1. Introduction
Lithium-ion batteries have a higher power density relative to conventional energy storage devices such as lead acid batteries and nickel-chromium batteries. They also have the advantages of long-lifetime and no memory effect, which are suitable for electric energy storage. Meanwhile, electric vehicles powered by lithium-ion batteries will not emit any pollutant emissions such as HC, CO, and NO
x during the working process, which is more environmentally friendly compared with traditional vehicles using internal combustion engines. However, the safety issues caused by lithium-ion batteries are serious and occur more frequently with the wide deployment of electric vehicles. Most fire accidents are the spontaneous combustion of lithium-ion batteries [
1]. The severe consequences of these accidents make the safety of lithium-ion batteries the focus of many investigations.
Combustion of lithium-ion batteries may be triggered by the thermal runaway process due to the mechanical, thermal, and electrical abuse of batteries. Thermal runaway normally refers to the uncontrollable temperature rise of a lithium-ion battery caused by internal exothermic reactions. Thermal abuse is one of the main reasons for the thermal runaway of lithium-ion batteries [
2]. If thermal runaway occurs, the internal reactions of the battery release a large amount of heat. The heat release rate might be as high as 8.3 kW for one 18650 lithium-ion cell [
3]. Thermal runaway also leads to the instability of the separator, electrode, and electrolyte under a high temperature such that a large amount of combustible gas is produced. Furthermore, a quick increase in the internal pressure will open the exhaust valve, which is likely to form a jet fire. A large number of electrolyte droplets, combustible gases, and solid particles are sprayed from the cell. The positive electrode oxides may decompose and oxygen can be generated, which will accelerate the reaction process in the cell [
4,
5]. These materials spread out and mix with the air in the battery pack, making it flammable due to the generation of partially oxidized species such as CO. Additionally, high-temperature cell walls and solid particles, or sometimes an electric arc, can provide the required ignition energy [
6,
7,
8]. Therefore, a fast flame propagation follows which can even cause an explosion.
The species of the venting gas are associated with the materials of the positive electrode, the liquid electrolyte, and the state of charge (SOC) of the cell. Sometimes, over 80% of them are H
2, CO, CO
2, CH
4, and C
2H
4 [
9,
10]. The detailed mass fractions are sensitive to the SOC. Essl et al. [
11] found that the content of CO was increased at 100% SOC compared with 0% SOC. Ethyl carbonate (EC), propylene carbonate (PC), ethyl methyl carbonate (EMC), dimethyl carbonate (DMC), and diethyl carbonate (DEC) are the main components of the liquid electrolyte. Henriksen et al. [
12] measured the laminar flame speed of DMC under various equivalence ratios. A maximum flame speed of 300 mm·s
−1 was recorded when the equivalence ratio was 1.04, which was much less than that of hydrogen [
13]. Qiao et al. [
14] analyzed the fire parameters for the electrolyte EC/PC/EMC (25/25/50). The heat release rate of this pool fire decreased as the pressure decreased.
Current investigations into the combustion characteristics of lithium-ion batteries were mainly focused on the heat release rate, accumulated heat release, flame height, and temperature. Chen et al. [
15] tested the combustion process of a module consisting of 32 cells of an 18650 battery. The peak heat release rate occurred at 442.6 kW. The effect of environmental pressure was also evaluated [
16]. The combustion characteristics of a 78 Ah soft pack battery (NCM811) were studied by Zou et al. [
17]. The heat release rate increased as the SOC rose, and a maximum of 154.32 kW was measured under 100% SOC.
The explosion of a lithium-ion battery is reflected by the maximum pressure, the pressure rising rate, and the explosion limit. According to the working conditions, explosions can be divided into constant-pressure explosions in open space [
18], limited explosions in semi-open space [
19], and constant-volume explosions in confined space [
20]. Chen et al. [
21] studied the pressure of an NCM 111 battery during the thermal runaway process and a maximum of 107.2 kPa was measured with a pressure rising rate of 697.4 kPa·s
−1. Baird et al. [
13] investigated the laminar flame speeds and maximum pressures of four typical venting gases. Henriksen et al. [
22] simulated the venting gas with a mixture of H
2, CO, CO
2, CH
4, C
2H
4, and C
2H
6. Chen et al. [
23] studied the explosion lower limit of the venting gas and found that the limit first increased and then decreased as the SOC ascended.
