1. Introduction
As to the wide application of heavy fuel oil (HFO) in utility boilers, industrial furnaces, and marine engines, the burning of HFO has been broadly tested in laboratory research and engineering development. With the gradual reduction of conventional light and medium oil resources, efficiently and economically recovering vast reserves of unconventional heavy oil and asphalt has attracted increasing attention. HFO has an energy density content similar to distilled fuel, but it has a very high viscosity and requires additional heating before spray combustion in the boiler [
1]. The specific characteristics of HFO are the high contents of asphaltenes, carbon residues, trace metals, such as vanadium and nickel, and fuel-bound nitrogen and sulfur. Asphaltenes are heavy polycyclic aromatic compounds with embedded heteroatoms, making the fuel difficult to burn, and the inefficient combustion process leads to the formation of large cenospheres (lightweight, inert, hollow spheres).
Asphaltenes are compounds in crude oil, heavy fuels (high-boiling and nonboiling petroleum fractions), and oil sand bitumen. They are insoluble in n-alkanes (e.g., n-heptane) and only soluble in aromatic solvents (e.g., toluene). Their chemical structures are still not fully understood, so asphaltenes are characterized by their solubility class instead of their chemical properties. Asphaltenes have a significant impact on the physicochemical properties of heavy fuels and residues. It has been shown that asphaltenes are the most aromatic part of heavy fuels and, therefore, increase the viscosity of the fuel [
2]. Thus, HFO with high asphaltene content requires the adding of solvent or the preheating of the fuel to improve fluidity. Asphaltenes in crude oil precipitate and deposit along the walls of the oil reservoir, thereby clogging the oil well and causing flowline fouling problems. For all these reasons, asphaltenes have been called ‘bad guys’ in petroleum fuels [
3].
The well-referred results established by HFO combustion were to distinguish two combustion stages. The first is the liquid phase of evaporating volatile substances, and, then, the solid phase of oxidized coke, carbonaceous cenosphere particles formed during the liquid phase. This liquid combustion stage is a complex process that involves heat and mass transfer and chemical reactions, such as pyrolysis (thermal cracking) and polymerization. The solid combustion phase occurs immediately after the liquid combustion phase and forms a hollow shell cenosphere [
4]. Most of the research on HFO combustion reported in the literature was carried out using suspended [
5,
6,
7] and falling droplet [
8,
9,
10] techniques, and many researchers used numerical simulations [
11,
12,
13]. Elbaz et al. [
7] studied the formation and oxidation of heavy oil, HFO, and particles produced by the combustion of droplets. They also used a scanning electron microscope (SEM) and an energy dispersive X-ray (EDX) to analyze the microstructure of the particles. The study concluded that the droplet ignition temperature is independent of the droplet size, but the liquid phase ignition delay time and droplet life are proportional to the initial droplet diameter. Kwack et al. [
14] burned No. 6 fuel in a closed burner, observed the combustion residue through a scanning electron microscope, and studied the qualitative relationship between the shape of these particles and the temperatures to which they were subjected. Xu et al. [
4] divided the process of heavy oil droplet combustion into four steps: ignition delay, flame lifetime, coke glowing delay, and coke ember time. Using the mixed oil of mixed heavy oil residue (HOR) and diesel light oil (LO), they further analyzed their composition characteristics in terms of oil composition and combustion conditions. They found that increasing the temperature of the combustion chamber significantly reduced the ignition delay and the coke luminescence delay, but hardly changed the flame lifetime. Ambalae et al. [
15] used a thermogravimetric analyzer (TGA) to obtain information on the pyrolysis and combustion behavior of crude oil (Neilburg) and its asphaltenes. The study found that asphaltenes contributed the most to coke formation among all saturated, aromatic, resin, and asphaltene fractions. They analyzed the temperature rise of whole oil and asphaltenes and conducted isothermal pyrolysis experiments to determine the temperature at which coke formation was maximized. In addition, they obtained isothermal combustion curves of coke derived from whole oil and asphaltenes. Atiku et al. [
16] explored the mechanism of forming fine particulate soot and cenospheres and studied the chemical structure of petroleum asphaltenes through pyrolysis technology. Jameel et al. [
17] used non-isothermal thermogravimetric analysis (TGA) and Fourier transform infrared (FTIR) spectrometers to study the pyrolysis and combustion of heavy oil in nitrogen and air, respectively, and deeply understood the three stages of heavy oil combustion.
Some work has been conducted on the influences of oil compositions on HFO burning. The effect of oil composition on HFO burning was studied by changing asphaltene content [
5,
18,
19], sulfur [
6,
20], metal (vanadium) [
21], viscosity [
22,
23], and fuel nitrogen [
24]. The viscosity of HFO largely depends on the volume fraction, chemical structure, and physicochemical properties of its asphaltenes. Asphaltenes are the most polar and heaviest components in HFO [
25]. Fakher et al. [
26] explained the main components of crude oil and its relationship with asphaltenes and the methods for quantifying asphaltenes in crude oils, showing more discussed models for asphaltene modeling, and mentioned the chemistry used to characterize and study asphaltene analysis methods. In addition, they also introduced the methods by which asphaltenes destroy oil recovery. The structure of asphaltenes was studied through atomic force microscopy with atomic resolution imaging and scanning tunnelling microscopy with molecular orbital imaging to study more than 100 asphaltene molecules [
27]. Peng et al. [
28] conducted experimental and theoretical studies on the specific effects of asphaltene content on the viscosity of heavy oil at different temperatures. They determined four important parameters to characterize the reconstituted heavy oil samples: solvation constant, shape factor, intrinsic viscosity, and maximum filling volume fraction. The study showed, through the results of nonlinear regression, that the state of asphaltene particles in heavy oil changed with the change of asphaltene content and temperature, and this change greatly influenced the viscosity of heavy oil. Bartle [
29] showed that asphaltene reduced the ignition delay time of heavy oil but did not affect the burning time of fuel droplets. The reduction in ignition delay was attributed to the volatiles produced by the pyrolysis of asphaltenes.
The current work is dedicated to showing how blending asphaltene influences the combustion of heavy fuel oil. The emission characteristics (gaseous emissions and particulate matter) of different asphaltene constituent oils were tested in the swirling flame burning system, while the inspected parameters were mainly about coking characteristics. This research used measurements to analyze blending fuel characteristics, combustion performance, and pollution, considered necessary for future energy supplies using diversified fuels. In this research, fuel samples with five different HFO blending fuels with asphaltene mass fractions of 4%, 6%, 8%, 16%, and 24% were compared in swirling flame experiments. Pure HFO containing 8% (by mass) asphaltenes was used as a basis for comparison. Low asphaltene content fuel oils were prepared by blending HFO with diesel light oil, and extra asphaltene was added to the HFO to produce high asphaltene oils. This work also intends to illustrate how the mixing of asphaltenes affects the emissions of HFO combustion.
2. Methodology and Fuel Characterization
In general, the burning behavior of different HFOs could lead to the economic development of energy systems with a wide range of fuel adaptability. Heavy crude oil’s chemical and physical properties change dramatically from one reservoir to another. Nevertheless, it is currently impossible to test all available HFO samples. In this regard, we recommend that any heavy oil is essentially a mixture of heavy oil, asphaltene, and light oil (diesel). Then, the critical oil characteristics, such as viscosity, density, volatility, asphaltene, and sulfur content, can be adjusted by changing the HFO asphaltene and diesel oil ratio, which determines the combustion characteristics of the oils. In this study, five different HFO samples were prepared by blending HFO with diesel and pure asphaltenes, with asphaltene mass fractions of 4%, 6%, 8%, 16%, and 24%, respectively, given in
Table 1. Mixing HFO changes the oil components so that the influence of asphaltene can be thoroughly analyzed. Therefore, this constitutes the main objective of the study. The original HFO sample used in this work featured 8% asphaltene in mass by the test standard IP 143, which was collected from the Shoaiba power plant in Saudi Arabia, and detailed information about these HFO properties can be found in Ref. [
30].
