A Review on Deactivation and Regeneration of Catalysts for Dimethyl Ether Synthesis
Abstract
:1. Introduction
2. Methods for DME Synthesis
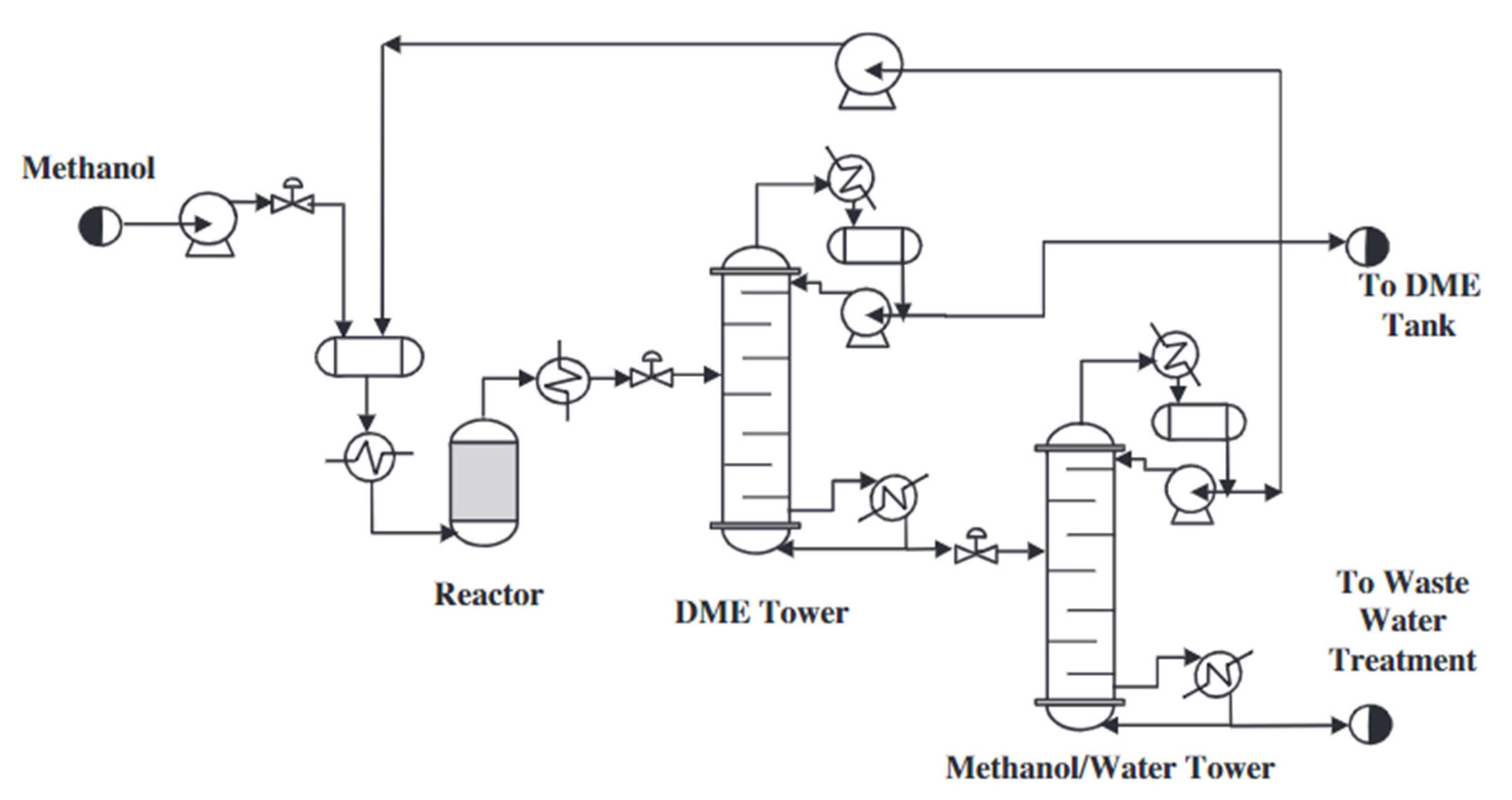
2.1. Indirect Method of Dimethyl Ether Synthesis
- (a)
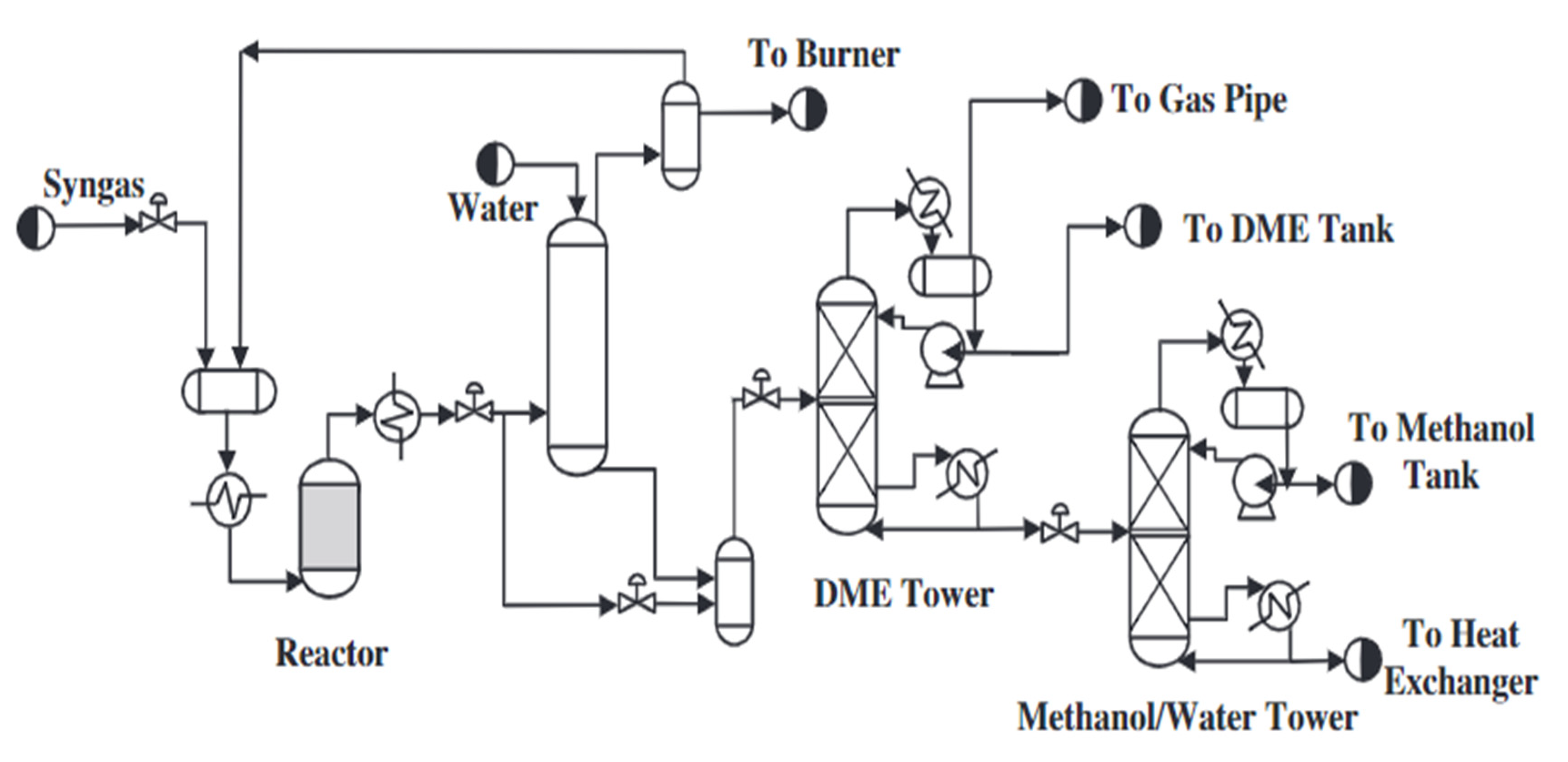
- Direct method of dimethyl ether synthesis
2.2. Synthesis of Dimethyl Ether from Methane
3. Structure and Modifications of DME Catalysts
