Dimethyl Carbonate: Review of Synthesis Routes and Catalysts Used
Abstract
:1. Introduction
2. Carbon Dioxide (CO2)-Based Synthesis of DMC
2.1. Direct Conversion of CO2 to DMC

| S. No | Catalysts | Dehydrating Agents Used | Reaction Conditions | MeOH Conversion, % | DMC Yield, % | DMC Selectivity, % | Source |
|---|---|---|---|---|---|---|---|
| 1 | Cu-Ni/TEG | - | T = 373 K; P = 1.2 MPa; | 4.97 | - | 89.3 | [26] |
| MeOH:CO2 ratio-2:1 | |||||||
| 2 | 20% Cu-Ni bimetal/0.4 mm molecular sieve | - | T = 393 K; P = 1.1 MPa; t = 5 h; n(CO2/MeOH) = 10; Gas space velocity of 510/h | 5 | 86 | [27] | |
| 3a | Cs-DMP-HMS | - | T = 323 K; P = 15 MPa; t = 5 h; Catalyst = 7.21 × 10−3 g/cm3; IL = 4.8 × 10−3 g/cm3; n(CH3OH/CO2) = 2 | 5.8 | - | 17.2 | [28] |
| 3b | Cs-DMP-HMS + Phos-IL | 8.1 | 23.6 | ||||
| 3c | Cs-DTP-HMS | 10.1 | 24.4 | ||||
| 3d | Cs-DTP-HMS + Phos-IL | 11.9 | 25.8 | ||||
| 3e | CHT-HMS + Phos-IL | 9.1 | 68.5 | ||||
| 3f | CHT-HMS | 9.2 | 82 | ||||
| 3g | Phos-IL | 0 | 0 | ||||
| 4a | Ce0.1Ti0.9O2 | - | T = 443 K, CO2/N2 volumetric ratio 1/7 | 4.2 | 2.3 | 55 | [29] |
| 4b | H3PW12O40/Ce0.1Ti0.9O2 | 5.5 | 5 | 91.4 | |||
| 5 | DEG[Vim]2[NTf2]2/MgO-CeO2 nanofiber sponge | - | T = 393 K; P = 3 MPa; t = 3 h; MeOH = 437.5 mmol; IL = 0.85 mmol; Catalyst = 0.27 mmol | 73.1b | - | 98.9 | [30] |
| 6a | Zn0.10Ce0.90O2/honeycomb ceramic (Monolithic) | - | T = 433 K; P = 2.4 MPa; MeOH = 0.145 mL/min; GHSV = 2880 mL gcat−1h−1 | 20.5 | - | 82.1 | [31] |
| 7a | CeO2-nanorod | - | T = 413 K; P = 3 MPa; t = 3 h; MeOH = 35 mL; Catalyst = 0.5 g | - | 1.5 c | - | [32] |
| 7b | CoO2/CeO2 | 1.4 c | |||||
| 7c | NiO/CeO2 | 0.9 c | |||||
| 7d | CaO/CeO2 | 0.8 c | |||||
| 7e | CuO/CeO2 | 0.2 c | |||||
| 8a | ZrO2-HX-C (calcination of zirconia hydroxide) | - | T = 433 K; P = 4.8 MPa; t = 5 h | - | 78 a | - | [33] |
| 8b | ZrO2-HT-(393 *) | 163 a | |||||
| 8c | ZrO2-HT-(433 *) | 140 a | |||||
| 8d | ZrO2-HT-(473 *) | 116 a | |||||
| 8e | ZrO2-HT-(493 *) | 115 a | |||||
| 8f | ZrO2-HT-(513 *) | 90 a | |||||
| 8g | meso-ZrO2 | 83 a | |||||
| 9 | 10% (w/w) Ce-Zr oxide/graphene nanocomposite | TMM | T = 383 K; P = 27.5 MPa; t = 16 h; 1:1 (w/w) TMM:MeOH | 58 | 33 | - | [34] |
| 10a | None | - | T = 383 K; P = 5 MPa (at room temp); t = 4 h; MeOH = 12 g; Cat = 1 g | - | 0 | 0 | [35] |
| 10b | ZrO2 | 0.12 b | 100 | ||||
| 10c | Fe0.3Zr0.7Oy | 0.24 b | 100 | ||||
| 10d | Fe0.5Zr0.5Oy | 0.35 b | 100 | ||||
| 10e | Fe0.7Zr0.3Oy | 0.44 b | 100 | ||||
| 10f | Fe0.9Zr0.1Oy | 0.28 b | 100 | ||||
| 10g | Fe2O3 | 0.04 b | 100 | ||||
| 11a | K2CO3 (0.0029 mol) | - | T = 353 K; P = 7.3 MPa; t = 6 h; MeOH = 0.85 mol, CH3I = 0.048 mol | - | 4.1 | - | [36] |
| 11b | KOH (0.054 mol) | 8.5 | |||||
| 11c | NaOH (0.054 mol) | 1.7 | |||||
| 11d | KHCO3 | 0.5 (10 h) | |||||
| 12a | CeO2 | 2-picolinamide | T = 393 K; P = 20 MPa | 17 | - | >99 | [37] |
| 12b | CeO2 | 2-cyanopyridine | T = 413 K; P = 20 MPa | 92.4 | >99 | ||
| 12c | CeO2 | 2-cyanopyridine | T = 353 K; P = 20 MPa | 94 | 98 | ||
| 13a | CH3OK and CH3I CH3OK and CH3I CH3OK and CH3I CH3OK and CH3I | Sieve/DMP | T = 353 K; P = 4 MPa; MeOH = 213 mmol; Catalyst = 10 mmol; Promoter = 20 mmol; Dehydrating agent = 10 mml; t = 24 h | 48.6 | 42.8 | 88 | [38] |
| 13b | Sieve/Na2SO4 | 14.8 | 14.8 | 100 | |||
| 13c | Sieve/butylene oxide | 2.5 | 0.3 | 15 | |||
| 13d | Sieve/MgO | 4.5 | 4.5 | 100 | |||
| 14a | 10 wt% Chitosan/IL#-NTF2 (anion) + DBU | - | T = 373 K; P = 7.5 MPa; MeOH = 618 mmol; Catalyst = 3 g; DBU = 1 g | 16.9 | - | 98.72 | [39] |
| 14b | 10 wt% Chitosan/IL#-Cl (anion) +DBU | 14.58 | 90.7 | ||||
| 14c | 10 wt% Chitosan/IL#-PF6 (anion) +DBU | 14.89 | 94.87 | ||||
| 14d | 10 wt% Chitosan/IL# without DBU | 10.21 | 98.12 | ||||
| 14e | Pure Chitosan | 0.13 | 99.4 | ||||
| 15a | [bmim][Cl] | 2,2-dimethoxypropane | T = 443 K; P = 4 MPa CO2; t = 24 h; MeOH = 250 mmol; Dehydrant = 25 mmol; IL = 2.5 mmol | 7.46 | - | 53.02 | [40] |
| 15b | [bmim][BF4] | 7.43 | 81.31 | ||||
| 15c | [bmim][PF6] | 7.19 | 46.9 | ||||
