossRef]
- Fox, W.K. Energy source. Access Sci. 2021. [Google Scholar] [CrossRef]
- Hora, H.; Eliezer, S.; Kirchhoff, G.; Nissim, N.; Wang, J.; Lalousis, P.; Xu, Y.; Miley, G.; Martinez-Val, J.; McKenzie, W. Road map to clean energy using laser beam ignition of boron-hydrogen fusion. Laser Part. Beams 2017, 35, 730–740. [Google Scholar] [CrossRef]
- Holmlid, L.; Kotarba, A.; Stelmachowski, P. Production of ultra-dense hydrogen H(0): A novel nuclear fuel. Int. J. Hydrogen Energy 2021, 46, 18466–18480. [Google Scholar] [CrossRef]
- Ibrahim, A.A. Hydrogen Production from Light Hydrocarbons. In Advances in Hydrogen Generation Technologies; Eyvaz, M., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-535-7. [Google Scholar]
- Mann, M.E. Do Global Warming and climate change represent a serious threat to our welfare and environment? Soc. Philos. Policy 2009, 26, 193–230. [Google Scholar] [CrossRef]
- Su, X.; Zhao, W.; Xia, D. The diversity of hydrogen-producing bacteria and methanogens within an in situ coal seam. Biotechnol. Biofuels 2018, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Rittmann, B.E.; Parra-Saldivar, R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third generation biofuels. J. Clean. Prod. 2015, 98, 53–65. [Google Scholar] [CrossRef]
- Davey, M.E.; O’Toole, G.A. Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef]
- Smith, N.W.; Shorten, P.R.; Altermann, E.; Roy, N.C.; McNabb, W.C. The Classification and Evolution of Bacterial Cross-Feeding. Front. Ecol. Evol. 2019, 7, 153. [Google Scholar] [CrossRef]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A. How Cells Obtain Energy from Food–Molecular Biology of the Cell–NCBI Bookshelf. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Awasthi, M.K.; Taherzadeh, M. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Detman, A.; Bucha, M.; Treu, L.; Chojnacka, A.; Pleśniak, Ł.; Salamon, A.; Łupikasza, E.; Gromadka, R.; Gawor, J.; Gromadka, A.; et al. Evaluation of acidogenesis products’ effect on biogas production performed with metagenomics and isotopic approaches. Biotechnol. Biofuels 2021, 14, 1–25. [Google Scholar] [CrossRef]
- Karekar, S.; Stefanini, R.; Ahring, B. Homo-Acetogens: Their Metabolism and Competitive Relationship with Hydrogenotrophic Methanogens. Microorganisms 2022, 10, 397. [Google Scholar] [CrossRef]
- González, J.; Sánchez, M.E.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C J. Carbon Res. 2018, 4, 59. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical background and biotechnological applications. AMB Express 2018, 8, 1–22. [Google Scholar] [CrossRef]
- Rodríguez-Reyes, J.J.; García-Depraect, O.; Castro-Muñoz, R.; León-Becerril, E. Dark Fermentation Process Response to the Use of Undiluted Tequila Vinasse without Nutrient Supplementation. Sustainability 2021, 13, 11034. [Google Scholar] [CrossRef]
- Nosek, D.; Cydzik-Kwiatkowska, A. Microbial Structure and Energy Generation in Microbial Fuel Cells Powered with Waste Anaerobic Digestate. Energies 2020, 13, 4712. [Google Scholar] [CrossRef]
- Bhagchandanii, D.D.; Babu, R.P.; Sonawane, J.M.; Khanna, N.; Pandit, S.; Jadhav, D.A.; Khilari, S.; Prasad, R. A Comprehensive Understanding of Electro-Fermentation. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers. Biorefin. 2020, 1–19. [Google Scholar] [CrossRef]
- Barghash, H.; Okedu, K.E.; Al Balushi, A. Bio-Hydrogen Production Using Landfill Leachate Considering Different Photo-Fermentation Processes. Front. Bioeng. Biotechnol. 2021, 9, 644065. [Google Scholar] [CrossRef]
- Moussa, R.N.; Moussa, N.; Dionisi, D. Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance. Processes 2022, 10, 156. [Google Scholar] [CrossRef]
