Energy-Efficient AnMBRs Technology for Treatment of Wastewaters: A Review
Abstract
:1. Introduction
2. AnMBRs Technology
2.1. Advantages of AnMBRs
2.2. MF and UF Membranes
| Membrane | MF | UF |
|---|---|---|
| Separation mechanism | sieving | sieving |
| Pore size [µm] | 0.1–1.0 | 0.01–0.1 |
| Type | porous, symmetric or asymetric | microporous, asymetric |
| Transmembrane pressure [bar] | 0.1–2.0 | 0.1–5.0 |
| Example applications | clarification, pretreatment | removal of macromolecules |
2.3. Configurations of AnMBRs
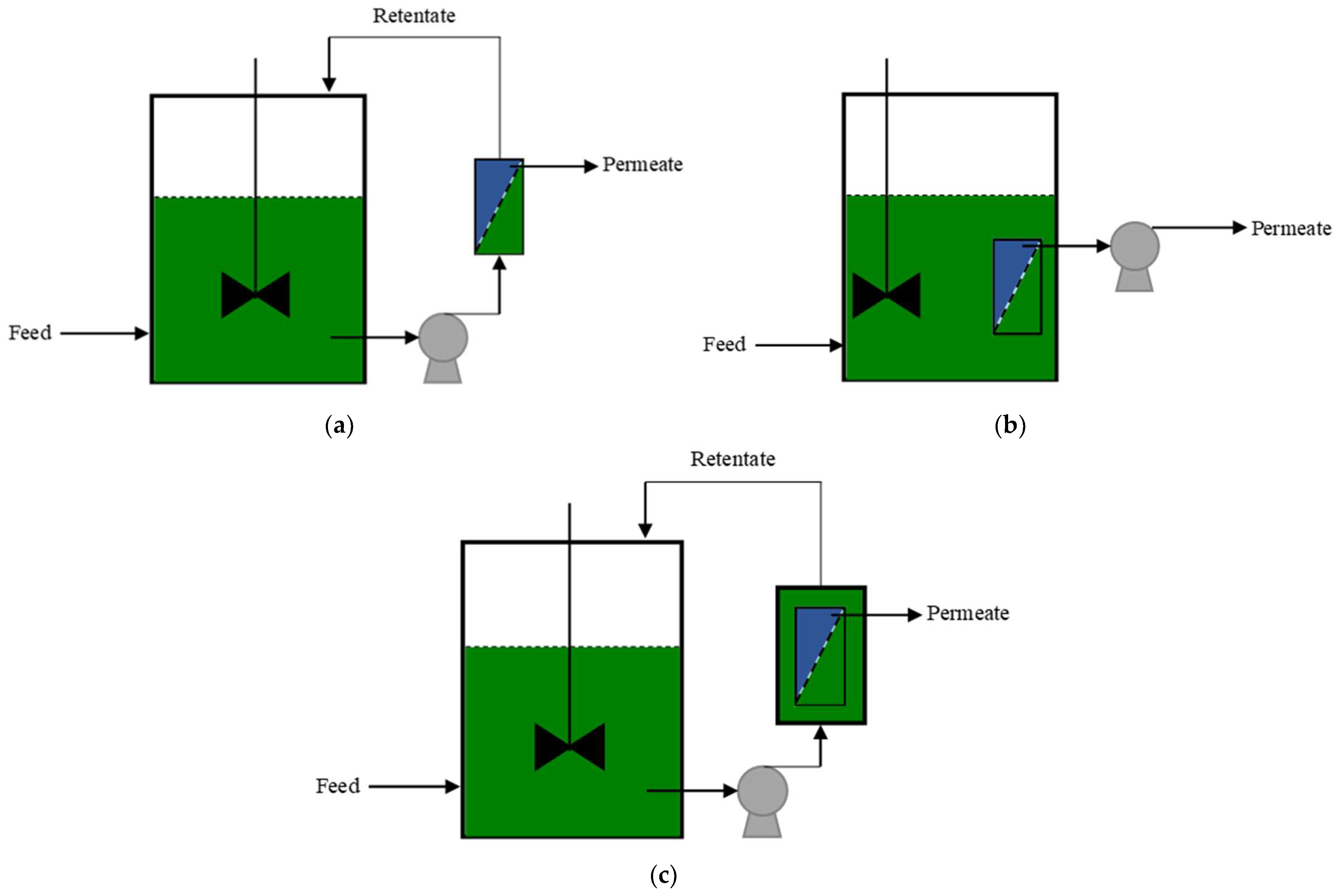
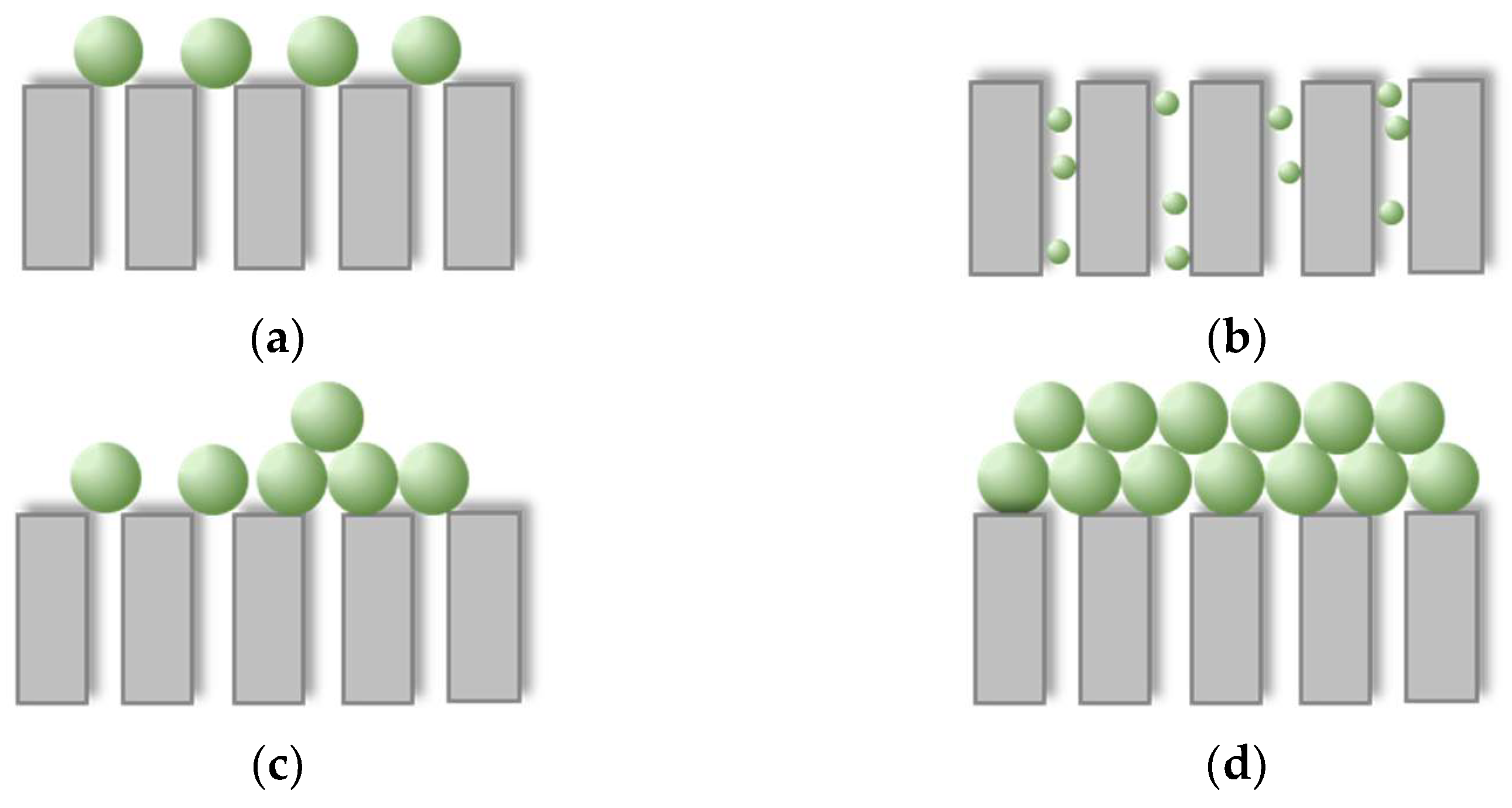
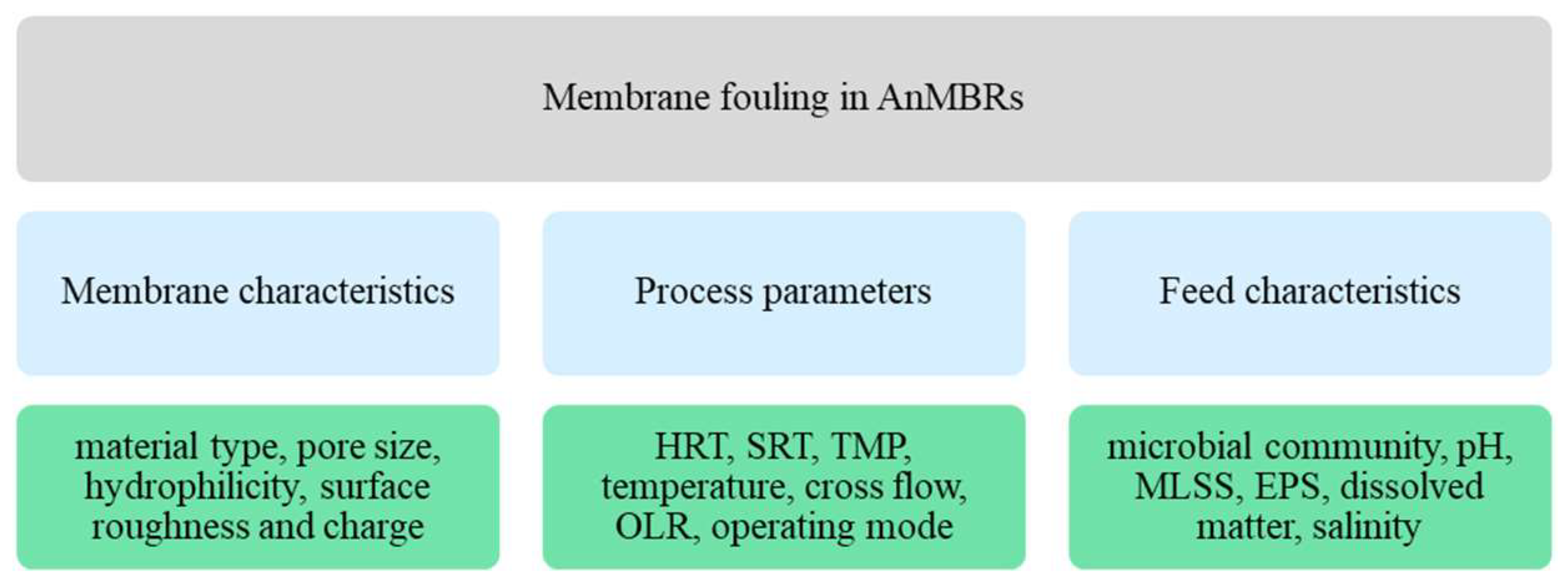
2.4. Fouling
3. Treatment of Municipal Wastewaters
3.1. Characteristics of Municipal Wastewaters
| City, Country | tCOD [mg/L] | sCOD [mg/L] | tBOD [mg/L] | TSS [mg/L] | VFA [mg/L] | VSS [mg/L] | TN [mg/L] | Ammonium Nitrogen [mg/L] | TP [mg/L] | pH | Alkalinity [mg CaCO3/L] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elmhurst, U.S. | NI | 84 ± 21 | NI | 120 ± 60 | NI | 65 ± 35 | NI | 27.3 ± 13.5 | NI | 7.5 ± 0.1 | NI | [40] |
| Valencia, Spain | 445 ± 95 | 73 ± 25 | NI | 186 ± 61 | 11 ± 7 | 150 ± 54 | NI | 27.0 ± 8.1 | NI | NI | 292.5 ± 37.2 | [44] |
| Valencia, Spain | 650 ± 147 | NI | NI | 313 ± 45 | NI | 257 ± 46 | NI | 35 ± 3 | NI | NI | NI | [47] |
| Jumilla, Spain | 1729 ± 914 | 372 ± 149 | NI | 964 ± 707 | 100 ± 80 | 675 ± 651 | 175 ± 106 | 56 ± 12 | NI | 8.2 ± 0.3 | 726 ± 141 | [67] |
| Falconara Marittima, Italy | 373 ± 148 | NI | NI | 232 ± 110 | NI | NI | 38.0 ± 7.0 | 31 ± 6 | 5.1 ± 1.5 | 7.7 ± 0.2 | NI | [75] |
| Villavaquerín, Spain | 892 ± 271 | 501 ± 165 | 573 ± 233 | 123 ± 35 | NI | 110 ± 30 | 92 ± 12 | 71 ± 14 | NI | NI | 450–550 | [120] |
| Villavaquerín, Spain | 978 ± 210 | 610 ± 146 | 474 ± 203 | 83.00 ± 8.63 | NI | 51.2 ± 8.13 | 92 ± 10 | 75 ± 16 | NI | 7.2 ± 0.6 | 494.7 ± 40.4 | [123] |
| Tagajo, Japan | 422 ± 74 | NI | 182 ± 67 | 197 ± 74 | NI | NI | 37.3 ± 1.3 | NI | 2.1 ± 0.3 | 7.08 ± 0.08 | NI | [149] |
| Tagajo, Japan | 411.9 ± 87.8 | NI | 159.8 ± 54.1 | 182.6 ± 71.3 | NI | NI | 37.3 ± 1.3 | NI | 2.1 ± 0.3 | 7.14 ± 0.07 | NI | [150] |
