Experimental Study on the Distribution Characteristics of CO2 in Methane Hydrate-Bearing Sediment during CH4/CO2 Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Experimental Procedure
2.2.1. Sample Preparation
2.2.2. CH4/CO2 Replacement
- (1)
- CO2 injection: After the formation of CH4 hydrate, the pressure vessel was cooled down to −20 °C and held for at least 1 h. CH4 gas in the vessel was released within 10 s and CO2 was re-injected until the pressure reached ~3.5 MPa. To avoid hydrate dissociation, CO2 was precooled in the cold-water buffer tank before injection. Then the vessel was heated to a certain temperature, and this specific point in time was recorded as the start of the replacement process.
- (2)
- In situ analysis: Straight after the re-injection of CO2, the valves between the pressure vessel and the GC were opened and closed in turn to allow a small volume of gas (less than 0.2% v/v of the pressure vessel) in the pressure vessel to be sampled, so that the initial gas composition in the pressure vessel could be determined. The same method was conducted to obtain the gas composition in the pressure vessel every 24 h until the end of the experiments, which lasted for 12 days.
- (3)
- Sample slicing: After replacement, hydrate-bearing sample in the pressure vessel was taken out and sliced into four pieces horizontally under the protection of liquid nitrogen (Figure 2). The composition of the decomposed gas for each sample piece was collected and analyzed by GC.
2.3. Calculation Methods
2.3.1. Hydrate Saturation
2.3.2. Replacement Efficiency
3. Results and Discussion
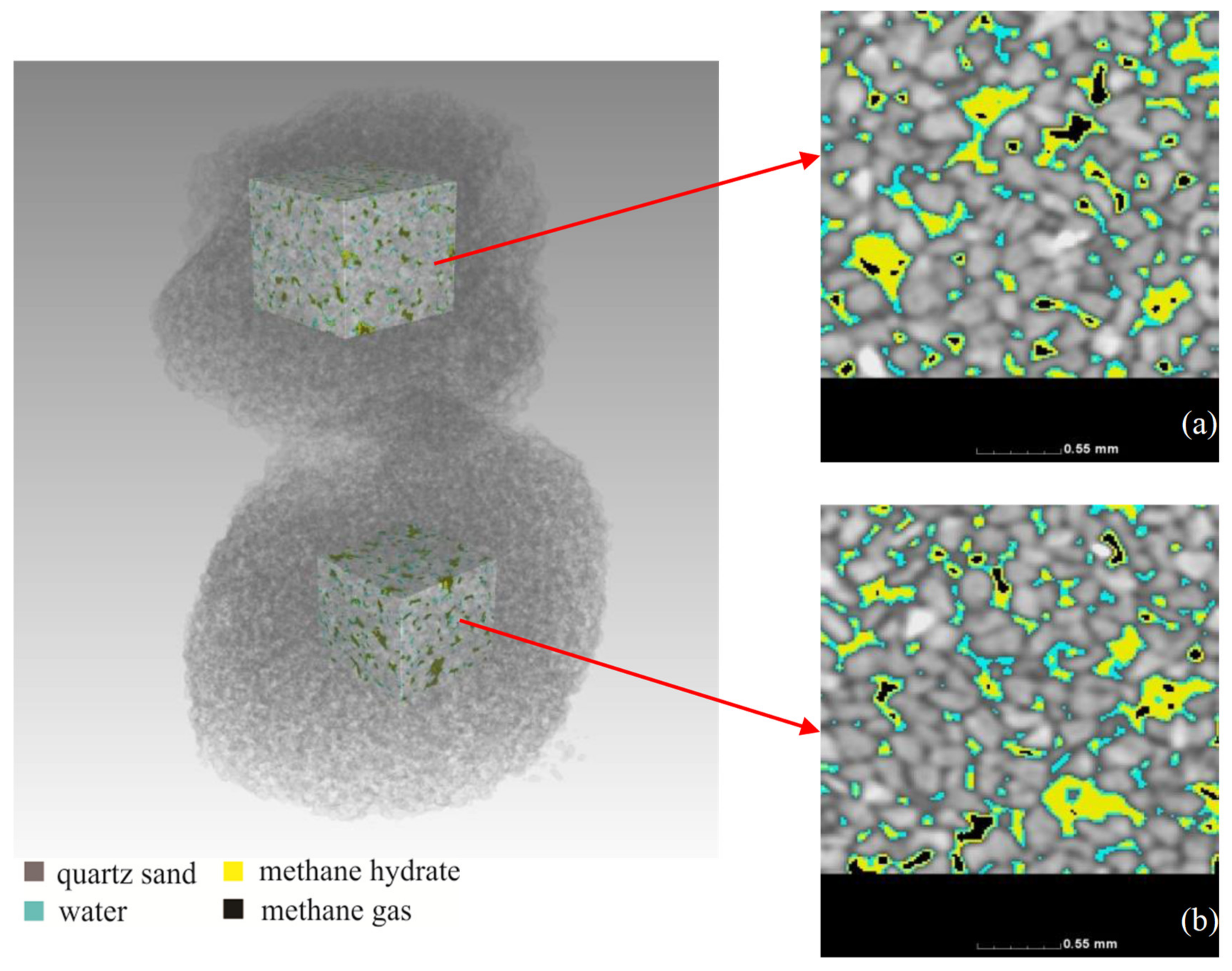
3.1. Distribution of Methane Hydrate before Replacement
3.2. Influencing Factor of Replacement Efficiency
3.2.1. Temperature
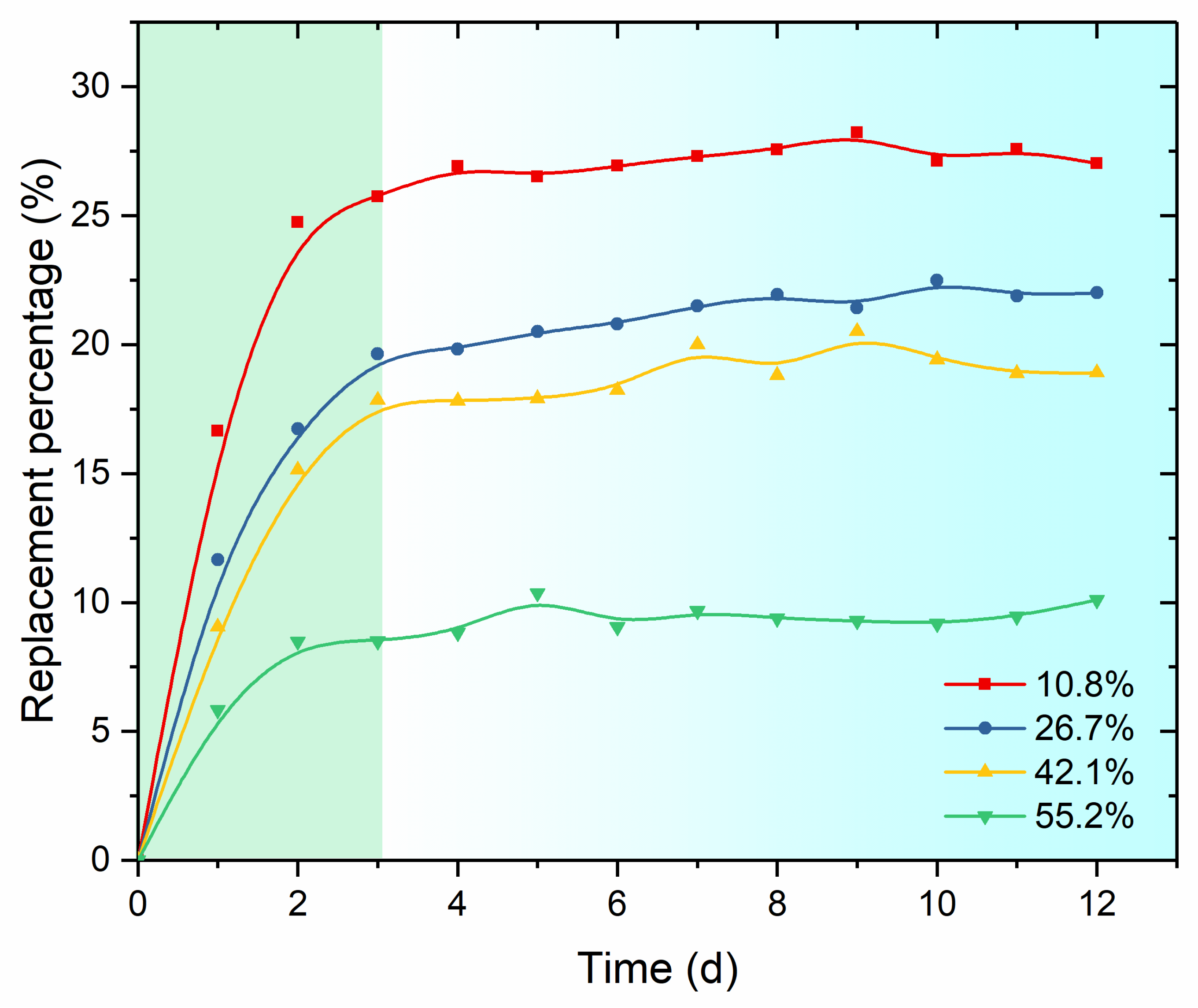
3.2.2. Initial Hydrate Saturation
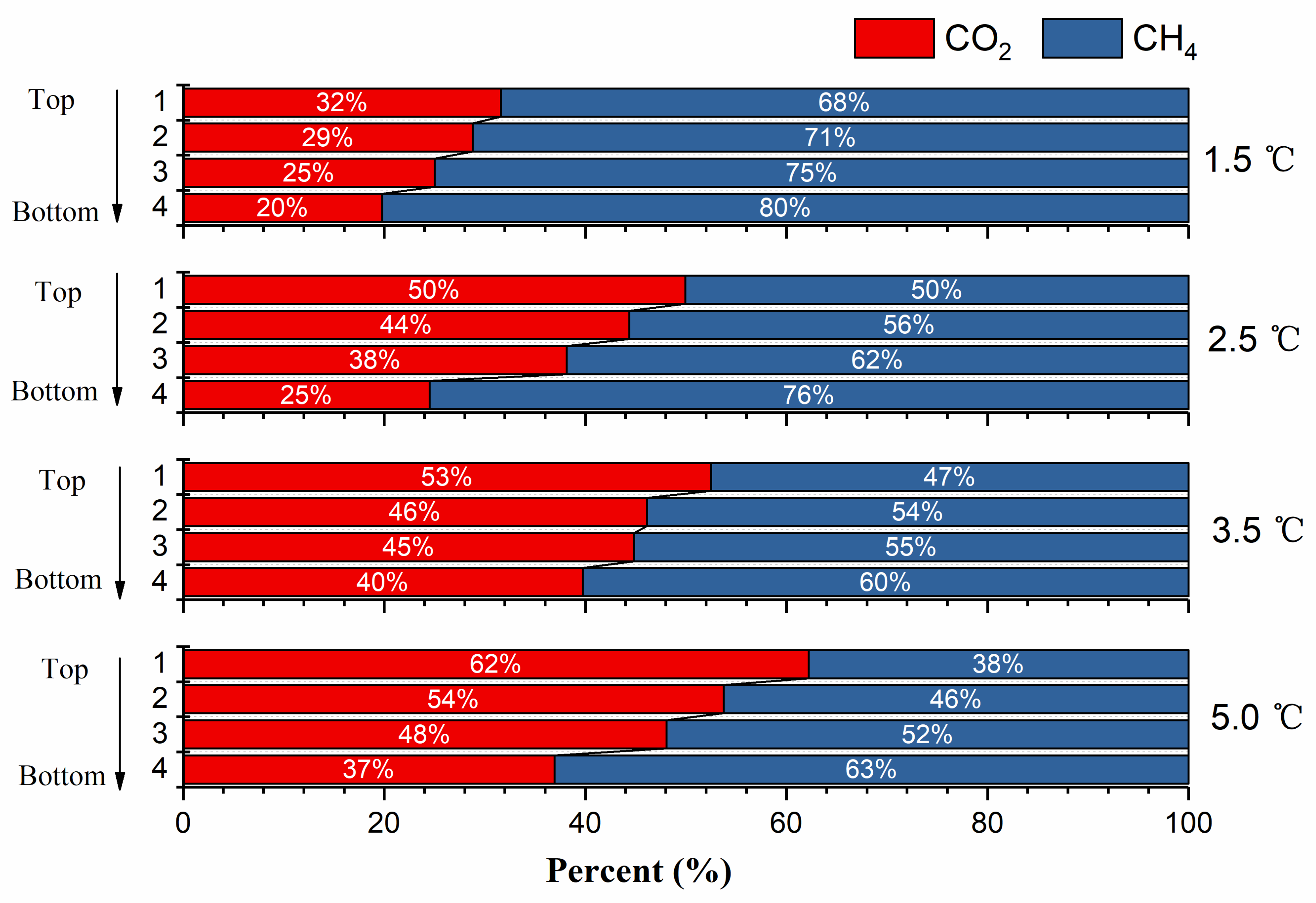
