Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Strains, Media, and Growth Conditions
2.3. Lipase Assay
2.4. Flow Cytometry Analysis
2.5. Analysis of Extracellular Free Fatty Acids
3. Results and Discussion
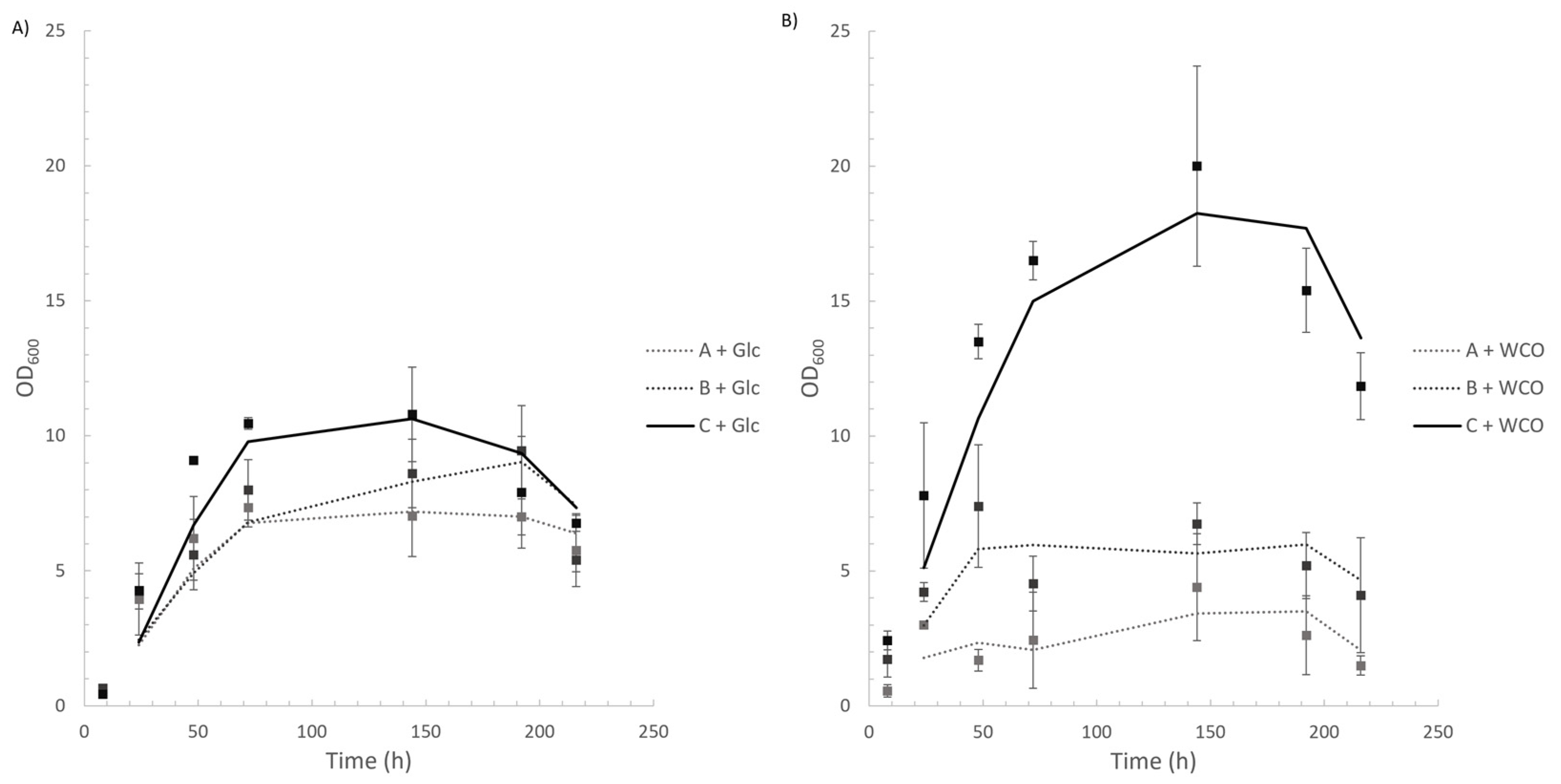
3.1. Small-Scale Batch Cultures
3.1.1. Cell Growth
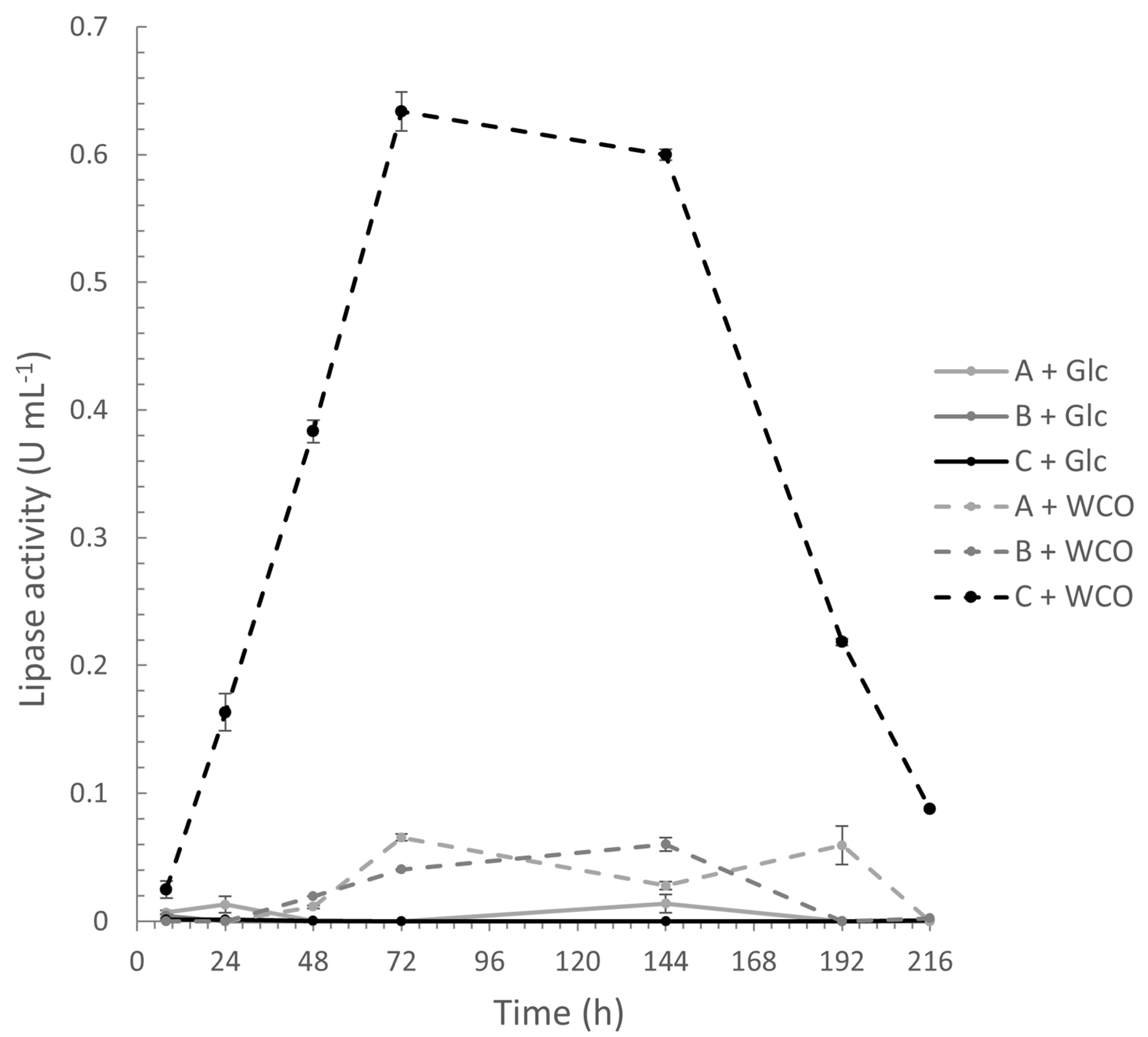
3.1.2. Lipase Activity Assay
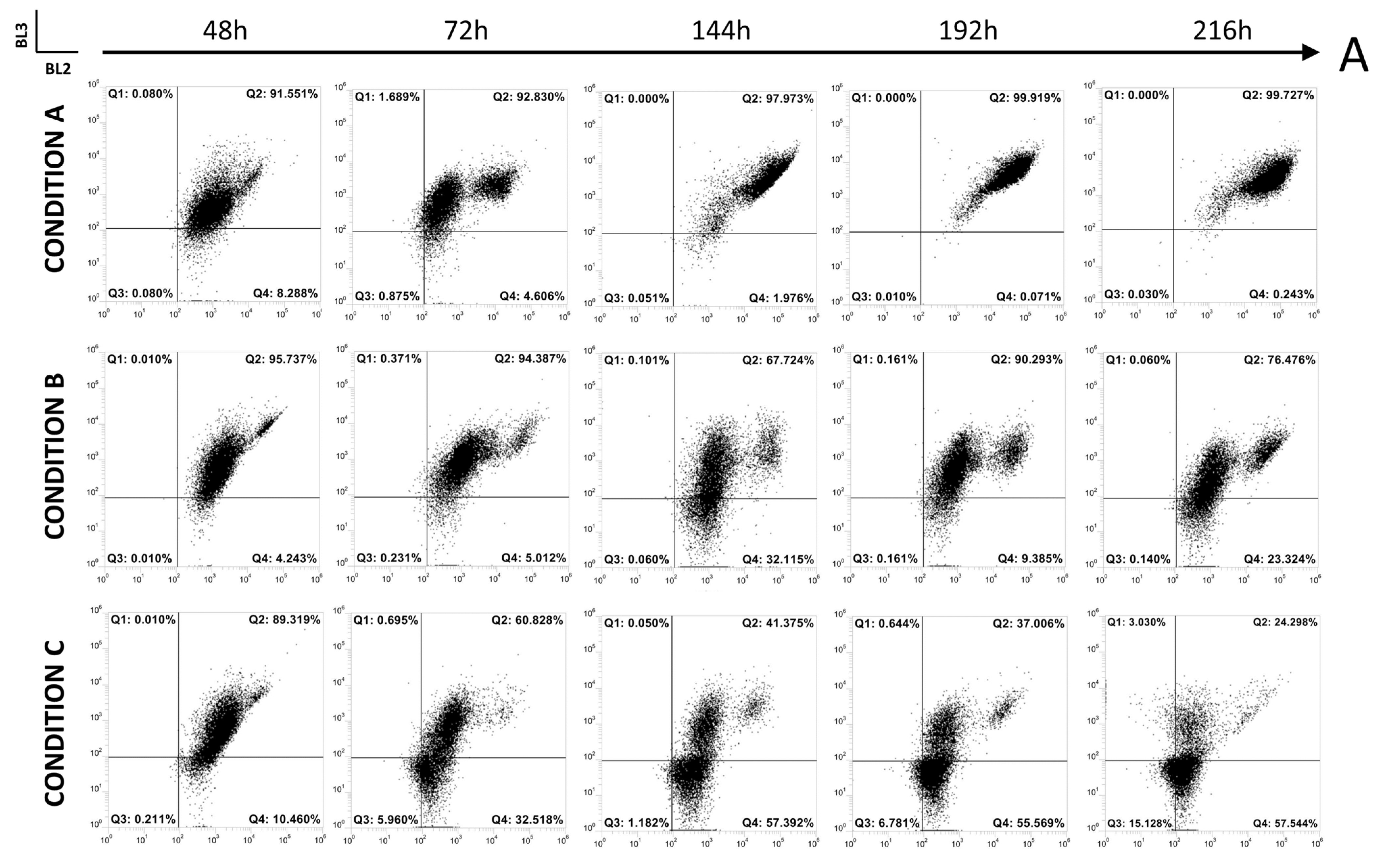
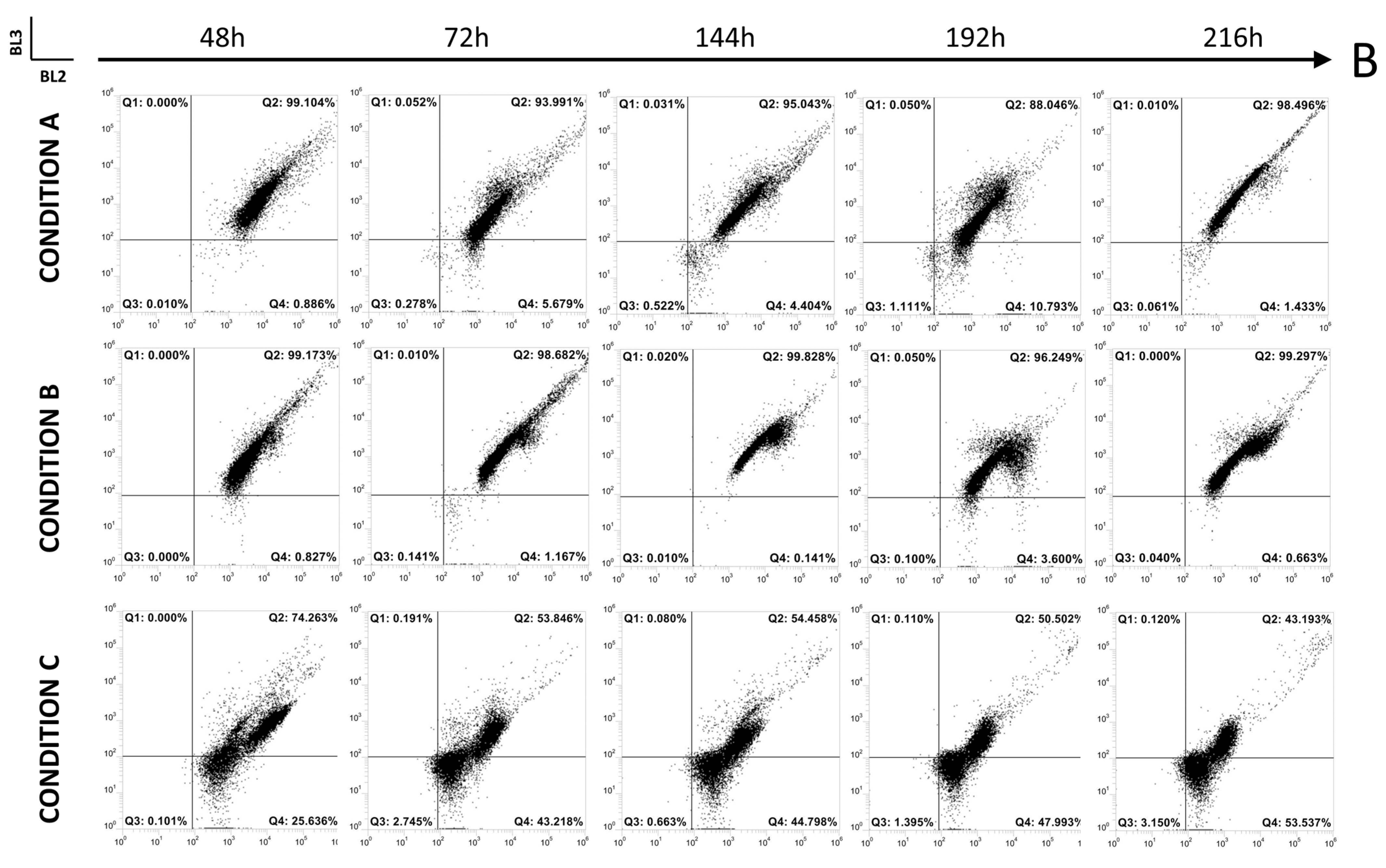
3.1.3. Flow Cytometry of Y. lipolytica W29 Cells
3.2. Up-Scale Batch Cultures
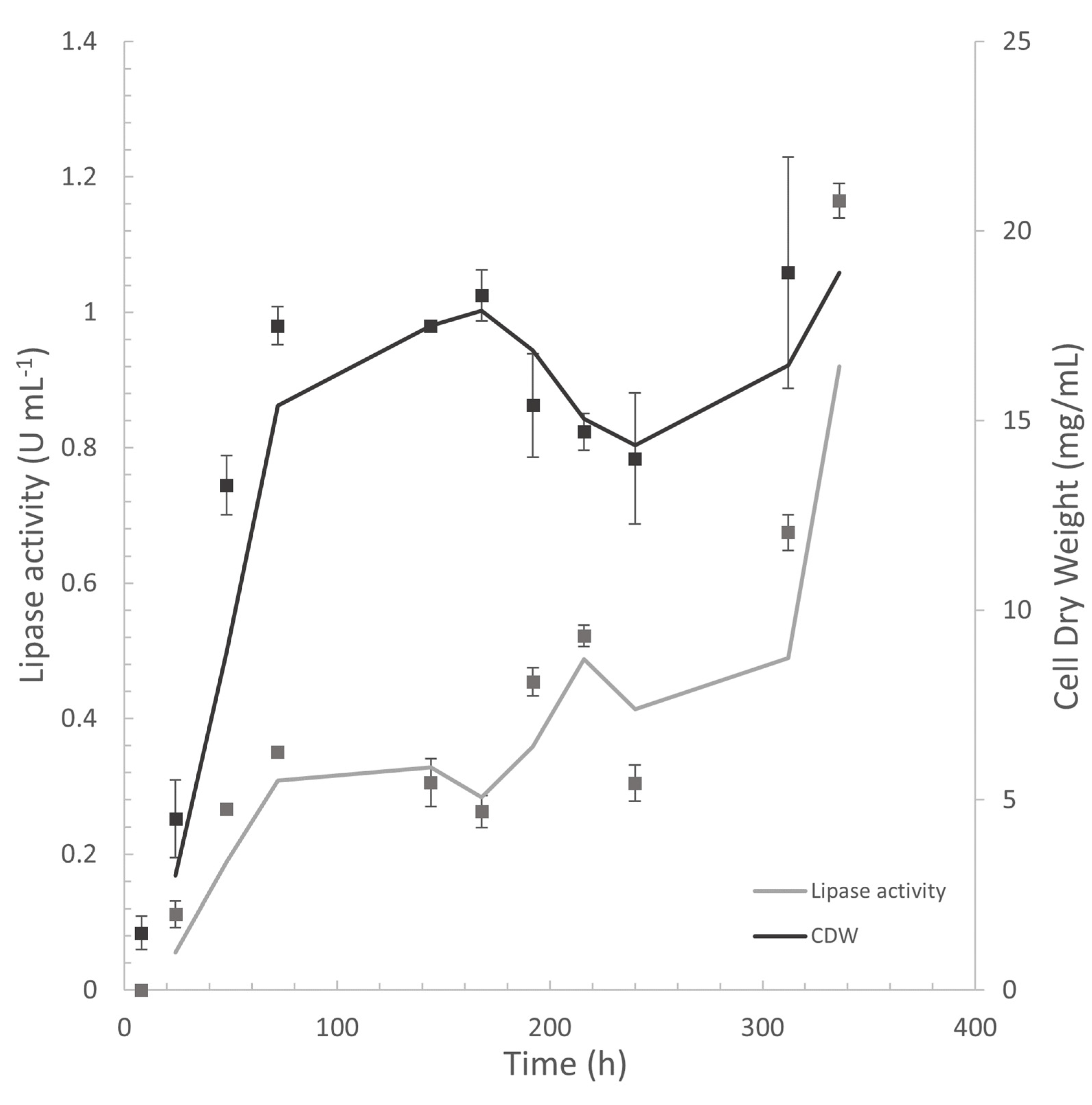
3.2.1. Cell Growth, Lipase Assay, and FFA Determination
3.2.2. Online Flow Cytometry Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Madzak, C. Yarrowia lipolytica Strains and Their Biotechnological Applications: How Natural Biodiversity and Metabolic Engineering Could Contribute to Cell Factories Improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef]
- Srinivasan, G.R.; Jambulingam, R. Comprehensive Study on Biodiesel Produced from Waste Animal Fats—A Review. J. Environ. Sci. Technol. 2018, 11, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Zha, L.; Abomohra, A.E.F.; Li, X.; Zhang, C.; Salama, E.S. Evaluation of Animal- and Plant-Based Lipidic Waste in Anaerobic Digestion: Kinetics of Long-Chain Fatty Acids Degradation. Crit. Rev. Biotechnol. 