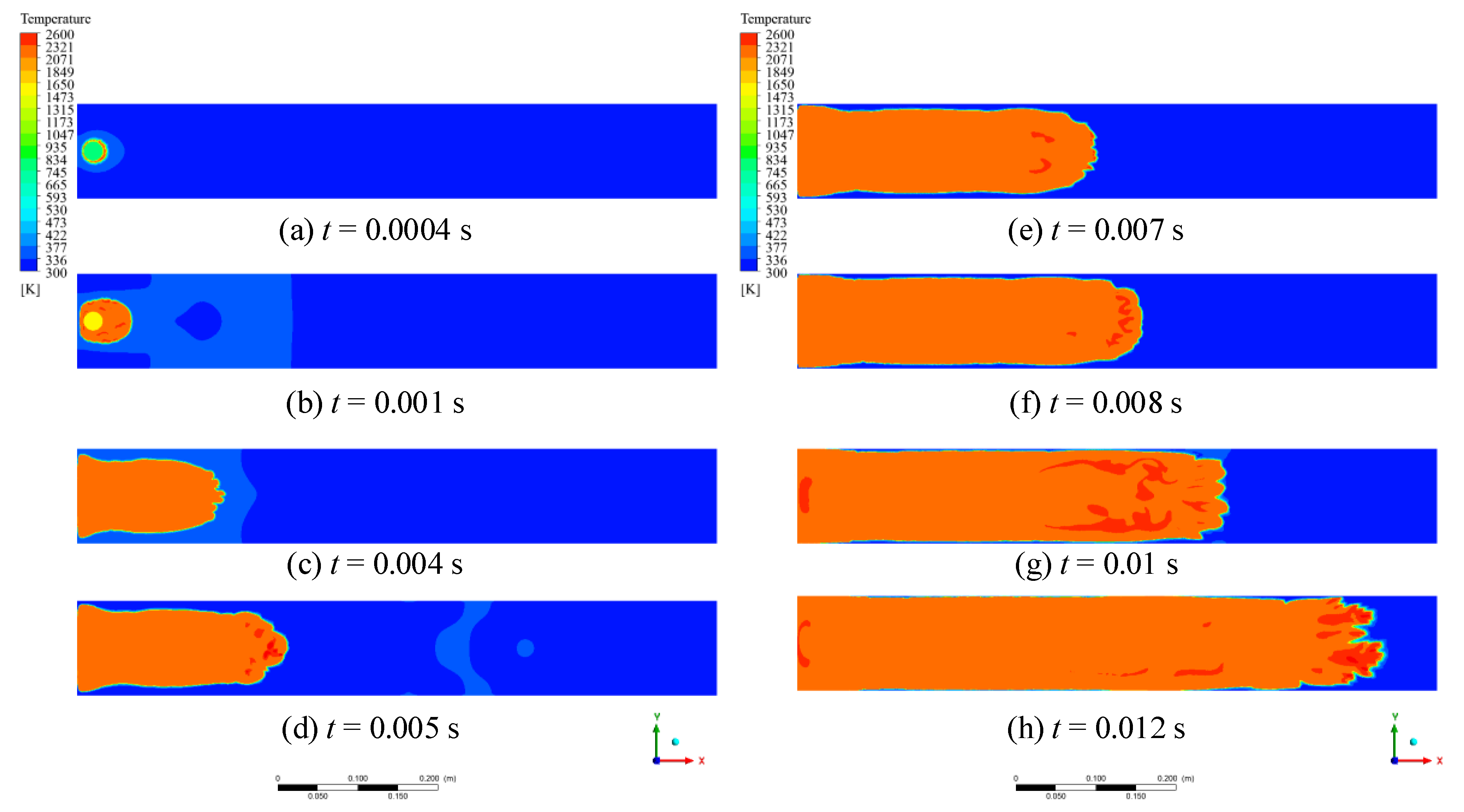
Current investigations concentrated on jet fires in an open space and deflagration in a closed constant-volume bomb, which could reflect the disastrous consequences of thermal runaway. However, practical combustion accidents occurring in electric vehicles are more likely in semi-confined spaces. A combustible mixture forms in a semi-confined space. If these venting gases are ignited, it is likely to have harmful consequences on external personnel or devices. At present, little research has discussed this kind of combustion of lithium-ion batteries. Pressure wave propagation and the interaction with the combustion flame are rarely studied. Therefore, in this study, the combustion characteristics of the venting gases in a semi-confined space are explored. The processes of pressure wave propagation and flame front spread are simulated. The possibility of detonation is discussed. First, based on the finite volume method and a finite rate model, a 2D channel is used to simulate the semi-confined space in a battery pack. The combustion characteristics of the stoichiometric mixture formed by the venting gases and the air are analyzed in the 2D channel. Subsequently, the effects of the initial pressure, temperature, and excess air coefficient on the flame speed and pressure variation are discussed. The possibility of deflagration is estimated. The results can provide some insights into the design of lithium-ion battery packs.
