Dry Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste: Biogas Production Optimization by Reducing Ammonia Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
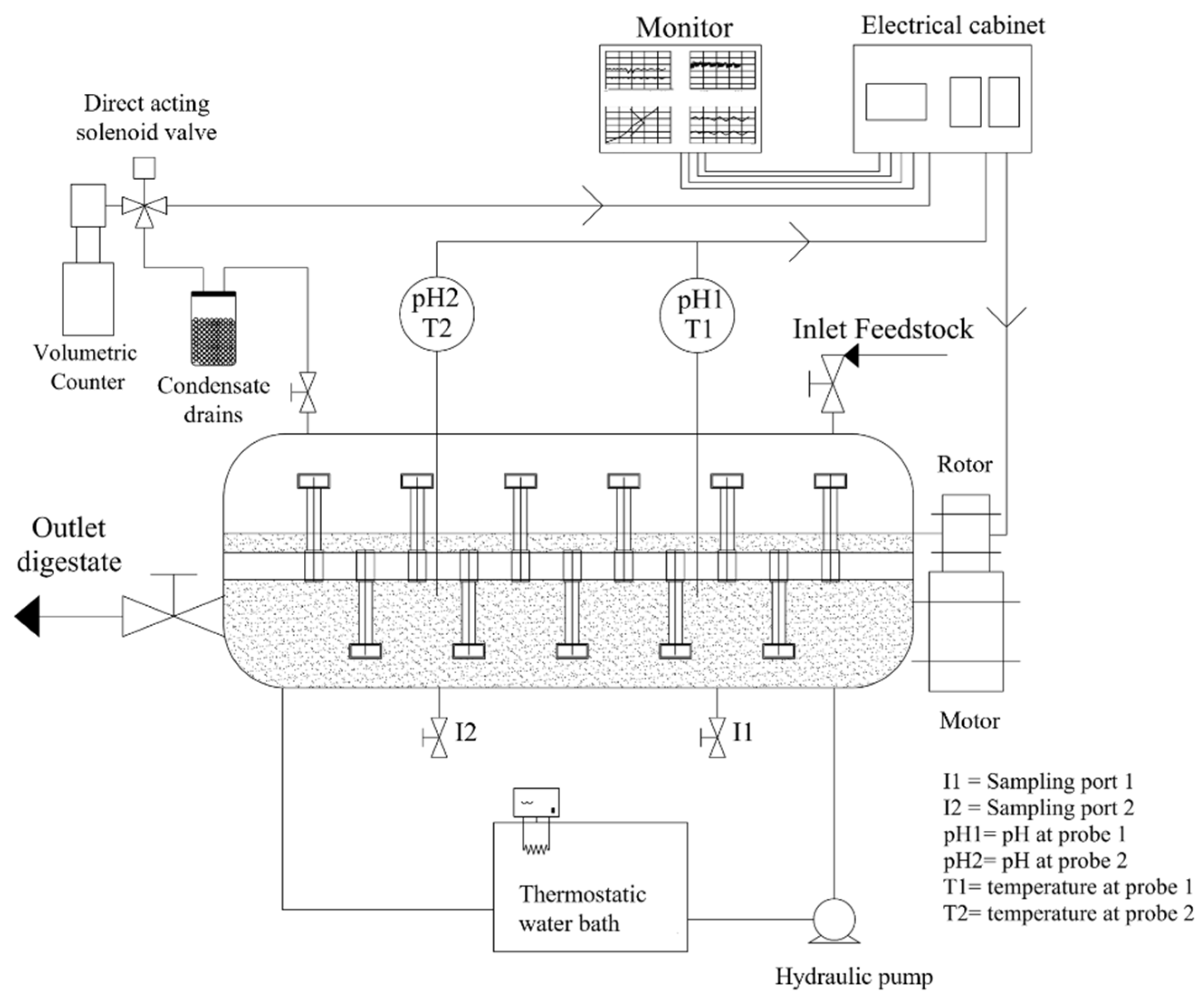
2.2. Reactor Configuration
2.3. Operating Conditions
2.4. Analytical Measurements
2.5. Peformance Evaluation
2.6. Statistical Analysis
3. Results
3.1. Substrate and Inoculum
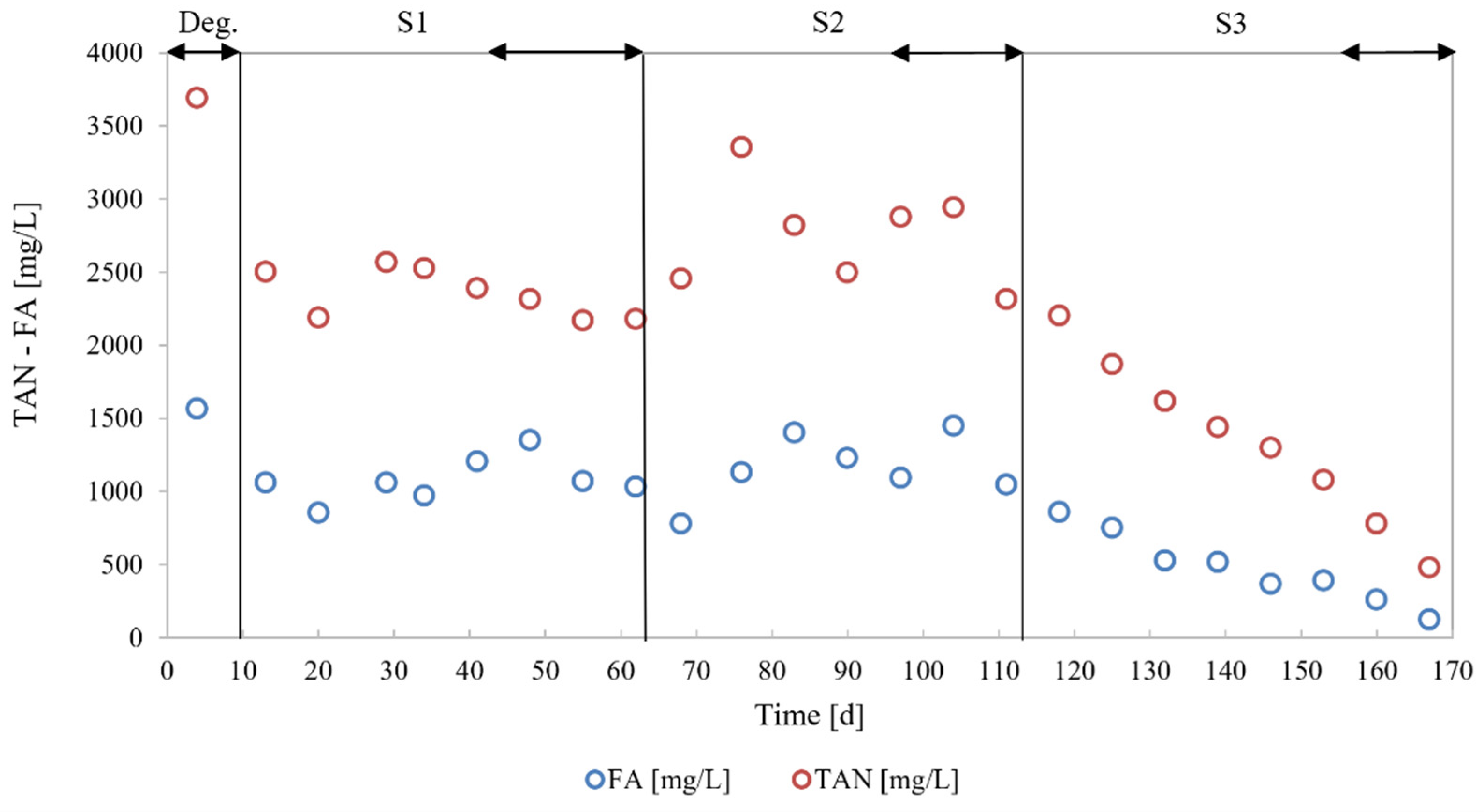
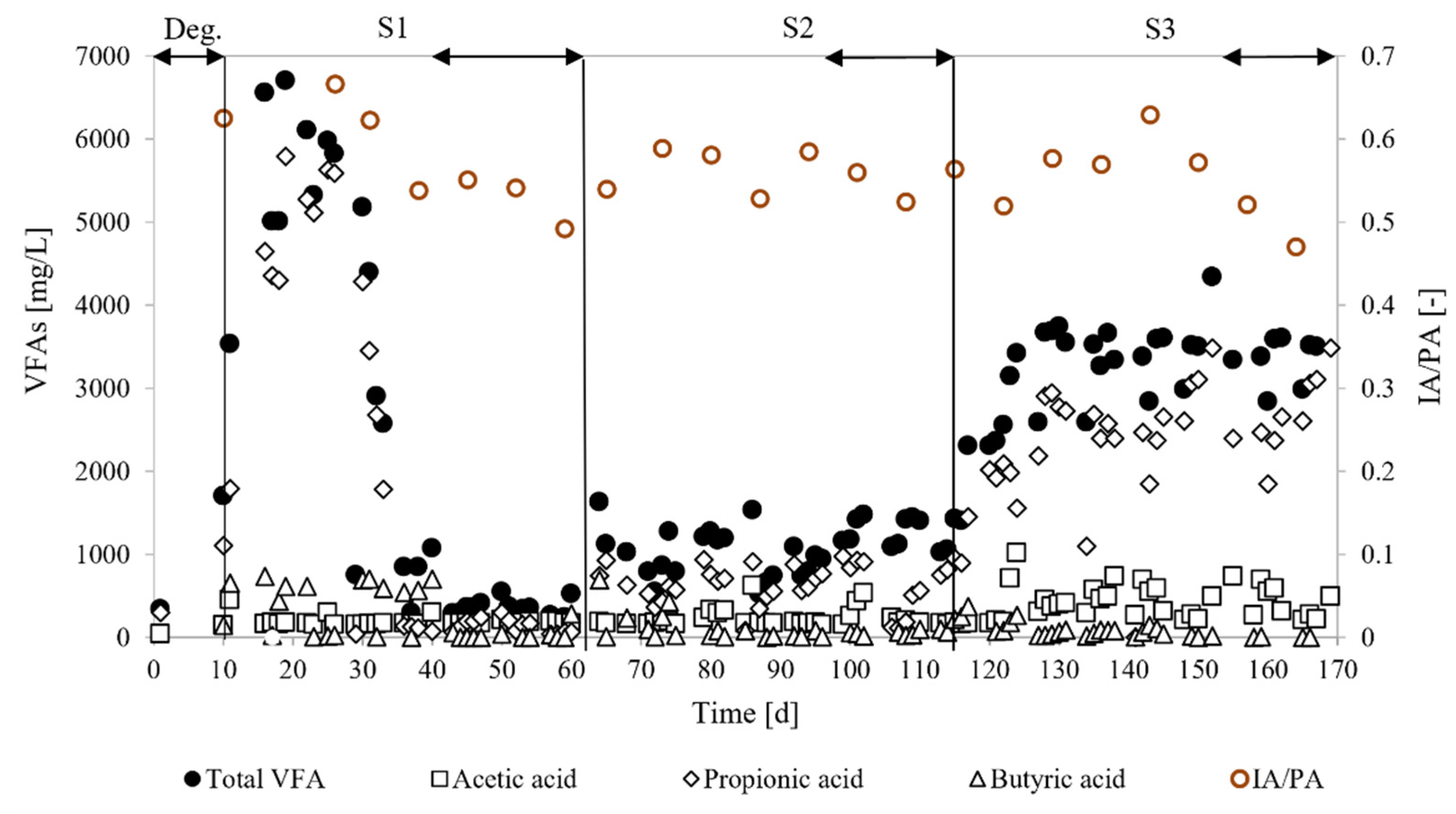
3.2. Process Stability
3.3. Process Performance
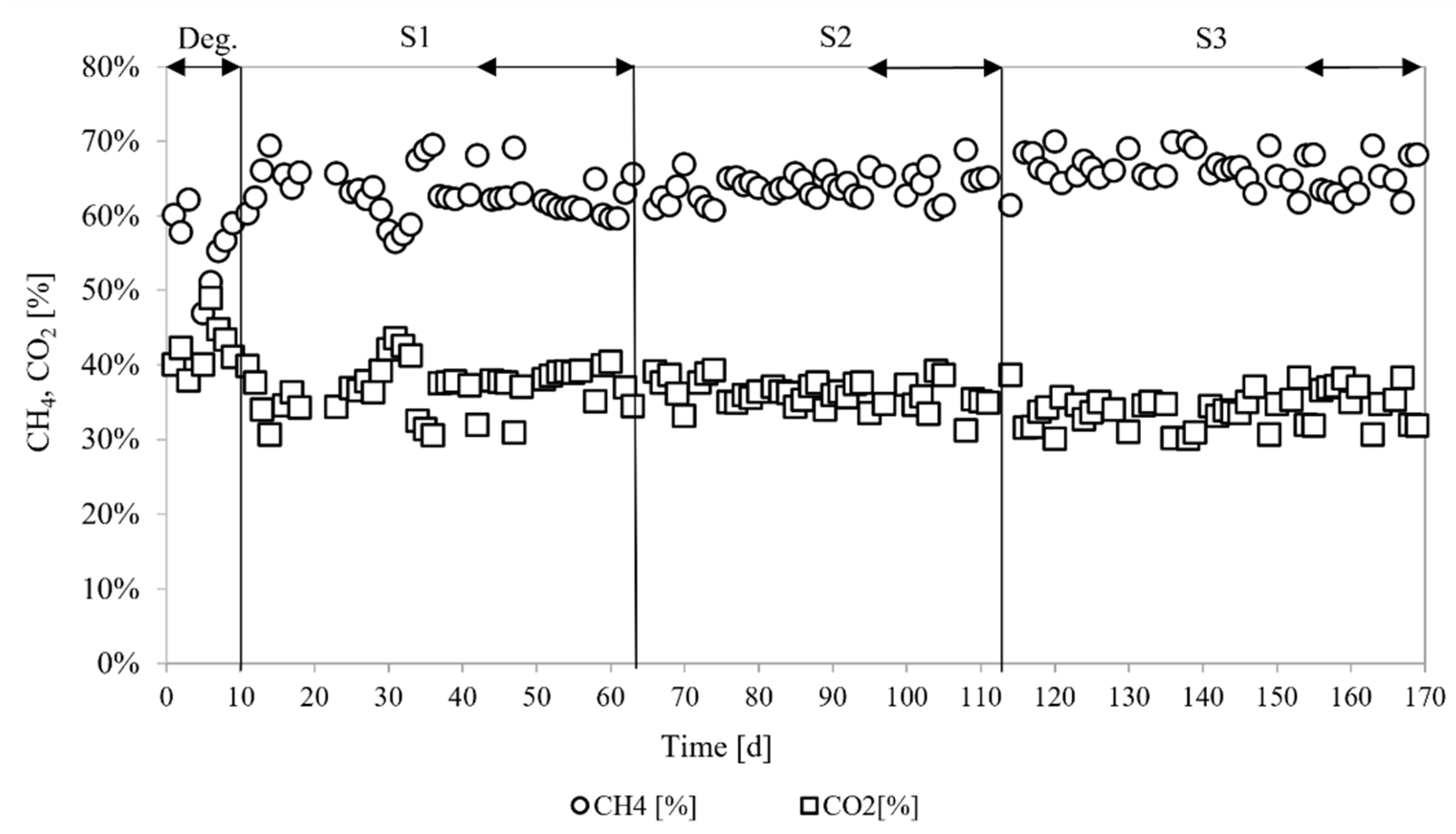
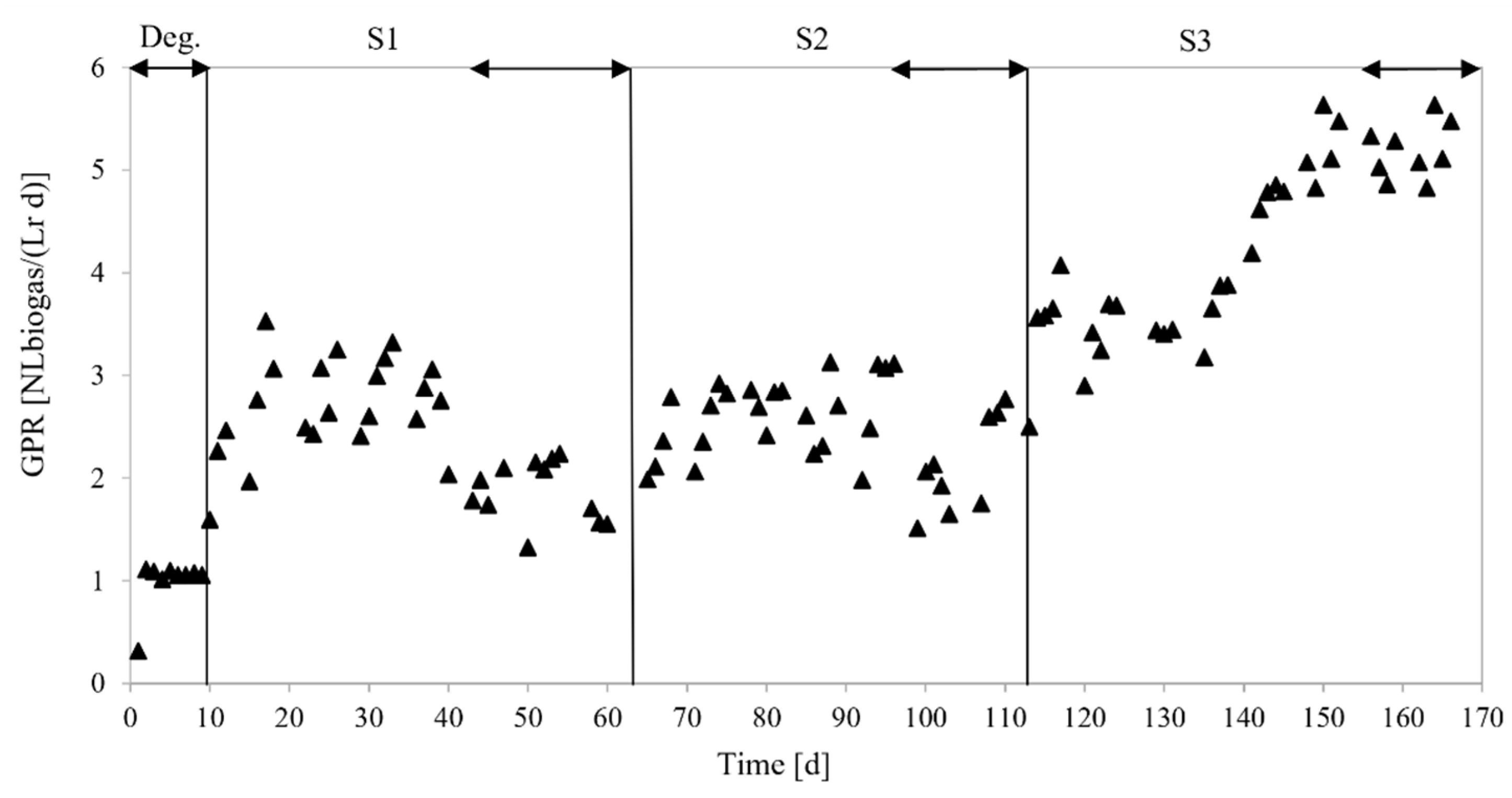
3.3.1. Biogas Yields
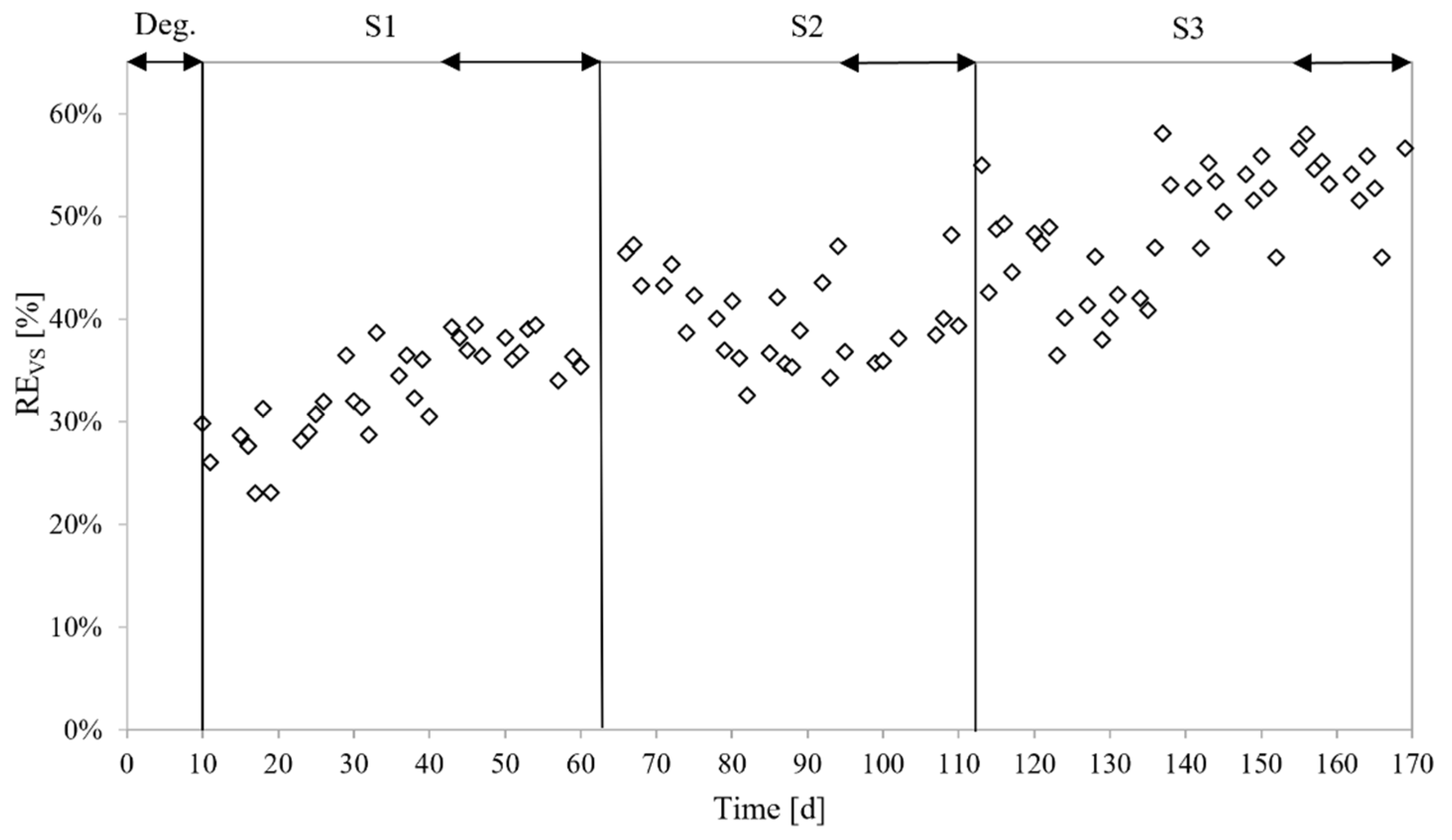
