Preparation and Characterization of Microencapsulated Phase Change Materials for Solar Heat Collection
Abstract
:1. Introduction
2. Experimental Method
2.1. Preparation Steps of MPCMs
- Add a certain mass fraction of docosane to 100 mL formamide solution, put it in a water bath, and heat it at a constant temperature (75 °C) until it melts, Then, subjected it to ultrasonic oscillation so that it is evenly distributed in the form of small droplets in formamide solution, and obtain docosane emulsion;
- Continuously stir the reaction solution kept at a constant temperature of 7 °C, and add a certain mass fraction of emulsifier to the fine emulsion of dodecane. Continuously stir magnetically for a certain amount of time at a rotational speed of 750 rpm and obtain a uniform and stable dodecane emulsion;
- Drop hydrochloric acid into the reaction solution to adjust its pH value;
- Continue to add a certain mass fraction of tetrabutyl titanate (TBT) solution to the reaction solution, and evenly disperse it around the emulsion droplets of doxyane by magnetic stirring;
- Add 60 mL formamide solution containing 5 g deionized water (water mass fraction of 12%) to the reaction solution drop by drop, with a titration duration of 2–3 h. At the same time, conduct magnetic stirring, adjusting the speed to 600 rpm, which takes 5 h;
- After the reaction, filter the solution with a vacuum pump to obtain the reaction precipitate, clean it with hot deionized water and hot anhydrous ethanol. After standing and drying for 24 h, titanium-dioxide-coated dodecane microcapsules can finally be obtained.
2.2. Microstructure Characterization
2.3. Thermal Conductivity Test
2.4. Measuring Method for Heat Storage Performance
3. Results and Discussion
3.1. Microstructure of MPCMs
3.2. Thermal Conductivity Test
