Evolution and Prospects in Managing Sewage Sludge Resulting from Municipal Wastewater Purification
Abstract
:1. Introduction
- Municipal wastewater (MWW) means the spent water of a community. From the standpoint of source, it may be a combination of the liquid- and water-carried waste from residences, commercial buildings, industrial plants, and institutions, together with any groundwater, surface water, and stormwater that may be present [1,2];
- Sewage sludge is the residue resulting from the treatment of wastewater released from various sources, including homes, industries, medical facilities, street runoff, and businesses [3,4]. Municipal sewage sludge (MSS) is the residual material produced as a by-product of MWW treatment plants (WWTPs);
2. Methodology
3. Sewer Networks and WWTPs
3.1. MWW Collection
- Towns and big cities
- Small towns or villages;
- Urban centers;
- Isolated houses.
3.2. WWTPs
- Preliminary and primary treatment;
- Secondary treatment;
- Advanced tertiary and quaternary treatment.
4. MSS Disposal and Nutrient and Chemical Recovery
4.1. On-Site Treatments
4.1.1. Stabilization and Thickening
4.1.2. Dewatering
4.2. Off-Site Treatments
4.2.1. Application to Agricultural Land and Reclamation Sites
4.2.2. Thermal Treatments (TT)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Law Insider. Municipal Wastewater Definition. Available online: https://www.lawinsider.com/dictionary/municipal-wastewater (accessed on 19 May 2022).
- Environment and Climate Change Canada. Canadian Environmental Sustainability Indicators: Greenhouse Gas Emissions; Environment and Climate Change Canada: Winnipeg, MB, Canada, 2020. [Google Scholar]
- Law Insider. Municipal Sewage Sludge Definition. Available online: https://www.lawinsider.com/dictionary/municipal-sewage-sludge (accessed on 18 May 2022).
- Zuloaga, O.; Navarro, P.; Bizkarguenaga, E.; Iparraguirre, A.; Vallejo, A.; Olivares, M.; Prieto, A. Overview of extraction, clean-up and detection techniques for the determination of organic pollutants in sewage sludge: A review. Anal. Chim. Acta 2012, 736, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Law Insider. Municipal Sewer System Definition. Available online: https://www.lawinsider.com/dictionary/municipal-sewer-system (accessed on 18 May 2022).
- Van Vuuren, S.J.; Van Dijk, M. Waterborne Sanitation Operation and Maintenance Guide; South African Water Research Commission: Gauteng, South Africa, 2011. [Google Scholar]
- Ehalt MacEdo, H.; Lehner, B.; Nicell, J.; Grill, G.; Li, J.; Limtong, A.; Shakya, R. Distribution and characteristics of wastewater treatment plants within the global river network. Earth Syst. Sci. Data 2022, 14, 559–577. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Hu, J.; Lee, D.J.; Chang, Y.; Lee, Y.J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef]
- EurEau. Wastewater Treatment—Sludge Management; EurEau: Hong Kong, China, 2021. [Google Scholar]
- EurEau. Europe’s Water in Figures—An Overview of the European Drinking Water and Waste Water Sectors; EurEau: Hong Kong, China, 2017. [Google Scholar]
- Ritchie, H.; Roser, M. Urbanization. Available online: https://ourworldindata.org/urbanization (accessed on 20 May 2022).
- Rashid, S.S.; Liu, Y.Q. Assessing environmental impacts of large centralized wastewater treatment plants with combined or separate sewer systems in dry/wet seasons by using LCA. Environ. Sci. Pollut. Res. 2020, 27, 15674–15690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burm, R.J.; Krawczyk, D.F.; Harlow, G.L. Chemical and physical comparison of combined and separate sewer discharges. J. Water Pollut. Control Fed. 1968, 40, 112–126. [Google Scholar]
- Burm, R.J.; Vaughan, R.D. Bacteriological comparison between combined and separate sewer discharges in southeastern Michigan. J. Water Pollut. Control Fed. 1966, 38, 400–409. [Google Scholar]
- De Toffol, S.; Engelhard, C.; Rauch, W. Combined sewer system versus separate system—A comparison of ecological and economical performance indicators. Water Sci. Technol. 2007, 55, 255–264. [Google Scholar] [CrossRef]
- Mannina, G.; Viviani, G. Separate and combined sewer systems: A long-term modelling approach. Water Sci. Technol. 2009, 60, 555–565. [Google Scholar] [CrossRef]
- Goffi, A.S.; Trojan, F.; de Lima, J.D.; Lizot, M.; Thesari, S.S. Economic feasibility for selecting wastewater treatment systems. Water Sci. Technol. 2018, 78, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Bachmann-Machnik, A.; Bruning, Y.; Bakhshipour, A.E.; Krauss, M.; Dittmer, U. Evaluation of combined sewer system operation strategies based on highly resolved online data. Water 2021, 13, 751. [Google Scholar] [CrossRef]
- Dittmer, U.; Bachmann-Machnik, A.; Launay, M.A. Impact of combined sewer systems on the quality of urban streams: Frequency and duration of elevated micropollutant concentrations. Water 2020, 12, 850. [Google Scholar] [CrossRef] [Green Version]
- Peters, P.E.; Zitomer, D.H. Current and future approaches to wet weather flow management: A review. Water Environ. Res. 2021, 93, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- EU Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31991L0271:EN:HTML (accessed on 22 May 2022).
- EU Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0098 (accessed on 7 June 2022).
- EU Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control) Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010L0075 (accessed on 7 June 2022).
- EU Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31986L0278 (accessed on 7 June 2022).
- EU Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000L0060 (accessed on 7 June 2022).
- EU Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:31991L0676 (accessed on 7 June 2022).
- Regulation (EC) No 401/2009 of the European Parliament and of the Council of 23 April 2009 on the European Environment Agency and the European Environment Information and Observation Network, Consolidated Text. Available online: https://eur-lex.europa.eu/eli/reg/2009/401/2021-07-29 (accessed on 20 May 2022).
- Commission Decision of 16 January 2001 Amending Decision 2000/532/EC as Regards the List of Wastes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32001D0118 (accessed on 22 May 2022).
- Havukainen, J.; Saud, A.; Astrup, T.F.; Peltola, P.; Horttanainen, M. Environmental performance of dewatered sewage sludge digestate utilization based on life cycle assessment. Waste Manag. 2022, 137, 210–221. [Google Scholar] [CrossRef]
- The European Critical Raw Materials Review. Available online: https://mycourses.aalto.fi/pluginfile.php/1176261/mod_folder/content/0/Theeuropeancriticalrawmaterialsreview_2014.pdf (accessed on 20 May 2022).
- Burian, S.J.; Nix, S.J.; Pitt, R.E.; Rocky Durrans, S. Urban Wastewater Management in the United States: Past, Present, and Future. J. Urban Technol. 2000, 7, 33–62. [Google Scholar] [CrossRef]
- Center for Sustainable Systems—University of Michigan US Wastewater Treatment Factsheet. Available online: https://css.umich.edu/sites/default/files/WastewaterTreatment_CSS04-14_e2021.pdf (accessed on 17 May 2022).
- American Society of Civil Engineers (ASCE) Infrastructure Report Card: Wastewater D+. Available online: https://infrastructurereportcard.org/cat-item/wastewater/ (accessed on 11 June 2022).
- Hashimoto, K. Institutional Mechanisms for Sustainable Sanitation: Lessons from Japan for Other Asian Countries; ADB Institute: Tokyo, Japan, 2019. [Google Scholar]
- Asian Development Bank. Sanitation and Sustainable Development in Japan; Asian Development Bank: Tokyo, Japan, 2016. [Google Scholar]
- GB Department for Environment Food & Rural Affair. National Policy Statement for Waste Water: A Framework Document for Planning Decisions on Nationally Significant Waste Water Infrastructure; Stationery Office: London, UK, 2010; ISBN 9780108511486. [Google Scholar]
- SACOSS. Towards Equitable Access to Clean Water and Sanitation for All South Australians; SACOSS: Unley, Australia, 2020. [Google Scholar]
- Christodoulou, A.; Stamatelatou, K. Overview of legislation on sewage sludge management in developed countries worldwide. Water Sci. Technol. 2016, 73, 453–462. [Google Scholar] [CrossRef] [PubMed]
- United Nations OECD Glossary of Environment Statistics. Available online: https://stats.oecd.org/Index.aspx (accessed on 20 May 2022).
- United Nations Educational Scientific and Cultural Organization. The United Nations World Water Development Report 2017; United Nations Educational Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Wei, L.; Zhu, F.; Li, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, S. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef] [PubMed]
- US Department of the Commerce—International Trade Administration (IN) China-Country Commercial Guide, Environmental Technology. Available online: https://www.trade.gov/country-commercial-guides/china-environmental-technology (accessed on 12 June 2022).