4. Characterisation Results Indicated Coke Formation
- (a)
- Thermogravimetric analysis—TGA
- (b)
- XPS
- (c)
- XAFS
- (d)
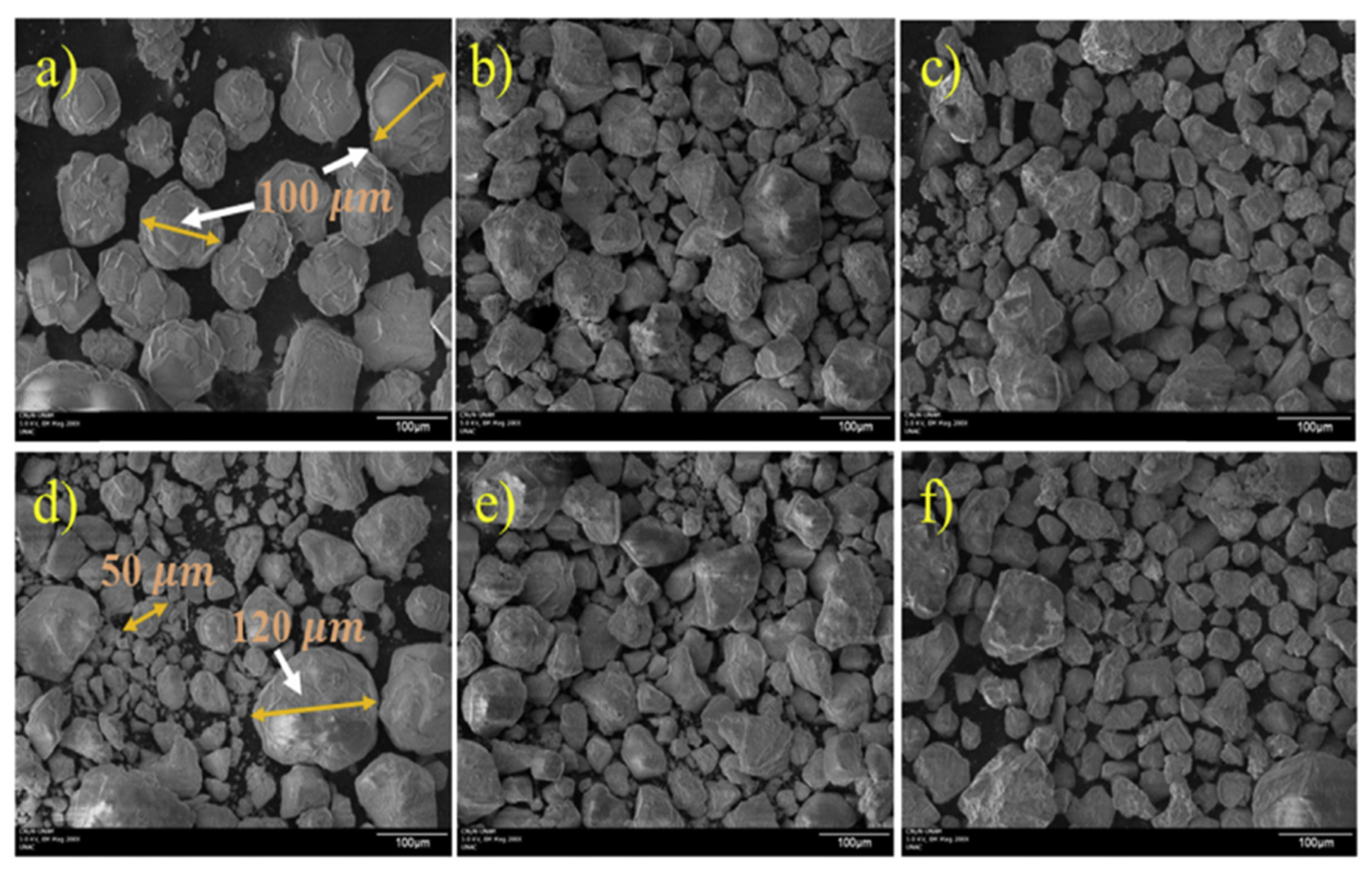
- SEM
- (e)
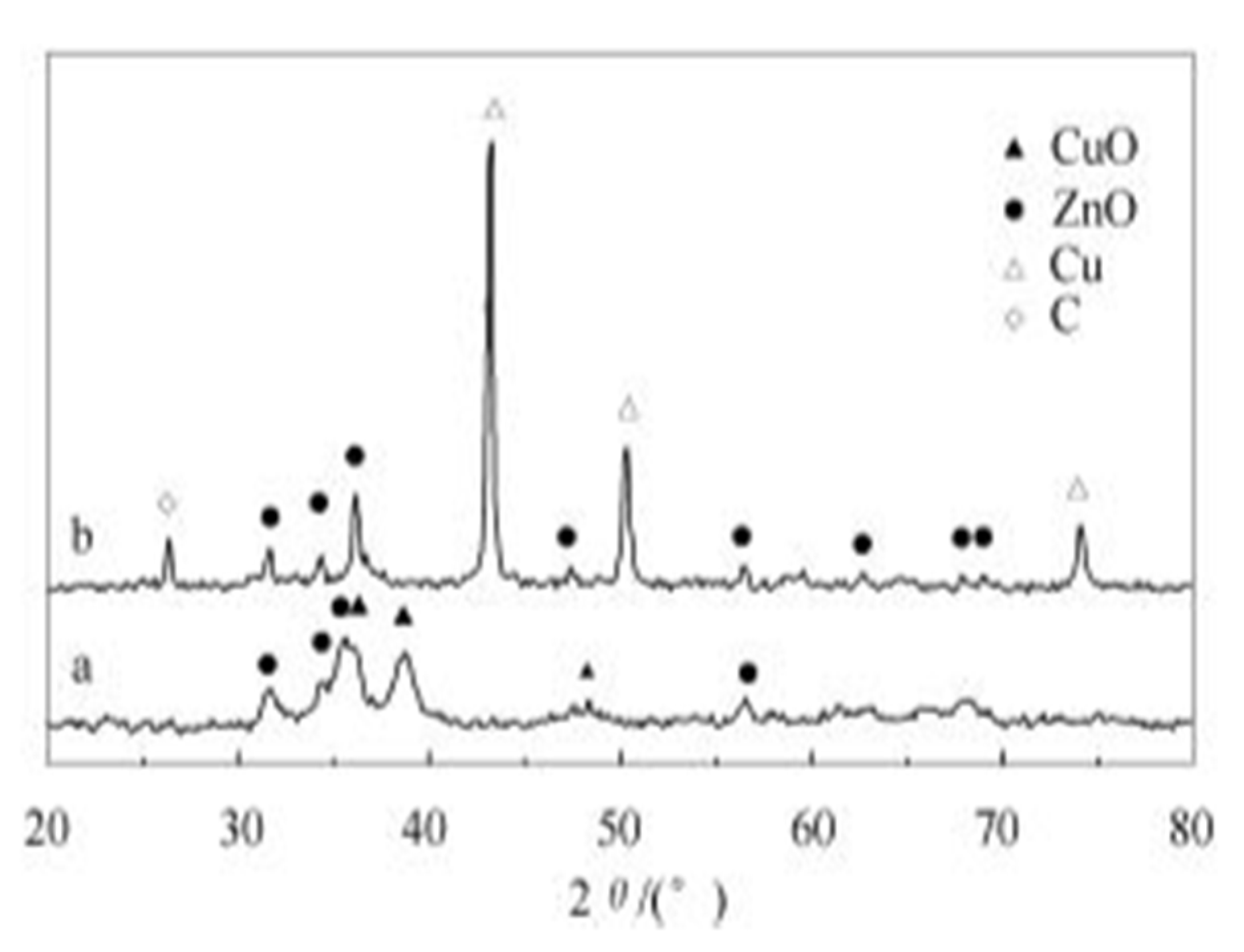
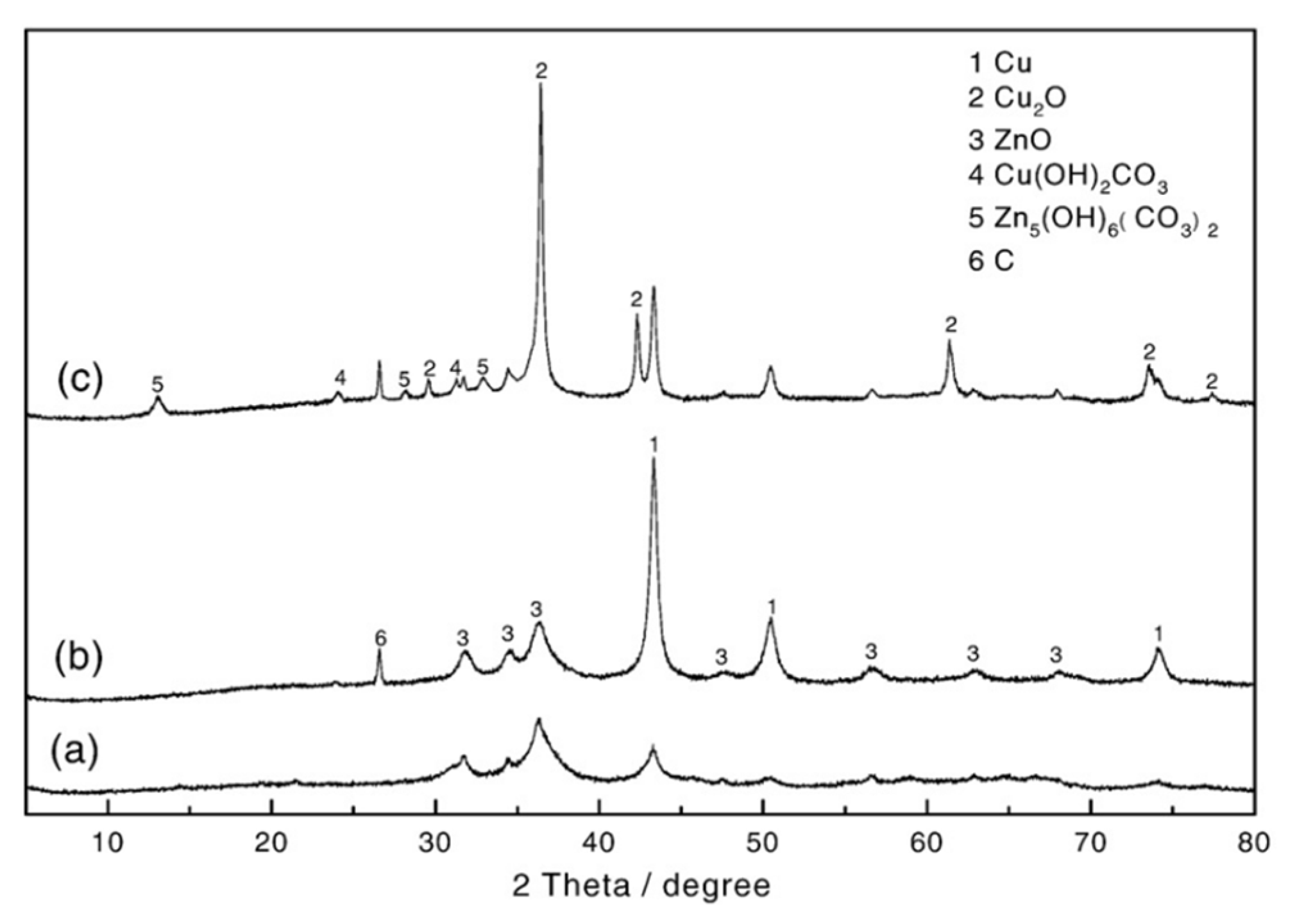
- XRD
- (f)
- In situ methods
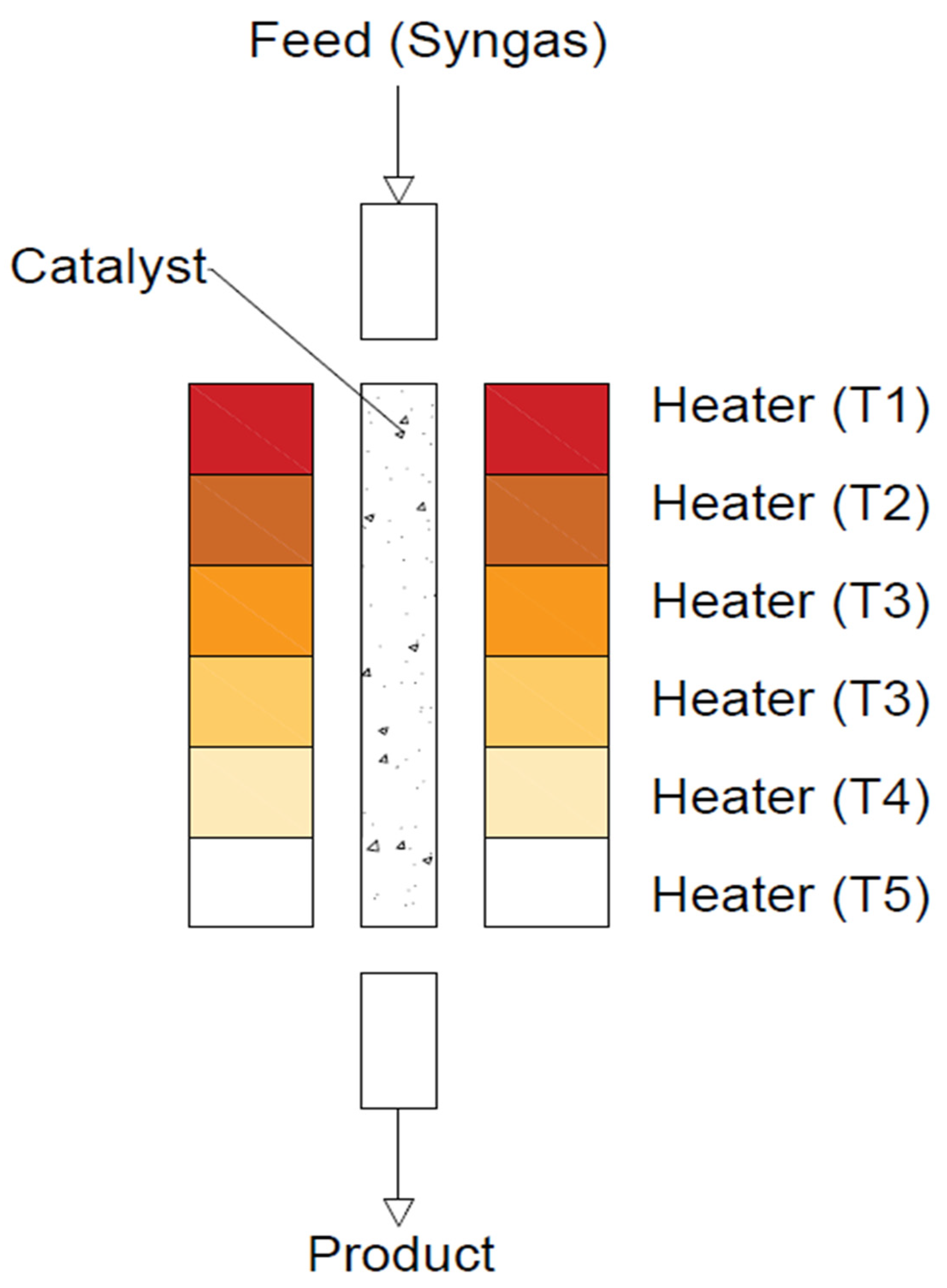
5. Reactors Configurations for DME Synthesis
- I.