5. Effects of Initial Conditions
When the combustible mixture is formed in the channel by the venting gas of a lithium-ion battery, its combustion and explosion characteristics will be affected by the initial state of the mixture. In the above section, a stoichiometric mixture with an equivalence ratio of 1 is assumed. Herein, the effects of the initial pressure, initial temperature, and initial equivalence ratio of the mixture are estimated during the flame propagation process in the channel.
5.1. Initial Pressure
The combustion characteristics are analyzed with an initial pressure of 81 kPa, 101 kPa, 154 kPa, and 174 kPa. The initial temperature is set to 329 K and the equivalence ratio is 1.
Figure 8a shows the results of the flame front position as a function of time under different initial pressures. The times that the flame front propagates to the right end are 0.0176 s, 0.0154 s, 0.0126 s, and 0.0114 s when the initial pressure is 81 kPa, 101 kPa, 154 kPa, and 174 kPa, respectively. As the initial pressure increases, the required propagation time decreases, and the flame velocity increases.
Figure 8b shows the results of the average flame velocity under different initial pressures. The maximum average flame velocities are 93.5 m·s
−1, 100 m·s
−1, 138.2 m·s
−1, and 151.5 m·s
−1, corresponding to an initial pressure of 81 kPa, 101 kPa, 154 kPa, and 174 kPa. A similar trend is displayed with the propagation time. However, no deflagration appears, and the combustion mode is not changed. According to the laminar premixed combustion theory, the laminar flame velocity
SL is proportional to the initial pressure
P0, which can be expressed as:
where n is the reaction order. For the combustion mechanism of the venting gas of a lithium-ion battery, the reaction order is greater than two. Therefore, the laminar flame velocity increases as the initial pressure rises. On the other hand, when the initial temperature and the equivalence ratio of the mixture remain unchanged, the increase in the initial pressure will increase the species mass in the fixed volume channel, and the corresponding chemical reaction rate will increase. The increase in the initial pressure will reduce the stability of the flame front. Under a high initial pressure, the wrinkles will occur earlier and more frequently on the flame front surface, and the roughness of the flame surface will increase, leading to an increase in the reaction surface area that accelerates the propagation of the flame front, as shown in
Figure 8c.
The results of the combustion pressure in the channel at x = 0.7 m are shown in
Figure 9. A similar trend is obtained for different initial pressures. One peak pressure occurs due to the ignition and the other appears because of the combustion wave. With regard to the considered initial pressures (81 kPa, 101 kPa, 154 kPa, and 174 kPa), the maximum overpressures are 10 kPa, 13.8 kPa, 23.1 kPa, and 25.6 kPa for the first peak while they are slightly reduced to 5 kPa, 8.2 kPa, 13.1 kPa, and 16.6 kPa for the second peak. As the initial pressure increases, the overpressures caused by the ignition and the combustion wave are all increased, indicating a more violent combustion.
5.2. Initial Temperature
The combustion characteristics of the venting gas with an initial temperature of 300 K, 329 K, 360 K, and 400 K are investigated subsequently. 329 K is close to the temperature of the stoichiometric mixture of the venting gas with the air in the pack at 300 K. 360 K approaches the boiling point of DMC (363 K) and 400 K is slightly less than the boiling point of DEC (401 K). Generally, the vent valve will open readily after the liquid electrolyte starts to boil. Therefore, these values are specified as the initial temperature, respectively. The initial pressure is set to 154 kPa with an equivalence ratio of 1. The results of the flame front position as a function of time are shown in
Figure 10a. The required times for the flame propagating to the right end are 0.0134 s, 0.0126 s, 0.008 s, and 0.00528 s for the four initial temperatures. As the initial temperature increases, the required time reduces and the average flame velocity increases. Compared with the result of an initial temperature of 300 K, the propagation time of an initial temperature of 329 K decreases by about 6%. Furthermore, the times for the cases with an initial temperature of 360 K and 400 K are decreased significantly by 40.3% and 60.6%, respectively. A large increase in the flame velocity indicates that such a great increase in the initial temperature may result in the variation of the combustion mode.