2020, 40, 733–749. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; de Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef] [Green Version]
- Radha, P.; Narayanan, S.; Chaudhuri, A.; Anjum, S.; Thomas, D.L.; Pandey, R.; Ramani, K. Synthesis of Single-Cell Oil by Yarrowia lipolytica MTCC 9520 Utilizing Slaughterhouse Lipid Waste for Biodiesel Production. Biomass Convers. Biorefinery 2020, 1–12. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Beopoulos, A.; Chardot, T.; Nicaud, J.M. Yarrowia lipolytica: A Model and a Tool to Understand the Mechanisms Implicated in Lipid Accumulation. Biochimie 2009, 91, 692–696. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia lipolytica. Yeast 2012, 29, 409–418. [Google Scholar] [CrossRef]
- Larroude, M.; Rossignol, T.; Nicaud, J.M.; Ledesma-Amaro, R. Synthetic Biology Tools for Engineering Yarrowia lipolytica. Biotechnol. Adv. 2018, 36, 2150–2164. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.C.; Strub, C.; Schorr-Galindo, S. Single Cell Oils (SCOs) from Oleaginous Yeasts and Moulds: Production and Genetics. Biomass Bioenergy 2014, 68, 135–150. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part I: Biochemistry of Single Cell Oil Production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial Production of Organic Acids: Expanding the Markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Belo, I. Microbial Valorization of Waste Cooking Oils for Valuable Compounds Production—A Review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2583–2616. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste Cooking Oils as Feedstock for Lipase and Lipid-Rich Biomass Production. Eur. J. Lipid Sci. Technol. 2019, 121, 1800188. [Google Scholar] [CrossRef] [Green Version]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Yarrowia lipolytica as a Model for Bio-Oil Production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Verger, R. ‘Interfacial Activation’ of Lipases: Facts and Artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Biundo, A.; Ribitsch, D.; Guebitz, G.M. Surface Engineering of Polyester-Degrading Enzymes to Improve Efficiency and Tune Specificity. Appl. Microbiol. Biotechnol. 2018, 102, 3551–3559. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The Lipases from Yarrowia lipolytica: Genetics, Production, Regulation, Biochemical Characterization and Biotechnological Applications. Biotechnol. Adv. 2011, 29, 632–644. [Google Scholar] [CrossRef]