| 15d | [bmim][Tf2N] | 6.89 | 39.3 | ||||
| 15e | [emim][BF4] | 9.22 | 83.3 | ||||
| 15f | [emim][Tf2N] | 8.98 | 43.01 | ||||
| 15g | [mbmim][Tf2N] | 6.81 | 35.13 | ||||
| 15h | [dmbmim][Tf2N] | 6.65 | 33.63 | ||||
| 15i | [bpy][Cl] | 7.73 | 53.44 | ||||
| 15j | [bpy][BF4] | 7.52 | 60.72 | ||||
| 15k | [bpy][PF6] | 7.57 | 41.4 | ||||
| 15l | [bpy][Tf2N] | 7.43 | 34.54 | ||||
| 15m | [epy][BF4] | 7.65 | 63.72 | ||||
| 15n | [epy][Tf2N] | 9.35 | 38.49 | ||||
| 15o | [dmbpy][Tf2N] | 6.78 | 28.81 | ||||
| 16a | [C1C4Im][HCO3], without base | DBU | T = RT; P = 1 MPa; t = 24 h; Base = 5 mmol | 14 | - | >99 | [41] |
| 16b | 5 mmol [C1C4Im][HCO3]Na2CO3 | 22 | 97 | ||||
| 16c | 5 mmol [C1C4Im][HCO3]NaHCO3 | 24 | >99 | ||||
| 16d | 5 mmol [C1C4Im][HCO3]K2CO3 | 61 | 54 | ||||
| 16e | 5 mmol [C1C4Im][HCO3]KHCO3 | 24 | >99 | ||||
| 16f | 5 mmol [C1C4Im][HCO3]Cs2CO3 | 45 | >99 | ||||
| 16g | 0.25 mmol [C1C4Im][HCO3]Cs2CO3 | 26 | >99 | ||||
| 16h | 2.5 mmol [C1C4Im][HCO3]Cs2CO3 | 37 | >99 | ||||
| 16i | 20 mmol [C1C4Im][HCO3]Cs2CO3 | 74 | 97 | ||||
| 16j | [C1C4Im][HCO3]Cs2CO3 | T = 323 K; P = 1 MPa; t = 24 h | 54 | >99 | |||
| 16k | 20 mmol [C1C4Im][HCO3]Cs2CO3 | T = 323 K; P = 1 MPa; t = 24 h | 82 | 94 |

2.1.1. Use of Dehydrating Agents
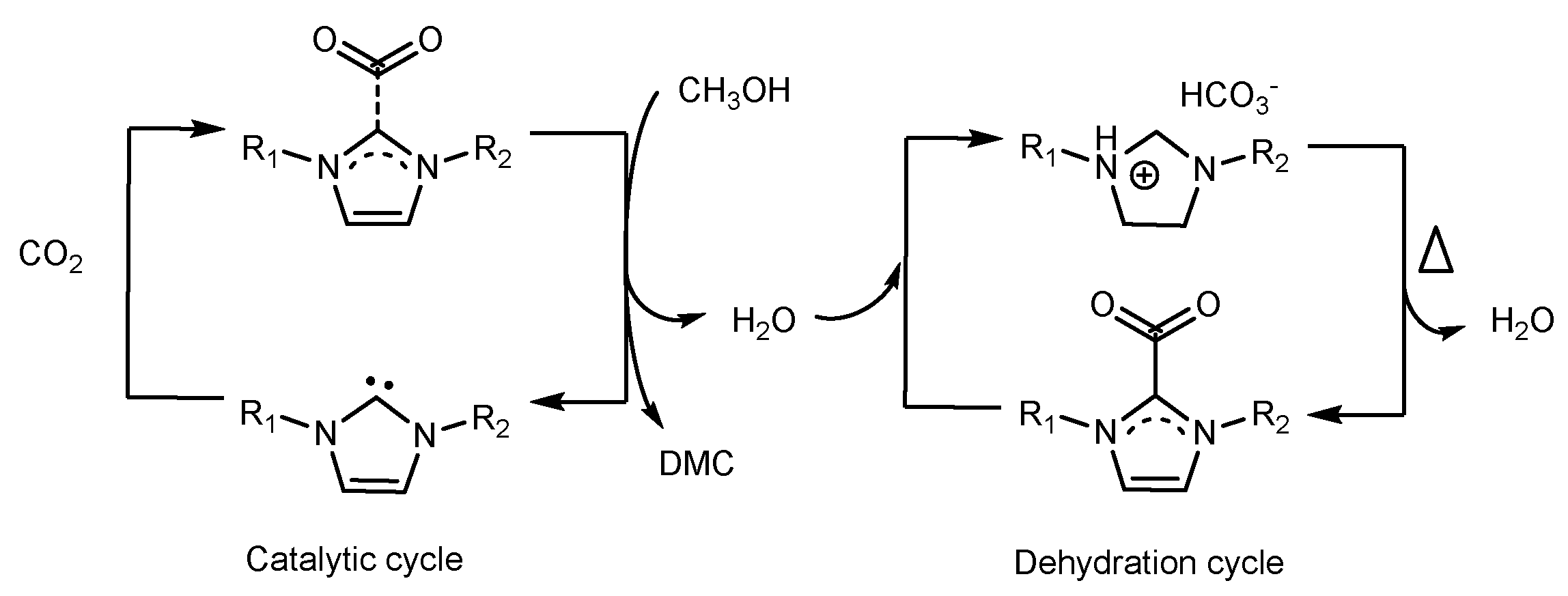
2.1.2. Use of Ionic Liquids (ILs)
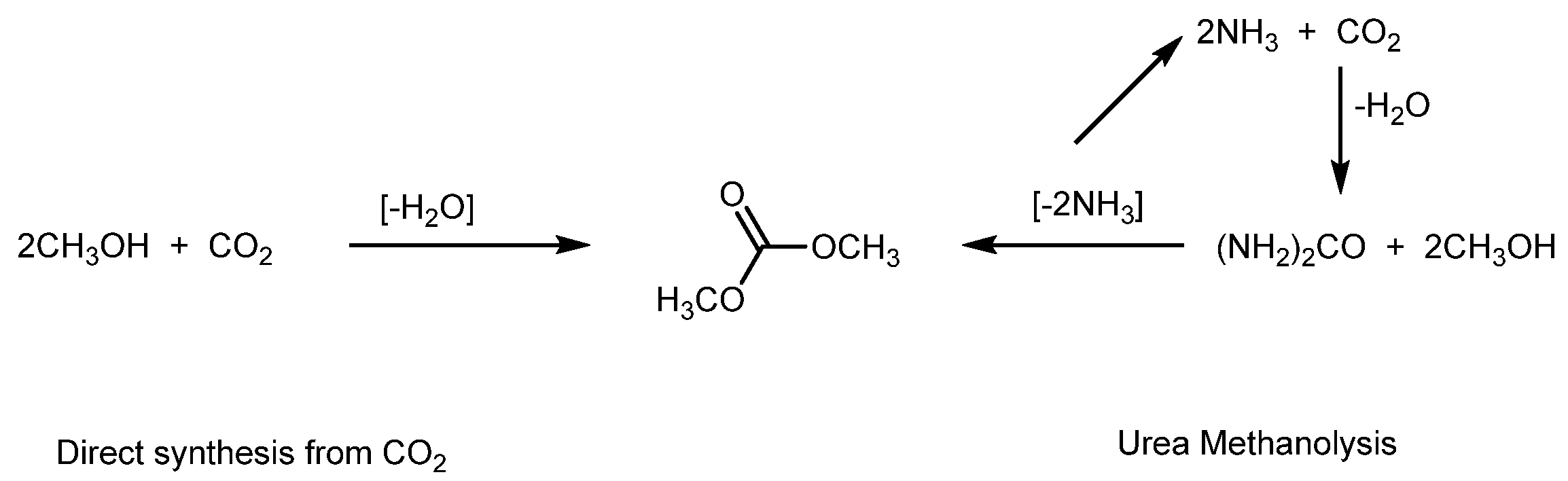
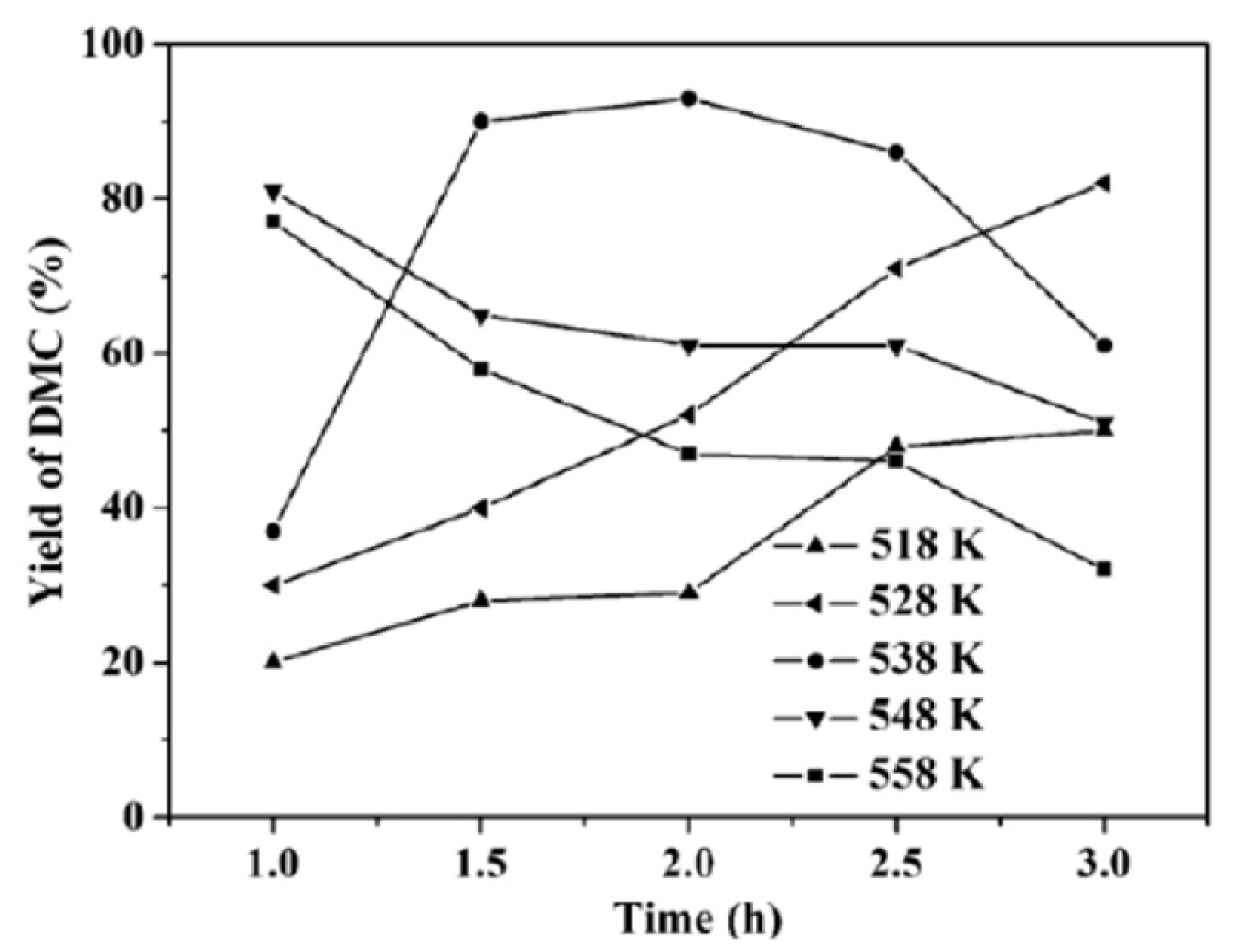
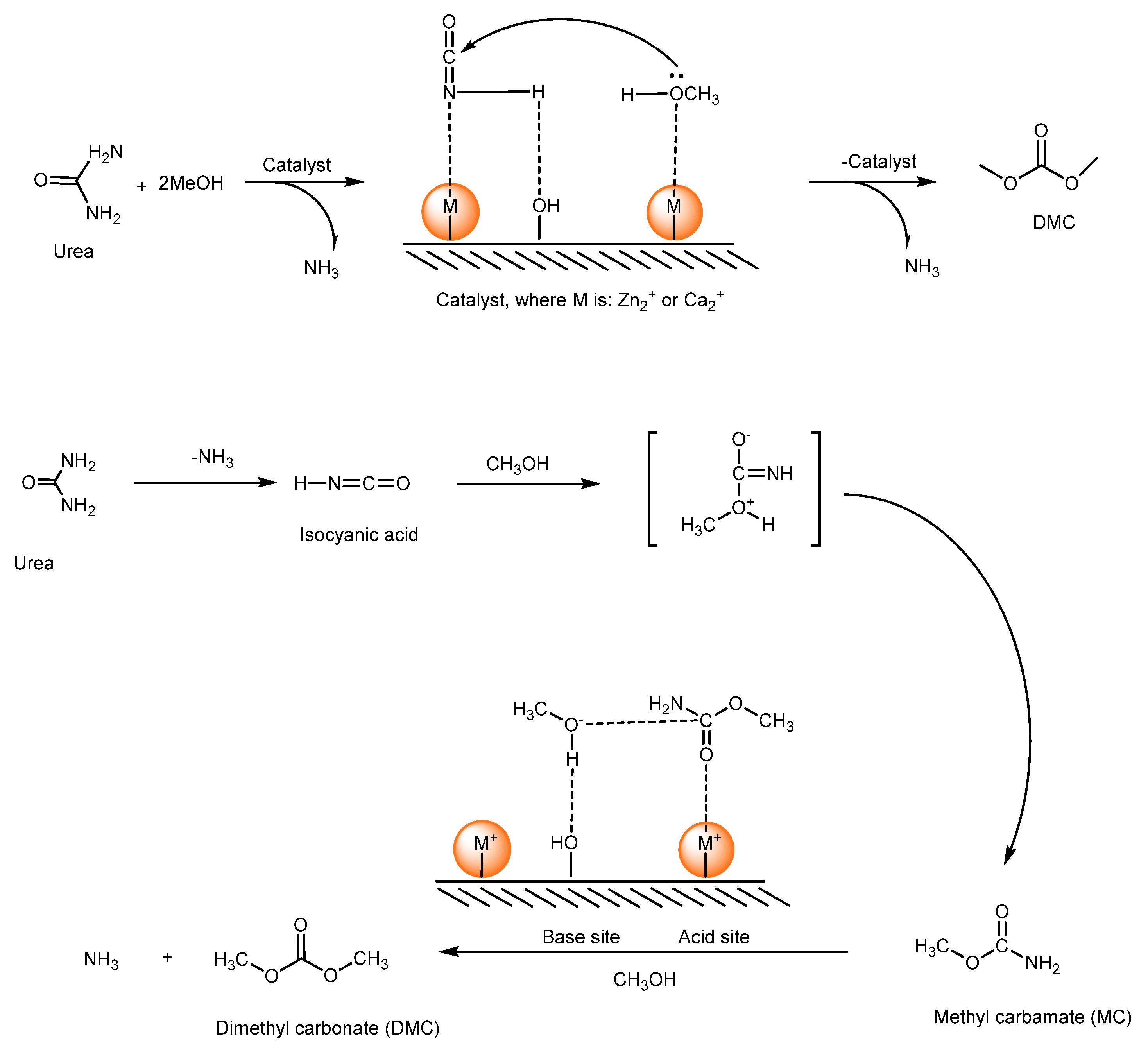
2.2. Conversion of Urea and Methanol to DMC


2.2.1. Homogeneous Catalysts for DMC Production from Urea
2.2.2. Heterogeneous Catalysts Used for DMC Production from Urea




3. Outlook and Future Perspectives
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Kim, W.B.; Joshi, A.U.; Lee, J.S. Making polycarbonates without employing phosgene: An overview on catalytic chemistry of intermediate and precursor syntheses for polycarbonate. Ind. Eng. Chem. Res. 2004, 43, 1897–1914. [Google Scholar] [CrossRef]
- Shiao, H.-C.; Chua, D.; Lin, H.; Slane, S.; Salomon, M. Low temperature electrolytes for Li-ion PVDF cells. J. Power Sources 2000, 87, 167–173. [Google Scholar] [CrossRef]
- Panchal, C.B.; Prindle, J.C.; Sturtz, R.; Doctor, R.D.; Miller, D.; Parker, S.; Peereboom, L. Utilization of captured CO2 for manufacturing alkyl carbonates. In Proceedings of the AIChE Spring Meeting, Orlando, FL, USA, 22–26 April 2018. [Google Scholar]
- Sturtz, R.; Gattinger, M.; Kuhl, H.; Peereboom, L.; Miller, D. Zinc-catalyzed formation of dimethyl carbonate from urea. In Proceedings of the AIChE Annual Meeting, San Francisco, CA, USA, 13–18 November 2016. [Google Scholar]
- Modak, A.; Bhanja, P.; Dutta, S.; Chowdhury, B.; Bhaumik, A. Catalytic reduction of CO2 into fuels and fine chemicals. Green Chem. 2020, 22, 4002. [Google Scholar] [CrossRef]
- Modak, A.; Ghosh, A.; Bhaumik, A.; Chowdhury, B. CO2 hydrogenation over functional nanoporous polymers and metal-organic frameworks. Adv. Colloid Interface Sci. 2021, 290, 102349. [Google Scholar] [CrossRef]
- Gosh, S.; Modak, A.; Samanta, A.; Kole, K.; Jana, S. Recent progress in materials development for CO2 conversion: Issues and challenges. Mater. Adv. 2021, 2, 3161–3187. [Google Scholar] [CrossRef]
- Tamboli, A.H.; Chaugule, A.A.; Kerm, H. Catalytic developments in the direct dimethyl carbonate synthesis from carbon dioxide and methanol. Chem. Eng. J. 2017, 323, 530–544. [Google Scholar] [CrossRef]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Cicha, W.V.; Manzer, L.E. Phosgene Manufacturing Process. U.S. Patent 6054612A, 25 April 2000. [Google Scholar]
- Zhang, P.; Huang, S.; Yang, Y.; Meng, Q.; Wang, S.; Ma, X. Effect of SSIE structure of Cu-exchanged β and Y on the selectivity for synthesis of diethyl carbonate by oxidative carbonylation of ethanol: A comparative investigation. Catal. Today 2010, 149, 202–206. [Google Scholar] [CrossRef]
- Nishihira, K.; Yoshida, S.; Tanaka, S. Continuous Process for Preparing Dimethyl Carbonate. EP0523728A2, 20 January 1993. [Google Scholar]