- Yates, M.; Strycharz-Glaven, S.; Golden, J.; Roy, J.; Tsoi, S.; Erickson, J.; El-Naggar, M.; Barton, S.C.; Tender, L. Characterizing Electron Transport through Living Biofilms. J. Vis. Exp. 2018, 2018, e54671. [Google Scholar] [CrossRef]
- Nawaz, A.; Hafeez, A.; Abbas, S.Z.; Haq, I.U.; Mukhtar, H.; Rafatullah, M. A state of the art review on electron transfer mechanisms, characteristics, applications and recent advancements in microbial fuel cells technology. Green Chem. Lett. Rev. 2020, 13, 101–117. [Google Scholar] [CrossRef]
- Yan, Z.; Hitt, J.L.; Turner, J.A.; Mallouk, T.E. Renewable electricity storage using electrolysis. Proc. Natl. Acad. Sci. USA 2020, 117, 12558–12563. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Osma, J.F. Microbial Electrochemical Systems: Deriving Future Trends from Historical Perspectives and Characterization Strategies. Front. Environ. Sci. 2020, 8, 44. [Google Scholar] [CrossRef]
- Schachinger, F.; Chang, H.; Scheiblbrandner, S.; Ludwig, R. Amperometric Biosensors Based on Direct Electron Transfer Enzymes. Molecules 2021, 26, 4525. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Dudek, M.; Zieliński, M.; Nowicka, A.; Kazimierowicz, J. Microalgal Hydrogen Production in Relation to Other Biomass-Based Technologies—A Review. Energies 2021, 14, 6025. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Chew, K.W.; Selvarajoo, A.; Chen, W.-H.; Chang, J.-S.; Show, P.L. Microalgae: The Future Supply House of Biohydrogen and Biogas. Front. Energy Res. 2021, 9, 158. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Lazaro, C.Z.; Sagir, E. CHAPTER 1. Photosynthesis and Hydrogen from Photosynthetic Microorganisms. Compr. Ser. Photochem. Photobiol. Sci. 2018, 16, 1–30. [Google Scholar] [CrossRef]
- Bayro-Kaiser, V.; Nelson, N. Microalgal hydrogen production: Prospects of an essential technology for a clean and sustainable energy economy. Photosynth. Res. 2017, 133, 49–62. [Google Scholar] [CrossRef]
- Burlacot, A.; Peltier, G. Photosynthetic Electron Transfer Pathways During Hydrogen Photoproduction in Green Algae: Mechanisms and Limitations. Compr. Ser. Photochem. Photobiol. Sci. 2018, 16, 189–212. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Nowicka, A.; Kazimierowicz, J.; Zieliński, M. The Effect of Autotrophic Cultivation of Platymonas subcordiformis in Waters from the Natural Aquatic Reservoir on Hydrogen Yield. Resources 2022, 11, 31. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current perspective on production and applications of microbial cellulases: A review. Bioresour. Bioprocess. 2021, 8, 1–34. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.; Kumar, G.; Yang, Y.-H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.-P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Zheng, J.-L.; Chen, T.-H.; Bhuyar, P. High Performance of Biohydrogen Production in Packed-Filter Bioreactor via Optimizing Packed-Filter Position. Int. J. Environ. Res. Public Health 2021, 18, 7462. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Mah, A.X.Y.; Ho, W.S.; Bong, C.P.C.; Hassim, M.H.; Liew, P.Y.; Asli, U.A.; Kamaruddin, M.J.; Chemmangattuvalappil, N.G. Review of hydrogen economy in Malaysia and its way forward. Int. J. Hydrogen Energy 2019, 44, 5661–5675. [Google Scholar] [CrossRef]
- Xie, Q.; Lu, Y.; Tang, L.; Zeng, G.; Yang, Z.; Fan, C.; Wang, J.; Atashgahi, S. The mechanism and application of bidirectional extracellular electron transport in the field of energy and environment. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1924–1969. [Google Scholar] [CrossRef]
