Pyrolysis of Chromated Copper Arsenate-Treated Wood: Investigation of Temperature, Granulometry, Biochar Yield, and Metal Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. CCA-Treated Wood Sampling and Characterization
2.2. Pyrolysis of CCA-Treated Wood
2.3. Characterization of Materials
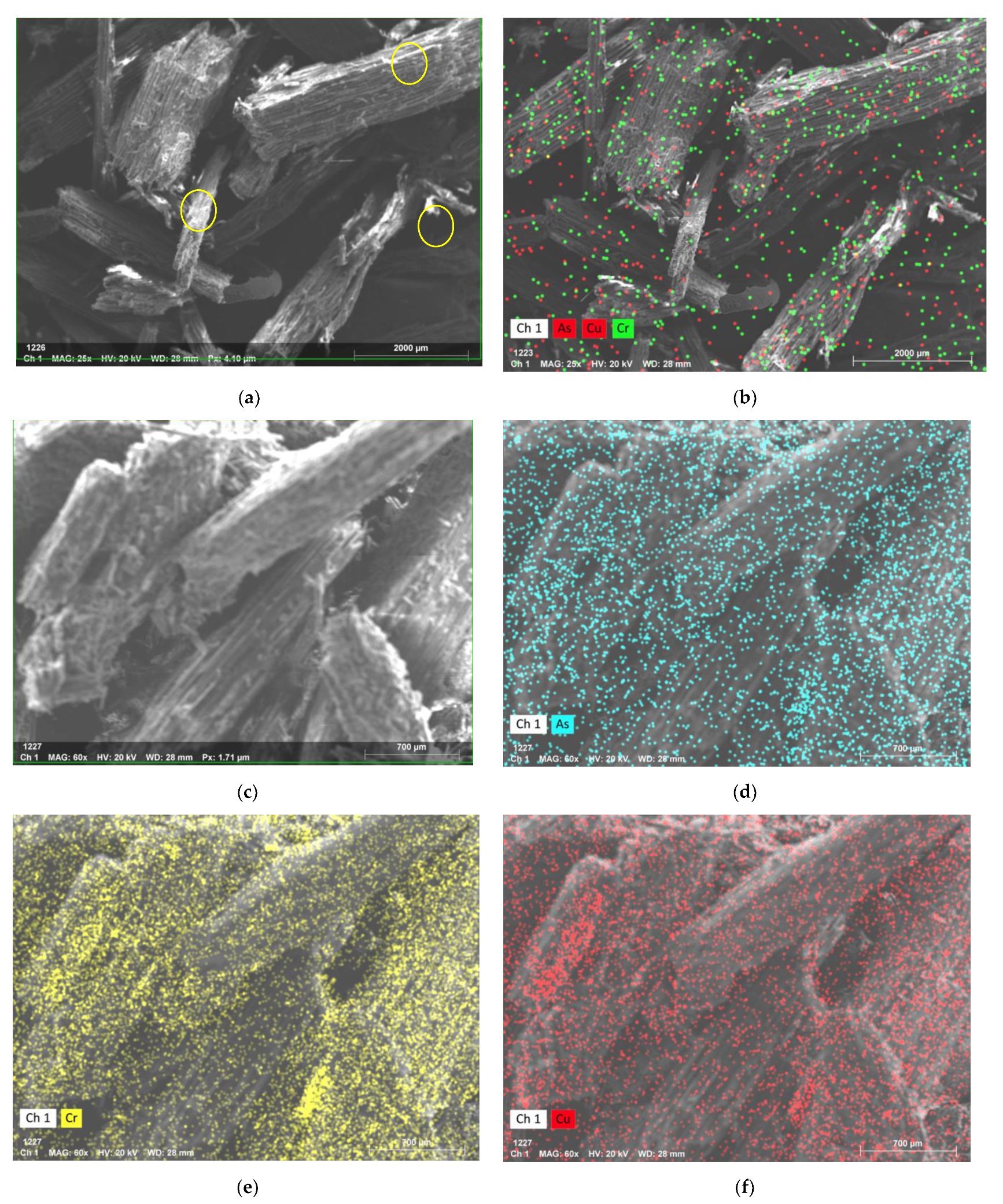
2.3.1. Microstructure