- Kizlink, K. Synthesis of dimethyl carbonate from carbon dioxide and methanol in the presence of organotin compounds. Collect. Czech. Chem. Commun. 1993, 58, 1399–1402. [Google Scholar] [CrossRef]
- Choi, J.-C.; Kohno, K.; Ohshima, Y.; Yasuda, H.; Sakakura, T. Tin- or titanium-catalyzed dimethyl carbonate synthesis from carbon dioxide and methanol: Large promotion by a small amount of triflate salts. Catal. Commun. 2008, 9, 1630–1633. [Google Scholar] [CrossRef]
- Fang, S.; Fujimoto, K. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol catalyzed by base. Appl. Catal. A Gen. 1996, 142, L1–L3. [Google Scholar] [CrossRef]
- Kizlink, J.; Pastucha, I. Preparation of dimethyl carbonate from methanol and carbon dioxide in the presence of Sn(IV) and Ti(IV) alkoxides and metal acetates. Collect. Czech. Chem. Commum. 1995, 60, 687–692. [Google Scholar] [CrossRef]
- Tomishige, K.; Sakaihori, T.; Ikeda, Y.; Fujimoto, K. A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia. Catal. Lett. 1999, 58, 225–229. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, Y.; Wang, C.; Hu, C.; Wu, Y.; Wang, E. Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions. Appl. Catal. A Gen. 2003, 256, 203–212. [Google Scholar] [CrossRef]
- Wu, X.L.; Xiao, M.; Meng, Y.Z.; Lu, Y.X. Direct synthesis of dimethyl carbonate on H3PO4 modified V2O5. J. Mol. Catal. A Chem. 2005, 238, 158–162. [Google Scholar] [CrossRef]
- Wang, X.J.; Xiao, M.; Wang, S.J.; Lu, Y.X.; Meng, Y.Z. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol using supported copper (Ni, V, O) catalyst with photo-assistance. J. Mol. Catal. A Chem. 2007, 278, 92–96. [Google Scholar] [CrossRef]
- Wu, X.L.; Meng, Y.Z.; Xiao, M.; Lu, Y.X. Direct synthesis of dimethyl carbonate (DMC) using Cu-Ni/VSO as catalyst. J. Mol. Catal. A Chem. 2006, 249, 93–97. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, T.; Liu, F. The effects of additives on the direct synthesis of dimethylcarbonate. Chin. J. Appl. Chem. 1999, 5, 115. [Google Scholar]
- Tomishige, K.; Kunimori, K. Catalytic and direct synthesis of dimethyl carbonate starting from carbon dioxide using CeO2-ZrO2 solid solution heterogeneous catalyst: Effect of H2O removal from the reaction system. Appl. Catal. A Gen. 2002, 237, 103–109. [Google Scholar] [CrossRef]
- Hou, Z.; Han, B.; Liu, Z.; Jiang, T.; Yang, G. Synthesis of dimethyl carbonate using CO2 and methanol: Enhancing the conversion by controlling the phase behavior. Green Chem. 2002, 4, 467–471. [Google Scholar] [CrossRef]
- Zhao, T.; Han, Y.; Sun, Y. Novel reaction route for dimethyl carbonate synthesis from CO2 and methanol. Fuel Process. Technol. 2000, 62, 187–194. [Google Scholar] [CrossRef]
- Bian, J.; Xiao, M.; Wang, S.J.; Lu, Y.X.; Meng, Y.Z. Novel application of thermally expanded graphite as the support of catalysts for direct synthesis of DMC from CH3OH and CO2. J. Colloid Interface Sci. 2009, 334, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, S.; Xiao, M.; Han, D.; Lu, Y.; Meng, Y. Direct synthesis of dimethyl carbonate from CO2 and CH3OH using 0.4 nm molecular sieve supported Cu-Ni bimetal catalyst. Chin. J. Chem. Eng. 2012, 20, 906–913. [Google Scholar] [CrossRef]
- Kabra, S.K.; Turpeinen, E.; Keiski, R.L.; Yadav, G.D. Direct synthesis of dimethyl carbonate from methanol and carbon dioxide: A thermodynamic and experimental study. J. Supercrit. Fluids 2016, 117, 98–107. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Lin, K.-S.; Yu, S.-H.; Lin, Y.-G. Synthesis and characterization of H3PW12O40/Ce0.1Ti0.9O2 for dimethyl carbonate formation via methanol carbonation. Int. J. Hydrogen Energy 2017, 42, 22108–22122. [Google Scholar] [CrossRef]
- Pawar, A.A.; Lee, D.; Chung, W.J.; Kim, H. Understanding the synergy between MgO-CeO2 as an effective promoter and ionic liquids for high dimethyl carbonate production from CO2 and methanol. Chem. Eng. J. 2020, 395, 124970. [Google Scholar] [CrossRef]