As shown in
Table 2, pure asphaltene was Chinese Standard No. 90 asphaltene, the properties of which are similar to those extracted from HFO [
20], and was applied to blending fuel to ensure high asphaltene contents of 16% and 24%. The light oil used was a commercially available diesel to produce low asphaltene content fuels of 4% and 6%. In
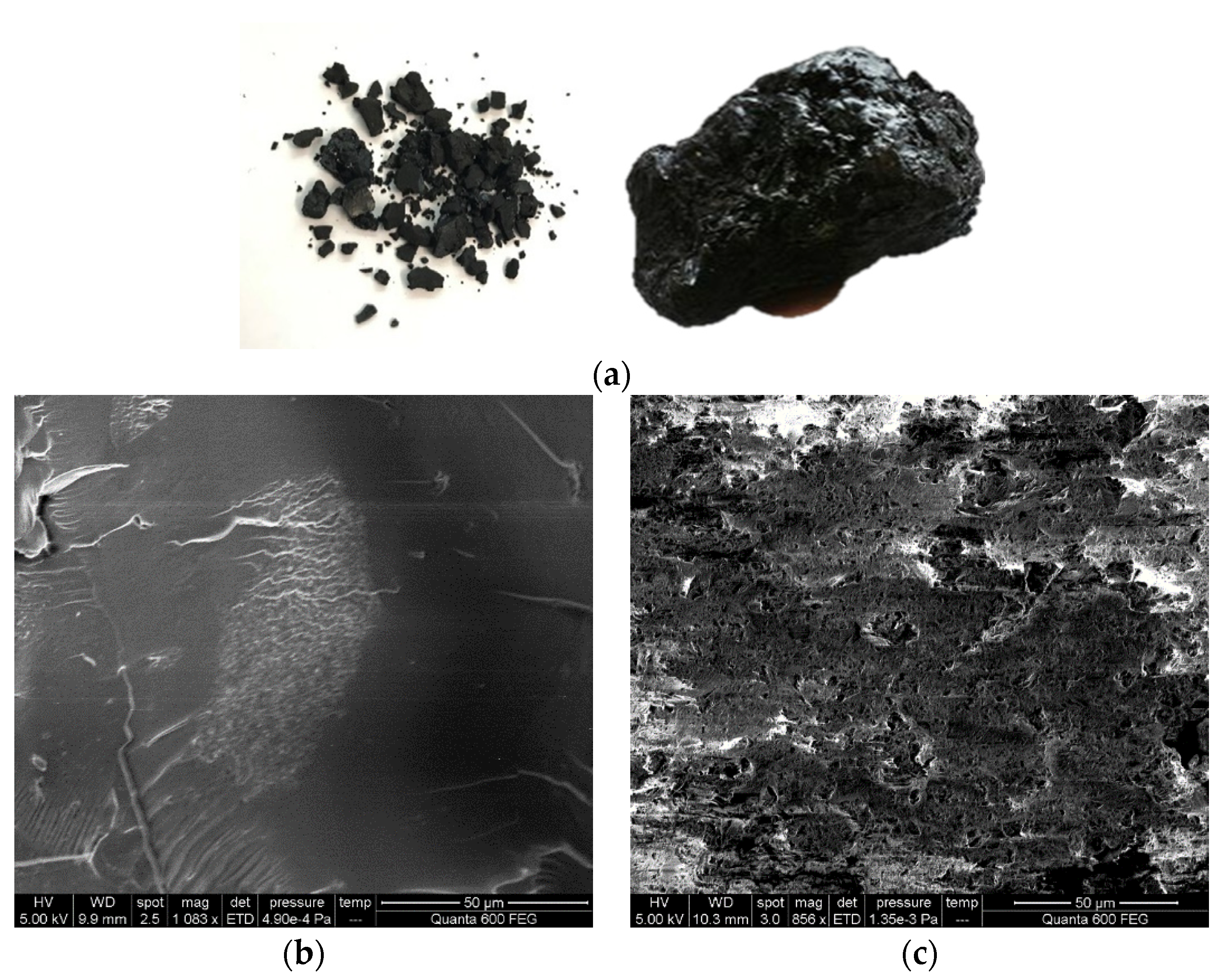
Figure 1, the asphaltene looked like pitch or soft bitumen at 27 °C, but it became of low liquidity when the temperature rose to 67 °C [
4]. Asphaltenes are a class of hydrocarbons with heteroatoms (such as S, N, and O), only defined by their precipitation in nonpolar solvents (such as pentane, hexane, or heptane). Typical asphaltenes include: (a) aromatic rings with alkyl chains up to C30, (b) Sulfur in the benzothiophene ring, (c) Nitrogen in Pyrrole and Pyridine, (d) Porphyrin compounded with vanadium, nickel, and nitrogen-containing pyrrole, (e) Ketones, carboxylic acids, phenols [
31].
Fuel viscosity is critical to fuel spray characteristics and further affects the combustion performance. Viscosity is a measurement of the internal flow resistance of a fluid when it is being deformed. A better understanding of the origin of the high viscosity of heavy oil can help greatly in finding more effective and economical methods to recover heavy oil and reduce the related capital and operating costs. Given this fact, after each blended heavy oil sample was prepared, the viscosity of the fuel was determined by an electromagnetically spinning viscometer (EMS-1000, KEM Kyoto Electronic Manufacturing Co., Ltd., Kyoto, Japan), shown in
Figure 2a,b. The viscosity measurement for the fuel sample was carried out three times, and the average value of the three measured viscosity data was noted. The measurement repeatability accuracy (RSD) was 3%. In
Figure 2a, the results showed that when the tested temperature ranged from 20.0 °C to 200 °C and the asphaltene fraction ranged from 4 to 24% (wt.%), the heavy oil sample behaved as a Newtonian fluid and its measured viscosity varied in a vast range of 2–568 mPa.s. The viscosities for the five samples decreased exponentially with increasing temperature. In
Figure 2a, asphaltene content in heavy oil played a decisive role at high viscosities, especially at low temperatures. Notably, as shown in
Figure 2b, for the viscosity of HFOs at 50 °C, the viscosity of fuel oil with 24% asphaltene content was equal to 568 mPa.s, while the viscosity of fuel oil with 4% asphaltene content was only 12 mPa.s at the same temperature, which was almost an order of magnitude lower than that of heavy fuel oil with more than 8% asphaltenes. At a high asphaltene content, the stably attractive interaction between asphaltene particles led to a sharp increase in the viscosity of heavy oil. An increased asphaltene content accompanied the higher viscosity due to the long-range hydrodynamic interactions between maltenes and asphaltene matter [
28]. Physically speaking, if solid particles or droplets are so densely dispersed and packed that there is no free space for them to move, the viscosity of the colloidal dispersion is close to infinity [
23].
The viscosity of heavy oil can be significantly reduced at higher temperatures, especially at high asphaltene contents, as the interparticle interactions among the dispersed asphaltene particles become weak. At a high-temperature range of 150 °C in
Figure 2b, the viscosities for the five fuel oils were insensitive to the asphaltene content, as the fuel viscosity came to be relatively low above 100 °C. Since the distance between the asphaltene particles was large enough, the interaction between them could be neglected under relatively high-temperature conditions. In conclusion, asphaltene content determined the high viscosity of heavy oil [
25], and these detailed nonlinear reduction results showed that the state of the asphaltene particles in the heavy oil changed with the asphaltene content and temperature, and this change in the state had a significant influence on the viscosity of the heavy oil.