| Tagajo, Japan | 408.0 ± 88.3 | NI | 172.1 ± 60.4 | 194.2 ± 98.6 | NI | NI | 24.3 ± 5.3 | NI | 8.5 ± 3.4 | 7.3 ± 0.3 | NI | [151] |
| NI | 364.4 ± 94.8 | 129.3 ± 30.9 | 165.7 ± 46.5 | 228.4 ± 46.9 | NI | 216.3 ± 45.6 | 44.5 ± 3.3 | 26.2 ± 2.5 | 6.4 ± 1.1 | 7.41 ± 0.25 | 201.8 ± 18.8 | [152] |
| Valencia, Spain | 510 ± 87 | 104 ± 13 | 305 ± 37 | 342 ± 75 | 3.9 ± 2.5 | 79.5 ± 2.5 | 52.8 ± 5.4 | 42.8 ± 3.4 | 10.2 ± 2.5 | NI | 453.2 ± 34.6 | [153] |
| Ksour-Essef, Tunisia | 685.0 ± 46.4 | NI | 356.0 ± 18.5 | 380.0 ± 9.3 | NI | NI | NI | - | 11.5 ± 0.6 | 7.2 ± 0.2 | NI | [154] |
| Mexico City, Mexico | 425 ± 138 | 212 ± 20 | NI | 70 ± 9 | NI | 54 ± 7 | NI | NI | NI | NI | NI | [155] |
| NI | 362.6 ± 46.0 | 150.1 ± 24.8 | - | 177.2 ± 34.9 | NI | 166.1 ± 32.8 | - | 26.5 ± 4.8 | - | 6.91 ± 0.17 | 177.1 ± 39.1 | [156] |
3.2. Performance of COD Removal
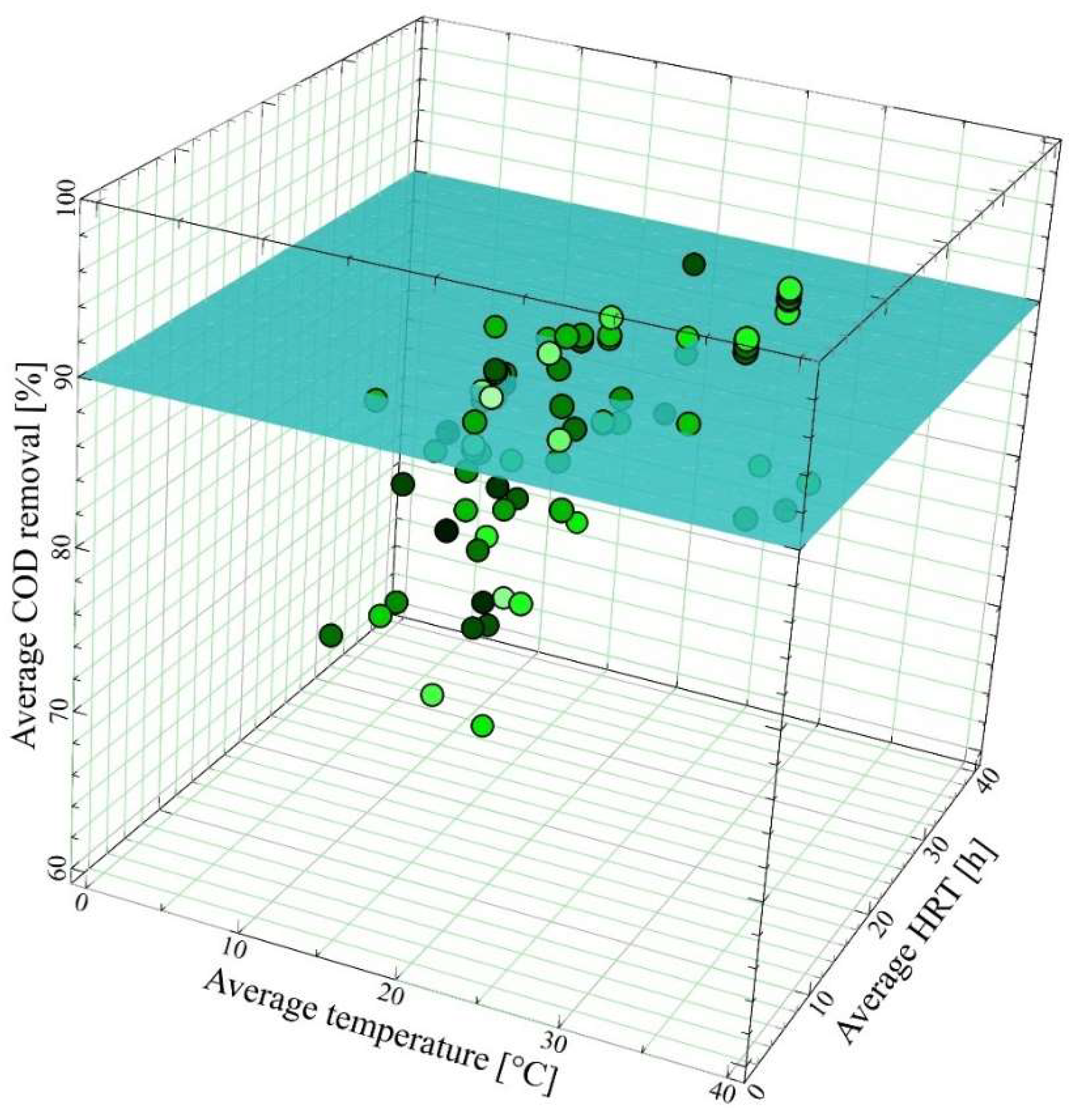
3.2.1. Impact of Temperature
3.2.2. Impact of Hydraulic Retention Time
3.2.3. Impact of Membrane Type
| Wastewater | AnMBR | Membrane | Process Conditions | Average Removal Performance | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Origin | Scale | Configuration | Type | Module | Material | COD [%] | BOD [%] | ||
| municipal | real | industrial | external submerged | UF | hollow fiber | NI | T = 33–17 °C; HRT = 6–26 h | ~85 | NI | [42] |
| municipal | real | industrial | external submerged | UF | hollow-fiber | NI | T = 25–30 °C; HRT = 60 h; 24 h; 40 h; SRT = 190 d; 120 d; 70 d | 92 | NI | [173] |
| municipal | real | semi-industrial | external | UF | hollow fiber | NI | T = 17–33 °C; SRT = 30–70 d | NI | NI | [47] |
| municipal | real | pilot | submerged | UF | hollow fiber | NI | T = 26–20 °C; HRT = 6–8 h; T = 10 °C; T = 25 °C | 85 ± 6.5%; 88 ± 9.7; 94 ± 3% | NI | [67] |
| municipal | real | pilot | submerged | NI 1 | flat sheet | PVDF | T = 23 ± 1 °C; 18 ± 4 °C; HRT = 2 d; 1 d, 0.5 d | from 69 ± 5 to 89 ± 2 | NI | [70] |
| municipal | real | pilot | submerged | UF | hollow fiber | PVDF | T = 18 ± 2 °C; HRT = 9.8–20.3 h | from 74.9 ± 9.9 to 90.0 ± 0.9 | NI | [123] |
| municipal | real | pilot | submerged | MF | hollow fiber | PVDF | T = 15 °C; HRT = 6–24 h; SRT = 20.7–515.7 d | 77.4; 90.5 | 81.9; 90.5 | [150] |