3.3. Vertical Distribution of CO2
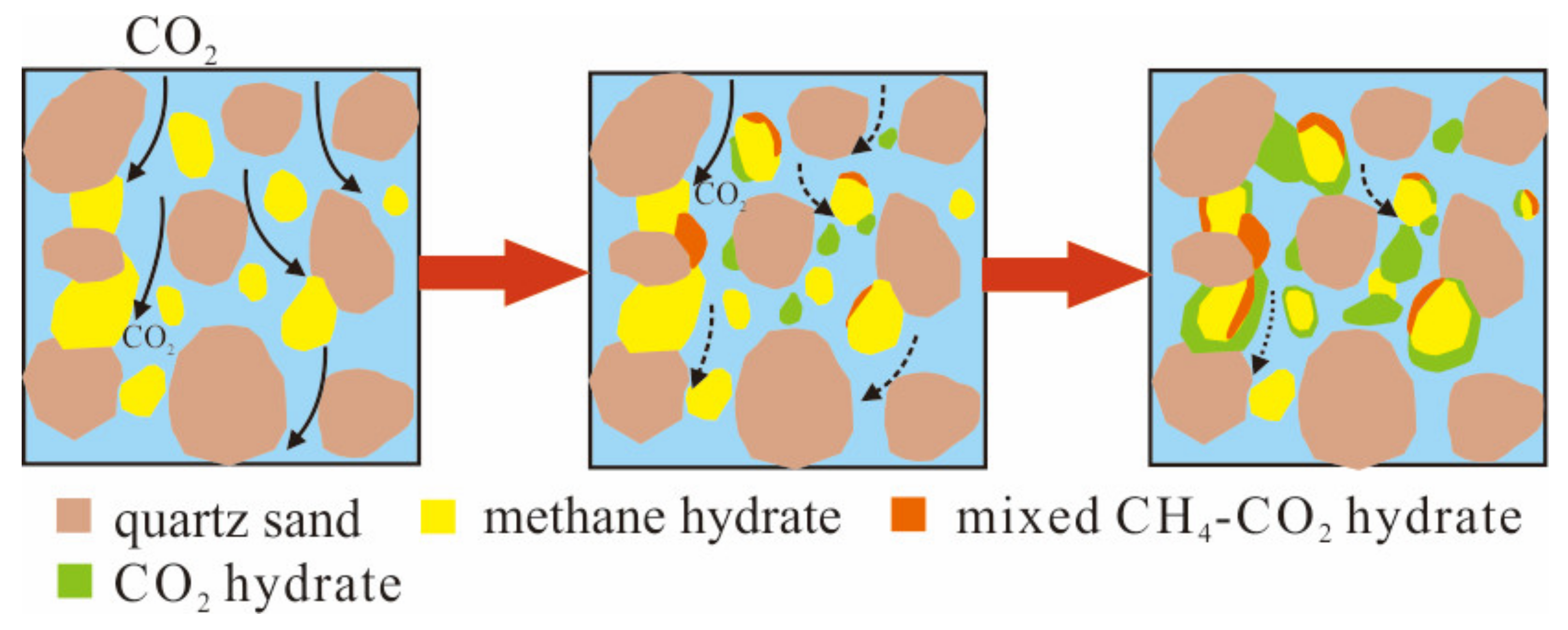
3.4. The Excess Consumption of CO2
4. Conclusions
- (1)
- Based on the replacement rates, the replacement process can be divided into a fast stage and a slow stage, which represent two different reaction processes. At the fast stage, replacement mainly occurred at the gas-hydrate interface, while at the slow stage, the occurrence of the replacement relied on the diffusion of CO2 and CH4 through the hydrate phase. Although the fast stage only lasted for about 3 days, the overall replacement percentage was mainly determined at this stage.
