Research Progress on Magnetic Catalysts and Its Application in Hydrogen Production Area
Abstract
:1. Introduction
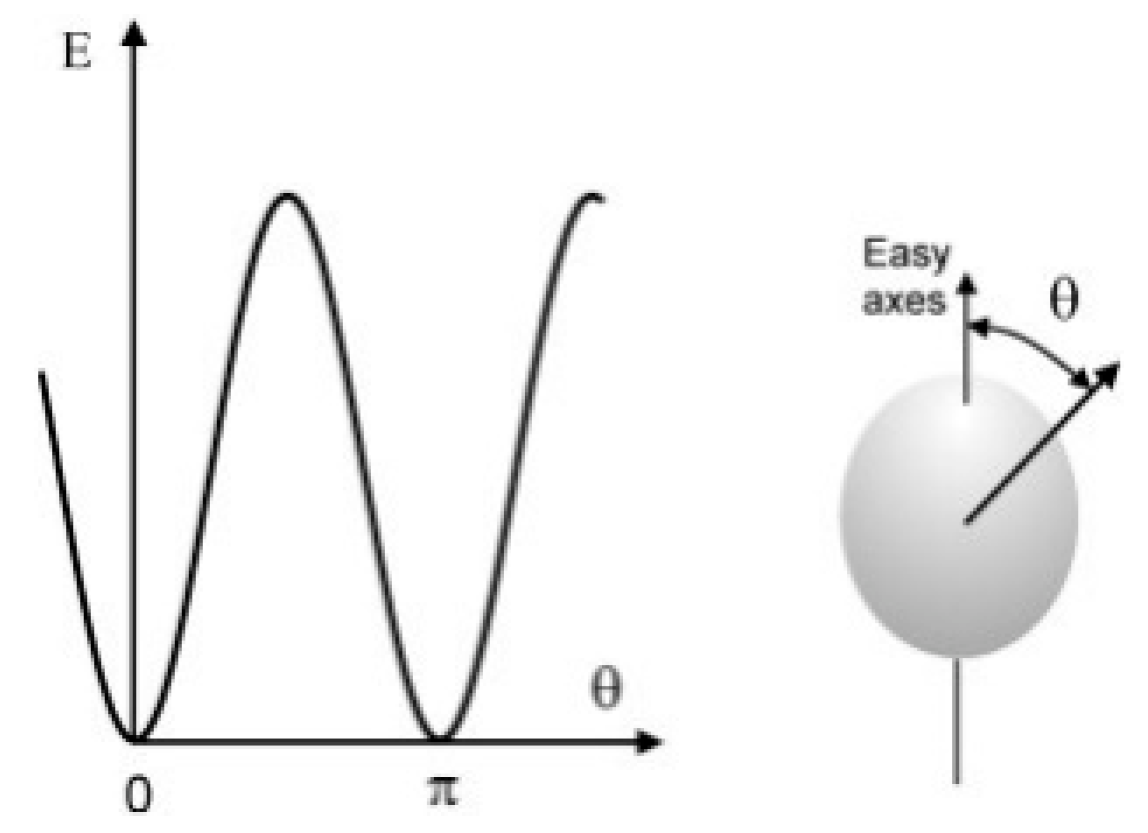
2. Magnetic Catalyst Induction Heating Theory and Its Influencing Factors
3. Synthesis of Magnetic Catalysts
3.1. Magnetic Catalyst Cores
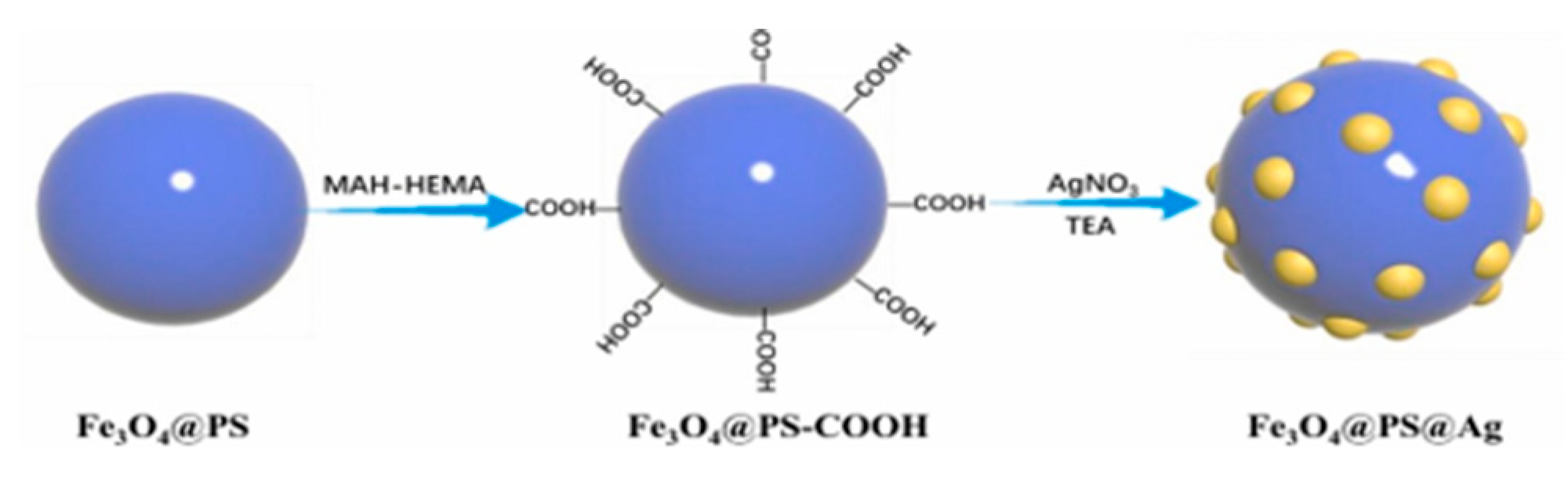
3.2. Surface Modified and Coated Magnetic Catalysts
3.3. Active Component Loaded Magnetic Catalyst
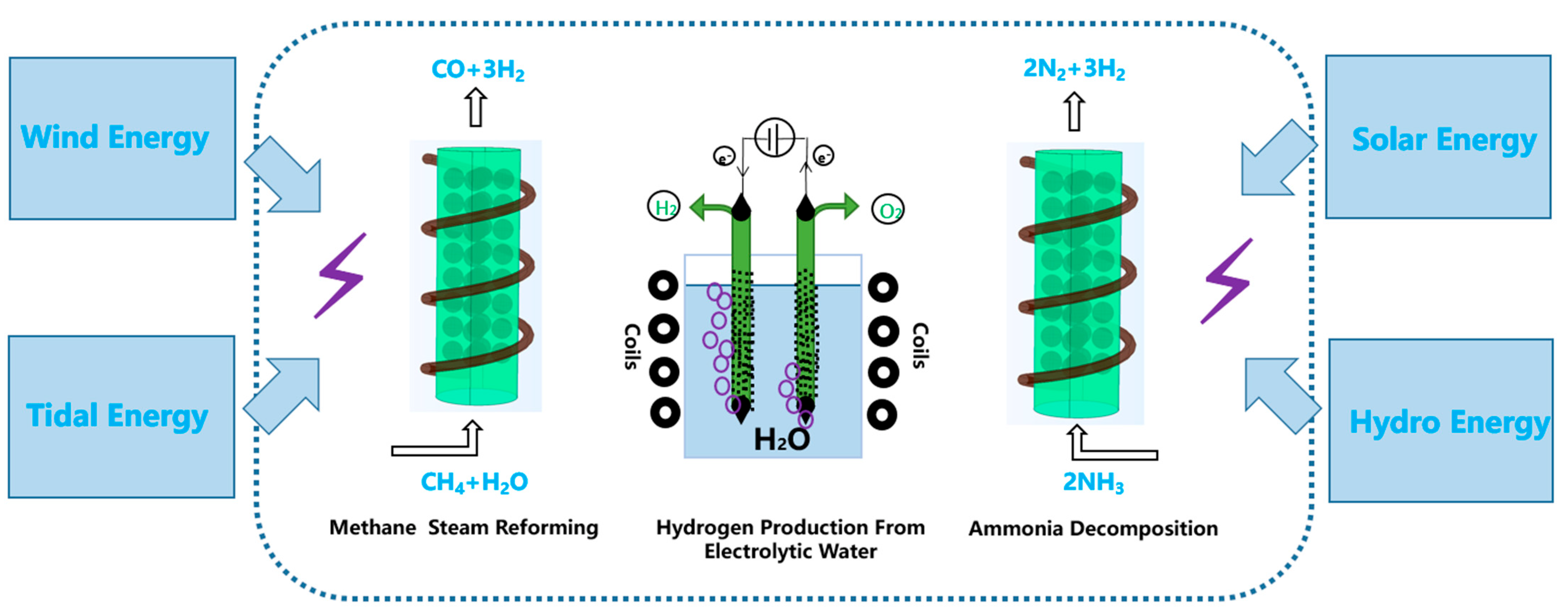
4. Application of Magnetic Catalyst
4.1. Catalysis Materials Synthesis
4.2. Endothermic Steam Reforming for Hydrogen Production
4.3. Hydrogen Production from Electrolytic Water
4.4. Other Hydrogen Production Technologies
4.5. Catalytic Wet Peroxide Oxidation
5. Conclusions
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, R.; Leach, M.; Bauen, A. Progress in renewable energy. Environ. Int. 2003, 29, 105–122. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Wismann, S.T.; Engbaek, J.S.; Vendelbo, S.B.; Eriksen, W.L.; Frandsen, C.; Mortensen, P.M.; Chorkendorff, I. Electrified methane reforming: Elucidating transient phenomena. Chem. Eng. J. 2021, 425, 131509. [Google Scholar] [CrossRef]
- Develey, G. Chauffage par induction électromagnétique: Technologie. Techniques de l’ingénieur. Génie Électrique 2000, 12, D5936.1–D5936.10. [Google Scholar] [CrossRef]
- Barglik, J.; Smagór, A.; Smalcerz, A.; Desisa, D.G. Induction Heating of Gear Wheels in Consecutive Contour Hardening Process. Energies 2021, 14, 3885. [Google Scholar] [CrossRef]
- Wang, D.; Xie, W.; Gao, Q.; Yan, H.; Zhang, J.X.; Lu, J.S.; Liaw, B.; Guo, Z.H.; Gao, F.; Yin, L.; et al. Non-magnetic injectable implant for magnetic field-driven thermochemotherapy and dual stimuli-responsive drug delivery: Transformable liquid metal hybrid platform for cancer theranostics. Small 2019, 15, 1900511. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Eng. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef] [Green Version]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [Google Scholar]
- Barua, R.; Datta, S.; Sengupta, P.; Chowdhury, A.R.; Datta, P. Advances in MEMS micropumps and their emerging drug delivery and biomedical applications. Adv. Chall. Pharm. Technol. 2021, 411–452. [Google Scholar] [CrossRef]
- Abd Elrahman, A.A.; Mansour, F.R. Targeted magnetic iron oxide nanoparticles: Preparation, functionalization and biomedical application. J. Drug Deliv. Sci. Technol. 2019, 52, 702–712. [Google Scholar] [CrossRef]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.; Ivkov, R. Clinical magnetic hyperthermia requires integrated magnetic particle imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef] [PubMed]
- IP Soares, P.; MM Ferreira, I.; AGBN Igreja, R.; MM Novo, C.; Borges, J.P.M.R. Application of hyperthermia for cancer treatment: Recent patents review. Recent Pat. Anti-Cancer Drug Discov. 2012, 7, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Asensio, J.M.; Miguel, A.B.; Fazzini, P.F.; Van Leeuwen, P.W.; Chaudret, B. Hydrodeoxygenation Using Magnetic Induction: High-Temperature Heterogeneous Catalysis in Solution. Angew. Chem. 2019, 131, 11428–11432. [Google Scholar]