- Chen, H.; Yan, S.; Ye, Z.; Meng, H.; Zhu, Y. Utilization of urban sewage sludge: Chinese perspectives. Environ. Sci. Pollut. Res. 2012, 19, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Jin, P.; Chen, D.; Liu, X.; Jin, H.; Wang, R.; Liu, Y. Resource reclamation of municipal sewage sludge based on local conditions: A case study in Xi’an, China. J. Clean. Prod. 2021, 316, 128189. [Google Scholar] [CrossRef]
- Zhou, G.; Gu, Y.; Yuan, H.; Gong, Y.; Wu, Y. Selecting sustainable technologies for disposal of municipal sewage sludge using a multi-criterion decision-making method: A case study from China. Resour. Conserv. Recycl. 2020, 161, 104881. [Google Scholar] [CrossRef]
- Chen, J.; Meng, X.Z.; Bergman, A.; Halden, R.U. Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard. Mater. 2019, 365, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, Z.; Liang, J.; Kuo, D.T.F.; Chen, S.; Hu, X.; Deng, M.; Zhang, H.; Lu, Y.H. Assessing pollution and risk of polycyclic aromatic hydrocarbons in sewage sludge from wastewater treatment plants in China’s top coal-producing region. Environ. Monit. Assess. 2019, 191, 102. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Cui, Y.; Zhang, W.; Zhuma, L.; Zhang, Z. Analysis on the Trend of Sewage Treatment Technology Patent in Dalian, China. In Proceedings of the 2020 5th International Conference on Advances in Energy and Environment Research (ICAEER 2020), Shanghai, China, 18–20 September 2020; EDP Sciences: Les Ulis, France, 2020; Volume 194. [Google Scholar]
- Huang, D.; Liu, X.; Jiang, S.; Wang, H.; Wang, J.; Zhang, Y. Current state and future perspectives of sewer networks in urban China. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Fu, B.; Wu, X.; Wang, Z.; Wu, X.; Wang, S. Coupling human and natural systems for sustainability: Experience from China’s Loess Plateau. Earth Syst. Dynam. 2022, 13, 795–808. [Google Scholar] [CrossRef]
- Shang, K. Mechanical Characteristics and Micro-Mechanism of Modified Dredged Sludge Based on Calcium-Containing Solid Waste Used as Landfill Cover Materials. Processes 2022, 10, 451. [Google Scholar] [CrossRef]
- Feng, L.; Luo, J.; Chen, Y. Dilemma of sewage sludge treatment and disposal in china. Environ. Sci. Technol. 2015, 49, 4781–4782. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Dai, X.; Hu, H.; Huang, X.; Chen, Z.; Li, T.; Yeshi, C.; Daigger, G. Emerging Trends and Prospects for Municipal Wastewater Management in China. ACS EST Eng. 2022, 2, 323–336. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, C.; Nakamura, S. Tracing the Consumption Origins of Wastewater and Sludge for a Chinese City Based on Waste Input-Output Analysis. Environ. Sci. Technol. 2020, 54, 12560–12567. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Z.; Venkatesan, A.K.; Ni, Y.L.; Steele, J.C.; Wu, L.L.; Bignert, A.; Bergman, Å.; Halden, R.U. Organic Contaminants in Chinese Sewage Sludge: A Meta-Analysis of the Literature of the Past 30 Years. Environ. Sci. Technol. 2016, 50, 5454–5466. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Lin, Y.; Hu, F.; Liu, R.; Ruan, T.; Jiang, G. Observation of Emerging Photoinitiator Additives in Household Environment and Sewage Sludge in China. Environ. Sci. Technol. 2016, 50, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, R.S.; Parmar, D. Wastewater Management Framework in India: Policy Challenges and Solutions for a Sustainable Future—India Water Portal. Available online: https://www.indiawaterportal.org/articles/wastewater-management-framework-india-policy-challenges-and-solutions-sustainable-future (accessed on 22 May 2022).
- Central Pollution Control Board—Ministry of Environment Forest and Climate Change Government of India National Inventory of Sewage Treatment Plants. 2021. Available online: https://www.cpcb.nic.in/status-of-stps/ (accessed on 21 May 2022).
- Durdević, D.; Trstenjak, M.; Hulenić, I. Sewage sludge thermal treatment technology selection by utilizing the analytical hierarchy process. Water 2020, 12, 1255. [Google Scholar] [CrossRef]
- The Economic Times Centre Looking to Monetise Treated Sewage, Dirty Water Removed from Ganga. Available online: https://economictimes.indiatimes.com/news/india/centre-looking-to-monetise-treated-sewage-dirty-water-removed-fromgan-ga/articleshow/90317707.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppstreported (accessed on 23 May 2022).
- India Science Wire NEERI Develops Eco-Friendly Phytorid Technology Sewage Treatment Plant. Available online: https://vigyanprasar.gov.in/isw/NEERI-develops-eco-friendly-Phytorid-Technology-Sewage-Treatment-Plant.html (accessed on 22 May 2022).
- U.S. Department of Commerce Russia—Country Commercial Guide, Water & Wastewater, International Trade Administration. 2021. Available online: https://www.trade.gov/country-commercial-guides/russia-water-wastewater (accessed on 22 May 2022).
- FanackWATER Wastewater Treatment and Reuse in MENA Countries. Water User in Most MENA… More. Available online: https://water.fanack.com/publications/wastewater-treatment-reuse-mena-countries/#:~:text= (accessed on 25 May 2022).
- AFRO Barometer AD503: Water and Sanitation: On-the-Ground Realities Challenge African Governments to Act. Available online: https://www.afrobarometer.org/publication/ad503-water-and-sanitation-ground-realities-challenge-african-governments-act/ (accessed on 25 May 2022).
- Martin-Hurtado, R.; Nolasco, D. Managing Wastewater as a Resource in Latin America and the Caribbean (LAC) towards a Circular Economy Approach. Available online: https://programme.worldwaterweek.org/Content/ProposalResources/allfile/managing_wastewater_as_a_resource_in_lac.pdf (accessed on 25 April 2022).
- Bertomeu Sanchez, S.; Serebrisky, T. Water and Sanitation in Latin America and the Caribbean: An Update on the State of the Sector. SSRN Electron. J. 2018, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.G.; Machado, F.; Datshkovsky, D. Water and Sanitation Services in Latin America: Access and Quality Outlook; Inter-American Development Bank: Washington, DC, USA, 2021. [Google Scholar]
- Wichelns, D.; Drechsel, P.; Qadir, M. Wastewater: Economic asset in an urbanizing world. In Wastewater: Economic Asset in an Urbanizing World; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Shah, M.; Rodriguez-Couto, S. Development in Wastewater Treatment Research and Processes—Microbial Degradation of Xenobiotics through Bacterial and Fungal Approach, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Pikaar, I.; Guest, J.; Ganigué, R.; Jensen, P.; Rabaey, K.; Seviour, T.; Trimmer, J.; Van Der Kolk, O.; Vaneeckhaute, C.; Verstraete, W. Resource Recovery from Water: Principles and Application; IWA Publishing: London, UK, 2022. [Google Scholar]
- Kehrein, P.; Van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants-market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef] [Green Version]
- Duque, A.F.; Campo, R.; Del Rio, A.V.; Amorim, C.L. Wastewater valorization: Practice around the world at pilot-and full-scale. Int. J. Environ. Res. Public Health 2021, 18, 9466. [Google Scholar] [CrossRef] [PubMed]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture—The effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B. New Technologies for Water and Wastewater Treatment: A Survey of Recent Patents. Recent Patents Chem. Eng. 2012, 1, 17–26. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. The potential of raw sewage sludge in construction industry—A review. J. Clean. Prod. 2018, 200, 342–356. [Google Scholar] [CrossRef]
- Di Costanzo, N.; Cesaro, A.; Di Capua, F.; Esposito, G. Exploiting the nutrient potential of anaerobically digested sewage sludge: A review. Energies 2021, 14, 8149. [Google Scholar] [CrossRef]
- Saravanan, V.S.; Mollinga, P.P.; Bogardi, J.J. Global change, wastewater and health in fast growing economies. Curr. Opin. Environ. Sustain. 2011, 3, 461–466. [Google Scholar] [CrossRef]
- Tassinari, G.; Boccaletti, S.; Soregaroli, C. The Socio-Economic Impacts of Wastewater Sludge Valorization: The Case of Biofertilizers in Italy. 2021. Available online: biomonitor.eu (accessed on 25 May 2022).
- Vu, M.T.; Nguyen, L.N.; Zdarta, J.; Mohammed, J.A.H.; Pathak, N.; Nghiem, L.D. Wastewater to R3—Resource recovery, recycling, and reuse efficiency in urban wastewater treatment plants. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Bohra, V.; Ahamad, K.U.; Kela, A.; Vaghela, G.; Sharma, A.; Deka, B.J. Energy and resources recovery from wastewater treatment systems. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Carvalheira, M.; Marreiros, B.C.; Reis, M.A. Acids (VFAs) and bioplastic (PHA) recovery. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 245–254. [Google Scholar]
- Libhaber, M.; Orozco-Jaramillo, Á. Sustainable Treatment and Reuse of Municipal Wastewater: For Decision Makers and Practising Engineers; IWA Publishing: London, UK, 2012; ISBN 9781780400167. [Google Scholar]
- Hernández-Flores, G.; Solorza-Feria, O.; Poggi-Varaldo, H.M. Bioelectricity generation from wastewater and actual landfill leachates: A multivariate analysis using principal component analysis. Int. J. Hydrogen Energy 2017, 42, 20772–20782. [Google Scholar] [CrossRef]
- Water Resources Division Municipal Wastewater Treatment: A Review of Treatment Technologies. Available online: https://www.gov.nl.ca/ecc/files/waterres-training-aww-04-domestic-wastewater-presentation-ver4.pdf (accessed on 25 May 2022).
- Jones, E.R.; Van Vliet, M.T.H.; Qadir, M.; Bierkens, M.F.P. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 2021, 13, 237–254. [Google Scholar] [CrossRef]
- Water Environment Federation. Operation of Municipal Wastewater Treatment Plants; Water Environment Federation: Alexandria, VA, USA, 2008; ISBN 0071597417. [Google Scholar]
- Collado, R.; Díez, R. Wastewater treatment facilities for isolated buildings. In Proceedings of the 37th IAHS World Congress on Housing Science, Santander, Spain, 26–29 October 2010; pp. 1–8. [Google Scholar]
- Centers for Disease Control and Prevention and U.S. Department of Housing and Urban Development. Healthy Housing Reference Manual; U.S. Department of Housing and Urban Development: Washington, DC, USA, 2006. [Google Scholar]
- TrenchLess Sewer Network. Available online: https://www.trenchlesspedia.com/definition/3006/sewer-network (accessed on 24 May 2022).