2.3.2. Physical and Chemical Properties
2.4. Toxicity Characterization and Leaching Procedure (TCLP)
2.5. Biochar Encapsulation
2.6. Statistical Analyses
3. Results and Discussion
3.1. Microstructure of CCA-Treated Wood and Biochar
3.2. Physical and Chemical Properties of CCA-Treated Wood and Biochar
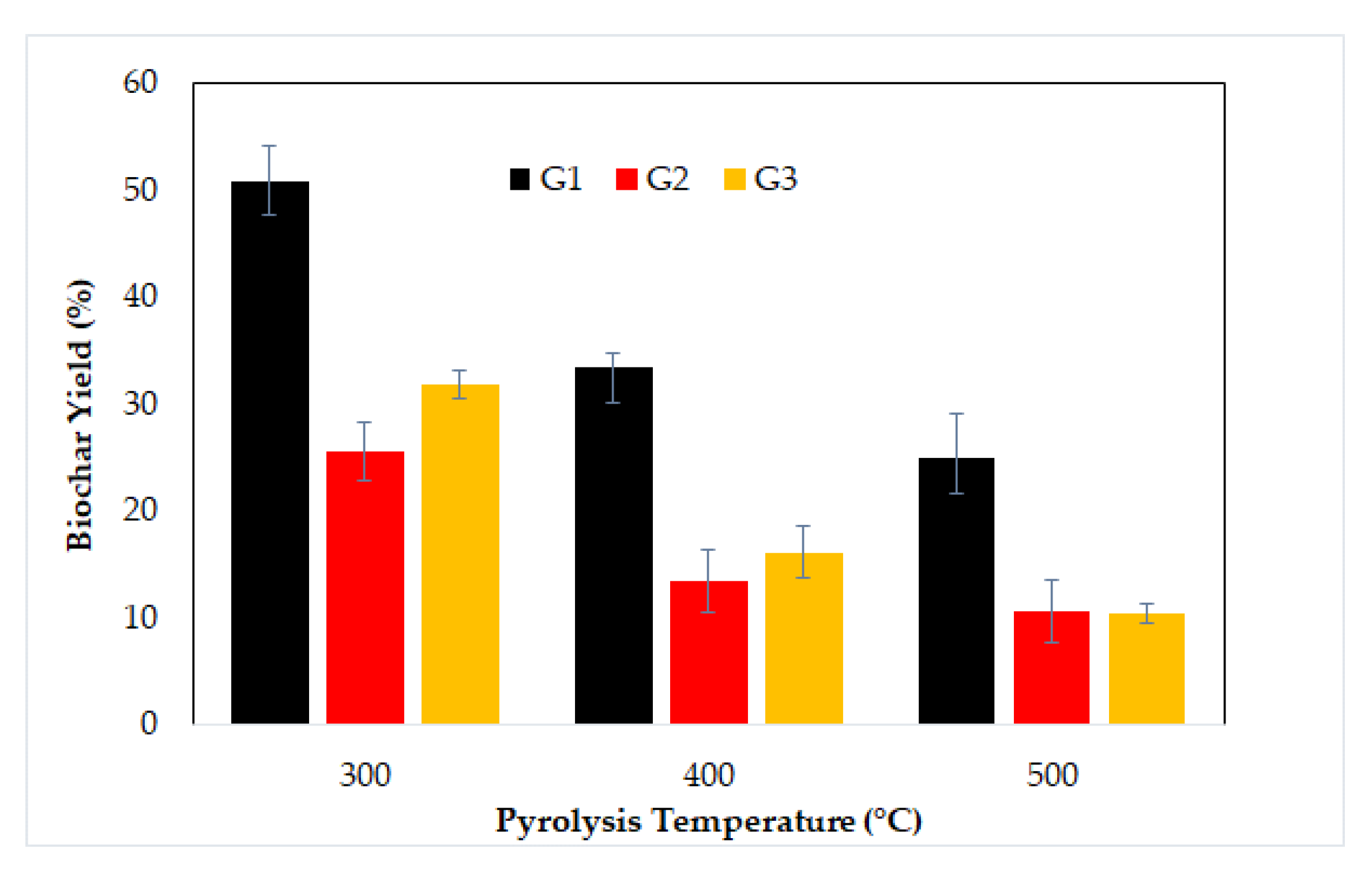