- Chen, Y.; Tiang, Q.; Ye, Z.; Li, Y.; Yang, Y.; Pu, H.; Li, G. Monolithic ZnxCe1−xO2 catalysts for catalytic synthesis of dimethyl carbonate from CO2 and methanol. New J. Chem. 2020, 44, 12522–12530. [Google Scholar] [CrossRef]
- Al-Darwish, J.; Senter, M.; Lawson, S.; Rezaei, F.; Rownaghi, A.A. Ceria nanostructured catalysts for conversion of methanol and carbon dioxide to dimethyl carbonate. Catal. Today 2020, 350, 120–126. [Google Scholar] [CrossRef]
- Akune, T.; Morita, Y.; Shirakawa, S.; Katagiri, K.; Inumaru, K. ZrO2 nanocrystals as catalyst for synthesis of dimethyl carbonate from methanol and carbon dioxide: Catalytic activity and elucidation of active sites. Langmuir 2018, 34, 23–29. [Google Scholar] [CrossRef]
- Saada, R.; Kellici, S.; Heil, T.; Morgan, D.; Saha, B. Greener synthesis of dimethyl carbonate using a novel ceria–zirconia oxide/graphene nanocomposite catalyst. Appl. Catal. B Environ. 2015, 168–169, 353–362. [Google Scholar] [CrossRef]
- Li, A.; Pu, Y.; Li, F.; Luo, J.; Zhao, N.; Xiao, F. Synthesis of dimethyl carbonate from methanol and CO2 over Fe–Zr mixed oxides. J. CO2 Util. 2017, 19, 33–39. [Google Scholar] [CrossRef]
- Cai, Q.; Lu, B.; Guo, L.; Shan, Y. Studies on synthesis of dimethyl carbonate from methanol and carbon dioxide. Catal. Commun. 2009, 10, 605–609. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Continuous DMC synthesis from CO2 and methanol over a CeO2 catalyst in a fixed bed reactor in the presence of a dehydrating agent. ACS Catal. 2014, 4, 3877–3880. [Google Scholar] [CrossRef] [Green Version]
- Faria, D.J.; Santos, L.M.; Bernard, F.L.; Pinto, I.S.; da Motta Resende, M.A.C.; Einloft, S. Dehydrating agent effect on the synthesis of dimethyl carbonate (DMC) directly from methanol and carbon dioxide. RSC Adv. 2020, 10, 34895–34902. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, A.H.; Chaugule, A.A.; Kim, H. Highly selective and multifunctional chitosan/ionic liquids catalyst for conversion of CO2 and methanol to dimethyl carbonates at mild reaction conditions. Fuel 2016, 166, 495–501. [Google Scholar] [CrossRef]
- Vieira, M.O.; Aquino, A.S.; Schütz, M.K.; Vecchia, F.D.; Ligabue, R.; Seferin, M.; Einloft, S. Chemical conversion of CO2: Evaluation of different ionic liquids as catalysts in dimethyl carbonate synthesis. Energy Procedia 2017, 114, 7141–7149. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, X.; Wu, D.; Li, R.; Yang, G.; Wu, Y. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol at room temperature using Imidazolium hydrogen carbonate ionic liquid as a recyclable catalyst and dehydrant. ChemSusChem 2017, 10, 2046–2052. [Google Scholar] [CrossRef]
- Bian, J.; Wei, X.E.; Jin, Y.R.; Wang, L.; Luan, D.C.; Guan, Z.P. Direct synthesis of dimethyl carbonate over activated carbon supported Cu-based catalysts. Chem. Eng. J. 2010, 165, 686–692. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.; Jung, J.C.; Song, I.K. Direct synthesis of dimethyl carbonate from methanol and carbon dioxide over H3PW12O40/CeXZr1−XO2 catalysts: Effect of acidity of the catalysts. Korean J. Chem. Eng. 2011, 28, 1518–1522. [Google Scholar] [CrossRef]
- Hofmann, H.J.; Brandner, A.; Claus, P. Direct synthesis of dimethyl carbonate by carboxylation of methanol on ceria-based mixed oxides. Chem. Eng. Technol. 2012, 35, 2140–2146. [Google Scholar] [CrossRef]
- Miguel, C.V.; Soria, M.A.; Mendes, A.; Madeira, L.M. Direct CO2 hydrogenation to methane or methanol from post-combustion exhaust streams—A thermodynamic study. J. Nat. Gas Sci. Eng. 2015, 22, 1–8. [Google Scholar] [CrossRef] [Green Version]
- La, K.W.; Youn, M.H.; Chung, J.S.; Baeck, S.H.; Song, I.K. Synthesis of dimethyl carbonate from methanol and carbon dioxide by heteropolyacid/metal oxide catalysts. Solid State Phenom. 2007, 119, 287–290. [Google Scholar] [CrossRef]
- Lee, H.J.; Joe, W.; Song, I.K. Direct synthesis of dimethyl carbonate from methanol and carbon dioxide over transition metal oxide/Ce0.6Zr0.4O2 catalysts: Effect of acidity and basicity of the catalysts. Korean J. Chem. Eng. 2012, 29, 317–322. [Google Scholar] [CrossRef]