- Conventional reactors
- (a)
- Fixed-bed reactor
- (b)
- Fluidised bed reactor
- (c)
- Isothermal adiabatic reactor
- (d)
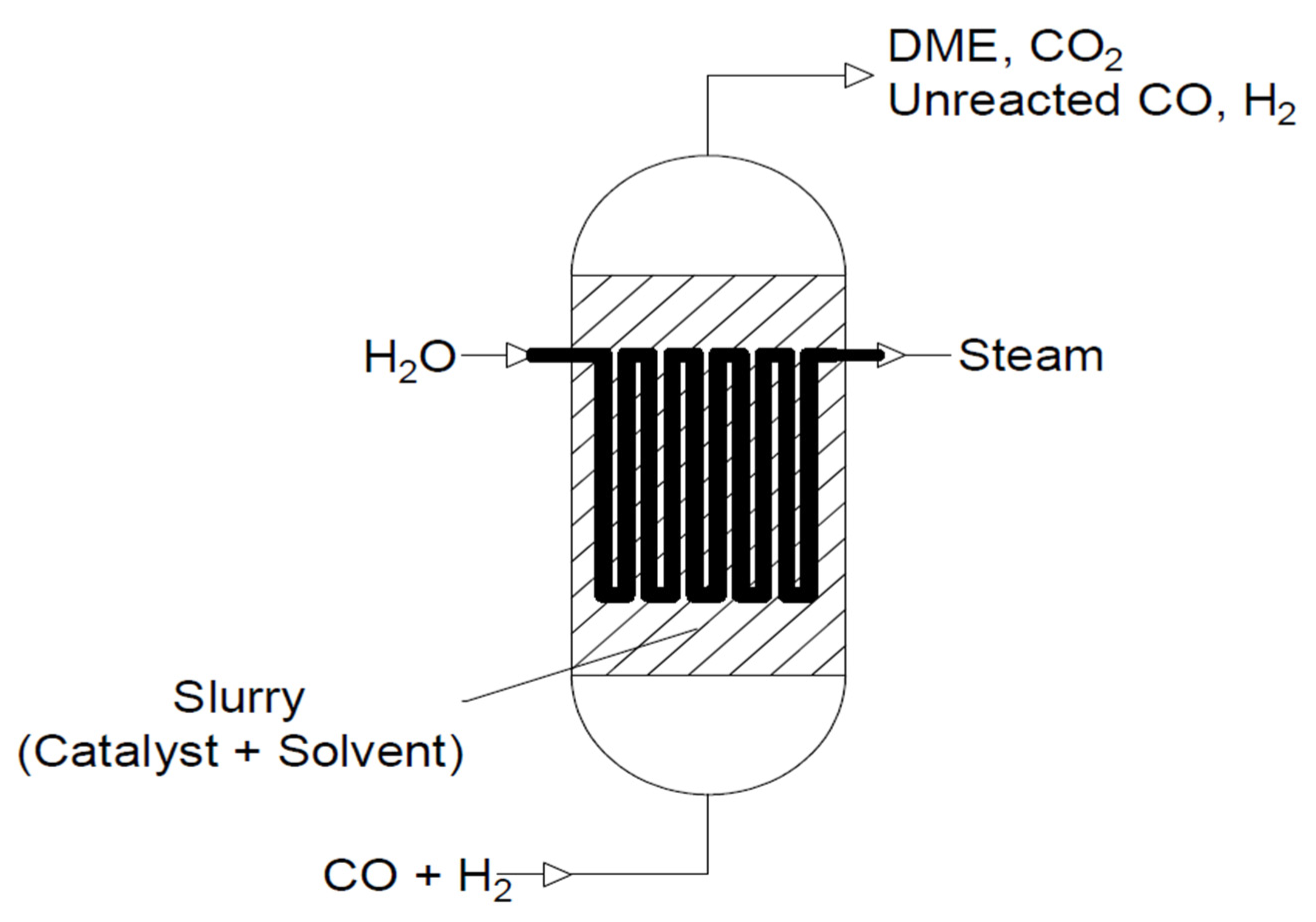
- Slurry reactors
6. Reasons for Catalysts Deactivation—Fixed Bed and Slurry Reactors
- (A)
- Fixed-bed reactors
| Catalyst | Ratio of Reagents | P (MPa) | T (°C) | Conclusions Regarding Deactivation | Ref. |
|---|---|---|---|---|---|
| CZA/γ-Al2O3 | CO:H2 = 1:1 | 40 | 245 | Catalyst deactivation strongly depends on the pressure and temperature of the process. | [105] |
| CZA/H-ZSM-5, core-shell structure SiO2/Al2O3 = 20.5–50.0 | CO2/H2 = 1:3 | 3.0 | 270 | Formation of coke was observed on the acid surface of the catalyst. | [77] |
| CZA/mesoporous alumina | H2/CO/CO2 = 50/10/40 | 50.0 | 275 | At 275 °C, the DME yield was 55%. The deactivation of the catalyst during the dehydration of methanol is influenced by water, which causes the catalyst to sinter. The problem with deactivation starts above 300 °C. | [106] |
| 5% Pd, 15% Zn/TiO2 and H-ZSM-5, SiO2/Al2O3 = 30, and γ-Al2O3 | CO2:H2:N2 = 1:3:1 | 2.0 | 270 | Temperatures above 270 °C caused the formation of oxygenates. The efficiency of PdZn/H-ZSM-5 catalysts is much higher compared to that of PdZn/TiO2-H-ZSM-5, which is mainly caused by the blocking of the main Bronsted acid sites. | [102] |
| CZA (20–40 mesh), commercial | H2/CO2 = 3 | 3.0 | 200 | The deactivation was caused by changes in the structure of ZnO and by the sintering of copper particles. | [80] |
| CZA/H-ZSM-5, 3:1 mass ratio, CZZA-HZSM-5, 1:1 mass ratio | H2:CO2 = 3:1 | 3.0 | 220–260 | In the case of the CZA/H-ZSM-5 catalyst, after 100 h, the CO2 conversion dropped from 26.8% to 24.0%, and the DME selectivity dropped from 17.5% to 14.3%. During the CZZA/H-ZSM-5 experiment, the methanol conversion dropped slightly from 20.9% to 20.4%, and the DME yield dropped from 13.0% to 12.2%. The main cause of catalyst deactivation was water, which affected coke formation on the H-ZSM5 surface due to the high methanol content of the CZZA layer | [103] |
| CZA/HSM-5, 52–65% CuO, 20–30% ZnO and 8–10% Al2O3. H-ZSM-5, and SiO2/Al2O3 = 40 | H2:CO = 1:1 | 3.0 | 230 | Two methods of catalyst reduction were used in this process. The difference was the reduction temperature. In method 1, it was 230 °C, and in method 2, it was 170 °C. In addition, in method 1, reduction was used for H2, and in the case of method 2, the catalyst was reduced with a mixture of H2/N2. The deactivation was associated with the sintering of copper particles and coking of the acid part. It has been shown that reduction with pure hydrogen causes faster catalyst deactivation due to temperature changes during the copper ions’ reduction, leading to sintering and, thus, the formation of larger Cu clusters. | [107] |
| CZA = 6:3:1, γ-Al2O3, NH4ZSM-5 SiO2/Al2O3 = 80, NH4ZSM-5 SiO2/Al2O3 = 23, HZSM-5 SiO2/Al2O3 = 80, HZSM-5 SiO2/Al2O3 = 23 10% Ag-γ-Al2O3, and η-Al2O3 CZA/ZSM-5 | H2:CO = 2:1, and 1–4% of CO2 | 2 | 200–260 | The deactivation of the catalyst was due to coke, which was formed during methanol formation. Coke formation is attributed to the degradation of methoxy ions (very important for the dehydrogenating capacity of the metallic function, which will contribute to activating the condensation step) and the dehydrocyclisation and aromatic condensation steps. | [21] |
| 10% Ag-γ-Al2O3 and η-Al2O3 CZA/ZSM-5 | 0.1 | 180–300 | Deactivation of heterogeneous catalysts is the result of poisoning, vapour/solid reactions, solid/solid reactions, fouling, and vapour compound formation. The most dangerous and most common are poisoning, sintering, and fouling. | [108] | |
| CZA/ZSM-5 | H2/CO/CO2/N2 = 61/30/5/4 volume ratios | 40 | 320 | The reason for deactivation is both coke deposition in the active sites of the metallic and quasi-catalytic functions, and the second reason for deactivation is the sintering of the CZA catalyst at temperatures above 325 °C. The results indicate that the decrease in the activity of the catalyst is related to the coking of the catalyst, but, ultimately, the activity is not affected. | [61] |
- (B)
- Slurry phase reactors
- (a)
- Reaction characteristics—the type and speed of major and side transformations, the shape and size of reactants and products, and reactor type;
- (b)
- Catalyst features—type, number, location, and strength of active sites, and size and shape of pores and holes;
- (c)
- Process conditions—temperature and pressure [112].
| Catalyst | Type of Solvent | Ratio of Gases | Type of Reactor | P (MPa) | T (°C) | Results and Discussion | Ref. |
|---|---|---|---|---|---|---|---|
| CZA-γ-Al2O3 | Liquid paraffin | H2:CO:CO2 = 68%:28%:3% | Stainless-steel high-pressure reactor, inside diameter of 16 mm, and total length of 400 mm | 5.0 | 260 | Methanol synthesis catalyst (MSC) deactivated more rapidly in the slurry reactor compared with the fixed-bed reactor. The released water was the direct cause of MSC catalyst deactivation. In the slurry reactor, the solvent created additional resistance for water removal from the reaction system. | [82] |
| Mg/ZSM-5, Si/Al = 30 | Polydimethylsiloxane and silicone oil (syltherm 800), | [-] | Fixed-bed: catalyst (0.4 mm-0.6 mm fraction)/inert quartz at a ratio of 1:1, 1 g of catalyst, and slurry reactor | 1.0 | 320 | Results from two reactors were presented: fixed-bed and slurry reactor. Coke formation was slower in the slurry reactor and amounted to 1.4 mg, compared with 1.7 mg in the fixed-bed reactor. On the external catalyst surface, the weight fraction of coke was higher, at about 11%. | [94] |
| CZA/HZSM-5, Si/Al = 40 | Solvent: inert liquid medium | H2:CO = 1:1 | Fixed-bed microreactor, i.d. 10 mm, length 300 mm, and 2 g of catalyst, and slurry reactor | 3.0 | 260 | The catalyst was prepared in two different ways. Methanol penetrated much faster from the metal surface of the catalyst to the acid part. Likewise, water was consumed much faster in the water–gas shift reaction. The contact area for the second method was much smaller; therefore, the diffusion of methanol and water on the catalyst’s surface became more difficult. The deactivation of the catalyst in this process was mainly due to the sintering of copper particles. | [107] |
| Commercial H-MFI, SiO2/Al2O3 = 80 | Solvent: oils, such as a Downtherm RP hydrocarbon oil, PMS-1000, Syltherm 800 silicone oils, and pentaerythritol ester | Slurry reactor, volume 250 mL | 0.1 | 260–280 | Commercial catalyst (H-MFI) was modified with magnesium, lanthanum, zirconium, and zinc. It was found that, at temperatures above 300 °C, the dispersion liquids decomposed significantly to form light hydrocarbons. It was confirmed that the decomposition of silicone oils, especially Syltherm 800, was much lower than those of hydrocarbon oils or pentaerythritol ester. The highest degree of DME conversion was achieved using Syltherm 800 solvent. About 90% DME conversion was obtained. Inert gas (to 10–20%) to avoid rapid deactivation of catalyst was used. The influence of different solvents on the DME synthesis process was studied. The reasons for deactivation were the formation of side reactions, C1–C4 reactions especially, and the presence of water and liquid organic products. | [94] | |
| CZA, CZAMn, and MnCZA | Liquid paraffin | H2/CO = 2.1 | Slurry reactor | 5.0 | 260 | The gas molar ratio H2/CO of 2.1 proved that the addition of manganese had a positive effect on the stability of the catalyst, but only in the case of the CZAMn catalyst synthesised by the co-precipitation method. Additionally, it prevented the quick sintering of the catalyst. Ultimately, 76.5% CO conversion and 66.7% selectivity for DME were achieved. | [117] |
| CZA | Liquid paraffin | CO:H2 = 1:2 | Slurry reactor | 5.0 | 200–300 | The temperature above the Tammann point (<190) was believed to be responsible for the deactivation of the copper-containing catalysts. It was found that the CZA catalyst retained its selectivity at the level of 75–80% at the temperature of 300 °C. The declining CO conversion was due to the accumulation of water in the reaction zone. This decomposed the zinc oxide and deactivated the catalyst. | [118] |
| CZA/Al2O3 | Liquid paraffin | H2:CO = 3:1 | Slurry reactor | 5.0 | 270 | Deactivation of catalysts was faster at higher pressures. The less water, the longer the life of the bifunctional catalyst. It was found that, if the amount of water exceeded 0.16 mol.% in the feed, then this factor played a negative role in the DME yield and the catalyst’s life was shortened. | [121] |
7. Strategies for Catalyst Regeneration
- II.