3.3. Effect of Particle Size and Pyrolysis Temperature on Biochar Characteristics
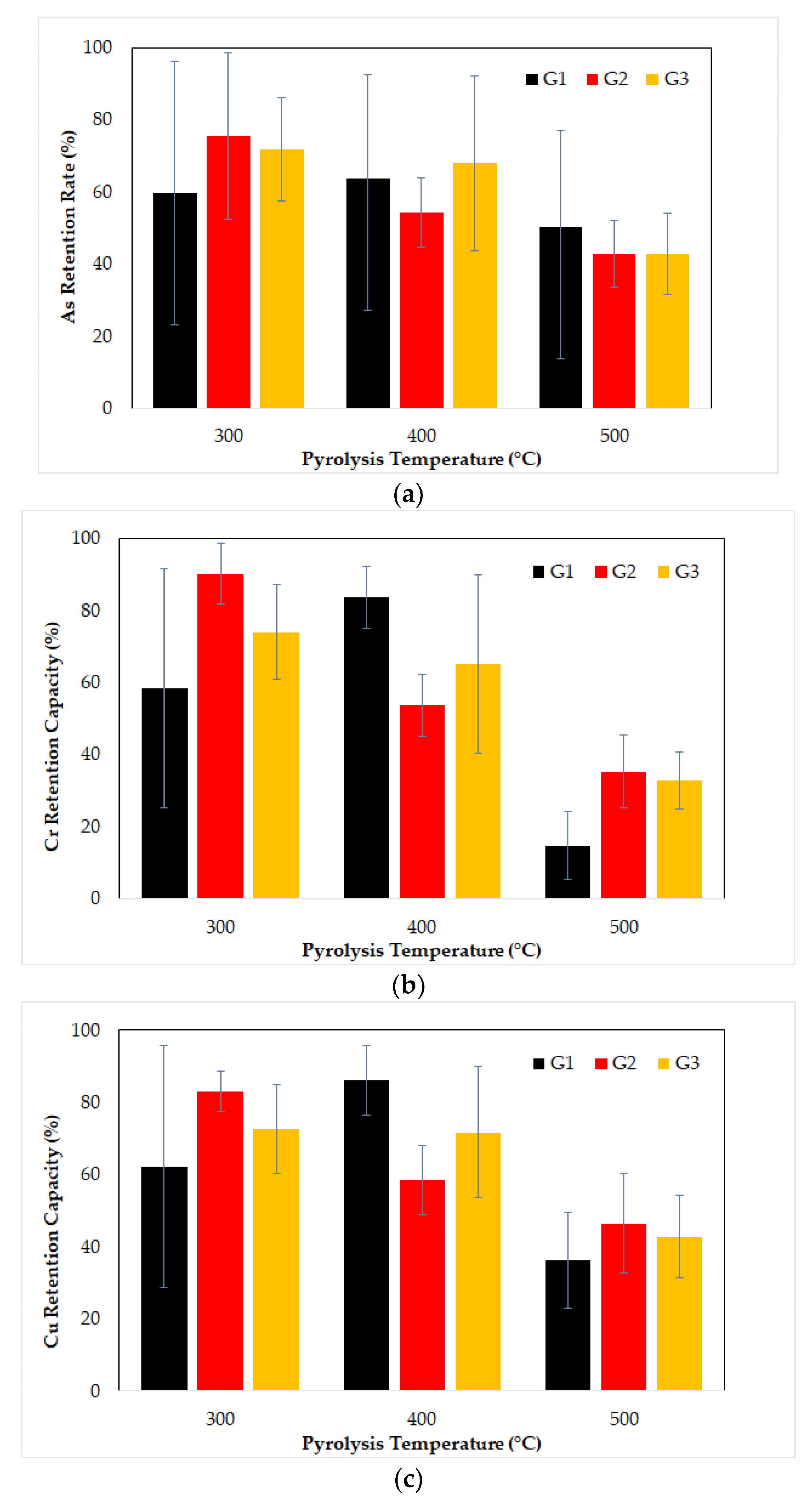
3.4. Effect of Pyrolysis Temperature on Metal Retention
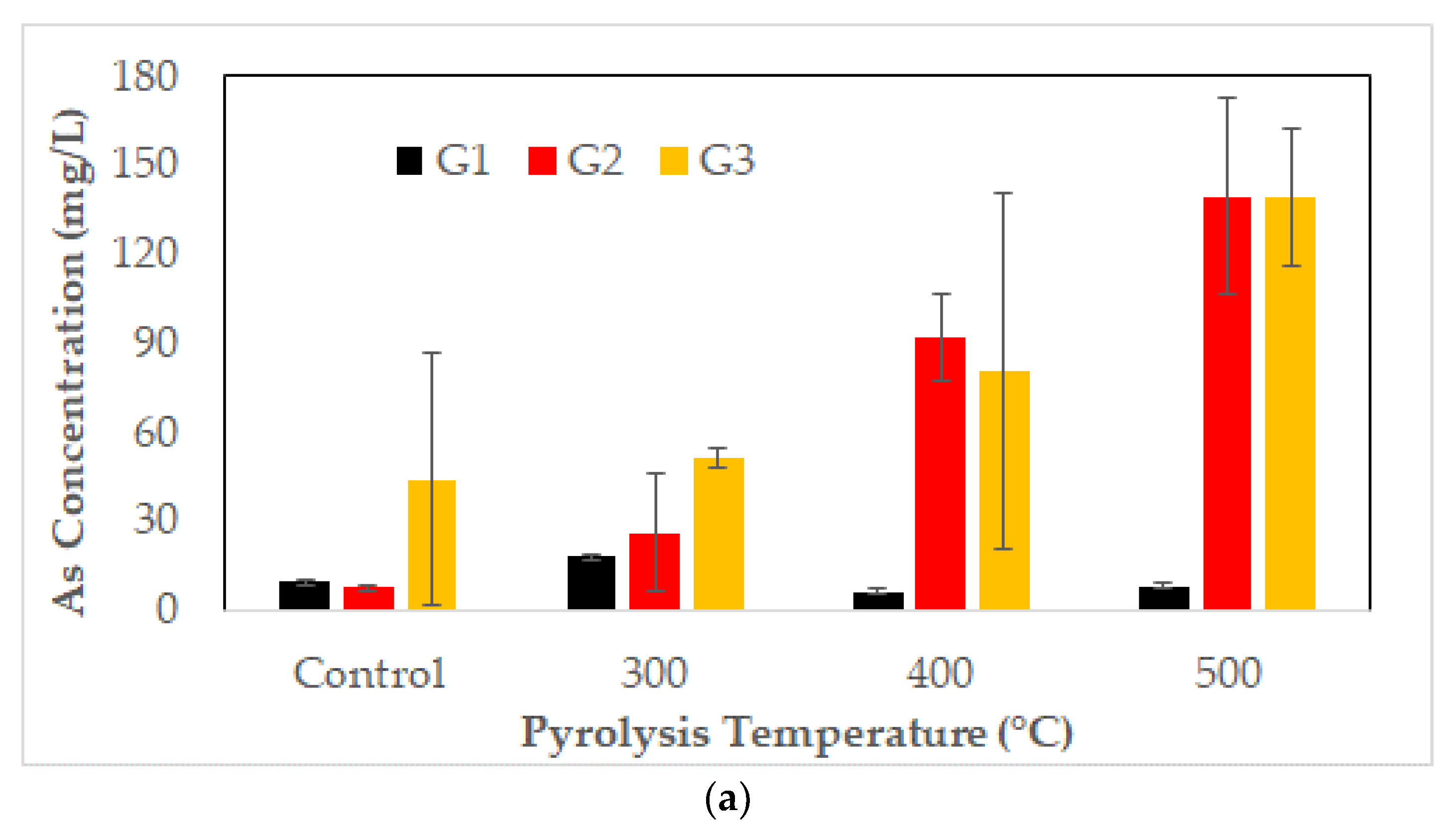
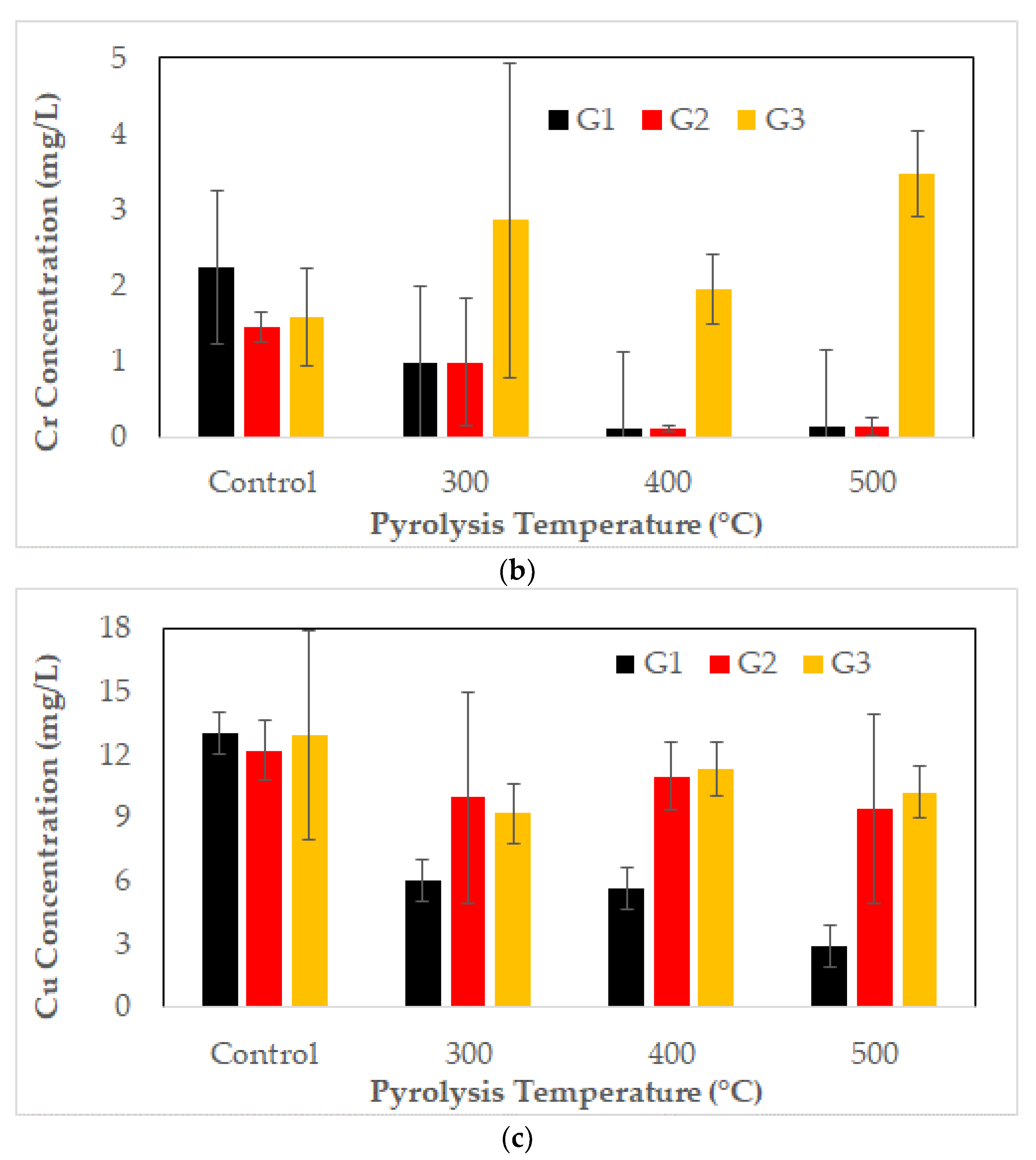
3.5. Metal Leaching of CCA-Contaminated Biochar
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groenier, J.S.; Lebow, S. Preservative-Treated Wood and Alternative Products in the Forest Service; USDA Forest Service: Missoula, MT, USA, 2006. [Google Scholar]
- Canadian Wood Council Durability by Treatment. Available online: https://cwc.ca/why-build-with-wood/durability/durability-by-treatment/ (accessed on 1 July 2021).