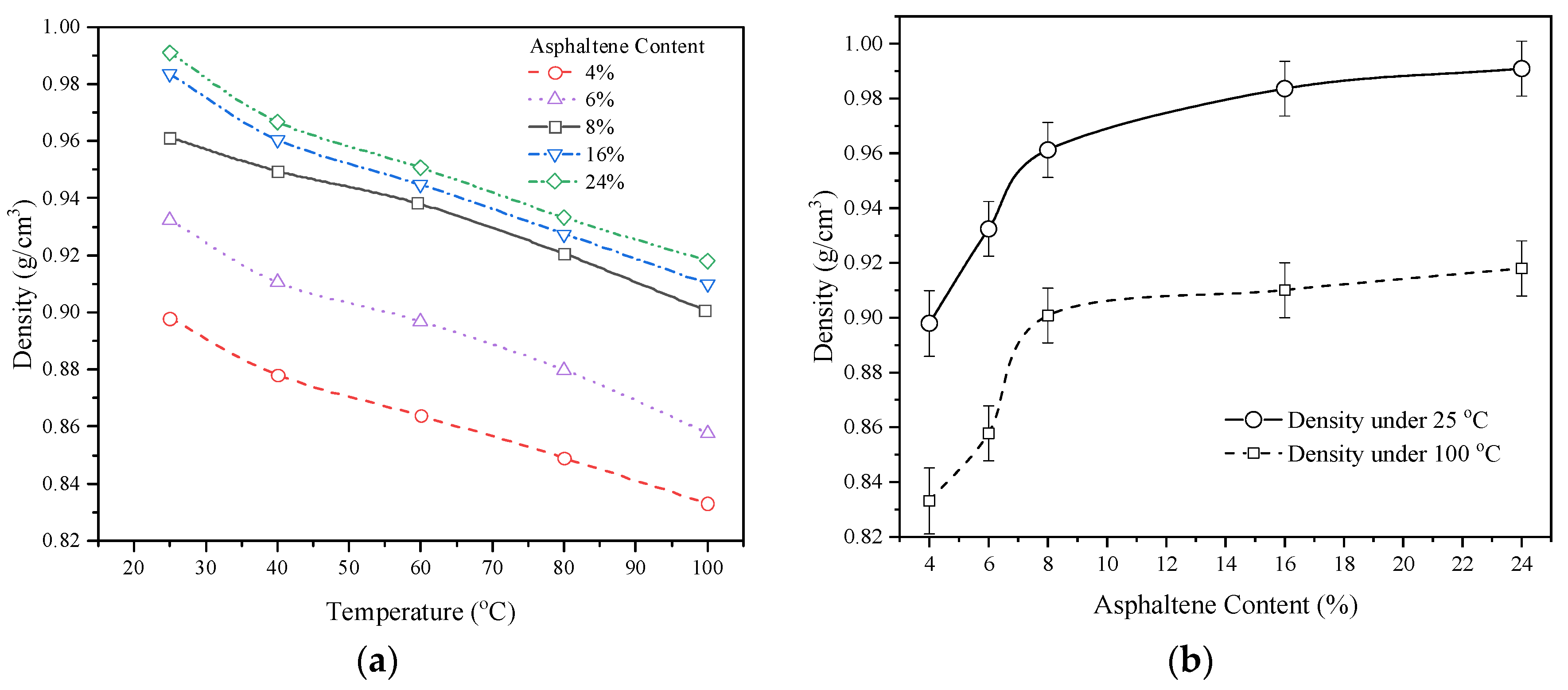
The density of various asphaltene contents heavy oil samples was measured by an electronic density meter (JN-300S/E, Shanghai Jenner Industrial Co., Ltd., Shanghai, China) with a temperature range of 25 °C to 100 °C, as shown in
Figure 3. The density of fuel with a high asphaltene content was higher than that for the combination of HFO and light oil. Asphaltenes are polar molecules. Due to their polar properties, they can be used as surfactants to stabilize the interface between the oil molecules in the fuel. Therefore, asphaltenes may result in higher density by tightening the mixture. Under each viscosity, the density basically decreased linearly with the increase of temperature, without a sudden drop process like that of the viscosity. The rate and trend of density decline were essentially constant. For example, as the temperature increased from 25 °C to 100 °C, the density decreased by 7.2% in 4% asphaltene oil and by 7.3% in 24% asphaltene oil. However, as shown in
Figure 3b, the density trend was not linear with the variation in asphaltene content. As the asphaltene content increased, the increase in density gradually became slow. For example, the density increased by 8.2% from 4% to 8% asphaltene content fuel, while it only increased by 2% from 8–24% asphaltene cotent fuel at 100 °C.
The heating values (gross calorific values) of HFO are reported in
Table 1 and were quantified by a Parr Instrument 6400 Automatic Isoperibol calorimeter. The heating values of the HFO samples decreased with increasing asphaltene content, due to the high heating value of light oil (diesel 46 MJ/kg). The highest value was up to 45.7 MJ/kg for the 4% asphaltene fuel, but no predictable linear trend was experienced. The heating value decreased by nearly 8% from 4% to 24% asphaltene fuels. There was a shape decrease trend occurring from 4% to 8% asphaltene fuels with a 6.8% reduction in the heating value, while there was only a 1.2% decrease in the heating values as the fuel asphaltene content increased from 8% to 24%. Notably, regarding the volumetric caloric value, the 24% fuel sample had the highest value of 41,915 MJ/m
3, due to the high-density characteristic of asphaltene.
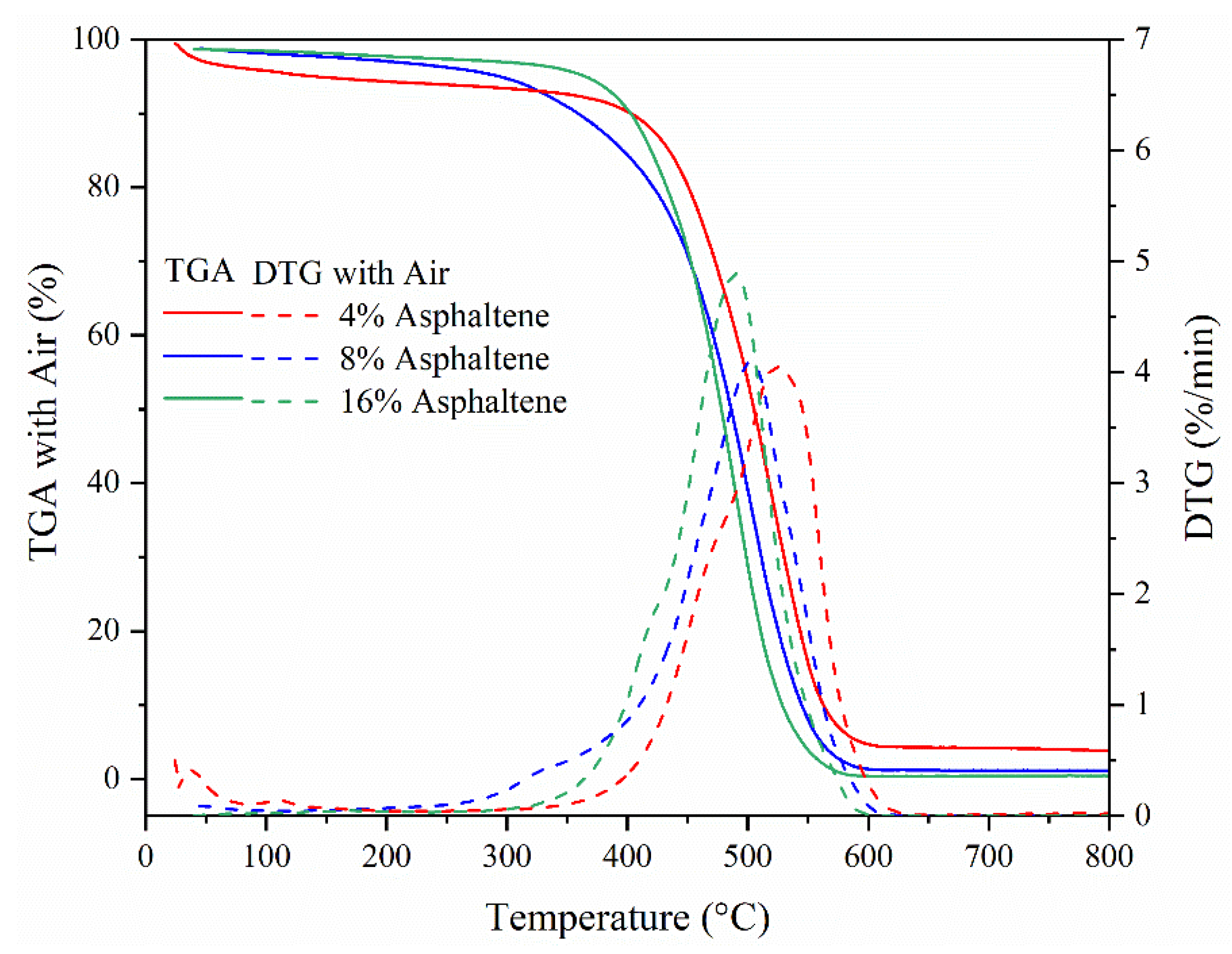
In
Figure 4, the thermogravimetric (TG) and differential thermogravimetric (DTG) curves of five heavy fuel oils under nitrogen and air atmospheres were measured using a Netzsch TG 209 F1Iris thermogravimetric analyzer, with a temperature rise rate of 5 °C/min. In the nitrogen atmosphere, the pyrolysis of HFO is a complex process involving many parallel reactions that coincide, due to the fuel’s highly distributed and multicomponent nature. As the fuel is heated, it undergoes devolatilization, including evaporation, distillation, and visbreaking. Due to the absence of oxygen, the HFO molecules decompose and release different hydrocarbon species at different temperatures. As the temperature increases, the rate of mass loss increases, and initially low boiling volatiles are given off, which results in the first peak at approximately 120 °C [
17].