- Çağatay, Ş.; Aksu, Z. Use of Different Kinds of Wastes for Lipase Production: Inductive Effect of Waste Cooking Oil on Activity. J. Biosci. Bioeng. 2021, 132, 234–240. [Google Scholar] [CrossRef]
- Ciliberti, C.; Biundo, A.; Colacicco, M.; Agrimi, G.; Pisano, I. Physiological characterization of Yarrowia lipolytica cultures grown on alternative carbon sources to develop microbial platforms for waste cooking oils valorization. Chem. Eng. Trans. 2022. [Google Scholar] [CrossRef]
- Colacicco, M.; Biundo, A.; Ciliberti, C.; Agrimi, G.; Pisano, I. Study of lipase production by Yarrowia lipolytica grown in high concentration of hydrophobic carbon sources. Chem. Eng. Trans. 2022. [Google Scholar] [CrossRef]
- Biundo, A.; Hromic, A.; Pavkov-Keller, T.; Gruber, K.; Quartinello, F.; Haernvall, K.; Perz, V.; Arrell, M.S.; Zinn, M.; Ribitsch, D.; et al. Characterization of a Poly(Butylene Adipate-Co-Terephthalate)-Hydrolyzing Lipase from Pelosinus Fermentans. Appl. Microbiol. Biotechnol. 2016, 100, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.P.; Bougaran, G. The Use of Fluorescent Nile Red and BODIPY for Lipid Measurement in Microalgae. Biotechnol. Biofuels 