| municipal | real | pilot | submerged | MF | hollow fiber | PVDF | T = 15 °C; 20 °C; 25 °C; HRT = 8 h | 84.5 ± 8.0 90.7 ± 2.6 90.0 ± 2.6 | 92.5 ± 3.0 90.9 ± 3.7 92.4 ± 5.1 | [152] |
| municipal | real | large pilot | submerged | MF | hollow fiber | PVDF | T = 25 °C; HRT = 24 h; 12 h; 8 h; 6 h | 89.5 ± 2.4; 90.0 ± 1; 93.2 ± 1.5; 91.1 ± 0.8 | 94.3 ± 1.9; 94.0 ± 0.9; 95.3 ± 1.4; 95.9 ± 0.6 | [156] |
| municipal | real | pilot | submerged | MF | tubular | PET | T = 15–20 °C; HRT = 2.6 h | 61.6 | NI | [160] |
| municipal | real | pilot | external submerged | UF | hollow fiber | NI | T = 33 °C; HRT = 20–6 h | 87 | NI | [44] |
| municipal | real | pilot | external submerged | UF | hollow fiber | PVDF | T = 18 ± 2 °C; HRT = 7–17.1 h | from 72.9 ± 7.8 to 89.8 ± 1.2 | NI | [120] |
| municipal | real | pilot | external submerged | UF | hollow fiber | PVDF | T = 23 ± 1 °C; HRT = 8.5 h; SRT = 100 d; 70 d; 40 d | 88.0 ± 3.9; 90.6 ± 3.0; 91.8 ± 1.9 | 94.0 ± 2.1; 94.6 ± 2.2; 93.8 ± 1.5 | [172] |
| municipal | real | pilot | external submerged | UF | hollow fiber | PVDF | T = 23 ± 1 °C; HRT = 8.5 h; SRT = 70 d | 79.9 ± 7.7; 93.7 ± 2.0 | 84.2 ± 5.0; 95.0 ± 1.5 | [177] |
| municipal | real | pilot | external submerged | MF | hollow fiber | NI | T = 35 °C; HRT = 2.2 h | 87 | NI | [178] |
| municipal | real | pilot | external submerged | UF | flat sheet | PES | T = 35 °C, 28 °C; 20 °C | ~90 | NI | [180] |
| municipal | real | pilot | external | UF | hollow fiber | NI | T = 30 °C; HRT = 5 h | 83 ± 1 | NI | [75] |
| municipal | real | pilot | external | UF | hollow fiber | NI | T = 27 °C; HRT = 24.4 ± 0.4 h | 88.0 ± 4.3 | 93 | [153] |
| municipal | real | pilot | external | UF | tubular | PVDF | HRT = 6 h | 92 | NI | [155] |
| municipal | real | pilot | external | UF | hollow fiber | PVDF | T = 12.5 ± 1.8 °C; 13.1 ± 1.8 °C, HRT = 8 h | 76 ± 4; 89 ± 4 | 89 ± 5; 91 ± 2 | [161] |
| municipal | real | mini-pilot | submerged | MF | hollow fiber | PVDF | T = 15 °C; 20 °C; 25 °C; HRT = 6 h | 77.4 ± 4.4; 90.0 ± 1.8; 90.1 ± 1.6 | 81.9 ± 8.5; 92.2 ± 2.4; 91.4 ± 1.7 | [149] |
