A Review of Thermochemical Conversion of Waste Biomass to Biofuels
Abstract
:1. Introduction
2. Classification of Waste Biomass and Biogenic Materials
2.1. Lignocellulosic Biomass
2.2. Oilseed Crops
2.3. Municipal Solid Waste
2.4. Food Waste
2.5. Animal Manure
3. Thermochemical Conversion Technologies
3.1. Torrefaction
3.2. Pyrolysis
3.3. Liquefaction
- (i)
- Hydrolysis of biomass → smaller monomer
- (ii)
- Smaller monomer → smaller compounds (by cleavage and decarboxylation)
- (iii)
- Recombination of the smaller fragments → new compounds (by condensation, polymerization)
3.4. Gasification
3.5. Transesterification
4. Major Biofuel Products
4.1. Biochar
4.2. Bio-Crude Oil
4.3. Bio-Oil
4.4. Syngas
4.5. Biodiesel
5. Strengths, Weaknesses, Opportunities and Threats Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, S.; Okolie, J.A.; Nanda, S.; Dalai, A.K. A review of biomass resources and thermochemical conversion technologies. Chem. Eng. Technol. 2022, 45, 791–799. [Google Scholar] [CrossRef]
- World Bioenergy Association. Global Bioenergy Statistics 2020; World Bioenergy Association: Stockholm, Sweden, 2020; Volume 3, p. 49. [Google Scholar]
- IEA. Renewables 2021: Biofuels. Available online: https://www.iea.org/reports/renewables-2021/biofuels?mode=transport®ion=World&publication=2021&flow=Consumption&product=Ethanol (accessed on 22 August 2022).
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.T.; Show, P.L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Xing, J.; Luo, K.; Wang, H.; Gao, Z.; Fan, J. A comprehensive study on estimating higher heating value of biomass from proximate and ultimate analysis with machine learning approaches. Energy 2019, 188, 116077. [Google Scholar] [CrossRef]
- Namkung, H.; Lee, Y.J.; Park, J.H.; Song, G.S.; Choi, J.W.; Kim, J.G.; Park, S.J.; Park, J.C.; Kim, H.T.; Choi, Y.C. Influence of herbaceous biomass ash pre-treated by alkali metal leaching on the agglomeration/sintering and corrosion behaviors. Energy 2019, 187, 115950. [Google Scholar] [CrossRef]
- Güleç, F.; Pekaslan, D.; Williams, O.; Lester, E. Predictability of higher heating value of biomass feedstocks via proximate and ultimate analyses—A comprehensive study of artificial neural network applications. Fuel 2022, 320, 123944. [Google Scholar] [CrossRef]
- Singh, A.; Nanda, S.; Guayaquil-Sosa, J.F.; Berruti, F. Pyrolysis of Miscanthus and characterization of value-added bio-oil and biochar products. Can. J. Chem. Eng. 2021, 99, S55–S68. [Google Scholar] [CrossRef]
- Khan, I.U.; Chen, H.; Yan, Z.; Chen, J. Extraction and quality evaluation of biodiesel from six familiar non-edible plants seeds. Processes 2021, 9, 840. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. An empirical model for prediction of household solid waste generation rate—A case study of Dhanbad, India. Waste Manag. 2017, 68, 3–15. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Municipal solid waste management and landfilling technologies: A review. Environ. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- Rajendran, N.; Gurunathan, B.; Han, J.; Krishna, S.; Ananth, A.; Venugopal, K.; Priyanka, R.S. Recent advances in valorization of organic municipal waste into energy using biorefinery approach, environment and economic analysis. Bioresour. Technol. 2021, 337, 125498. [Google Scholar] [CrossRef]
- Gunarathne, V.; Ashiq, A.; Ramanayaka, S.; Wijekoon, P.; Vithanage, M. Biochar from municipal solid waste for resource recovery and pollution remediation. Environ. Chem. Lett. 2019, 17, 1225–1235. [Google Scholar] [CrossRef]