- Li, X.; Abu-Reesh, I.M.; He, Z. Development of Bioelectrochemical Systems to Promote Sustainable Agriculture. Agriculture 2015, 5, 367–388. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Al-Ghouti, M.; Hassan, M.K. From Waste to Watts: Updates on Key Applications of Microbial Fuel Cells in Wastewater Treatment and Energy Production. Sustainability 2022, 14, 955. [Google Scholar] [CrossRef]
- Santoro, C.; Garcia, M.J.S.; Walter, X.A.; You, J.; Theodosiou, P.; Gajda, I.; Obata, O.; Winfield, J.; Greenman, J.; Ieropoulos, I. Urine in Bioelectrochemical Systems: An Overall Review. ChemElectroChem 2020, 7, 1312–1331. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Jose, V. Photobiological hydrogen as a renewable fuel. Holist. Approach Environ. 2021, 11, 67–71. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef]
- Sakurai, H.; Masukawa, H.; Kitashima, M.; Inoue, K. Photobiological hydrogen production: Bioenergetics and challenges for its practical application. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 1–25. [Google Scholar] [CrossRef]
- Vardar-Schara, G.; Maeda, T.; Wood, T. Metabolically engineered bacteria for producing hydrogen via fermentation. Microb. Biotechnol. 2008, 1, 107–125. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Okoh, A.I.; Makaka, G.; Simon, M. Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy. Int. J. Environ. Res. Public Health 2013, 10, 4390–4417. [Google Scholar] [CrossRef]
- Bozdag, G.O.; Libby, E.; Pineau, R.; Reinhard, C.T.; Ratcliff, W.C. Oxygen suppression of macroscopic multicellularity. Nat. Commun. 2021, 12, 2838. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Navarro, P.X.; Poggi-Varaldo, H.M. Hydrogen from Dark Fermentation of the Organic Fraction of Waste Diapers: Optimization Based on Response Surface Experiments. Front. Energy Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Marshall, I.P.; Berggren, D.R.; Azizian, M.F.; Burow, L.C.; Semprini, L.; Spormann, A.M. The Hydrogenase Chip: A tiling oligonucleotide DNA microarray technique for characterizing hydrogen-producing and -consuming microbes in microbial communities. ISME J. 2011, 6, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.; Lindblad, P. Cyanobacterial Hydrogenases and Hydrogen Metabolism Revisited: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2015, 16, 10537–10561. [Google Scholar] [CrossRef]
- Veaudor, T.; Blanc-Garin, V.; Chenebault, C.; Diaz-Santos, E.; Sassi, J.-F.; Cassier-Chauvat, C.; Chauvat, F. Recent Advances in the Photoautotrophic Metabolism of Cyanobacteria: Biotechnological Implications. Life 2020, 10, 71. [Google Scholar] [CrossRef]
- Lima-Melo, Y.; Kılıç, M.; Aro, E.-M.; Gollan, P.J. Photosystem I Inhibition, Protection and Signalling: Knowns and Unknowns. Front. Plant Sci. 2021, 12, 791124. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Thavasi, V.; Zharmukhamedov, S.K.; Klimov, V.V.; Nagata, T.; Nishihara, H.; Ramakrishna, S. Hydrogen photoproduction by use of photosynthetic organisms and biomimetic systems. Photochem. Photobiol. Sci. 2009, 8, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zimorski, V.; Mentel, M.; Tielens, A.G.M.; Martin, W.F. Energy metabolism in anaerobic eukaryotes and Earth’s late oxygenation. Free Radic. Biol. Med. 2019, 140, 279–294. [Google Scholar] [CrossRef]
- Key, S.; Ma, J.K.-C.; Drake, P.M. Genetically modified plants and human health. J. R. Soc. Med. 2008, 101, 290–298. [Google Scholar] [CrossRef]
- Gregory, T.R. Understanding Natural Selection: Essential Concepts and Common Misconceptions. Evol. Educ. Outreach 2009, 2, 156–175. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Carvajal, A.I.; Wilson, R.H.; Cai, Z.; Li, Y. Discovery of a readily heterologously expressed Rubisco from the deep sea with potential for CO2 capture. Bioresour. Bioprocess. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Bay, S.K.; Waite, D.W.; Dong, X.; Gillor, O.; Chown, S.L.; Hugenholtz, P.; Greening, C. Chemosynthetic and photosynthetic bacteria contribute differentially to primary production across a steep desert aridity gradient. ISME J. 2021, 15, 3339–3356. [Google Scholar] [CrossRef] [PubMed]