3.3.2. Bioenergy Recovery
3.4. Digestate Characterization
4. Discussion
4.1. Process Stability
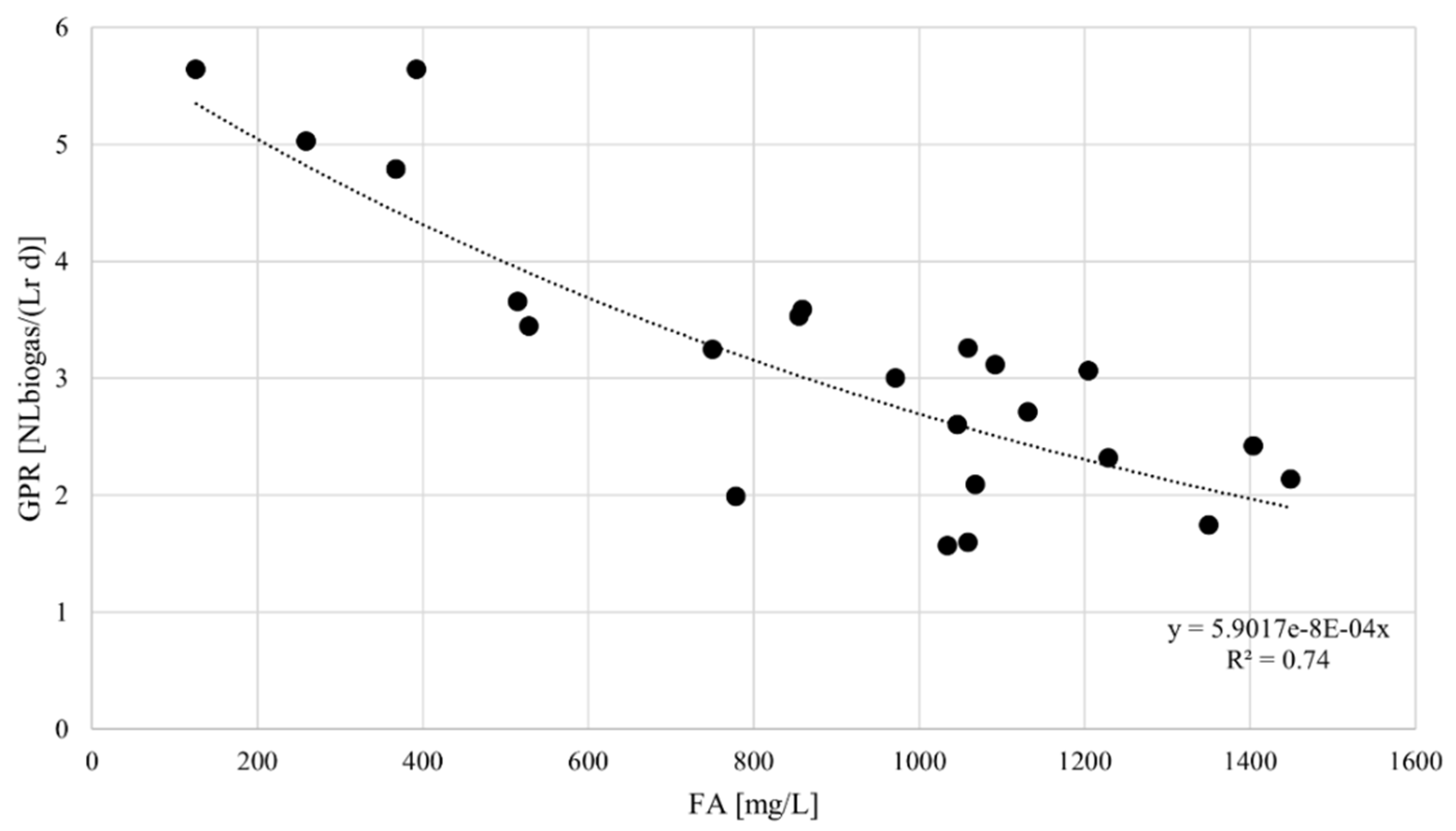
4.2. Biogas Yields
4.3. Bioenergy Recovery
4.4. Digestate Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Unit of Measurement | S1 ° | S2 ° | S3 * | |
|---|---|---|---|---|
| pH | - | 7.96 ± 0.4 | 7.99 ± 0.4 | 7.62 ± 0.22 |
| TOC | % | 7 ± 1.1 | 8.2 ± 1.2 | 8.7 ± 0.79 |
| Organic nitrogen | % | 0.33 ± 0.05 | 0.15 ± 0.02 | 0.45 ± 0.06 |
| TAN | mg/kg | 3420 ± 510 | 3490 ± 520 | 2366 ± 823 |
| Total alkalinity | mgCaCO3/kg | 14,500 ± 1700 | 14,100 ± 1700 | 11,533 ± 288 |
| TS | % | 19 ± 1.3 | 23.9 ± 1.7 | 26.63 ± 3.82 |