Figure 10b shows the profiles of the average flame velocity as a function of time under different initial temperatures. At the initial temperature of 300 K, 329 K, 360 K, and 400 K, the maximum average flame velocity is 109.2 m·s
−1, 138.2 m·s
−1, 264.7 m·s
−1, and 402.5 m·s
−1, respectively. The results imply a high sensitivity of the initial temperature to the flame velocity of the venting gas. Normally, the laminar flame velocity
SL has an Arrhenius relation with the initial temperature
T0 as:
The reaction rate increases exponentially with the increase in the initial temperature because the activation energy normally is not influenced by the temperature. The average flame velocity at an initial temperature of 329 K is close to that of 300 K. When the initial temperature continues to increase, the average flame velocity increases significantly.
When the initial temperature is 400 K, the flame propagation shows a different variation compared with the other three initial temperatures. The flame velocity exceeds the local sonic speed, and the deflagration mode occurs for the combustion of the venting gas mixture. Normally, the pressure wave propagates in the unburned gas at the local sound speed. The pressure wave originally generated during the ignition should decay rapidly and will not have an evident acceleration impact on the front unburned gas. However, when the initial temperature is 400 K, the shock wave and its reflected wave generated by the flame combustion spread forward in a more obvious cell shape, as shown in
Figure 11. The pressure wave continuously imposes on the unburned gas, making its pressure and temperature rise evidently. The flame propagates and accelerates in the channel. When the flame reaches the local sound velocity of the unburned gas, the flame front will catch up with the forward propagation speed of the pressure wave, forming a deflagration wave structure where the precursor shock wave is superimposed on the subsequent rapid chemical reaction surface. Accordingly, the temperature and pressure of the unburned gas mixture in front of the flame surface are further increased. The combustion mode changes from slow deflagration (less than the local sonic speed) to fast deflagration (greater than the local sonic speed). Therefore, if the venting gas of a lithium-ion battery mixes with the air and the temperature is higher than 400 K, there is a high risk of fast deflagration and even the explosion of the battery pack.
The results of the pressure variation as a function of time are shown in
Figure 12 under different initial temperatures. The pressure is measured at the location X = 0.7 m. When the initial temperature is 300 K, 329 K, 360 K, and 400 K, the maximum overpressure caused by the ignition is 17 kPa, 23 kPa, 17 kPa, and 48 kPa, respectively. The corresponding maximum overpressure caused by the combustion wave is 13 kPa, 13 kPa, 22 kPa, and 42 kPa, respectively. At the initial temperature of 400 K, the maximum overpressure when reaching the fast deflagration state is significantly greater than the cases of the other initial temperatures.
5.3. Excess Air Coefficient
Finally, the effects of the equivalence ratio of the gas mixture on the flame propagation are evaluated. Assuming that the initial amount of air in the channel before mixing with the venting gas remains constant, the quantity of venting gas erupted from the battery determines the initial equivalence ratio of the mixture. In this study, the excess air coefficient α is used to assess the effects of the initial concentration of the venting gas. α is defined as the ratio of the actual air quantity in the channel to the air mass required to completely burn the venting gas. Before mixing, the temperature and pressure of the air in the channel are 298 K and 1 bar. Combustible mixtures with different excess air coefficients are determined by changing the molar value of the venting gas. The detailed initial states under different excess air coefficients are shown in
Table 6. The α value is specified to cover the typical range for premixed combustion, from stochiometric to power mixtures with slightly rich concentrations and economic mixtures with slightly lean concentrations, respectively. In this range, the flame propagation process is the most dramatic.
