Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review
Abstract
:1. Introduction
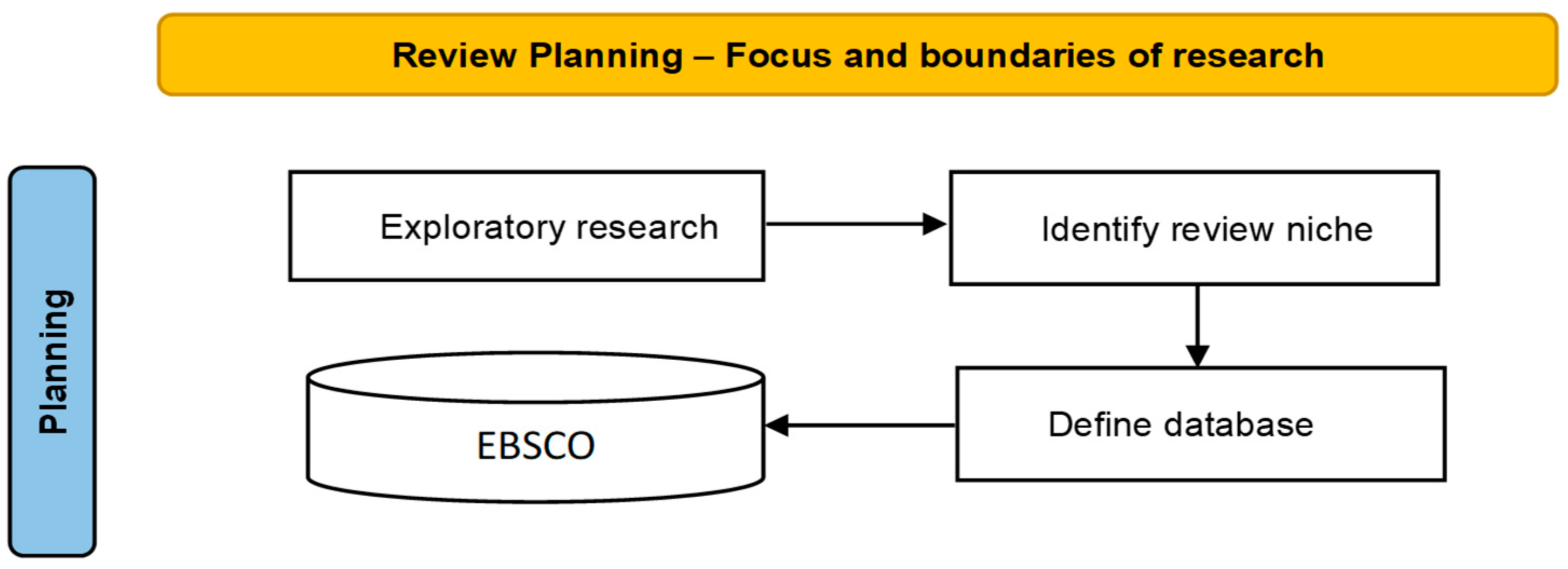
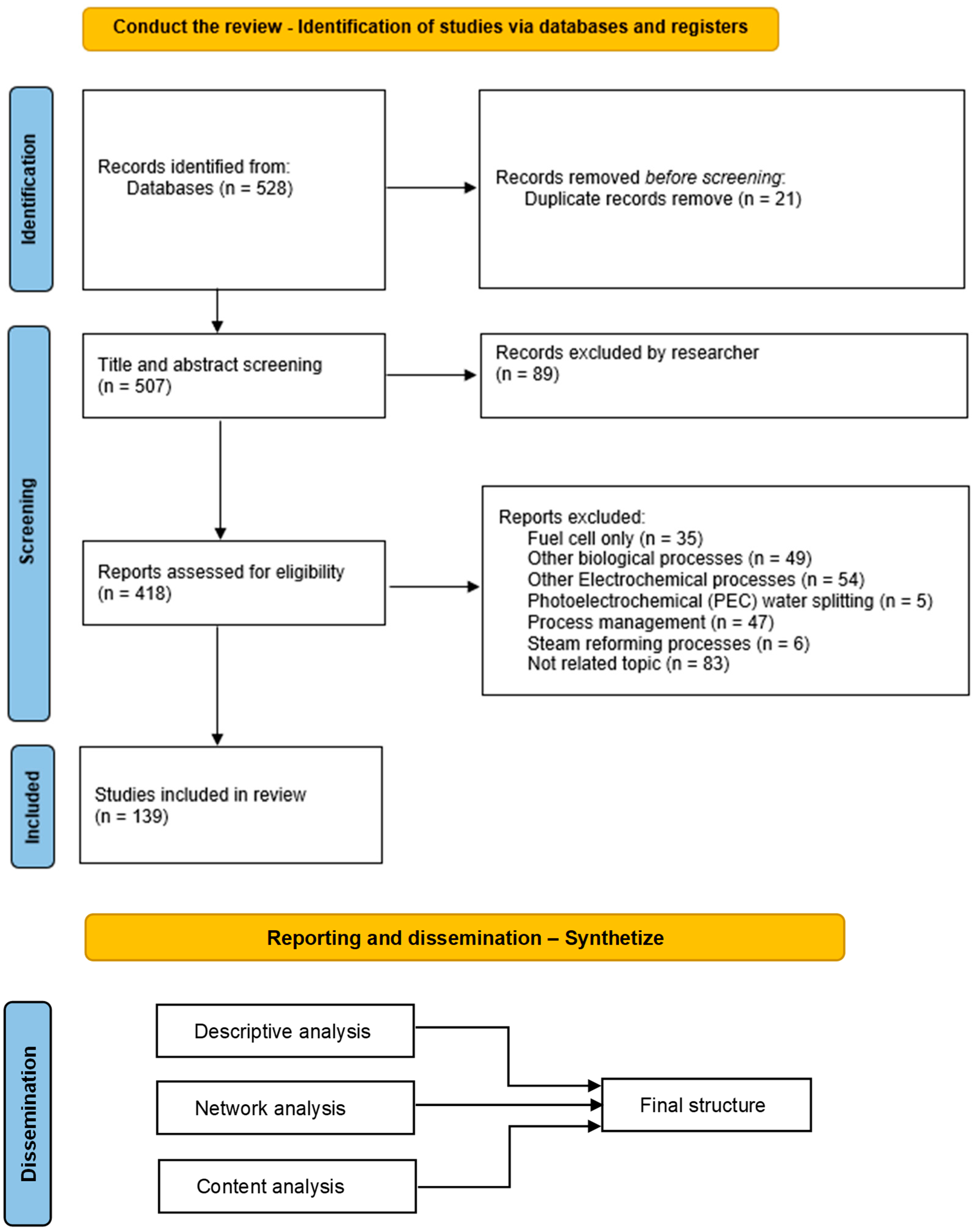
2. Materials and Methods
- RQ1: What are the main characteristics and clusters that research focuses on?
- RQ2: What are the main key performance indicators (KPIs) of the publish studies?
- RQ3: How the KPIs have evolved over the last years?
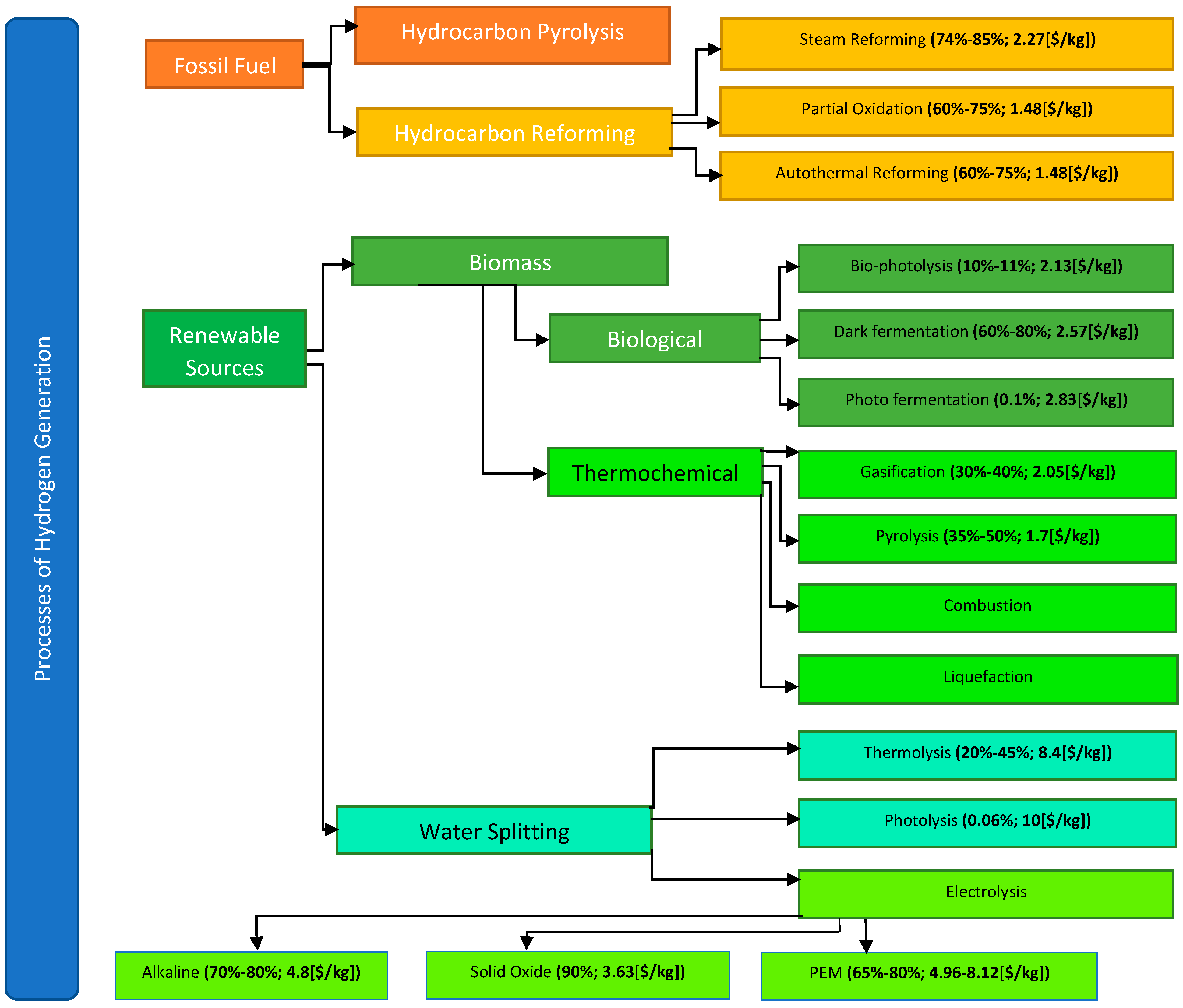
- Have no pollution output;
- Can integrate renewable energies;
- Have low CAPEX;
- Have low OPEX;
- Integrate technologies with long life;
- Delivers high purity H2;
- Have not reached technology maturity.