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarola, A.M.; Girelli, A.M.; Lorusso, S. High Performance Liquid Chromatography Determination of Fatty Acids in Drying Oils Following Lipase Action. J. Chromatogr. Sci. 2012, 50, 294–300. [Google Scholar] [CrossRef]
- Hudson, J.A.; MacKenzie, C.A.M.; Joblin, K.N. Conversion of Oleic Acid to 10-Hydroxystearic Acid by Two Species of Ruminal Bacteria. Appl. Microbiol. Biotechnol. 1995, 44, 1–6. [Google Scholar] [CrossRef]
- Girardi, E.; Agrimi, G.; Goldmann, U.; Fiume, G.; Lindinger, G.; Sedlyarov, V.; Srndic, I.; Gurtl, B.; Agerer, B.; Kartnig, F.; et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat. Commun. 2020, 11, 6145. [Google Scholar] [CrossRef]
- Kuttiraja, M.; Dhouha, A.; Tyagi, R.D. Harnessing the Effect of PH on Lipid Production in Batch Cultures of Yarrowia lipolytica SKY7. Appl. Biochem. Biotechnol. 2018, 184, 1332–1346. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Xia, J.; He, A.; Zhang, T.; Li, X.; Xu, J. Effects of Osmotic Pressure and PH on Citric Acid and Erythritol Production from Waste Cooking Oil by Yarrowia lipolytica. Eng. Life Sci. 2018, 18, 344–352. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Zhang, T.; Deng, Y.; He, J. Citric Acid Production in Yarrowia lipolytica SWJ-1b Yeast When Grown on Waste Cooking Oil. Appl. Biochem. Biotechnol. 