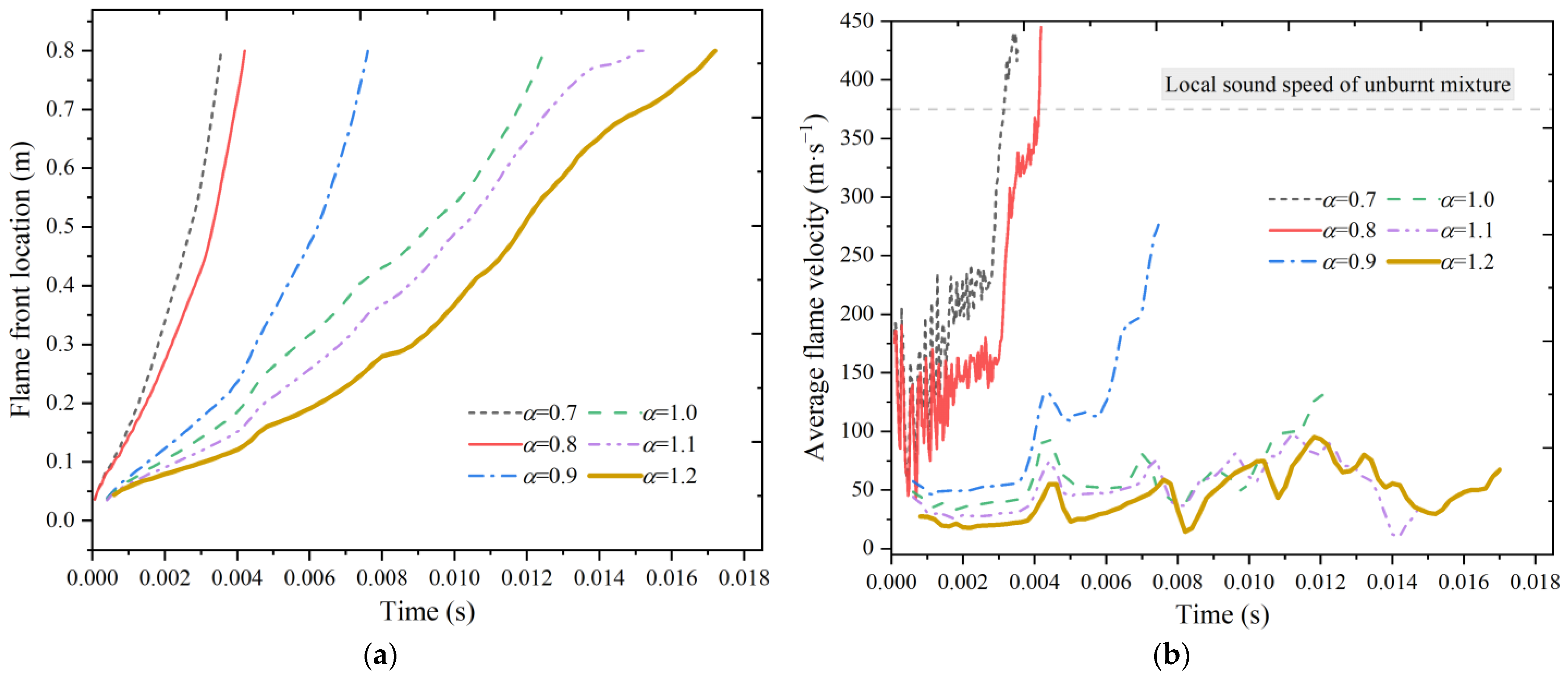
Figure 13a shows the results of the flame front position as a function of time under different excess air coefficients. When the excess air coefficient decreases from 1.2 to 0.7, the time required for the flame front to propagate to the right end is 0.0172 s, 0.0152 s, 0.0126 s, 0.0076 s, 0.00418 s, and 0.00354 s, respectively. With the decrease in the excess air coefficient, the molar amount of the venting gas in the channel increases, the time required for flame propagation decreases, and the average propagation velocity increases.
Figure 13b shows the profile of the average flame velocity under different excess air coefficients. When the excess air coefficient decreases from 1.2 to 0.7, the maximum average flame velocity increases from 95.25 m·s
−1 to 437.5 m·s
−1. The average flame velocity is larger in a slightly fuel-rich mixture. The maximum velocity arrives at 445 m·s
−1 when the excess air coefficient is 0.8.