- Scarfiello, C.; Bellusci, M.; Pilloni, L.; Pietrogiacomi, D.; La Barbera, A.; Varsano, F. Supported catalysts for induction-heated steam reforming of methane. Int. J. Hydrogen Energy 2021, 46, 134–145. [Google Scholar] [CrossRef]
- Kuhwald, C.; Türkhan, S.; Kirschning, A. Inductive heating and flow chemistry–a perfect synergy of emerging enabling technologies. Beilstein J. Org. Chem. 2022, 18, 688–706. [Google Scholar] [CrossRef]
- Kim, D.K.; Woo, Y.Y.; Park, K.S.; Sim, W.J.; Moon, Y.H. Advanced induction heating system for hot stamping. Int. J. Adv. Manuf. Technol. 2018, 99, 583–593. [Google Scholar] [CrossRef]
- Sharma, P.; Holliger, N.; Pfromm, P.H.; Liu, B.; Chikan, V. Size-controlled synthesis of iron and iron oxide nanoparticles by the rapid inductive heating method. ACS Omega 2020, 5, 19853–19860. [Google Scholar] [CrossRef]
- Lucía, O.; Maussion, P.; Dede, E.J.; Burdío, J.M. Induction heating technology and its applications: Past developments, current technology, and future challenges. IEEE Trans. Ind. Electron. 2013, 61, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Sakata, S.; Lee, S.; Morita, H.; Johzaki, T.; Sawada, H.; Iwasa, Y.; Matsuo, k.; Law, K.F.F.; Yao, A.; Hata, M.; et al. Magnetized fast isochoric laser heating for efficient creation of ultra-high-energy-density states. Nat. Commun. 2018, 9, 3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Tuci, G.; Duong-Viet, C.; Liu, Y.; Rossin, A.; Luconi, L.; Nhut, J.M.; Nguyen-Dinh, L.; Pham-Huu, C.; Giambastiani, G. Induction heating: An enabling technology for the heat management in catalytic processes. ACS Catal. 2019, 9, 7921–7935. [Google Scholar] [CrossRef]
- Lai, G.H.; Lak, J.H.; Tsai, D.H. Hydrogen production via low-temperature steam–methane reforming using Ni–CeO2–Al2O3 hybrid nanoparticle clusters as catalysts. ACS Appl. Energy Mater. 2019, 2, 7963–7971. [Google Scholar] [CrossRef]
- Ovenston, A.; Walls, J.R. Generation of heat in a single catalyst pellet placed in an electromagnetic field for endothermic reforming of hydrocarbons. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1983, 79, 1073–1084. [Google Scholar] [CrossRef]
- Pérez-Camacho, M.N.; Abu-Dahrieh, J.; Rooney, D.; Sun, K. Biogas reforming using renewable wind energy and induction heating. Catal. Today 2015, 242, 129–138. [Google Scholar] [CrossRef]
- Vinum, M.G.; Almind, M.R.; Engbæk, J.S.; Vendelbo, S.B.; Hansen, M.F.; Frandsen, C.; Bendix, J.; Mortensen, P.M. Dual-Function Cobalt–Nickel Nanoparticles Tailored for High-Temperature Induction-Heated Steam Methane Reforming. Angew. Chem. 2018, 130, 10729–10733. [Google Scholar] [CrossRef] [Green Version]
- Almind, M.R.; Vendelbo, S.B.; Hansen, M.F.; Vinum, M.G.; Frandsen, C.; Mortensen, P.M.; Engbæk, J.S. Improving performance of induction-heated steam methane reforming. Catal. Today 2020, 342, 13–20. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen production technologies: Current state and future developments. Conf. Papers Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lin, G.; Chen, J. Evaluation and calculation on the efficiency of a water electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 10851–10858. [Google Scholar] [CrossRef]