- High-Tech Systems
- (a)
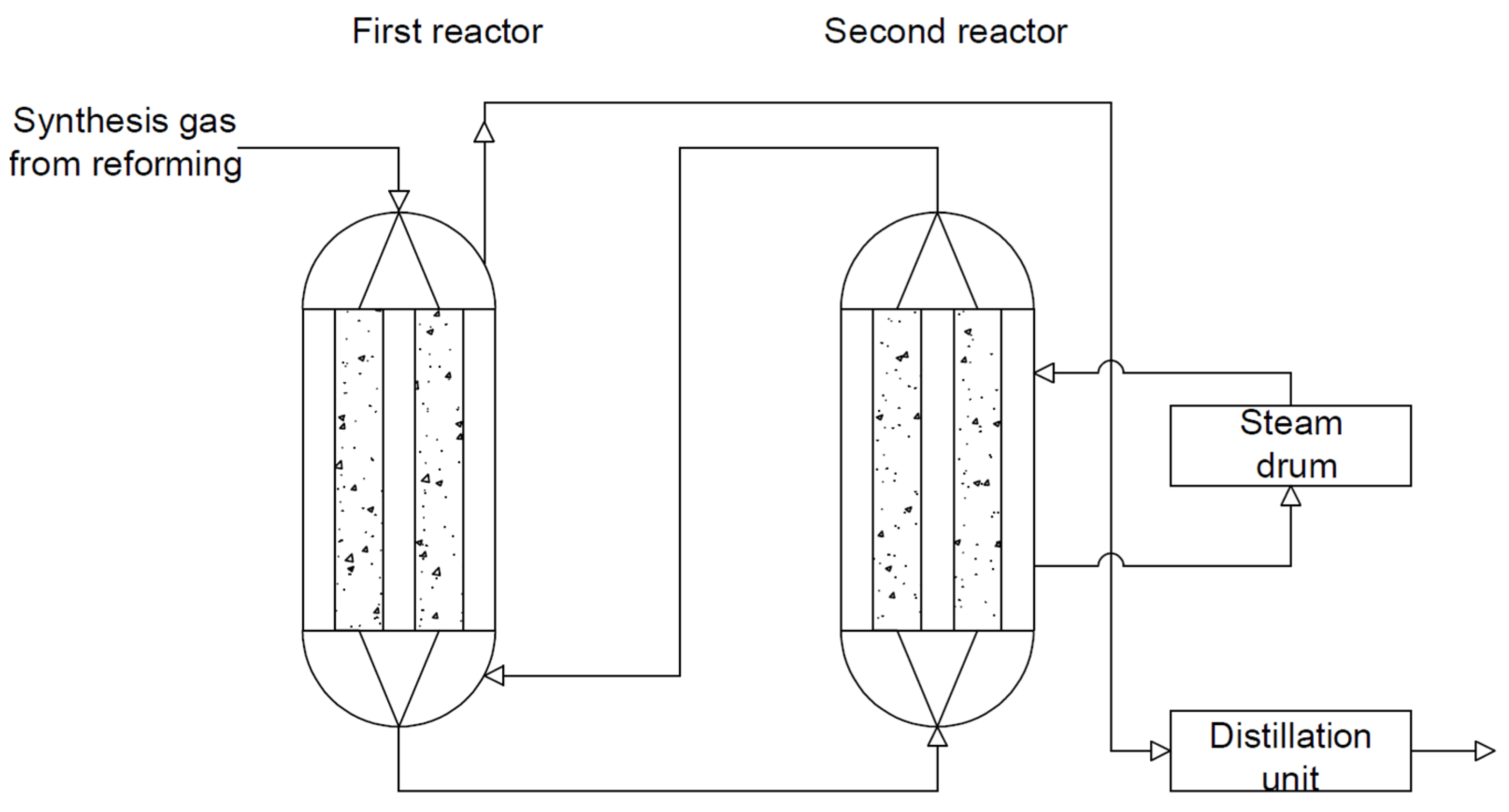
- Double-reactor system
- (b)
- Tubular membrane reactors
- (c)
- Microstructural reactors
- (d)
- Catalytic distillation
- (e)
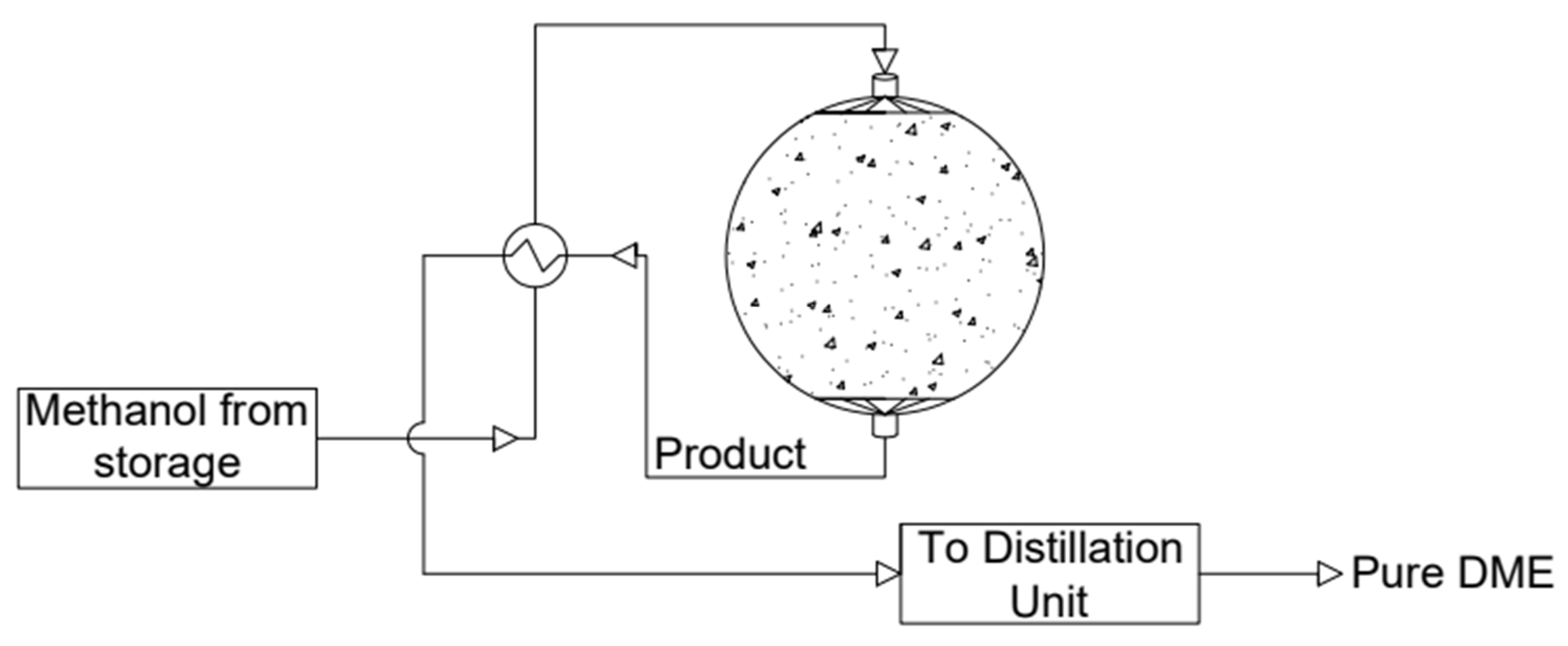
- Reactor with a spherical membrane
- (f)
- Temperature-gradient reactor
- (1)
- Restore the activity of the catalyst;
- (2)
- Use the catalyst for another process;
- (3)
- Recover and recycle the important and/or expensive catalytic components;
- (4)
- Remove the catalyst.
- (a)
- Heating at 250 °C for four cycles, with each cycle lasting 10 h;
- (b)
- Rinsing at three different temperatures of 250 °C, 300 °C, or 350 °C for three cycles.
