Methane Hydrate Behavior for Water–Oil Systems Containing CTAB and Synperonic PE/F127 Surfactants
Abstract
:1. Introduction
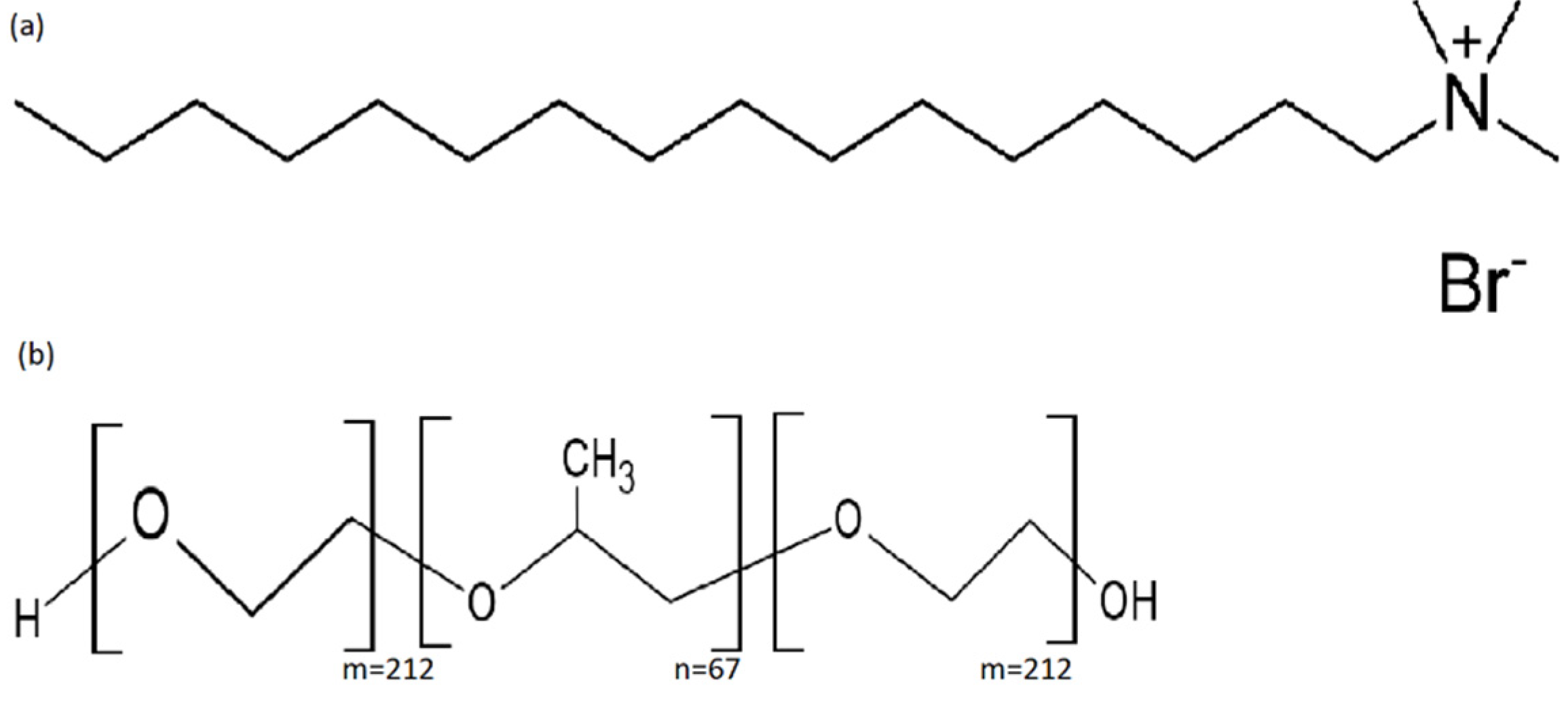
2. Materials and Methods
3. Results and Discussion
3.1. Dissociation
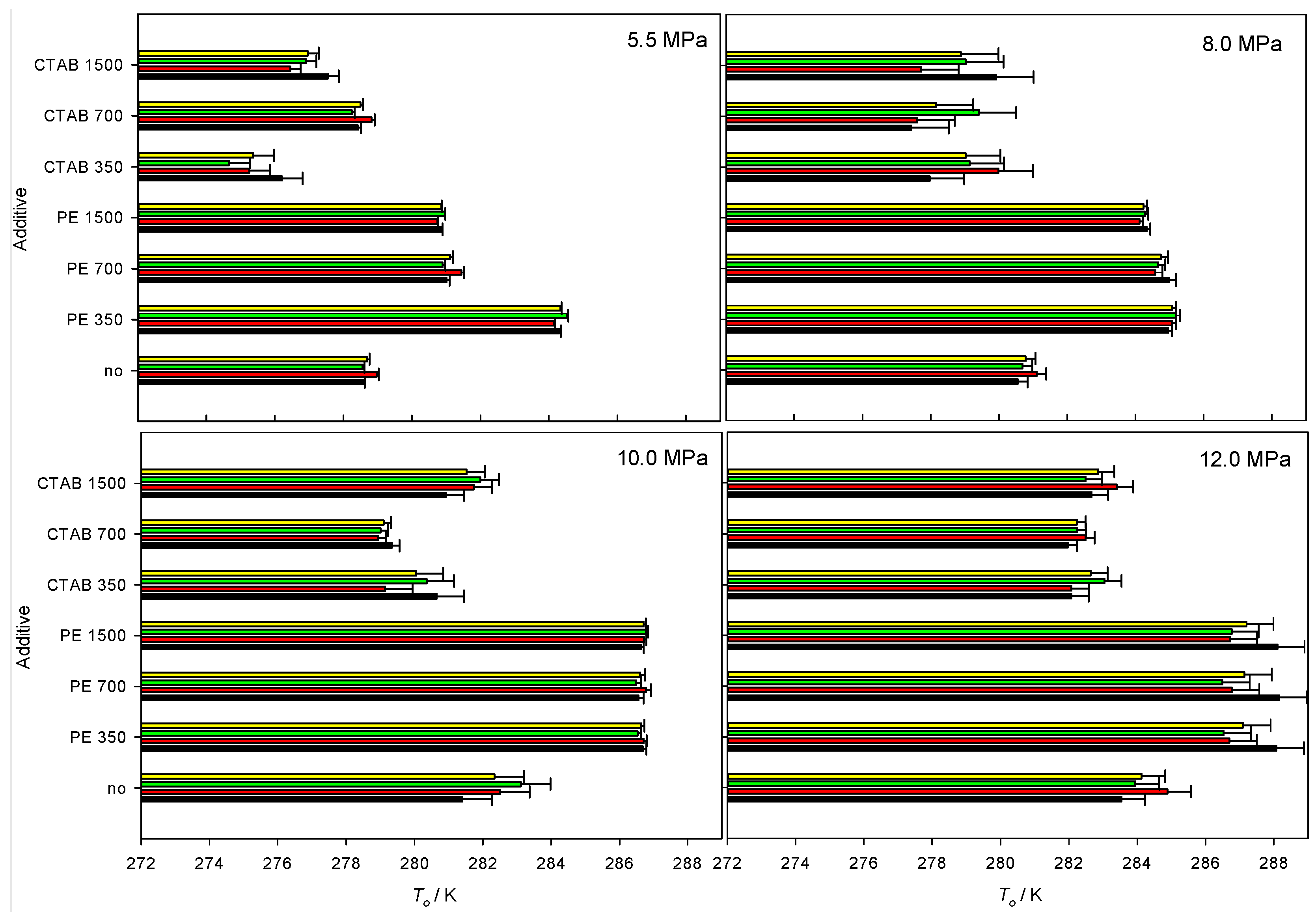
3.2. Temperature Onset
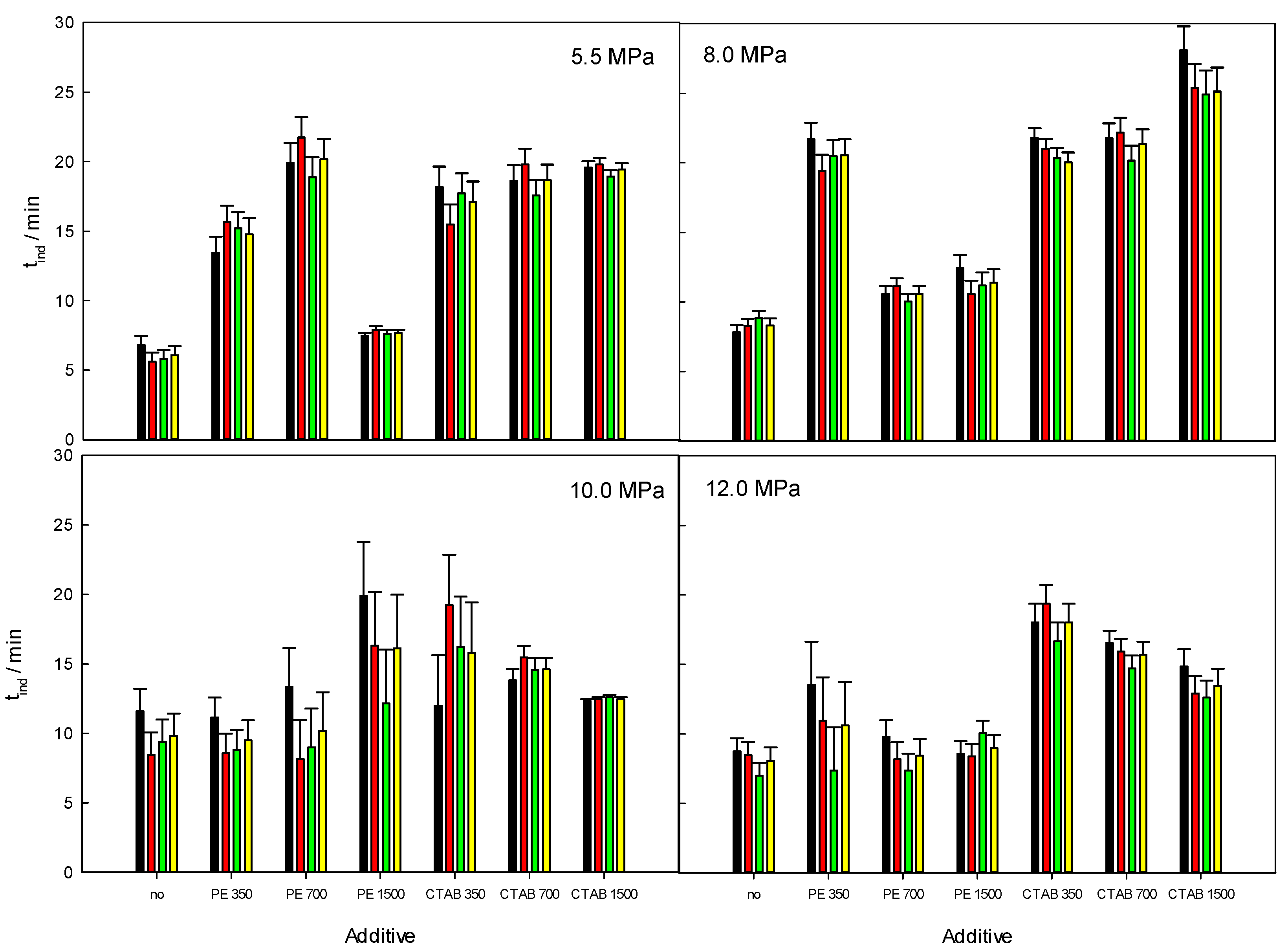
3.3. Induction Time (tind)
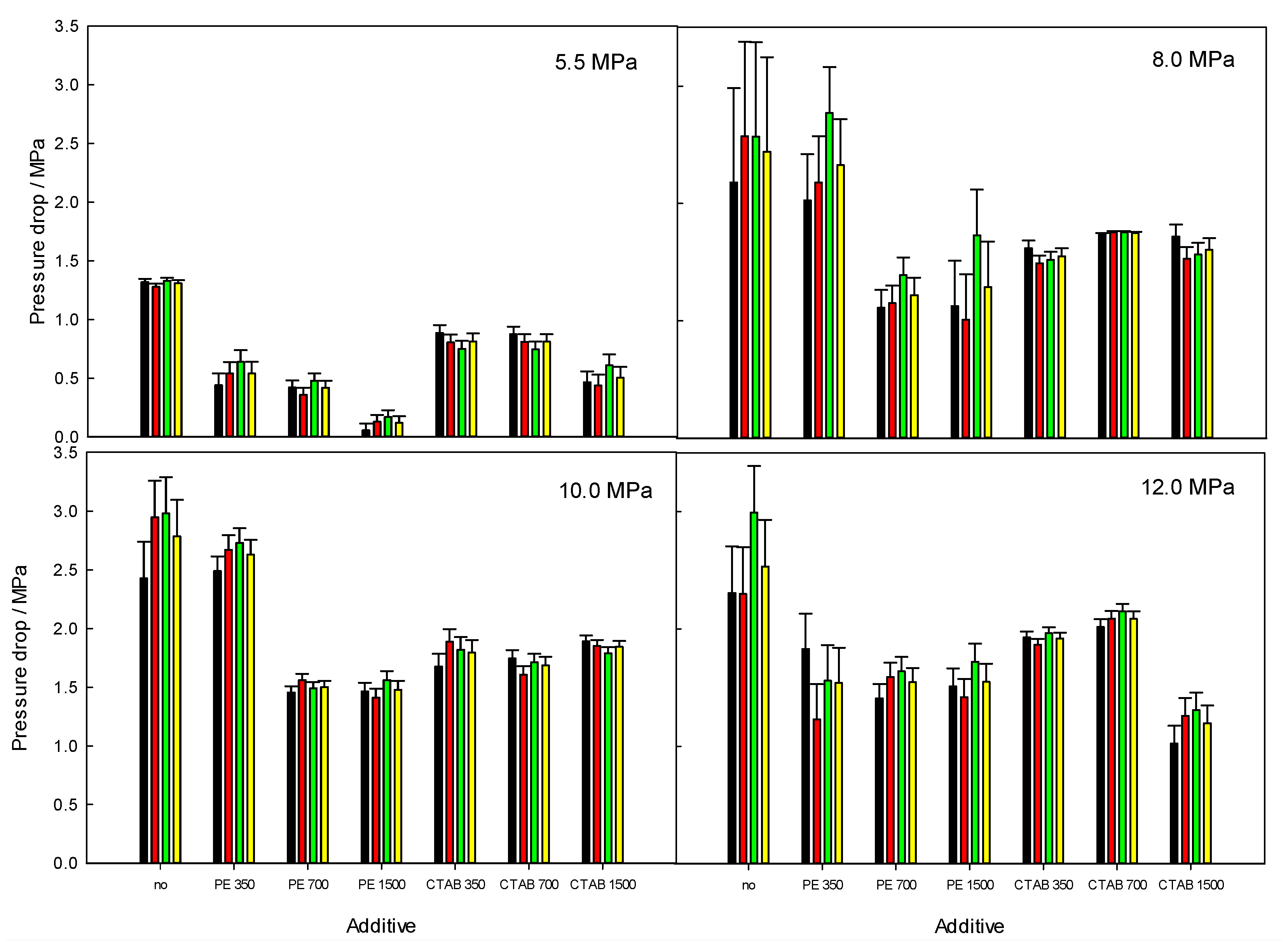
3.4. Pressure Drop
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Makogon, T.Y. Handbook of Multiphase Flow Assurance; Elsevier Science & Technology: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Carroll, J.J. Natural Gas Hydrates, A Guide for Engineers, 2nd ed.; Elsevier Gulf Professional Publishing: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Elechi, V.U.; Ikiensikimama, S.S.; Ajienka, J.A.; Akaranta, O.; Okon, O.E. Mitigation capacity of an eco-friendly locally sourced surfactant for gas hydrate inhibition in an offshore environment. J. Pet. Explor. Prod. Technol. 2021, 11, 1797–1808. [Google Scholar] [CrossRef]