- Marin, C.M.; Li, L.; Bhalkikar, A.; Doyle, J.E.; Zeng, X.C.; Cheung, C.L. Kinetic and mechanistic investigations of the direct synthesis of dimethyl carbonate from carbon dioxide over ceria nanorod catalysts. J. Catal. 2016, 340, 295–301. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Li, Y.; Huang, S.; An, J.; Shi, R.; Pei, Y.; Li, Z.; Ren, J. Effects of surface acid-base properties of ZrO2 on the direct synthesis of DMC from CO2 and methanol: A combined DFT and experimental study. Chem. Eng. Sci. 2021, 229, 116018. [Google Scholar] [CrossRef]
- Bian, J.; Xiao, M.; Wang, S.; Lu, Y.; Meng, Y. Carbon nanotubes supported Cu-Ni bimetallic catalysts and their properties for the direct synthesis of dimethyl carbonate from methanol and carbon dioxide. Appl. Surf. Sci. 2009, 255, 7188–7196. [Google Scholar] [CrossRef]
- Merza, G.; Laszlo, B.; Oszko, A.; Potari, G.; Baan, K.; Erdohelyi, A. The direct synthesis of dimethyl carbonate by the oxicarbonylation of methanol over Cu supported on carbon nanotube. J. Mol. Catal. A Chem. 2014, 393, 117–124. [Google Scholar] [CrossRef]
- Daniel, C.; Schuurman, Y.; Farrusseng, D. Surface effect of nano-sized cerium-zirconium oxides for the catalytic conversion of methanol and CO2 into dimethyl carbonate. J. Catal. 2021, 394, 486–494. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Catalytic CO2 conversion to organic carbonates with alcohols in combination with dehydration system. Catal. Sci. Technol. 2014, 4, 2830–2845. [Google Scholar] [CrossRef]
- Choi, J.C.; He, L.N.; Yasuda, H.; Sakakura, T. Selective and high yield synthesis of dimethyl carbonate directly from carbon dioxide and methanol. Green Chem. 2002, 4, 230–234. [Google Scholar] [CrossRef]
- Eta, V.; Maki-Arvela, P.; Warna, J.; Salmi, T.; Mikkola, J.P.; Murzin, D.Y. Kinetics of dimethyl carbonate synthesis from methanol and carbon dioxide over ZrO2–MgO catalyst in the presence of butylene oxide as additive. Appl. Catal. A Gen. 2011, 404, 39–46. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, A.; Noorjahan, B.; Fujimoto, K.I.; Suzuki, K.; Tomishige, K. Low pressure CO2 to dimethyl carbonate by the reaction with methanol promoted by acetonitrile hydration. Chem. Commun. 2009, 30, 4596–4598. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Wakasugi, H.; Shimizu, K.; Satsuma, A. Efficient and substrate-specific hydration of nitriles to amides in water by using a CeO2 catalyst. Chem. A Eur. J. 2011, 17, 11428–11431. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Nakao, K.; Suzuki, K.; Tomishige, K. Organic carbonate synthesis from CO2 and alcohol over CeO2 with 2-cyanopyridine: Scope and mechanistic studies. J. Catal. 2014, 318, 95–107. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Sonehara, S.; Suzuki, K.; Fujimoto, K.; Tomishige, K. Ceria-catalyzed conversion of carbon dioxide into dimethyl carbonate with 2-cyanopyridine. ChemSusChem 2013, 6, 1341–1344. [Google Scholar] [CrossRef]
- Wang, S.P.; Zhou, J.J.; Zhao, S.Y.; Zhao, Y.J.; Ma, X.B. Enhancements of dimethyl carbonate synthesis from methanol and carbon dioxide: The in situ hydrolysis of 2-cyanopyridine and crystal face effect of ceria. Chin. Chem. Lett. 2015, 26, 1096–1100. [Google Scholar] [CrossRef]
- Du, J.; Shi, J.; Li, Z.; Liu, Z.; Fan, X.; Tao, C. Ionic liquid mediated CO2 activation for DMC synthesis. J. Nat. Gas Chem. 2012, 21, 476–479. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Z.; Zhang, H.; Ma, J.; Jiang, B.; Zhang, L. Highly efficient and reversible CO2 capture by task-specific deep eutectic solvents. Ind. Eng. Chem. Res. 2019, 58, 13321–13329. [Google Scholar] [CrossRef]