| VS | % | 13.7 ± 1.3 | 15.4 ± 1.7 | 17.76 ± 3.75 |
| Ash | % | 5.3 ± 0.37 | 8.5 ± 0.6 | 8.86 ± 0.45 |
| C | % °° | 37.05 ± 4.42 | 34.3 ± 4.1 | 32.81 ± 1.61 |
| H | % °° | 4.52 ± 0.45 | 3.97 ± 0.39 | 3.87 ± 0.2 |
| N ** | % °° | 3.15 ± 0.37 | 1.79 ± 0.21 | 2.43 ± 0.4 |
| S | % °° | 0.13 ± 0.02 | 0.26 ± 0.04 | 0.24 ± 0.1 |
| C/N | - | 11.73 ± 0 | 19.06 ± 7.94 | 13.63 ± 1.71 |
| Lignin | % VS | 32.84 ± 2.33 | 39.61 ± 2.79 | 36.44 ± 3.86 |
| Cellulose | % VS | 22.62 ± 1.82 | 21.42 ± 1.68 | 18.82 ± 1.87 |
| Carbohydrates 1 | % VS | 18.24 ± 2.18 | 19.48 ± 2.33 | 22.99 ± 3.24 |
| Proteins | % VS | 15.32 ± 2.77 | 16.88 ± 3.05 | 16.94 ± 1.81 |