- Solo-Gabriele, H.; Townsend, T. Disposal Practices and Management Alternatives for CCA-Treated Wood Waste. Waste Manag. Res. 1999, 17, 378–389. [Google Scholar] [CrossRef]
- Shalat, S.L.; Solo-Gabriele, H.M.; Fleming, L.E.; Buckley, B.T.; Black, K.; Jimenez, M.; Shibata, T.; Durbin, M.; Graygo, J.; Stephan, W. A Pilot Study of Children’s Exposure to CCA-Treated Wood from Playground Equipment. Sci. Total Environ. 2006, 367, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Health Canada; Pest Management Regulatory Agency. Heavy Duty Wood Preservatives: Creosote, Pentachlorophenol, Chromated Copper Arsenate (CCA) and Ammoniacal Copper Zinc Arsenate (ACZA); Health Canada Pest Management Regulatory Agency: Ottawa, ON, Canada, 2010; ISBN 978-1-100-15147-2. [Google Scholar]
- Coudert, L. Décontamination de Déchets de Bois Traité à Base de Composés Cuivrés En Vue de Leur Revalorisation. Ph.D. Thesis, Université du Québec, Institut National de la Recherche Scientifique, Québec, QC, Canada, 2013. [Google Scholar]
- AWPA American Wood Protection Association. Book of Standards. American Wood Protection Association: Granbury, TX, USA, 1997. [Google Scholar]
- Helsen, L.; Van den Bulck, E. Metal Behavior during the Low-Temperature Pyrolysis of Chromated Copper Arsenate-Treated Wood Waste. Environ. Sci. Technol. 2000, 34, 2931–2938. [Google Scholar] [CrossRef]
- Janin, A. Développement d’un Procédé Chimique de Décontamination de Bois Usagé Traité à l’arseniate de Cuivre Chromaté. Ph.D. Thesis, Université du Québec, Institut National de la Recherche Scientifique, Québec, QC, Canada, 2009. [Google Scholar]
- Nicholas, D.D.; Loos, W.E. Wood Deterioration and Its Prevention by Preservative Treatments. 1: Degradation and Protection of Wood; Syracuse wood science series; 2. print; Syracuse University Press: Syracuse, NY, USA, 1982; ISBN 978-0-8156-2285-7. [Google Scholar]
- Freeman, M.H.; Shupe, T.F.; Vlosky, R.P.; Barnes, H. Past, Present, and Future of the Wood Preservation Industry: Wood Is a Renewable Natural Resource That Typically Is Preservative Treated to Ensure Structural Integrity in Many Exterior Applications. For. Prod. J. 2003, 53, 8–16. [Google Scholar]
- Ribeiro, A.B.; Mateus, E.P.; Ottosen, L.M.; Bech-Nielsen, G. Electrodialytic Removal of Cu, Cr, and As from Chromated Copper Arsenate-Treated Timber Waste. Environ. Sci. Technol. 2000, 34, 784–788. [Google Scholar] [CrossRef]
- Christensen, I.V.; Pedersen, A.J.; Ottosen, L.M.; Ribeiro, A.B. Electrodialytic Remediation of CCA-Treated Waste Wood in a 2 M3 Pilot Plant. Sci. Total Environ. 2006, 364, 45–54. [Google Scholar] [CrossRef]
- Sierra-Alvarez, R. Fungal Bioleaching of Metals in Preservative-Treated Wood. Process Biochem. 2007, 42, 798–804. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Choi, D.; Kikuchi, S. Enhanced Extraction of Heavy Metals in the Two-Step Process with the Mixed Culture of (Lactobacillus bulgaricus) and (Streptococcus thermophilus). Bioresour. Technol. 2012, 103, 477–480. [Google Scholar] [CrossRef] [Green Version]
- Kakitani, T.; Hata, T.; Kajimoto, T.; Imamura, Y. Designing a Purification Process for Chromium-, Copper- and Arsenic-Contaminated Wood. Waste Manag. 2006, 26, 453–458. [Google Scholar] [CrossRef]
- Botomé, M.L.; Poletto, P.; Junges, J.; Perondi, D.; Dettmer, A.; Godinho, M. Preparation and Characterization of a Metal-Rich Activated Carbon from CCA-Treated Wood for CO2 Capture. Chem. Eng. J. 2017, 321, 614–621. [Google Scholar] [CrossRef]
- Chen, X.; Liaw, S.B.; Wu, H. Effect of Water Vapour on Particulate Matter Emission during Oxyfuel Combustion of Char and in Situ Volatiles Generated from Rapid Pyrolysis of Chromated-Copper-Arsenate-Treated Wood. Proc. Combust. Inst. 2019, 37, 4319–4327. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Jakab, E.; Faix, O.; Till, F.; Székely, T. Thermogravimetry/Mass Spectrometry Study of Six Lignins within the Scope of an International Round Robin Test. J. Anal. Appl. Pyrolysis 1995, 35, 167–179. [Google Scholar] [CrossRef]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and Kinetics of Homogeneous Secondary Reactions of Tar from Continuous Pyrolysis of Wood Chips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]