- Key words:
| Hydrogen production | <AND> |
| Hydrogen Electrolysis | <AND> |
| PEM | <AND> |
| Exchange Membrane | <AND> |
- Disciplines:
- Applied Sciences
- Chemistry
- Engineering
- Environmental Sciences
- Information Technology
- Life Sciences
- Physics
- Power & Energy
- Science
- Technology
- Expanders:
- Also search within the full text of the articles
- Search modes:
- Find all my search terms
- Apply related words
- Also search within the full text of the article
- Apply equivalent subjects
- Results limits:
- Full text
- Peer reviewed
- Published date:
- January 2020–February 2022
- Language:
- English
3. Results
3.1. RQ1: What Are the Main Characteristics and Clusters That Research Focuses on?
- Electrodes (anode/cathode) materials;
- Electrodes (anode/cathode) shape enhancing;
- Electrolysis cell;
- Electrolyte;
- Exchange membrane.
3.2. RQ2: What Are the Main Key Performance Indicators (KPIs) of the Publish Studies?
- Efficiency of the electrolysis cell [%];
- Faradaic efficiency [%];
- High frequency resistance HFR [mΩ cm²];
- Current density [mA/cm2];
- Electrode deposition decay time [h];
- Voltage [V];
- Temperature [°C];
- Pressure [atm];
- Electrochemical resistance [mΩ/cm2];
- Electrolyte pH;
- Electrolyte flow rate [mL/min];
- Anode loading [mg/cm2];
- Cathode loading [mg/cm2];
- Membrane film thickness [μm];
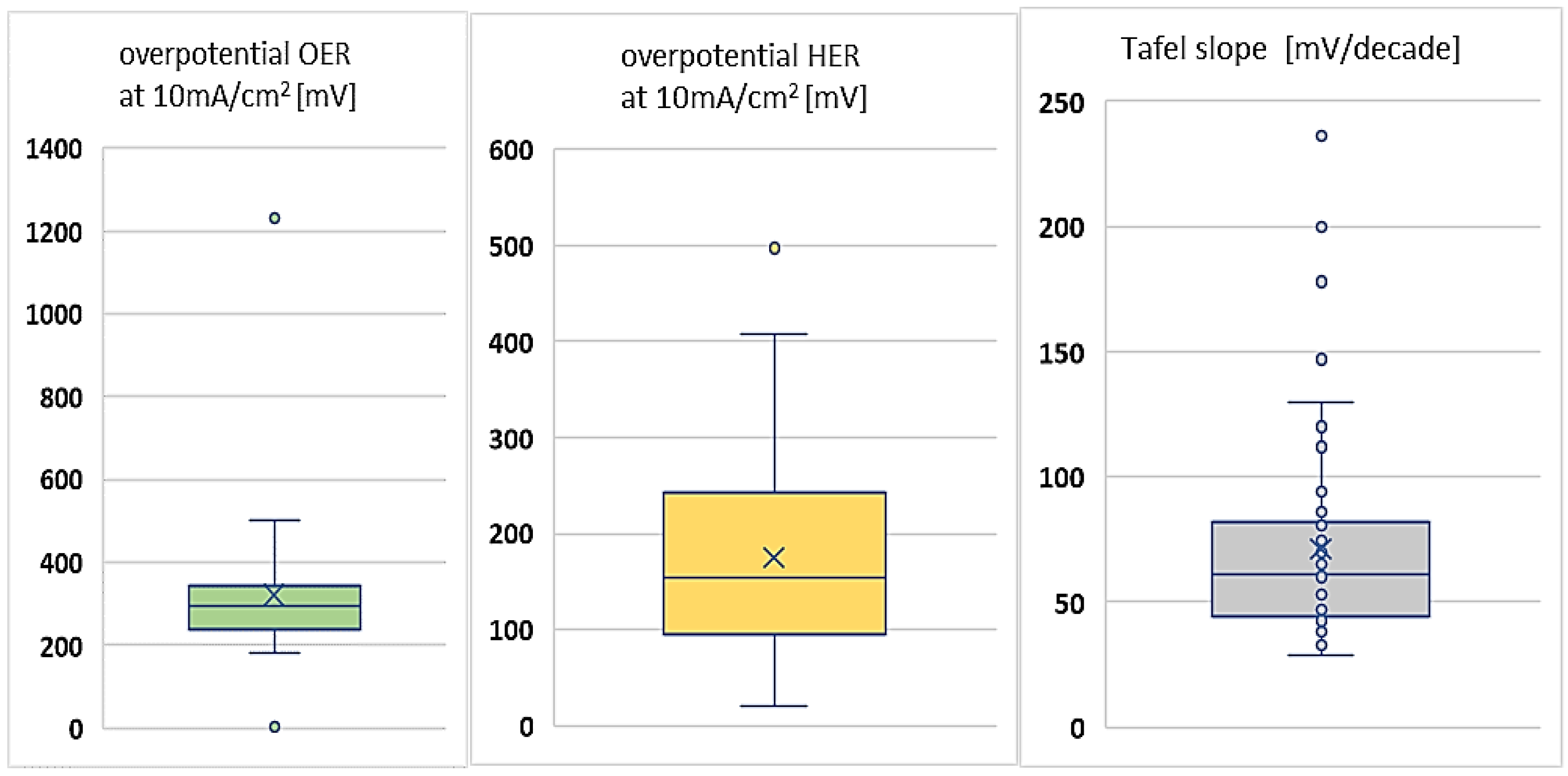
- Overpotential (voltage efficiency) HER at 10 mA/cm2 [mV];
- Overpotential (voltage efficiency) OER at 10 mA/cm2 [mV];
- H2 production [Nm3/h];
- Power consumption for H2 production [kWh/Nm3];
- Electrolysis system size [kW];
- Ion exchange capacity [meq/g];
- Ionic conductivity [mS/cm];
- Electrolyte resistivity [MΩ cm];
- Hydrogen crossover [mA cm−²];
- Degradation rate [μV/h];
- H2 purity [%];
- Tafel slope [mV dec−1];
- System lifetime [years];
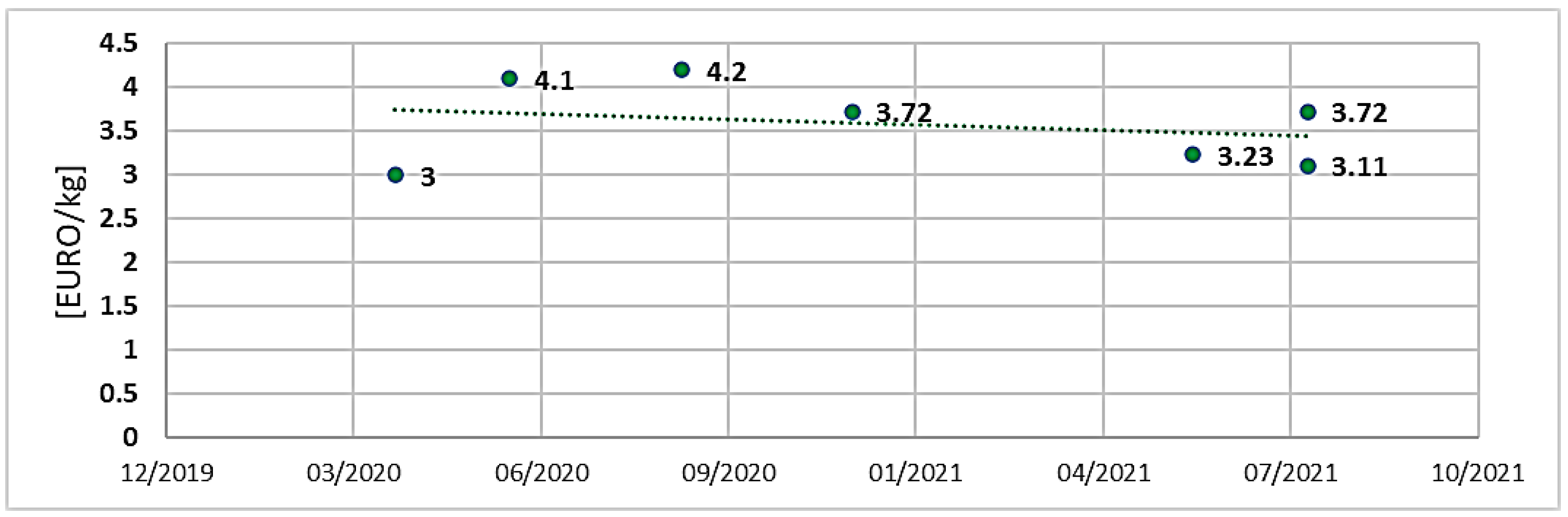
- Production cost [EURO/kg].