| Fats, oil and grease | % VS | 0.71 ± 0.04 | 0.64 ± 0.04 | 0.38 ± 0 |
| Aluminum | mg/kg | 480 ± 140 | 920 ± 260 | 946.66 ± 77.67 |
| Calcium | mg/kg | 8000 ± 1300 | 8800 ± 1400 | 9633.33 ± 550.75 |
| Magnesium | mg/kg | 760 ± 230 | 1060 ± 310 | 1110 ± 43.58 |
| Manganese | mg/kg | 48 ± 16 | 55 ± 18 | 68 ± 10 |
| Potassium | mg/kg | 2640 ± 400 | 2560 ± 380 | 2670 ± 120 |
| Sodium | mg/kg | 1210 ± 390 | 1120 ± 370 | 1053 ± 55 |
| Arsenic | mg/kg °° | <RL | 4.18 ± 0.62 | <RL |
| Cadmium | mg/kg °° | <RL | <RL | <RL |
| Chrome | mg/kg °° | 152.63 ± 23.15 | 175.73 ± 26.35 | 189.52 ± 18.87 |
| Mercury | mg/kg °° | <RL | <RL | <RL |
| Nickel | mg/kg °° | 89.47 ± 13.68 | 100.41 ± 15.06 | 107.56 ± 12.41 |
| Copper | mg/kg °° | 73.68 ± 11.05 | 66.94 ± 10.04 | 65.86 ± 12.86 |
| Zinc | mg/kg °° | 163.15 ± 24.73 | 150.62 ± 22.59 | 152.94 ± 16.15 |
| OUR | (mmolO2/(kgVS h)) | 42.53 ± 2.85 | 40.25 ± 6.57 | 29.46 ± 12.26 |
| RBP | (NLbiogas/gVS) | 0.11 ± 0 | 0.1 ± 0 | 0.11 ± 0 |
References
- EUR-Lex—52019DC0640—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52019DC0640 (accessed on 24 June 2021).
- IRENA. Global Renewables Outlook: Energy Transformation 2050. Available online: https://irena.org/publications/2020/Apr/Global-Renewables-Outlook-2020 (accessed on 9 July 2021).