| Catalyst | Ratio of Gases | Type of Reactor | Pressure (MPa) | T (°C) | Results and Discussion | Ref. |
|---|---|---|---|---|---|---|
| NH4-ZSM-5 | [-] | Tubular reactor | 0.1 | 300 | Regeneration was carried out with the use of air for 10 h, with a flow of 0.5l pm, pressure of 0.10 MPa, and temperature of 570 °C. The regeneration carried out in this way allowed the structure of the catalyst to remain unchanged after six regenerations. Moreover, it allowed reducing the content of aromatics from 56.6% vol. to 30.2 vol.% | [134] |
| CuO/γ-Al2O3 modified with hematite | [-] | U-type tubular reactor | 0.1 | 290 | It was concluded that hematite addition caused a decrease in by-product formation when Cu:Fe = 1:1 Regeneration was carried out in air flow at 600 °C for 2 h. After regeneration, the maximum value of conversion was 66%, and that before was 70%. | [81] |
| HZSM-5, Si/Al = 15 and 140 | [-] | Fixed-bed reactor | 0.15 | 325–400 | Oligomerisation, condensation, and aromatisation pathways had high impacts on coke formation. Temperatures of 350–550 °C were best for totally removing coke structures. This is very useful for industries that carry out processes in reactions similar to the MTO process. | [40] |
| CZZA/HZSM-5, | H2:CO2 = 3:1 | Fixed-bed reactor | 2.8 | 240 | The best temperature for regeneration was 250 °C. Higher temperatures caused losses of copper activity and irreversible losses of the active surface of copper. Even though the lowering of the coke removal temperature resulted in the preservation of copper dispersion, heavier compounds, such as oligomers and aromatics, could not be removed. They accumulated in the catalyst structure and then agglomerated into larger and heavier compounds that were increasingly hard to remove at relatively low temperatures. | [103] |
| ZSM-5/γ-Al2O3 | [-] | Fixed-bed reactor | 0.1 | 280 | The water content of the raw methanol solution reached 20%. The deactivation rate of γ-Al2O3 was approximately 12.5 times higher than that of pure methanol. They also prepared ZSM-5/γ-Al2O3/LC by liquid-phase coating, and some of the skeleton incurred desiliconisation and dealumination in the raw materials. As a consequence, the acid strength was increased by modulating the Si/Al ratio of the ZSM-5 skeleton, and the mesoporous ratio of catalysts was improved. | [137] |
| CZA/γ-Al2O3 | H2:CO2 = 4:1 | Slurry reactor | 0.4 | 275 | The coke was burnt with air flow at a temperature below 325 °C. The main cause of deactivation was coke. The experiment showed that there was no sintering at temperatures below 325 °C. | [140] |
| Cu–Fe2O4/γ-Al2O3 | [-] | Fixed-bed reactor | 0.1 | 290 | This regeneration was carried out at 600 °C for 2 h in air. In this article, there was no information about the results of this regeneration. | [25] |
| HZSM-5, Si/Al = 140 | [-] | Fixed-bed reactor | [-] | [-] | Catalyst regeneration was carried out in 10 cycles. A clear effect of the catalyst acidity and reaction conditions on the amount and composition of the coke formed was observed. An increase in the temperature and/or catalyst acidity favoured coke deposition, which was explained by the higher activity of acid sites in secondary reactions. The slight development of coke structures was explained by their complete combustion during a temperature increase from 350 to 550 °C. The catalyst completely recovered its activity, which enabled its use on an industrial scale. | [40] |
| CZA/γ-Al2O3, CZA/Na-HZSM-5 | H2/CO = 4/1 | Fixed-bed reactor | 40 | 275 | After regeneration, it was found that the CZA/γ-Al2O3 catalyst did not regenerate; consequently, the DME selectivity and yield decreased. As the amount of regeneration increased, the yield reached an equilibrium state, resulting in yield and selectivity that were half of those of the fresh catalyst. | [132] |
| CZA/γ-Al2O3-H-ZS-5, | H2/CO = 2/1 | Fixed-bed reactor | 3.0 | 220 | The best regeneration results were obtained with a 5% O2–He mixture. This mixture allows for the redispersion of copper particles, which is impossible with the other two mixtures. From the experiments carried out, it was concluded that the deactivation of the catalyst was due to the sintering of the copper particles. It was found that the copper particles could be dispersed again. | [131] |
| CZA/γ-Al2O3 | H2/CO = 3/1 | Fixed-bed reactor | 0.3 | 275 | A temperature of 325 °C was the limiting condition to avoid irreversible deactivation by the sintering of the metallic part. Regeneration with a 5% O2/He mixture allowed the complete combustion of the coke. The treatment prior to burning the coke was of particular importance, as this was intended to age the coke by unifying its structure. The fraction of coke that required a higher temperature, between 330 and 380 °C, settled on the γ-Al2O3 no-bearer and its combustion was not catalysed. It was confirmed that the combustion of coke deposited on the metal could start at temperatures above 150 °C. The phenomenon of coke heterogeneity was observed in acidic catalysts with a bi-modal pore structure, in which more eluted coke (with a lower H/C ratio) was generated in the mesopores, and its combustion was slower than that in less-eluted coke, i.e., more hydrogenated coke was generated in the micropores. | [55] |
8. Conclusions and Outlook
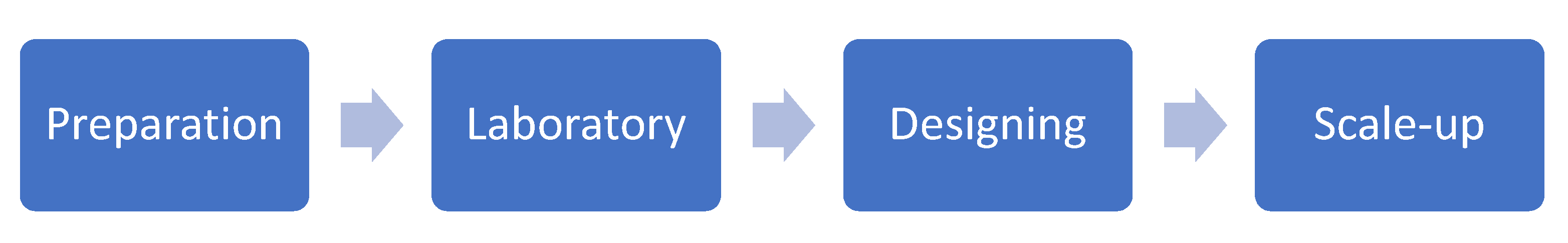
8.1. Preparation
- Preparation of a test system with strictly defined parameters—specified reactor and bed length;
- Optimisation of the operation of the system with a commercially available catalyst with known properties—achieve optimum conversion/selectivity.
8.2. Catalyst Development in Laboratory
- Modification of catalysts and testing on a test system;
- Selecting catalysts that are more advantageous than a commercial catalyst;
- Confirmation of the chosen catalysts in long-term tests (minimum 7 days);
- During research, a detailed analysis of the composition of the products and the analysis of the available technologies necessary for the separation of DME and the recycling of unreacted reagents should be carried out. Problems with the purification of the product stream may be crucial for the applicability of the technology.
8.3. Model Installation Designing
- (1)
- Preparation of a process design for a model installation, including flow calculations, mass and energy exchange, technological diagrams, and a list of apparatus and devices;
- (2)
- Designing the automation and analytics system for the model installation; Scale-up
- (3)
- Purchase, construction, and testing of a model installation on inert media—checking the tightness and stability of the system;
- (4)
- Testing the installation on a selected catalyst along with the possibility of purifying the product stream;
- (5)
- Confirmation of the catalyst’s improvement for DME synthesis and preliminary economic analysis of its introduction to the market.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl Ether: A Review of Technologies and Production Challenges. Chem. Eng. Process. Process Intensif. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Masudi, A.; Che Jusoh, N.W.; Muraza, O. Recent Progress on Low Rank Coal Conversion to Dimethyl Ether as Clean Fuel: A Critical Review. J. Clean. Prod. 2020, 277, 124024. [Google Scholar] [CrossRef]
- Sousa-Aguiar, E.F.; Appel, L.G.; Mota, C. Natural Gas Chemical Transformations: The Path to Refining in the Future. Catal. Today 2005, 101, 3–7. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Catalysis Chemistry of Dimethyl Ether Synthesis. ACS Catal. 2014, 4, 3346–3356. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Ezhova, N.N.; Yashina, O.V. Catalysis in the Dispersed Phase: Slurry Technology in the Synthesis of Dimethyl Ether (Review). Pet. Chem. 2017, 57, 553–570. [Google Scholar] [CrossRef]
- Fei, J.; Hou, Z.; Zhu, B.; Lou, H.; Zheng, X. Synthesis of Dimethyl Ether (DME) on Modified HY Zeolite and Modified HY Zeolite-Supported Cu-Mn-Zn Catalysts. Appl. Catal. A Gen. 2006, 304, 49–54. [Google Scholar] [CrossRef]
- Ahmad, R.; Schrempp, D.; Behrens, S.; Sauer, J.; Döring, M.; Arnold, U. Zeolite-Based Bifunctional Catalysts for the Single Step Synthesis of Dimethyl Ether from CO-Rich Synthesis Gas. Fuel Process. Technol. 2014, 121, 38–46. [Google Scholar] [CrossRef]
- Laugel, G.; Nitsch, X.; Ocampo, F.; Louis, B. Methanol Dehydration into Dimethylether over ZSM-5 Type Zeolites: Raise in the Operational Temperature Range. Appl. Catal. A Gen. 2011, 402, 139–145. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Coking and Deactivation of Zeolites Influence of the Pore Structure. Appl. Catal. 1989, 54, 1–27. [Google Scholar] [CrossRef]
- Moradi, G.R.; Ahmadpour, J.; Yaripour, F. Intrinsic Kinetics Study of LPDME Process from Syngas over Bi-Functional Catalyst. Chem. Eng. J. 2008, 144, 88–95. [Google Scholar] [CrossRef]
- Joelsson, J.M.; Gustavsson, L. Reductions in greenhouse gas emissions and oil use by DME (di-methyl ether) and FT (Fischer-Tropsch) diesel production in chemical pulp mills. Energy 2012, 39, 363–374. [Google Scholar] [CrossRef]
- Mao, D.; Xia, J.; Chen, Q.; Lu, G. Highly Effective Conversion of Syngas to Dimethyl Ether over the Hybrid Catalysts Containing High-Silica HMCM-22 Zeolites. Catal. Commun. 2009, 10, 620–624. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.; Wang, W.; Xiao, Y.P.; Liu, C.; Yang, F. Solid-Solid Reaction of CuFe2O4 with C in Chemical Looping System: A Comprehensive Study. Fuel 2020, 267, 117163. [Google Scholar] [CrossRef]