- Ahmedi, F.; Kusari, L. Possibilities of Converting the Existing Wastewater Collection System to a Separate or a Combined System. In Proceedings of the Eleventh International Conference on Urban Drainage–Edinburgh International Conference Centre, Edinburgh, UK, 31 August–5 September 2008; pp. 1–6. [Google Scholar]
- Office of Water-Sacramento California State University Impacts of Sanitary Sewer Overflows and Combined Sewer Overflows on Human Health and on the Environment: A Literature Review Sacramento Area Sewer District. Available online: https://www.owp.csus.edu/research/wastewater/papers/SSO-Lit-Review.pdf (accessed on 26 June 2022).
- Ong, S.K. Wastewater Engineering; Keystone Environmental Ltd.: Burnaby, BC, Canada, 2007; ISBN 9780471739043. [Google Scholar]
- Guyer, J.P.; Asce, F.; Aei, F. Introduction to Preliminary Wastewater Treatment. 2018. Available online: https://books.google.co.jp/books/about/An_Introduction_to_Preliminary_Wastewate.html?id=7UFJDwAAQBAJ&printsec=frontcover&source=kp_read_button&hl=en&redir_esc=y#v=onepage&q&f=false (accessed on 25 June 2022).
- Duncan, M. Domestic Wastewater Treatment in Developing Countries; Earthscan: London, UK, 2003; ISBN 1-84407-019-0. [Google Scholar]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal; IWA Publishing: London, UK, 2007; ISBN 1 84339 161 9. [Google Scholar]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Tayal, A. Primary Treatment of Wastewater. Available online: https://livelywatt.com/primary-treatment-of-wastewater/ (accessed on 22 May 2022).
- Orhon, D.; Artan, N. Modeling of Activated Sludge Systems; Technomic: Lancaster, PA, USA, 1997; ISBN 1-56676-101-8. [Google Scholar]
- Orhon, D. Evolution of the activated sludge process: The first 50 years. J. Chem. Technol. Biotechnol. 2015, 90, 608–640. [Google Scholar] [CrossRef]
- Wang, L.K.; Wu, Z.; Shammas, N.K. Activated Sludge Processes. In Biological Treatment Processes. Handbook of Environmental Engineering; Humana Press: Totowa, NJ, USA, 2009; Volume 8, p. 75. [Google Scholar]
- Ouyang, J.; Li, C.; Wei, L.; Wei, D.; Zhao, M.; Zhao, Z.; Zhang, J.; Chang, C.C. Activated sludge and other aerobic suspended culture processes. Water Environ. Res. 2020, 92, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, Y.; Dai, X.; Chen, H. Characterization of organic fractions in wastewater for high-rate activated sludge process. Chinese J. Environ. Eng. 2020, 14, 1471–1480. [Google Scholar] [CrossRef]
- McCarty, P.L.; Brodersen, C.F. Theory of extended aeration activated sludge. Water Pollut. Control Fed. 1962, 34, 1095–1103. [Google Scholar]
- Middlebrooks, E.J.; Garland, C.F. Kinetics of model and field extended-aeration wastewater treatment units. Water Pollut. Control Fed. 1968, 40, 586–612. [Google Scholar]
- Middlebrooks, E.J.; Jenkins, D.; Neal, R.C.; Phillips, J.L. Kinetics and effluent quality in extended aeration. Water Res. 1969, 3, 39–46. [Google Scholar] [CrossRef]
- Kraus, L.S. The use of digested sludge and digester overflow to control bulking activated sludge. Sewage Work. J. 1945, 17, 1177–1190. [Google Scholar]
- Kraus, L.S. Digested sludge—An aid to the activated sludge process. Sewage Work. J. 1946, 18, 1099–1112. [Google Scholar] [PubMed]
- McKinney, R.E. Research and current development in the activated sludge process. J. Water Pollut. Control Fed. 1965, 37, 1696–1704. [Google Scholar]
- Ullrich, A.H.; Smith, M.W. Operating experience with activated sludge—Biosorption at Austin. Sewage Ind. Waste. 1957, 29, 400–413. [Google Scholar]
- Ullrich, A.H.; Smith, M.W. The biosorption process of sewage and waste treatment. Sewage Ind. Waste. 1951, 23, 1248–1253. [Google Scholar]
- Grich, E. Operating experience with activated sludge reaeration. Water Pollut. Control Fed. 1961, 33, 856–863. [Google Scholar]
- Jones, E.L.; Alspaugh, T.A.; Stokes, H.B. Aerobic treatment of textile mill waste. Water Pollut. Control Fed. 1962, 34, 495–512. [Google Scholar]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.D.; Li, J.; Van Loosdrecht, M.C.M.; Li, T.Y. A sustainability-based evaluation of membrane bioreactors over conventional activated sludge processes. J. Environ. Chem. Eng. 2018, 6, 2597–2605. [Google Scholar] [CrossRef]
- Arabi, S.; Pellegrin, M.L.; Aguinaldo, J.; Sadler, M.E.; McCandless, R.; Sadreddini, S.; Wong, J.; Burbano, M.S.; Koduri, S.; Abella, K.; et al. Membrane processes. Water Environ. Res. 2020, 92, 1447–1498. [Google Scholar] [CrossRef] [PubMed]
- Mace, S.; Mata-Alvarez, J. Utilization of SBR technology for wastewater treatment: An overview. Ind. Eng. Chem. Res. 2002, 41, 5539–5553. [Google Scholar] [CrossRef]
- Gupta, R.K.; Poddar, B.J.; Nakhate, S.P.; Chavan, A.R.; Singh, A.K.; Purohit, H.J.; Khardenavis, A.A. Role of heterotrophic nitrifiers and aerobic denitrifiers in simultaneous nitrification and denitrification process: A nonconventional nitrogen removal pathway in wastewater treatment. Lett. Appl. Microbiol. 2022, 74, 159–184. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, R.K. Sequencing batch reactor technology for biological wastewater treatment: A review. Asia-Pacific J. Chem. Eng. 2011, 6, 3–13. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A review on anaerobic-aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- d.lgs. n. 152/2006 (T.U. Ambiente)—Allegato 3: Rilevamento delle Caratteristiche dei Bacini Idrografici e Analisi Dell’impatto Esercitato Dall’Attività Antropica; Italy, 2006. Available online: https://www.bosettiegatti.eu/info/norme/statali/2006_0152.htm (accessed on 25 May 2022).
- Zykova, I.; Maksimuk, N.; Rebezov, M.; Kuznetsova, E.; Derkho, M.; Sereda, T.; Kazhibayeva, G.; Somova, Y.; Zaitseva, T. Interaction between heavy metals and microorganisms during wastewater treatment by activated sludge. ARPN J. Eng. Appl. Sci. 2019, 14, 2139–2145. [Google Scholar]
- Wastewater Treatment across the World: Which Countries Recycle the Most and Where Is the Best Water Quality? Available online: https://www.hydrotech-group.com/blog/wastewater-treatment-across-the-world-which-countries-recycle-the-most-and-where-is-the-best-water-quality#:~:text=Intermsofglobalwastewatertreatment%2Citis,So%2Cthepercentageishigherthanpreviouslyexpecte (accessed on 30 March 2022).
- European Environment Agency Urban Waste Water Treatment in Europe. Available online: https://www.eea.europa.eu/data-and-maps/indicators/urban-waste-water-treatment/urban-waste-water-treatment-assessment-5 (accessed on 30 March 2022).
- Eurostat—Statistic Explained Glossary: European Environment Agency (EEA). Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:European_Environment_Agency_(EEA)#:~:text=Glossary%3AEuropeanEnvironmentAgency%28EEA%29SeeEEAdisambiguationpage,EUanditsmembercountriesinmaking (accessed on 21 May 2022).
- S.I. No. 419/1994—Environment Protection Agency Act, 1992 (Urban Waste Water Treatment) Regulations. 1994. Available online: https://www.irishstatutebook.ie/eli/1994/si/419/made/en/print (accessed on 18 May 2022).
- Nsavyimana, G.; Kaboneka, S.; Bigumandondera, P.; Ngahane, E.L.; Ndikumana, T.; Vasel, J.L. Exploring a New Approach of the Population Equivalent Concept through a Detailed Characterization of Grey and Black Waters. Int. J. Adv. Sci. Res. Eng. 2020, 6, 32–49. [Google Scholar] [CrossRef]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge production from municipal wastewater treatment in sewage treatment plant. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Karlikanovaite-Balikci, A.; Ozbayram, E.G.; Yagci, N.; Ince, O. Microbial community shifts in the oxic-settling-anoxic process in response to changes to sludge interchange ratio. Heliyon 2019, 5, e1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, K.; Gadhamshetty, V.; Tysklind, M.; Bhattacharyya, D.; Upadhyayula, V.K.K. A sustainable performance assessment framework for circular management of municipal wastewater treatment plants. J. Clean. Prod. 2022, 339, 130657. [Google Scholar] [CrossRef]
- Medaoud, S.; Mokrani, L.; Mezhoud, S.; Ziane, S. Characterization of Stabilised Sewage Sludge for Reuse in Road Pavement. Civ. Environ. Eng. Reports 2022, 32, 201–217. [Google Scholar] [CrossRef]
- Eurostat Sewage Sludge Production and Disposal from Urban Wastewater. Available online: https://ec.europa.eu/eurostat/databrowser/view/ten00030/default/table?lang=en (accessed on 21 June 2022).
- EPA Basic Information about Biosolids. Available online: https://www.epa.gov/biosolids/basic-information-about-biosolids (accessed on 17 June 2022).