- Fazeli, A.; Bakhtvar, F.; Jahanshaloo, L.; Sidik, N.A.C.; Bayat, A.E. Malaysia’s stand on municipal solid waste conversion to energy: A review. Renew. Sustain. Energy Rev. 2016, 58, 1007–1016. [Google Scholar] [CrossRef]
- Ouda, O.K.; Cekirge, H.M.; Raza, S.A. An assessment of the potential contribution from waste-to-energy facilities to electricity demand in Saudi Arabia. Energy Convers. Manag. 2013, 75, 402–406. [Google Scholar] [CrossRef]
- Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Townsend, T.G. Opportunities and challenges associated with using municipal waste incineration ash as a raw ingredient in cement production—A review. Resour. Conserv. Recycl. 2020, 160, 104888. [Google Scholar] [CrossRef]
- Mishra, S.; Tiwary, D.; Ohri, A.; Agnihotri, A.K. Impact of municipal solid waste landfill leachate on groundwater quality in Varanasi, India. Groundw. Sustain. Dev. 2019, 9, 100230. [Google Scholar] [CrossRef]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]
- Kiran, E.U.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; He, Z.; Qin, Y.; Li, Y.Y. Enhanced biomethanation of lipids by high-solid co-digestion with food waste: Biogas production and lipids degradation demonstrated by long-term continuous operation. Bioresour. Technol. 2022, 348, 126750. [Google Scholar] [CrossRef]
- Kiran, E.U.; Liu, Y. Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel 2015, 159, 463–469. [Google Scholar] [CrossRef]
- Qin, Z.; Duns, G.J.; Pan, T.; Xin, F. Consolidated processing of biobutanol production from food wastes by solventogenic Clostridium sp. strain HN4. Bioresour. Technol. 2018, 264, 148–153. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A.; Rahman, N.A. Food waste and food processing waste for biohydrogen production: A review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.R.; Nanda, S.; Dalai, A.K.; Meda, V. Slow pyrolysis of agro-food wastes and physicochemical characterization of biofuel products. Chemosphere 2021, 285, 131431. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.R.; Nanda, S.; Dalai, A.K.; Meda, V. Taguchi-based process optimization for activation of agro-food waste biochar and performance test for dye adsorption. Chemosphere 2021, 285, 131531. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Trinh, N.N.; Bach, Q.V. Methane emissions and associated microbial activities from paddy salt-affected soil as influenced by biochar and cow manure addition. Appl. Soil Ecol. 2020, 152, 103531. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Duan, N.; Tsapekos, P.; Awasthi, M.K.; Liu, Z.; Mohammadi, A.; Angelidaki, I.; Tsang, D.C.; Zhang, Z.; Pan, J.; et al. A critical review on livestock manure biorefinery technologies: Sustainability, challenges, and future perspectives. Renew. Sustain. Energy Rev. 2021, 135, 110033. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, G.; An, C.; Chen, X.; Zhang, P.; Xin, X.; Shen, J.; Agnew, J. Anaerobic digestion of livestock manure in cold regions: Technological advancements and global impacts. Renew. Sustain. Energy Rev. 2020, 119, 109494. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Zafiri, C.; Kornaros, M. Dark fermentation of sweet sorghum stalks, cheese whey and cow manure mixture: Effect of pH, pretreatment and organic load. Processes 2021, 9, 1017. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, X.; Wang, Y.; Li, H.; Li, Z.; Zhou, L.; Qu, Y.; Li, Z.; Bao, X. Cow manure as a lignocellulosic substrate for fungal cellulase expression and bioethanol production. AMB Exp. 2018, 8, 190. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Zulkifli, N.W.M.; Ibrahim, S.; Chen, W.H.C.; Chong, C.T.; Ok, Y.S. Valorization of animal manure via pyrolysis for bioenergy: A review. J. Clean. Prod. 2022, 343, 130965. [Google Scholar] [CrossRef]