When the excess air coefficient is around 0.9, the combustion mode changes. When the excess air coefficient is decreased to 0.8, the average flame velocity has exceeded the local sonic speed, and the combustion mode has changed from slow deflagration to fast deflagration, which significantly increases the risk of a combustion accident. The local sonic velocity is a criterion to distinguish the combustion mode. Once the flame propagation velocity reaches the sound velocity, the pressure disturbance generated by the combustion wave will be behind the flame front surface and will no longer affect the flame surface. The flame continues to be accelerated during the propagation, forming shock waves at the flame front. The unburned gases will become directly combusted under the action of shock waves and energy is released to maintain the flame propagation speed. In such circumstances, deflagration waves will turn into detonation waves. Obviously, it is difficult to produce detonation in a channel with an open end. However, the detonation may still occur under fuel-rich conditions.
The results of combustion pressure measured at the location X = 0.7 m under different excess air coefficients are shown in
Figure 14. The maximum overpressure caused by the ignition pressure wave is 10.9 kPa, 20.3 kPa, 22.9 kPa, 22 kPa, 61.7 kPa, and 91.14 kPa while the maximum overpressure caused by the combustion wave is 11.8 kPa, 12.8 kPa, 13 kPa, 53 kPa, 91.3 kPa, and 94.9 kPa, respectively.
6. Conclusions
In this study, the combustion characteristics of the venting gas from a lithium-ion battery are simulated and analyzed in a 2D channel with an open end. The flame propagation velocity and pressure variation are estimated for a stoichiometric mixture at first. The effects of the initial states, including the initial pressure, temperature, and equivalence ratio, are then assessed.
The pressure variation in the channel is mainly caused by the ignition pressure wave and the following combustion wave. For the combustion process with a stoichiometric mixture, the peak overpressure from the ignition pressure wave is 22.9 kPa, which is greater than the peak overpressure produced by the combustion wave at 15.6 kPa at the initial stage of ignition. Increasing the initial pressure in the channel will reduce the stability of the flame front, and more wrinkles will appear earlier, leading to an increase in the flame surface area. The maximum flame velocity will increase accordingly in the channel. Under an initial pressure of 175 kPa, the maximum flame velocity is 151 m·s−1, and the maximum overpressure is 25.6 kPa. The maximum overpressure increases with the increase in the initial pressure.
The combustion of the mixture in the channel is sensitive to the initial temperature. Increasing the initial temperature will increase the flame propagation velocity. When the initial temperature is 400 K, the maximum flame velocity is 400 m·s−1, which is greater than the local sound velocity of the unburned gas, and the combustion mode changes to fast deflagration. The maximum overpressure of the ignition pressure wave at the initial temperature of 400 K is 48 kPa, and the maximum overpressure of the combustion wave is 42 kPa, which is twice the overpressure at the initial temperature of 360 K. When the initial temperature is above 400 K, the explosion risk is high.
With the increase in the amount of venting gas, the excess air coefficient in the channel decreases, and the maximum flame velocity increases first and then decreases. When the excess air coefficient is 0.8, the maximum flame speed is achieved with a slightly rich mixture, which is about 445 m·s−1. When the excess air coefficient is below 0.9, the combustion mode changes from slow deflagration to fast deflagration, which has a high risk of explosion. When the excess air coefficient is 0.8, the peak overpressure caused by the ignition pressure wave is 61.7 kPa, which is less than that caused by the combustion wave of 91.3 kPa. The maximum combustion overpressure increases with the decrease in excess air coefficient, and a high risk of explosion exists when the venting gas mixture is rich.
In practice, to avoid dramatic explosions when thermal runaway occurs, some control methods can be employed. First, a relief valve can be used to control the pressure in the battery pack. If the pressure exceeds a certain value over the ambient pressure, the relief valve opens automatically to limit the combustion pressure. Second, nonflammable phase-change materials can be inserted into the pack to reduce the combustion temperature. Third, fire retardants can be released if the venting gas is detected in the pack. The amount of venting gas, the equivalence ratio, and the ignition point are three key factors. Because high-temperature solid particles are sprayed together with the venting gas and sometimes even an electric arc occurs in the venting gas due to charged particles, it is difficult to control the ignition point, and more investigation is required.
This study only considers the propagation process in a 2D channel. In the future, a more precise 3D simulation needs to be performed to give a more precise evaluation. Meanwhile, it is difficult to measure the pressure variation in such a high-temperature environment. Further experimental facility needs to be developed and experimental validation is also required.