2015, 175, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.-E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; van Heuvel, M.; Misset, O. Bacterial lipases. FEMS Microbiol. Rev. 1994, 15, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Overview, A.; Houde, A.; Kademi, A.; Leblanc, D. Lipases and Their Industrial Applications. Appl. Biochem. Biotechnol. 2004, 118, 155–170. [Google Scholar]
- Treichel, H.; de Oliveira, D.; Mazutti, M.A.; di Luccio, M.; Oliveira, J.V. A Review on Microbial Lipases Production. Food Bioprocess Technol. 2010, 3, 182–196. [Google Scholar] [CrossRef]
- Corzo, G.; Revah, S. Production and Characteristics of the Lipase from Yarrowia lipolytica 681. Bioresour. Technol. 1999, 70, 173–180. [Google Scholar] [CrossRef]
- Domínguez, A.; Costas, M.; Longo, M.A.; Sanromán, A. A Novel Application of Solid State Culture: Production of Lipases by Yarrowia lipolytica. Biotechnol. Lett. 2003, 25, 1225–1229. [Google Scholar] [CrossRef]
- Lopes, M.; Gomes, A.S.; Silva, C.M.; Belo, I. Microbial Lipids and Added Value Metabolites Production by Yarrowia lipolytica from Pork Lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Lazar, Z.; Liu, N.; Stephanopoulos, G. Holistic Approaches in Lipid Production by Yarrowia lipolytica. Trends Biotechnol. 2018, 36, 1157–1170. [Google Scholar] [CrossRef]