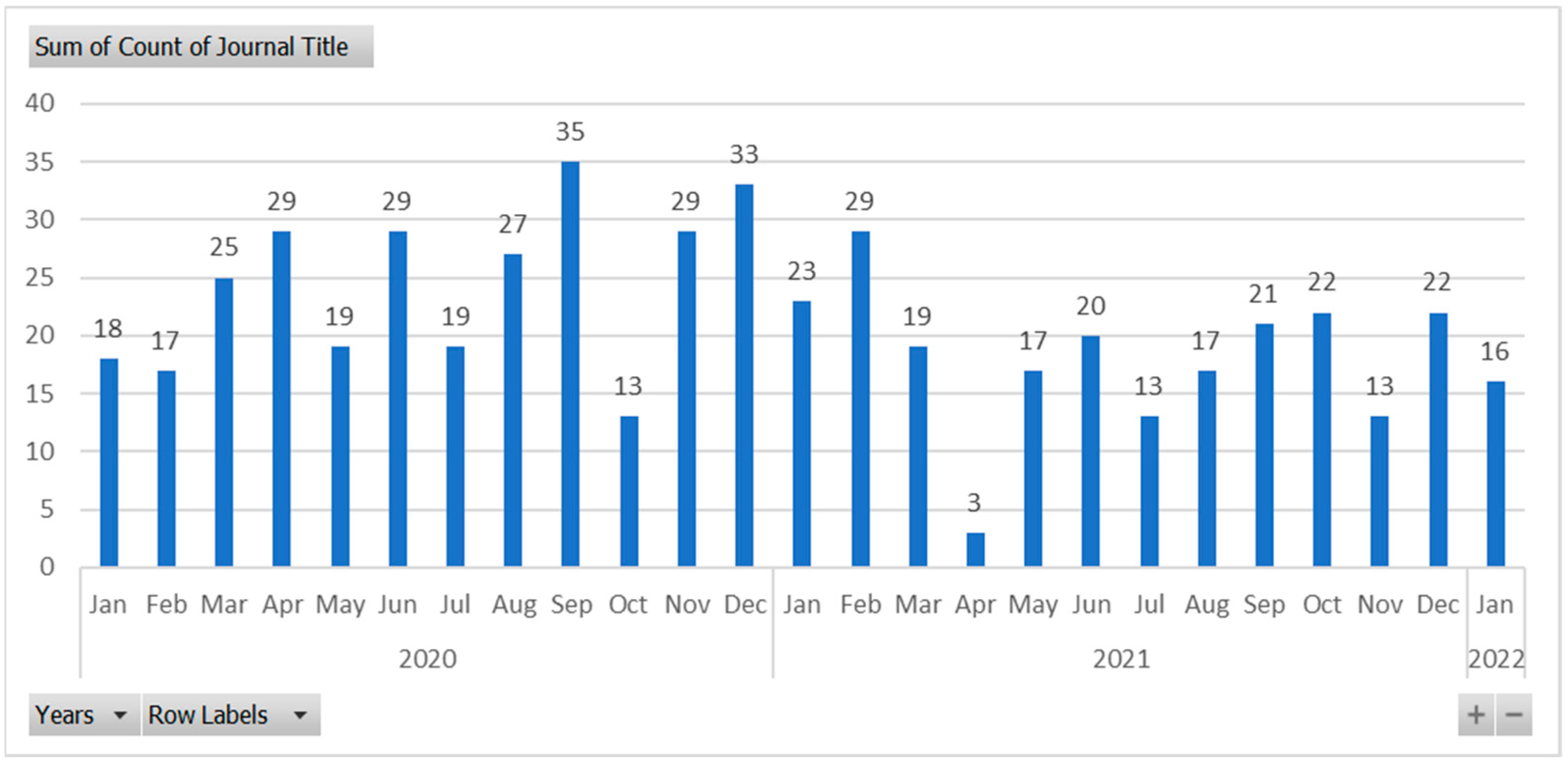
3.3. RQ3: How the KPIs have Evolved over the Last Years?
4. Discussion
5. Conclusions
- Expensive catalysts are showing a reduced presence in the research trend for HER (Figure 9). Research made progresses in improving the stability and activity by using sustainable materials, but a gap still remains to be filled for complying with the economical requirements of the market readiness. [149] PGM catalysts need to be replaced in order to be able to deploy large PEM electrolyzers or to achieve economical market readiness. The candidates for this replacement are Ni, Co, Fe, Mo, W and Cu, but not as single metal catalyst. Research is reaching for a compound with high catalytic activity through altering the electronic structures or by increasing the number of active sites [4];
- OER catalytic processes in PEM mainly focuses on IrOx compounds that deliver high stability but with a high production cost, Ru based catalysts prove high activity with low costs but a low operational stability. [149] It is observed in Ref. [4] that OER activity increases with increasing oxygen—metal bond strength (oxophilicity) but the durability decreases on the same order or materials: Au << Pt < Ir < Ru << Os [4];
- Ni based catalysts have a distinctive role in our review as they are reflected a large number or articles for HER and OER in AWE. Pure Ni electrodes covering have a low stability and activity during water splitting processes so various alloying are aiming to solving this vulnerability. In HER Ni based materials show a trend to replace the expensive and rare Pt in AWE [4];
- Cu based catalysts for OER activity in AWE shows some obvious advantages: low toxicity compared with other metal-based materials, low cost and excellent electrical conductivity;
- Co based catalysts (phosphide-coupled Co) research shows an excellent HER activity in AWE [4].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AEM | Anion Exchange Membrane |
| Au | Gold |
| AWE | Alkaline Water Electrolysis |
| B | Boron |
| BW | Box and Whiskers chart |
| CAPEX | Capital Expenditures |
| Co | Cobalt |
| Cu | Copper |
| DI | Deionized |
| Fe | Iron |
| H2 | Hydrogen |
| H2SO4 | Sulfuric acid |
| HER | Hydrogen Evolution Reaction |
| Ir | Iridium |
| KOH | Potassium hydroxide |
| KPI | Key Performance Indicator |
| Mo | Molybdenum |
| NAF | Not Accounted For |
| Ni | Nickel |
| OER | Oxygen Evolution Reaction |
| OPEX | Operating Expenses |
| Os | Osmium |
| PEMWE | Polymer Electrolyte Membrane Water Electrolysis |
| PO3 | Phosphite ion |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Pt | Platinum |
| PTG | Platinum Group Metals |
| PV | Photovoltaic |
| RQ | Research Questions |
| Ru | Ruthenium |
| SLR | Systematic Literature Review |
| W | Tungsten |
References
- DiChristopher, T. Hydrogen technology faces efficiency disadvantage in power storage race. In S&P Global Market Intelligence; S&P Global: New York, NY, USA, 2021; Available online: www.spglobal.com (accessed on 26 April 2022).
- Fonseca, J.D.; Camargo, M.; Commenge, J.-M.; Falk, L.; Gil, I.D. Trends in design of distributed energy systems using hydrogen as energy vector: A systematic literature review. Int. J. Hydrogen Energy 2018, 44, 9486–9504. [Google Scholar] [CrossRef]
- ShivaKumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Kim, H.; Park, H.; Bang, H.; Kim, S.-K. Electrodeposition-fabricated catalysts for polymer electrolyte water electrolysis. Korean J. Chem. Eng. 2020, 37, 1275–1294. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Corporate Author. Kyoto Protocol. 2017. Available online: http://unfccc.int/kyoto_protocol/items/2830.php (accessed on 30 May 2022).
- Paris Agreement to the United Nations Framework Convention on Climate Change. T.I.A.S. No. 16-1104. 12 December 2015. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 17 August 2022).