- Qin, H.-B.; Zhang, Z.-Y.; Sun, C.-Y.; Chen, G.-J.; Ma, Q.-L.; Ning, Z.-F. Interfacial Tension between Methane and Water Containing Kinetic Hydrate Inhibitor PVP Ramification and Its Emulsification Property. J. Chem. Eng. Data 2017, 62, 2770–2775. [Google Scholar] [CrossRef]
- Lv, Y.-N.; Jia, M.-L.; Chen, J.; Sun, C.-Y.; Gong, J.; Chen, G.-J.; Liu, B.; Ren, N.; Guo, S.-D.; Li, Q.-P. Self-Preservation Effect for Hydrate Dissociation in Water + Diesel Oil Dispersion Systems. Energy Fuels 2015, 29, 5563–5572. [Google Scholar] [CrossRef]
- Chen, J.; Yan, K.-L.; Jia, M.-L.; Sun, C.-Y.; Zhang, Y.-Q.; Si, S.; Ma, Q.-L.; Yang, L.-Y.; Wang, X.-Q.; Chen, G.-J. Memory Effect Test of Methane Hydrate in Water + Diesel Oil + Sorbitan Monolaurate Dispersed Systems. Energy Fuels 2013, 27, 7259–7266. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Q.; Li, R.; Wang, W.; Zhu, X.; Li, H.; Wang, Y. Effects of waxes on hydrate behaviors in water-in-oil emulsions containing asphaltenes. Chem. Eng. Sci. 2021, 244, 116831. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, K.; Lang, X.; Fan, S. Investigation on hydrate-based methane storage properties in water-in-oil emulsion with high water content. CIESC J. 2021, 72, 4872. [Google Scholar] [CrossRef]
- Lv, X.; Shi, B.; Zhou, S.; Peng, H.; Lei, Y.; Yu, P. Study on the growth rate of natural gas hydrate in water-in-oil emulsion system using a high-pressure flow loop. RSC Adv. 2018, 8, 36484–36492. [Google Scholar] [CrossRef] [Green Version]
- Talatori, S.; Barth, T. Rate of hydrate formation in crude oil/gas/water emulsions with different water cuts. J. Pet. Sci. Eng. 2011, 80, 32–40. [Google Scholar] [CrossRef]
- Akhfash, M.; Aman, Z.; Ahn, S.Y.; Johns, M.L.; May, E.F. Gas hydrate plug formation in partially-dispersed water–oil systems. Chem. Eng. Sci. 2016, 140, 337–347. [Google Scholar] [CrossRef]
- Stoporev, A.S.; Ogienko, A.G.; Sizikov, A.A.; Semenov, A.P.; Kopitsyn, D.; Vinokurov, V.; Svarovskaya, L.I.; Altunina, L.K.; Manakov, A.Y. Unexpected formation of sII methane hydrate in some water-in-oil emulsions: Different reasons for the same phenomenon. J. Nat. Gas Sci. Eng. 2018, 60, 284–293. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Q.; Zheng, H.; Wang, Q.; Zhang, D.; Cheng, X.; Li, R. Effect of wax on hydrate formation in water-in-oil emulsions. J. Dispers. Sci. Technol. 2019, 41, 1821–1830. [Google Scholar] [CrossRef]
- Turner, D.; Miller, K.; Sloan, E. Direct conversion of water droplets to methane hydrate in crude oil. Chem. Eng. Sci. 2009, 64, 5066–5072. [Google Scholar] [CrossRef]
- Shestakov, V.; Sagidullin, A.; Stoporev, A.; Grachev, E.; Manakov, A. Analysis of methane hydrate nucleation in water-in-oil emulsions: Isothermal vs constant cooling ramp method and new method for data treatment. J. Mol. Liq. 2020, 318, 114018. [Google Scholar] [CrossRef]