The low asphaltene content case of 4% had the highest mass loss rate in the first peak of DTG due to the fuel blended with the light fuel of diesel, in which the rate of light components was much higher than that in pure HFO. The high asphaltene content case of 24% needed a higher temperature to reach the peak of DTG accompanying the lowest mass loss rate in the first peak. With a further increase in temperature up to 250 °C, the rate of mass loss briefly decreased because the bulk of the low boiling volatiles were depleted. There is a second steep increase peaking at 425 °C, due to the release of high boiling volatiles. The high asphaltene cases of 8% and 24% had the same higher mass loss in this peak. Beyond 500 °C, no further apparent mass loss from the fuel was observed. However, the high asphaltene case of 24% had the highest mass left, due to the effect of asphaltene and the high rate of metal compositions in asphaltene. Clearly, the carbon residues were also consistent with the changing trend of asphaltene content. This meant that asphaltene was the origin for carbon formation; thus, the quantity of carbons formed increased with increasing asphaltene content in the oil blend. This result further indicated that the formation of cenospheres was caused by asphaltene during HFO combustion.
In addition, the thermogravimetric (TGA) level in the air condition also corresponded to the asphaltene content in the fuel, shown in
Figure 4b. Before the droplet was ignited, the volatiles evaporated considerably. In
Figure 4b, under air condition, two peaks were induced by the combustion of low boiling point volatiles and high boiling point matter. These profiles corresponded to those in N
2 with similar temperature regions at approximately 120 °C and 450 °C. It was proven that the fuel components defined the combustion process of HFO in air. The burning of HFO included both the combustion of flammable volatiles and the burn-up of cenospheres and coke residue. Thus, there was no residue after 500 °C in
Figure 4b at the end of the test. The typical classified three reaction stages can be identified, in
Figure 4b, as LTO (low-temperature oxidation), FD (fuel deposition), and HTO (high-temperature oxidation) [
5,
32]. As to the pure HFO (8% asphaltene) case, LTO occurred at temperatures below 390 °C with three main processes. Below about 250 °C, the main gaseous products were hydrocarbons with saturated and unsaturated C–H bonds, ascribed to the evaporation of low-boiling point hydrocarbons. Below about 340 °C, oxygen addition reactions occurred with alkyl radicals to generate hydroperoxides; In the temperature range of 340 to 390 °C, the formation of hydroperoxide continued, and the decomposition and isomerization reactions of the hydroperoxides were the dominating reactions releasing carbonyl group, H
2O, CO
2, and CO. In FD between 390 and 460 °C, oxygen-containing compounds produced during the LTO were consumed to produce coke and through cracking or pyrolysis reactions. In HTO, at above 460 °C, the combustion of the coke and solid residues formed in FD process was the dominating reaction. The organic matter was completely oxidized, producing water and carbon oxides along with a production of sulfur and nitrogen oxides depending on the composition of the fuel.
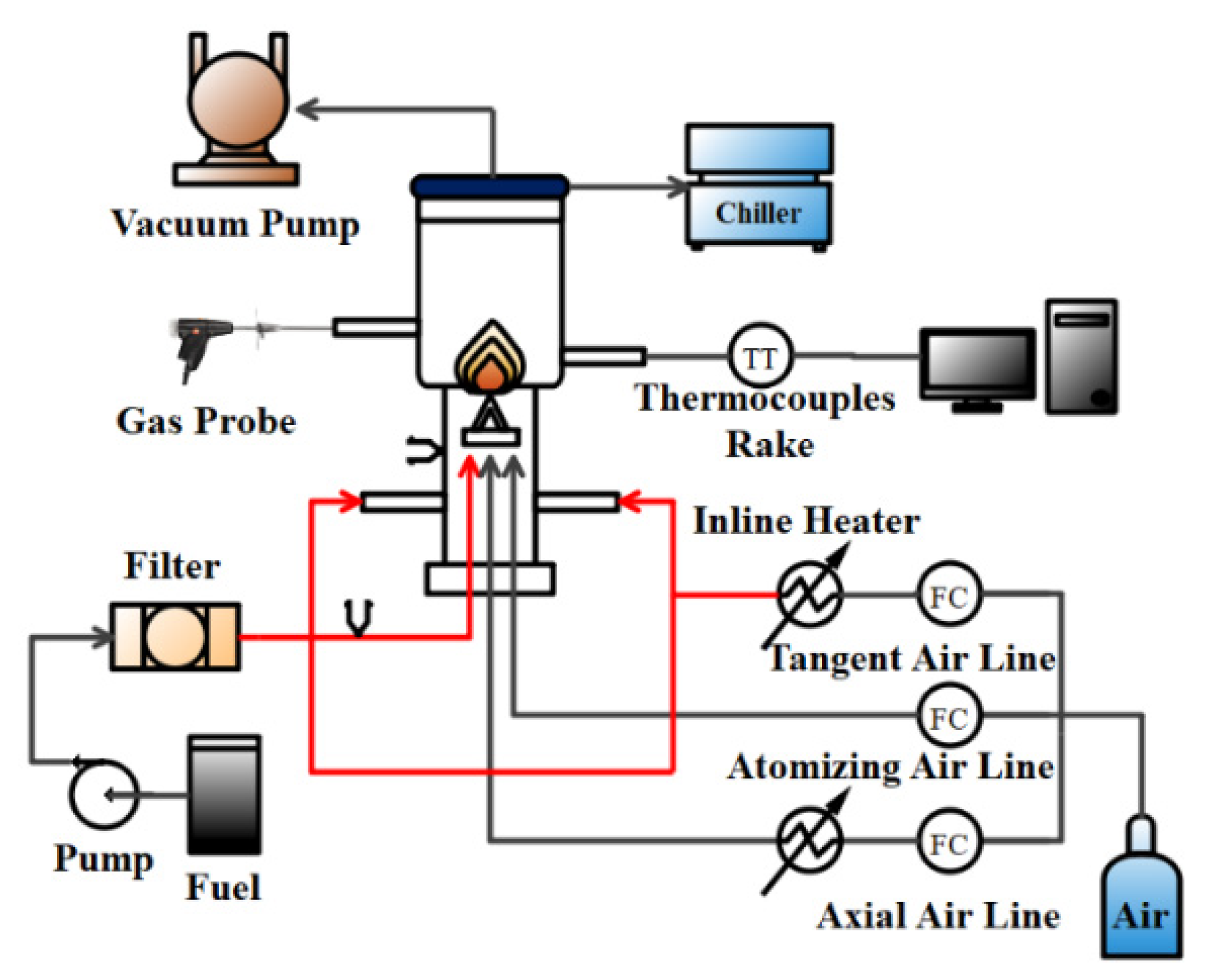
Due to the specific characteristics of asphaltene, heavy fuel oil requires more time and heat to be sufficiently combusted than light distillate oils, such as diesel. Therefore, a swirling flame combustion system was applied to produce a turbulent jet diffusion flame with high vortex stability to burn fuel efficiently [
33], shown in
Figure 5. The current burner configuration involved relatively simple geometry but replicated many of the fundamental swirling flame behaviors of real gas turbine combustors and boilers.