- Liu, Q.; Xua, R.; Yan, C.; Han, L.; Lei, H.; Ruan, R.; Zhang, X. Fast hydrothermal co-liquefaction of corn stover and cow manure for biocrude and hydrochar production. Bioresour. Technol. 2021, 340, 125630. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Valorization of horse manure through catalytic supercritical water gasification. Waste Manag. 2016, 52, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Iwabuchi, K.; Maemoku, N.; Sasaki, I.; Taniguro, K. A new torrefaction system employing spontaneous self-heating of livestock manure under elevated pressure. Waste Manag. 2019, 85, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cheong, D.Y.; Harvey, J.T.; Kim, J.; Lee, C. Improving biomethanation of chicken manure by co-digestion with ethanol plant effluent. Int. J. Env. Res. Public Health 2019, 16, 5023. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.L.K.; Matsumura, Y. Catalytic gasification of poultry manure and eucalyptus wood mixture in supercritical water. Ind. Eng. Chem. Res. 2012, 51, 5685–5690. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Dalai, A.K.; Meda, V. A review of torrefaction technology for upgrading lignocellulosic biomass to solid biofuels. BioEnergy Res. 2021, 14, 645–669. [Google Scholar] [CrossRef]
- Basu, P.; Sadhukhan, A.K.; Gupta, P.; Rao, S.; Dhungana, A.; Acharya, B. An experimental and theoretical investigation on torrefaction of a large wet wood particle. Bioresour. Technol. 2014, 159, 215–222. [Google Scholar] [CrossRef]
- Chen, W.H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Minaret, J. Review on comparative study of dry and wet torrefaction. Sustain. Energy Technol. Assess. 2015, 12, 26–37. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Torrefaction of Acacia nilotica: Oxygen distribution and carbon densification mechanism based on in-depth analyses of solid, liquid, and gaseous products. Energy Fuels 2020, 34, 12586–12597. [Google Scholar] [CrossRef]
- Amer, M.; Nour, M.; Ahmed, M.; Ookawara, S.; Nada, S.; Elwardany, A. The effect of microwave drying pretreatment on dry torrefaction of agricultural biomasses. Bioresour. Technol. 2019, 286, 121400. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A. Fuel property enhancement of lignocellulosic and nonlignocellulosic biomass through torrefaction. Biomass Convers. Bioref. 2016, 6, 139–149. [Google Scholar] [CrossRef]
- Lê Thành, K.; Commandré, J.M.; Valette, J.; Volle, G.; Meyer, M. Detailed identification and quantification of the condensable species released during torrefaction of lignocellulosic biomasses. Fuel Process. Technol. 2015, 139, 226–235. [Google Scholar] [CrossRef]
- Xu, G.; Li, M.; Lu, P. Experimental investigation on flow properties of different biomass and torrefied biomass powders. Biomass Bioenergy 2019, 122, 63–75. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Dutta, A.; Abhi, T.D. Correlations to predict properties of torrefied biomass using mass loss fraction and experimental validation. Energy Fuels 2020, 34, 11091–11102. [Google Scholar] [CrossRef]