- Tong, S.; Wang, Z.; Xie, K.; Liu, J.; Zhang, J.; Fu, W.; Sun, B. Experimental Study on the Methane Hydrate Formation in Water-In-Oil Emulsions with Dissolved Wax. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 9–12 November 2020. [Google Scholar] [CrossRef]
- Daraboina, N.; Pachitsas, S.; von Solms, N. Natural gas hydrate formation and inhibition in gas/crude oil/aqueous systems. Fuel 2015, 148, 186–190. [Google Scholar] [CrossRef]
- Stoporev, A.; Manakov, A.Y.; Altunina, L.; Strelets, L.A.; Kosyakov, V.I. Nucleation rates of methane hydrate from water in oil emulsions. Can. J. Chem. 2015, 93, 882–887. [Google Scholar] [CrossRef]
- Zi, M.; Chen, D.; Wang, J.; Hu, P.; Wu, G. Kinetic and rheological study of methane hydrate formation in water-in-oil emulsion: Effects of emulsion composition and silica sands. Fuel 2019, 255, 115708. [Google Scholar] [CrossRef]
- Mu, L.; Li, S.; Ma, Q.-L.; Zhang, K.; Sun, C.-Y.; Chen, G.-J.; Liu, B.; Yang, L.-Y. Experimental and modeling investigation of kinetics of methane gas hydrate formation in water-in-oil emulsion. Fluid Phase Equilibria 2014, 362, 28–34. [Google Scholar] [CrossRef]
- Informe de Labores de la CNH, Gobierno de México. Available online: https://www.gob.mx/cms/uploads/attachment/file/65479/informe_labores_14_15.pdf (accessed on 13 May 2022).
- Kaiser, M.J.; Siddhartha, N. Gulf of Mexico Decommissioning Trends and Operating Cost Estimation; OCS Study BOEM 2019-023; US Department of the Interior, Bureau of Ocean Energy Management (BOEM): Washington, DC, USA, 2018. [Google Scholar]
- ASTM D2007; Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils and Other Petrole-Um-derived Oils by the Clay-Gel Absorption Chromatographic Metho. ASTM International: Conshohocken, PA, USA, 2016.
- ASTM D1298-12b; Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: Conshohocken, PA, USA, 2017.
- ASTM D3230; Standard Test Method for Salts in Crude Oil (Electrometric Method). ASTM International: Conshohocken, PA, USA, 2019.