- Adam, N.; Perner, M. Microbially Mediated Hydrogen Cycling in Deep-Sea Hydrothermal Vents. Front. Microbiol. 2018, 9, 2873. [Google Scholar] [CrossRef]
- Jordaan, K.; Lappan, R.; Dong, X.; Aitkenhead, I.J.; Bay, S.K.; Chiri, E.; Wieler, N.; Meredith, L.K.; Cowan, D.A.; Chown, S.L.; et al. Hydrogen-Oxidizing Bacteria Are Abundant in Desert Soils and Strongly Stimulated by Hydration. mSystems 2020, 5, e01131-20. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Klavins, L.; Vaseashta, A. Microbial Life on the Surface of Microplastics in Natural Waters. Appl. Sci. 2021, 11, 11692. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Lovley, D.R. Role of the NiFe Hydrogenase Hya in Oxidative Stress Defense in Geobacter sulfurreducens. J. Bacteriol. 2012, 194, 2248–2253. [Google Scholar] [CrossRef]
- Kruse, S.; Goris, T.; Wolf, M.; Wei, X.; Diekert, G. The NiFe Hydrogenases of the Tetrachloroethene-Respiring Epsilonproteobacterium Sulfurospirillum multivorans: Biochemical Studies and Transcription Analysis. Front. Microbiol. 2017, 8, 444. [Google Scholar] [CrossRef]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural Transformation of Heterogeneous Materials for Electrocatalytic Oxygen Evolution Reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, P. Future Microbial Applications for Bioenergy Production: A Perspective. Front. Microbiol. 2017, 8, 450. [Google Scholar] [CrossRef]
- Sahni, S.; Singh, M.K.; Narang, A. Sustainable Solution for Future Energy Challenges Through Microbes. Energy 2021, 231–249. [Google Scholar] [CrossRef]
- Park, S.-G.; Rajesh, P.; Sim, Y.-U.; Jadhav, D.A.; Noori, T.; Kim, D.-H.; Al-Qaradawi, S.Y.; Yang, E.; Jang, J.-K.; Chae, K.-J. Addressing scale-up challenges and enhancement in performance of hydrogen-producing microbial electrolysis cell through electrode modifications. Energy Rep. 2022, 8, 2726–2746. [Google Scholar] [CrossRef]
- Germscheidt, R.L.; Moreira, D.E.B.; Yoshimura, R.G.; Gasbarro, N.P.; Datti, E.; dos Santos, P.L.; Bonacin, J.A. Hydrogen Environmental Benefits Depend on the Way of Production: An Overview of the Main Processes Production and Challenges by 2050. Adv. Energy Sustain. Res. 2021, 2, 2100093. [Google Scholar] [CrossRef]
- Pulyaeva, V.N.; A Kharitonova, N.; Kharitonova, E.N. Advantages and Disadvantages of the Production and Using of Liquid Biofuels. IOP Conf. Ser. Mater. Sci. Eng. 2020, 976, 012031. [Google Scholar] [CrossRef]

| Microorganism | Substrate | % | Ref. | |
|---|---|---|---|---|
| Bacteria | Zymomonas mobilis | Corn steep liquor | 98% | [37] |
| Saccharomyces cerevisiae | Barley straw | 82% | [38] | |
| S. cerevisiae | Coffee grounds | 87.2% | [39] | |
| Yeasts | Kluyveromyces marxianus SUB-80-S | Poplar and eucalyptus biomass | 50–72% | [40] |
| K. marxianus IMB3 | Wild millet | 86% | [41] | |
| Candida shehatae | SX media (3% xylose and 0.67% YNB (nitrogen base without yeast) without amino acid) | 71.6% | [42] | |
| Mold | Fusarium oxysporum | Beer trope | 60% of the theoretical yield | [43] |
| Microalgae | Substrate | Mass Proportion of Oil (%) | Ref. |
|---|---|---|---|
| Botryococcus braunii | dry matter | 25–75 | [94] |
| Chlorella sp. | dry matter | 28–32 | [94] |
| Crypthecodinium cohnii | dry matter | 20 | [94] |
| Nannochloropsis sp. | dry matter | 31–68 | [94] |
| Phaeodactylum tricornutum | dry matter | 20–30 | [94] |
| Schizochytrium sp. | dry matter | 50–77 | [94] |