- (2)
- Higher replacement temperature and lower initial methane hydrate saturation resulted in higher overall CO2 replacement percentage. Meanwhile, the CO2 content decreased, and the methane content increased with the increase of sediment depth. At the same sediment depth, the variation of the relative CO2/CH4 contents showed an increasing trend with the increase of experimental temperature/initial methane hydrate saturation.
- (3)
- The CH4/CO2 replacement reaction mainly occurs in the fast reaction stage while the CO2 hydrate formation by CO2 and pore water almost runs through the whole experimental cycle at almost the same rate. Under current experimental conditions and duration, the permeability of the sediments during replacement remained feasible for the diffusion of the gaseous phase. The major hinderance of CH4 recovery should be the diffusion of CO2 through the mix CH4-CO2 layer formed at the surface of methane hydrate.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and applications of gas hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef]
- Ye, J.; Qin, X.; Xie, W.; Lu, H.; Ma, B.; Qiu, H.; Liang, J.; Lu, J.A.; Kuang, Z.; Lu, C.; et al. Main progress of the second gas hydrate trial production in the South China Sea. Geol. China 2020, 47, 557–568. [Google Scholar]
- Dallimore, S.R.; Collett, T.S. Scientific Results from the Mallik 2002 Gas Hydrate Production Research Well Program, Mackenzie Delta, Northwest Territories, Canada; Geological Survey of Canada: Vancouver, BC, Canada, 2005. [Google Scholar]
- Dubreuil-Boisclair, C.; Gloaguen, E.; Bellefleur, G.; Marcotte, D. Non-Gaussian gas hydrate grade simulation at the Mallik site, Mackenzie Delta, Canada. Mar. Pet. Geol. 2012, 35, 20–27. [Google Scholar] [CrossRef]
- Judith, M.S. From lab to field, from micro to macro—Test of technologies for the production of hydrate bonded CH4 via CO2 sequestration in hydrates. In Proceedings of the 9th International Conference on Gas Hydrates, Denver, CO, USA, 25–30 June 2017. [Google Scholar]
- Ebinuma, T. Washington, DC: U.S. Patent and Trademark Office. U.S. Patent No. 5,261,490, 11 May 1993. [Google Scholar]
- Geng, C.Y.; Wen, H.; Zhou, H. Molecular simulation of the potential of methane reoccupation during the replacement of methane hydrate by CO2. J. Phys. Chem. A 2009, 113, 5463–5469. [Google Scholar] [CrossRef]
- Jadhawar, P.; Yang, J.H.; Chapoy, A.; Tohidi, B. Subsurface carbon dioxide sequestration and storage in methane hydrate reservoirs combined with clean methane energy recovery. Energy Fuel 2021, 35, 1567–1579. [Google Scholar] [CrossRef]
- Pan, D.; Zhong, X.; Zhu, Y.; Zhai, L.; Zhang, H.; Li, X.; Wang, Y.; Chen, C. CH4 recovery and CO2 sequestration from hydrate-bearing clayey sediments via CO2/N2 injection. J. Nat. Gas Sci. Eng. 2020, 83, 103503. [Google Scholar] [CrossRef]
- Panda, D.; Saini, C.; Singh, S.K.; Kumar, E.A. In situ casting of rice husk ash in metal organic frameworks induces enhanced CO2 capture performance. Sci. Rep. 2020, 10, 20219. [Google Scholar] [CrossRef] [PubMed]