- Elizalde-Solis, O.; Díaz-Ibarra, J.A.; Mendo-Sánchez, R.D.; Vega-Díaz, Y.B.; Pérez-López, H.I.; Zúñiga-Moreno, A. Phase Equilibria for Gas Hydrates Formed with Methane or Ethane + Tetra-n-Butylphosphonium Bromide + Water. J. Chem. Eng. Data 2020, 65, 5428–5436. [Google Scholar] [CrossRef]
- Sangwai, J.; Oellrich, L. Phase equilibrium of semiclathrate hydrates of methane in aqueous solutions of tetra-n-butyl ammonium bromide (TBAB) and TBAB–NaCl. Fluid Phase Equilibria 2014, 367, 95–102. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Mohammadi, A.H. Phase equilibria of semiclathrate hydrates for methane+tetra n-butylammonium chloride (TBAC), carbon dioxide+TBAC, and nitrogen+TBAC aqueous solution systems. Fluid Phase Equilibria 2014, 381, 102–107. [Google Scholar] [CrossRef]
- Cha, M.; Hu, Y.; Sum, A. Methane hydrate phase equilibria for systems containing NaCl, KCl, and NH 4 Cl. Fluid Phase Equilibria 2016, 413, 2–9. [Google Scholar] [CrossRef]
- Turner, D.J.; Miller, K.T.; Sloan, E.D. Methane hydrate formation and an inward growing shell model in water-in-oil dispersions. Chem. Eng. Sci. 2009, 64, 3996–4004. [Google Scholar] [CrossRef]
- Lv, X.; Shi, B.; Wang, Y.; Gong, J. Study on Gas Hydrate Formation and Hydrate Slurry Flow in a Multiphase Transportation System. Energy Fuels 2013, 27, 7294–7302. [Google Scholar] [CrossRef]
- Lafarge, T.; Possolo, A. The NIST Uncertainty Machine. NCSLI Meas. 2015, 10, 20–27. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Anderson, R.; Tohidi, B. Carbon monoxide clathrate hydrates: Equilibrium data and thermodynamic modeling. AIChE J. 2005, 51, 2825–2833. [Google Scholar] [CrossRef]
- Nakamura, T.; Makino, T.; Sugahara, T.; Ohgaki, K. Stability boundaries of gas hydrates helped by methane—structure-H hydrates of methylcyclohexane and cis-1,2-dimethylcyclohexane. Chem. Eng. Sci. 2003, 58, 269–273. [Google Scholar] [CrossRef]
- Kharrat, M.; Dalmazzone, D. Experimental determination of stability conditions of methane hydrate in aqueous calcium chloride solutions using high pressure differential scanning calorimetry. J. Chem. Thermodyn. 2003, 35, 1489–1505. [Google Scholar] [CrossRef]
- Yang, S.; Cho, S.; Lee, H.; Lee, C. Measurement and prediction of phase equilibria for water + methane in hydrate forming conditions. Fluid Phase Equilibria 2001, 185, 53–63. [Google Scholar] [CrossRef]
- Clarke, M.; Bishnoi, P.R. Determination of the activation energy and intrinsic rate constant of methane gas hydrate decomposition. Can. J. Chem. Eng. 2001, 79, 143–147. [Google Scholar] [CrossRef]
- Smelik, E.A.; King, H.E. Crystal-growth studies of natural gas clathrate hydrates using a pressurized optical cell. Am. Miner. 1997, 82, 88–98. [Google Scholar] [CrossRef]
- Nixdorf, J.; Oellrich, L.R. Experimental determination of hydrate equilibrium conditions for pure gases, binary and ternary mixtures and natural gases. Fluid Phase Equilibria 1997, 139, 325–333. [Google Scholar] [CrossRef]
- Mei, D.-H.; Liao, J.; Yang, J.-T.; Guo, T.-M. Experimental and Modeling Studies on the Hydrate Formation of a Methane + Nitrogen Gas Mixture in the Presence of Aqueous Electrolyte Solutions. Ind. Eng. Chem. Res. 1996, 35, 4342–4347. [Google Scholar] [CrossRef]
- Hütz, U.; Englezos, P. Measurement of structure H hydrate phase equilibrium and the effect of electrolytes. Fluid Phase Equilibria 1996, 117, 178–185. [Google Scholar] [CrossRef]
- Dickens, G.R.; Quinby-Hunt, M.S. Methane hydrate stability in seawater. Geophys. Res. Lett. 1994, 21, 2115–2118. [Google Scholar] [CrossRef]
- Adisasmito, S.; Frank, R.J.; Sloan, E.D., Jr. Hydrates of carbon dioxide and methane mixtures. J. Chem. Eng. Data 1991, 36, 68–71. [Google Scholar] [CrossRef]
- Kroenlein, K.; Muzny, C.D.; Kazakov, A.; Diky, V.V.; Chirico, R.D.; Sloan, E.D.; Frenkel, M. Clathrate Hydrate Physical Property Database, NIST Standard Reference Database. Available online: http://gashydrates.nist.gov (accessed on 22 March 2022).