| municipal | real | laboratory | submerged | MF | hollow fiber | NI | T = 25 °C; HRT = 12–24 h | from 88.1 ± 1.7 to 89.0 ± 2.7 | from 91.4 ± 4.3 to 93.9 ± 1.3 | [151] |
| municipal | real | laboratory | submerged | UF | hollow fiber | NI | T = 25 °C; HRT = 12–24 h | from 88.9 ± 2.1 to 89.5 ± 3.0 | from 92.4 ± 2.3 to 94.0 ± 1.3 | [151] |
| municipal | real | laboratory | submerged | UF | flat sheet | PVDF | T = 30 ± 3 °C; HRT = 10 h | 90 | NI | [179] |
| municipal | real | laboratory | external submerged | UF | hollow fiber | PVDF | T = 23 ± 1 °C; HRT = 12.5 h; SRT = 40 d; 70 d | 92.3 ± 1.5 | 94.6 ± 2.2 | [172] |
| municipal | real | laboratory | external | MF | tubular | PVDF | T = 25 °C; HRT = 0.5–2 d; SRT = 19–233 d | from 55 ± 18 to 68 ± 8 2 | NI | [40] |
| municipal | real | laboratory | external | MF | NI | NI | HRT = 0.5–2 d, SRT = 40–217 d | from 55 to 72 2 | NI | [41] |
| municipal | real | NI | external | UF | NI | NI | T = 37 °C; HRT = 15 h | 88 | 90 | [154] |
| municipal | syntethic | pilot | submerged | MF | hollow fiber | PP | T = 22 ± 2 °C; SRT = 10–40 d | from 98.13 to 99.35 2 | NI | [181] |
| municipal | syntethic | semi-pilot | submerged | MF | flat sheet | PVDF | T = 37 ± 1 °C; HRT = 47 d | 98.84; 98.75 | NI | [182] |
| municipal | syntethic | laboratory | external submerged | MF | flat sheet | PVDF | T = 35 °C; HRT = 7 h | NI | NI | [158] |
| municipal | syntethic | laboratory | external submerged | MF | flat tubular | ceramic | T = 35 °C; HRT = 7 h | NI | NI | [158] |
| municipal | syntethic | laboratory | external | MF | tubular | PVDF | T = 35 ± 1 °C; HRT = 0.8 d | 85 ± 8.9 | NI | [115] |
| municipal | syntethic | laboratory | external | UF | tubular | PES | T = 15 °C; 25 °C; HRT = 6 h | 90; 92% | NI | [157] |
| municipal | syntethic | laboratory | external | MF | tubular | ceramic | T = 35 °C; HRT = 0.4–13.8 h | NI | NI | [159] |
| municipal | syntethic | laboratory | external | MF | tubular | PTFE | T = 25 ± 1 °C; 15 ± 1 °C; HRT = 12 h | >95; >85 | NI | [162] |
| municipal | syntethic | laboratory | external | MF | tubular | PTFE | T = 25 ± 1 °C; HRT = 6 h; 8 h; 12 h | 93.8 ± 1.9; 95.9 ± 1.0; 95.0 ± 0.8 | NI | [171] |
| municipal | syntethic | laboratory | external | UF | hollow fiber | PVDF | T = 35 ± 1 °C; HRT = 6 h; 12 h | ~98 | NI | [174] |