3.3. The Influence of Different Variables on the Thermal Properties of MPCMs
3.3.1. Effect of the Type of Emulsifier on MPCMs
3.3.2. Effect of Ultrasonic Oscillation Time on MPCMs
3.3.3. Effect of pH on MPCMs
3.3.4. Effect of Emulsifier Mass Fraction on MPCMs
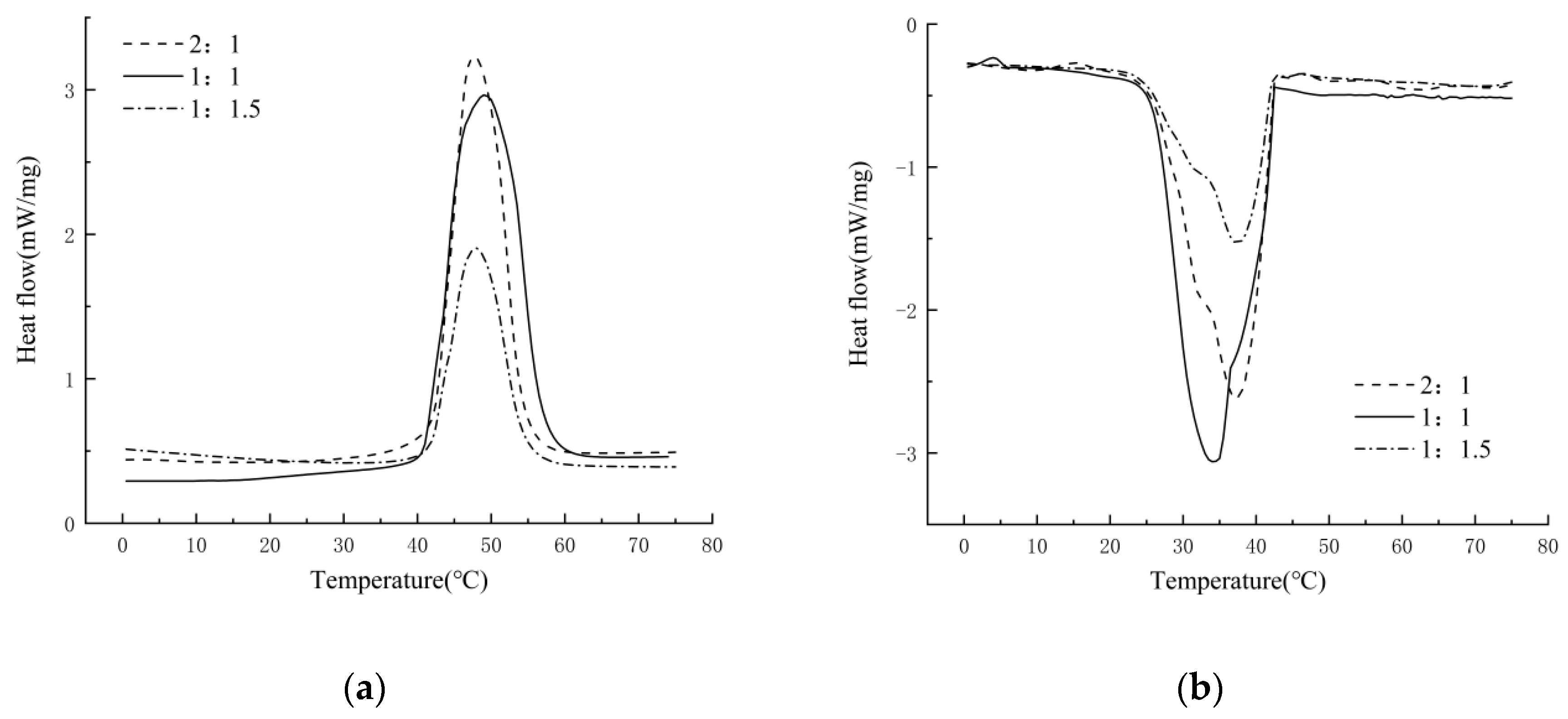
3.3.5. Effect of the Core–Shell Ratio on MPCMs
4. Conclusions
- The thermal conductivity of the MPCMs prepared in this experiment was significantly higher than that of several commonly used PCMs. It gradually increased with the increase in the specific gravity of the shell material and the increase in the phase change temperature;
- Among the three groups of phase change microcapsules prepared with SDS, OP-10, and sodium alginate as emulsifiers, the MPCMs with SDS as a dispersant had better heat storage capacity. When the added amount of SDS was 0.45 g, the latent heat of phase change was 166.7 J/g;
- The thermal properties of MPCMs prepared under four different ultrasonic oscillation times (i.e., 6, 8, 10, and 12 min) were tested. When the ultrasonic time was set to 10 min, the size of the docosane emulsion droplets was suitable, which could completely mix and react with the emulsifier, resulting in a good emulsification effect. The latent heat of phase change was 96.3 J/g, and the nucleation rate was better;
- The effects of different pH values (i.e., 2.5, 3.5, 4.5, and 5.5) on the preparation of MPCMs were analyzed. When the pH value was 3.5, the latent heat of phase change was 101.4 J/g, and the MPCMs had a superior shell structure;
- Three kinds of core–shell ratio (i.e., 2:1, 1:1, and 1:1.5) microcapsules were tested by DSC. When the core–shell ratio of docosane and titanium dioxide was 1:1, the latent heat of phase change was 166.7 J/g and had a good coating rate.