- de Kleijne, K.; Hanssen, S.V.; van Dinteren, L.; Huijbregts, M.A.; van Zelm, R.; de Coninck, H. Limits to Paris compatibility of CO2 capture and utilization. One Earth 2022, 5, 168–185. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane exploitation by carbon dioxide from gas hydrates-Phase equilibria for CO2-CH4 mixed hydrate system. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Uchida, T.; Ikeda, I.Y.; Takeya, S.; Kamata, Y.; Ohmura, R.; Nagao, J.; Zatsepina, O.Y.; Buffett, B.A. Kinetics and stability of CH4-CO2 mixed gas hydrates during formation and long-term storage. Chem. Phys. Chem. 2005, 6, 646–654. [Google Scholar] [CrossRef]
- Smith, D.H.; Seshadri, K.; Wilder, J.W. Assessing the thermodynamic feasibility of the conversion of methane hydrate into carbon dioxide hydrate in porous media. In Proceedings of the First National Conference on Carbon Sequestration, Washington, DC, USA, 14–17 May 2001; pp. 1–16. [Google Scholar]
- Yezdimer, E.M.; Cummings, P.T.; Chialvo, A.A. Determination of the Gibbs free energy of gas replacement in SI clathrate hydrates by molecular simulation. J. Phys. Chem. 2002, 106, 7982–7987. [Google Scholar] [CrossRef]
- Lee, Y.; Deusner, C.; Kossel, E.; Choi, W.; Seo, Y.; Haeckel, M. Influence of CH4 hydrate exploitation using depressurization and replacement methods on mechanical strength of hydrate-bearing sediment. Appl. Energy 2020, 277, 115569. [Google Scholar] [CrossRef]
- Jung, J.; Ryou, J.E.; Al-Raoush, R.I.; Alshibli, K.; Lee, J.Y. Effects of CH4-CO2 replacement in hydrate-bearing sediments on S-wave velocity and electrical resistivity. J. Nat. Gas Sci. Eng. 2020, 82, 103506. [Google Scholar] [CrossRef]
- Schoderbek, D.; Farrell, H.; Howard, J.; Raterman, K.; Silpngarmlert, S.; Martin, K.; Smith, B.; Klein, P. ConocoPhillips Gas Hydrate Production Test; ConocoPhillips Co.: Houston, TX, USA, 2013. [Google Scholar]
- Kvamme, B. Feasibility of simultaneous CO2 storage and CH4 production from natural gas hydrate using mixtures of CO2 and N2. Can. J. Chem. 2015, 10, 21–30. [Google Scholar] [CrossRef]
- Sun, J.Y.; Liu, L.L.; Wang, X.W.; Wang, F.F.; Liu, C.L. Experimental study on the process of CO2 replacement of CH4 from methane hydrate in sediments. Nat. Gas Ind. 2015, 35, 56–62. [Google Scholar]
- Ota, M.; Morohashi, K.; Abe, Y.; Watanabe, M.; Smith, R.L., Jr.; Inomata, H. Replacement of CH4 in the hydrate by use of liquid CO2. Energy Convers. Manag. 2005, 46, 1680–1691. [Google Scholar] [CrossRef]
- Mok, J.; Choi, W.; Lee, J.; Seo, Y. Effects of pressure and temperature conditions on thermodynamic and kinetic guest exchange behaviors of CH4- CO2 + N2 replacement for energy recovery and greenhouse gas storage. Energy 2022, 239, 122153. [Google Scholar] [CrossRef]
- Le, Q.D.; Rodriguez, C.T.; Legoix, L.N.; Pirim, C.; Chazallon, B. Influence of the initial CH4-hydrate system properties on CO2 capture kinetics. Appl. Energy 2020, 280, 115843. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Zhao, Y.; Chen, L.; Dong, C.; Sun, B. Experimental investigation of different factors influencing the replacement efficiency of CO2 for methane hydrate. Appl. Energy 2018, 228, 309–316. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Dai, Z.; Jiang, S.; Sun, Y. Concurrent decomposition and replacement of marine gas hydrate with the injection of CO2-N2. Chem. Eng. J. 2021, 420, 129936. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yao, Z.; Li, J.; Wu, Q.; Wang, Y. Experimental study on the effect of pressure on the replacement process of CO2-CH4 hydrate below the freezing point. Energy Fuels 2018, 32, 646–650. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, Y.J.; Zheng, T.; Yuan, Q.; Sun, C.Y.; Yang, L.Y.; Chen, G.J. Replacement in CH4-CO2 hydrate below freezing point based on abnormal self-preservation differences of CH4 hydrate. Chem. Eng. J. 2021, 403, 126283. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, S.; Liang, D.; Du, J. Replacement of methane from quartz sand-bearing hydrate with carbon dioxide-in-water emulsion. Energy Fuels 2008, 22, 1759–1764. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, X.H.; Dandekar, A.; Sun, C.Y.; Li, Q.P.; Ma, Z.W.; Liu, B.; Chen, G.J. Replacement of methane from hydrates in porous sediments with CO2-in-water emulsions. Ind. Eng. Chem. Res. 2014, 53, 12476–12484. [Google Scholar] [CrossRef]
- Ota, M.; Abe, Y.; Watanabe, M.; Smith, R.L.; Inomata, H. Methane recovery from methane hydrate using pressurized CO2. Fluid Phase Equilib. 2005, 228, 553–559. [Google Scholar] [CrossRef]
- McGrail, B.P.; Zhu, T.; Hunter, R.B. A new method for enhanced production of gas hydrates with CO2. In Proceedings of the AAPG Hedberg Conference, Vancouver, BC, Canada, 12–16 September 2004. [Google Scholar]
- Ota, M.; Saito, T.; Aida, T.; Watanabe, M.; Sato, Y.; Smith, R.L., Jr.; Inomata, H. Macro and Microscopic CH4-CO2 Replacement in CH4 Hydrate Under Pressurized CO2. Environ. Energy Eng. AIChE J. 2007, 53, 2715–2721. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D.Y.; Lee, J.W.; Huh, D.G.; Park, K.P.; Lee, J.; Lee, H. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates. Proc. Natl. Acad. Sci. USA 2006, 103, 12690–12694. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, Y.; Lee, J.; Lee, H.; Seo, Y. CH4 recovery and CO2 sequestration using flue gas in natural gas hydrates as revealed by a micro-differential scanning calorimeter. Appl. Energy 2015, 150, 120–127. [Google Scholar] [CrossRef]
- Mok, J.; Choi, W.; Seo, Y. The dual-functional roles of N2 gas for the exploitation of natural gas hydrates: An inhibitor for dissociation and an external guest for replacement. Energy 2021, 232, 121054. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, L.; Wang, X.; Liu, K.; Sun, B. Experimental investigation of the behavior of methane gas hydrates during depressurization-assisted CO2 replacement. J. Nat. Gas Sci. Eng. 2019, 61, 284–292. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Wang, J.; Zhao, J.; Dong, H.; Yang, M.; Liu, Y.; Song, Y. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO2 replacement of methane hydrate. Chem. Eng. J. 2017, 308, 40–49. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Natural gas hydrates: Comparison between two different applications of thermal stimulation for performing CO2 replacement. Energy 2019, 172, 423–434. [Google Scholar] [CrossRef]
- Ouyang, Q.; Fan, S.; Wang, Y.; Lang, X.; Wang, S.; Zhang, Y.; Yu, C. Enhanced Methane Production Efficiency with In Situ Intermittent Heating Assisted CO2 Replacement of Hydrates. Energy Fuels 2020, 34, 12476–12485. [Google Scholar] [CrossRef]
- Lv, J.; Cheng, Z.; Duan, J.; Wang, S.; Xue, K.; Liu, Y.; Mu, H. Enhanced CH4 recovery from hydrate-bearing sand packs via CO2 replacement assisted thermal stimulation method. J. Nat. Gas Sci. Eng. 2021, 96, 104326. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. The use of sodium chloride as strategy for improving CO2/CH4 replacement in natural gas hydrates promoted with depressurization methods. Arab. J. Geosci. 2020, 13, 898. [Google Scholar] [CrossRef]