| Cylindrotheca sp. | dry matter | 16–37 | [95] |
| Nitzschia sp. | dry matter | 45–47 | [95] |
| Feedstock | Production Method | Energy Efficiency (%) | Ref. |
|---|---|---|---|
| Bioelectrolysis (microbial electrolysis) | 70–80 | [103] | |
| Biothermolysis (co-fermentation hydrolysis) | 35–45 | [103] | |
| Biomass | Thermolysis (pyrolysis) | 35–50 | [153] |
| Thermolysis (gasification) | 35–50 | [103] | |
| Thermolysis (partial oxidation) | 60–75 | [103] | |
| Biophotolysis (photofermentation) | <1 | [153] | |
| Microalgae | Biophotolysis (photofermentation) | <1 | [154] |
| Microorganism | Biolysis (dark fermentation) | 60–80 | [155] |
| Process | Energy Source | Feedstock | Capital Cost (M USD) | Hydrogen Cost (USD/kg) | Ref. |
|---|---|---|---|---|---|
| Biomass pyrolysis | Generated steam | Biomass | 53.4–3.1 | 1.25–2.20 | [102] |
| Biomass gasification | Generated steam | Biomass | 149.3–6.4 | 1.77–2.05 | [102] |
| Direct biophotolysis | Solar | Water + algae | 50 USD/m2 | 2.13 | [102] |
| Indirect biophotolysis | Solar | Water + algae | 135 USD/m2 | 1.42 | [102] |
| Dark fermentation | - | Biomass | - | 2.57 | [102] |
| Photo-fermentation | Solar | Biomass | - | 2.83 | [102] |
| Biofuel | Advantage | Disadvantage | Ref. |
|---|---|---|---|
| Bioethanol | Renewable sources; low cost; algae can rapidly absorb carbon dioxide, accumulate high concentrations of lipid and carbohydrates, be easily cultivated, and require less land than terrestrial plants | High costs of lignocellulosic feedstock; inputs of energy and water; requirements for large volume bioreactors and distillation columns; generation of large volumes of waste or low-value coproducts | [35] |
| Biodiesel | Renewable, sustainable, environmentally friendly, and biodegradable sources; low cost and high conversion rate; ideal replacement for petrol; reducing greenhouse gases; less harmful carbon emission; ecologically and economically sustainable bioprocess; use of existing engines without changes | High energy consumption; environmentally unfriendly processing including chemical catalysts, high cost, and limited supply of feedstocks; complex production processes; downstream technology; simultaneously produced waste; production is dependent on large quantities of water and oil | [79] |
| Biohydrogen | Renewable sources; cleanliness; low greenhouse gas emissions; biohydrogen has the advantage of being able to use a wide range of substrates to produce hydrogen; the first stage of the waste treatment and valorization process uses mild temperatures and does not need the external addition of metal catalysts; clear environmental benefits | Low performance; high capital cost investment; expensive materials; complex maintenance; variable energy loss; decreasing hydrogen production with the increase in the volume of the reactor; hydrogen storage; global-warming potential; land use; terrestrial- and freshwater-ecotoxicity potential; ecotoxicity potential; human-toxicity potential | [103,186,187] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talapko, J.; Talapko, D.; Matić, A.; Škrlec, I. Microorganisms as New Sources of Energy. Energies 2022, 15, 6365. https://doi.org/10.3390/en15176365
Talapko J, Talapko D, Matić A, Škrlec I. Microorganisms as New Sources of Energy. Energies. 2022; 15(17):6365. https://doi.org/10.3390/en15176365
Chicago/Turabian StyleTalapko, Jasminka, Domagoj Talapko, Anita Matić, and Ivana Škrlec. 2022. "Microorganisms as New Sources of Energy" Energies 15, no. 17: 6365. https://doi.org/10.3390/en15176365
APA StyleTalapko, J., Talapko, D., Matić, A., & Škrlec, I. (2022). Microorganisms as New Sources of Energy. Energies, 15(17), 6365. https://doi.org/10.3390/en15176365