- Wang, X.; Shang, D.; Zeng, S.; Wang, Y.; Zhang, X.; Zhang, X.; Liu, J. Enhanced CO2 capture by binary systems of pyridinium-based ionic liquids and porous ZIF-8 particles. J. Chem. Thermodyn. 2019, 128, 415–423. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, L.; Bai, Y.; Li, F.; Dong, H.; Wang, H.; Zhang, X.; Zeng, S. Superbase ionic liquid-based deep eutectic solvents for improving CO2 absorption. ACS Sustain. Chem. Eng. 2020, 8, 2523–2530. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Gelbein, A.P. Process and Catalyst for Making Dialkyl Carbonates. U.S. Patent 6392078, 21 May 2002. [Google Scholar]
- Yamazaki, N.; Nakahama, S. Polymers derived from carbon dioxide and carbonates. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 249–252. [Google Scholar] [CrossRef]
- Lin, H.; Yang, B.; Sun, J.; Wang, X.; Wang, D. Kinetics studies for the synthesis of dimethyl carbonate from urea and methanol. Chem. Eng. J. 2004, 103, 21–27. [Google Scholar] [CrossRef]
- Gui, X.; Cao, F.; Liu, D.; Fang, D. Synthesis of dimethyl carbonate from carbon dioxide under supercritical condition. J. Chem. Eng. Chin. Univ. 1998, 12, 152. [Google Scholar]
- Wu, C.; Zhao, X.; Wang, Y. Effect of reduction treatment on catalytic performance of Zn-based catalyst for the alcoholysis of urea to dimethyl carbonate. Catal. Commun. 2005, 6, 694–698. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, N.; Wei, W.; Sun, Y. Synthesis of dimethyl carbonate from urea and methanol over ZnO. Ind. Eng. Chem. Res. 2005, 44, 7596–7599. [Google Scholar] [CrossRef]
- Zhao, W.; Peng, W.; Wang, D.; Zhao, N.; Li, J.; Xiao, F.; Wei, W.; Sun, Y. Zinc oxide as the precursor of homogenous catalyst for synthesis of dialkyl carbonate from urea and alcohols. Catal. Commun. 2009, 10, 655–658. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Zhao, W.; Wei, W.; Sun, Y. Reaction of zinc oxide with urea and its role in urea methanolysis. React. Kinet. Mech. Catal. 2010, 99, 381–389. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Gao, Y.; FXiao, F.; Wei, W.; Sun, Y. Synthesis of dimethyl carbonate from methyl carbamate and methanol over lanthanum compounds. Fuel Process. Technol. 2010, 91, 1081–1086. [Google Scholar] [CrossRef]
- Xin, S.; Wang, L.; Li, H.; Huang, K.; Li, F. Synthesis of diethyl carbonate from urea and ethanol over lanthanum oxide as a heterogeneous basic catalyst. Fuel Process. Technol. 2014, 126, 453–459. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, W.; Zhao, N.; Wei, W.; Sun, Y. A DFT study on the reaction mechanism for dimethyl carbonate synthesis from methyl carbamate and methanol. J. Mol. Catal. A Chem. 2011, 351, 29–40. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A.; Fasciano, S.; Pàpai, I.; Curulla-Ferré, D.; Aresta, M. The reaction mechanism in the ethanolysis of urea with transition metal-based catalysts: DFT calculations and experiments. J. CO2 Util. 2014, 8, 27–33. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Wei, W.; Sun, Y. Synthesis of dimethyl carbonate from methyl carbamate and methanol using a fixed-bed reactor. Chem. Eng. Technol. 2012, 35, 2183–2188. [Google Scholar] [CrossRef]
- Joe, W.; Lee, H.J.; Hong, U.G.; Ahn, Y.S.; Song, C.J.; Kwon, B.J.; Song, I.K. Urea methanolysis to dimethyl carbonate over ZnO–CeO2–MO (MO: La2O3, Y2O3, Co2O3, Ga2O3, and ZrO2) catalysts. J. Ind. Eng. Chem. 2012, 18, 1730–1735. [Google Scholar] [CrossRef]
- Asghari, S.; Ghiaci, M. Dimethyl carbonate synthesis from urea methanolysis over ZnO-Nb2O5-TiO2 mixed oxide catalysts. Ind. Eng. Chem. Res. 2020, 59, 6405–6415. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, B.; Wang, X.; Zhao, J.; Cai, Q. Selective synthesis of dimethyl carbonate from urea and methanol over Fe2O3/HMCM-49. Catal. Sci. Technol. 2012, 2, 305–309. [Google Scholar] [CrossRef]
- Wu, X.; Kang, M.; Yin, Y.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. Synthesis of dimethyl carbonate by urea alcoholysis over Zn/Al bi-functional catalysts. Appl. Catal. A Gen. 2014, 473, 13–20. [Google Scholar] [CrossRef]