- Chaturvedi, E.; Laik, S.; Mandal, A. A comprehensive review of the effect of different kinetic promoters on methane hydrate formation. Chin. J. Chem. Eng. 2020, 32, 1–16. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Miraglia, D.B.; Rodríguez, J.L.; Minardi, R.M.; Schulz, P.C. Critical Micelle Concentration and HLB of the Sodium Oleate–Hexadecyltrimethylammonium Bromide Mixed System. J. Surfactants Deterg. 2011, 14, 401–408. [Google Scholar] [CrossRef]
- Saikia, T.; Mahto, V. Evaluation of Soy Lecithin as Eco-Friendly Biosurfactant Clathrate Hydrate Antiagglomerant Additive. J. Surfactants Deterg. 2018, 21, 101–111. [Google Scholar] [CrossRef]
- Doolaanea, A.A.; Ismail, A.F.H.; Mansor, N.; Nor, N.H.M.; Mohamed, F. Effect of Surfactants on Plasmid DNA Stability and Release from Poly (d,l-lactide-co-glycolide) Microspheres. Trop. J. Pharm. Res. 2015, 14, 1769. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, H.; Ripmeester, J.; Walker, V.K.; Englezos, P. Kinetic inhibition of natural gas hydrates in saline solutions and heptane. Fuel 2014, 117, 109–117. [Google Scholar] [CrossRef]
- Kar, S.; Kakati, H.; Mandal, A.; Laik, S. Experimental and modeling study of kinetics for methane hydrate formation in a crude oil-in-water emulsion. Pet. Sci. 2016, 13, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Ning, Y.; Guo, P.; Li, Y.; Wang, W. Investigation on Hydrate Growth at the Oil–Water Interface: In the Presence of Wax and Surfactant. Langmuir 2021, 37, 6838–6845. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, S.; Samanta, S.; Milano, G.; Roccatano, D. Molecular dynamics simulation study of solvent effects on conformation and dynamics of polyethylene oxide and polypropylene oxide chains in water and in common organic solvents. J. Chem. Phys. 2012, 136, 124901. [Google Scholar] [CrossRef] [PubMed]




| SARA | wt% |
|---|---|
| Saturates | 13.53 |
| Aromatics | 15.86 |
| Resins | 42.83 |
| Asphaltenes | 27.78 |
| (g·cm−3) | 0.9863 |
| °API | 14.63 |
| MW (g·mol−1) | 485.25 |
| Salt content (ppm) | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavón-García, A.; Zúñiga-Moreno, A.; García-Morales, R.; Pérez-López, H.I.; Elizalde-Solis, O. Methane Hydrate Behavior for Water–Oil Systems Containing CTAB and Synperonic PE/F127 Surfactants. Energies 2022, 15, 5213. https://doi.org/10.3390/en15145213
Pavón-García A, Zúñiga-Moreno A, García-Morales R, Pérez-López HI, Elizalde-Solis O. Methane Hydrate Behavior for Water–Oil Systems Containing CTAB and Synperonic PE/F127 Surfactants. Energies. 2022; 15(14):5213. https://doi.org/10.3390/en15145213
Chicago/Turabian StylePavón-García, Antonio, Abel Zúñiga-Moreno, Ricardo García-Morales, Hugo I. Pérez-López, and Octavio Elizalde-Solis. 2022. "Methane Hydrate Behavior for Water–Oil Systems Containing CTAB and Synperonic PE/F127 Surfactants" Energies 15, no. 14: 5213. https://doi.org/10.3390/en15145213
APA StylePavón-García, A., Zúñiga-Moreno, A., García-Morales, R., Pérez-López, H. I., & Elizalde-Solis, O. (2022). Methane Hydrate Behavior for Water–Oil Systems Containing CTAB and Synperonic PE/F127 Surfactants. Energies, 15(14), 5213. https://doi.org/10.3390/en15145213