The swirling flow was generated by a swirl generator with air entering tangentially and axially to cause a swirling air injection, which is referred to the burner of Driscoll [
34,
35]. The nozzle of Siphon (P/N 30609-2) was used to produce a spray specially designed for viscous liquids. An R-type thermocouple (Pt/13%RhPt, Omega Engineering P13R-010) rake was controlled by a linear translation to measure the radial flame temperature distribution. For more detailed information about this experimental system, please refer to our previous work in Refs. [
30,
36,
37]. In
Table 3, the experimental conditions for the five different fuel samples were kept constant and provided with a swirling number of 19.2 calculated by Equation (1):
The axial and tangential air inlets were applied to control the swirl number. The axial air stream entered through the bottom of the plenum, and its flow rate was
Qa with diameter as
Da. The four tangential air inlets were oriented to the burner tube to impart the swirling momentum with the flow rate represented as
Qt and diameter of
Dt. This condition was selected based on the optimization work of the HFO combustion performance evaluation in various swirling flows. We tested the performance of the HFO combustion, under various swirling flame conditions, for different global equivalence ratios, swirling numbers, tangent, and axial airflow rates [
37].
3. Results and Discussion
The in-flame temperature distribution of the centerline of the flame of the experiments is given in
Figure 6. The uncertainties are given as the standard deviation of the flame temperature profiles. The low asphaltene content case of 4% had the highest temperature distribution, ascribed to complete combustion performance due to the fine spray characteristic (low viscosity and density) and high rate of the light components (in
Figure 4) of the fuel. In addition, the heating value of diesel (46 MJ/kg) was slightly higher than that of pure HFO (42.4 MJ/kg). The high H level in 4% asphaltene content in
Table 1 also increased the temperature. The fuels with 6% and 8% asphaltene contents exhibited almost the same level of temperature profiles, due to the similar fuel properties introduced in the fuel characteristics investigation above, in
Table 2. The fuel sample with 16% asphaltene was in the state between 8% and 24% asphaltene conditions, which could be regarded as the critical condition for sufficient combustion. The results of the gaseous and solid particle emission in the next sections further confirmed that the 16% asphaltene was a critical value for the acceptable concertation of high asphaltene fuel combustion in the range of asphaltene contents in this study.
The 24% asphaltene fuel oil had an obviously low-temperature distribution in comparison to the other cases. Asphaltene is the main factor that induces incomplete combustion. In the observation of the experiment of 24% asphaltene HFO, the fuel was very difficult to combust, and the flame was very unstable during the experiment, which meant that the heat could not accumulate well. The incomplete combustion of the high asphaltene experiment could be ascribed to the following factors: (a) The density of the asphaltene was higher than that of pure HFO, and the density of the high asphaltene fuel was higher than that of pure HFO. In our experiment, the flow rates were constant in volume at 15 mL/min, and more fuel entered the burner in the high asphaltene case, which brought about changes in the global equivalence ratio, approaching the fuel-rich condition. (b) The high viscosity of 24% asphaltene fuel inducing low-quality spray flow also made the combustion incomplete. (c) According to the results from our previous investigation on suspended droplet experiments, the ignition delay time of high asphaltene content was higher than that of low asphaltene fuel under the same droplet size [
5]. Therefore, the high asphaltene fuel droplets needed more time to be combusted.
In
Figure 6a, before the droplet was ignited, the volatiles in the fuel needed to evaporate first by absorbing a large amount of heat. These factors resulted in the relatively low temperature location in the root of the flame, especially for the 24% asphaltene case. In
Figure 6b, the average measurement flame temperature of fuel oils was subject to a linear relationship with respect to the asphaltene content. This research might point to a viable way to burn these residues by mixing them with low-viscosity and high-volatility fuels. In this case, the burning characteristics of the blending fuels introduced in this work are essential for technology development.
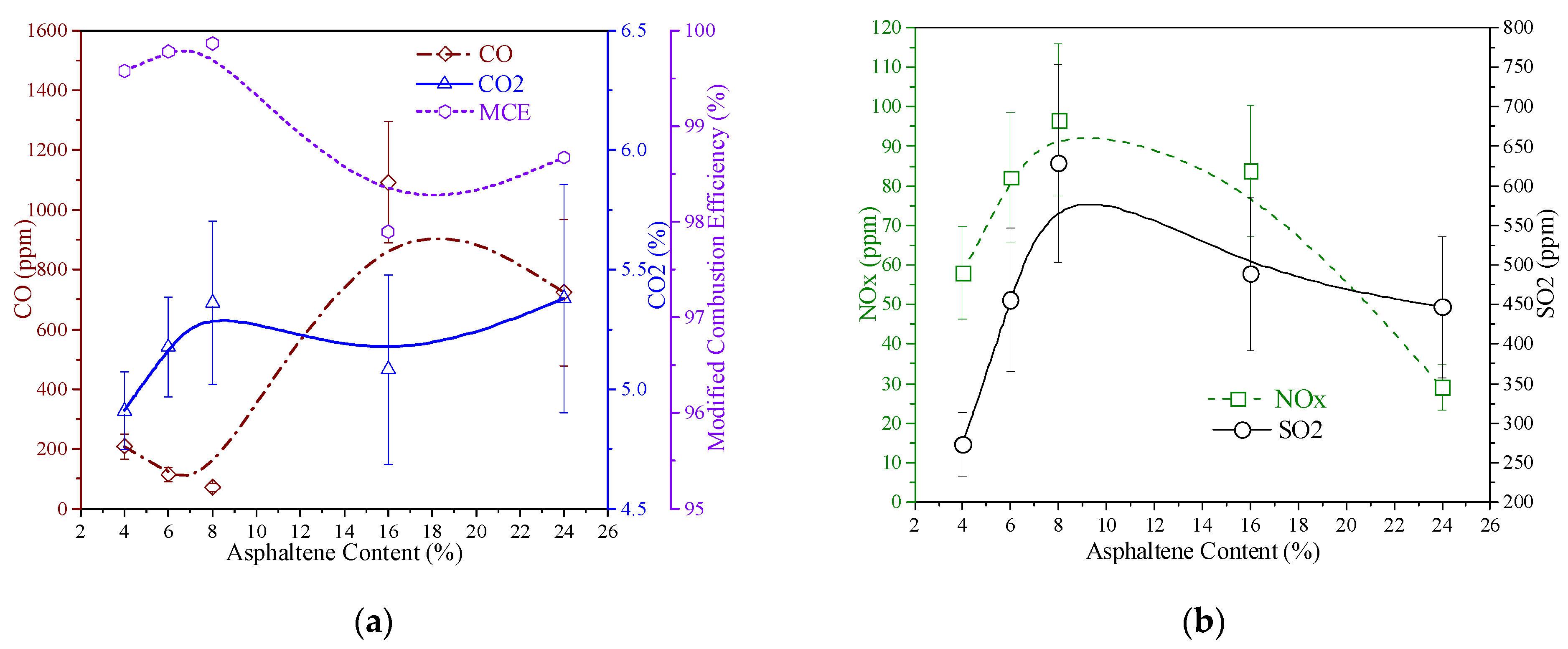
The flue gas emissions of the five different asphaltene content swirling flame experiments are given in
Figure 7. When a stable HFO swirl flame was obtained, a gas analyzer (TESTO 350) was used to measure the gaseous emissions of CO, SO
2, NO
x, O
2 and CO
2 from the top of the furnace in the overfire area, a method which has been widely applied to the studies of pollutants measurement of combustion devices [
38,
39,
40]. Each component’s gas emission measurement result was averaged from the data within 10 min and normalized to 15% oxygen applying Equation (2) to eliminate the influence of the dilution air as:
where
C[@15%O
2] was the calculated concertation eliminating the dilution air effect,
Cm was the gaseous pollution measurement content [
41]. The detailed measurement method description, validation and uncertainties can be found in our previous work [
30,
37]. HFO is characterized by a high asphaltene content (approximately 8% by mass), which leads to incomplete combustion. Asphaltenes are also responsible for the high metal content of fuels because of the presence of embedded heteroatoms. In
Figure 7a, the general trend of the CO and CO
2 profiles rose with increasing asphaltene content. The Modified Combustion Efficiency (MCE) was also calculated by CO
2/(CO + CO
2) to determine the combustion efficiency at different asphaltene concentrations. The 24% asphaltene case had a high level of oxygen (up to 13%) left which proved the incomplete combustion of high asphaltene content experiment compared to the low asphaltene cases, which also resulted in the high-level emission of CO. The CO emissions of 4% and 6% asphaltene case were higher than that of the 8% asphaltene case, due to the higher carbon concentration in the 4% asphaltene fuel sample, as shown in
Table 1.