- Kovalev, A.A.; Mikheeva, E.R.; Kovalev, D.A.; Katraeva, I.V.; Zueva, S.; Innocenzi, V.; Panchenko, V.; Zhuravleva, E.A.; Litti, Y.V. Feasibility Study of Anaerobic Codigestion of Municipal Organic Waste in Moderately Pressurized Digesters: A Case for the Russian Federation. Appl. Sci. 2022, 12, 2933. [Google Scholar] [CrossRef]
- Kalyuzhnyi, S.V. Wastewater Sludge Management in the Russian Federation: The Current Status and Perspectives. Water Pract. Technol. 2007, 2, wpt2007085. [Google Scholar] [CrossRef]
- Apollo, S. A review of sludge production in South Africa municipal wastewater treatment plants, analysis of handling cost and potential minimization methods. Phys. Sci. Rev. 2022. [Google Scholar] [CrossRef]
- Evans, T.D. SEWAGE SLUDGE—Operational and Environmental Issue. Available online: http://www.fwr.org/sludge.pdf (accessed on 17 June 2022).
- Danso-Boateng, E. Sewage Sludge: Assessment, Treatment and Environmental Impact; Nova Science: Leicestershire, UK, 2017; ISBN 978-1-53611-072-2. [Google Scholar]
- Mwanza, B.G.; Mbohwa, C.; Telukdarie, A. Municipal solid waste management in Kitwe City: An engineering management perspective. Manag. Environ. Qual. An Int. J. 2018, 29, 1075–1092. [Google Scholar] [CrossRef]
- Jenkins, D.; Wanner, J. (Eds.) Activated Sludge—100 Years and Counting; IWA Publishing: London, UK, 2014; p. 13. [Google Scholar] [CrossRef]
- Burms, H.; Gremminger, L. Method for Lime Stabilization of Wastewater Treatment Plant Sludges. U.S. Patent 5277826, 11 January 1994. [Google Scholar]
- Mackenzie, D. Water and Wastewater Engineering: Design Principles and Practice, Second Edition. J. Franklin Inst. 2020, 284, 1344. [Google Scholar]
- Papastergiadis, E.; Sklari, S.; Zouboulis, A.; Chasiotis, A.; Samaras, P. The use of steelmaking slag for sewage sludge stabilization. Desalin. Water Treat. 2015, 55, 1697–1702. [Google Scholar] [CrossRef]
- Samaras, P.; Papadimitriou, C.A.; Haritou, I.; Zouboulis, A.I. Investigation of sewage sludge stabilization potential by the addition of fly ash and lime. J. Hazard. Mater. 2008, 154, 1052–1059. [Google Scholar] [CrossRef]
- Kosowski, P.; Szostek, M.; Pieniazek, R.; Antos, P.; Skrobacz, K.; Piechowiak, T.; Zaczek, A.; Józefczyk, R.; Balawejder, M. New approach for sewage sludge stabilization with ozone. Sustainability 2020, 12, 886. [Google Scholar] [CrossRef] [Green Version]
- Ki, Y.P.; Ahn, K.H.; Sung, K.M.; Jong, H.H.; Jae, H.K. Feasibility of sludge ozonation for stabilization and conditioning. Ozone Sci. Eng. 2003, 25, 73–80. [Google Scholar] [CrossRef]
- Ki, D.; Kupferer, R.; Torres, C.I. High-rate stabilization of primary sludge in a single-chamber microbial hydrogen peroxide producing cell. Environ. Sci. Water Res. Technol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef]
- Semblante, G.U.; Hai, F.I.; Dionysiou, D.D.; Fukushi, K.; Price, W.E.; Nghiem, L.D. Holistic sludge management through ozonation: A critical review. J. Environ. Manag. 2017, 185, 79–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuncay, S.; Akcakaya, M.; Icgen, B. Ozonation of sewage sludge prior to anaerobic digestion led to Methanosaeta dominated biomethanation. Fuel 2022, 313, 122690. [Google Scholar] [CrossRef]
- Badalians Gholikandi, G.; Zakizadeh, N.; Masihi, H. Application of peroxymonosulfate-ozone advanced oxidation process for simultaneous waste-activated sludge stabilization and dewatering purposes: A comparative study. J. Environ. Manag. 2018, 206, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Wu, W.; Li, G.; Wang, Y.; Li, G.; Dong, Y.; Yuan, H.; Zhu, N. Application of CaO2-enhanced peroxone process to adjust waste activated sludge characteristics for dewaterability amelioration: Molecular transformation of dissolved organic matters and realized mechanism of deep-dewatering. Chem. Eng. J. 2022, 437, 135306. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Zhang, D.; Fan, X.; Guo, Y.; Li, E.; Zheng, H. Insight to peroxone-Fe(III) joint conditioning-horizontal electro-dewatering process on water reduction in activated sludge: Performance and mechanisms. J. Hazard. Mater. 2021, 402, 123441. [Google Scholar] [CrossRef]
- Geng, N.; Wang, Y.; Zhang, D.; Fan, X.; Li, E.; Han, Z.; Zhao, X. An electro-peroxone oxidation-Fe(III) coagulation sequential conditioning process for the enhanced waste activated sludge dewatering: Bound water release and organics multivariate change. Sci. Total Environ. 2022, 883, 155272. [Google Scholar] [CrossRef]
- Matsch, L.C.; Drnevich, R.F. Autothermal aerobic digestion. J. Water Pollut. Control Fed. 1977, 49, 296–310. [Google Scholar]
- Bernard, S.; Gray, N.F. Aerobic digestion of pharmaceutical and domestic wastewater sludges at ambient temperature. Water Res. 2000, 34, 725–734. [Google Scholar] [CrossRef]
- Kozak, J.; Wlodarczyk-Makula, M.; Popenda, A. Impact of Aerobic Stabilization of Sewage Sludge on PAHs Concentration in Reject Waters. J. Ecol. Eng. 2021, 22, 27–35. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Pakou, C.; Lyberatos, G. Occurrence, toxicity, and biodegradation of selected emerging priority pollutants in municipal sewage sludge. In Comprehensive Biotechnology; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Jamal, A.; Norieh, N.; Farzadkia, M. Comparison of Aerobic and Lime Stabilization Methods for Evaluation of Sewage Sludge Reuse. J. Environ. Sci. Technol. 2011, 4, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. Microbiology and biochemistry of anaerobic digesters: An overview. In Bioreactors: Sustainable Design and Industrial Applications in Mitigation of GHG Emissions; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Jenicek, P.; Bartacek, J.; Kutil, J.; Zabranska, J.; Dohanyos, M. Potentials and limits of anaerobic digestion of sewage sludge: Energy self-sufficient municipal wastewater treatment plant? Water Sci. Technol. 2012, 66, 1277–1281. [Google Scholar] [CrossRef]
- Steinberg, L.M.; Regan, J.M. Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef] [Green Version]
- Hanum, F.; Yuan, L.C.; Kamahara, H.; Aziz, H.A.; Atsuta, Y.; Yamada, T.; Daimon, H. Treatment of sewage sludge using anaerobic digestion in Malaysia: Current state and challenges. Front. Energy Res. 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Arthurson, V. Proper sanitization of sewage sludge: A critical issue for a sustainable society. Appl. Environ. Microbiol. 2008, 74, 5267–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmborg, J.; Magnér, J. Pharmaceutical residues in sewage sludge: Effect of sanitization and anaerobic digestion. J. Environ. Manag. 2015, 153, 1–10. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Energy and Resource Recovery From Sludge: Full-Scale Experiences. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Méndez-Contreras, J.M.; Rendón-Sagardi, J.A.; Ruiz-Espinoza, J.E.; Alvarado-Lassman, A.; Martínez-Delgadillo, S.A. Behavior of the mesophilic and thermophilic anaerobic digestion in the stabilization of municipal wastewater sludge (part 1). Rev. Mex. Ing. Quim. 2009, 8, 283–290. [Google Scholar]
- Parkin, G.F.; Owen, W.F. Fundamentals of Anaerobic Digestion of Wastewater Sludges. J. Environ. Eng. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Dilek Erdirencelebi; Cansu Bayhan Feasibility and potential of separate anaerobic digestion of municipal sewage sludge fractions. Water SA 2020, 46, 123–130. [CrossRef] [Green Version]
- Water Environment Federation. Design of Municipal Wastewater Treatment Plants; Water Environment Federation: Alexandria, VA, USA, 2009; Volume 30. [Google Scholar]
- Tony Pembroke, J.; Ryan, M.P. Autothermal thermophilic aerobic digestion (ATAD) for heat, gas, and production of a class a biosolids with fertilizer potential. Microorganisms 2019, 7, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartkowska, I.; Biedka, P.; Tałałaj, I.A. Production of biosolids by autothermal thermophilic aerobic digestion (ATAD) from a municipal sewage sludge: The polish case study. Energies 2020, 13, 6258. [Google Scholar] [CrossRef]
- Bolzonella, D.; Innocenti, L.; Cecchi, F. Biological nutrient removal wastewater treatments and sewage sludge anaerobic mesophilic digestion performances. Water Sci. Technol. 2002, 46, 199–208. [Google Scholar] [CrossRef]
- Judd, S. Watermaths: Process Fundamentals for the Design and Operation of Water and Wastewater Treatment Technologies; IWA Publishing: London, UK, 2019. [Google Scholar]
- Introduction to Sludge Dewatering—Overview of Sludge Thickening and Dewatering, Sludge Processing. Available online: https://www.sludgeprocessing.com/sludge-dewatering/introduction-to-sludge-dewatering/ (accessed on 17 June 2022).
- Andreoli, C.V.; Von Sperling, M.; Fernandes, F. Sludge Treatment and Disposal; Encyclopedia Britannica, Inc.: Chicago, IL, USA, 1982; Volume 25, ISBN 9781843391661. [Google Scholar]
- Zikakis, D.; Chauzy, J.; Droubogianni, I.; Georgakopoulos, A. Thermal hydrolysis of waste activated sludge only at the psyttalia wwtp in athens: Operation feedbacks. In Proceedings of the 22nd European Biosolids & Organic Resources Conference, Leeds, UK, 20–21 November 2017. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- Report Market Research Biosolids Market —Biosolids Market Growth to Spur with Rising Application of Biosolids in Agriculture during 2021–2031. Available online: https://www.factmr.com/report/biosolids-market (accessed on 22 May 2022).