- Pires, A.L.G.; Rotella Junior, P.; Morioka, S.N.; Rocha, L.C.S.; Bolis, I. Main Trends and Criteria Adopted in Economic Feasibility Studies of Offshore Wind Energy: A Systematic Literature Review. Energies 2022, 15, 12. [Google Scholar] [CrossRef]
- Kovács, K.E.; Dan, B.; Hrabéczy, A.; Bacskai, K.; Pusztai, G. Is Resilience a Trait or a Result of Parental Involvement? The Results of a Systematic Literature Review. Educ. Sci. 2022, 12, 372. [Google Scholar] [CrossRef]
- Feng, C.; Wang, F.; Liu, Z.; Nakabayashi, M.; Xiao, Y.; Zeng, Q.; Fu, J.; Wu, Q.; Cui, C.; Han, Y.; et al. A self-healing catalyst for electrocatalytic and photoelectrochemical oxygen evolution in highly alkaline conditions. Nat. Commun. 2021, 12, 5980. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Shi, R.; Zhang, T.; Chiovoloni, S.; Lu, J.Q.; Chen, W.; Worsley, M.A.; Wood, B.C.; Baker, S.E.; et al. Periodic Porous 3D Electrodes Mitigate Gas Bubble Traffic during Alkaline Water Electrolysis at High Current Densities. Adv. Energy Mater. 2020, 10, 2002955. [Google Scholar] [CrossRef]
- Vincent, I.; Lee, E.C.; Kim, H.M. Highly cost-effective platinum-free anion exchange membrane electrolysis for large scale energy storage and hydrogen production. RSC Adv. 2020, 10, 37429–37438. [Google Scholar] [CrossRef]
- Srinivasa, N.; Hughes, J.P.; Adarakatti, P.S.; Manjunatha, C.; Rowley-Neale, S.J.; Ashoka, S.; Banks, C.E. Facile synthesis of Ni/NiO nanocomposites: The effect of Ni content in NiO upon the oxygen evolution reaction within alkaline media. RSC Adv. 2021, 11, 14654–14664. [Google Scholar] [CrossRef]
- Hu, K.; Ohto, T.; Nagata, Y.; Wakisaka, M.; Aoki, Y.; Fujita, J.-I.; Ito, Y. Catalytic activity of graphene-covered non-noble metals governed by proton penetration in electrochemical hydrogen evolution reaction. Nat. Commun. 2021, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Zhang, G.; Huang, H.; Lu, W.T.; Cao, F.F.; Shao, Z.G. Ni17W3–W Interconnected Hybrid Prepared by Atmosphere- and Thermal-Induced Phase Separation for Efficient Electrocatalysis of Alkaline Hydrogen Evolution. Small 2020, 16, 2005184. [Google Scholar] [CrossRef] [PubMed]
- Danilovic, N.; Subbaraman, R.; Chang, K.-C.; Chang, S.H.; Kang, Y.J.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.-T.; Myers, D.; et al. Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Peng, M.; Sun, X.; Luo, Y.; Asiri, A.M. Efficient electrochemical water splitting catalyzed by electrodeposited NiFe nanosheets film. Int. J. Hydrogen Energy 2016, 41, 8785–8792. [Google Scholar] [CrossRef]
- Pu, Z.; Luo, Y.; Asiri, A.B.; Sun, X. Efficient Electrochemical Water Splitting Catalyzed by Electrodeposited Nickel Diselenide Nanoparticles Based Film. ACS Appl. Mater. Interfaces 2016, 8, 4718–4723. [Google Scholar] [CrossRef]
- Swesi, A.T.; Masud, J.; Nath, M. Nickel selenide as a high-efficiency catalyst for oxygen evolution reaction. Energy Environ. Sci. 2016, 9, 1771–1782. [Google Scholar] [CrossRef]
- Jiang, N.; You, B.; Sheng, M.; Sun, Y. Bifunctionality and Mechanism of Electrodeposited Nickel-Phosphorous Films for Efficient Overall Water Splitting. ChemCatChem 2016, 8, 106–112. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, S.; Li, C.M. Electrodeposition of nickel-phosphorus nanoparticles film as a Janus electrocatalyst for electro-splitting of water. Power Sources 2015, 299, 342–346. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.-K.; Cho, S.K.; Kim, J.J. Enhanced catalytic activity of electrodeposited Ni-Cu-P toward oxygen evolution reaction. Appl. Catal. B-Environ. 2018, 237, 409–415. [Google Scholar] [CrossRef]
- Zang, Z.; Liu, S.; Xiao, J.; Wang, S. Fiber-based multifunctional nickel phosphide electrodes for flexible energy conversion and storage. J. Mater. Chem. A 2016, 4, 9691–9699. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, F.; Li, C.; Huang, W.; Lin, Y. Formation of Prussian blue analog on Ni foam via in-situ electrodeposition method and conversion into Ni-Fe-mixed phosphates as efficient oxygen evolution electrode. Electrochim. Acta 2019, 313, 91. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Bagal, I.V.; Ryu, S.-W.; Han, Y.-K.; Kim, D.-H. Nano-Micro-Structured Nickel-Cobalt Hydroxide/Ni2P2O7 Assembly on Nickel Foam: An Outstanding Electrocatalyst for Alkaline Oxygen Evolution Reaction. ChemCatChem 2019, 11, 4256–4261. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Yu, Y.W.; Hua, Y.X.; Li, Y.; Dong, P. Electrochemical fabrication of porous Ni-Cu alloy nanosheets with high catalytic activity for hydrogen evolution. Dong Electrochim. Acta 2016, 215, 609–616. [Google Scholar] [CrossRef]
- Rad, P.J.; Aliofkhazraei, M.; Darband, G.B. Ni-W nanostructure well-marked by Ni selective etching for enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 880–894. [Google Scholar]
- Sun, T.; Cao, J.; Dong, J.; Du, H.; Zhang, H.; Chen, J.; Xu, L. Ordered mesoporous Ni Co alloys for highly efficient electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 6637–6645. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, J.; Lei, H.; Yang, W.; Zhang, Q. Direct Electrodeposition of Phosphorus-Doped Nickel Superstructures from Choline Chloride–Ethylene Glycol Deep Eutectic Solvent for Enhanced Hydrogen Evolution Catalysis. ACS Sustain. Chem. Eng. 2018, 7, 1529–1537. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Chong, Z.; Omanovic, S. Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. A Chem. 2005, 226, 179–197. [Google Scholar] [CrossRef]
- Damian, A.; Omanovic, S. Ni and NiMo hydrogen evolution electrocatalysts electrodeposited in a polyaniline matrix. J. Power Sources 2006, 158, 464–476. [Google Scholar] [CrossRef]
- Martinez, S.; Metikoš-Huković, M.; Valek, L. Electrocatalytic properties of electrodeposited Ni–15Mo cathodes for the HER in acid solutions: Synergistic electronic effect. J. Mol. Catal. A Chem. 2006, 245, 114–121. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.; Kim, H.; Kim, J.; Ahn, S.H. Facile fabrication of nanostructured NiMo cathode for high-performance proton exchange membrane water electrolyzer. J. Ind. Eng. Chem. 2019, 79, 255–260. [Google Scholar] [CrossRef]
- Badawy, W.; Nady, H.; Negem, M. Cathodic hydrogen evolution in acidic solutions using electrodeposited nano-crystalline Ni–Co cathodes. Int. J. Hydrogen Energy 2014, 39, 10824–10832. [Google Scholar] [CrossRef]
- Nady, H.; Negem, M. Ni–Cu nano-crystalline alloys for efficient electrochemical hydrogen production in acid water. RSC Adv. 2016, 6, 51111–51119. [Google Scholar] [CrossRef]
- Nady, H.; Negem, M. Electroplated Zn–Ni nanocrystalline alloys as an efficient electrocatalyst cathode for the generation of hydrogen fuel in acid medium. Int. J. Hydrogen Energy 2018, 43, 4942–4950. [Google Scholar] [CrossRef]