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.; Zhao, X.; Ji, J. Conceptual development of a novel photovoltaic-thermoelectric system and preliminary economic analysis. Energy Convers. Manag. 2016, 126, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Armstrong, A.; Burney, J.; Ryan, G.; Moore-O’Leary, K.; Diédhiou, I.; Kammen, D.M. Techno-ecological synergies of solar energy for global sustainability. Nat. Sustain. 2019, 2, 560–568. [Google Scholar] [CrossRef]
- Yuan, M.; Ye, F.; Xu, C. Supercooling study of erythritol/EG composite phase change materials. Energy Procedia 2019, 158, 4629–4634. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Sugeno, T.; Ishige, T.; Takeuchi, H.; Pyatenko, A.T. An evaluation of microencapsulated PCM for use in cold energy transportation medium. In Proceedings of the 31st Intersociety Energy Conversion Engineering Conference, Washington, DC, USA, 11–16 August 1996. [Google Scholar]
- Tan, P.; Lindberg, P.; Eichler, K.; Löveryd, P.; Johansson, P.; Kalagasidis, A.S. Effect of phase separation and supercooling on the storage capacity in a commercial latent heat thermal energy storage: Experimental cycling of a salt hydrate PCM. J. Energy Storage 2020, 29, 101266. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, L.; Shan, F.; Fang, G. Preparation and characteristics of microencapsulated stearic acid as composite thermal energy storage material in buildings. Energy Build. 2013, 62, 469–474. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellon, C.; Nogues, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Bahrar, M.; Djamai, Z.I.; Mankibi, M.E.; Larbi, A.S.; Salvia, M. Numerical and experimental study on the use of microencapsulated phase change materials (PCMs) in textile reinforced concrete panels for energy storage. Sustain. Cities Soc. 2018, 41, 455–468. [Google Scholar] [CrossRef]
- Mankel, C.; Caggiano, A.; Ukrainczyk, N.; Koenders, E. Thermal energy storage characterization of cement-based systems containing microencapsulated-PCMs. Constr. Build. Mater. 2019, 199, 307–320. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Al-Shannaq, R.; Fernández, A.I.; Farid, M.M. Preparation and characterization of microencapsulated phase change materials for use in building applications. Materials 2015, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Nejman, A.; Cieslak, M.; Gajdzicki, B.; Goetzendorf-Grabowska, B.; Karaszewska, A. Methods of PCM microcapsules application and the thermal properties of modified knitted fabric. Thermochim. Acta 2014, 589, 158–163. [Google Scholar] [CrossRef]