- Yu, J.; Adingo, S.; Liu, X.; Li, X.; Sun, J.; Zhang, X. Micro plastics in soil ecosystem—A review of sources, fate, and ecological impact. Plant Soil Environ. 2022, 68, 1–17. [Google Scholar] [CrossRef]
- Mohajerani, A.; Karabatak, B. Microplastics and pollutants in biosolids have contaminated agricultural soils: An analytical study and a proposal to cease the use of biosolids in farmlands and utilise them in sustainable bricks. Waste Manag. 2020, 107, 252–265. [Google Scholar] [CrossRef]
- Hušek, M.; Moško, J.; Pohořelý, M. Sewage sludge treatment methods and P-recovery possibilities: Current state-of-the-art. J. Environ. Manag. 2022, 315, 115090. [Google Scholar] [CrossRef]
- Li, S.; Li, R.; Tang, Y.; Chen, G. Microwave-induced heavy metal removal from dewatered biosolids for cost-effective composting. J. Clean. Prod. 2019, 241, 118342. [Google Scholar] [CrossRef]
- Fu, Q.; Malchi, T.; Carter, L.J.; Li, H.; Gan, J.; Chefetz, B. Pharmaceutical and Personal Care Products: From Wastewater Treatment into Agro-Food Systems. Env. Sci. Technol. 2019, 53, 14083–14090. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Colón, J.; Alarcón, M.; Healy, M.G.; Namli, A.; Sanin, F.D.; Tayà, C.; Ponsà, S. Producing sludge for agricultural applications. In Innovative Wastewater Treatment & Resource Recovery Technologies: Impacts on Energy, Economy and Environment; IWA Publishing: London, UK, 2017. [Google Scholar]
- De Bertoldi-Schnappinger, U. Compost Science and Technology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 8. [Google Scholar]
- Treybal, R.E. Mass Transfer Operations, 3rd ed.; McGraw-Hill Book Company: New York, NY, USA, 1981; ISBN 0070651760. [Google Scholar]
- Lowe, P. Developments in the Thermal Drying of Sewage Sludge. Water Environ. J. 1995, 9, 306–316. [Google Scholar] [CrossRef]
- Gross, T.S.C. Thermal Drying of Sewage Sludge. Water Environ. J. 1993, 7, 255–261. [Google Scholar] [CrossRef]
- An-nori, A.; Ezzariai, A.; El Mejahed, K.; El Fels, L.; El Gharous, M.; Hafidi, M. Solar Drying as an Eco-Friendly Technology for Sewage Sludge Stabilization: Assessment of Micropollutant Behavior. Front. Environ. Sci. 2022. [Google Scholar] [CrossRef]
- Camelin, E.; Cristina, G.; Simelton, E.; Fino, D.; Tommasi, T. The Bioenergy-Fertilizer Nexus: A Challenge Achievable from Municipal Wastewater. In Innovations in Land, Water and Energy for Vietnam’s Sustainable Development; Springer: Cham, Switzerland, 2021; pp. 143–166. [Google Scholar]
- Masmoudi, A.; Ben Sik Ali, A.; Dhaouadi, H.; Mhiri, H. Comparison Between Two Solar Drying Techniques of Sewage Sludge: Draining Solar Drying and Drying Bed. Waste Biomass Valorization 2020, 12, 4089–4102. [Google Scholar] [CrossRef]
- Acharya, B.; Sule, I.; Dutta, A. A review on advances of torrefaction technologies for biomass processing. Biomass Convers. Biorefinery 2012, 2, 349–369. [Google Scholar] [CrossRef]
- Ribeiro Nunes, L.J.; De Oliveira Matias, J.C.; Da Silva Catalao, J.P. Torrefaction of Biomass for Energy Applications: From Fundamentals to Industrial Scale; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-809462-4. [Google Scholar]
- Nguyen, Q.; Nguyen, D.D.; Vothi, H.; He, C.; Goodarzi, M.; Bach, Q.V. Isothermal torrefaction kinetics for sewage sludge pretreatment. Fuel 2020, 277, 118103. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Huang, Z.; Yuan, X.; Tan, M.; Jiang, L.; Wu, Z.; Qin, X.; Li, H. Comparison of atmospheric pressure and gas-pressurized torrefaction of municipal sewage sludge: Properties of solid products. Energy Convers. Manag. 2020, 213, 112793. [Google Scholar] [CrossRef]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of sewage sludge: Kinetics and fuel properties of biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef] [Green Version]
- Tic, W.J.; Guziałowska-Tic, J.; Pawlak-Kruczek, H.; Wóznikowski, E.; Zadorozny, A.; Niedźwiecki, Ł.; Wnukowski, M.; Krochmalny, K.; Czerep, M.; Ostrycharczyk, M.; et al. Novel concept of an installation for sustainable thermal utilization of sewage sludge. Energies 2018, 11, 748. [Google Scholar] [CrossRef] [Green Version]
- Białowiec, A.; Pulka, J.; Styczyńska, M.; Koziel, J.A.; Kalka, J.; Jureczko, M.; Felis, E.; Manczarski, P. Is biochar from the torrefaction of sewage sludge hazardous waste? Materials 2020, 13, 3544. [Google Scholar] [CrossRef]
- Pulka, J.; Manczarski, P.; Stepien, P.; Styczyńska, M.; Koziel, J.A.; Białowiec, A. Waste-to-carbon: Is the torrefied sewage sludge with high ash content a better fuel or fertilizer? Materials 2020, 13, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, G.; Romano, P. Evolution of the Olive Oil Industry along the Entire Production Chain and Related Waste Management. Energies 2022, 15, 465. [Google Scholar] [CrossRef]
- Hu, B.; Yu, S.H.; Wang, K.; Liu, L.; Xu, X.W. Functional carbonaceous materials from hydrothermal carbonization of biomass: An effective chemical process. Dalt. Trans. 2008. [Google Scholar] [CrossRef] [PubMed]
- Gallifuoco, A.; Taglieri, L.; Scimia, F.; Papa, A.A.; Di Giacomo, G. Hydrothermal conversions of waste biomass: Assessment of kinetic models using liquid-phase electrical conductivity measurements. Waste Manag. 2018, 77, 586–592. [Google Scholar] [CrossRef]
- Vallejo, F.; Díaz-Robles, L.; Vega, R.; Cubillos, F.; Espinoza, A.P.; Pinilla, F.; Pino-Cortés, E. An experimental study for municipal organic waste and sludge treated by hydrothermal carbonization. Chem. Eng. Trans. 2020, 81, 355–360. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Wheatley, A.D.; Martin, S.J.; Shama, G. Hydrothermal carbonization of primary sewage sludge and synthetic faeces: Effect of reaction temperature and time on filterability. Environ. Prog. Sustain. Energy 2015, 34, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- TerraNova Energy Technology. Available online: https://www.terranova-energy.com/en/technology/ (accessed on 17 June 2022).
- Data Obtained from Gran Sasso Acqua SpA (GSA) Which Manages the Water Service of L’Aquila (IT) and, in Particular the WWTP of “Ponte Rosarolo”. Available online: http://www.gransassoacqua.it/ (accessed on 17 June 2022).
- Xu, Z.; Bai, X. Microplastic Degradation in Sewage Sludge by Hydrothermal Carbonization: Efficiency and Mechanisms. Chemosphere 2022, 297, 1342203. [Google Scholar] [CrossRef]
- Alipour, M.; Asadi, H.; Chen, C.; Besalatpour, A.A. Fate of organic pollutants in sewage sludge during thermal treatments: Elimination of PCBs, PAHs, and PPCPs. Fuel 2022, 319, 123864. [Google Scholar] [CrossRef]
- Ekanthalu, V.S.; Narra, S.; Ender, T.; Antwi, E.; Nelles, M. Influence of Post-and Pre-Acid Treatment during Hydrothermal Carbonization of Sewage Sludge on P-Transformation and the Characteristics of Hydrochar. Processes 2022, 10, 151. [Google Scholar] [CrossRef]
- Knötig, P.; Etzold, H.; Wirth, B. Article model-based evaluation of hydrothermal treatment for the energy efficient dewatering and drying of sewage sludge. Processes 2021, 9, 1346. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of hydrothermal carbonisation with anaerobic digestion; Opportunities for valorisation of digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef] [Green Version]
- Paneque, M.; Knicker, H.; Kern, J.; De la Rosa, J.M. Hydrothermal carbonization and pyrolysis of sewage sludge: Effects on Lolium perenne Germination and Growth. Agronomy 2019, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Paneque, M.; De la Rosa, J.M.; Kern, J.; Reza, M.T.; Knicker, H. Hydrothermal carbonization and pyrolysis of sewage sludges: What happen to carbon and nitrogen? J. Anal. Appl. Pyrolysis 2017, 128, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef]
- Yahav Spitzer, R.; Mau, V.; Gross, A. Using hydrothermal carbonization for sustainable treatment and reuse of human excreta. J. Clean. Prod. 2018, 205, 955–963. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- SLUDGE 4.0 Economia Circolare per il Trattamento e la Trasformazione dei Fanghi Biologici in Biofertilizzanti—Consorzio Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali Progetto Cofinanziato nel Quadro del POR FESR Toscana 2014–2020. Available online: https://www.instm.it/public/02/05/Sludge.pdf (accessed on 17 June 2022).