- Mollamahale, Y.B.; Jafari, N.; Hosseini, D. Electrodeposited Ni-W nanoparticles: Enhanced catalytic activity toward hydrogen evolution reaction in acidic media. Mater. Lett. 2018, 213, 15–18. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Kim, D.-K.; Oh, S.; Choi, I.; Kim, S.-K. Electrochemically Fabricated NiW on a Cu Nanowire as a Highly Porous Non-Precious-Metal Cathode Catalyst for a Proton Exchange Membrane Water Electrolyzer. ACS Sustain. Chem. Eng. 2019, 7, 8265–8273. [Google Scholar] [CrossRef]
- Wang, X.; Su, R.; Aslan, H.; Kibsgaard, J.; Wendt, S.; Meng, L.; Dong, M.; Huang, Y.; Besenbacher, F. Tweaking the composition of NiMoZn alloy electrocatalyst for enhanced hydrogen evolution reaction performance. Nano Energy 2015, 12, 9–18. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Kim, D.-K.; Choi, I.; Kim, S.-K. Pulse-electrodeposited nickel phosphide for high-performance proton exchange membrane water electrolysis. J. Alloys Compd. 2019, 785, 296–304. [Google Scholar] [CrossRef]
- Walter, C.; Menezes, P.W.; Driess, M. Perspective on intermetallics towards efficient electrocatalytic water-splitting. Chem. Sci. 2021, 12, 8603–8631. [Google Scholar] [CrossRef]
- Yu, C.; Lu, J.; Luo, L.; Xu, F.; Shen, P.K.; Tsiakaras, P.; Yin, S. Bifunctional catalysts for overall water splitting: CoNi oxyhydroxide nanosheets electrodeposited on titanium sheets. Electrochim. Acta 2019, 301, 449–457. [Google Scholar] [CrossRef]
- Nan, K.; Du, H.; Su, L.; Li, C.M. Directly Electrodeposited Cobalt Sulfide Nanosheets as Advanced Catalyst for Oxygen Evolution Reaction. ChemistrySelect 2018, 3, 7081–7088. [Google Scholar] [CrossRef]
- Liu, T.; Liang, Y.; Liu, Q.; Sun, X.; He, Y.; Asiri, A.M. Electrodeposition of cobalt-sulfide nanosheets film as an efficient electrocatalyst for oxygen evolution reaction. Electrochem. Commun. 2015, 60, 92–96. [Google Scholar] [CrossRef]
- Jiang, N.; You, B.; Sheng, M.; Sun, Y. Electrodeposited Cobalt-Phosphorous-Derived Films as Competent Bifunctional Catalysts for Overall Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 6251–6254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.-P.; Liu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Self-Supported Cobalt Phosphide Mesoporous Nanorod Arrays: A Flexible and Bifunctional Electrode for Highly Active Electrocatalytic Water Reduction and Oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347. [Google Scholar] [CrossRef]
- Rapson, T.D.; Ju, H.; Marshall, P.; Devilla, R.; Jackson, C.J.; Giddey, S.; Sutherland, T.D. Engineering a solid-state metalloprotein hydrogen evolution catalyst. Sci. Rep. 2020, 10, 3774. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Rao, G.K.; Saha, S.; Ganguli, A.K. Cu-Based Nanocomposites as Multifunctional Catalysts. ChemPhysChem 2015, 17, 155–161. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Jiang, N.; Liu, X.; Sun, Y. Simultaneous H2 Generation and Biomass Upgrading in Water by an Efficient Noble-Metal-Free Bifunctional Electrocatalyst. Angew. Chem. Int. Ed. 2016, 55, 9913–9917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Yin, J.; Abou-Hamad, E.; Liu, X.; Chen, W.; Yao, T.; Mohammed, O.F.; Alshareef, H.N. Highly Stable Phosphonate-Based MOFs with Engineered Bandgaps for Efficient Photocatalytic Hydrogen Production. Adv. Mater. 2020, 32, 1906368. [Google Scholar] [CrossRef]
- Themuwara, A.C.; Dheer, L.; Attanayake, N.H.; Yan, Q.; Waghmare, U.V.; Strongin, D.R. Co-Mo-P Based Electrocatalyst for Superior Reactivity in the Alkaline Hydrogen Evolution Reaction. ChemCatChem 2018, 10, 4832. [Google Scholar] [CrossRef]
- Bai, N.; Li, Q.; Mao, D.; Li, D.; Dong, H. One-Step Electrodeposition of Co/CoP Film on Ni Foam for Efficient Hydrogen Evolution in Alkaline Solution. ACS Appl. Mater. Interfaces 2016, 8, 29400–29407. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; Che, Q.; Chen, Y.; Xu, X. Interface Engineering of Crystalline/Amorphous Co2P/CoMoPx Nanostructure as Efficient Electrocatalysts for Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 7, 2437–2445. [Google Scholar] [CrossRef]
- Wu, H.; Zuo, X.; Wang, S.-P.; Yin, J.-W.; Zhang, Y.-N.; Chen, J. Theoretical and experimental design of Pt-Co(OH)2 electrocatalyst for efficient HER performance in alkaline solution. Prog. Nat. Sci. Mater. Int. 2019, 29, 356–361. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, W.; Jia, F.; Wu, T.; Han, D.; Niu, L. Controlled electrodeposition of CoMoSx on carbon cloth: A 3D cathode for highly-efficient electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2016, 41, 3811–3819. [Google Scholar] [CrossRef]
- Saadi, F.H.; Carim, A.I.; Verlage, E.; Hemminger, J.C.; Lewis, N.S.; Soriaga, M.P. CoP as an Acid-Stable Active Electrocatalyst for the Hydrogen-Evolution Reaction: Electrochemical Synthesis, Interfacial Characterization and Performance Evaluation. J. Phys. Chem. C 2014, 118, 29294–29300. [Google Scholar] [CrossRef] [Green Version]
- Djara, R.; Lacour, M.-A.; Merzouki, A.; Cambedouzou, J.; Cornu, D.; Tingry, S.; Holade, Y. Iridium and Ruthenium Modified Polyaniline Polymer Leads to Nanostructured Electrocatalysts with High Performance Regarding Water Splitting. Polymers 2021, 13, 190. [Google Scholar] [CrossRef]
- Dang, Q.; Lin, H.; Fan, Z.; Ma, L.; Shao, Q.; Ji, Y.; Zheng, F.; Geng, S.; Yang, S.-Z.; Kong, N.; et al. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021, 12, 6007. [Google Scholar] [CrossRef]
- Su, H.; Zhou, W.; Zhou, W.; Li, Y.; Zheng, L.; Zhang, H.; Liu, M.; Zhang, X.; Sun, X.; Xu, Y.; et al. In-situ spectroscopic observation of dynamic-coupling oxygen on atomically dispersed iridium electrocatalyst for acidic water oxidation. Nat. Commun. 2021, 12, 6118. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, A.; Kim, J.M.; Lim, D.; Chae, K.H.; Cho, E.N.; Han, H.J.; Jeon, K.U.; Kim, M.; Lee, G.H.; et al. Highly efficient oxygen evolution reaction via facile bubble transport realized by three-dimensionally stack-printed catalysts. Nat. Commun. 2020, 11, 4921. [Google Scholar] [CrossRef]
- Elezović, N.R.; Zabinski, P.; Lačnjevac, U.; Pajić, M.N.K.; Jović, V.D. Electrochemical deposition and characterization of iridium oxide films on Ti2AlC support for oxygen evolution reaction. J. Solid State Electrochem. 2020, 25, 351–363. [Google Scholar] [CrossRef]
- Knöppel, J.; Möckl, M.; Escalera-López, D.; Stojanovski, K.; Bierling, M.; Böhm, T.; Thiele, S.; Rzepka, M.; Cherevko, S. On the limitations in assessing stability of oxygen evolution catalysts using aqueous model electrochemical cells. Nat. Commun. 2021, 12, 2231. [Google Scholar] [CrossRef]
- Li, R.; Wang, H.; Hu, F.; Chan, K.C.; Liu, X.; Lu, Z.; Wang, J.; Li, Z.; Zeng, L.; Li, Y.; et al. IrW nanochannel support enabling ultrastable electrocatalytic oxygen evolution at 2 A cm−2 in acidic media. Nat. Commun. 2021, 12, 3540. [Google Scholar] [CrossRef]
- Lee, B.-S.; Ahn, S.H.; Park, H.-Y.; Choi, I.; Yoo, S.J.; Kim, H.-J.; Henkensmeier, D.; Kim, J.Y.; Park, S.; Nam, S.W.; et al. Development of electrodeposited IrO2 electrodes as anodes in polymer electrolyte membrane water electrolysis. Appl. Catal. B Environ. 2015, 179, 285–291. [Google Scholar] [CrossRef]