- Blanco López, M.C.; Blanco, C.G.; Martı́nez-Alonso, A.; Tascón, J.M.D. Composition of Gases Released during Olive Stones Pyrolysis. J. Anal. Appl. Pyrolysis 2002, 65, 313–322. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A Review on Pyrolysis of Biomass Constituents: Mechanisms and Composition of the Products Obtained from the Conversion of Cellulose, Hemicelluloses and Lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Wei, L.; Xu, S.; Zhang, L.; Zhang, H.; Liu, C.; Zhu, H.; Liu, S. Characteristics of Fast Pyrolysis of Biomass in a Free Fall Reactor. Fuel Process. Technol. 2006, 87, 863–871. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, Kinetics and Endothermicity of the Biomass Pyrolysis Reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and Prediction of Biomass Pyrolysis Products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Darvell, L.I.; Jones, J.M.; Yates, N.; Thain, S.; Donnison, I.S. The Effect of Alkali Metals on Combustion and Pyrolysis of Lolium and Festuca Grasses, Switchgrass and Willow. Fuel 2007, 86, 1560–1569. [Google Scholar] [CrossRef]
- Helsen, L.; van den Bulck, E.; Mullens, S.; Mullens, J. Low-Temperature Pyrolysis of CCA-Treated Wood: Thermogravimetric Analysis. J. Anal. Appl. Pyrolysis 1999, 52, 65–86. [Google Scholar] [CrossRef]
- Helsen, L.; van den Bulck, E. Metal Retention in the Solid Residue after Low-Temperature Pyrolysis of Chromated Copper Arsenate (CCA)-Treated Wood. Environ. Eng. Sci. 2003, 20, 569–580. [Google Scholar] [CrossRef]
- Henke, K.R. Arsenic: Environmental Chemistry, Health Threats, and Waste Treatment; John Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-470-02758-5. [Google Scholar]
- Larfeldt, J. Modelling and Measurements of the Pyrolysis of Large Wood Particles. Fuel 2000, 79, 1637–1643. [Google Scholar] [CrossRef]
- Seebauer, V.; Petek, J.; Staudinger, G. Effects of Particle Size, Heating Rate and Pressure on Measurement of Pyrolysis Kinetics by Thermogravimetric Analysis. Fuel 1997, 76, 1277–1282. [Google Scholar] [CrossRef]
- Şensöz, S.; Angın, D.; Yorgun, S. Influence of Particle Size on the Pyrolysis of Rapeseed (Brassica napus L.): Fuel Properties of Bio-Oil. Biomass Bioenergy 2000, 19, 271–279. [Google Scholar] [CrossRef]
- Peters, B.; Bruch, C. Drying and Pyrolysis of Wood Particles: Experiments and Simulation. J. Anal. Appl. Pyrolysis 2003, 70, 233–250. [Google Scholar] [CrossRef]
- Sadhukhan, A.K.; Gupta, P.; Saha, R.K. Modelling and Experimental Studies on Pyrolysis of Biomass Particles. J. Anal. Appl. Pyrolysis 2008, 81, 183–192. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.-S.; Garcia-Perez, M.; Mourant, D.; Rhodes, M.J.; Li, C.-Z. Effects of Particle Size on the Fast Pyrolysis of Oil Mallee Woody Biomass. Fuel 2009, 88, 1810–1817. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Dimin, M.F.; Juoi, J.M.; Mohd Husin, M.H.; Mitan, N.M.M. Influence of Heating Temperature and Holding Time on Biochars Derived from Rubber Wood Sawdust via Slow Pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 31–39. [Google Scholar] [CrossRef]
- Cuypers, F.; Helsen, L. Pyrolysis of Chromated Copper Arsenate (CCA) Treated Wood Waste at Elevated Pressure: Influence of Particle Size, Heating Rate, Residence Time, Temperature and Pressure. J. Anal. Appl. Pyrolysis 2011, 92, 111–122. [Google Scholar] [CrossRef]
- Fu, Q.; Argyropoulos, D.S.; Tilotta, D.C.; Lucia, L.A. Understanding the Pyrolysis of CCA-Treated Wood. J. Anal. Appl. Pyrolysis 2008, 82, 140–144. [Google Scholar] [CrossRef]
- Kinata, S.E.; Loubar, K.; Bouslamti, A.; Belloncle, C.; Tazerout, M. Influence of Impregnation Method on Metal Retention of CCB-Treated Wood in Slow Pyrolysis Process. J. Hazard. Mater. 2012, 233, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Gauvin, F.; Brouwers, H.J.H. The Recycling Potential of Wood Waste into Wood-Wool/Cement Composite. Constr. Build. Mater. 2020, 260, 119786. [Google Scholar] [CrossRef]
- Spence, R.D.; Shi, C. Stabilization and Solidification of Hazardous, Radioactive, and Mixed Wastes; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-367-39341-0. [Google Scholar]
- Ministère de l’environnement et de la Lutte Contre les Changements Climatiques Lignes Directrices Sur La Gestion Des Matières Résiduelles et Des Sols Contaminés Traités Par Stabilisation et Solidification; Ministère de l’Environnement et de la Lutte Contre les Changements Climatiques: Québec, QC, Canada, 2021. Available online: https://www.environnement.gouv.qc.ca/matieres/mat_res/ld-gestion-matres-sols-stabilisation-solid.pdf (accessed on 8 July 2022).