- Salimbeni, A. 6-Organic waste streams upgrading for gasification process optimization. In Substitute Natural Gas from Waste: Technical Assessment and Industrial Applications of Biochemical and Thermochemical Processes; Materazzi, M., Foscolo, P.U., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 73–103. ISBN 9780128155547. [Google Scholar]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Badrolnizam, R.S.; Elham, O.S.J.; Hadzifah, S.N.; Husain, M.H.N.; Hidayu, A.R.; Mohammad, N.F.; Mohamad Daud, A.R. Sewage sludge conversion via hydrothermal liquefaction (HTL)—A preliminary study. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1349, pp. 1–8. [Google Scholar]
- Kapusta, K. Effect of ultrasound pretreatment of municipal sewage sludge on characteristics of bio-oil from hydrothermal liquefaction process. Waste Manag. 2018, 78, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Jahromi, H.; Roy, P.; Adhikari, S.; Hassani, E.; Oh, T.S. Hydrothermal liquefaction of municipal sewage sludge: Effect of red mud catalyst in ethylene and inert ambiences. Energy Convers. Manag. 2021, 245, 114615. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Jaiswal, K.K.; Kumar, V.; Kumar, S.; Vlaskin, M.S.; Grigorenko, A.V.; Rindin, K.G. Recent advances and viability in sustainable thermochemical conversion of sludge to bio-fuel production. Fuel 2022, 316, 123351. [Google Scholar] [CrossRef]
- Acelas, N.Y.; López, D.P.; Wim Brilman, D.W.F.; Kersten, S.R.A.; Kootstra, A.M.J. Supercritical water gasification of sewage sludge: Gas production and phosphorus recovery. Bioresour. Technol. 2014, 174, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ruya, P.M.; Purwadi, R.; Lim, S.S. Supercritical water gasification of sewage sludge for power generation– thermodynamic study on auto-thermal operation using Aspen Plus. Energy Convers. Manag. 2020, 206, 112458. [Google Scholar] [CrossRef]
- Amrullah, A.; Matsumura, Y. Supercritical water gasification of sewage sludge in continuous reactor. Bioresour. Technol. 2018, 249, 276–283. [Google Scholar] [CrossRef]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2020, 391, 123499. [Google Scholar] [CrossRef]
- Fan, C.; Jin, H. Numerical simulation of gasification of a shrinking char particle in supercritical water. Fuel 2022, 318, 123692. [Google Scholar] [CrossRef]
- Gong, W.; Zhou, Z.; Liu, Y.; Wang, Q.; Guo, L. Catalytic Gasification of Sewage Sludge in Supercritical Water: Influence of K2CO3 and H2O2 on Hydrogen Production and Phosphorus Yield. ACS Omega 2020, 5, 3389–3396. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhu, W.; Chen, C.; Zhang, H.; Lin, N.; Su, Y. Influence of reaction conditions on the catalytic activity of a nickel during the supercritical water gasification of dewatered sewage sludge. J. Supercrit. Fluids 2018, 140, 356–363. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S. Supercritical Water Oxidation for Environmentally Friendly Treatment of Organic Wastes. In Advanced Supercritical Fluids Technologies; Pioro, I., Ed.; Intechopen: London, UK, 2020. [Google Scholar]
- Borella, M.; Casazza, A.A.; Garbarino, G.; Riani, P.; Busca, G. A Study of the Pyrolysis Products of Kraft Lignin. Energies 2022, 15, 991. [Google Scholar] [CrossRef]
- Ławińska, O.; Korombel, A.; Zajemska, M. Pyrolysis-Based Municipal Solid Waste Management in Poland—SWOT Analysis. Energies 2022, 15, 510. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, S.; Xiao, B.; Yu, D.; Jia, X. Mechanism of wet sewage sludge pyrolysis in a tubular furnace. Int. J. Hydrogen Energy 2011, 36, 355–363. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Díaz, R.M. Influence of sewage sludge treatment on pyrolysis and combustion of dry sludge. Energy 2013, 55, 426–435. [Google Scholar] [CrossRef]
- Xue, X.; Chen, D.; Song, X.; Dai, X. Hydrothermal and Pyrolysis Treatment for Sewage Sludge: Choice from Product and from Energy Benefit. Energy Procedia 2015, 66, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Inguanzo, M.; Pis, J.J. Microwave-induced pyrolysis of sewage sludge. Water Res. 2002, 36, 3261–3264. [Google Scholar] [CrossRef]
- Januševičius, T.; Mažeikienė, A.; Danila, V.; Paliulis, D. The characteristics of sewage sludge pellet biochar prepared using two different pyrolysis methods. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Hämäläinen, A.; Kokko, M.; Chatterjee, P.; Kinnunen, V.; Rintala, J. The effects of digestate pyrolysis liquid on the thermophilic anaerobic digestion of sewage sludge—Perspective for a centralized biogas plant using thermal hydrolysis pretreatment. Waste Manag. 2022, 147, 73–82. [Google Scholar] [CrossRef]
- Brunetti, N.; Bonfitto, E.; Di Giacomo, G.; Del Re, G.; Iacoboni, S. Pyrolysis of exhausted olive oil husks coupled with a two-stages thermal decomposition of aqueous olive oil mill effluents. In Proceedings of the International Conference on Pyrolysis and Gasification, Luxembourg, 23–25 May 1989; pp. 586–590. [Google Scholar]
- Parsley, D.; Ciora, R.J.; Flowers, D.L.; Laukaitaus, J.; Chen, A.; Liu, P.K.T.; Yu, J.; Sahimi, M.; Bonsu, A.; Tsotsis, T.T. Field evaluation of carbon molecular sieve membranes for the separation and purification of hydrogen from coal- and biomass-derived syngas. J. Memb. Sci. 2014, 450, 81–92. [Google Scholar] [CrossRef]
- Gai, C.; Chen, M.; Liu, T.; Peng, N.; Liu, Z. Gasification characteristics of hydrochar and pyrochar derived from sewage sludge. Energy 2016, 113, 957–965. [Google Scholar] [CrossRef]
- Gai, C.; Guo, Y.; Liu, T.; Peng, N.; Liu, Z. Hydrogen-rich gas production by steam gasification of hydrochar derived from sewage sludge. Int. J. Hydrogen Energy 2016, 41, 3363–3372. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Hydrogen and syngas production from sewage sludge via steam gasification. Int. J. Hydrogen Energy 2010, 35, 11738–11745. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, L.; Li, S.; Yin, J.; Jin, H. Catalytic gasification of sewage sludge in near and supercritical water with different catalysts. Chem. Eng. J. 2020, 388, 124292. [Google Scholar] [CrossRef]
- Herbert, L. Centenary History and Waste Managers in London and South East England. Available online: https://www.ciwm.co.uk/Custom/BSIDocumentSelector/Pages/DocumentViewer.aspx?id=QoR7FzWBtitMKLGdXnS8mUgJfkM0vi6KMAYwUqgqau3ztZeoed%252bsdmKIqDzPOm8yAXgBZR%252fn1fYhL%252bTNdjUq9g2xwY63C2g8GcAQQyfpf3SImIrrED%252bTfsUM91bKsogr (accessed on 21 June 2022).
- Zhang, L.; Xu, C.C.; Champagne, P.; Mabee, W. Biological and thermo-chemical processes for energy and product recovery from sludges: A review. Waste Manag. Res. 2014, 32, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yuan, S.; Xu, Y.; Qian, G. Effects of phosphorus and iron on the composition and property of Portland cement clinker utilized incinerated sewage sludge ash. Constr. Build. Mater. 2022, 341, 127754. [Google Scholar] [CrossRef]
- Edelstein, K. Incinerators: Dinosaurs in the World of Energy Generation. Available online: https://www.fractracker.org/2020/10/incinerators-dinosaurs-in-the-world-of-energy-generation/ (accessed on 21 June 2022).