- Burton, N.A.; Padilla, R.V.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021, 135, 110255. [Google Scholar] [CrossRef]
- Chisholm, G.; Zhao, T.; Cronin, L. Hydrogen from water electrolysis. In Storing Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 559–591. [Google Scholar]
- Li, W.; Tian, H.; Ma, L.; Wang, Y.; Liu, X.; Gao, X. Low-Temperature Water Electrolysis: Fundamentals, Progress, and New Strategies. Mater. Adv. 2022, 3, 5598–5644. [Google Scholar] [CrossRef]
- Kaneko, H.; Gokon, N.; Hasegawa, N.; Tamaura, Y. Solar thermochemical process for hydrogen production using ferrites. Energy 2005, 30, 2171–2178. [Google Scholar] [CrossRef]
- Cui, B.; Zhang, J.; Liu, S.; Liu, X.; Zhang, Z.; Sun, J. A low-temperature electro-thermochemical water-splitting cycle for hydrogen production based on LiFeO2/Fe redox pair. Int. J. Hydrogen Energy 2020, 45, 20800–20807. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A study on the Fe–Cl thermochemical water splitting cycle for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 18867–18875. [Google Scholar] [CrossRef]
- Ponikvar, Z.; Likozar, B.; Gyergyek, S. Electrification of Catalytic Ammonia Production and Decomposition Reactions: From Resistance, Induction, and Dielectric Reactor Heating to Electrolysis. ACS Appl. Energy Mater. 2022, 5, 5457–5472. [Google Scholar] [CrossRef]
- Houlding, T.K.; Rebrov, E.V. Application of alternative energy forms in catalytic reactor engineering. Green Process. Synth. 2012, 1, 19–31. [Google Scholar] [CrossRef]
- Appino, C.; Bottauscio, O.; de la Barriere, O.; Fiorillo, F.; Manzin, A.; Ragusa, C. Computation of eddy current losses in soft magnetic composites. IEEE Trans. Magn. 2012, 48, 3470–3473. [Google Scholar] [CrossRef] [Green Version]
- Vallejo-Fernandez, G.; O’Grady, K. Effect of the distribution of anisotropy constants on hysteresis losses for magnetic hyperthermia applications. Appl. Phys. Lett. 2013, 103, 142417. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- De la Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.P.; Rivero, G.; Hernando, A. Study of Heating Efficiency as a Function of Concentration, Size, and Applied Field in γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 2012, 116, 25602–25610. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Ruta, S.; Chantrell, R.; Hovorka, O. Unified model of hyperthermia via hysteresis heating in systems of interacting magnetic nanoparticles. Sci. Rep. 2015, 5, 9090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie-Pelecky, D.L.; Rieke, R.D. Magnetic properties of nanostructured materials. Chem. Mater. 1996, 8, 1770–1783. [Google Scholar] [CrossRef]