- Zaimes, G.G.; Soratana, K.; Harden, C.L.; Landis, A.E.; Khanna, V. Biofuels via fast pyrolysis of perennial grasses: A life cycle evaluation of energy consumption and greenhouse gas emissions. Environ. Sci. Technol. 2015, 49, 10007–10018. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V. Thermochemical production of bio-oil: A review of downstream processing technologies for bio-oil upgrading, production of hydrogen and high value-added products. Renew. Sustain. Energy Rev. 2021, 135, 110152. [Google Scholar] [CrossRef]
- Suresh, A.; Alagusundaram, A.; Kumar, P.S.; Vo, D.V.N.; Christopher, F.C.; Balaji, B.; Viswanathan, V.; Sankar, S. Microwave pyrolysis of coal, biomass and plastic waste: A review. Environ. Chem. Lett. 2021, 19, 3609–3629. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Yu, S.; Park, J.; Kim, M.; Ryu, C.; Park, J. Characterization of biochar and byproducts from slow pyrolysis of hinoki cypress. Bioresour. Technol. Rep. 2019, 6, 217–222. [Google Scholar] [CrossRef]
- Wang, W.C.; Lee, A.C. The study of producing “drop-in” fuels from agricultural waste through fast pyrolysis and catalytic hydro-processing. Renew. Energy 2019, 133, 1–10. [Google Scholar] [CrossRef]
- Yi, L.; Liu, H.; Li, S.; Li, M.; Wang, G.; Man, G.; Yao, H. Catalytic pyrolysis of biomass wastes over Org-CaO/Nano-ZSM-5 to produce aromatics: Influence of catalyst properties. Bioresour. Technol. 2019, 294, 122186. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chang, X.; Wang, J.; Zhang, Z. Catalytic pyrolysis of agricultural and forestry wastes in a fixed-bed reactor using K2CO3 as the catalyst. Waste Manag. Res. J. Sustain. Circular Econ. 2020, 38, 78–87. [Google Scholar] [CrossRef]
- de Almeida, S.G.; Tarelho, L.A.; Hauschild, T.; Costa, M.A.M.; Dussán, K.J. Biochar production from sugarcane biomass using slow pyrolysis: Characterization of the solid fraction. Chem. Eng. Process. Process Intens. 2022, 179, 109054. [Google Scholar] [CrossRef]
- Gao, L.; Goldfarb, J.L. Solid waste to biofuels and heterogeneous sorbents via pyrolysis of wheat straw in the presence of fly ash as an in situ catalyst. J. Anal. Appl. Pyrolysis 2019, 137, 96–105. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic hydrogenation of cellulose-hemicellulose-lignin and biomass agricultural wastes for synthetic natural gas production. J. Anal. Appl. Pyrolysis 2020, 145, 104753. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, S.; Bassi, A.; Xu, C.C. Improvement in bio-crude yield and quality through co-liquefaction of algal biomass and sawdust in ethanol-water mixed solvent and recycling of the aqueous by-product as a reaction medium. Energy Convers. Manag. 2018, 171, 618–625. [Google Scholar] [CrossRef]
- Singh, R.; Krishna, B.B.; Bhaskar, T. Hydrothermal liquefaction of lignocellulosic biomass components: Effect of alkaline catalyst. In Biofuels; Agarwal, A.K., Agarwal, R.A., Gupta, T., Gurjar, B.R., Eds.; Springer: Singapore, 2017; pp. 69–84. [Google Scholar]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupta, R. Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: Parametric study and products characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Saba, A.; Lopez, B.; Lynam, J.G.; Reza, M.T. Hydrothermal liquefaction of loblolly pine: Effects of various wastes on produced biocrude. ACS Omega 2018, 3, 3051–3059. [Google Scholar] [CrossRef]
- Goswami, G.; Makut, B.B.; Das, D. Sustainable production of bio-crude oil via hydrothermal liquefaction of symbiotically grown biomass of microalgae-bacteria coupled with effective wastewater treatment. Sci. Rep. 2019, 9, 15016. [Google Scholar] [CrossRef] [PubMed]