NO
x refers to oxides of nitrogen and can induce smog formation, acid rain, and ozone depletion in the upper atmosphere. In
Figure 7b, the trend of NO
x decreased with the increase in asphaltene content in the fuel, due to the declined average temperature profiles, in
Figure 6a, having a suppression effect on the combustion temperature of high asphaltene fuel. The formation of NO
x can be classified into three mechanisms: thermal, prompt, and fuel thermal. Thermal NO
x is the dominant mechanism in combustion processes above 1100 °C, which is common in most high-temperature heating applications [
42]. In
Figure 6b, the average flame temperature of low-asphaltene heavy oil (4%, 6%, 8%) combustion was above 1100 °C, and its maximum temperature could reach 1125 °C, while the average temperatures of the other two cases were below 1100 °C. Prompt NO
x usually predominates in fuel-rich conditions formed by relatively fast reactions between nitrogen, oxygen, and hydrocarbon radicals. In our experimental study, the global equivalence ratio of pure HFO (8% asphaltenes) was 0.91. Therefore, the combustion of low-asphaltene fuels (4% and 6%) occurred under fuel-lean conditions, while the combustion of high-asphaltene fuels was under fuel-rich conditions. Fuel NO
x comes from the direct oxidation reaction of organic-nitrogen compounds in the fuel. HFO contains a large amount of bound nitrogen that increased as the asphaltene content increased, as shown in
Table 1, with the maximum value up to 0.431% in mass for the 24% case. The conversion of fuel-bound nitrogen to NO
x was between 15% and 100%. In general, the higher the conversion efficiency is, the lower the nitrogen content in the fuel. Therefore, in this study, the mechanism of NO
x formation varied with the fuel asphaltene content. This was the reason for the high level of NO
x emissions from 6% and 8% asphaltene fuel combustion.
In
Figure 7b, the trend of SO
2 slightly increased with increasing asphaltene content in the fuel. Additionally, SO
x reactions may inhibit fuel NO
x formation. The low SO
2 emission in the 4% asphaltene experiment was caused by the low concentration of sulfur in the fuel sample, as shown in
Table 1. The incomplete combustion of high asphaltene fuel of 24% was the reason for the low SO
2 emission of the fuel, which also showed that some residual fuel attached to the filter, as shown in
Figure 8e. Sulfur oxide emission is a critical problem with HFO combustion. The primary source of SO