- Pinkaew, K.; Yang, G.; Vitidsant, T.; Jin, Y.; Zeng, C.; Yoneyama, Y.; Tsubaki, N. A New Core-Shell-like Capsule Catalyst with SAPO-46 Zeolite Shell Encapsulated Cr/ZnO for the Controlled Tandem Synthesis of Dimethyl Ether from Syngas. Fuel 2013, 111, 727–732. [Google Scholar] [CrossRef]
- Phienluphon, R.; Pinkaew, K.; Yang, G.; Li, J.; Wei, Q.; Yoneyama, Y.; Vitidsant, T.; Tsubaki, N. Designing Core (Cu/ZnO/Al2O3)-Shell (SAPO-11) Zeolite Capsule Catalyst with a Facile Physical Way for Dimethyl Ether Direct Synthesis from Syngas. Chem. Eng. J. 2015, 270, 605–611. [Google Scholar] [CrossRef]
- Grigor’eva, N.G.; Filippova, N.A.; Agliullin, M.R.; Kutepov, B.I.; Narender, N. Crystalline and amorphous aluminosilicates with different pore structures for the synthesis of pyridines. J. Chem. Res. 2017, 41, 253–261. [Google Scholar] [CrossRef]
- Maity, A.; Chaudhari, S.; Titman, J.J.; Polshettiwar, V. Catalytic Nanosponges of Acidic Aluminosilicates for Plastic Degradation and CO2 to Fuel Conversion. Nat. Commun. 2020, 11, 3828. [Google Scholar] [CrossRef]
- Zha, F.; Ding, J.; Chang, Y.; Ding, J.; Wang, J.; Ma, J. Cu-Zn-al Oxide Cores Packed by Metal-Doped Amorphous Silica-Alumina Membrane for Catalyzing the Hydrogenation of Carbon Dioxide to Dimethyl Ether. Ind. Eng. Chem. Res. 2012, 51, 345–352. [Google Scholar] [CrossRef]
- Palčić, A.; Jaén, S.N.; Wu, D.; Cai, M.; Liu, C.; Pidko, E.A.; Khodakov, A.Y.; Ordomsky, V.; Valtchev, V. Embryonic Zeolites for Highly Efficient Synthesis of Dimethyl Ether from Syngas. Microporous Mesoporous Mater. 2021, 322, 111138. [Google Scholar] [CrossRef]
- Bahadori, F.; Oshnuie, M.N. Exergy Analysis of Indirect Dimethyl Ether Production Process. Sustain. Energy Technol. Assess. 2019, 31, 142–145. [Google Scholar] [CrossRef]
- Abu-Dahrieh, J.; Rooney, D.; Goguet, A.; Saih, Y. Activity and Deactivation Studies for Direct Dimethyl Ether Synthesis Using CuO-ZnO-Al2O3 with NH4ZSM-5, HZSM-5 or γ-Al2O3. Chem. Eng. J. 2012, 203, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Inoue, N.; Shikada, T.; Ohno, Y. Direct Dimethyl Ether Synthesis. J. Nat. Gas Chem. 2003, 12, 219–227. [Google Scholar]
- Yagi, H.; Ohno, Y.; Inoue, N.; Okuyama, K.; Aoki, S. Slurry Phase Reactor Technology for DME Direct Synthesis. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent Progress for Direct Synthesis of Dimethyl Ether from Syngas on the Heterogeneous Bifunctional Hybrid Catalysts. Appl. Catal. B Environ. 2017, 217, 494–522. [Google Scholar] [CrossRef]
- Mota, N.; Ordoñez, E.M.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Direct Synthesis of Dimethyl Ether from Co2: Recent Advances in Bifunctional/Hybrid Catalytic Systems. Catalysts 2021, 11, 411. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Studt, F.; Abild-Pedersen, F.; Schlögl, R.; Behrens, M. Hydrogenation of CO2 to Methanol and CO on Cu/ZnO/Al2O3: Is There a Common Intermediate or Not? J. Catal. 2015, 328, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Vargheese, V.; Kobayashi, Y.; Oyama, S.T. The Direct Partial Oxidation of Methane to Dimethyl Ether over Pt/Y2O3 Catalysts Using an NO/O2 Shuttle. Angew. Chem. Int. Ed. 2020, 59, 16644–16650. [Google Scholar] [CrossRef]
- Vargheese, V.; Murakami, J.; Bando, K.K.; Tyrone Ghampson, I.; Yun, G.N.; Kobayashi, Y.; Ted Oyama, S. The Direct Molecular Oxygen Partial Oxidation of CH4 to Dimethyl Ether without Methanol Formation over a Pt/Y2O3 Catalyst Using an NO/NO2 Oxygen Atom Shuttle. J. Catal. 2020, 389, 352–365. [Google Scholar] [CrossRef]
- Atkins, M.P.; Earle, M.J.; Seddon, K.R.; Swadźba-Kwaśny, M.; Vanoye, L. Selective Homogeneous Synthesis of Dimethyl Ether from Methanol. Chem. Commun. 2010, 46, 1745–1747. [Google Scholar] [CrossRef]
- Grzybowska-Świerkosz, B. Elementy Katalizy Heterogenicznej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1993; pp. 45–48, 163–166. ISBN 83-01-10511-9. [Google Scholar]
- Wen, Z.; Wang, C.; Wei, J.; Sun, J.; Guo, L.; Ge, Q.; Xu, H. Isoparaffin-Rich Gasoline Synthesis from DME over Ni-Modified HZSM-5. Catal. Sci. Technol. 2016, 6, 8089–8097. [Google Scholar] [CrossRef]
- Xu, M.; Lunsford, J.H.; Goodman, D.W.; Bhattacharyya, A. Synthesis of Dimethyl Ether (DME) from Methanol over Solid-Acid Catalysts; Elsevier: Amsterdam, The Netherlands, 1997; Volume 149. [Google Scholar]
- Liuzzi, D.; Peinado, C.; Peña, M.A.; van Kampen, J.; Boon, J.; Rojas, S. Increasing Dimethyl Ether Production from Biomass-Derived Syngas: Via Sorption Enhanced Dimethyl Ether Synthesis. Sustain. Energy Fuels 2020, 4, 5674–5681. [Google Scholar] [CrossRef]
- Khandan, N.; Kazemeini, M.; Aghaziarati, M. Determining an Optimum Catalyst for Liquid-Phase Dehydration of Methanol to Dimethyl Ether. Appl. Catal. A Gen. 2008, 349, 6–12. [Google Scholar] [CrossRef]
- Samimi, F.; Bayat, M.; Rahimpour, M.R.; Keshavarz, P. Mathematical Modeling and Optimization of DME Synthesis in Two Spherical Reactors Connected in Series. J. Nat. Gas Sci. Eng. 2014, 17, 33–41. [Google Scholar] [CrossRef]
- Long, X.; Song, Y.H.; Liu, Z.T.; Liu, Z.W. Insights into the Long-Term Stability of the Magnesia Modified H-ZSM-5 as an Efficient Solid Acid for Steam Reforming of Dimethyl Ether. Int. J. Hydrogen Energy 2019, 44, 21481–21494. [Google Scholar] [CrossRef]
- Li, H.; He, S.; Ma, K.; Wu, Q.; Jiao, Q.; Sun, K. Micro-Mesoporous Composite Molecular Sieves H-ZSM-5/MCM-41 for Methanol Dehydration to Dimethyl Ether: Effect of SiO2/Al2O3ratio in H-ZSM-5. Appl. Catal. A Gen. 2013, 450, 152–159. [Google Scholar] [CrossRef]
- Alharbi, W.; Kozhevnikova, E.F.; Kozhevnikov, I.v. Dehydration of Methanol to Dimethyl Ether over Heteropoly Acid Catalysts: The Relationship between Reaction Rate and Catalyst Acid Strength. ACS Catal. 2015, 5, 7186–7193. [Google Scholar] [CrossRef]
- Budiman, A.; Ridwan, M.; Min, S.; Choi, J.; Won, C.; Ha, J.; Jin, D.; Suh, Y. Applied Catalysis A: General Design and Preparation of High-Surface-Area Cu/ZnO/Al2O3 Catalysts Using a Modified Co-Precipitation Method for the Water-Gas Shift Reaction. Appl. Catal. A Gen. 2013, 462–463, 220–226. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Ateka, A.; Pérez-Uriarte, P.; Castaño, P.; Aguayo, A.T.; Bilbao, J. Insight into the Deactivation and Regeneration of HZSM-5 Zeolite Catalysts in the Conversion of Dimethyl Ether to Olefins. Ind. Eng. Chem. Res. 2018, 57, 13689–13702. [Google Scholar] [CrossRef]
- Wang, L.; Huang, L.; Liang, F.; Liu, S.; Wang, Y.; Zhang, H. Preparation, Characterization and Catalytic Performance of Single-Atom Catalysts. Chin. J. Catal. 2017, 38, 1528–1539. [Google Scholar] [CrossRef]
- An, W.; Chuang, K.T.; Sanger, A.R. Dehydration of Methanol to Dimethyl Ether by Catalytic Distillation. Can. J. Chem. Eng. 2004, 82, 948–955. [Google Scholar] [CrossRef]
- Tokay, K.C.; Dogu, T.; Dogu, G. Dimethyl Ether Synthesis over Alumina Based Catalysts. Chem. Eng. J. 2012, 184, 278–285. [Google Scholar] [CrossRef]
- Velázquez-Herrera, F.D.; Fetter, G.; Rosato, V.; Pereyra, A.M.; Basaldella, E.I. Effect of Structure, Morphology and Chemical Composition of Zn-Al, Mg/Zn-Al and Cu/Zn-Al Hydrotalcites on Their Antifungal Activity against A. Niger. J. Environ. Chem. Eng. 2018, 6, 3376–3383. [Google Scholar] [CrossRef]
- Previtali, D.; Longhi, M.; Galli, F.; di Michele, A.; Manenti, F.; Signoretto, M.; Menegazzo, F.; Pirola, C. Low Pressure Conversion of CO2 to Methanol over Cu/Zn/Al Catalysts. The Effect of Mg, Ca and Sr as Basic Promoters. Fuel 2020, 274, 117804. [Google Scholar] [CrossRef]
- Sun, M.; Yu, L.; Ye, F.; Diao, G.; Yu, Q.; Hao, Z.; Zheng, Y.; Yuan, L. Transition Metal Doped Cryptomelane-Type Manganese Oxide for Low-Temperature Catalytic Combustion of Dimethyl Ether. Chem. Eng. J. 2013, 220, 320–327. [Google Scholar] [CrossRef]
- Kosova, N.I.; Vodorezova, O.Y.; Kurzina, I.A.; Kurina, L.N.; Vorobeva, V.M.; Shtertser, N.V.; Godymchuk, A.Y. The Catalysts Synthesis Methanol for Direct Synthesis of Dimethyl Ether from Synthesis Gas. Adv. Mater. Res. 2015, 1085, 29–33. [Google Scholar] [CrossRef]
- Fan, X.; Jin, B.; Ren, S.; Li, S.; Yu, M.; Liang, X. Roles of Interaction between Components in CZZA/HZSM-5 Catalyst for Dimethyl Ether Synthesis via CO2 Hydrogenation. AIChE J. 2021, 67, e17353. [Google Scholar] [CrossRef]
- Fan, X.; Ren, S.; Jin, B.; Li, S.; Yu, M.; Liang, X. Enhanced Stability of Fe-Modified CuO-ZnO-ZrO2-Al2O3/HZSM-5 Bifunctional Catalysts for Dimethyl Ether Synthesis from CO2 Hydrogenation. Chin. J. Chem. Eng. 2021, 38, 106–113. [Google Scholar] [CrossRef]
- Venugopal, A.; Palgunadi, J.; Deog, J.K.; Joo, O.S.; Shin, C.H. Dimethyl Ether Synthesis on the Admixed Catalysts of Cu-Zn-Al-M (M = Ga, La, Y, Zr) and γ-Al2O3: The Role of Modifier. J. Mol. Catal. A Chem. 2009, 302, 20–27. [Google Scholar] [CrossRef]
- Zuo, H.; Mao, D.; Guo, X.; Yu, J. Highly Efficient Synthesis of Dimethyl Ether Directly from Biomass-Derived Gas over Li-Modified Cu-ZnO-Al2O3/HZSM-5 Hybrid Catalyst. Renew. Energy 2018, 116, 38–47. [Google Scholar] [CrossRef]
- Hua, Y.; Guo, X.; Mao, D.; Lu, G.; Rempel, G.L.; Ng, F.T.T. Single-Step Synthesis of Dimethyl Ether from Biomass-Derived Syngas over CuO-ZnO-MOx (M = Zr, Al, Cr, Ti)/HZSM-5 Hybrid Catalyst: Effects of MOx. Appl. Catal. A Gen. 2017, 540, 68–74. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced Catalytic Performance of Zr Modified CuO/ZnO/Al2O3 Catalyst for Methanol and DME Synthesis via CO2 Hydrogenation. J. CO2 Util. 2020, 36, 82–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhang, S.; Wang, K.; Wu, J. CO2 Hydrogenation to Dimethyl Ether over CuO-ZnO-Al2O3/HZSM-5 Prepared by Combustion Route. RSC Adv. 2014, 4, 16391–16396. [Google Scholar] [CrossRef]