- Nejman, A.; Cieslak, M. The impact of the heating/cooling rate on the thermoregulating properties of textile materials modified with PCM microcapsules. Appl. Therm. Eng. 2017, 127, 212–223. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Fang, G. Synthesis and properties of microencapsulated stearic acid/silica composites with graphene oxide for improving thermal conductivity as novel solar thermal storage materials. Sol. Energy Mater. Sol. Cells 2018, 189, 197–205. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Wu, D. Design and fabrication of bifunctional microcapsules for solar thermal energy storage and solar photocatalysis by encapsulating paraffin phase change material into cuprous oxide. Sol. Energy Mater. Sol. Cells 2017, 168, 146–164. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, J.; Wang, H.; Song, G.; Liu, Y.; Tang, G. Enhancement in thermal property of phase change microcapsules with modified silicon nitride for solar energy. Sol. Energy Mater. Sol. Cells 2016, 151, 89–95. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, J.; Zhang, R.; Han, X.; Wu, J. Photo-thermal performance evaluation on MWCNTs-dispersed microencapsulated PCM slurries for direct absorption solar collectors. J. Energy Storage 2019, 26, 100793. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Fang, X.; Zhang, Z. Preparation of graphite nanoparticles-modified phase change microcapsules and their dispersed slurry for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 159, 159–166. [Google Scholar] [CrossRef]
- Xu, Y.L.; Han, X.G.; Wu, Y.B. Preparation and influence factors of microcapsules. In Proceedings of the Gansu Chemical Society Chemistry Teaching Experience Exchange Conference of Gansu Middle School, Lanzhou City College, Lanzhou, China, 23 December 2007. [Google Scholar]