| municipal | syntethic | laboratory | external | MF | flat sheet | PVDF | T = 35 °C; HRT = 26 h | from 90 to 96 | NI | [175] |
| municipal | syntethic | laboratory | external | MF | flat sheet | PES | T = 35 °C; HRT = 26 h | from 90 to 96 | NI | [175] |
| municipal | syntethic | laboratory | external | UF | tubular | PVDF | HRT = 4 h; 8 h; 12 h | ~80 | NI | [176] |
| municipal | syntethic | laboratory | external | UF | flat sheet | PVDF | HRT = 2.4 ± 0.6–3.6 ± 1.1 d | from 90.9 ± 6.0 to 95.9 ± 0.7 | NI | [183] |
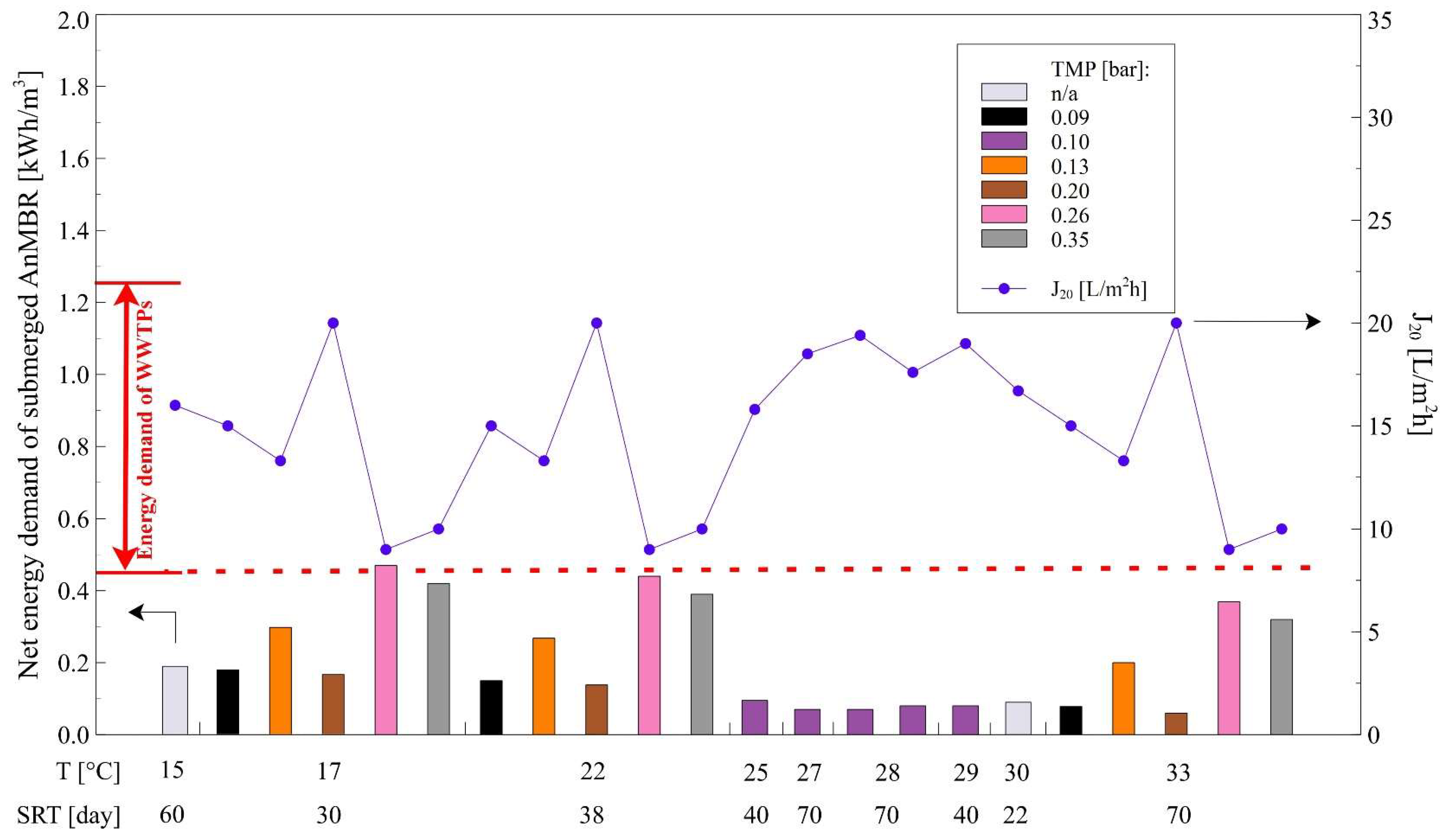
3.3. Energy Demand
4. Treatment of Domestic Wastewaters
4.1. Characteristics of Domestic Wastewater
4.2. Performance of COD Removal
4.2.1. Impact of Temperature
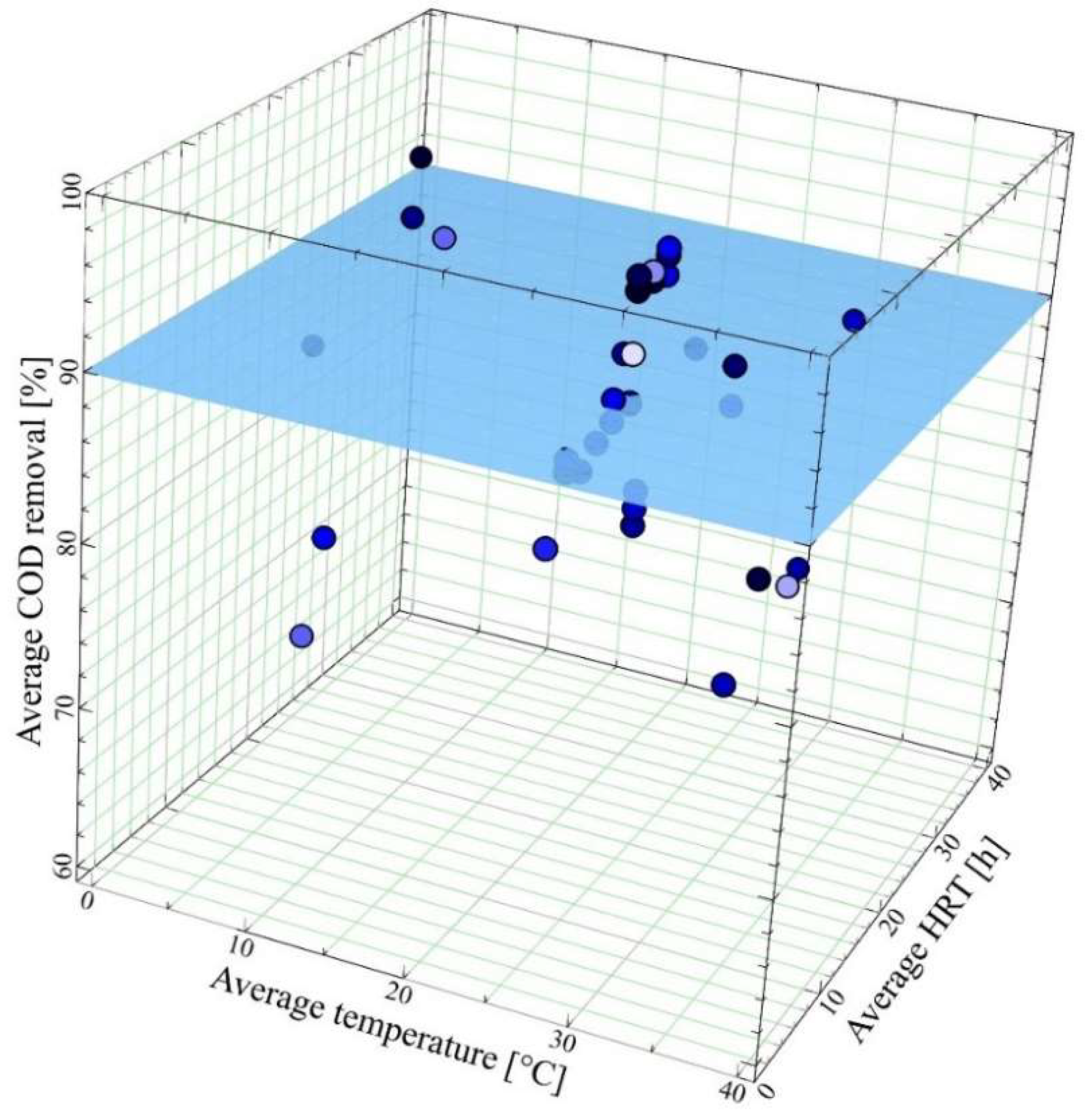
| Wastewater | AnMBR | Membrane | Process Conditions | Average Removal Performance | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Origin | Scale | Configuration | Type | Module | Material | COD [%] | BOD [%] | ||
| domestic | real | pilot | submerged | UF | hollow fiber | PVDF | without external temperature control, HRT = 16 h | 84 | 93 | [48] |
| domestic | real | pilot | external submerged | UF | hollow fiber | PVDF | without external temperature control, HRT = 16 h | 86 | 95 | [210] |
| domestic | real | pilot | external | MF | hollow fiber | NI | T = 25 °C; HRT = 6 h | 88 | NI | [111] |
| domestic | real | pilot | external | UF | NI | NI | T = 37 °C | >76 | >84 | [194] |
| domestic | real | mini-pilot | submerged | MF | hollow fiber | PVDF | T = 25 °C; HRT = 4 h; 6–12 h; SRT = 53 d, 65 d, 76 d, ∞ | 84.4 ± 3.7; 88.7–89.3 | 87.3; 92–94 | [195] |
| domestic | real | laboratory | submerged | MF; UF | NI | ceramic | T = 25–30 °C; HRT = 7.5 h; SRT = 60 d | 88.6 ± 9.0; 87.9 ± 7.4; 86.3 ± 9.7 | NI | [198] |
| domestic | real | laboratory | submerged | UF | hollow fiber | PE | T = 12–27 °C; 18.5–22.5 °C; HRT = 6; 4 h | 60–95 | NI | [203] |
| domestic | real | laboratory | submerged | MF | flat sheet | PES | T = 15 °C, HRT = 16–24 h | 69 ± 10 | NI | [206] |
| domestic | real | laboratory | submerged | MF | hollow fiber | PVDF | T = 35 ± 1 °C; HRT = 15; 10; 6 h | 80.23; 82.69; 78.19 | NI | [207] |