- Sierra, I.; Ereña, J.; Aguayo, A.T.; Arandes, J.M.; Bilbao, J. Regeneration of CuO-ZnO-Al2O3/γ-Al2O3 catalyst in the Direct Synthesis of Dimethyl Ether. Appl. Catal. B Environ. 2010, 94, 108–116. [Google Scholar] [CrossRef]
- Ereña, J.; Sierra, I.; Olazar, M.; Gayubo, A.G.; Aguayo, A.T. Deactivation of a CuO-ZnO-Al2O3/γ-Al2O3 Catalyst in the Synthesis of Dimethyl Ether. Ind. Eng. Chem. Res. 2008, 47, 2238–2247. [Google Scholar] [CrossRef]
- Aguayo, T.; Ereña, J.; Mier, D.; Arandes, M.; Olazar, M.; Bilbao, J. Kinetic Modeling of Dimethyl Ether Synthesis in a Single Step on a CuO-ZnO-Al2O3/Gamma-Al2O3 Catalyst. Industrial 2007, 46, 5522–5530. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.T.; Chen, T.; Chen, Q.; He, M.Y. Ion-Exchange Resin-Catalyzed Synthesis of Polyoxymethylene Dimethyl Ethers: A Practical and Environmentally Friendly Way to Diesel Additive. Chem. Eng. Commun. 2014, 201, 709–717. [Google Scholar] [CrossRef]
- Wang, W.; Gao, X.; Yang, Q.; Wang, X.; Song, F.; Zhang, Q.; Han, Y.; Tan, Y. Vanadium Oxide Modified H-Beta Zeolite for the Synthesis of Polyoxymethylene Dimethyl Ethers from Dimethyl Ether Direct Oxidation. Fuel 2019, 238, 289–297. [Google Scholar] [CrossRef]
- Haltenort, P.; Hackbarth, K.; Oestreich, D.; Lautenschütz, L.; Arnold, U.; Sauer, J. Heterogeneously Catalyzed Synthesis of Oxymethylene Dimethyl Ethers (OME) from Dimethyl Ether and Trioxane. Catal. Commun. 2018, 109, 80–84. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lee, H.M.; Huang, M.H. One-Step Synthesis of Dimethyl Ether from the Gas Mixture Containing CO2 with High Space Velocity. Appl. Energy 2012, 98, 92–101. [Google Scholar] [CrossRef]
- Kornas, A.; Śliwa, M.; Ruggiero-Mikołajczyk, M.; Samson, K.; Podobiński, J.; Karcz, R.; Duraczyńska, D.; Rutkowska-Zbik, D.; Grabowski, R. Direct Hydrogenation of CO2 to Dimethyl Ether (DME) over Hybrid Catalysts Containing CuO/ZrO2 as a Metallic Function and Heteropolyacids as an Acidic Function. React. Kinet. Mech. Catal. 2020, 130, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Tan, Y.; Xie, H.; Zhang, Q.; Han, Y. Direct Synthesis of Dimethyl Ether from Biomass-Derived Syngas over Cu-ZnO-Al2O3-ZrO2(x)/γ-Al2O3bifunctional Catalysts: Effect of Zr-Loading. Fuel Process. Technol. 2014, 126, 88–94. [Google Scholar] [CrossRef]
- Liu, D.; Yao, C.; Zhang, J.; Fang, D.; Chen, D. Catalytic Dehydration of Methanol to Dimethyl Ether over Modified γ-Al2O3 catalyst. Fuel 2011, 90, 1738–1742. [Google Scholar] [CrossRef]
- Nie, R.; Lei, H.; Pan, S.; Wang, L.; Fei, J.; Hou, Z. Core-Shell Structured CuO-ZnO@H-ZSM-5 Catalysts for CO Hydrogenation to Dimethyl Ether. Fuel 2012, 96, 419–425. [Google Scholar] [CrossRef]
- Wei, Y.; de Jongh, P.E.; Bonati, M.L.M.; Law, D.J.; Sunley, G.J.; de Jong, K.P. Enhanced Catalytic Performance of Zeolite ZSM-5 for Conversion of Methanol to Dimethyl Ether by Combining Alkaline Treatment and Partial Activation. Appl. Catal. A Gen. 2015, 504, 211–219. [Google Scholar] [CrossRef]
- Aboul-Fotouh, S.M.K.; Aboul-Gheit, N.A.K.; Naghmash, M.A. Dimethylether Production on Zeolite Catalysts Activated by Cl-, F- and/or Ultrasonication. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2016, 44, 428–436. [Google Scholar] [CrossRef]
- Kianfar, E.; Salimi, M.; Pirouzfar, V.; Koohestani, B. Synthesis and Modification of Zeolite ZSM-5 Catalyst with Solutions of Calcium Carbonate (CaCO3) and Sodium Carbonate (Na2CO3) for Methanol to Gasoline Conversion. Int. J. Chem. React. Eng. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Bakare, I.A.; Muraza, O.; Sanhoob, M.A.; Miyake, K.; Hirota, Y.; Yamani, Z.H.; Nishiyama, N. Dimethyl Ether-to-Olefins over Aluminum Rich ZSM-5: The Role of Ca and La as Modifiers. Fuel 2018, 211, 18–26. [Google Scholar] [CrossRef]
- Magomedova, M.; Galanova, E.; Davidov, I.; Afokin, M.; Maximov, A. Dimethyl Ether to Olefins over Modified Zsm-5 Based Catalysts Stabilized by Hydrothermal Treatment. Catalysts 2019, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Sheng, H.; Ma, H.; Qian, W.; Fei, N.; Zhang, H.; Ying, W. Platinum-Copper Bimetallic-Modified Nanoprism Mordenite for Carbonylation of Dimethyl Ether. Energy Fuels 2019, 33, 10159–10166. [Google Scholar] [CrossRef]
- Wang, Q.; Han, W.; Lyu, J.; Zhang, Q.; Guo, L.; Li, X. In Situ Encapsulation of Platinum Clusters within H-ZSM-5 Zeolite for Highly Stable Benzene Methylation Catalysis. Catal. Sci. Technol. 2017, 7, 6140–6150. [Google Scholar] [CrossRef]
- Golubev, K.B.; Zhang, K.; Su, X.; Kolesnichenko, N.V.; Wu, W. Dimethyl Ether Aromatization over Nanosized Zeolites: Effect of Preparation Method and Zinc Modification on Catalyst Performance. Catal. Commun. 2021, 149, 106176. [Google Scholar] [CrossRef]
- Pedram, M.Z.; Kazemeini, M.; Fattahi, M.; Amjadian, A. A Physicochemical Evaluation of Modified HZSM-5 Catalyst Utilized for Production of Dimethyl Ether from Methanol. Pet. Sci. Technol. 2014, 32, 904–911. [Google Scholar] [CrossRef]
- Pekmezci Karaman, B.; Oktar, N.; Doğu, G.; Doğu, T. Bifunctional Silicotungstic Acid and Tungstophosphoric Acid Impregnated Cu–Zn–Al & Cu–Zn–Zr Catalysts for Dimethyl Ether Synthesis from Syngas. Catal. Lett. 2020, 150, 2744–2761. [Google Scholar] [CrossRef]
- Obukhova, T.; Stashenko, A.N.; Batova, T.I.; Kolesnichenko, N.V. Dimethyl Ether Conversion to Light Olefins in Slurry and Fixed-Bed Reactors: Coke Nature and Location on Mg/ZSM-5 Catalyst. J. Chem. Technol. Biotechnol. 2021, 96, 2696–2703. [Google Scholar] [CrossRef]
- Liu, R.; Tian, H.; Yang, A.; Zha, F.; Ding, J.; Chang, Y. Preparation of HZSM-5 Membrane Packed CuO-ZnO-Al2O3 Nanoparticles for Catalysing Carbon Dioxide Hydrogenation to Dimethyl Ether. Appl. Surf. Sci. 2015, 345, 1–9. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Dolsiririttigul, N.; Chanlek, N.; Poo-arporn, Y.; Cheng, C.K.; Ayodele, B.V.; Chareonpanich, M.; Limtrakul, J. Enhanced Activity and Stability of SO42−/ZrO2 by Addition of Cu Combined with CuZnOZrO2 for Direct Synthesis of Dimethyl Ether from CO2 Hydrogenation. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Chiang, C.L.; Lin, K.S. Preparation and Characterization of CuO–Al2O3 Catalyst for Dimethyl Ether Production via Methanol Dehydration. Int. J. Hydrogen Energy 2017, 42, 23526–23538. [Google Scholar] [CrossRef]
- Liang, B.; Ma, J.; Su, X.; Yang, C.; Duan, H.; Zhou, H.; Deng, S.; Li, L.; Huang, Y. Investigation on Deactivation of Cu/ZnO/Al2O3 Catalyst for CO2 Hydrogenation to Methanol. Ind. Eng. Chem. Res. 2019, 58, 9030–9037. [Google Scholar] [CrossRef] [Green Version]
- Armenta, M.A.; Valdez, R.; Quintana, J.M.; Silva-Rodrigo, R.; Cota, L.; Olivas, A. Highly Selective CuO/Γ–Al2O3 Catalyst Promoted with Hematite for Efficient Methanol Dehydration to Dimethyl Ether. Int. J. Hydrogen Energy 2018, 43, 6551–6560. [Google Scholar] [CrossRef]
- Wang, D.S.; Tan, Y.S.; Han, Y.Z.; Noritatsu, T. Study on Deactivation of Hybrid Catalyst for Dimethyl Ether Synthesis in Slurry Reactor. J. Fuel Chem. Technol. 2008, 36, 171–175. [Google Scholar] [CrossRef]
- Wang, D.; Han, Y.; Tan, Y.; Tsubaki, N. Effect of H2O on Cu-Based Catalyst in One-Step Slurry Phase Dimethyl Ether Synthesis. Fuel Process. Technol. 2009, 90, 446–451. [Google Scholar] [CrossRef]
- Kabir, K.B.; Maynard-Casely, H.E.; Bhattacharya, S. In Situ Studies of Structural Changes in DME Synthesis Catalyst with Synchrotron Powder Diffraction. Appl. Catal. A Gen. 2014, 486, 49–54. [Google Scholar] [CrossRef]
- Miletto, I.; Catizzone, E.; Bonura, G.; Ivaldi, C.; Migliori, M.; Gianotti, E.; Marchese, L.; Frusteri, F.; Giordano, G. In Situ FT-IR Characterization of CuZnZr/Ferrierite Hybrid Catalysts for One-Pot CO2-to-DME Conversion. Materials 2018, 11, 2275. [Google Scholar] [CrossRef] [Green Version]
- Farsi, M.; Jahanmiri, A.; Eslamloueyan, R. Modeling and optimization of MeOH to DME in isothermal fixed-bed reactor. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Kekpugile, D.K.; Sylvanus, H.U. Modeling of a Tubular Fixed-Bed Reactor for the Production of Dimethyl Ether Using Alumina Catalyst. Int. J. Chem. Process Eng. Res. 2016, 3, 23–34. [Google Scholar] [CrossRef]
- Zapater, D.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. MTO with SAPO-34 in a Fixed-Bed Reactor: Deactivation Profiles. Ind. Eng. Chem. Res. 2021, 60, 16162–16170. [Google Scholar] [CrossRef]
- Abashar, M.E.E. Dimethyl Ether Synthesis in a Multi-Stage Fluidized Bed Reactor. Chem. Eng. Process. Process Intensif. 2017, 122, 172–180. [Google Scholar] [CrossRef]
- Vicente, J.; Ereña, J.; Oar-Arteta, L.; Olazar, M.; Bilbao, J.; Gayubo, A.G. Effect of Operating Conditions on Dimethyl Ether Steam Reforming in a Fluidized Bed Reactor with a CuO-ZnO-Al2O3 and Desilicated ZSM-5 Zeolite Bifunctional Catalyst. Ind. Eng. Chem. Res. 2014, 53, 3462–3471. [Google Scholar] [CrossRef]
- Yousefi, A.; Eslamloueyan, R.; Kazerooni, N.M. Optimal Conditions in Direct Dimethyl Ether Synthesis from Syngas Utilizing a Dual-Type Fluidized Bed Reactor. Energy 2017, 125, 275–286. [Google Scholar] [CrossRef]
- Fluidized Bed Reactor—Refinery Feedstocks. Available online: https://ebrary.net/131855/engineering/fluidized_reactor (accessed on 18 July 2022).