- Din, M.A.U.; Irfan, S.; Dar, S.U.; Rizwan, S. Synthesis of 3D IrRuMn Sphere as a Superior Oxygen Evolution Electrocatalyst in Acidic Environment. Chem.–A Eur. J. 2020, 26, 5662–5666. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.; Kim, H.Y.; Hwang, E.; Ahn, S.H.; Kim, S.-K. Activity and stability of the oxygen evolution reaction on electrodeposited Ru and its thermal oxides. Appl. Surf. Sci. 2015, 359, 227–235. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.; Kim, H.; Hwang, E.; Ahn, S.H.; Kim, S.-K. Electrochemical Preparation of Ru/Co Bi-layered Catalysts on Ti Substrates for the Oxygen Evolution Reaction. Bull. Korean Chem. Soc. 2016, 37, 1270–1277. [Google Scholar] [CrossRef]
- Cross, M.W.; Smith, R.P.; Varhue, W.J. RuO2 Nanorods as an Electrocatalyst for Proton Exchange Membrane Water Electrolysis. Micromachines 2021, 12, 1412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, C.; Li, P.; Yao, K.; Zhao, Z.; Fan, J.; Li, H.; Wang, H. Benchmarking Phases of Ruthenium Dichalcogenides for Electrocatalysis of Hydrogen Evolution: Theoretical and Experimental Insights. Small 2021, 17, 2007333. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Suliman, M.H.; Rehman, A.; Hakeem, A.S.; Al Ghanim, A.; Qamar, M. Fabrication of platinum thin films for ultra-high electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 15076–15085. [Google Scholar] [CrossRef]
- Huan, T.N.; Rousse, G.; Zanna, S.; Lucas, I.T.; Xu, X.; Menguy, N.; Mougel, V.; Fontecave, M. A Dendritic Nanostructured Copper Oxide Electrocatalyst for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 4792–4796. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Cu2O–Cu Hybrid Foams as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Catal. 2016, 7, 986–991. [Google Scholar] [CrossRef]
- Deng, S.; Shen, Y.; Xie, D.; Lu, Y.; Yu, X.; Yang, L.; Wang, X.; Xia, X.; Tu, J. Directional construction of Cu2S branch arrays for advanced oxygen evolution reaction. J. Energy Chem. 2019, 39, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Bo, X.; Li, M.; Guo, L. Facile electrodeposition fabrication of molybdenum-tungsten sulfide on carbon cloth for electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 15479–15488. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Jin, M.; Xiong, Q.; Wang, G.; Zhang, H.; Zhao, H. Robust enhanced hydrogen production at acidic conditions over molybdenum oxides-stabilized ultrafine palladium electrocatalysts. Nano Res. 2020, 14, 268–274. [Google Scholar] [CrossRef]
- Blackstone, C.; Ignaszak, A. Van der Waals Heterostructures—Recent Progress in Electrode Materials for Clean Energy Applications. Materials 2021, 14, 3754. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Patel, M.K.; Miotello, A.; Patel, N. Metal Boride-Based Catalysts for Electrochemical Water-Splitting: A Review. Adv. Funct. Mater. 2019, 30, 1906481. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rowley-Neale, S.; Banks, C. Enhancing the efficiency of the hydrogen evolution reaction utilising Fe3P bulk modified screen-printed electrodes via the application of a magnetic field. RSC Adv. 2021, 11, 8073–8079. [Google Scholar] [CrossRef]
- Yu, J.; Le, T.A.; Tran, N.Q.; Lee, H. Earth-Abundant Transition-Metal-Based Bifunctional Electrocatalysts for Overall Water Splitting in Alkaline Media. Chem.–A Eur. J. 2020, 26, 6423–6436. [Google Scholar] [CrossRef]
- Antonyshyn, I.; Jimenez, A.M.B.; Sichevych, O.; Burkhardt, U.; Veremchuk, I.; Schmidt, M.; Ormeci, A.; Spanos, I.; Tarasov, A.; Teschner, D.; et al. Al2Pt for Oxygen Evolution in Water Splitting: A Strategy for Creating Multifunctionality in Electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 16770–16776. [Google Scholar] [CrossRef]
- Le Bideau, D.; Chocron, O.; Mandin, P.; Kiener, P.; Benbouzid, M.; Sellier, M.; Kim, M.; Ganci, F.; Inguanta, R. Evolutionary Design Optimization of an Alkaline Water Electrolysis Cell for Hydrogen Production. Appl. Sci. 2020, 10, 8425. [Google Scholar] [CrossRef]
- Hu, Q.; Lin, J.; Zeng, Q.; Fu, C.; Li, J. Optimal control of a hydrogen microgrid based on an experiment validated P2HH model. IET Renew. Power Gener. 2019, 14, 364–371. [Google Scholar] [CrossRef]
- Xue, F.; Su, J.; Li, P.; Zhang, Y. Application of Proton Exchange Membrane Electrolysis of Water Hydrogen Production Technology in Power Plant. IOP Conf. Ser. Earth Environ. Sci. 2021, 631, 012079. [Google Scholar] [CrossRef]
- Jianghao, N. Research on the Hydrogen Production Technology. IOP Conf. Ser. Earth Environ. Sci. 2021, 813, 012004. [Google Scholar] [CrossRef]
- An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G.G.; Fisher, A.C.; Xi, P.; Xu, Z.J.; Yan, C.H. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Adv. Mater. 2021, 33, 2006328. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.V.; Nguyen, X.L.; Kim, Y.; Yu, S. Characteristics of Water Transport of Membrane Electrolyte over Selected Temperature for Proton Exchange Membrane Fuel Cell. Polymers 2022, 14, 2972. [Google Scholar] [CrossRef] [PubMed]
- Arminio-Ravelo, J.A.; Quinson, J.; Pedersen, M.A.; Kirkensgaard, J.J.K.; Arenz, M.; Escudero-Escribano, M. Synthesis of Iridium Nanocatalysts for Water Oxidation in Acid: Effect of the Surfactant. ChemCatChem 2019, 12, 1282–1287. [Google Scholar] [CrossRef]
- Klose, C.; Saatkamp, T.; Münchinger, A.; Bohn, L.; Titvinidze, G.; Breitwieser, M.; Kreuer, K.; Vierrath, S. All-Hydrocarbon MEA for PEM Water Electrolysis Combining Low Hydrogen Crossover and High Efficiency. Adv. Energy Mater. 2020, 10, 1903995. [Google Scholar] [CrossRef]
- Matienzo, D.; Settipani, D.; Instuli, E.; Kallio, T. Active IrO2 and NiO Thin Films Prepared by Atomic Layer Deposition for Oxygen Evolution Reaction. Catalysts 2020, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Takatsu, N.; Farzaneh, H. Techno-Economic Analysis of a Novel Hydrogen-Based Hybrid Renewable Energy System for Both Grid-Tied and Off-Grid Power Supply in Japan: The Case of Fukushima Prefecture. Appl. Sci. 2020, 10, 4061. [Google Scholar] [CrossRef]
- Kalbasi, R.; Jahangiri, M.; Tahmasebi, A. Comprehensive Investigation of Solar-Based Hydrogen and Electricity Production in Iran. Int. J. Photoenergy 2021, 2021, 6627491. [Google Scholar] [CrossRef]
- Li, S.; Kang, Q.; Baeyens, J.; Zhang, H.L.; Deng, Y.M. Hydrogen Production: State of Technology. IOP Conf. Ser. Earth Environ. Sci. 2020, 544, 012011. [Google Scholar] [CrossRef]
- Holzapfel, P.K.R.; Bühler, M.; Escalera-López, D.; Bierling, M.; Speck, F.D.; Mayrhofer, K.J.J.; Cherevko, S.; Pham, C.V.; Thiele, S. Fabrication of a Robust PEM Water Electrolyzer Based on Non-Noble Metal Cathode Catalyst: [Mo3S13]2− Clusters Anchored to N-Doped Carbon Nanotubes. Small 2020, 16, 2003161. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Su, J.; Li, P. Technical Research on Hydrogen Supply Chain Industry. IOP Conf. Ser. Earth Environ. Sci. 2020, 558, 022040. [Google Scholar] [CrossRef]