- Wu, X.; Kang, M.; Zhao, N.; Wei, W.; Sun, Y. Dimethyl carbonate synthesis over ZnO–CaO bi-functional catalysts. Catal. Commun. 2014, 46, 46–50. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A.; Aresta, M.; Fasciano, S.; Cucciolito, M.E.; Ruffo, F.; Aresta, B.M.; Curulla-Ferré, D.; De Giglio, E. Synthesis of diethyl carbonate by ethanolysis of urea: A study on the recoverability and recyclability of new Zn-based heterogeneous catalysts. Appl. Catal. A Gen. 2015, 493, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, X.; Zhang, X.; Fang, X. Synthesis of dimethyl carbonate from urea and methanol catalyzed by various metal oxides and salts. J. Fuel Chem. Technol. 2015, 43, 1375–1379. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S.; Zhou, F.; Yang, B.; Alshammari, A.S.; Lu, L.; Deng, Y. Two-step synthesis of dimethyl carbonate from urea, ethylene glycol and methanol using acid-base bifunctional zinc-yttrium oxides. Fuel Process. Technol. 2014, 126, 359–365. [Google Scholar] [CrossRef]
- An, H.; Zhang, G.; Zhao, X.; Wang, Y. Preparation of highly stable Ca-Zn-Al oxide catalyst and its catalytic performance for one-pot synthesis of dimethyl carbonate. Catal. Today 2018, 316, 185–192. [Google Scholar] [CrossRef]
- Lakshmi, D.D.; Rao, S.B.; Lingaiah, N. Synthesis of dimethyl carbonate from methanol and urea over zinc-strontia mixed oxide catalysts. Catal. Commun. 2019, 122, 1–4. [Google Scholar] [CrossRef]
- He, H.; Wang, T.; Zhu, S. Continuous production of biodiesel fuel from vegetable oil using supercritical methanol process. Fuel 2007, 86, 442–447. [Google Scholar] [CrossRef]
- Hou, Z.; Luo, L.; Liu, K.; Liu, C.; Wang, Y.; Dai, L. High-yield synthesis of dimethyl carbonate from the direct alcoholysis of urea in supercritical methanol. Chem. Eng. J. 2014, 236, 415–418. [Google Scholar] [CrossRef]

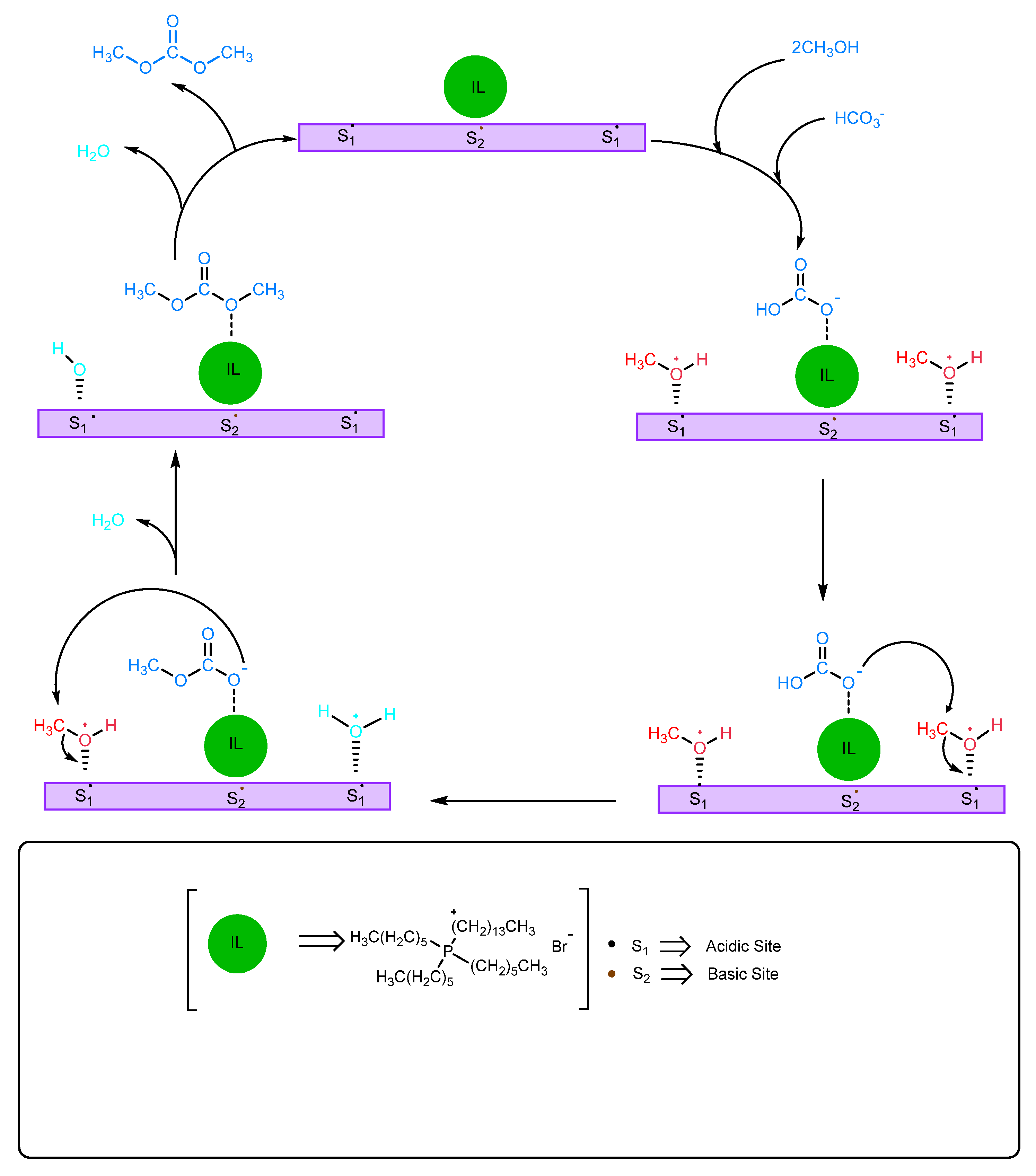
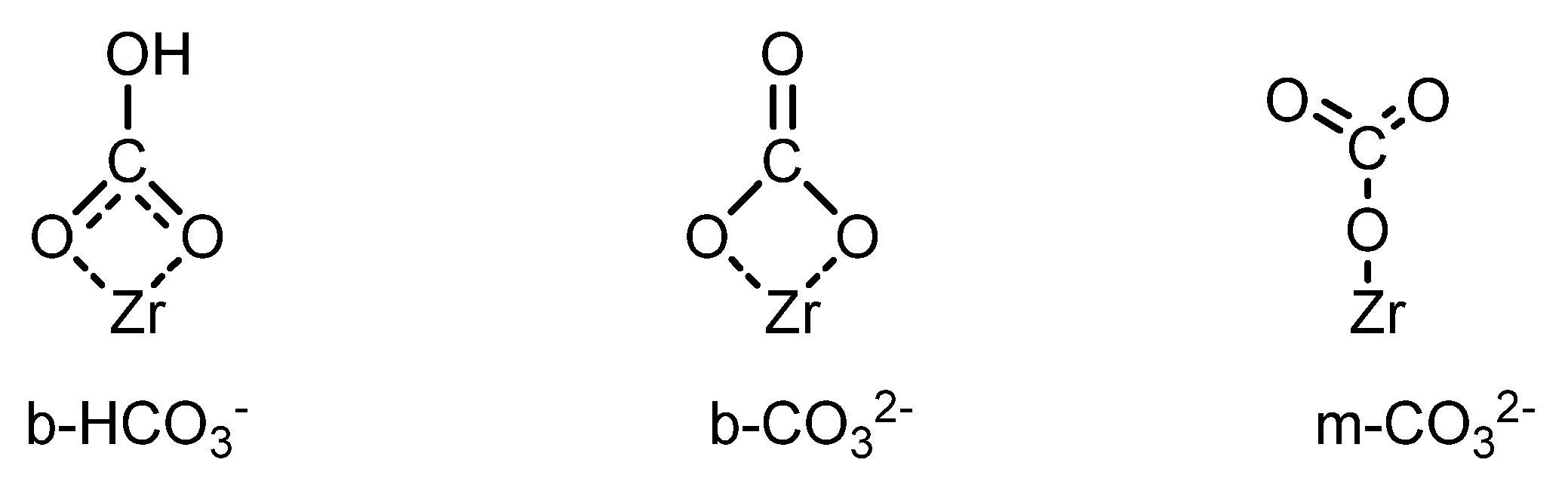




| Ionic Liquids | Bond Distance, Å | Bond Angle, ° | |
|---|---|---|---|
| O(1)-C(2) | C(2)-O(3) | O(1)-C(2)-O(3) | |
| CO2 (gas phase) | 1.169 | 1.692 | 180 |
| [bmim][PF6] | 1.172 | 1.166 | 176.730 |
| [bmim][BF4] | 1.173 | 1.165 | 175.462 |
| [bmim][Cl] | 1.175 | 1.166 | 174.207 |
| S. No | Catalysts | Reaction Conditions | Urea/MC # Conversion, % | DMC/DEC * Yield, % | Source |
|---|---|---|---|---|---|
| 1a | Zn powder | T = 443 K; P = 1.6 MPa; t = 6 h; Urea:MeOH = 1:46; Zn = 2.4 wt% | - | 12.7 x | [69] |
| 1b | ZnO/Al2O3 | T = 443 K; P = 1.6 MPa; t = 6 h; Urea:MeOH = 1:46; Zn = 10 wt% | 8.9 x | ||
| 2a | None | T = 453–473 K; t = 8–10 h; Urea/MC = 0.1 mol; MeOH = 64 g; Catalyst = 1 g | - | 0.8 x, 2.6 y | [71] |
| 2b | PbO | 22 x, 23.8 y | |||
| 2c | Zn(CH3COO)2 | 24 x, 21.1 y | |||
| 2d | MgO | 16.9 x, 17.5 y | |||
| 2e | CaO | 15.8 x, 18.2 y | |||
| 2f | ZnO | 34 x, 4.2 y | |||
| 3a | None | T = 453 K; t = 10 h; Urea/MC = 0.2 mol; MeOH = 4 mol; Catalyst = 2 g | - | 0.8 x, 1.0 y | [72] |
| 3b | ZnO-I from Zn(CO3)2 | 29 x, 4.3 y | |||
| 3c | ZnO-II (CMR) | 29 x, 5.5 y | |||
| 3d | CaO from (CaCO3)2 | 16 x, 15 y | |||
| 4a | None | T = 453 K; t = 8 h; MC = 7.5 g; MeOH = 64 g; Catalyst = 1 g | 4.00 # | 2.1 y | [73] |
| 4b | La2O3 | 10.2 # | 5.9 y | ||
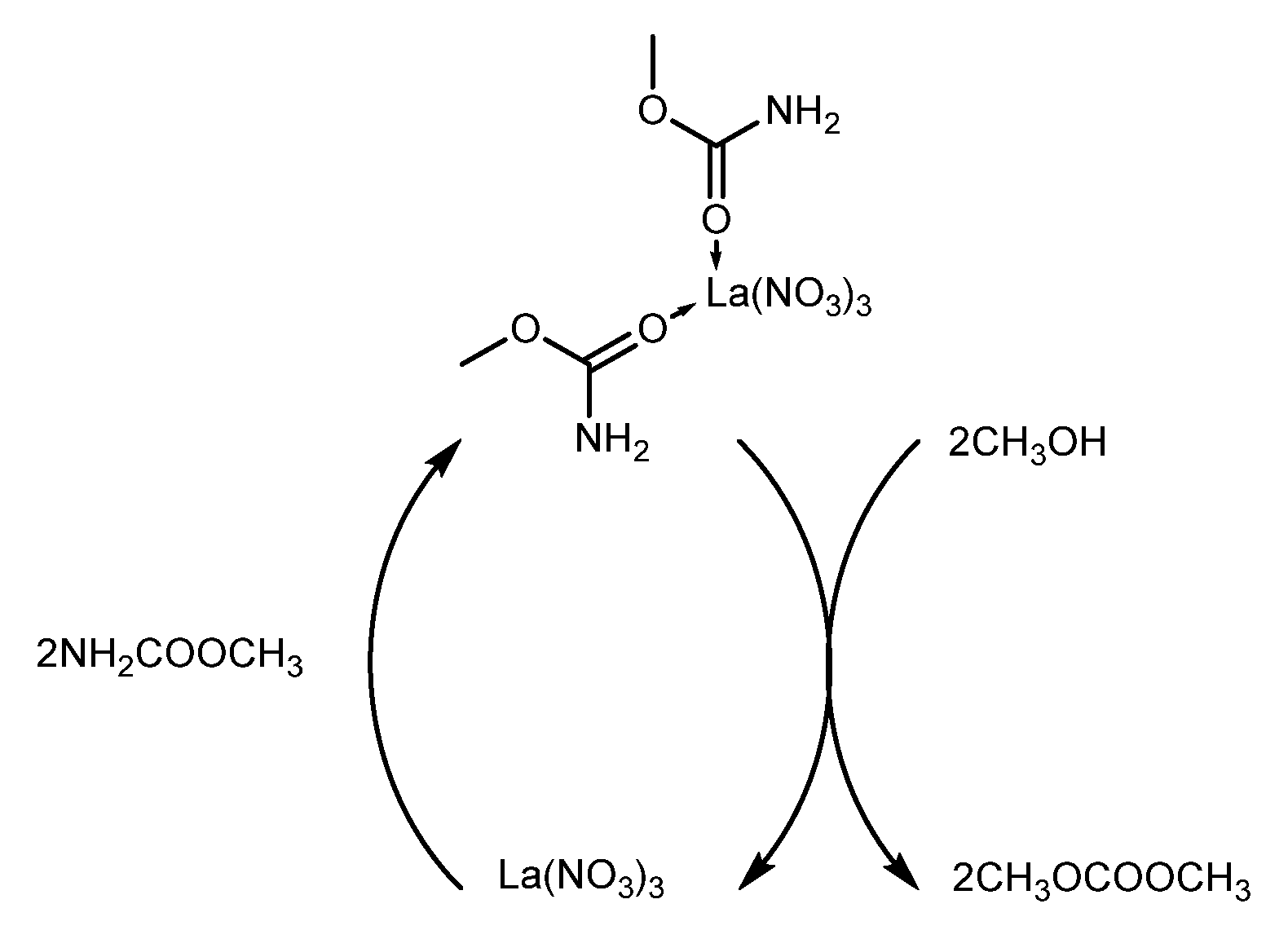
| 4c | LaCl3 | 73.9 # | 28.1 y | ||
| 4d | LaF3 | 20.0 # | 6.1 y | ||
| 4e | La2(CO3)3 | 12.4 # | 8.2 y | ||
| 4f | LaPO4 | 12.2 # | 7.6 y | ||
| 4g | La(NO3)3 | 84.8 # | 53.7 y | ||
| 4h | NaNO3 | 9.90 # | 4.5 y | ||
| 4i | Cu(NO3)2 | 11.4 # | 5.2 y | ||
| 5a | None | T = 473 K; t = 3 h; Urea:EtOH = 1:10; Catalyst = 17 wt% | - | 3.00 * | [74] |
| 5b | La2O3- calcination temp. 773 K | 29.2 * | |||
| 5c | Al2O3 | 5.30 * | |||
| 5d | MgO | 3.60 * | |||
| 5e | TiO2 | 4.50 * | |||
| 5f | CaO | 8.40 * | |||
| 5g | ZnO | 30.6 * | |||
| 6a | None | T = 453 K; t = 10 h; MC = 7.5 g; MeOH = 64 g; Catalyst = 2 g | 4.10 # | 2.6 | [77] |
| 6b | ZnO | 5.30 # | 3.5 | ||
| 6c | Al2O3 | 5.90 # | 3.3 | ||
| 6d | ZnO-Al2O3, physically mixed | 5.40 # | 3.1 | ||
| 6e | ZnO-Cr2O3 | 36.8 # | 23.5 | ||
| 6f | ZnO-Fe2O3 | 47.6 # | 30.4 | ||
| 6g | ZnO-Al2O3 | 56.4 # | 34.6 | ||
| 6h | ZnAl2O4 | 9.30 # | 4.2 | ||
| 7a | ZnO(0.7)-CeO2(0.3) | T = 443 K; t = 4 h; P = 2 MPa CO2; Urea = 3 g; MeOH = 40 mL; Catalyst = 0.75 g | 100 | 39.2 x, 49.1 z, 7.1 NM | [78] |