- Fursina, A.A.; Sofin, R.G.S.; Shvets, I.V.; Natelson, D. Origin of hysteresis in resistive switching in magnetite is Joule heating. Phys. Rev. B 2009, 79, 245131. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Moura, N.; Bajgiran, K.R.; Melvin, A.T.; Dooley, K.M.; Dorman, J.A. Direct Probing of Fe3O4 Nanoparticle Surface Temperatures during Magnetic Heating: Implications for Induction Catalysis. ACS Appl. Nano Mater. 2021, 4, 13778–13787. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, S.; Bi, Q.; Yang, L.; Xing, H.; Liu, H. Preparation of ferrite magnetic nanocatalysts and their applications in the field of resources and energy. Adv. Chem. 2019, 31, 381–393. [Google Scholar]
- Zhao, F.; Zhang, B.; Feng, L. Preparation and magnetic properties of magnetite nanoparticles. Mater. Lett. 2012, 68, 112–114. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Wang, H.M.; Hsieh, S.L.; Wu, C.H.; Chou, H.H.; Hsieh, S. Role of Néel and brownian relaxation mechanisms for water-based Fe3O4 nanoparticle ferrofluids in hyperthermia. Biomed. Eng. Appl. Basis Commun. 2010, 22, 393–399. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Wang, X.Z.; Chen, Y.Q.; Dai, L.Y. NiCo2O4 nanoparticles: An efficient and magnetic catalyst for Knoevenagel condensation. J. Zhejiang Univ. Sci. A 2020, 21, 74–84. [Google Scholar] [CrossRef]
- Malik, R.; Reddy, V.R.; Vas, J.V.; Medwal, R.; Annapoorni, S. Designed synthesis of FexCo100-x alloy nanoparticles by polyol reduction: An evolution of structural, morphological and magnetic properties. IEEE Trans. Magn. 2022. [Google Scholar] [CrossRef]
- Rafienia, M.; Bigham, A.; Hassanzadeh-Tabrizi, S.A. Solvothermal synthesis of magnetic spinel ferrites. J. Med. Signals Sens. 2018, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhou, L.; Hui, X.; Zhao, G.; Li, Y. Application of superparamagnetic nanocatalysts in organic synthesis. Adv. Chem. 2012, 24, 327–337. [Google Scholar]
- Ceylan, S.; Friese, C.; Lammel, C.; Mazac, K.; Kirschning, A. Inductive heating for organic synthesis by using functionalized magnetic nanoparticles inside microreactors. Angew. Chem. Int. Ed. 2008, 47, 8950–8953. [Google Scholar] [CrossRef]
- Morales, F.; Márquez, G.; Sagredo, V.; Torres, T.E.; Denardin, J.C. Structural and magnetic properties of silica-coated magnetite nanoaggregates. Phys. B Condens. Matter 2019, 572, 214–219. [Google Scholar] [CrossRef]
- Fajaroh, F.; Setyawan, H.; Nur, A.; Lenggoro, I.W. Thermal stability of silica-coated magnetite nanoparticles prepared by an electrochemical method. Adv. Powder Technol. 2013, 24, 507–511. [Google Scholar] [CrossRef]
- Deng, Y.H.; Wang, C.C.; Hu, J.H.; Yang, W.L.; Fu, S.K. Investigation of formation of silica-coated magnetite nanoparticles via sol–gel approach. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 87–93. [Google Scholar] [CrossRef]
- Houlding, T.K.; Gao, P.; Degirmenci, V.; Tchabanenko, K.; Rebrov, E.V. Mechanochemical synthesis of TiO2/NiFe2O4 magnetic catalysts for operation under RF field. Mater. Sci. Eng. B 2015, 193, 175–180. [Google Scholar] [CrossRef]
- Cervantes, O.; Lopez, Z.R.; Casillas, N.; Knauth, P.; Checa, N.; Cholico, F.A.; Hernandez- Gutiérrez, R.; Quintero, L.H.; Paz, J.A.; Cano, M.E. A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production. Molecules 2022, 27, 544. [Google Scholar] [CrossRef]