- Pandey, J.S.; Karantonidis, C.; Karcz, A.P.; Solms, N. Enhanced CH4-CO2 Hydrate Swapping in the Presence of Low Dosage Methanol. Energies 2020, 13, 5238. [Google Scholar] [CrossRef]
- Lee, B.R.; Koh, C.A.; Sum, A.K. Quantitative measurement and mechanisms for CH4 production from hydrates with the injection of liquid CO2. Phys. Chem. Chem. Phys. 2014, 16, 14922–14927. [Google Scholar] [CrossRef]
- Bai, D.; Zhang, X.; Chen, G.; Wang, W. Replacement mechanism of methane hydrate with carbon dioxide from microsecond molecular dynamics simulations. Energy Environ. Sci. 2012, 5, 7033–7041. [Google Scholar] [CrossRef]
- Li, C.; Hu, G.; Ye, Y.; Liu, C.; Cheng, J.; Zhang, L.; Zheng, R. Microscopic distribution of gas hydrate in sediment determined by X-ray computerized tomography. J. Optoelectron. Laser 2013, 24, 551–557. [Google Scholar]
- Li, C.; Liu, C.; Hu, G.; Sun, J.; Hao, X.; Liu, L.; Meng, Q. Investigation on the multi-parameter of hydrate-bearing sands using nano-focus X-ray computed tomography. J. Geophys. Res. Solid Earth 2019, 124, 2286–2296. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhao, J.; Zhang, L.; Chen, X.; Zhang, Y.; Liu, Y.; Song, Y. Combined replacement and depressurization methane hydrate recovery method. Energy Explor. Exploit. 2016, 34, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Sun, C.-Y.; Liu, B.; Wang, X.; Ma, Z.-W.; Ma, Q.-L.; Yang, L.-Y.; Chen, G.-J.; Li, Q.-P.; Li, S.; et al. Methane recovery from natural gas hydrates in porous sediment using pressurized liquid CO2. Energy Convers Manag. 2013, 67, 257–264. [Google Scholar] [CrossRef]







| Run1 | Run2 | Run3 | Run4 | Run5 | Run6 | Run7 | Run8 | |
|---|---|---|---|---|---|---|---|---|
| Replacement Temperature (T) | 1.5 | 2.5 | 3.5 | 5.0 | 2.5 | 2.5 | 2.5 | 2.5 |
| Initial hydrate saturation (Sh) | 46.5 | 46.9 | 45.8 | 46.2 | 10.8 | 26.7 | 42.1 | 55.2 |
| Pressure of CO2 injection | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Hao, X.; Li, C.; Wu, N.; Chen, Q.; Liu, C.; Li, Y.; Meng, Q.; Huang, L.; Bu, Q. Experimental Study on the Distribution Characteristics of CO2 in Methane Hydrate-Bearing Sediment during CH4/CO2 Replacement. Energies 2022, 15, 5634. https://doi.org/10.3390/en15155634
Sun J, Hao X, Li C, Wu N, Chen Q, Liu C, Li Y, Meng Q, Huang L, Bu Q. Experimental Study on the Distribution Characteristics of CO2 in Methane Hydrate-Bearing Sediment during CH4/CO2 Replacement. Energies. 2022; 15(15):5634. https://doi.org/10.3390/en15155634
Chicago/Turabian StyleSun, Jianye, Xiluo Hao, Chengfeng Li, Nengyou Wu, Qiang Chen, Changling Liu, Yanlong Li, Qingguo Meng, Li Huang, and Qingtao Bu. 2022. "Experimental Study on the Distribution Characteristics of CO2 in Methane Hydrate-Bearing Sediment during CH4/CO2 Replacement" Energies 15, no. 15: 5634. https://doi.org/10.3390/en15155634
APA StyleSun, J., Hao, X., Li, C., Wu, N., Chen, Q., Liu, C., Li, Y., Meng, Q., Huang, L., & Bu, Q. (2022). Experimental Study on the Distribution Characteristics of CO2 in Methane Hydrate-Bearing Sediment during CH4/CO2 Replacement. Energies, 15(15), 5634. https://doi.org/10.3390/en15155634