- Peters, D.; van der Leun, K.; van Tiliburg, J.; Berg, T.; Schimmel, M.; van der Hoorn, I.; Buseman, M.; Staats, M.; Schenkel, M.; Ur Rehaman Mir, G. Gas Decarbonisation Pathways 2020–2050: Gas for Climate; Guidehouse: Utrecht, The Netherlands, 2020. [Google Scholar]
- European Environment Agency. Bio-Waste in Europe: Turning Challenges into Opportunities; Publications Office: Luxembourg, 2020. [Google Scholar]
- Directive 2008/98/EC of the European Parliament and of The Council of 19 November 2008 on Waste and Repealing Certain Directives; Publications Office of the European Union: Luxembourg, 2008; pp. 3–30.
- Campuzano, R.; González-Martínez, S. Characteristics of the organic fraction of municipal solid waste and methane production: A review. Waste Manag. 2016, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L.; Cossu, R. Composition variability of the organic fraction of municipal solid waste and effects on hydrogen and methane production potentials. Waste Manag. 2015, 36, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sailer, G.; Eichermüller, J.; Poetsch, J.; Paczkowski, S.; Pelz, S.; Oechsner, H.; Müller, J. Characterization of the separately collected organic fraction of municipal solid waste (OFMSW) from rural and urban districts for a one-year period in Germany. Waste Manag. 2021, 131, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, I.; Rossi, E.; Iannelli, R. Bromatological, Proximate and Ultimate Analysis of OFMSW for Different Seasons and Collection Systems. Sustainability 2020, 12, 2639. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Biotechnol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Baere, L.D.; Mattheeuws, B. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Europe. Waste Manag. 2012, 3, 517–526. [Google Scholar]
- Rosenheim, H.; De, I.; Hyvedemm, S. Anaerobic Digestion—Factsheet; European Bioplastics e.V.: Berlin, Germany, 2015; 8p. [Google Scholar]
- Fagbohungbe, M.O.; Dodd, I.C.; Herbert, B.M.J.; Li, H.; Ricketts, L.; Semple, K.T. High solid anaerobic digestion: Operational challenges and possibilities. Environ. Technol. Innov. 2015, 4, 268–284. [Google Scholar] [CrossRef]
- Pecorini, I.; Peruzzi, E.; Albini, E.; Doni, S.; Macci, C.; Masciandaro, G.; Iannelli, R. Evaluation of MSW Compost and Digestate Mixtures for a Circular Economy Application. Sustainability 2020, 12, 3042. [Google Scholar] [CrossRef] [Green Version]
- EU Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 27 April 2020).
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Strategies for the anaerobic digestion of the organic fraction of municipal solid waste: An overview. Water Sci. Technol. 2006, 53, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Ruiz, D.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Accumulation of propionic acid during consecutive batch anaerobic digestion of commercial food waste. Bioresour. Technol. 2017, 245, 724–733. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Nisman, B. The Stickland Reaction. Bacteriol. Rev. 1954, 18, 16–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Li, L.; Qin, Y.; Kong, Z.; Wu, J.; Kubota, K.; Li, Y.-Y. Characterization of microbial community and main functional groups of prokaryotes in thermophilic anaerobic co-digestion of food waste and paper waste. Sci. Total. Environ. 2019, 652, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, M.; Zeshan; Yousaf, S.; Haider, M.R.; Malik, R.N. High-solids anaerobic co-digestion of food waste and rice husk at different organic loading rates. Int. Biodeterior. Biodegrad. 2015, 102, 149–153. [Google Scholar] [CrossRef]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Xie, D.; Guan, W.; Yang, M.; Zhao, P.; Gao, M.; Wang, Q.; Wu, C. Adding activated carbon to the system with added zero-valent iron further improves anaerobic digestion performance by alleviating ammonia inhibition and promoting DIET. J. Environ. Chem. Eng. 2021, 9, 106616. [Google Scholar] [CrossRef]
- Takashima, M.; Yaguchi, J. High-solids thermophilic anaerobic digestion of sewage sludge: Effect of ammonia concentration. J. Mater. Cycles Waste Manag. 2021, 23, 205–213. [Google Scholar] [CrossRef]
- Zhang, W.; Heaven, S.; Banks, C.J. Continuous operation of thermophilic food waste digestion with side-stream ammonia stripping. Bioresour. Technol. 2017, 244, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Heaven, S.; Banks, C.J. Thermophilic Digestion of Food Waste by Dilution: Ammonia Limit Values and Energy Considerations. Energy Fuels 2017, 31, 10890–10900. [Google Scholar] [CrossRef]
- Yirong, C.; Zhang, W.; Heaven, S.; Banks, C.J. Influence of ammonia in the anaerobic digestion of food waste. J. Environ. Chem. Eng. 2017, 5, 5131–5142. [Google Scholar] [CrossRef] [Green Version]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Nappi, P.; Valenzano, F.; Consiglio, M. Analisi Merceologica dei Rifiuti Urbani Rassegna di Metodologie e Definizione di una Metodica di Riferimento; Agenzia Nazionale per la Protezione dell’Ambiente: Rome, Italy, 2000. [Google Scholar]
- Rossi, E.; Becarelli, S.; Pecorini, I.; Di Gregorio, S.; Iannelli, R. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Plug-Flow Reactors: Focus on Bacterial Community Metabolic Pathways. Water 2022, 14, 195. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, E.; Pecorini, I.; Paoli, P.; Iannelli, R. Plug-flow reactor for volatile fatty acid production from the organic fraction of municipal solid waste: Influence of organic loading rate. J. Environ. Chem. Eng. 2022, 10, 106963. [Google Scholar] [CrossRef]