| domestic | real | laboratory | submerged | MF | NI | NI | T = 25 ± 0.2 °C, HRT = 24–4 h | from 88.9 to 84.4 | NI | [208] |
| domestic | real | laboratory | submerged | UF | NI | NI | T = 25 ± 0.2 °C, HRT = 24–10 h | from 88.9 to 88.2 | NI | [208] |
| domestic | real | laboratory | external submerged | MF | flat sheet | ceramic | T = 30 ± 3 °C HRT = 17 h; SRT = 30 d | 88.97 ± 4.13; 91.33 ± 4.24 | 77.38 ± 8.19; 80.27 ± 7.79 | [134] |
| domestic | real | laboratory | external submerged | MF | flat sheet | PVDF | T = 25 °C | NI | NI | [140] |
| domestic | real | laboratory | external submerged | MF | frame | PES | T = 25–30 °C; HRT = 10 h; SRT = 30 d; 60 d; 90 d | 84 ± 6; 85 ± 3; 86 ± 3 | NI | [193] |
| domestic | real | laboratory | submerged | UF | flat sheet | PES, PAN, PVDF | T = 25 ± 0.3 °C | 89.7 ± 3 | NI | [49] |
| domestic | real and synthetic | pilot | external | UF | NI | NI | T = 37 °C | NI | NI | [197] |
| domestic | synthetic | pilot | external | MF | tubular | ceramic | T = 35 ± 1 °C; HRT = 4 d | 84 | NI | [200] |
| domestic | synthetic | laboratory | submerged | MF | flat sheet | alumina-based ceramic | HRT = 44 ± 3.1 h; 18 ± 1.3 h | 90.5 ± 6.8; 96.1 ± 5.1 | NI | [201] |
| domestic | synthetic | laboratory | submerged | MF | flat sheet | pyrophyllite-based ceramic | HRT = 43 ± 1.6 h; 18 ± 1.6 h | 92.9 ± 5.5; 42.6 ± 19.2 | NI | [201] |
| domestic | synthetic | laboratory | submerged | MF | flat sheet | alumina-based ceramic | T = 33 ± 2 °C; HRT = 28 h | 91.0 ± 13.8 | NI | [202] |
| domestic | synthetic | laboratory | submerged | UF | flat sheet | PVDF | T = 33 ± 2 °C; HRT = 22.5 h | 77.8 ± 20.5 | NI | [202] |
| domestic | synthetic | laboratory | submerged | MF | hollow fiber | PE | T = 25; 20; 15; 11 °C; HRT = 3.5; 4.6 and 5.7 h | from 76 to 96 | NI | [204] |
| domestic | synthetic | laboratory | submerged | MF | flat sheet | PES | T = 15–3 °C; HRT = 17 ± 0.79–29 ± 2.2 h | from 95 ± 1.6 to 86 ± 4.0 | NI | [205] |
| domestic | synthetic | laboratory | submerged | MF | flat sheet | PES | T = 15 °C; HRT = 16–24 h | 92 ± 5.0 | 92 | [206] |