- Wang, F.; Wang, H.; Jin, F.; Al-Tabbaa, A. The Performance of Blended Conventional and Novel Binders in the In-Situ Stabilisation/Solidification of a Contaminated Site Soil. J. Hazard. Mater. 2015, 285, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Centre d’expertise en Analyse Environnementale du Québec Determination of Metals: Method by Mass Spectrometry with an Argon Plasma Ionizing Source, MA. 200–Mét 1.1, Rév. 3 Ministère du Développement Durable, de l’Environnement, de la Faune et des Parcs du Québec 2014, 13 Pages, 4. Available online: https://www.ceaeq.gouv.qc.ca/methodes/pdf/MA203MetRP10.pdf (accessed on 8 July 2022).
- SAS Institute Inc. Statistical Analysis Software. Users’ Guide Statistics Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Helsen, L.; Hacala, A. Formation of metal agglomerates during carbonisation of chromated copper arsenate (CCA) treated wood waste: Comparison between a lab scale and an industrial plant. J. Hazard. Mater. 2006, 137, 1438–1452. [Google Scholar] [CrossRef]
- Kemiha, M.; Nzihou, A.; Mateos, D. Thermal valorization of wood waste by pyrolysis at low temperature. Chem. Eng. Trans. 2011, 25, 545–550. [Google Scholar]
- Ottosen, L.M.; Pedersen, A.J.; Christensen, I.V. Characterization of residues from thermal treatment of treated wood and extraction of Cu, Cr, As and Zn. Wood Sci. Technol. 2005, 39, 87–98. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Renewable Fuels and Chemicals by Thermal Processing of Biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, T.-S.; Eom, I.-Y.; Kang, S.M.; Cho, T.-S.; Choi, I.G.; Choi, J.W. Characterization of Pyrolytic Products Obtained from Fast Pyrolysis of Chromated Copper Arsenate (CCA)- and Alkaline Copper Quaternary Compounds (ACQ)-Treated Wood Biomasses. J. Hazard. Mater. 2012, 227, 445–452. [Google Scholar] [CrossRef]
- Mercer, T.G.; Frostick, L.E. Leaching Characteristics of CCA-Treated Wood Waste: A UK Study. Sci. Total Environ. 2012, 427, 165–174. [Google Scholar] [CrossRef]
- Shiau, R.J.; Smith, R.L.; Avellar, B. Effects of Steam Explosion Processing and Organic Acids on CCA Removal from Treated Wood Waste. Wood Sci. Technol. 2000, 34, 377–388. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of Inorganic Salts on the Primary Pyrolysis Products of Cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Giudicianni, P.; Pindozzi, S.; Grottola, C.M.; Stanzione, F.; Faugno, S.; Fagnano, M.; Fiorentino, N.; Ragucci, R. Effect of Feedstock and Temperature on the Distribution of Heavy Metals in Char from Slow Steam Pyrolysis of Contaminated Biomasses. Chem. Eng. Trans. 2017, 58, 505–510. [Google Scholar] [CrossRef]
- Ayadi, R.; Koubaa, A.; Braghiroli, F.; Migneault, S.; Wang, H.; Bradai, C. Effect of the Pyro-Gasification Temperature of Wood on the Physical and Mechanical Properties of Biochar-Polymer Biocomposites. Materials 2020, 13, 1327. [Google Scholar] [CrossRef] [Green Version]
- Conner, J.; Lear, P. Treatment of Land and Arsenic Wastes at TSDFs. Pollut. Technol. Rev. 1993, 214, 110. [Google Scholar]
- Rechichi, D. Encapsulation of Hazardous Waste Materials. U.S. Patent 6,399,848 B1, 4 June 2002. 8p. Available online: https://patentimages.storage.googleapis.com/10/44/c4/05a54a30064fda/US6399848.pdf (accessed on 25 June 2022).