- Alberti, A.; Kappius, L.; Mantl, S. The effect of the reaction temperature on the thermal stability of polycrystalline CoSi2 layers on Si (001). Microelectron. Eng. 2001, 55, 151–156. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, T.; Jiao, S.; Guo, C. Preparation of reduced graphene oxide modified magnetic phase change microcapsules and their application in direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2020, 216, 110695. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Karaipekli, A. Preparation, characterization and thermal properties of PMMA/n-heptadecane microcapsules as novel solid–liquid microPCM for thermal energy storage. Appl. Energy 2010, 87, 1529–1534. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Romero-Cano, M.; Vincent, B. Controlled release of 4-nitroanisole from poly(lactic acid) nanoparticles. J. Control. Release 2002, 82, 127–135. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Hu, L.; Wang, Y.; Gao, L. Fabrication and properties of microencapsulated paraffin@ SiO2 phase change composite for thermal energy storage. ACS Sustain. Chem. Eng. 2013, 1, 374–380. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Wang, X.; Wu, D. Fabrication of microencapsulated phase change materials based on n-octadecane core and silica shell through interfacial polycondensation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 104–117. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Tsai, P.-S.; Yang, Y.-M. Preparation of silica microspheres encapsulating phase-change material by sol-gel method in O/W emulsion. J. Microencapsul. 2006, 23, 3–14. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Cao, L.; Tang, F.; Fang, G. Preparation and characteristics of microencapsulated palmitic acid with TiO2 shell as shape-stabilized thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2014, 123, 183–188. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium dioxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- Zhao, A.; An, J.; Yang, J.; Yang, E.-H. Microencapsulated phase change materials with composite titania-polyurea (TiO2-PUA) shell. Appl. Energy 2018, 215, 468–478. [Google Scholar] [CrossRef]
- Cahill, D.G.; Allen, T.H. Thermal conductivity of sputtered and evaporated SiO2 and TiO2 optical coatings. Appl. Phys. Lett. 1994, 65, 309–311. [Google Scholar] [CrossRef]


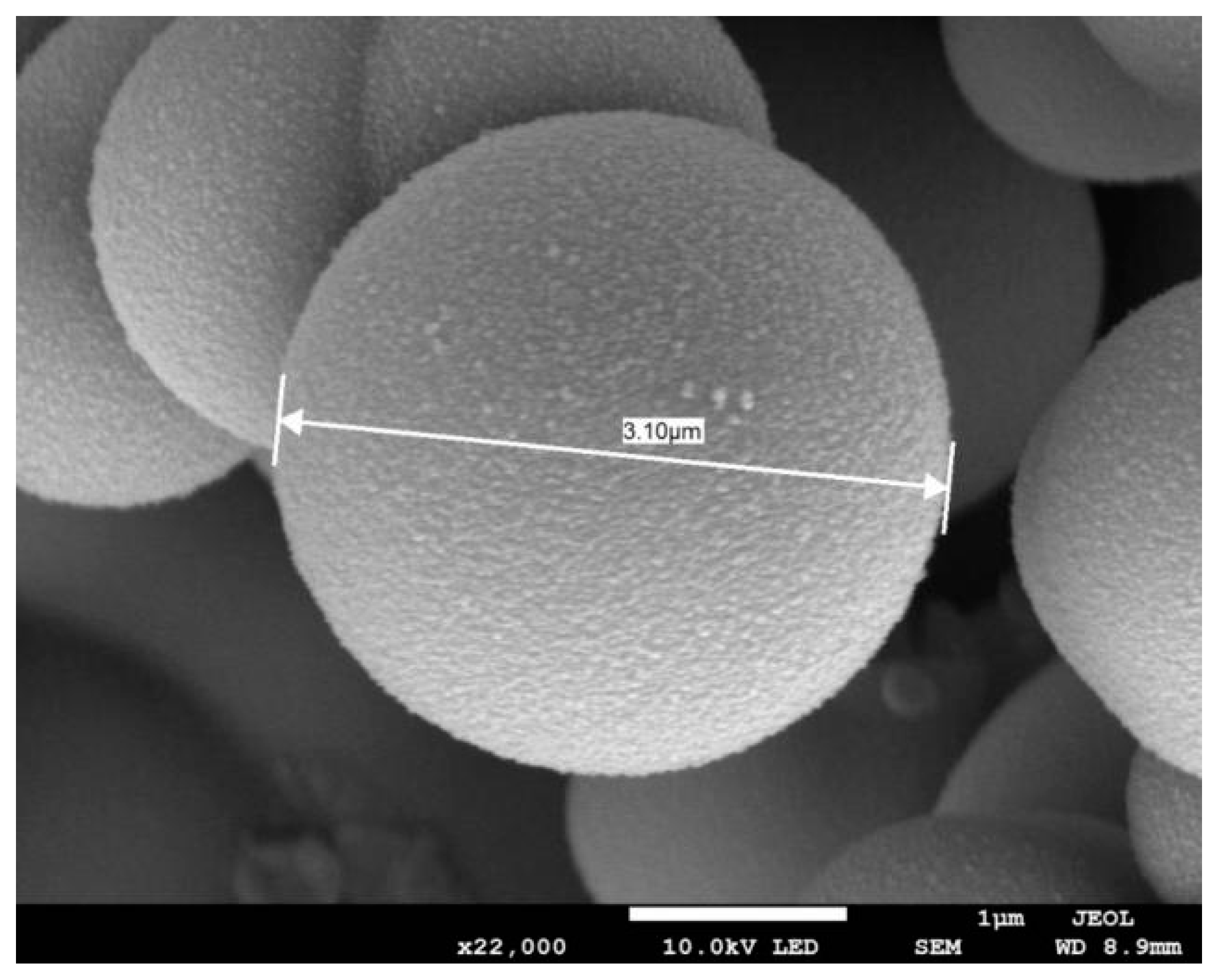
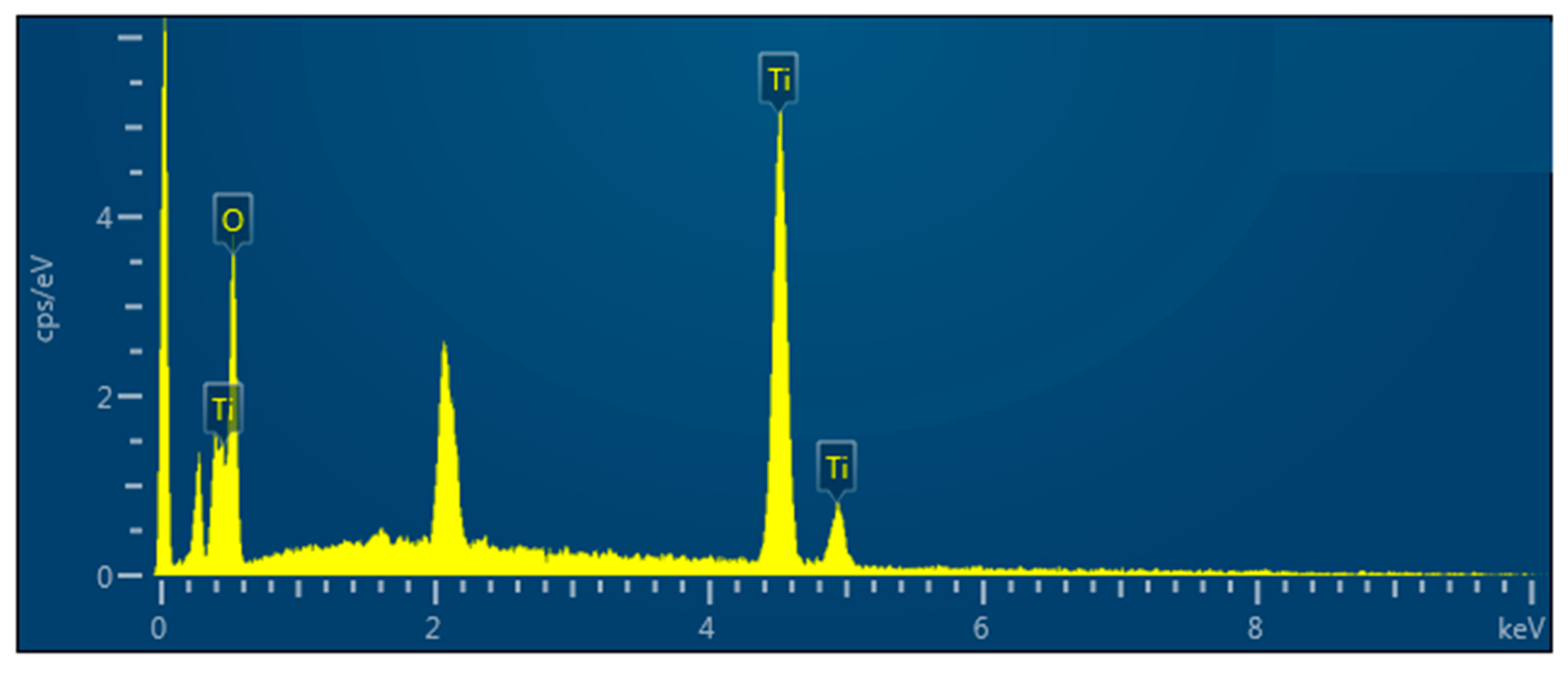




| Raw Material Name | Chemical Grade or Purity | Manufacturer |
|---|---|---|
| Tetrabutyl titanate | 98 wt% | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. |