| domestic | synthetic | laboratory | submerged | MF | plate and frame | PES | T = 25–30 °C; HRT = 12 h; 10 h; 8 h; SRT = 30; 60 d; ∞ | >97 | NI | [209] |
| domestic | synthetic | laboratory | submerged | MF | flat sheet | polyolefin | HRT = 12 h | ~83% | NI | [211] |
| domestic | synthetic | laboratory | submerged | UF | flat sheet | PES | T = 25 °C; HRT = 13 h | 92.3 ± 4.1 | NI | [199] |
| domestic | synthetic | laboratory | submerged | MF | NI | NI | T = 15 °C | NI | NI | [212] |
| domestic | synthetic | laboratory | submerged | MF | hollow fiber | NI | NI | 97.1 ± 0.3 | NI | [213] |
4.2.2. Impact of Hydraulic Retention Time
4.2.3. Impact of Membrane Type
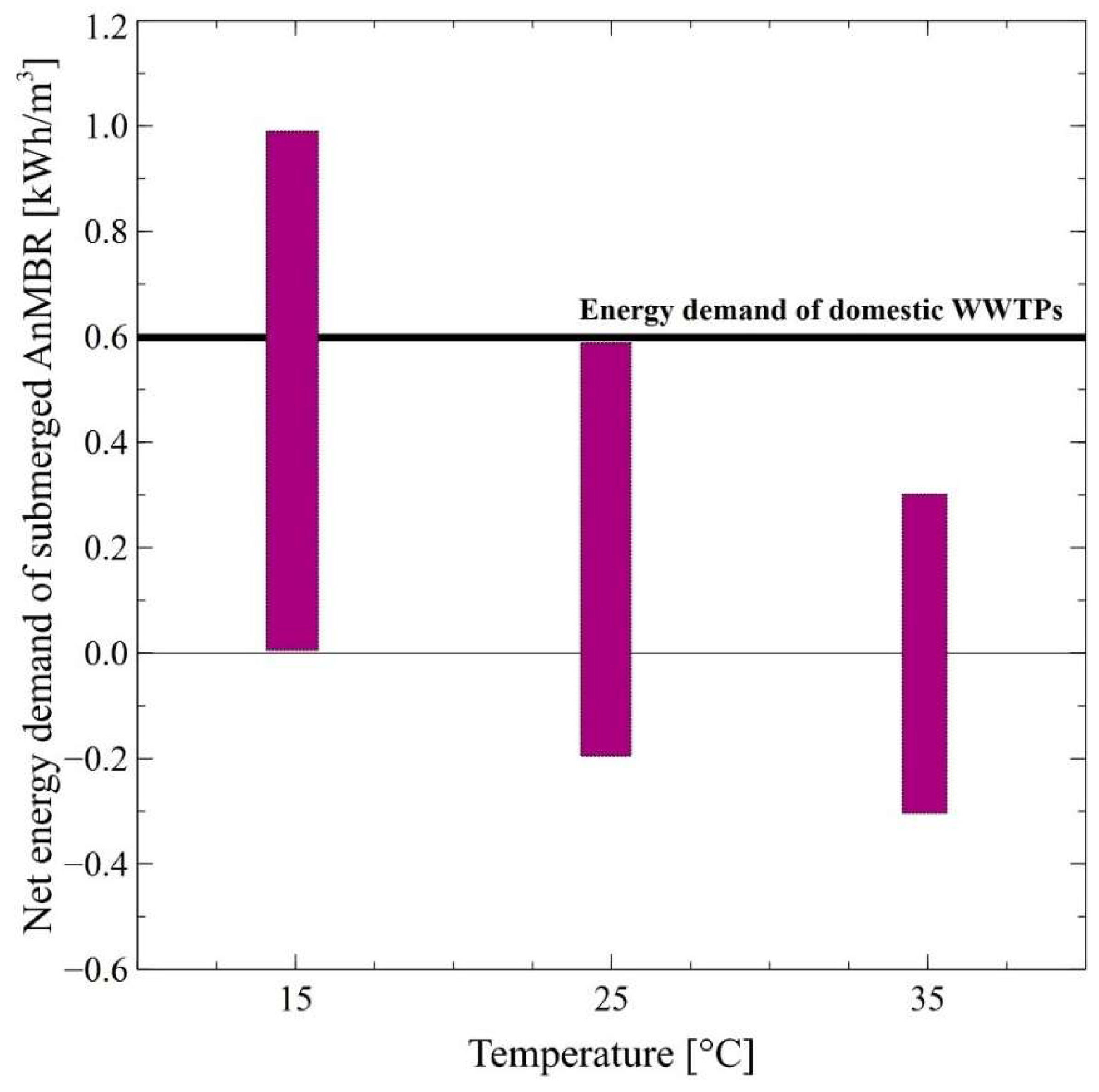
4.3. Energy Demand
5. Conclusions
6. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AeMBR | aerobic membrane bioreactors |
| AnMBR | anaerobic membrane bioreactor |
| BOD | biological oxygen demand |
| CAS | classical activated sludge |
| COD | chemical oxygen demand |
| EPS | extracellular polymeric substance |
| HRT | hydraulic retention time |
| J20 | 20 °C-standardized critical flux |
| MF | microfiltration |
| MBR | membrane bioreactor |
| MLSS | mixed liquor suspended solid |
| N | nitrogen |
| NF | nanofiltration |
| NTU | nephelometric turbidity unit |
| OLR | organic loading rate |
| P | phosphorus |
| PAN | polyacrylonitrile |
| PE | polyethylene |
| PES | polyethersulfone |
| PET | polyethylene terephthalate |
| PTFE | poly-tetrafluoroethylene |
| PVDF | polyvinylidene fluoride |
| RO | reverse osmosis |
| SRT | solid retention time |
| sCOD | soluble chemical oxygen demand |
| SS | suspended solids |
| T | temperature |
| tBOD | total biochemical oxygen demand |
| tCOD | total chemical oxygen demand |
| TMP | transmembrane pressure |
| TN | total nitrogen |
| TP | total phosphorus |
| TSS | total suspended solids |
| UASB | upflow anaerobic sludge blanket |
| UF | ultrafiltration |
| VFA | volatile fatty acids |
| VSS | volatile suspended solids |
| WWTP | wastewater treatment plant |
References
- Sonune, A.; Ghate, R. Developments in Wastewater Treatment Methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.unwater.org/water-facts/quality-and-wastewater/ (accessed on 26 May 2022).