- Yin, Z.-C.; Zhang, Y.-G. Preparation of nickel-cobalt alloy/carbon nanotube nanocomposites and their catalytic properties. J. Anqing Norm. Univ. Nat. Sci. Ed. 2019, 25, 82–92. [Google Scholar]
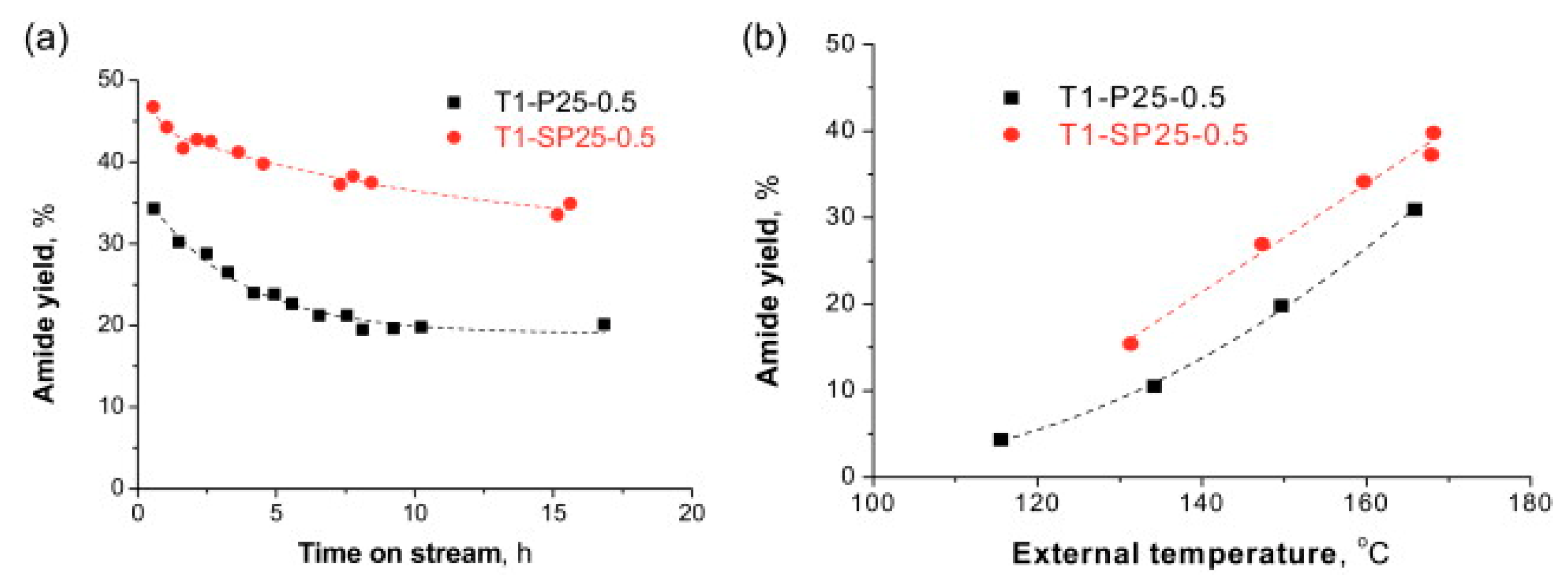
- Liu, Y.; Gao, P.; Cherkasov, N.; Rebrov, E.V. Direct amide synthesis over core–shell TiO2@NiFe2O4 catalysts in a continuous flow radiofrequency-heated reactor. RSC Adv. 2016, 6, 100997–101007. [Google Scholar] [CrossRef] [Green Version]
- Marbaix, J.; Mille, N.; Lacroix, L.M.; Asensio, J.M.; Fazzini, P.F.; Soulantica, K.; Carrey, J.; Chaudret, B. Tuning the composition of FeCo nanoparticle heating agents for magnetically induced catalysis. ACS Appl. Nano Mater. 2020, 3, 3767–3778. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, X.; Hua, R.; Zhang, R.; Yao, Y.; Zhao, B.; Liu, T.; Zheng, J.; Lu, G. Remarkably catalytic activity in the reduction of 4-nitrophenol and methylene blue by Fe3O4@COF supported noble metal nanoparticles. Appl. Catal. B Environ. 2020, 260, 118142. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Song, S.; Liu, D.; Zhang, H. Selectively deposited noble metal nanoparticles on Fe3O4/graphene composites: Stable, recyclable, and magnetically separable catalysts. Chem. A Eur. J. 2012, 18, 7601–7607. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, K.; Liu, P.; Chen, M. Construction of detachable core/shell Fe3O4@C supported noble metal catalysts and their catalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123729. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Wei, Y.; Jin, Y.; Sun, S.; Wang, Z.; Jiang, Y. Silver nanoparticles decorated magnetic polymer composites (Fe3O4@PS@Ag) as highly efficient reusable catalyst for the degradation of 4-nitrophenol and organic dyes. J. Environ. Manag. 2021, 278, 111473. [Google Scholar] [CrossRef]
- Piner, R.; Li, H.; Kong, X.; Tao, L.; Kholmanov, I.N.; Ji, H.; Lee, W.H.; Suk, J.W.; Ye, J.; Chen, S.; et al. Graphene synthesis via magnetic inductive heating of copper substrates. ACS Nano 2013, 7, 7495–7499. [Google Scholar] [CrossRef]
- García-Aguilar, J.; Fernández-García, J.; Rebrov, E.V.; Lees, M.R.; Gao, P.; Cazorla-Amorós, D.; Berenguer-Murcia, Á. Magnetic zeolites: Novel nanoreactors through radiofrequency heating. Chem. Commun. 2017, 53, 4262–4265. [Google Scholar] [CrossRef] [PubMed]
- Zadražil, A.; Štěpánek, F. Remote control of reaction rate by radiofrequency heating of composite catalyst pellets. Chem. Eng. Sci. 2015, 134, 721–726. [Google Scholar] [CrossRef]
- Chaudhuri, S.R.; Hartwig, J.; Kupracz, L.; Kodanek, T.; Wegner, J.; Kirschning, A. Oxidations of Allylic and Benzylic Alcohols under Inductively-Heated Flow Conditions with Gold-Doped Superparamagnetic Nanostructured Particles as Catalyst and Oxygen as Oxidant. Adv. Synth. Catal. 2014, 356, 3530–3538. [Google Scholar] [CrossRef]