x comes from HFOs that contain the highest sulfur, with 3.36% in 24% asphaltene fuel, as shown in
Table 1. Notably, in the five oil samples, the sulfur content in the fuel increased nonlinearly with the increase in asphaltenes. The sulfur content decreased significantly after adding light oil, but by adding pure asphaltene, the effect of increasing sulfur content was not pronounced, which indicated that the source of sulfur in the heavy fuel oil was not all asphaltene. Sulfur dioxide (SO
2) tends to be the preferential product at higher temperatures, while sulfur trioxide (SO
3) is more likely to form at lower temperatures. Since most combustion processes are at high temperatures, SO
2 is the most common form of SO
x emitted from systems containing sulfur. According to the sulfur balance calculation, most sulfur comes to flue gas emissions forming SO
2 and SO
3, and the remaining sulfur goes into the PM.
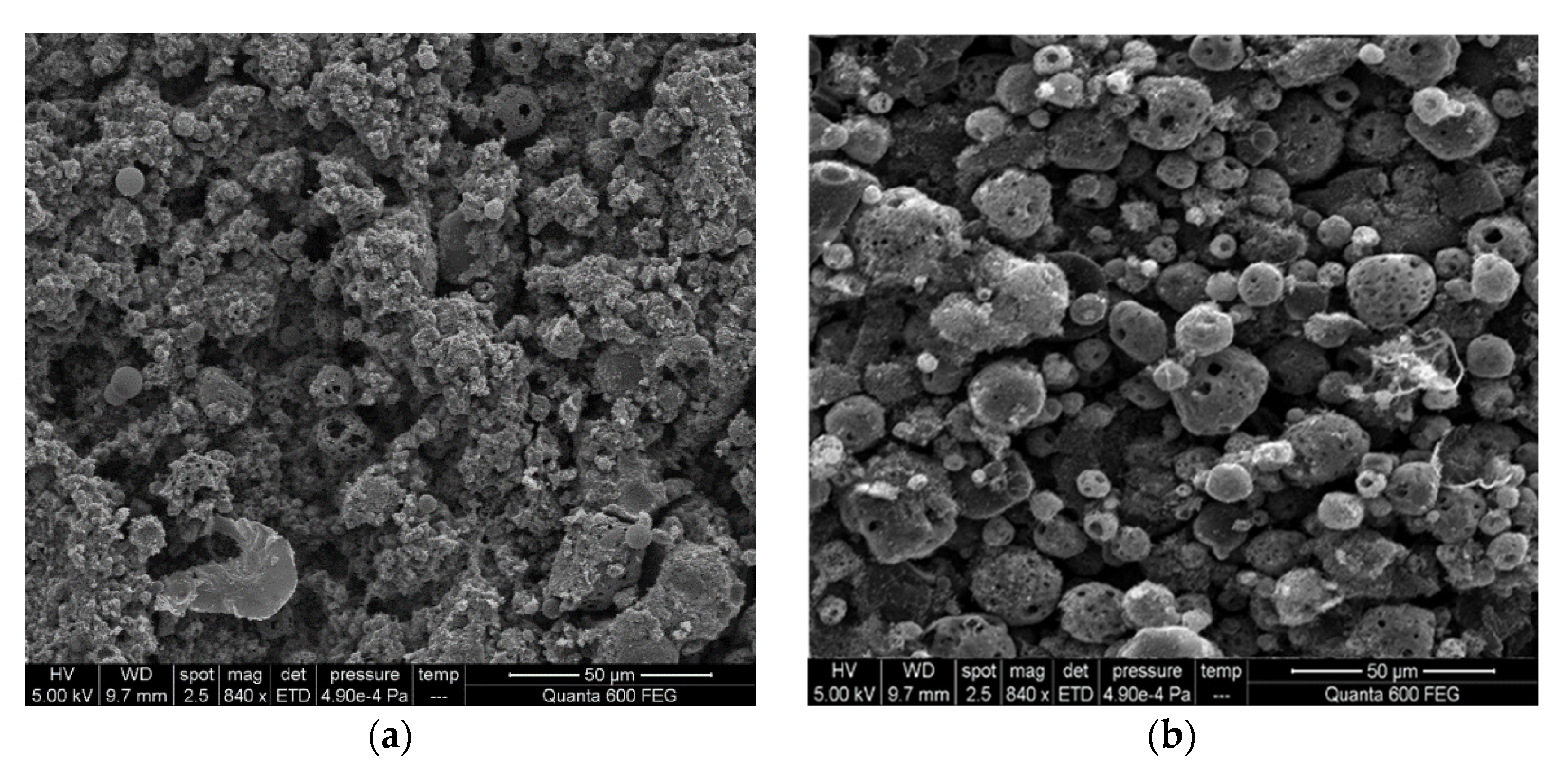
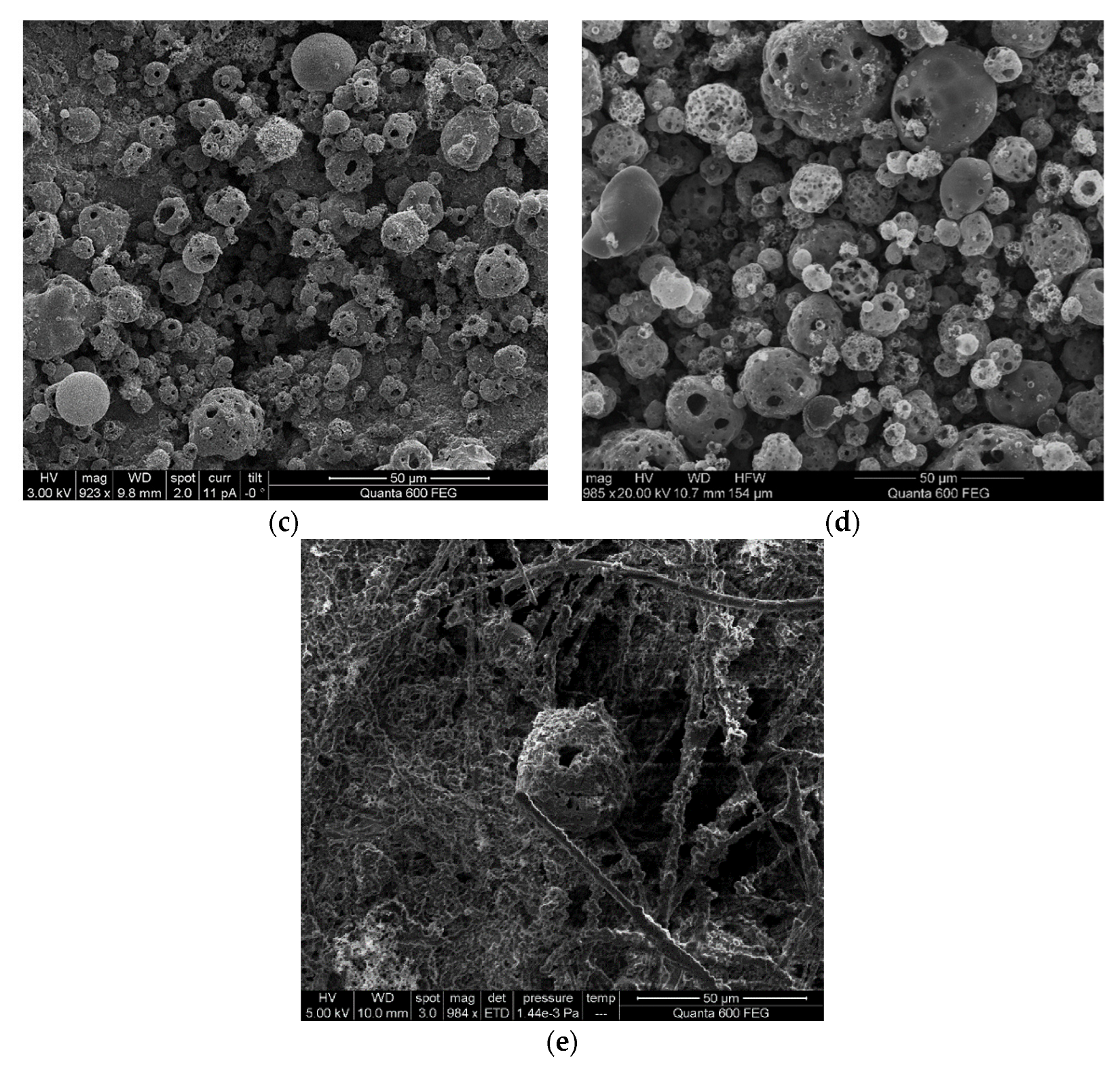
A comparison of the morphologies of five asphaltene samples is provided in
Figure 8. The cenospheres were obviously distinguished in the SEM measurement results. The general characteristics of burning heavy oil droplets can be clearly identified, such as micro-explosions, the formation of smoke and coke, and coke residue smoldering. Note that combustion in the solid phase is actually carried out without flame generation [
4]. For coke formation, the cenosphere is where the evolution of volatile substances abruptly ends, and the droplets collapse to form hard carbonaceous residues. Oils with 6%, 8%, and 16% asphaltene morphology showed minimal shrinkage and formed large thin-walled coke shells, similar in size to the original spray droplets.
In general, compared to the 8% asphaltene sample, the 4% asphaltene sample had an accumulation distribution that was more powdery and more small particles but fewer cenospheres, which could be ascribed to the small spray droplet size, fine spray, and good combustion performance. In
Figure 8e, the SEM result of 24% asphaltene illustrated the incomplete combustion of the high asphaltene experiment in which the cenosphere and filter fiber were all covered by unburned fuel. The relatively long ignition delay time of the high asphaltene sample made the fuel absorb heat and vapor but not combust, and further attached to the filter due to the cooling device above the filter.