- Han, X.; Niu, M.; Jiang, X.; Liu, J. Combustion characteristics of sewage sludge in a fluidized bed. Ind. Eng. Chem. Res. 2012, 51, 10565–10570. [Google Scholar] [CrossRef]
- Roskosch, A.; Heidecke, P. Sewage Sludge Disposal in the Federal Republic of Germany; German Environment Agency: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Dichtl, N.; Rogge, S.; Bauerfeld, K. Novel strategies in sewage sludge treatment. Clean Soil Air Water 2007, 35, 473–479. [Google Scholar] [CrossRef]
- Schnell, M.; Horst, T.; Quicker, P. Thermal treatment of sewage sludge in Germany: A review. J. Environ. Manag. 2020, 263, 110367. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Nath, D. 11—Microbial electrochemical technologies for wastewater treatment: Insight into theory and reality. In Clean Energy and Resource Recovery; An, A., Tyagi, V., Kumar, M., Cetecioglu, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 179–200. ISBN 9780323901789. [Google Scholar]
- Faragò, M.; Damgaard, A.; Madsen, J.A.; Andersen, J.K.; Thornberg, D.; Andersen, M.H.; Rygaard, M. From wastewater treatment to water resource recovery: Environmental and economic impacts of full-scale implementation. Water Res. 2021, 204, 117554. [Google Scholar] [CrossRef]
- Olivares, J.A.; Melero, J.A.; Puyol, D.; Dufour, J. Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Goswami, R.; Thakur, R. 26—Valorizing sludge: A biorefinery perspective prospecting for sustainable development. In Clean Energy and Resource Recovery; An, A., Tyagi, V., Kumar, M., Cetecioglu, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323901789. [Google Scholar]
- Cakmak, E.K.; Atasoy, M.; Owusu-Agyeman, I.; Khatami, K.; Cetecioglu, Z. Circular city concept for future biorefineries. In Clean Energy and Resource Recovery; An, A., Tyagi, V., Kumar, M., Cetecioglu, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 335–352. ISBN 9780323901789. [Google Scholar]
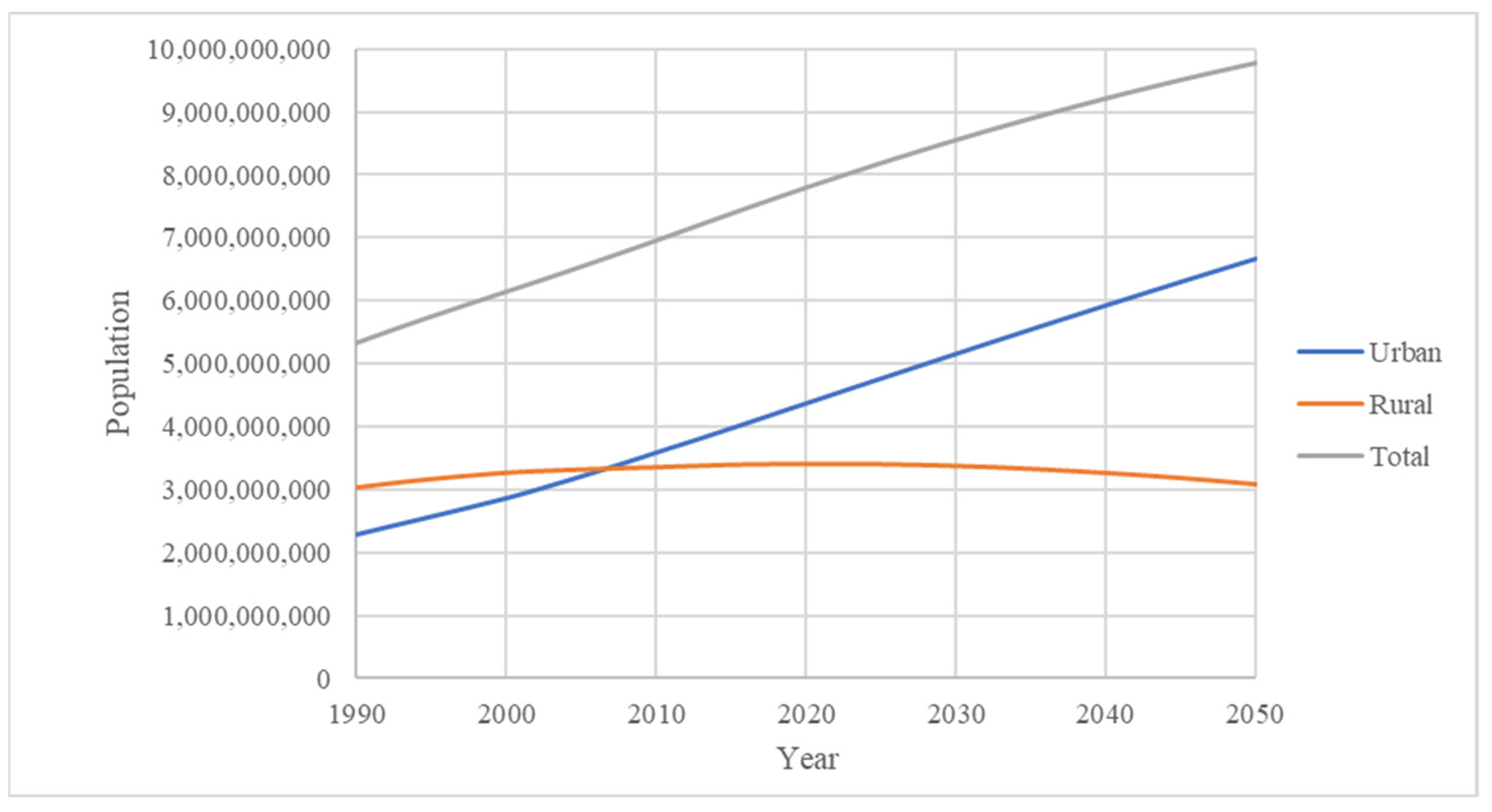
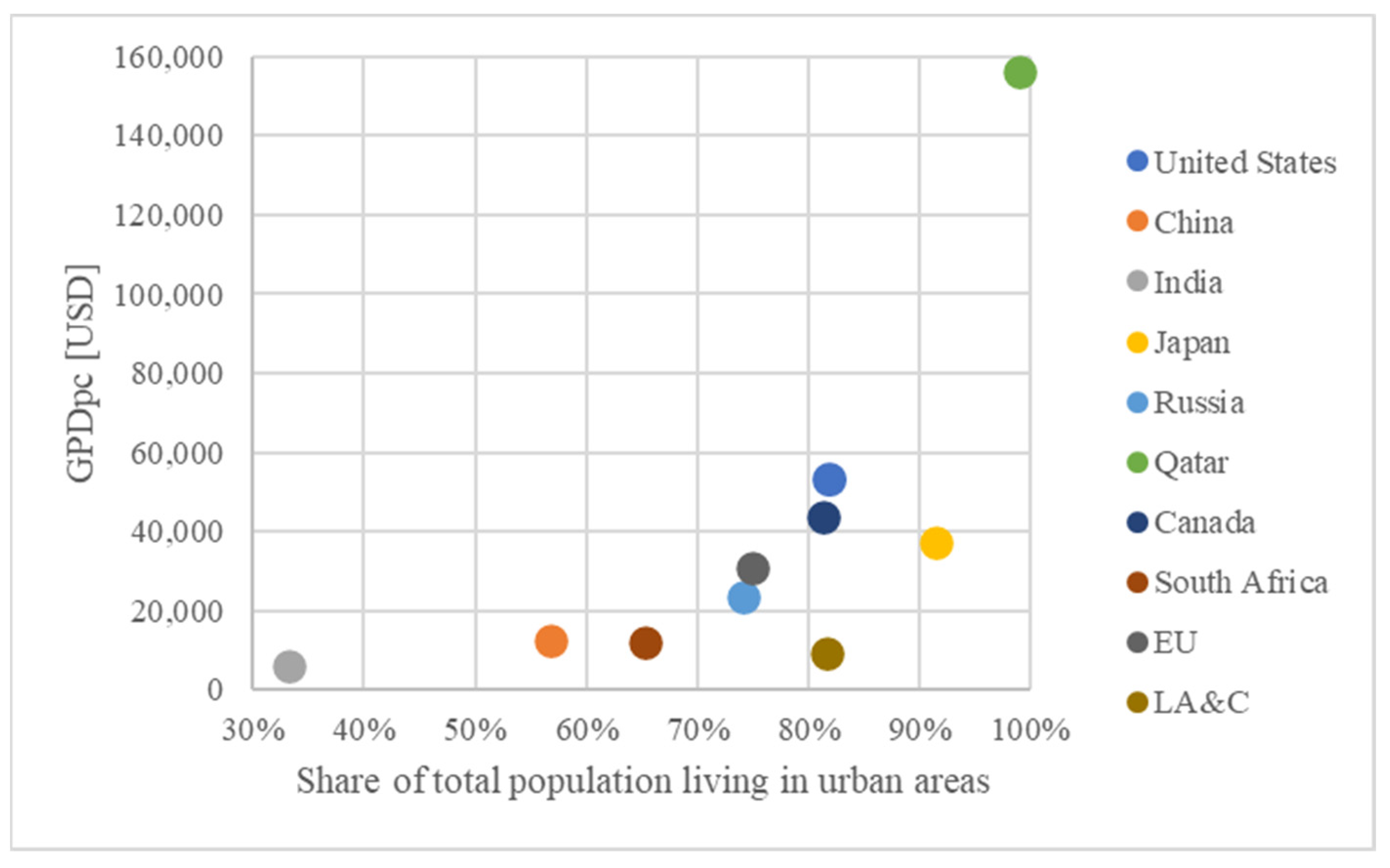
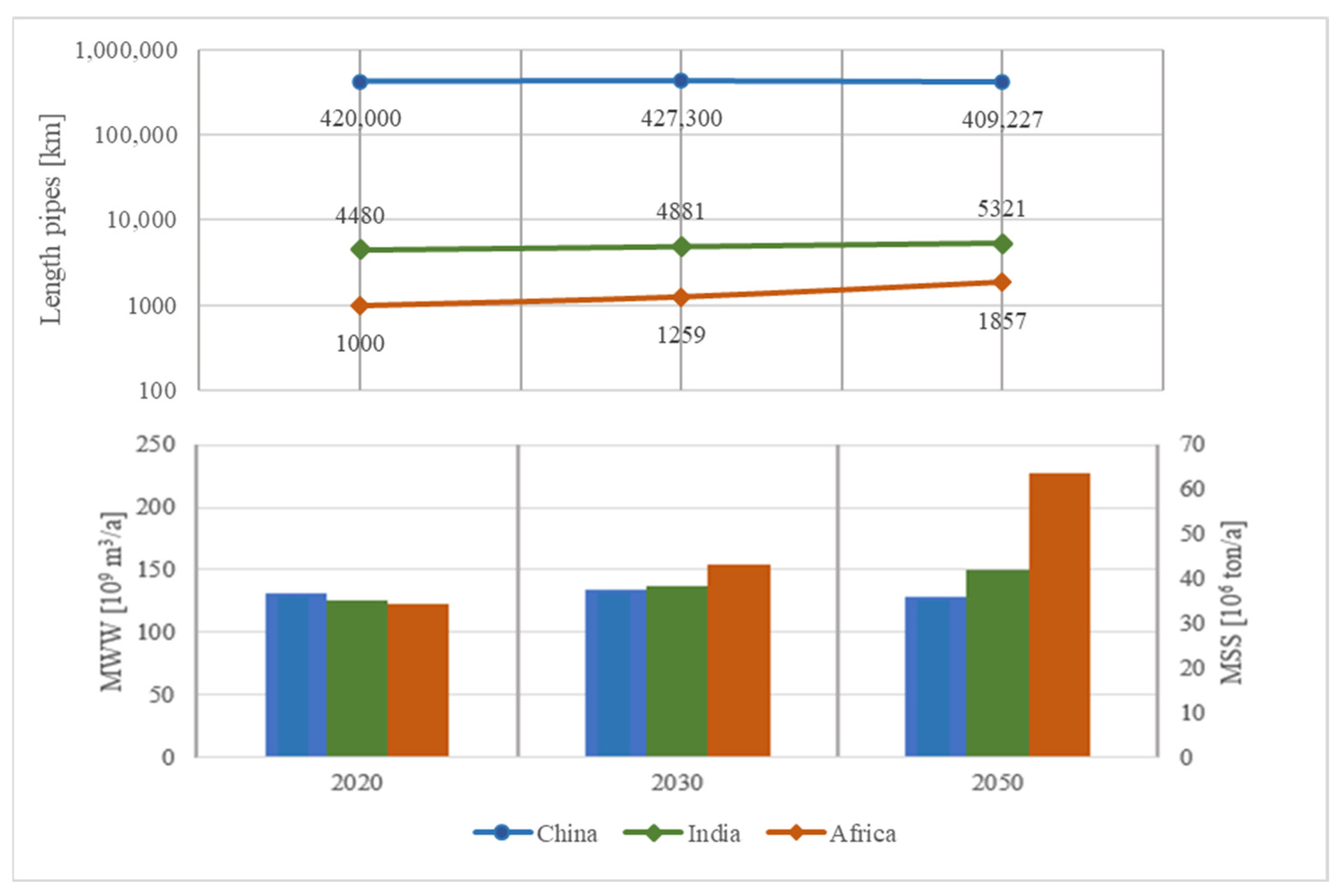


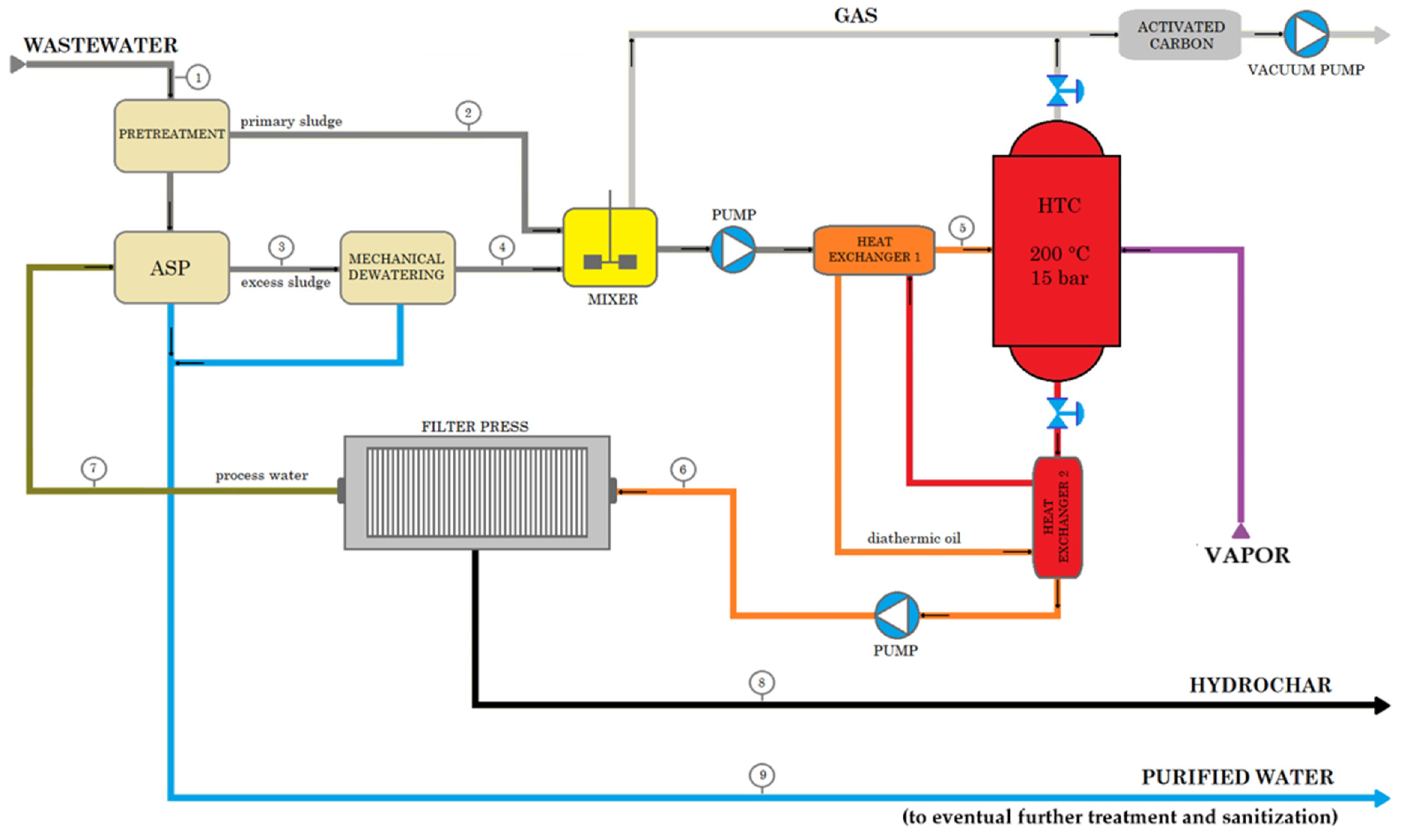
| Name | Number | Ref. | |
|---|---|---|---|
| Collection | Urban Waste Water Treatment Directive (UWWTD) | 91/271/EEC | [22] |
| Processing | UWWTD | - | - |
| Waste Framework Directive (Waste FD) | 2008/98/EC | [23] | |
| Industrial Emissions Directive (IED) | 2010/75/EU | [24] | |
| Transporting | Waste FD | - | - |
| Outlets | Sewage Sludge Directive (SSD) | 86/278/EEC | [25] |
| Waste FD | - | - | |
| IED | - | - | |
| Environmental protection | SSD | - | - |
| Water Framework Directive and the daughter Directives | 2000/60/EC | [26] | |
| Nitrate Directive | 91/676/EEC | [27] |
| Type of ASP | Ref. |
|---|---|
| CAS | [100,101,102,103] |
| Step aeration | [100,101,102] |
| Modified aeration (with or without primary settling) | [100,101,102] |
| High-rate ASP | [100,101,102,104] |
| Extended aeration | [105,106,107] |
| Hatfield and Krauss processes | [108,109,110] |
| Contact stabilization | [111,112,113,114] |
| Membrane bioreactor (MBR) | [115,116,117] |