- Gollakota, A.; Savage, P.E. Hydrothermal liquefaction of model food waste biomolecules and ternary mixtures under isothermal and fast conditions. ACS Sustain. Chem. Eng. 2018, 6, 9018–9027. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagoz, S. Hydrothermal liquefaction of lignocellulosic biomass using potassium fluoride-doped alumina. Energy Fuels 2019, 33, 3248–3256. [Google Scholar] [CrossRef]
- Zhang, Y.; Minaret, J.; Yuan, Z.; Dutta, A.; Xu, C. Mild hydrothermal liquefaction of high water content agricultural residue for bio-crude oil production: A parametric study. Energies 2018, 11, 3129. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, J.H.; Choi, I.G.; Choi, J.W. Comprehensive characterization of hydrothermal liquefaction products obtained from woody biomass under various alkali catalyst concentrations. Environ. Technol. 2019, 40, 1657–1667. [Google Scholar] [CrossRef]
- Makwana, J.P.; Pandey, J.; Mishra, G. Improving the properties of producer gas using high temperature gasification of rice husk in a pilot scale fluidized bed gasifier (FBG). Renew. Energy 2019, 130, 943–951. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Chemistry and energy beyond fossil fuels. A perspective view on the role of syngas from waste sources. Catal. Today 2020, 342, 4–12. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M. Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: A review. Renew. Sustain. Energy Rev. 2019, 105, 462–475. [Google Scholar] [CrossRef]
- Gupta, P.; Velazquez-Vargas, L.G.; Fan, L.S. Syngas Redox (SGR) Process to produce hydrogen from coal derived syngas. Energy Fuels 2007, 21, 2900–2908. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; Dieringer, P.; Epple, B. Biomass-based chemical looping gasification: Overview and recent developments. Appl. Sci. 2021, 11, 7069. [Google Scholar] [CrossRef]
- Gopirajan, P.V.; Gopinath, K.P.; Sivaranjani, G.; Arun, J. Optimization of hydrothermal gasification process through machine learning approach: Experimental conditions, product yield and pollution. J. Clean. Prod. 2021, 306, 127302. [Google Scholar] [CrossRef]
- Hamad, M.A.; Radwan, A.M.; Heggo, D.A.; Moustafa, T. Hydrogen rich gas production from catalytic gasification of biomass. Renew. Energy 2016, 85, 1290–1300. [Google Scholar] [CrossRef]
- Vamvuka, D.; Karouki, E.; Sfakiotakis, S.; Salatino, P. Gasification of waste biomass chars by carbon dioxide via thermogravimetry—Effect of catalysts. Combust. Sci. Technol. 2012, 184, 64–77. [Google Scholar] [CrossRef]
- Gökkaya, D.S.; Çokkuvvetli, T.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal gasification of poplar wood chips with alkali, mineral, and metal impregnated activated carbon catalysts. J. Supercrit. Fluids 2019, 152, 104542. [Google Scholar] [CrossRef]
- Hai, I.U.; Sher, F.; Yaqoob, A.; Liu, H. Assessment of biomass energy potential for SRC willow woodchips in a pilot scale bubbling fluidized bed gasifier. Fuel 2019, 258, 116143. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Veljković, V.B.; Banković-Ilić, I.B.; Stamenković, O.S. Purification of crude biodiesel obtained by heterogeneously-catalyzed transesterification. Renew. Sustain. Energy Rev. 2015, 49, 500–516. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Gebeyehu, K.B.; Asfaw, B.T.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef] [PubMed]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Negm, N.A.; Betiha, M.A.; Alhumaimess, M.S.; Hassan, H.M.; Rabie, A.M. Clean transesterification process for biodiesel production using heterogeneous polymer-heteropoly acid nanocatalyst. J. Clean. Prod. 2019, 238, 117854. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. 2017, 59, 165–188. [Google Scholar] [CrossRef]