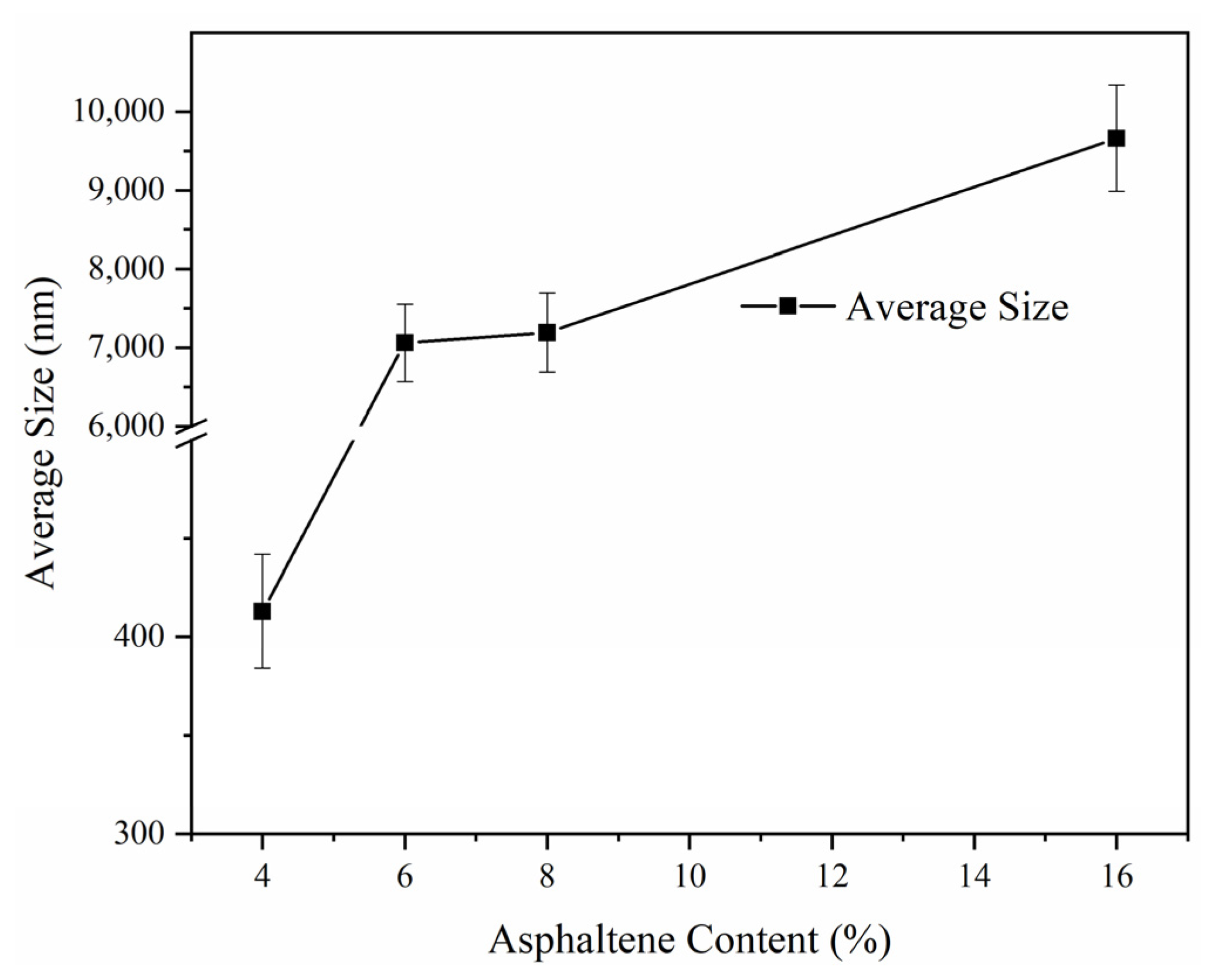
The averaged sizes of solid particles collected from the filter of the four experiments measured by the Zetasizer Nano ZS90 are compared in
Figure 9, except for the 24% one because the particles of 24% fuel were all stuck on the filter and were hard to collect. Based on the morphology of the 24% sample, we could not ascribe the increase in the deposition rate in the particle emissions because a lot of fuel attached to the filter. For this reason, we could not scrape the solid deposition on the filter from the sample of 24% asphaltene content fuel, and it was also impossible to analyze the composition by ICP/ODS. The average size of the particle increased with the increase in the asphaltene content of fuel, due to a strong correspondence between the spray droplet size and the diameter of the cenosphere [
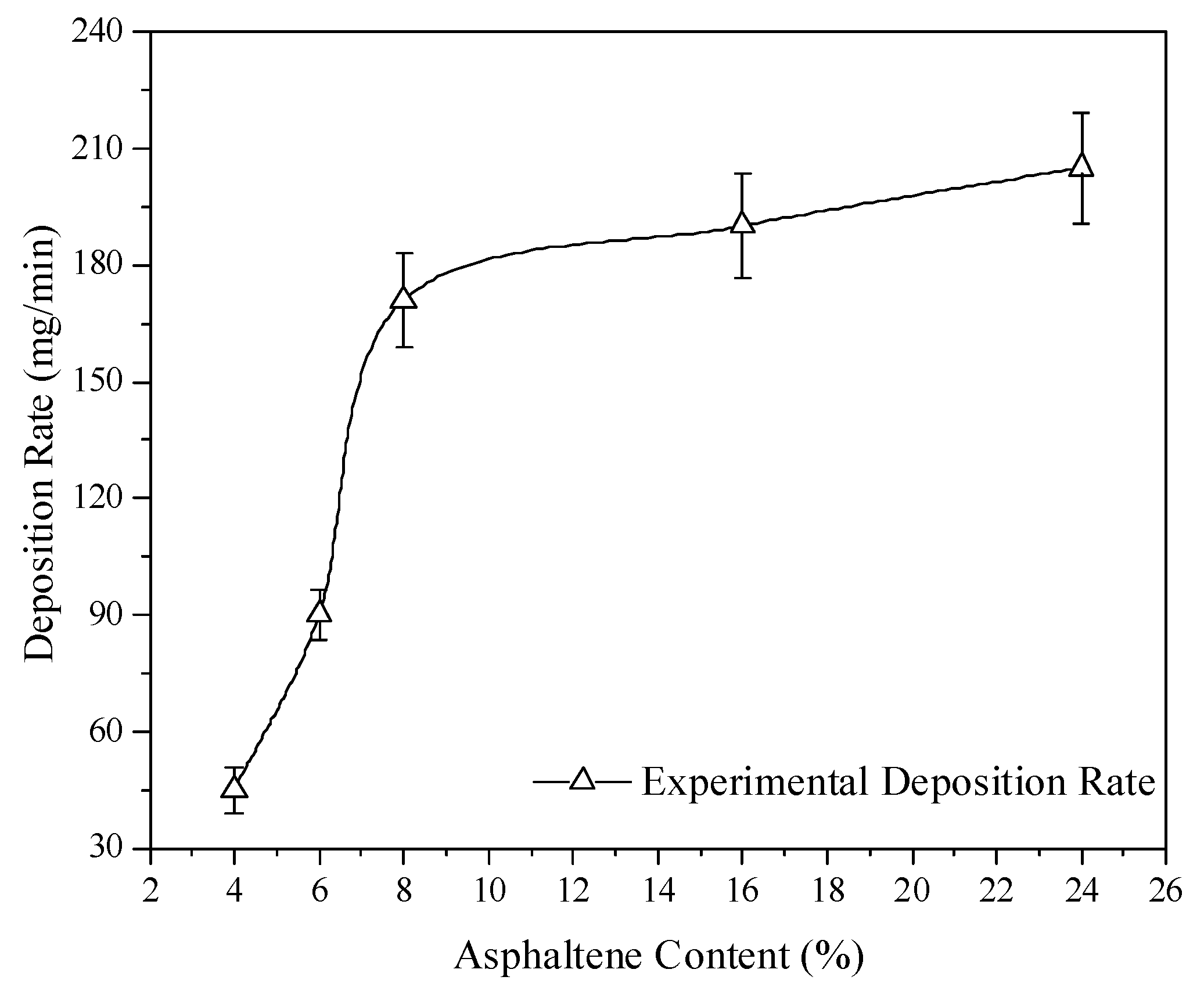
30]. The size of 16% asphaltene fuel was nearly 23 times larger than that of the 4% asphaltene case. The deposition rates are given in
Figure 10. The low asphaltene experiment of 4% had the lowest deposition rate, which could be ascribed to fine combustion and the low metal compositions of a low level in the 4% asphaltene fuel sample.
The components of solid particle emissions from the representative cases of 4%, 8%, and 16% asphaltene experiments are compared in
Table 4. The sulfur content in solid particulates increased with increasing asphaltene content in the fuel. The high level of metal components in the solid particle emissions corresponded to the composition of pure asphaltene. High levels of sulfur and vanadium induced cold corrosion and hot corrosion for burners, which are unacceptable for fuel applications. Thus, asphaltene caused the formation of cenospheres. The TG diagrams of the blended oil combustion particles are compared in
Figure 11. Unlike the TGA results of the fuel in air, the TG results of the particles after combustion were similar, indicating fine combustion performance with these three asphaltene fuels.