- Gunawan, T.A.; Singlitico, A.; Blount, P.; Burchill, J.; Carton, J.G.; Monaghan, R.F.D. At What Cost Can Renewable Hydrogen Offset Fossil Fuel Use in Ireland’s Gas Network? Energies 2020, 13, 1798. [Google Scholar] [CrossRef] [Green Version]
- Peksen, M. Hydrogen Technology towards the Solution of Environment-Friendly New Energy Vehicles. Energies 2021, 14, 4892. [Google Scholar] [CrossRef]
- Aubras, F.; Damour, C.; Benne, M.; Boulevard, S.; Bessafi, M.; Grondin-Perez, B.; Kadjo, A.; Deseure, J. A Non-Intrusive Signal-Based Fault Diagnosis Method for Proton Exchange Membrane Water Electrolyzer Using Empirical Mode Decomposition. Energies 2021, 14, 4458. [Google Scholar] [CrossRef]
- Niaz, H.; Lakouraj, M.M.; Liu, J. Techno-economic feasibility evaluation of a standalone solar-powered alkaline water electrolyzer considering the influence of battery energy storage system: A Korean case study. Korean J. Chem. Eng. 2021, 38, 1617–1630. [Google Scholar] [CrossRef]
- Phoumin, H.; Kimura, F.; Arima, J. Potential Renewable Hydrogen from Curtailed Electricity to Decarbonize ASEAN’s Emissions: Policy Implications. Sustainability 2020, 12, 10560. [Google Scholar] [CrossRef]
- Samani, A.E.; D’Amicis, A.; De Kooning, J.D.; Bozalakov, D.; Silva, P.; Vandevelde, L. Grid balancing with a large-scale electrolyser providing primary reserve. IET Renew. Power Gener. 2020, 14, 3070–3078. [Google Scholar] [CrossRef]
- Kopteva, A.; Kalimullin, L.; Tcvetkov, P.; Soares, A. Prospects and Obstacles for Green Hydrogen Production in Russia. Energies 2021, 14, 718. [Google Scholar] [CrossRef]
- Marzi, E.; Morini, M.; Gambarotta, A. Analysis of the Status of Research and Innovation Actions on Electrofuels under Horizon 2020. Energies 2022, 15, 618. [Google Scholar] [CrossRef]
- Nguyen, H.; Han, J.; Nguyen, X.; Yu, S.; Goo, Y.-M.; Le, D. Review of the Durability of Polymer Electrolyte Membrane Fuel Cell in Long-Term Operation: Main Influencing Parameters and Testing Protocols. Energies 2021, 14, 4048. [Google Scholar] [CrossRef]
- Boulmrharj, S.; Khaidar, M.; Bakhouya, M.; Ouladsine, R.; Siniti, M.; Zine-Dine, K. Performance Assessment of a Hybrid System with Hydrogen Storage and Fuel Cell for Cogeneration in Buildings. Sustainability 2020, 12, 4832. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G. Performance Prediction of Proton Exchange Membrane Fuel Cells (PEMFC) Using Adaptive Neuro Inference System (ANFIS). Sustainability 2020, 12, 4952. [Google Scholar] [CrossRef]
- D’Ovidio, G.; Ometto, A.; Villante, C. A Novel Optimal Power Control for a City Transit Hybrid Bus Equipped with a Partitioned Hydrogen Fuel Cell Stack. Energies 2020, 13, 2682. [Google Scholar] [CrossRef]
- Gül, M.; Akyüz, E. Hydrogen Generation from a Small-Scale Solar Photovoltaic Thermal (PV/T) Electrolyzer System: Numerical Model and Experimental Verification. Energies 2020, 13, 2997. [Google Scholar] [CrossRef]
- Li, K.; Fan, Q.; Chuai, H.; Liu, H.; Zhang, S.; Ma, X. Revisiting Chlor-Alkali Electrolyzers: From Materials to Devices. Trans. Tianjin Univ. 2021, 27, 202–216. [Google Scholar] [CrossRef]
- Lee, H.I.; Dung, D.T.; Kim, J.; Pak, J.H.; Kim, S.K.; Cho, H.S.; Cho, W.C.; Kim, C.H. The synthesis of a Zirfon-type porous separator with reduced gas crossover for alkaline electrolyzer. Int. J. Energy Res. 2019, 44, 1875–1885. [Google Scholar] [CrossRef]
- Dawood, F.; Shafiullah, G.; Anda, M. Stand-Alone Microgrid with 100% Renewable Energy: A Case Study with Hybrid Solar PV-Battery-Hydrogen. Sustainability 2020, 12, 2047. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Stropnik, R.; Sekavčnik, M.; Lotrič, A. Criticality and Life-Cycle Assessment of Materials Used in Fuel-Cell and Hydrogen Technologies. Sustainability 2021, 13, 3565. [Google Scholar] [CrossRef]
- Londono, J.V.; Mazza, A.; Pons, E.; Lok, H.; Bompard, E. Modelling and Control of a Grid-Connected RES-Hydrogen Hybrid Microgrid. Energies 2021, 14, 1540. [Google Scholar] [CrossRef]
- Ying, Z.; Wang, Y.; Zheng, X.; Geng, Z.; Dou, B.; Cui, G. Modeling and experimental assessment of the novel HI-I2-H2O electrolysis for hydrogen generation in the sulfur-iodine cycle. Int. J. Energy Res. 2020, 44, 6285–6296. [Google Scholar] [CrossRef]
- Yan, X.; Biemolt, J.; Zhao, K.; Zhao, Y.; Cao, X.; Yang, Y.; Wu, X.; Rothenberg, G.; Yan, N. A membrane-free flow electrolyzer operating at high current density using earth-abundant catalysts for water splitting. Nat. Commun. 2021, 12, 4143. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kim, M.J.; Brown, M.; Wiley, B.J. Alkaline Water Electrolysis at 25 A cm−2 with a Microfibrous Flow-through Electrode. Adv. Energy Mater. 2020, 10, 2001174. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- Iqbal, W.; Yumei, H.; Abbas, Q.; Hafeez, M.; Mohsin, M.; Fatima, A.; Jamali, M.A.; Jamali, M.; Siyal, A.; Sohail, N. Assessment of Wind Energy Potential for the Production of Renewable Hydrogen in Sindh Province of Pakistan. Processes 2019, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Tachikawa, T.; Beniya, A.; Shigetoh, K.; Higashi, S. Relationship between OER Activity and Annealing Temperature of Sputter-Deposited Flat IrO2 Thin Films. Catal. Lett. 2020, 150, 1976–1984. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Yang, W.; Liu, W.; Min, F.; Mao, S.S.; Xie, J. Catalyst-coated proton exchange membrane for hydrogen production with high pressure water electrolysis. Appl. Phys. Lett. 2021, 119, 123903. [Google Scholar] [CrossRef]
- Waribam, P.; Jaiyen, K.; Samart, C.; Ogawa, M.; Guan, G.; Kongparakul, S. MXene potassium titanate nanowire/sulfonated polyether ether ketone (SPEEK) hybrid composite proton exchange membrane for photocatalytic water splitting. RSC Adv. 2021, 11, 9327–9335. [Google Scholar] [CrossRef]
- Tian, D.; Gu, T.; Yellamilli, S.N.; Bae, C. Phosphoric Acid-Doped Ion-Pair Coordinated PEMs with Broad Relative Humidity Tolerance. Energies 2020, 13, 1924. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite Polymers Development and Application for Polymer Electrolyte Membrane Technologies—A Review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yamasaki, K.; Ishimoto, H.; Takata, Y. Ultrathin Electrolyte Membranes with PFSA-Vinylon Intermediate Layers for PEM Fuel Cells. Polymers 2020, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Mazzapioda, L.; Sgambetterra, M.; Tsurumaki, A.; Navarra, M.A. Different approaches to obtain functionalized alumina as additive in polymer electrolyte membranes. J. Solid State Electrochem. 2021, 26, 17–27. [Google Scholar] [CrossRef]
- Panchenko, U.; Arlt, T.; Manke, I.; Müller, M.; Stolten, D.; Lehnert, W. Synchrotron Radiography for a Proton Exchange Membrane (PEM) Electrolyzer. Fuel Cells 2020, 20, 300–306. [Google Scholar] [CrossRef]