- Marin, I.M.; De Masi, D.; Lacroix, L.M.; Fazzini, P.F.; van Leeuwen, P.W.; Asensio, J.M.; Chaudret, B. Hydrodeoxygenation and hydrogenolysis of biomass-based materials using FeNi catalysts and magnetic induction. Green Chem. 2021, 23, 2025–2036. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, Y.; Xia, S.; Yu, J.; Ding, B. Direct magnetic reinforcement of electrocatalytic ORR/OER with electromagnetic induction of magnetic catalysts. Adv. Mater. 2021, 33, 2007525. [Google Scholar] [CrossRef] [PubMed]
- Yassine, S.R.; Fatfat, Z.; Darwish, G.H.; Karam, P. Localized catalysis driven by the induction heating of magnetic nanoparticles. Catal. Sci. Technol. 2020, 10, 3890–3896. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sadhukhan, J. Process intensification aspects for steam methane reforming: An overview. AIChE J. 2009, 55, 408–422. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Advances in hydrogen production from natural gas reforming. Adv. Energy Sustain. Res. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies–A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.; Song, W.; Xu, D. Methane steam reforming thermally coupled with catalytic combustion in catalytic microreactors for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 664–680. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, W.; Zhong, Y.; Yang, Y.; Hong, M.; Shen, Z. 3D-Printed Regular-Porous Structure with Trapezoidal Multiple Microchannels as Combustion Reaction Support for the Autothermal Methanol Steam Reforming Microreactor for Hydrogen Production. Ind. Eng. Chem. Res. 2022, 61, 2443–2454. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Engbæk, J.S.; Vendelbo, S.B.; Hansen, M.F.; Østberg, M. Direct hysteresis heating of catalytically active Ni–Co nanoparticles as steam reforming catalyst. Ind. Eng. Chem. Res. 2017, 56, 14006–14013. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Phan, C.M.; Liu, S.; Pham-Huu, C.; Nguyen-Dinh, L. Radio-frequency induction heating powered low-temperature catalytic CO2 conversion via bi-reforming of methane. Chem. Eng. J. 2022, 430, 132934. [Google Scholar] [CrossRef]
- Varsano, F.; Bellusci, M.; La Barbera, A.; Petrecca, M.; Albino, M.; Sangregorio, C. Dry reforming of methane powered by magnetic induction. Int. J. Hydrogen Energy 2019, 44, 21037–21044. [Google Scholar] [CrossRef]
- Fache, A.; Marias, F.; Chaudret, B. Catalytic reactors for highly exothermic reactions: Steady-state stability enhancement by magnetic induction. Chem. Eng. J. 2020, 390, 124531. [Google Scholar] [CrossRef]
- Almind, M.R.; Vinum, M.G.; Wismann, S.T.; Hansen, M.F.; Vendelbo, S.B.; Engbæk, J.S.; Mortensen, P.M.; Chorkendorff, I.; Frandsen, C. Optimized CoNi Nanoparticle Composition for Curie-Temperature-Controlled Induction-Heated Catalysis. ACS Appl. Nano Mater. 2021, 4, 11537–11544. [Google Scholar] [CrossRef]
- Almind, M.R. Induction-Heated Catalytic Hydrogen Production: A Magnetic Investigation. Ph.D. Thesis, Department of Physics, Technical University of Denmark, Kongens Lyngby, Denmark, 2020. [Google Scholar]