- Canet, A.; Bonet-Ragel, K.; Benaiges, M.D.; Valero, F. Lipase-catalysed transesterification: Viewpoint of the mechanism and influence of free fatty acids. Biomass Bioenergy 2016, 85, 94–99. [Google Scholar] [CrossRef]
- Pikula, K.; Zakharenko, A.; Stratidakis, A.; Razgonova, M.; Nosyrev, A.; Mezhuev, Y.; Tsatsakis, A.; Golokhvas, K. The advances and limitations in biodiesel production: Feedstocks, oil extraction methods, production, and environmental life cycle assessment. Green Chem. Lett. Rev. 2020, 13, 275–294. [Google Scholar] [CrossRef]
- Katre, G.; Raskar, S.; Zinjarde, S.; Kumar, V.R.; Kulkarni, B.D.; RaviKumar, A. Optimization of the in situ transesterification step for biodiesel production using biomass of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil. Energy 2018, 142, 944–952. [Google Scholar] [CrossRef]
- Bashiri, H.; Pourbeiram, N. Biodiesel production through transesterification of soybean oil: A kinetic Monte Carlo study. J. Mol. Liquids 2016, 223, 10–15. [Google Scholar] [CrossRef]
- Mohadesi, M.; Aghel, B.; Gouran, A.; Razmehgir, M.H. Transesterification of waste cooking oil using Clay/CaO as a solid base catalyst. Energy 2022, 242, 122536. [Google Scholar] [CrossRef]
- Duti, I.J.; Maliha, M.; Ahmed, S. Biodiesel production from waste frying oil and its process simulation. J. Mod. Sci. Technol. 2016, 4, 50–62. [Google Scholar]
- Hu, W.; Zhou, X.; Tan, J.; Hou, J.; Xie, Y.; Wang, X.; Wang, Y.; Zhang, Y. In situ transesterification of wet sewage sludge via hydrothermal process: Biodiesel production and residue utilization. Biomass Bioenergy 2020, 141, 105715. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valor. 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A review of non-soil biochar applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Kang, K.; Nanda, S.; Hu, Y. Current trends in biochar application for catalytic conversion of biomass to biofuels. Catal. Today 2022. [Google Scholar] [CrossRef]
- Koveria, A.; Kieush, L.; Svietkina, O.; Perkov, Y. Metallurgical coke production with biomass additives. Part 1. A review of existing practices. Can. Metal. Quarter. 2020, 59, 417–429. [Google Scholar] [CrossRef]
- Salimi, M.; Safari, F.; Tavasoli, A.; Shakeri, A. Hydrothermal gasification of different agricultural wastes in supercritical water media for hydrogen production: A comparative study. Int. J. Ind. Chem. 2016, 7, 277–285. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Yuan, R.; Wang, P. Biomass pyrolysis with alkaline-earth-metal additive for co-production of bio-oil and biochar-based soil amendment. Sci. Total Environ. 2020, 743, 140760. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Chand, R.; Borugadda, V.B.; Qiu, M.; Dalai, A.K. Evaluating the potential for bio-fuel upgrading: A comprehensive analysis of bio-crude and bio-residue from hydrothermal liquefaction of agricultural biomass. Appl. Energy 2019, 254, 113679. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae-A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhang, Y.; Zhang, J.; Yu, G.; Schideman, L.C.; Zhang, P.; Minarick, M. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Bioresour. Technol. 2014, 152, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Nakhshiniev, B.; Yoshikawa, K. A review of production and upgrading of algal bio-oil. Renew. Sustain. Energy Rev. 2016, 58, 918–930. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, D.; Feng, X.; Zhu, J.; Lu, X.; Mu, L.; Qian, H. Machine learning prediction of bio-oil characteristics quantitatively relating to biomass compositions and pyrolysis conditions. Fuel 2022, 312, 122812. [Google Scholar] [CrossRef]