- Veh, P.; Britton, B.; Holdcroft, S.; Zengerle, R.; Vierrath, S.; Breitwieser, M. Improving the water management in anion-exchange membrane fuel cells via ultra-thin, directly deposited solid polymer electrolyte. RSC Adv. 2020, 10, 8645–8652. [Google Scholar] [CrossRef] [Green Version]
- Arunachalam, M.; Sinopoli, A.; Aidoudi, F.; Creager, S.E.; Smith, R.; Merzougui, B.; Aïssa, B. Investigating the suitability of poly tetraarylphosphonium based anion exchange membranes for electrochemical applications. Sci. Rep. 2021, 11, 13841. [Google Scholar] [CrossRef]
- Zignani, S.C.; Faro, M.L.; Trocino, S.; Aricò, A.S. Investigation of NiFe-Based Catalysts for Oxygen Evolution in Anion-Exchange Membrane Electrolysis. Energies 2020, 13, 1720. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Sinha-Ray, S.; Sinha-Ray, S. Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review. Polymers 2020, 12, 238. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.; Cebola, M.-J.; Santos, D. Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers. Energies 2021, 14, 3193. [Google Scholar] [CrossRef]
- Deng, W.; Li, Y.; Wang, F.; Ma, Q.; Min, S. Palladium Nanoparticles Supported on Basswood-Derived Porous Carbon Membrane as Free-Standing Cathodes for Efficient pH-Universal Electrocatalytic H2 Evolution. Electrocatalysis 2021, 12, 340–349. [Google Scholar] [CrossRef]
- Naito, T.; Shinagawa, T.; Nishimoto, T.; Takanabe, K. Water Electrolysis in Saturated Phosphate Buffer at Neutral pH. ChemSusChem 2020, 13, 5921–5933. [Google Scholar] [CrossRef]
- Vidales, A.G.; Sridhar, D.; Meunier, J.-L.; Omanovic, S. Nickel oxide on directly grown carbon nanofibers for energy storage applications. J. Appl. Electrochem. 2020, 50, 1217–1229. [Google Scholar] [CrossRef]
- Guo, W.; Kim, J.; Kim, H.; Ahn, S.H. Direct electrodeposition of Ni-Co-S on carbon paper as an efficient cathode for anion exchange membrane water electrolysers. Int. J. Energy Res. 2020, 45, 1918–1931. [Google Scholar] [CrossRef]
- Ahn, J.; Park, Y.S.; Lee, S.; Yang, J.; Pyo, J.; Lee, J.; Kim, G.H.; Choi, S.M.; Seol, S.K. 3D-printed NiFe-layered double hydroxide pyramid electrodes for enhanced electrocatalytic oxygen evolution reaction. Sci. Rep. 2022, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Pierozynski, B.; Mikolajczyk, T.; Wojciechowski, B.; Luba, M. An Innovative 500 W Alkaline Water Electrolyser System for the Production of Ultra-Pure Hydrogen and Oxygen Gases. Energies 2021, 14, 526. [Google Scholar] [CrossRef]
- Vincent, I.; Lee, E.-C.; Kim, H.-M. Comprehensive impedance investigation of low-cost anion exchange membrane electrolysis for large-scale hydrogen production. Sci. Rep. 2021, 11, 293. [Google Scholar] [CrossRef]
- Sdanghi, G.; Dillet, J.; Didierjean, S.; Fierro, V.; Maranzana, G. Feasibility of Hydrogen Compression in an Electrochemical System: Focus on Water Transport Mechanisms. Fuel Cells 2019, 20, 370–380. [Google Scholar] [CrossRef]
- Ahshan, R. Potential and Economic Analysis of Solar-to-Hydrogen Production in the Sultanate of Oman. Sustainability 2021, 13, 9516. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Z.; Qiu, S.; Luo, Y.; Liu, Z.; Yang, F.; Liu, H.; Ge, S.; Zou, X.; Ding, B.; et al. A Ta-TaS2 monolith catalyst with robust and metallic interface for superior hydrogen evolution. Nat. Commun. 2021, 12, 6051. [Google Scholar] [CrossRef]
- Santos, J.E.L.; da Silva, D.R.; Martínez-Huitle, C.A.; dos Santos, E.V.; Quiroz, M.A. Cathodic hydrogen production by simultaneous oxidation of methyl red and 2,4-dichlorophenoxyacetate in aqueous solutions using PbO2, Sb-doped SnO2 and Si/BDD anodes. Part 2: Hydrogen production. RSC Adv. 2020, 10, 37947–37955. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chen, C.-H.; Jung, G.-B.; Li, S.-C.; Zeng, Y.-Z. Internal Microscopic Diagnosis of Accelerated Aging of Proton Exchange Membrane Water Electrolysis Cell Stack. Micromachines 2020, 11, 1078. [Google Scholar] [CrossRef]
- Darvishi, Y.; Hassan-Beygi, S.R.; Zarafshan, P.; Hooshyari, K.; Malaga-Toboła, U.; Gancarz, M. Numerical Modeling and Evaluation of PEM Used for Fuel Cell Vehicles. Materials 2021, 14, 7907. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhao, S.; Mi, Y.; Han, L.; Liu, X.; Qi, D.; Sun, J.; Liu, Y.; Bao, H.; Zhuo, L.; et al. Trifunctional Single-Atomic Ru Sites Enable Efficient Overall Water Splitting and Oxygen Reduction in Acidic Media. Small 2020, 16, 2002888. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yu, L.; Gao, M.; Chen, X.; Zhou, W.; Ma, C.; Wu, L.; Zhu, J.; Meng, X.; Hu, J.; et al. Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer. Nat. Commun. 2020, 11, 3351. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, P.; Lacour, M.-A.; Masquelez, N.; Cambedouzou, J.; Tingry, S.; Cornu, D.; Holade, Y. Insights on the Electrocatalytic Seawater Splitting at Heterogeneous Nickel-Cobalt Based Electrocatalysts Engineered from Oxidative Aniline Polymerization and Calcination. Molecules 2021, 26, 5926. [Google Scholar] [CrossRef]
- EPO; IRENA. Patent Insight Report. Innovation Trends in Electrolysers for Hydrogen Production; EPO: Vienna, Austria, 2022. [Google Scholar]
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21. [Google Scholar] [CrossRef]
- Pozio, A.; Bozza, F.; Nigliaccio, G.; Platter, M.; Monteleone, G. Development perspectives on low-temperature electrolysis. Energ. Ambiente Innov. 2021, 1, 66–72. [Google Scholar] [CrossRef]

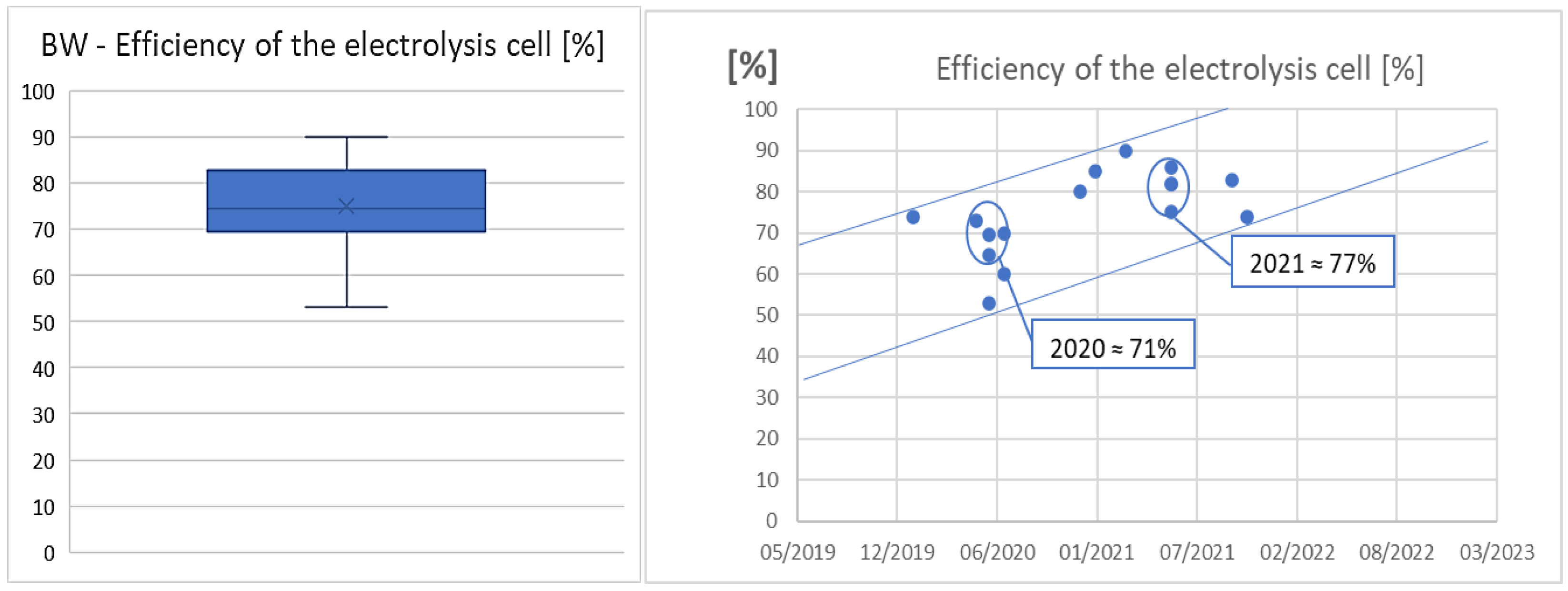
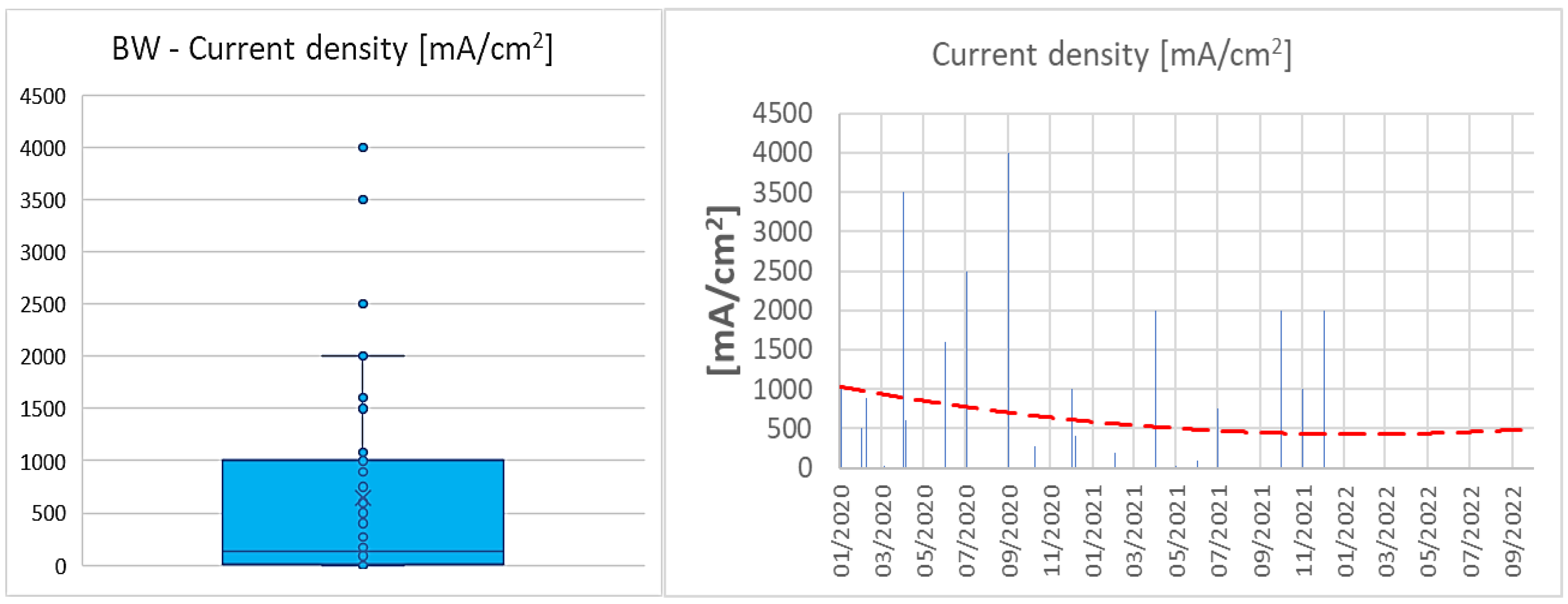
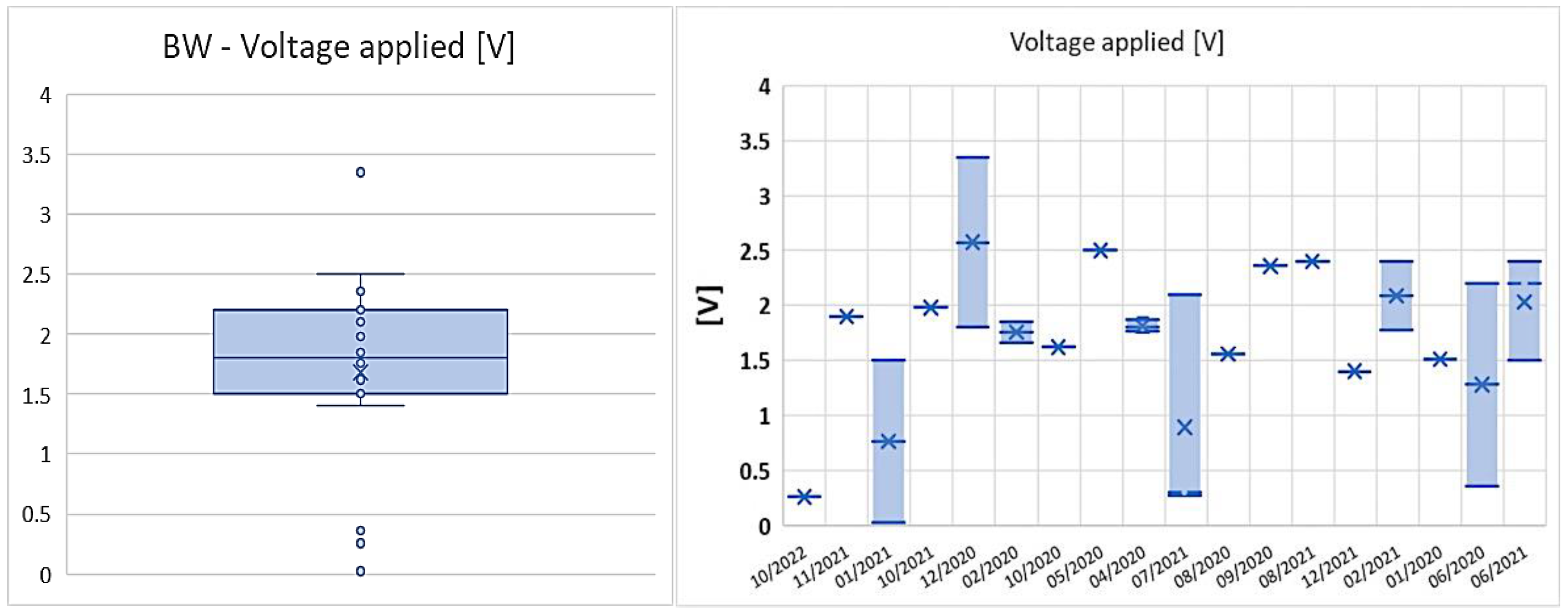
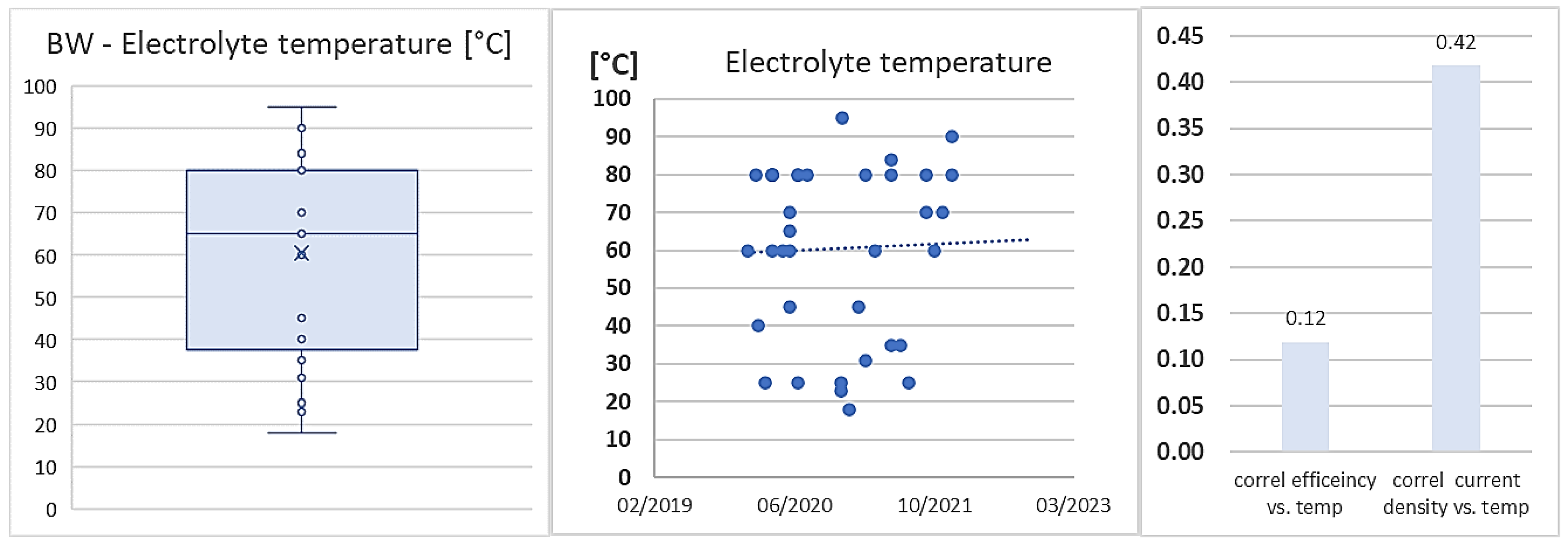

| Parameter | AWE | PEM |
|---|---|---|
| Cell efficiency [%] | ≈70 ÷ 89 | ≈53 ÷ 90 |
| Current density [mA/cm2] | ≈50 ÷ 2500 | ≈100 ÷ 4000 |
| Charge carrier | OH− | H⁺ |
| Temperature range [°C] | ≈60 ÷ 90 | ≈25 ÷ 80 |
| Electrolyte | ≈1.1 ÷ 9.5 M KOH | ≈H2O/DI H2O |
| Pressure [bar] | ≈11.8 ÷ 29 | ≈1 ÷ 29.6 |
| Estimated lifetime [h] [150] | ≈90,000 | <20,000 |
| H2 purity [%] | ≈99.1 ÷ 99.8 | ≈99.99 |
| Energy for H2 gen [kWh/Nm3] | ≈3.8 ÷ 4.4 | ≈3.54 ÷ 4.5 |
| Production [Nm3/h] | ≈40 ÷ 12,550 | ≈30 ÷ 5000 |
| Operating cost [EURO/kg] | ≈3.72 ÷ 4.2 | ≈3 ÷ 8.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hora, C.; Dan, F.C.; Rancov, N.; Badea, G.E.; Secui, C. Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies 2022, 15, 6076. https://doi.org/10.3390/en15166076
Hora C, Dan FC, Rancov N, Badea GE, Secui C. Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies. 2022; 15(16):6076. https://doi.org/10.3390/en15166076
Chicago/Turabian StyleHora, Cristina, Florin Ciprian Dan, Nicolae Rancov, Gabriela Elena Badea, and Calin Secui. 2022. "Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review" Energies 15, no. 16: 6076. https://doi.org/10.3390/en15166076
APA StyleHora, C., Dan, F. C., Rancov, N., Badea, G. E., & Secui, C. (2022). Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies, 15(16), 6076. https://doi.org/10.3390/en15166076