- Abd Ghani, N.A.; Azapour, A.; Muhammad, A.F.S.; Abdullah, B. Dry reforming of methane for hydrogen production over NiCo catalysts: Effect of NbZr promoters. Int. J. Hydrogen Energy 2019, 44, 20881–20888. [Google Scholar] [CrossRef]
- Beheshti Askari, A.; Al Samarai, M.; Morana, B.; Tillmann, L.; Pfänder, N.; Wandzilak, A.; Watts, B.; Belkhou, R.; Muhler, M.; DeBeer, S. In situ X-ray microscopy reveals particle dynamics in a NiCo dry methane reforming catalyst under operating conditions. ACS Catal. 2020, 10, 6223–6230. [Google Scholar] [CrossRef] [PubMed]
- Horlyck, J.; Lawrey, C.; Lovell, E.C.; Amal, R.; Scott, J. Elucidating the impact of Ni and Co loading on the selectivity of bimetallic NiCo catalysts for dry reforming of methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Neither, C.; Faure, S.; Bordet, A.; Deseure, J.; Chatenet, M.; Carrey, J.; Chaudret, B.; Rouet, A. Improved water electrolysis using magnetic heating of FeC–Ni core–shell nanoparticles. Nat. Energy 2018, 3, 476–483. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Idriss, H. Hydrogen production from water: Past and present. Curr. Opin. Chem. Eng. 2020, 29, 74–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; Li, L.; Zhan, C.; Xiao, F.; Quan, X.; Li, W. Effect of the thickness of nickel electrode with aligned porous structure on hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 13552–13560. [Google Scholar] [CrossRef]
- Su, M.; Wenda, Z.; Lin, L.; Chen, M.; Jiang, Z.; Luo, X.; Yang, Y.; Yu, T.; Lei, W.; Yuan, C. Micro Eddy Current Facilitated by Screwed MoS2 Structure for Enhanced Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2111067. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, Y.; Zhou, Z.; Liu, F.; Liu, X.; Yang, L.; Liu, Q.; Sang, Y.; Liu, H.; Zhang, X.; et al. Rapid Synthesis of Various Electrocatalysts on Ni Foam Using a Universal and Facile Induction Heating Method for Efficient Water Splitting. Adv. Funct. Mater. 2021, 31, 2009580. [Google Scholar] [CrossRef]
- Li, T.; Kang, S.; Zhang, X.; Fu, X.; Feng, S.; Hu, Z.; Zhu, D.; Lu, W. Improved hydrogen evolution at high temperature using an electro-thermal method. J. Phys. D Appl. Phys. 2020, 53, 185302. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, B.; Nichols, F.; Ko, J.; Mercado, R.; Bridges, F.; Chen, S. Rapid preparation of carbon-supported ruthenium nanoparticles by magnetic induction heating for efficient hydrogen evolution reaction in both acidic and alkaline media. SusMat 2022, 2, 335–346. [Google Scholar] [CrossRef]
- Badakhsh, A.; Kwak, Y.; Lee, Y.J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; Park, C.W.; Jo, Y.S. A compact catalytic foam reactor for decomposition of ammonia by the Joule-heating mechanism. Chem. Eng. J. 2021, 426, 130802. [Google Scholar] [CrossRef]
- Wu, L.; Ma, H.; Mei, J.; Li, Y.; Xu, Q.; Li, Z. Low energy consumption and high quality bio-fuels production via in-situ fast pyrolysis of reed straw by adding metallic particles in an induction heating reactor. Int. J. Hydrogen Energy 2022, 47, 5828–5841. [Google Scholar] [CrossRef]