- Baldi, F.; Pecorini, I.; Iannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Patinvoh, R.J.; Kalantar Mehrjerdi, A.; Sárvári Horváth, I.; Taherzadeh, M.J. Dry fermentation of manure with straw in continuous plug flow reactor: Reactor development and process stability at different loading rates. Bioresour. Technol. 2017, 224, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massé, D.I.; Gilbert, Y.; Saady, N.M.C.; Liu, C. Low-temperature anaerobic digestion of swine manure in a plug-flow reactor. Environ. Technol. 2013, 34, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Chinellato, G.; Battista, F.; Bolzonella, D.; Cavinato, C. Single-phase anaerobic digestion of the organic fraction of municipal solid waste without dilution: Reactor stability and process performance of small, decentralised plants. Waste Manag. 2021, 125, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, Z.; Feng, J.; Zhao, L.; Yu, J. Effects of digestate recirculation ratios on biogas production and methane yield of continuous dry anaerobic digestion. Bioresour. Technol. 2020, 316, 123963. [Google Scholar] [CrossRef] [PubMed]
- Agenzia Nazionale per Protezione dell’Ambiente. Metodi di Analisi del Compost—Manuale; ANPA: Rome, Italy, 2001. [Google Scholar]
- Pecorini, I.; Baldi, F.; Carnevale, E.A.; Corti, A. Biochemical methane potential tests of different autoclaved and microwaved lignocellulosic organic fractions of municipal solid waste. Waste Manag. 2016, 56, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, I.; Iannelli, R. Characterization of Excavated Waste of Different Ages in View of Multiple Resource Recovery in Landfill Mining. Sustainability 2020, 12, 1780. [Google Scholar] [CrossRef] [Green Version]
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved Alkalimetric Monitoring for Anaerobic Digestion of High-Strength Wastes. J. (Water Pollut. Control. Fed.) 1986, 58, 406–411. [Google Scholar]
- Ente Nazionale Italiano di Unificazione. Ammendanti e Substrati di Coltivazione—Determinazione Dell’attività Biologica Aerobica—Parte 1: Tasso di Assorbimento Dell’ossigeno (OUR); Ente Nazionale Italiano di Unificazione: Rome, Italy, 2020. [Google Scholar]
- Ente Nazionale Italiano di Unificazione. Metodo per la Misura della Produzione Potenziale di Metano da Digestione Anaerobica ad Umido-Matrici in Alimentazione; Ente Nazionale Italiano di Unificazione: Rome, Italy, 2018. [Google Scholar]
- Baldi, F.; Iannelli, R.; Pecorini, I.; Polettini, A.; Pomi, R.; Rossi, A. Influence of the pH control strategy and reactor volume on batch fermentative hydrogen production from the organic fraction of municipal solid waste. Waste Manag. Res. 2019, 37, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Cesaro, A.; Belgiorno, V. Anaerobic digestion of mechanically sorted organic waste: The influence of storage time on the energetic potential. Sustain. Chem. Pharm. 2021, 20, 100373. [Google Scholar] [CrossRef]
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the variability of food waste quality: A need for efficient valorisation through anaerobic digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, R.; González-Martínez, S. Influence of process parameters on the extraction of soluble substances from OFMSW and methane production. Waste Manag. 2017, 62, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; Khanal, S.K. (Ed.) Wiley-Blackwell: Ames, IA, USA, 2008; ISBN 978-0-8138-2346-1. [Google Scholar]
- Drosg, B. Process Monitoring in Biogas Plants; IEA bioenergy: Paris, France, 2013; pp. 1–38. [Google Scholar]
- Veluchamy, C.; Gilroyed, B.H.; Kalamdhad, A.S. Process performance and biogas production optimizing of mesophilic plug flow anaerobic digestion of corn silage. Fuel 2019, 253, 1097–1103. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2018, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Lossie, U.; Pütz, P. Targeted Control of Biogas Plants with the Help of FOS/TAC; Practice Report; Hach-Lange: Loveland, CO, USA, 2008. [Google Scholar]
- Arias, D.E.; Veluchamy, C.; Habash, M.B.; Gilroyed, B.H. Biogas production, waste stabilization efficiency, and hygienization potential of a mesophilic anaerobic plug flow reactor processing swine manure and corn stover. J. Environ. Manag. 2021, 284, 112027. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, B.K. Dry Continuous Anaerobic Digestion of Municipal Solid Waste in Thermophilic Conditions. Master’s Thesis, Asian Institute of Technology, School of Environment, Resources and Development, Khlong Nueng, Thailand, 2008. [Google Scholar]
- Kuglarz, M.; Karakashev, D.; Angelidaki, I. Microwave and thermal pretreatment as methods for increasing the biogas potential of secondary sludge from municipal wastewater treatment plants. Bioresour. Technol. 2013, 134, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Wen, Z.; Huang, S.; De Clercq, D. Mechanical biological treatment of municipal solid waste: Energy efficiency, environmental impact and economic feasibility analysis. J. Clean. Prod. 2018, 178, 731–739. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, L.; Nithya, K.; Loh, K.-C.; Dai, Y.; He, Y.; Wah Tong, Y. Optimizing mixing strategy to improve the performance of an anaerobic digestion waste-to-energy system for energy recovery from food waste. Appl. Energy 2019, 249, 28–36. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