| Docosane | 98 wt% | Shanghai Aladdin Bio-Chem., Shanghai, China. |
| Formamide | analytically pure | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. |
| Hydrochloric acid | analytically pure | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. |
| Anhydrous ethanol | 99.8 wt% | Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China. |
| Deionized water | / | Produced in the Lab |
| Device | Model | Manufacturer |
|---|---|---|
| Magnetic stirrer | ZNCL-GS130*60 | Gongyi Yuhua Instrument Co., Ltd., Henan, China. |
| Electronic balance | YH-A 10002 | Ruian Yingheng Electric Co., Ltd., Zhejiang, China. |
| Ultrasonic cell crusher | SCIENTZ-IID | Ningbo Xinzhi Biological Technology Co., Ltd., Ningbo, China. |
| Intelligent peristaltic pump | STP-F01A Micro peristaltic pump | Carmel Fluid Technology Co., Ltd., Shanghai, China. |
| Oil-free diaphragm vacuum pump | YM-10A | Platinum Instruments Co., Ltd., Shanghai, China. |
| Variables | Emulsifier Type | Ultrasonic Oscillation Time (min) | pH | Emulsifier Mass Fraction (%) | Core–Shell Ration |
|---|---|---|---|---|---|
| Emulsifier type | SDS/sodium alginate/OP-10 | SDS | SDS | SDS | SDS |
| Ultrasonic oscillation time (min) | 10 min | 6/8/10/12 | 10 min | 10 min | 10 min |
| pH | 4.5 | 4.5 | 2.5/3.5/4.5/5.5 | 3.5 | 3.5 |
| Emulsifier mass fraction (%) | 0.22 | 0.22 | 0.22 | 0.22/0.40/0.57/0.75 | 0.4 |
| Core–shell ration | 1:1 | 1:1 | 1:1 | 1:1 | 2:1/1:1/1:1.5 |
| Core–Shell Ratio (2:1) | Core–Shell Ratio (1:1) | Core–Shell Ratio (1:1.5) | |||
|---|---|---|---|---|---|
| Temperature °C | Thermal Conductivity W/(m·K) | Temperature °C | Thermal Conductivity W/(m·K) | Temperature °C | Thermal Conductivity W/(m·K) |
| 25 | 0.808 | 25 | 0.869 | 25 | 0.913 |
| 40 | 0.856 | 40 | 0.914 | 40 | 0.983 |
| 60 | 0.907 | 60 | 0.958 | 60 | 1.012 |
| Variable Parameter | Melting | Crystallization | ||
|---|---|---|---|---|
| Type of Emulsifier | Latent Heat | Phase Change Onset Temperature | Latent Heat | Phase Change Onset Temperature |
| SDS | 91.1 J/g | 42.3 °C | 89.7 J/g | 35.5 °C |
| Sodium alginate | 41.8 J/g | 42.1 °C | 42.5 J/g | 35.3 °C |
| OP-10 | 70.1 J/g | 42.5 °C | 69.8 J/g | 35.1 °C |
| Variable Parameter | Melting | Crystallization | ||
|---|---|---|---|---|
| Ultrasound Time | Latent Heat | Phase Change Onset Temperature | Latent Heat | Phase Change Onset Temperature |