- Dias, C.; Silva, C.; Freitas, C.; Reis, A.; da Silva, T.L. Effect of Medium PH on Rhodosporidium Toruloides NCYC 921 Carotenoid and Lipid Production Evaluated by Flow Cytometry. Appl. Biochem. Biotechnol. 2016, 179, 776–787. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Erten, H. Production of Oils and Fats by Oleaginous Microorganisms with an Emphasis given to the Potential of the Nonconventional Yeast Yarrowia lipolytica. Crit. Rev. Biotechnol. 2018, 38, 1230–1243. [Google Scholar] [CrossRef]
- Katre, G.; Joshi, C.; Khot, M.; Zinjarde, S.; Ravikumar, A. Evaluation of Single Cell Oil (SCO) from a Tropical Marine Yeast Yarrowia lipolytica NCIM 3589 as a Potential Feedstock for Biodiesel. AMB Express 2012, 2, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez, A.; Deive, F.J.; Sanromán, M.A.; Longo, M.A. Effect of Lipids and Surfactants on Extracellular Lipase Production by Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 2003, 78, 1166–1170. [Google Scholar] [CrossRef]
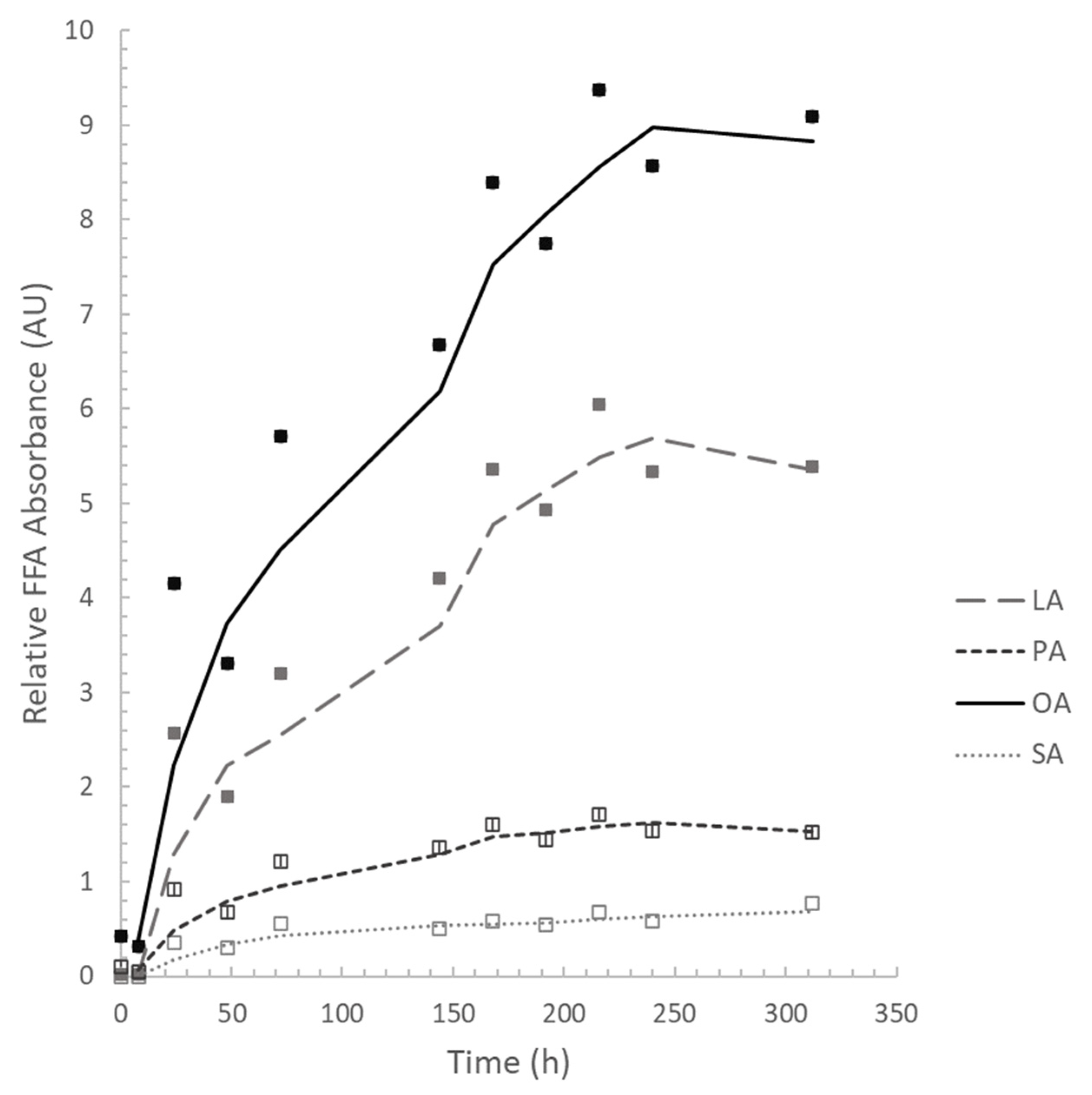