- Salehi, E.; Abedi, J.; Harding, T. Bio-oil from sawdust: Effect of operating parameters on the yield and quality of pyrolysis products. Energy Fuels 2011, 25, 4145–4154. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Slow pyrolysis of chemically treated walnut shell for valuable products: Effect of process parameters and in-depth product analysis. Energy 2019, 181, 665–676. [Google Scholar] [CrossRef]
- Joshi, N.; Lawal, A. Hydrodeoxygenation of pyrolysis oil in a microreactor. Chem. Eng. Sci. 2012, 74, 1–8. [Google Scholar] [CrossRef]
- Adnan, M.A.; Xiong, Q.; Muraza, O.; Hossain, M.M. Gasification of wet microalgae to produce H2-rich syngas and electricity: A thermodynamic study considering exergy analysis. Renew. Energy 2020, 147, 2195–2205. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Tian, M.W.; Yuen, H.C.; Yan, S.R.; Huang, W.L. The multiple selections of fostering applications of hydrogen energy by integrating economic and industrial evaluation of different regions. Int. J. Hydrogen Energy 2019, 44, 29390–29398. [Google Scholar] [CrossRef]
- Alam, S.S.; Churkunti, P.R.; Depcik, C. Comparison of waste plastic fuel, waste cooking oil biodiesel, and ultra-low sulfur diesel using a Well-to-Exhaust framework. Int. J. Environ. Sci. Technol. 2022, 19, 5857–5876. [Google Scholar] [CrossRef]
- Ling, J.S.J.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Saptoro, A.; Nolasco-Hipolito, C. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appl. Sci. 2019, 1, 810. [Google Scholar] [CrossRef] [Green Version]
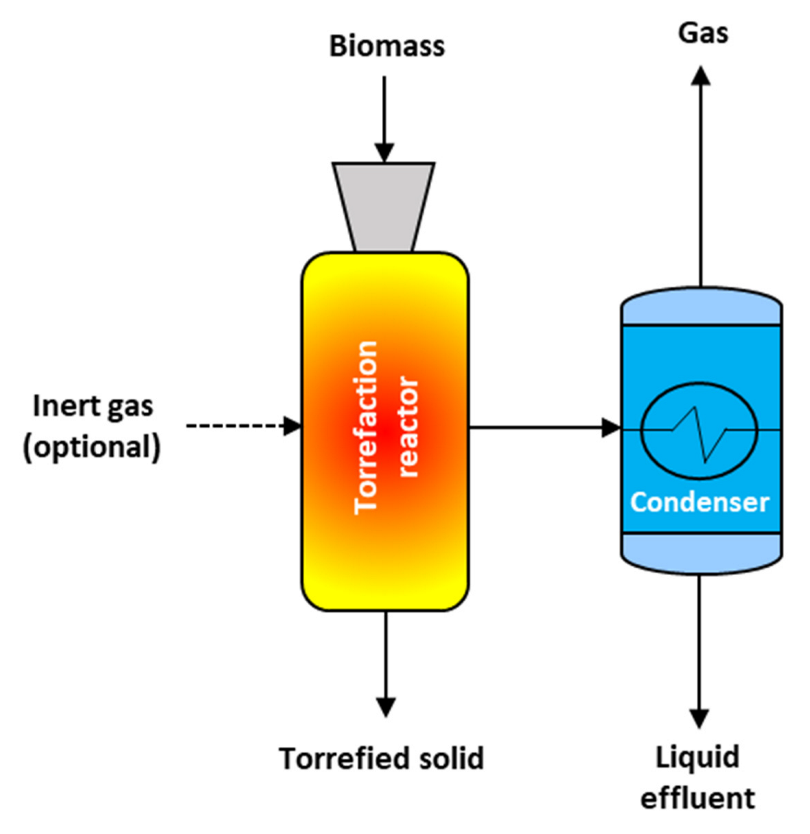


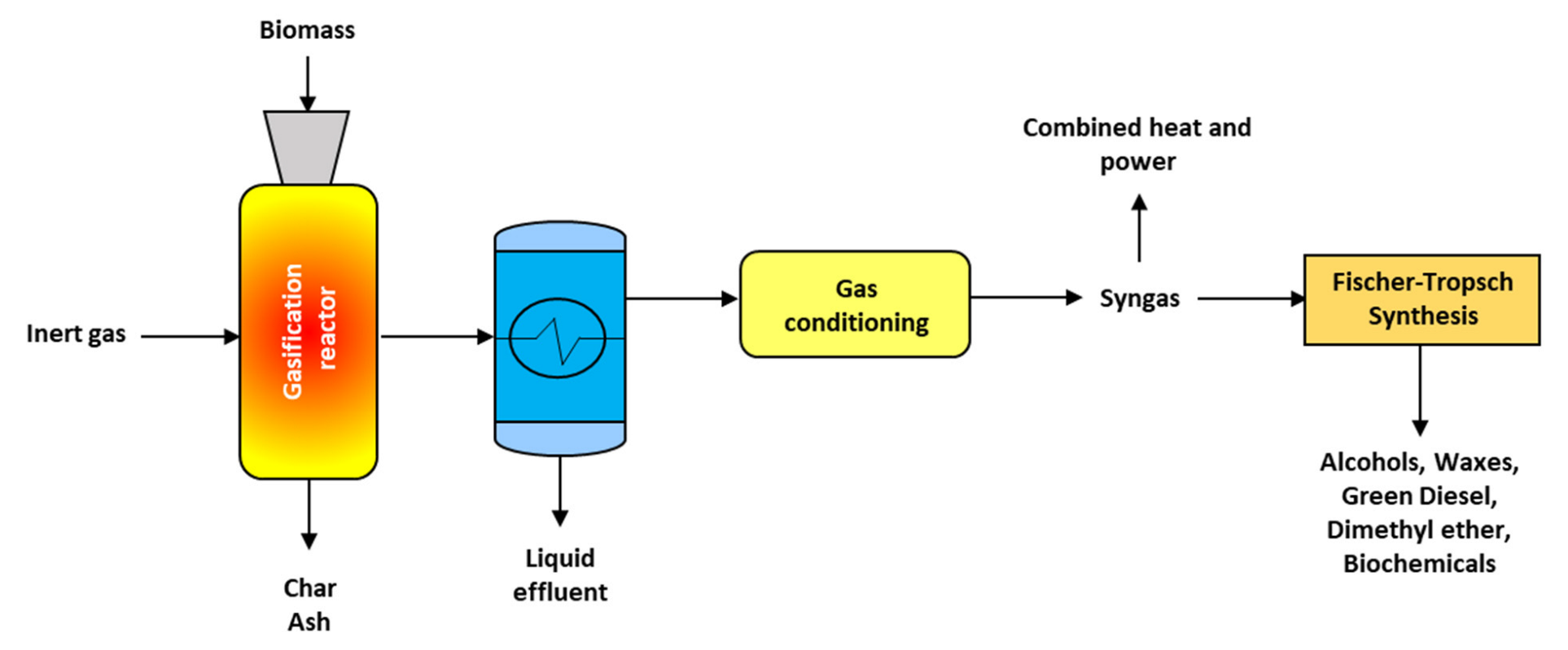
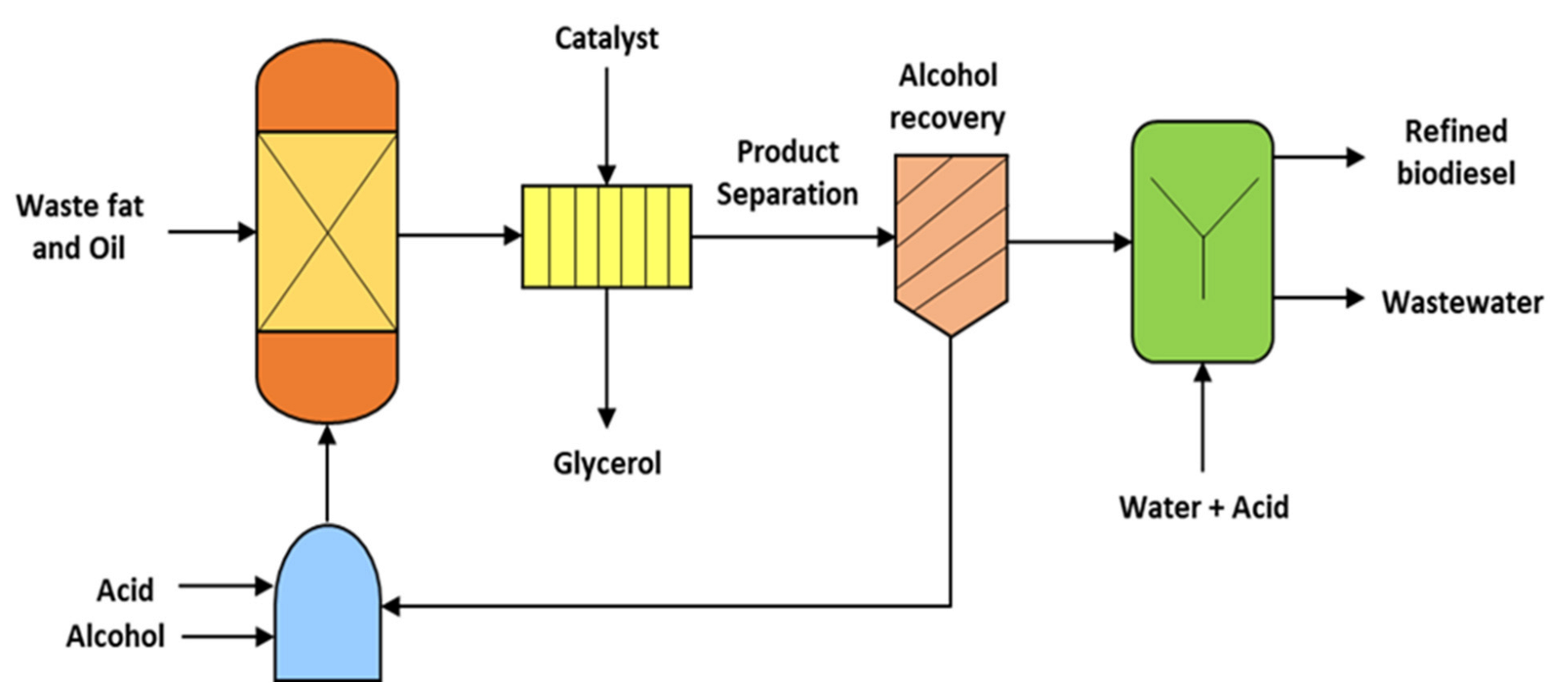
| Feedstock | Temperature (°C) | Residence Time (min) | Observations | Reference |
|---|---|---|---|---|
| Acacia nilotica (Babul) | 225–300 | 15–60 |
| Singh et al. [41] |
| Cotton stalk, sugarcane bagasse, rice straw and rice husk | 250–300 | 30 |
| Amer et al. [42] |
| Oats, willow and poultry litter | 200–300 | 15–45 |
| Acharya and Dutta [43] |
| Pinewood, Miscanthus, and wheat straw | 250–300 | 45 |
| Lê Thành et al. [44] |
| Soybean straw, corn straw, rice straw and rice husk | 300 | - |
| Xu et al. [45] |
| Switchgrass | 200–400 | - |
| Nhuchhen et al. [46] |
| Process | Temperature (°C) | Heating Rate (°C/min) | Vapor Residence Time | Bio-Oil (wt.%) | Biochar (wt.%) | Gas (wt.%) |
|---|---|---|---|---|---|---|
| Slow pyrolysis | 300–500 | <50 | >30 min | 20–50 | 25–35 | 20–50 |
| Intermediate pyrolysis | 400–600 | 200–300 | 10 min | 35–50 | 25–40 | 20–30 |
| Fast/Flash pyrolysis | 400–900 | 10–1000 | <2 s | 60–75 | 10–25 | 10–30 |
| Feedstock | Catalyst | Reactor | Temperature (°C) | Observations | Reference |
|---|---|---|---|---|---|
| Bamboo and pigeon pea stalk biomass | No catalyst | Muffle furnace | 400–600 |
| Sahoo et al. [50] |
| Coconut shell, palm kernel shell, rice husk, cotton stalk, wheat straw, sugarcane bagasse and biomass model compounds (hemicellulose, cellulose and lignin) | 10 wt.% NiAl2O3 | Two-stage fixed bed reactor | 550 |
| Akubo et al. [51] |
| Food waste, canola hull and oat hull | No catalyst | Tubular reactor | 300–600 |
| Patra et al. [24] |
| Hinoki cypress | No catalyst | Fixed bed reactor | 350–600 |
| Yu et al. [52] |
| Miscanthus | 5 wt.% Pd/C | Fluidized bed and fixed bed reactor | 350–550 |
| Wang and Lee [53] |