| 6 min | 46.2 J/g | 41.6 °C | 45.1 J/g | 31.8 °C |
| 8 min | 66.8 J/g | 41.9 °C | 61.3 J/g | 31.6 °C |
| 10 min | 96.3 J/g | 42.1 °C | 86.3 J/g | 32.2 °C |
| 12 min | 90.3 J/g | 42.3 °C | 75.1 J/g | 32.1 °C |
| Variable Parameter | Melting | Crystallization | ||
|---|---|---|---|---|
| pH | Latent Heat | Phase Change Onset Temperature | Latent Heat | Phase Change Onset Temperature |
| 2.5 | 68.8 J/g | 41.9 °C | 68.3 J/g | 30.6 °C |
| 3.5 | 101.4 J/g | 41.5 °C | 94.6 J/g | 31.2 °C |
| 4.5 | 96.3 J/g | 41.6 °C | 86.3 J/g | 32.2 °C |
| 5.5 | 68.8 J/g | 41.8 °C | 69.1 J/g | 32.6 °C |
| Variable Parameter | Melting | Crystallization | ||
|---|---|---|---|---|
| Emulsifier Mass Fraction | Latent Heat | Phase Change Onset Temperature | Latent Heat | Phase Change Onset Temperature |
| 0.22% | 101.4 J/g | 41.5 °C | 94.6 J/g | 31.2 °C |
| 0.40% | 166.7 J/g | 42.4 °C | 164.2 J/g | 31.2 °C |
| 0.57% | 159.7 J/g | 41.9 °C | 155.3 J/g | 30.4 °C |
| 0.75% | 84.6 J/g | 41.9 °C | 79.1 J/g | 31.9 °C |
| Variable Parameter | Melting | Crystallization | ||
|---|---|---|---|---|
| Core–Shell Ration | Latent Heat | Phase Change Temperature | Latent Heat | Phase Change Temperature |
| 2:1 | 68.2 J/g | 41.9 °C | 94.6 J/g | 28.9 °C |
| 1:1 | 166.7 J/g | 42.4 °C | 164.2 J/g | 31.2 °C |
| 1:1.5 | 132.9 J/g | 42.3 °C | 79.1 J/g | 42.9 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhao, R.; Wang, C.; Feng, L.; Li, S.; Gong, Y. Preparation and Characterization of Microencapsulated Phase Change Materials for Solar Heat Collection. Energies 2022, 15, 5354. https://doi.org/10.3390/en15155354
Chen H, Zhao R, Wang C, Feng L, Li S, Gong Y. Preparation and Characterization of Microencapsulated Phase Change Materials for Solar Heat Collection. Energies. 2022; 15(15):5354. https://doi.org/10.3390/en15155354
Chicago/Turabian StyleChen, Hongbing, Rui Zhao, Congcong Wang, Lianyuan Feng, Shuqian Li, and Yutong Gong. 2022. "Preparation and Characterization of Microencapsulated Phase Change Materials for Solar Heat Collection" Energies 15, no. 15: 5354. https://doi.org/10.3390/en15155354
APA StyleChen, H., Zhao, R., Wang, C., Feng, L., Li, S., & Gong, Y. (2022). Preparation and Characterization of Microencapsulated Phase Change Materials for Solar Heat Collection. Energies, 15(15), 5354. https://doi.org/10.3390/en15155354