| 7b | ZnO(0.64)-CeO2(0.26)-La2O3(0.1) | 100 | 50.4 x, 40.2 z, 6.7 NM | ||
| 7c | ZnO(0.64)-CeO2(0.26)-Y2O3(0.1) | 100 | 45.6 x, 44.4 z, 6.8 NM | ||
| 7d | ZnO(0.64)-CeO2(0.26)-Co2O3(0.1) | 100 | 41.8 x, 49.6 z, 4.9 NM | ||
| 7e | ZnO(0.64)-CeO2(0.26)-Ga2O3(0.1) | 100 | 29.9 x, 65.6 z, 2.4 NM | ||
| 7f | ZnO(0.64)-CeO2(0.26)-ZrO2(0.1) | 100 | 34.9 x, 57.4 z, 6.3 NM | ||
| 8a | ZnO(0.64)-TiO2 (0.36) | T = 413 K; t = 4 h; Urea = 16.6 mmol; MeOH = 333 mmol; Catalyst = 200 mg; | 39.2 | 24.5 x | [79] |
| 8b | ZnO(0.64)-Nb2O5 (0.10) TiO2 (0.26) | 52.8 | 33 x | ||
| 8c | ZnO(0.60)-Nb2O5 (0.14)-TiO2 (0.26) | 58.2 | 36.4 x | ||
| 8d | ZnO(0.54)-Nb2O5(0.20)-TiO2 (0.26) | 62.5 | 39.1 x | ||
| 8e | ZnO(0.50)-Nb2O5(0.24)-TiO2 (0.26) | 59.3 | 37.1 x | ||
| 8f | Nb2O5(0.64)-TiO2 (0.36) | 44.6 | 27.9 x | ||
| 9a | SBA-15 | T = 453 K; t = 8 h; Urea = 0.0105 mol; MeOH = 1.68 mol; Catalyst = 0.3 g | - | 0 | [80] |
| 9b | MCM-49 | 3.7 | |||
| 9c | HMCM-49 | 4.1 | |||
| 9d | Fe2O3 | 2.4 | |||
| 9e | Fe2O3/SBA-15 | 10 | |||
| 9f | Fe2O3/MCM-49 | 14.7 | |||
| 9g | Fe2O3/HMCM-49 | 20.5 | |||
| 9h | Fe2O3/HY | 7 | |||
| 9i | Fe2O3/HZSM-5 | 3.2 | |||
| 10a | None | T = 453 K; t = 10 h; Urea = 0.1 mol; MeOH = 64 g; Catalyst = 0.5 g | - | 6.50, 84.8 z | [81] |
| 10b | ZnO | 29.5, 84.8 z | |||
| 10c | Zn/Al hydrotalcites (Zn/Al 2.47 mol ratio) | 36.5, 55.1 z | |||
| 11a | ZnO | T = 453 K; t = 10 h; Urea = 0.1 mol; MeOH = 64 g; Cat = 0.4 g; Zn/Ca = 2.72 molar ratio | - | 30.8, 59.0 z | [82] |
| 11b | ZnO + CaO Physically mixed | 22.9, 70.9 z | |||
| 11c | ZnO + CaO Calcined at 1073 K | 41.2, 50.5 z | |||
| 12a | ZnO | T = 453 K; t = 7 h; Urea:EtOH = 1:10; Urea:Cat = 7:1 | - | 17.0 * | [83] |
| 12b | CuO/ZnO | 10.3 * | |||
| 12c | In2O3/ZnO | 7.40 * | |||
| 12d | SiO2/ZnO | 1.20 * | |||
| 12e | ZIF-8 | 15.20 * at 15 h | |||
| 12f | Tethered Zn(NCO)2 | 1.01 * | |||
| 12g | Tethered ZnCl2 | 0.71 * | |||
| 13a | Zn(NO3)2.6H2O | T = 443 K; t = 4 h; Urea:MeOH = 1:15; Catalyst = 2 wt% | - | 3.5, 45.7 z | [84] |
| 13b | Zn(AC)2.2H2O | 3.0, 48.8 z | |||
| 13c | Ce(NO3)2.6H2O | 3.2, 45.9 z | |||
| 13d | CeCl3.7H2O | 3.4, 50.1 z | |||
| 13e | ZnBr2 | 2.4, 57.9 z | |||
| 13f | ZnCl2 | 2.4, 59.2 z | |||
| 13g | Zr(NO3)2 | 1.6, 51.2 z | |||
| 13h | CaO | 1.3, 44.9 z | |||
| 13i | ZnO | 2.8, 51.2 z | |||
| 13j | ZnO-CeO2 | 3.1, 55.7 z | |||
| 13k | ZnO-CeCl3.7H2O | 3.1, 44.7 z | |||
| 13l | ZnO-Ce(NO3)3.6H2O | 2.6, 47.8 z | |||
| 13m | Zn(OH)2; SiO2; Al(OH)3 | 0 | |||
| 13n | Zn(NO3)2-Al(OH)3 | 2.7 | |||
| 13o | Zn(NO3)2-Zn(OH)3 | 4.05 | |||
| 14a | Y2O3 | T = 423 K; t = 3 h; Urea = 6 g; EG = 9.3 g; Catalyst = 0.3 g | 27 | [85] | |
| 14b | ZnO | 75 | |||
| 14c | Zn/Y = 3:1 673 K | 92 | |||
| 15a | Ca-Zn-Al oxide | T = 443 K; t = 8 h; Urea:PG = 2, Cat = 2.7 wt% | 82.4 | [86] | |
| 16a | ZnO-SrO2 molar ratio 3:1 | T = 443 K; t = 6 h; Urea:MeOH = 1:15; Catalyst = 2.6 wt% | - | 16 x, 72 z, 12 NM | [87] |
| 16b | ZnO-SrO2 molar ratio 2:1 | 18 x, 63 z, 19 NM | |||
| 16c | ZnO-SrO2 molar ratio 1:1 | 35 x, 42 z, 23 NM | |||
| 16d | ZnO-SrO2 molar ratio 1:2 | 24 x, 45 z, 31 NM | |||
| 16e | ZnO-SrO2 molar ratio 1:3 | 19 x, 44 z, 37 NM | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohli, K.; Sharma, B.K.; Panchal, C.B. Dimethyl Carbonate: Review of Synthesis Routes and Catalysts Used. Energies 2022, 15, 5133. https://doi.org/10.3390/en15145133
Kohli K, Sharma BK, Panchal CB. Dimethyl Carbonate: Review of Synthesis Routes and Catalysts Used. Energies. 2022; 15(14):5133. https://doi.org/10.3390/en15145133
Chicago/Turabian StyleKohli, Kirtika, Brajendra K. Sharma, and Chandrakant B. Panchal. 2022. "Dimethyl Carbonate: Review of Synthesis Routes and Catalysts Used" Energies 15, no. 14: 5133. https://doi.org/10.3390/en15145133
APA StyleKohli, K., Sharma, B. K., & Panchal, C. B. (2022). Dimethyl Carbonate: Review of Synthesis Routes and Catalysts Used. Energies, 15(14), 5133. https://doi.org/10.3390/en15145133