| Peanut shell, corncob Jatropha seeds de-oiled cake and bagasse | Org-CaO/Nano-ZSM-5 | A dual-catalyst fixed-bed reactor system | 50–320 |
| Yi et al. [54] |
| Pinewood, peanut shell and rice straw | K2CO3 | Fixed bed reactor | 300–700 |
| Fan et al. [55] |
| Sugarcane bagasse, straw and acid-treated biomass | No catalyst | Fixed bed reactor | 450–650 |
| de Almeida et al. [56] |
| Switchgrass and Miscanthus | Trace minerals | Fast pyrolysis reactor | 500 |
| Zaimes et al. [47] |
| Wheat straw | 1–10 wt.% coal fly ash | 450–750 |
| Gao et al. [57] | |
| Willow, sugarcane bagasse, Ugu plant and rice straw | 10 wt.% Ni/Al2O3 | Two-stage pyrolysis-catalytic hydrogenation reactor | 500 |
| Jaffar et al. [58] |
| Feedstock | Catalyst | Reactor | Temperature (°C) | Observations | Reference |
|---|---|---|---|---|---|
| Cellulose and lignin | KOH | High pressure autoclave | 280 |
| Singh et al. [61] |
| Corn stover | - | Autoclave reactor | 250–375 |
| Mathanker et al. [62] |
| Loblolly pine, sewage sludge and cow manure | - | Bench-top reactor | 250–300 |
| Saba et al. [63] |
| Microbial biomass | - | - | 200–300 |
| Goswami et al. [64] |
| Potato starch, Casein and sunflower oil | - | Stainless-steel batch reactors | 350–600 |
| Gollakota and Savage [65] |
| Spruce wood | Potassium fluoride doped alumina catalyst | Bench-top reactor | 250–350 |
| Alper et al. [66] |
| Tomato plant waste | H2SO4 and KOH | Autoclave reactor | 220–280 |
| Zhang et al. [67] |
| Wood biomass (larch and Mongolian oak) | K2CO3 | Batch reactor | 300 |
| Hwang et al. [68] |
| Feedstock | Catalyst | Reactor | Temperature (°C) | Observations | Reference |
|---|---|---|---|---|---|
| Cotton stalks, corn stalks and rice straw | Marly clay, calcium hydroxide, dolomite, and cement kiln dust | Bench-scale fixed-bed reactor | 700–850 |
| Hamad et al. [76] |
| MSW and wastepaper | K2CO3, Li2CO3, Rb2CO3, CaCO3, CsCO3, CaSO4 and Na2CO3 | - | 800–950 |
| Vamvuka et al. [77] |
| Poplar wood chips | Ru/activated carbon, Ni/activated carbon, KOH, Dolomite, Trona and Borax | Batch reactor | 300–600 |
| Gökkaya et al. [78] |
| Willow wood chips | - | Bubbling fluidized bed reactor | 600–850 |
| Hai et al. [79] |
| Feedstock | Catalyst | Reactor | Temperature (°C) | Observations | Reference |
|---|---|---|---|---|---|
| Biomass from the oleaginous yeast Yarrowia lipolytica cultivated on waste cooking oil | H2SO4 | - | 65–110 |
| Katre et al. [91] |
| Soyabean oil | NaOH | Stirred tank reactor | 50 |
| Bashiri and Pourbeiram [92] |
| Waste cooking oil | CaO and Clay | Heater equipped with a magnetic agitator | 45–55 |
| Mohadesi et al. [93] |
| Waste frying oil | NaOH | Shake flask | 55 |
| Duti et al. [94] |
| Wet sewage sludge | H2SO4 | Hydrothermal reactor | 70–160 |
| Hu et al. [95] |
| Technology | Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|---|
| Torrefaction |
|
|
|
|
| Pyrolysis |
|
|
|
|
| Liquefaction |
|
|
|
|
| Gasification |
|
|
|
|
| Transesterification |
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. https://doi.org/10.3390/en15176352
Jha S, Nanda S, Acharya B, Dalai AK. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies. 2022; 15(17):6352. https://doi.org/10.3390/en15176352
Chicago/Turabian StyleJha, Shivangi, Sonil Nanda, Bishnu Acharya, and Ajay K. Dalai. 2022. "A Review of Thermochemical Conversion of Waste Biomass to Biofuels" Energies 15, no. 17: 6352. https://doi.org/10.3390/en15176352
APA StyleJha, S., Nanda, S., Acharya, B., & Dalai, A. K. (2022). A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies, 15(17), 6352. https://doi.org/10.3390/en15176352