| FFA * | 0 h | 8 h | 24 h | 48 h | 72 h | 144 h | 168 h | 192 h | 216 h | 240 h | 312 h |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | 3.85 | 4.28 | 32.13 | 30.64 | 29.93 | 33.01 | 33.61 | 33.64 | 33.92 | 33.29 | 32.11 |
| PA | 17.55 | 13.58 | 11.50 | 10.95 | 11.41 | 10.67 | 10.04 | 9.83 | 9.64 | 9.57 | 9.09 |
| OA | 78.60 | 82.14 | 51.92 | 53.47 | 53.46 | 52.33 | 52.67 | 52.82 | 52.67 | 53.47 | 54.17 |
| SA | 0 | 0 | 4.45 | 4.93 | 5.20 | 3.99 | 3.67 | 3.69 | 3.77 | 3.66 | 4.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colacicco, M.; Ciliberti, C.; Agrimi, G.; Biundo, A.; Pisano, I. Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils. Energies 2022, 15, 5217. https://doi.org/10.3390/en15145217
Colacicco M, Ciliberti C, Agrimi G, Biundo A, Pisano I. Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils. Energies. 2022; 15(14):5217. https://doi.org/10.3390/en15145217
Chicago/Turabian StyleColacicco, Mattia, Cosetta Ciliberti, Gennaro Agrimi, Antonino Biundo, and Isabella Pisano. 2022. "Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils" Energies 15, no. 14: 5217. https://doi.org/10.3390/en15145217
APA StyleColacicco, M., Ciliberti, C., Agrimi, G., Biundo, A., & Pisano, I. (2022). Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils. Energies, 15(14), 5217. https://doi.org/10.3390/en15145217