| Sequencing batch reactor (SBR) | [118,119,120,121] |
| Filtration | Disinfection |
|---|---|
| Bag filters | Dichlorination |
| Drum filters | UV treatment |
| Disc filters | Ozone treatment |
| Membrane | Advanced ozone oxidation treatment |
| Country/Region | MSS [Million tons/a] | Ref. |
|---|---|---|
| EU | 12–14 | [30,60,133] |
| USA | 14 | [33,134] |
| China | 12–18 | [42,43] |
| India | 10–15 | [59] |
| Canada | 0.7–1.2 | [2] |
| Australia | 0.3 | [38] |
| Japan | 2.3 | [35,36] |
| Russia | 5–15 | [135,136] |
| South Africa | 1.2–2 | [137] |
| UK | 1.6–1.8 | [37] |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | °C | 25 | 25 | 25 | 25 | 150 | 50 | 30 | 30 | 25 |
| Pressure | bar | 1 | 1 | 1 | 1 | 20 | 10–15 | 1 | 1 | 1 |
| Mass Flow | ||||||||||
| Total | kg/h | 1000 | 2.0 | 9.8 | 3.9 | 5.9 | 5.9 | 4.5 | 1.4 | 999 |
| Water | kg/h | 999 | 1.9 | 8.8 | 2.9 | 4.9 | 4.9 | 4.5 | 0.4 | 999 |
| Solid | kg/h | 1 | 0.02 | 0.98 | 0.98 | 1 | 1 | - | 1 | - |
| Mass Fraction | ||||||||||
| Water | - | 0.999 | 0.99 | 0.9 | 0.75 | 0.83 | 0.83 | 1 | 0.3 | 1 |
| Solid | - | 0.001 | 0.01 | 0.1 | 0.25 | 0.17 | 0.17 | - | 0.7 | - |
| RMSS Management | Energetic Performance | Mass and Volume Reduction | Required Skill | Development Degree |
|---|---|---|---|---|
| Anaerobic Digestion | WTE technology | Significant | High | Consolidated and diffused Medium–large plants |
| Composting | Aeration is energy-demanding | Moderate | Low | Consolidated and diffused |
| Application on agricultural land and reclamation sites | - | - | Low | Consolidated and diffused Product often out-specification Conflicting with new regulations |
| Drying | Energy-intensive | Significant | Low | Consolidated |
| Pyrolysis | Dehydration pretreatment is energy-intensive | Significant | From low to high depending on operating parameters | Consolidated |
| Gasification | Influenced by the initial mixture content | High | High Severe operating conditions | Consolidated Large plants |
| Incineration | WTE technology but dehydration pretreatment or co-incineration is required | High | High Severe operating conditions | Consolidated Large plants |
| HTC and HTL | Directly applicable to RMSS Almost self-sufficient | High | Low Mild operating conditions | Ready for full-scale applications |
| Process integration and biorefinery | Hybrid ASP–HTC–AD is a WTE technology | High | High | Ready for full-scale applications |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giacomo, G.; Romano, P. Evolution and Prospects in Managing Sewage Sludge Resulting from Municipal Wastewater Purification. Energies 2022, 15, 5633. https://doi.org/10.3390/en15155633
Di Giacomo G, Romano P. Evolution and Prospects in Managing Sewage Sludge Resulting from Municipal Wastewater Purification. Energies. 2022; 15(15):5633. https://doi.org/10.3390/en15155633
Chicago/Turabian StyleDi Giacomo, Gabriele, and Pietro Romano. 2022. "Evolution and Prospects in Managing Sewage Sludge Resulting from Municipal Wastewater Purification" Energies 15, no. 15: 5633. https://doi.org/10.3390/en15155633