- Mondal, U.; Yadav, G.D. Perspective of Dimethyl Ether as Fuel: Part II—Analysis of Reactor Systems and Industrial Processes. J. CO2 Util. 2019, 32, 321–338. [Google Scholar] [CrossRef]
- Ezhova, N.N.; Yashina, O.V.; Stashenko, A.N.; Khivrich, E.N.; Kolesnichenko, N.V. Dimethyl Ether Conversion into Light Olefins in a Slurry Reactor: Entrainment and Decomposition of Dispersion Liquid. Kinet. Catal. 2019, 60, 681–687. [Google Scholar] [CrossRef]
- Papari, S.; Kazemeini, M.; Fattahi, M. Modelling-Based Optimisation of the Direct Synthesis of Dimethyl Ether from Syngas in a Commercial Slurry Reactor. Chin. J. Chem. Eng. 2013, 21, 611–621. [Google Scholar] [CrossRef]
- Zuo, Z.J.; Wang, L.; Han, P.-D.; Huang, W. Methanol Synthesis by CO and CO2 Hydrogenation on Cu/γ-Al2O3 Surface in Liquid Paraffin Solution. Appl. Surf. Sci. 2014, 290, 398–404. [Google Scholar] [CrossRef]
- Sun, K.; Wang, P.; Bian, Z.; Huang, W. An Investigation into the Effects of Different Existing States of Aluminum Isopropoxide on Copper-Based Catalysts for Direct Synthesis of Dimethyl Ether from Syngas. Appl. Surf. Sci. 2018, 428, 534–540. [Google Scholar] [CrossRef]
- Gogate, M.R. The Direct Dimethyl Ether (DME) Synthesis Process from Carbon-Based Feed Stocks: Current Status and Future Prospects II. Kinetic Studies and Catalyst Deactivation. Prog. Petrochem. Sci. 2018, 2. [Google Scholar] [CrossRef]
- Li, G.; Zhao, W.; Chai, M.; Li, Y.; Jia, Q.; Chen, Y. Liquid–Liquid Phase-Change Absorption of SO2 Using N, N-Dimethylcyclohexylamine as Absorbent and Liquid Paraffin as Solvent. J. Hazard. Mater. 2018, 360, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tavan, Y.; Hosseini, S.H. From Laboratory Experiments to Simulation Studies of Methanol Dehydration to Produce Dimethyl Ether Reaction—Part II: Simulation and Cost Estimation. Chem. Eng. Process. Process Intensif. 2013, 73, 151–157. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Perspective of Dimethyl Ether as Fuel: Part I. Catalysis. J. CO2 Util. 2019, 32, 299–320. [Google Scholar] [CrossRef]
- Bahruji, H.; Armstrong, R.D.; Ruiz Esquius, J.; Jones, W.; Bowker, M.; Hutchings, G.J. Hydrogenation of CO2 to Dimethyl Ether over Brønsted Acidic PdZn Catalysts. Ind. Eng. Chem. Res. 2018, 57, 6821–6829. [Google Scholar] [CrossRef]
- Fan, X. Catalytic Carbon Dioxide Hydrogenation to Methanol/Dimethyl Ether over Copper-Based Catalysts. Ph.D. Thesis, Faculty of the Graduate School of the Missouri University of Science and Technology, Rolla, MO, USA, 2021; pp. 77–81. [Google Scholar]
- Kim, S.; Kim, Y.T.; Zhang, C.; Kwak, G.; Jun, K.W. Effect of Reaction Conditions on the Catalytic Dehydration of Methanol to Dimethyl Ether Over a K-Modified HZSM-5 Catalyst. Catal. Lett. 2017, 147, 792–801. [Google Scholar] [CrossRef]
- Peláez, R.; Marín, P.; Díez, F.V.; Ordóñez, S. Direct Synthesis of Dimethyl Ether in Multi-Tubular Fixed-Bed Reactors: 2D Multi-Scale Modelling and Optimum Design. Fuel Process. Technol. 2018, 174, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Bayat, A.; Dogu, T. Optimization of CO2/CO Ratio and Temperature for Dimethyl Ether Synthesis from Syngas over a New Bifunctional Catalyst Pair Containing Heteropolyacid Impregnated Mesoporous Alumina. Ind. Eng. Chem. Res. 2016, 55, 11431–11439. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Xiao, W. Studies on Deactivation of the Catalyst in Direct Dimethyl Ether Synthesis. Int. J. Chem. React. Eng. 2012, 10. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Papari, S.; Kazemeini, M.; Fattahi, M. Mathematical Modeling of a Slurry Reactor for DME Direct Synthesis from Syngas. J. Nat. Gas Chem. 2012, 21, 148–157. [Google Scholar] [CrossRef]
- Gadek, M.; Kubica, R.; Jedrysik, E. Production of Methanol and Dimethyl Ether from Biomass Derived Syngas—A Comparison of the Different Synthesis Pathways by Means of Flowsheet Simulation. Comput. Aided Chem. Eng. 2013, 32, 55–60. [Google Scholar] [CrossRef]
- Kung, H.H. Deactivation of Methanol Synthesis Catalysts—A Review. Catal. Today 1992, 11, 443–453. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-Liquid via Synthesis of Methanol, DME or Fischer–Tropsch-Fuels: A Review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Pacheco, M.E.; Martins Salim, V.M.; Pinto, J.C. Accelerated Deactivation of Hydrotreating Catalysts by Coke Deposition. Ind. Eng. Chem. Res. 2011, 50, 5975–5981. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Wang, K.; Ding, F.; Jia, S.; Liu, G.; Tan, L. Investigation on the Promotional Role of Ga2O3 on the CuO-ZnO/HZSM-5 Catalyst for CO2 hydrogenation. RSC Adv. 2021, 11, 14426–14433. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. On the Effectiveness of Zeolite-Based Catalysts in Perspectives. Chem. Eng. 2017. [Google Scholar] [CrossRef] [Green Version]
- Pekmezci Karaman, B.; Oktar, N. Tungstophosphoric Acid Incorporated Hierarchical HZSM-5 Catalysts for Direct Synthesis of Dimethyl Ether. Int. J. Hydrogen Energy 2020, 45, 34793–34804. [Google Scholar] [CrossRef]
- Tan, Y.; Xie, H.; Cui, H.; Han, Y.; Zhong, B. Modification of Cu-Based Methanol Synthesis Catalyst for Dimethyl Ether Synthesis from Syngas in Slurry Phase. Catal. Today 2005, 104, 25–29. [Google Scholar] [CrossRef]
- Ivantsov, M.I.; Kulikova, M.V.; Gubanov, M.A.; Dement’eva, O.S.; Chudakova, M.V.; Bondarenko, G.N.; Khadzhiev, S.N. Methanol Synthesis in a Three-Phase Slurry Reactor with Ultrafine Catalysts. Pet. Chem. 2017, 57, 571–575. [Google Scholar] [CrossRef]
- Dadgar, F.; Myrstad, R.; Pfeifer, P.; Holmen, A.; Venvik, H.J. Direct Dimethyl Ether Synthesis from Synthesis Gas: The Influence of Methanol Dehydration on Methanol Synthesis Reaction. Catal. Today 2016, 270, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.M.; Lee, D.W.; Kim, D.K.; Lee, J.S.; Lee, K.Y. The Heterogeneous Catalyst System for the Continuous Conversion of Free Fatty Acids in Used Vegetable Oils for the Production of Biodiesel. Catal. Today 2008, 131, 238–243. [Google Scholar] [CrossRef]
- Papari, S.; Kazemeini, M.; Fattahi, M.; Fatahi, M. Dme Direct Synthesis from Syngas in A Large-Scale Three-Phase Slurry Bubble Column Reactor: Transient Modeling. Chem. Eng. Commun. 2014, 201, 612–634. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Haghbakhsh, R.; Rahimpour, M.R. Investigation of Thermally Double Coupled Double Membrane Heat Exchanger Reactor to Produce Dimethyl Ether and Methyl Formate. J. Nat. Gas Sci. Eng. 2016, 32, 185–197. [Google Scholar] [CrossRef]
- Vakili, R.; Pourazadi, E.; Setoodeh, P.; Eslamloueyan, R.; Rahimpour, M.R. Direct Dimethyl Ether (DME) Synthesis through a Thermally Coupled Heat Exchanger Reactor. Appl. Energy 2011, 88, 1211–1223. [Google Scholar] [CrossRef]
- Method for Producing Dimethyl Ether Using Separation Membrane Reactor. Patent JP3892413B2, 14 March 2007.
- Farsi, M. DME Production in Multi-Stage Radial Flow Spherical Membrane Reactors: REACTOR Design and Modeling. J. Nat. Gas Sci. Eng. 2014, 20, 366–372. [Google Scholar] [CrossRef]
- Allahyari, S.; Haghighi, M.; Ebadi, A. Direct Synthesis of DME over Nanostructured CuO-ZnO-Al2O3/HZSM-5 Catalyst Washcoated on High Pressure Microreactor: Effect of Catalyst Loading and Process Condition on Reactor Performance. Chem. Eng. J. 2015, 262, 1175–1186. [Google Scholar] [CrossRef]
- Allahyari, S.; Haghighi, M.; Ebadi, A. Direct Conversion of Syngas to DME as a Green Fuel in a High Pressure Microreactor: Influence of Slurry Solid Content on Characteristics and Reactivity of Washcoated CuO-ZnO-Al2O3/HZSM-5 Nanocatalyst. Chem. Eng. Process. Process Intensif. 2014, 86, 53–63. [Google Scholar] [CrossRef]
- Müller, M.; Hübsch, U. Dimethyl Ether. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Ben Arnor, H.; Halloin, V.L. Methanol Synthesis in a Multifunctional Reactor. Chem. Eng. Sci. 1999, 54, 1419–1423. [Google Scholar] [CrossRef]
- Mills, G. Status and Future Opportunities for Conversion of Synthesis Gas to Liquid Energy Fuels: Final Report; NREL: Golden, CO, USA, 1993. [Google Scholar]
- Luan, Y.; Xu, H.; Yu, C.; Li, W.; Hou, S. In-Situ Regeneration Mechanisms of Hybrid Catalysts in the One-Step Synthesis of Dimethyl Ether from Syngas. Catal. Lett. 2007, 115, 23–26. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Ereña, J.; Sierra, I.; Olazar, M.; Bilbao, J. Deactivation and Regeneration of Hybrid Catalysts in the Single-Step Synthesis of Dimethyl Ether from Syngas and CO2. Catal. Today 2005, 106, 265–270. [Google Scholar] [CrossRef]
- Bonura, G.; Todaro, S.; Frusteri, L.; Majchrzak-Kucęba, I.; Wawrzyńczak, D.; Pászti, Z.; Tálas, E.; Tompos, A.; Ferenc, L.; Solt, H.; et al. Inside the Reaction Mechanism of Direct CO2 Conversion to DME over Zeolite-Based Hybrid Catalysts. Appl. Catal. B Environ. 2021, 294, 120255. [Google Scholar] [CrossRef]
- Liang, K.C.; Yeh, F.M.; Wu, C.G.; Lee, H.M. Gasoline Production by Dehydration of Dimethyl Ether with NH4-ZSM-5 Catalyst. Energy Procedia 2015, 75, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Daligaux, V.; Richard, R.; Manero, M.H. Deactivation and Regeneration of Zeolite Catalysts Used in Pyrolysis of Plastic Wastes—A Process and Analytical Review. Catalysts 2021, 11, 770. [Google Scholar] [CrossRef]
- Babic, V. Increasing the Porosity of Zeolites. Ph.D. Thesis, Normandie Université, Rouen, France, 2021. [Google Scholar]
- Zeng, L.; Wang, Y.; Mou, J.; Liu, F.; Yang, C.; Zhao, T.; Wang, X.; Cao, J. Promoted Catalytic Behavior over γ-Al2O3 Composited with ZSM-5 for Crude Methanol Conversion to Dimethyl Ether. Int. J. Hydrogen Energy 2020, 45, 16500–16508. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Abdelkader, A.; Hassan, N.M.; Laffir, F.; Mclaren, M.; Rooney, D.W. Silver Modified η-Al2O3 Catalyst for DME Production. J. Phys. Chem. C 2017, 121, 25018–25032. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Bongard, H.; Schmidt, W.; Schüth, F. One-Pot Synthesis of Mesoporous Cu-γ-Al2O3 as Bifunctional Catalyst for Direct Dimethyl Ether Synthesis. Microporous Mesoporous Mater. 2012, 164, 3–8. [Google Scholar] [CrossRef]
- Ereña, J.; Sierra, I.; Aguayo, A.T.; Ateka, A.; Olazar, M.; Bilbao, J. Kinetic Modelling of Dimethyl Ether Synthesis from (H2 + CO2) by Considering Catalyst Deactivation. Chem. Eng. J. 2011, 174, 660–667. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, J.; Wysocka, I.; Murgrabia, S.; Rogala, A. A Review on Deactivation and Regeneration of Catalysts for Dimethyl Ether Synthesis. Energies 2022, 15, 5420. https://doi.org/10.3390/en15155420
Sobczak J, Wysocka I, Murgrabia S, Rogala A. A Review on Deactivation and Regeneration of Catalysts for Dimethyl Ether Synthesis. Energies. 2022; 15(15):5420. https://doi.org/10.3390/en15155420
Chicago/Turabian StyleSobczak, Joanna, Izabela Wysocka, Stanisław Murgrabia, and Andrzej Rogala. 2022. "A Review on Deactivation and Regeneration of Catalysts for Dimethyl Ether Synthesis" Energies 15, no. 15: 5420. https://doi.org/10.3390/en15155420
APA StyleSobczak, J., Wysocka, I., Murgrabia, S., & Rogala, A. (2022). A Review on Deactivation and Regeneration of Catalysts for Dimethyl Ether Synthesis. Energies, 15(15), 5420. https://doi.org/10.3390/en15155420