| Reactor Type (Wv) | Substrate (C/N) | TS [%] | HRT (d) | T (°C) | FA (mg/L) | TAN (mg/L) | SMP (NLCH4/gVS) | Strategy to Reduce Ammonia Inhibition | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CSTR (4 L) | Source segregated domestic waste (C/N = 16.5) | 23.9 | n.n | 55 | 700–1100 | 2500 | Digester failure | - °° | [31] |
| Vegetables, fruits, meat, dairy products, and bread (C/N = 46.6) | 22.5 | 55 | 700–1000 | 1000–2000 | 0.39 | ||||
| CSTR (4 L) | Source segregated domestic waste | n.a | 75 | 55 | n.a. | 5000 | 0.39 | Dilution W/S = 0:1 | [30] |
| 3100 | 0.44 | Dilution W/S = 0.5:1 | |||||||
| 25 | 1500 | Dilution W/S = 2:1 | |||||||
| 500 | 2300 | n.a. | Dilution W/S = 1:1 | ||||||
| CSTR (2 L) | Sewage sludge | 7–8 | 20 | 55 | 310 | 2170 | 0.33 | - | [28] |
| 9–10 | 350 | >2500 | Digester failure | ||||||
| 220 | 1720 | n.a. | Ammonia stripping of digestate | ||||||
| CSTR (35 L) | Food waste (C/N = 13.6) | 23.8 | n.a. | 55 | n.a. | <2500 | 0.5 | Ammonia stripping of digestate = 2.1 kg of digestate twice a week | [29] |
| 1000–2000 | Ammonia stripping of digestate = 2.5 kg of digestate twice a week | ||||||||
| 1500 | Ammonia stripping of digestate = 5 kg of digestate twice a week |
| Unit of Measurement | Inoculum * | OFMSW * | GW * | |
|---|---|---|---|---|
| pH | - | 7.26 ± 0.36 | 4.4 ± 0.24 | 6.37 ± 0.3 |
| TOC | % | 8.9 ± 1.3 | 11.05 ± 1.06 | 22.56 ± 2.96 |
| Organic nitrogen | % | 0.37 ± 0.05 | 0.55 ± 0 | 1.15 ± 0.17 |
| TAN (N-NH4+) | mg/kg | 3690 ± 550 | 390 ± 395 | 266.67 ± 81.54 |
| Total alkalinity | mgCaCO3/kg | 15,800 ± 1900 | 3515 ± 21 | 3769 ± 454 |
| Solid content (TS) | % | 22.8 ± 1.6 | 26.4 ± 2.26 | 69.83 ± 4.5 |
| Volatile solid (VS) | % | 17.12 ± 0.4 | 20.71 ± 2.1 | 51.03 ± 3.16 |
| Ash | % | 5.68 ± 0.4 | 5.68 ± 0.16 | 23.19 ± 1.37 |
| C | % °° | 39.03 ± 1.1 | 41.85 ± 4.01 | 33.05 ± 8.89 |
| H | % °° | 4.91 ± 0.11 | 5.66 ± 0.08 | 4.37 ± 0.4 |
| N ** | % °° | 2.91 ± 0.1 | 2.34 ± 0.1 | 1.66 ± 0.2 |
| S | % °° | 0.19 ± 0.08 | 0.21 ± 0.04 | 0.68 ± 0.12 |
| C/N | - | 13.38 ± 1.3 | 18.1 ± 2.68 | 20.83 ± 1.53 |
| Lignin | % VS | 40.3 ± 2.8 | 16.65 ± 2.38 | 34.51 ± 3.04 |
| Cellulose | % VS | 22.78 ± 1.81 | 22.2 ± 2.73 | 26.31 ± 2.03 |
| Carbohydrates ° | % VS | 22.19 ± 2.68 | 36.03 ± 2.49 | 27.32 ± 2.46 |
| Proteins | % VS | 10.51 ± 1.86 | 17.37 ± 2.04 | 14.08 ± 2.35 |
| Fats, oil, and grease | % VS | 0.81 ± 0.05 | 2.09 ± 0.23 | 0.69 ± 0.11 |
| Aluminum | mg/kg | 550 ± 160 | 610 ± 226 | 550 ± 432 |
| Calcium | mg/kg | 12,100 ± 1500 | 6600 ± 141 | 5962 ± 3605 |
| Magnesium | mg/kg | 940 ± 280 | 785 ± 190 | 528 ± 633 |
| Manganese | mg/kg | 58 ± 19 | 32 ± 7.07 | 43 ± 66 |
| Potassium | mg/kg | 2990 ± 450 | 2425 ± 7 | 1563 ± 1026 |
| Sodium | mg/kg | 1380 ± 450 | 1045 ± 77 | 268 ± 170 |
| Arsenic | mg/kg °° | <RL | <RL ± <RL | 0.17 ± 1.31 |
| Cadmium | mg/kg °° | 4.38 ± <RL | <RL ± <RL | - |
| Chrome | mg/kg °° | 131.57 ± 19.73 | 22.72 ± 5.35 | 40.05 ± 53.93 |
| Mercury | mg/kg °° | <RL ± <RL | <RL ± <RL | - |
| Nickel | mg/kg °° | 83.33 ± 12.71 | 7.57 ± <RL | 8.81 ± 14.39 |
| Copper | mg/kg °° | 65.78 ± 10.08 | 28.4 ± 8.03 | 28.05 ± 52.33 |
| Zinc | mg/kg °° | 122.8 ± 18.42 | 115.53 ± 13.39 | 38.46 ± 108.3 |
| Iron | mg/kg °° | 4666 ± 425 | 3308 ± 254 | 2198.39 ± 4642.36 |
| S1 | S2 | S3 | |
|---|---|---|---|
| pHout (-) | 8.41 ± 0.12a | 8.32 ± 0.1b | 8.11 ± 0.08c |
| pH1 (-) | 8.41 ± 0.03a | 8.63 ± 0.05b | 8.35 ± 0.09a |
| pH2 (-) | 8.51 ± 0.03a | 8.74 ± 0.07b | 8.44 ± 0.12a |
| T1 (°C) | 53.56 ± 0.31a | 53.08 ± 3.78b | 52.75 ± 0.36a |
| T2 (°C) | 53.36 ± 0.28a | 52.82 ± 3.72b | 52.47 ± 0.34a |
| TA (mgCaCO3/L) | 20,037 ± 660 | 21,130 ± 406 | 16,011 ± 50 |
| IA (mgCaCO3/L) | 6922 ± 324 | 7555 ± 391 | 5320 ± 220 |
| IA/PA (-) | 0.52 ± 0.03 | 0.55 ± 0.03 | 0.49 ± 0.03 |
| Total VFAs (mg/L) | 394 ± 210a | 1162 ± 244b | 3345 ± 81c |
| TAN (mg/L) 1 | 2220 ± 80 | 2711 ± 346 | 631 ± 211 |
| FA (mg/L) | 1151 ± 173 | 1196 ± 220 | 193 ± 94 |
| CH4 (%) | 62.63 ± 2.61a | 64.34 ± 2.19a | 64.97 ± 2.73b |
| CO2 (%) | 37.2 ± 2.59a | 35.65 ± 2.19a | 35.02 ± 2.73b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, E.; Pecorini, I.; Ferrara, G.; Iannelli, R. Dry Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste: Biogas Production Optimization by Reducing Ammonia Inhibition. Energies 2022, 15, 5515. https://doi.org/10.3390/en15155515
Rossi E, Pecorini I, Ferrara G, Iannelli R. Dry Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste: Biogas Production Optimization by Reducing Ammonia Inhibition. Energies. 2022; 15(15):5515. https://doi.org/10.3390/en15155515
Chicago/Turabian StyleRossi, Elena, Isabella Pecorini, Giovanni Ferrara, and Renato Iannelli. 2022. "Dry Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste: Biogas Production Optimization by Reducing Ammonia Inhibition" Energies 15, no. 15: 5515. https://doi.org/10.3390/en15155515
APA StyleRossi, E., Pecorini, I., Ferrara, G., & Iannelli, R. (2022). Dry Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste: Biogas Production Optimization by Reducing Ammonia Inhibition. Energies, 15(15), 5515. https://doi.org/10.3390/en15155515