- Chen, H.; Lee, J.; Zheng, Y.; Duan, Q. A non-traditional energy transfer process in CWPO heterogeneous reaction for wastewater treatment. Chem. Eng. Res. Des. 2016, 114, 142–147. [Google Scholar] [CrossRef]
- Márquez, J.J.R.; Levchuk, I.; Sillanpää, M. Application of catalytic wet peroxide oxidation for industrial and urban wastewater treatment: A review. Catalysts 2018, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Melero, J.A.; Martínez, F.; Botas, J.A.; Molina, R.; Pariente, M.I. Heterogeneous catalytic wet peroxide oxidation systems for the treatment of an industrial pharmaceutical wastewater. Water Res. 2009, 43, 4010–4018. [Google Scholar] [CrossRef]
- Munoz, M.; Nieto-Sandoval, J.; Serrano, E.; de Pedro, Z.M.; Casas, J.A. CWPO intensification by induction heating using magnetite as catalyst. J. Environ. Chem. Eng. 2020, 8, 104085. [Google Scholar] [CrossRef]
- Jing, J.; Jianchao, L.; Yong, M.; Na, X. Preparation and performance of catalysts for dye wastewater treatment using CWPO in induction heating fixed bed (IHFB) reactor. Chin. J. Environ. Eng. 2014, 8, 3125–3131. [Google Scholar]





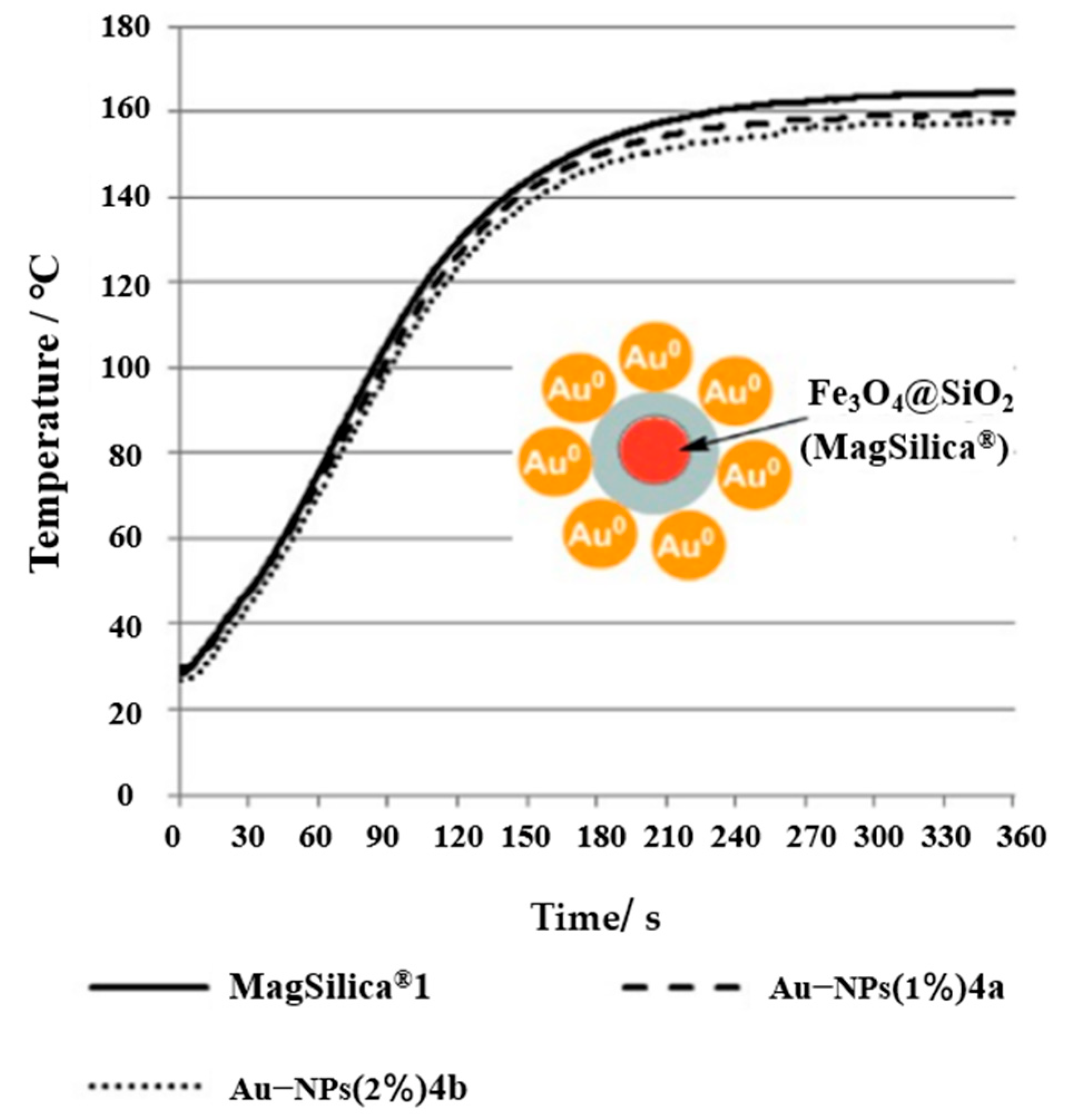
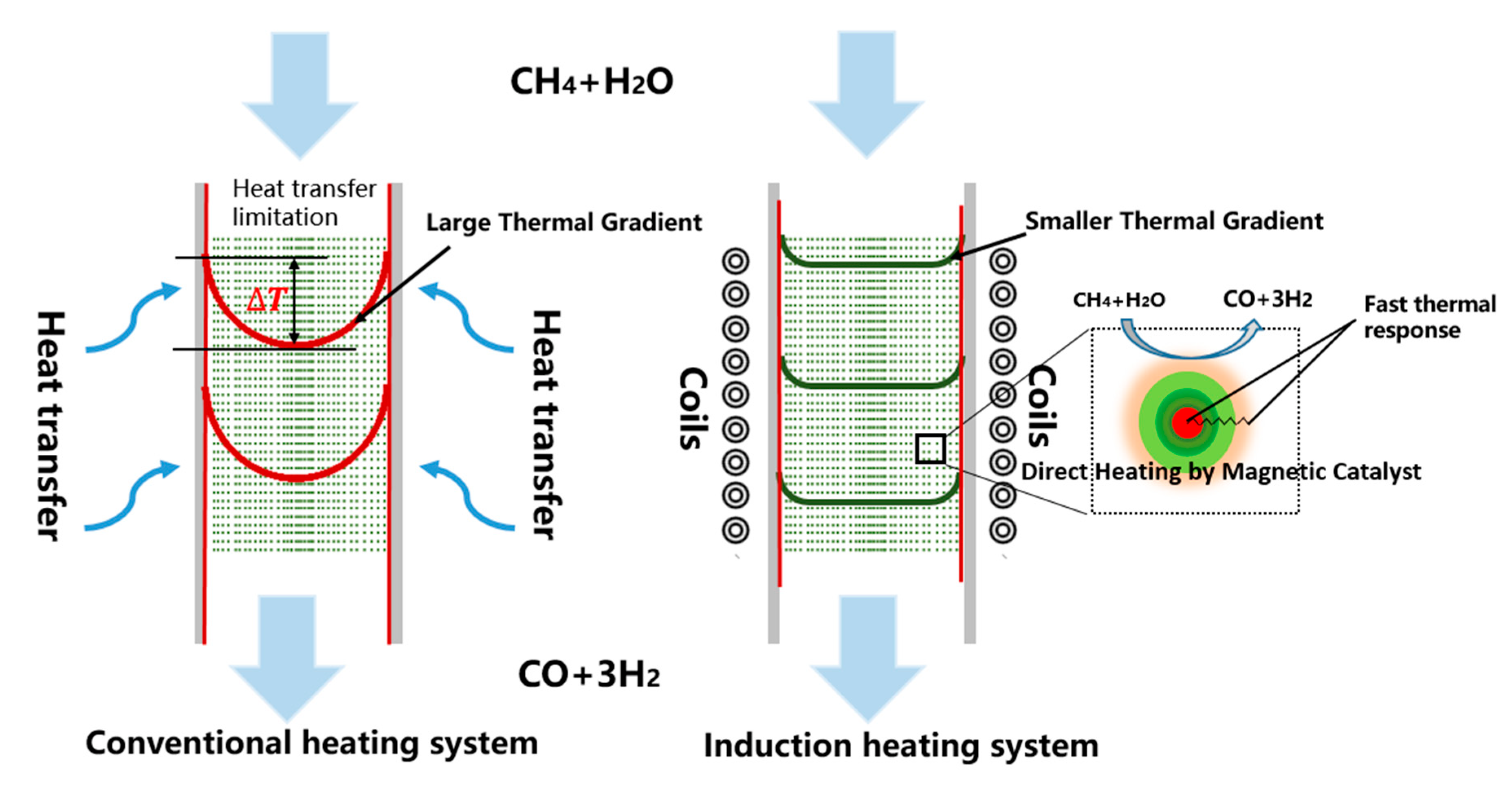
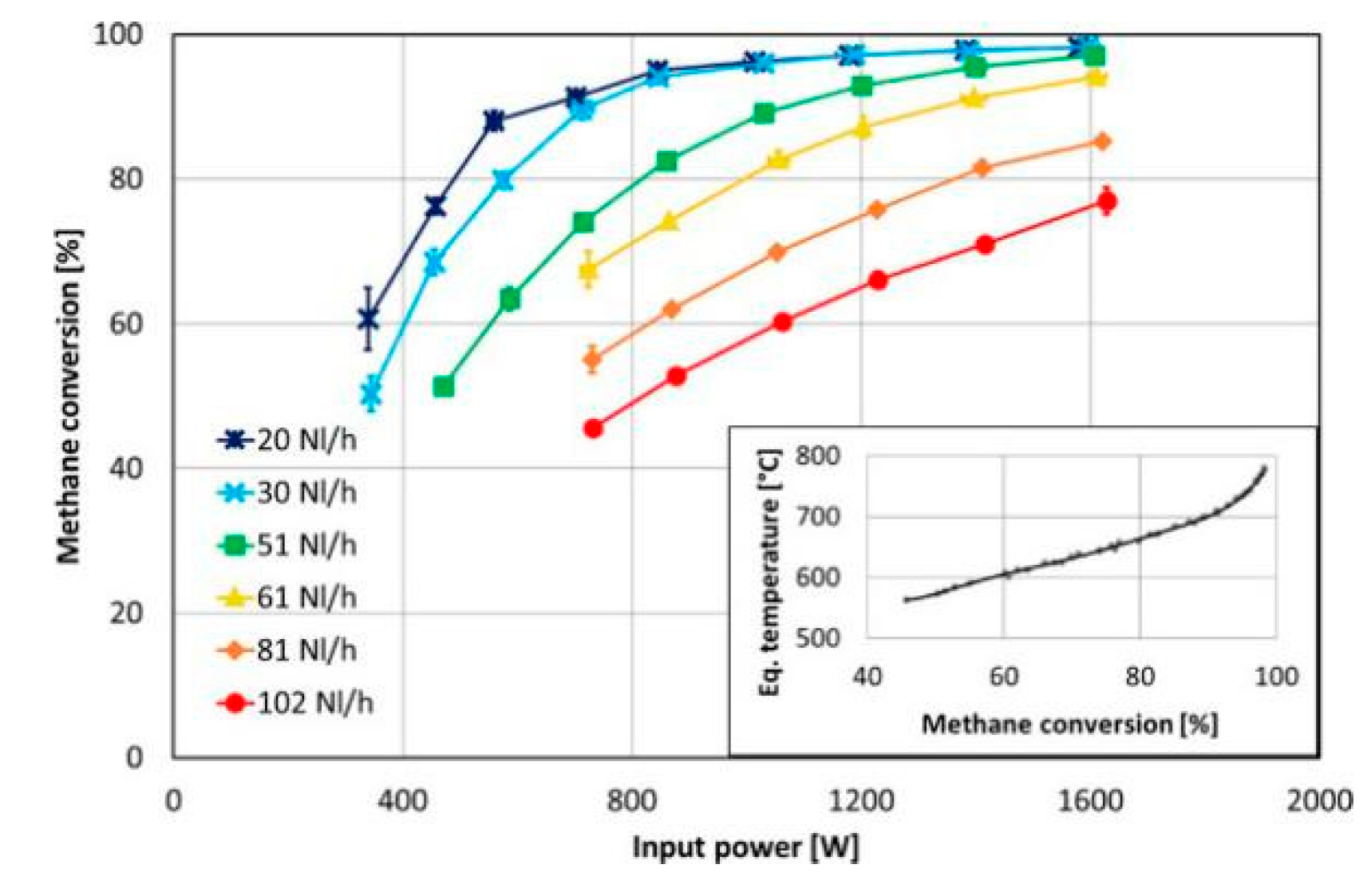
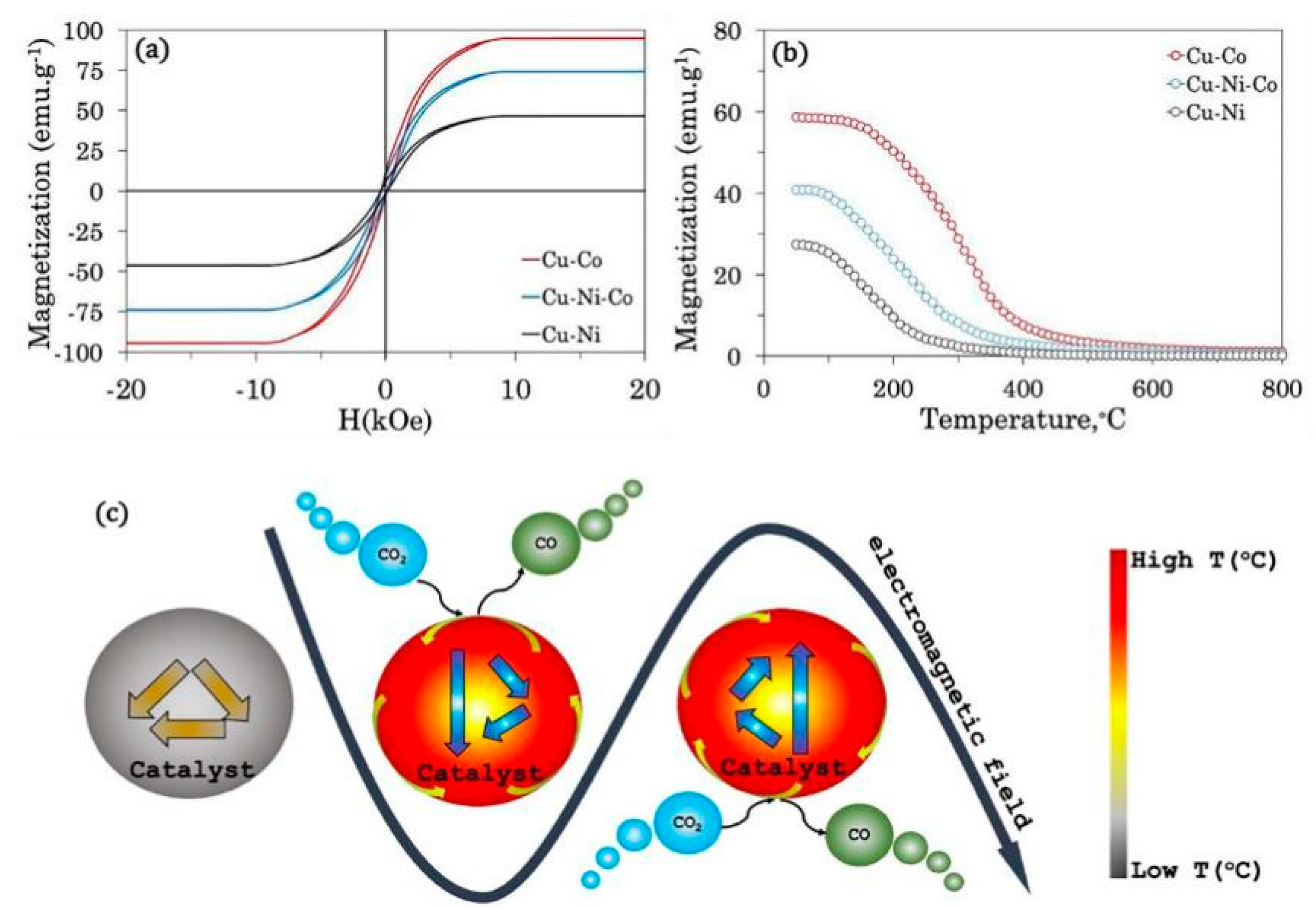
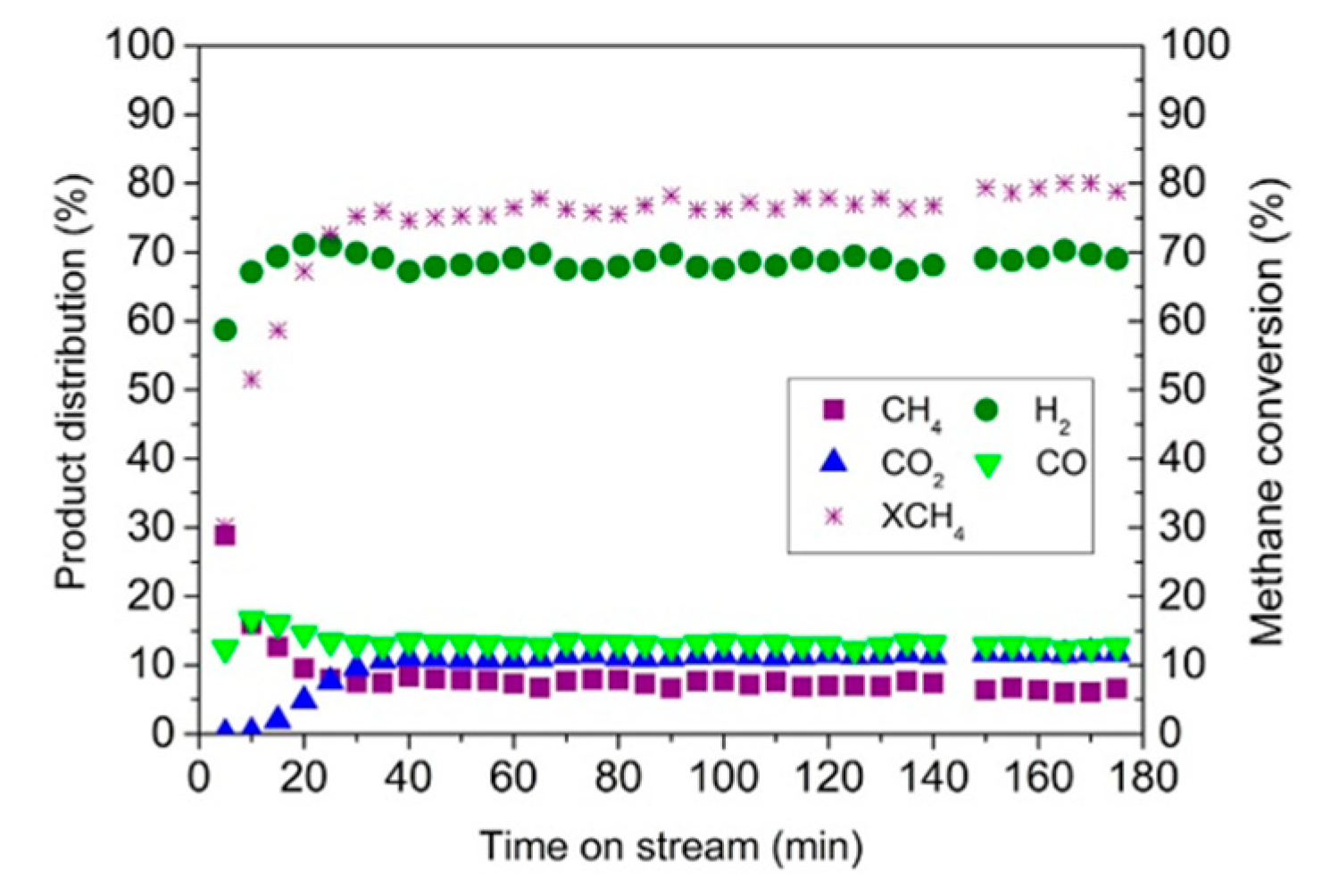
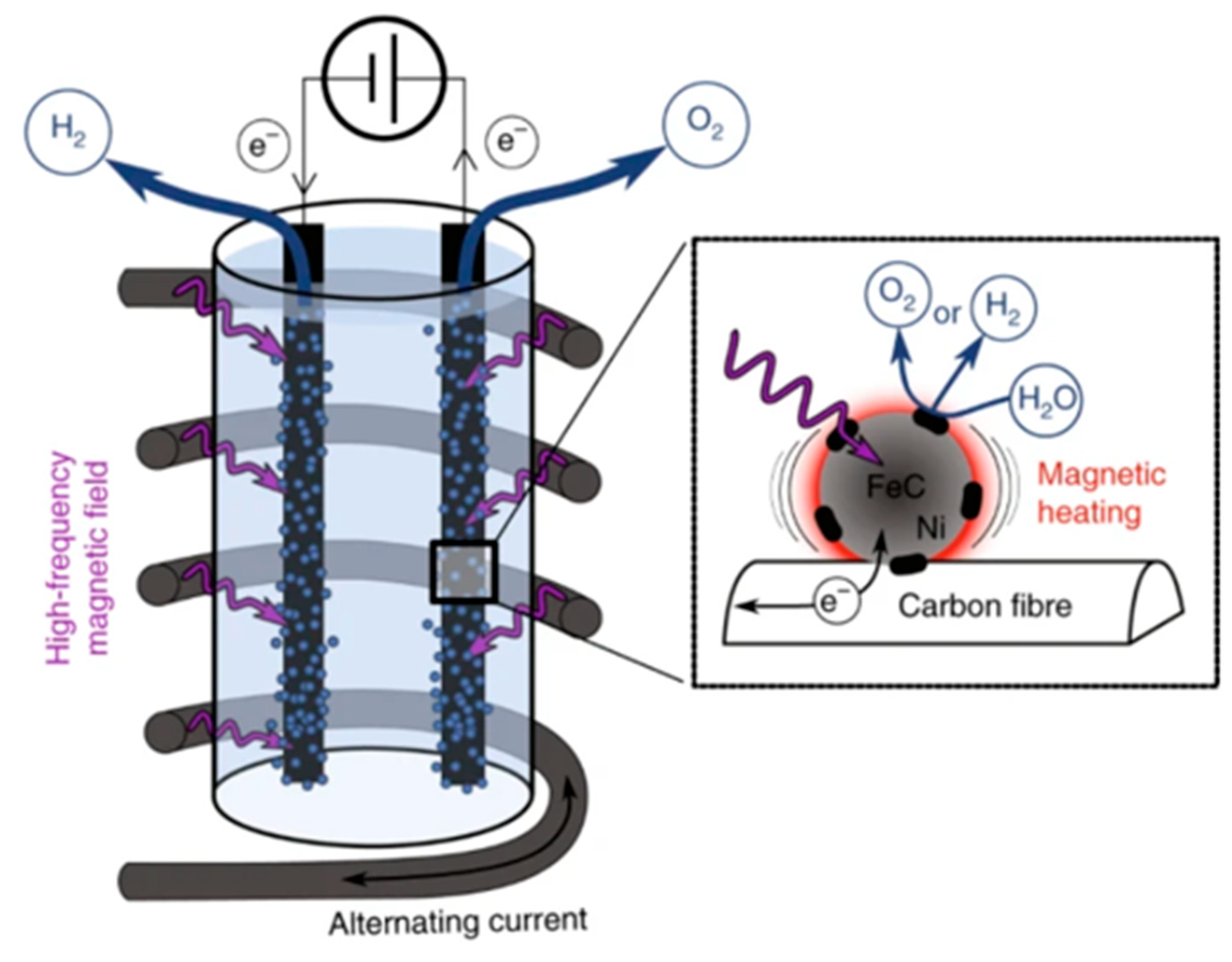

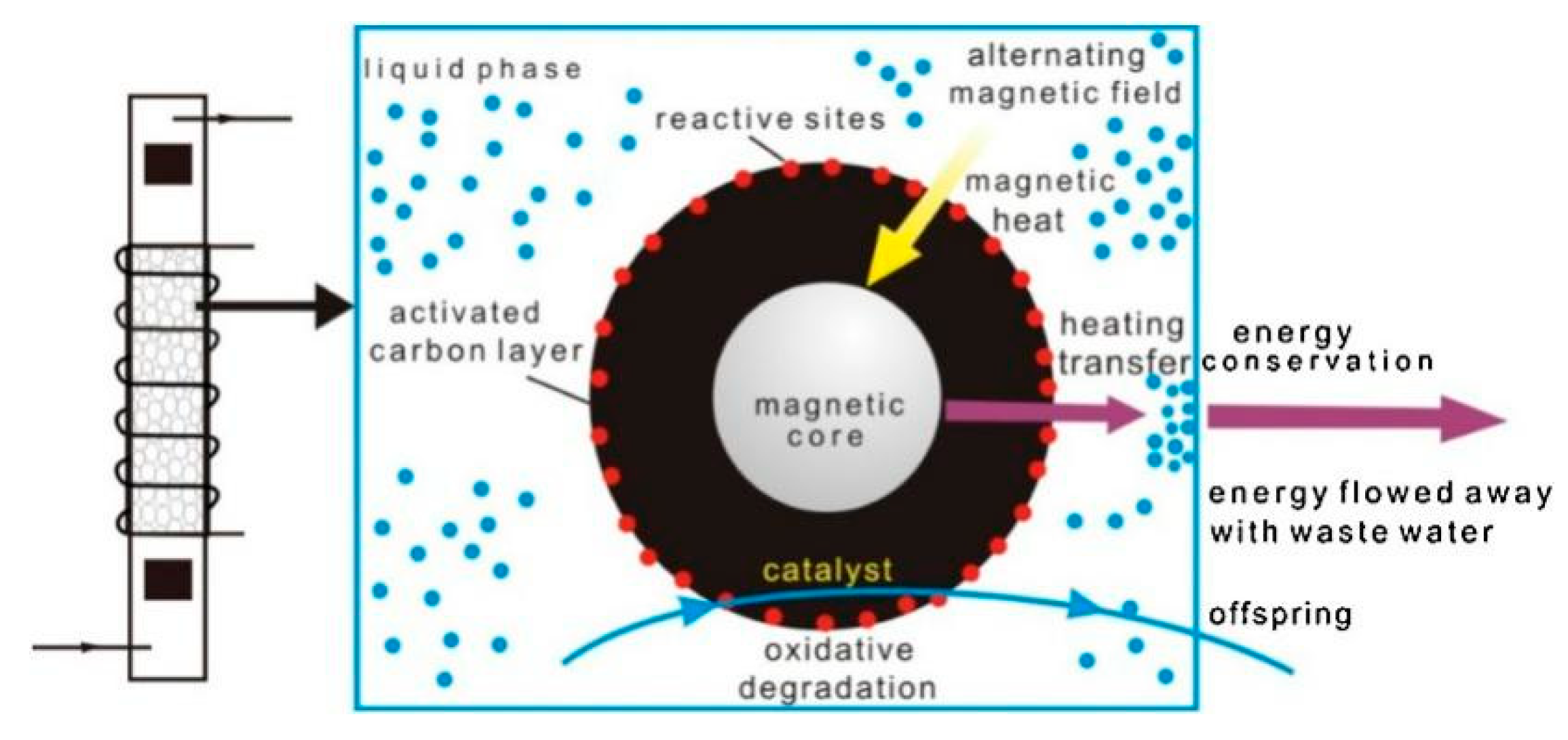
| Catalyst | Reaction Temperature | Methane Conversion Rate | Advantages | Literature Sources |
|---|---|---|---|---|
| Ni60Co40 | 720 °C | 80% | Easily scalable to mass production | Scarfiello et al. [16] |
| CoNi Cu⊂CoNi | 715 °C | 90.95% | Cu-doped catalyst has achieved 95% methane conversion | Pérez- Vinum et al. [26] |
| CoNi | 800 °C | 90% | Easy to prepare and High activity | Almind et al. [27] |
| NiCo/MgAl2O4 | 780 °C | 98% | Direct heating, High catalytic activity and High methane conversion rate | Mortensen et al. [79] |
| Cu-Co | 500 °C | 62% | The Cu-Co catalyst preserved its catalytic stability for at least 50 h at 500 °C. | Nguyen et al. [80] |
| Ni60Co40 | 850 °C | 70% | Minimize catalyst deactivation | Varsano et al. [81] |
| Co50Ni50 | 767 °C | 98% | High methane conversion rate and No support material required | Almind et al. [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Guan, D.; Li, Y.; Zhong, J. Research Progress on Magnetic Catalysts and Its Application in Hydrogen Production Area. Energies 2022, 15, 5327. https://doi.org/10.3390/en15155327
Wang F, Guan D, Li Y, Zhong J. Research Progress on Magnetic Catalysts and Its Application in Hydrogen Production Area. Energies. 2022; 15(15):5327. https://doi.org/10.3390/en15155327
Chicago/Turabian StyleWang, Feng, Delun Guan, Yatian Li, and Jingxuan Zhong. 2022. "Research Progress on Magnetic Catalysts and Its Application in Hydrogen Production Area" Energies 15, no. 15: 5327. https://doi.org/10.3390/en15155327
APA StyleWang, F., Guan, D., Li, Y., & Zhong, J. (2022). Research Progress on Magnetic Catalysts and Its Application in Hydrogen Production Area. Energies, 15(15), 5327. https://doi.org/10.3390/en15155327