| Degree of Oxidation | Chemical Formula | Type A (%) | Type B (%) | Type C (%) |
|---|---|---|---|---|
| Cr (VI) | CrO3 | 65.5 | 35.3 | 47.5 |
| Cu (II) | CuO | 18.1 | 19.6 | 18.5 |
| As (V) | As2O5 | 16.4 | 45.1 | 34.0 |
| Elements/Properties | Sample Type | ||
|---|---|---|---|
| Untreated Wood | CCA-Treated Wood | Biochar Produced at 300 °C | |
| Elementary Analysis (%wt) | |||
| N | 0.17 ± 0.04 | 0.32 ± 0.03 | 0.27 ± 0.02 |
| C | 49.6 ± 0.10 | 46.1 ± 0.15 | 58.27 ± 0.21 |
| H | 6.74 ± 0.04 | 6.05 ± 0.02 | 4.04 ± 0.03 |
| O * | 35.2 ± 0.07 | 38.4 ± 0.09 | 28.94 ± 2.37 |
| S | 1.26 ± 0.07 | 0.94 ± 0.06 | 0.58 ± 0 01 |
| Moisture content (%) | 6.91 ± 0.03 | 6.85 ± 0.03 | 1.86 ± 0.01 |
| Ash (%) | 0.08 ± 0.02 | 1.42 ± 0.02 | 4.80 ± 0.01 |
| Particle Size (mm) | Concentration (mg/kg) | ||
|---|---|---|---|
| As | Cr | Cu | |
| 0.85 < G1 < 1.4 | 1539 ± 842 | 2856 ± 171 | 1585 ± 118 |
| 1.4 < G2 < 2 | 2850 ± 498 | 3580 ± 601 | 2107 ± 353 |
| 2 < G3 < 3.35 | 2478 ± 839 | 3461 ± 379 | 2019 ± 310 |
| Source | DF 1 | Biochar | Retention Capacity | Metal Concentration | Metal Leaching | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | As | Cr | Cu | As | Cr | Cu | As | Cr | Cu | ||
| Model | 8 | 74.6 ** | 1.17 ns | 8.09 ** | 4.03 ** | 14.6 ** | 22.2 ** | 22.8 ** | 11.9 ** | 7.07 ** | 3.49 * |
| Size (G) | 2 | 145 ** | 0.97 ns | 0.20 ns | 0.27 ns | 72.6 ** | 7.80 ** | 72.6 ** | 25.2 ** | 23.6 ** | 12.0 ** |
| Temperature (T) | 2 | 146 ** | 3.27 ns | 26.5 ** | 13.3 ** | 11.3 ** | 5.82 * | 11.3 ** | 12.9 ** | 2.58 ns | 1.02 ns |
| TxG | 4 | 3.25 * | 0.23 ns | 2.82 ns | 1.28 ns | 3.62 * | 3.63 * | 3.62 * | 4.67 ** | 1.04 ns | 0.47 ns |
| Error | 18 | - | - | - | - | - | - | ||||
| R2 | 0.97 | 0.34 | 0.78 | 0.64 | 0.87 | 0.91 | 0.91 | 0.84 | 0.76 | 0.61 | |
| Concentration of Metals (mg/L) | |||
|---|---|---|---|
| As | Cr | Cu | |
| Before pyrolysis | 9.23 ± 1.04 | 2.24 ± 0.27 | 13.0 ± 1.44 |
| Before encapsulation | 25.95 ± 19.73 | 0.99 ± 0.84 | 9.96 ± 5.20 |
| After encapsulation | 0.63 ± 0.10 | 0.05 ± 0.02 | 0.52 ± 0.04 |
| Reduction (%) | 88.4 | 95.0 | 94.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gmar, M.; Bouafif, H.; Bouslimi, B.; Braghiroli, F.L.; Koubaa, A. Pyrolysis of Chromated Copper Arsenate-Treated Wood: Investigation of Temperature, Granulometry, Biochar Yield, and Metal Pathways. Energies 2022, 15, 5071. https://doi.org/10.3390/en15145071
Gmar M, Bouafif H, Bouslimi B, Braghiroli FL, Koubaa A. Pyrolysis of Chromated Copper Arsenate-Treated Wood: Investigation of Temperature, Granulometry, Biochar Yield, and Metal Pathways. Energies. 2022; 15(14):5071. https://doi.org/10.3390/en15145071
Chicago/Turabian StyleGmar, Mouna, Hassine Bouafif, Besma Bouslimi, Flavia L. Braghiroli, and Ahmed Koubaa. 2022. "Pyrolysis of Chromated Copper Arsenate-Treated Wood: Investigation of Temperature, Granulometry, Biochar Yield, and Metal Pathways" Energies 15, no. 14: 5071. https://doi.org/10.3390/en15145071
APA StyleGmar, M., Bouafif, H., Bouslimi, B., Braghiroli, F. L., & Koubaa, A. (2022). Pyrolysis of Chromated Copper Arsenate-Treated Wood: Investigation of Temperature, Granulometry, Biochar Yield, and Metal Pathways. Energies, 15(14), 5071. https://doi.org/10.3390/en15145071