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. Npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 97, pp. 63–119. ISBN 978-0-12-804816-0. [Google Scholar]
- Jasim, N.A. The Design for Wastewater Treatment Plant (WWTP) with GPS X Modelling. Cogent Eng. 2020, 7, 1723782. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Gu, J.; Liu, Y. Energy Self-Sufficient Biological Municipal Wastewater Reclamation: Present Status, Challenges and Solutions Forward. Bioresour. Technol. 2018, 269, 513–519. [Google Scholar] [CrossRef]
- Bohra, V.; Ahamad, K.U.; Kela, A.; Vaghela, G.; Sharma, A.; Deka, B.J. Energy and Resources Recovery from Wastewater Treatment Systems. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 17–36. ISBN 978-0-323-90178-9. [Google Scholar]
- Lehtoranta, S.; Malila, R.; Särkilahti, M.; Viskari, E.-L. To Separate or Not? A Comparison of Wastewater Management Systems for the New City District of Hiedanranta, Finland. Environ. Res. 2022, 208, 112764. [Google Scholar] [CrossRef]
- Renuka, N.; Ratha, S.K.; Kader, F.; Rawat, I.; Bux, F. Insights into the Potential Impact of Algae-Mediated Wastewater Beneficiation for the Circular Bioeconomy: A Global Perspective. J. Environ. Manag. 2021, 297, 113257. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, S.; Hans, N.; Goswami, L.; Devi, G.; Deshavath, N.N.; Yadav, M.K.; Lall, A.M. An Insight into Biological and Chemical Technologies for Micropollutant Removal from Wastewater. In Fate and Transport of Subsurface Pollutants; Gupta, P.K., Bharagava, R.N., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2021; Volume 24, pp. 199–226. ISBN 9789811565632. [Google Scholar]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M.; Yang, S.J.; Wang, Q.; Wang, X.C.; Ao, D. Current Status of Urban Wastewater Treatment Plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef]
- Zaibel, I.; Arnon, S.; Zilberg, D. Treated Municipal Wastewater as a Water Source for Sustainable Aquaculture: A Review. Rev. Aquacult 2022, 14, 362–377. [Google Scholar] [CrossRef]
- Musabandesu, E.; Loge, F. Load Shifting at Wastewater Treatment Plants: A Case Study for Participating as an Energy Demand Resource. J. Clean. Prod. 2021, 282, 124454. [Google Scholar] [CrossRef]
- Herrera-Navarrete, R.; Arellano-Wences, H.J.; Colín-Cruz, A.; Sampedro-Rosas, M.L.; Rosas-Acevedo, J.L.; Rodríguez-Herrera, A.L. Thematic and Geographical Trend in Scientific Research Applied in Municipal Wastewater Treatment Plants: An Overview. Water Air Soil Pollut 2021, 232, 318. [Google Scholar] [CrossRef]
- Longo, S.; d’Antoni, B.M.; Bongards, M.; Chaparro, A.; Cronrath, A.; Fatone, F.; Lema, J.M.; Mauricio-Iglesias, M.; Soares, A.; Hospido, A. Monitoring and Diagnosis of Energy Consumption in Wastewater Treatment Plants. A State of the Art and Proposals for Improvement. Appl. Energy 2016, 179, 1251–1268. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.L.; Valenzuela-Heredia, D.; Pedrouso, A.; Val del Río, A.; Belmonte, M.; Mosquera-Corral, A. Greenhouse Gases Emissions from Wastewater Treatment Plants: Minimization, Treatment, and Prevention. J. Chem. 2016, 2016, 3796352. [Google Scholar] [CrossRef] [Green Version]
- Parravicini, V.; Svardal, K.; Krampe, J. Greenhouse Gas Emissions from Wastewater Treatment Plants. Energy Procedia 2016, 97, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Shan, X.; Xiao, X.; Cai, R.; Zhang, Y.; Jiao, N. Excessive Greenhouse Gas Emissions from Wastewater Treatment Plants by Using the Chemical Oxygen Demand Standard. Sci. China Earth Sci. 2022, 65, 87–95. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Circular Economy Is Game-Changing Municipal Wastewater Treatment Technology towards Energy and Carbon Neutrality. Chem. Eng. J. 2022, 429, 132114. [Google Scholar] [CrossRef]
- Goliopoulos, N.; Mamais, D.; Noutsopoulos, C.; Dimopoulou, A.; Kounadis, C. Energy Consumption and Carbon Footprint of Greek Wastewater Treatment Plants. Water 2022, 14, 320. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater Treatment in Microbial Fuel Cells—An Overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, S.; Sun, D. Assessment of Greenhouse Gas Emission from A/O and SBR Wastewater Treatment Plants in Beijing, China. Int. Biodeterior. Biodegrad. 2016, 108, 108–114. [Google Scholar] [CrossRef]
- Fighir (Arsene), D.; Teodosiu, C.; Fiore, S. Environmental and Energy Assessment of Municipal Wastewater Treatment Plants in Italy and Romania: A Comparative Study. Water 2019, 11, 1611. [Google Scholar] [CrossRef] [Green Version]
- Tumendelger, A.; Alshboul, Z.; Lorke, A. Methane and Nitrous Oxide Emission from Different Treatment Units of Municipal Wastewater Treatment Plants in Southwest Germany. PLoS ONE 2019, 14, e0209763. [Google Scholar] [CrossRef] [PubMed]
- Crone, B.C.; Garland, J.L.; Sorial, G.A.; Vane, L.M. Significance of Dissolved Methane in Effluents of Anaerobically Treated Low Strength Wastewater and Potential for Recovery as an Energy Product: A Review. Water Res. 2016, 104, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Wang, X.; Wu, J.; Li, F. Energy Self-Sufficient Wastewater Treatment Plants: Feasibilities and Challenges. Energy Procedia 2017, 105, 3741–3751. [Google Scholar] [CrossRef]
- Neczaj, E.; Grosser, A. Circular Economy in Wastewater Treatment Plant–Challenges and Barriers. In Proceedings of the EWaS3, Lefkada Island, Greece, 31 July 2018; MDPI: Basel, Switzerland, 2018; p. 614. [Google Scholar]
- Gandiglio, M.; Lanzini, A.; Soto, A.; Leone, P.; Santarelli, M. Enhancing the Energy Efficiency of Wastewater Treatment Plants through Co-Digestion and Fuel Cell Systems. Front. Environ. Sci. 2017, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Vaccari, M.; Foladori, P.; Nembrini, S.; Vitali, F. Benchmarking of Energy Consumption in Municipal Wastewater Treatment Plants—A Survey of over 200 Plants in Italy. Water Sci. Technol. 2018, 77, 2242–2252. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iea.org/reports/water-energy-nexus/ (accessed on 26 May 2022).
- Gude, V.G. Energy and Water Autarky of Wastewater Treatment and Power Generation Systems. Renew. Sustain. Energy Rev. 2015, 45, 52–68. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.G.A. Membrane Bioreactors for Wastewater Reclamation: Cost Analysis. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 311–322. ISBN 978-0-12-819854-4. [Google Scholar]
- Pabi, S.; Amarnath, A.; Goldstein, R.; Reekie, L. Electricity Use and Management in the Municipal Water Supply and Wastewater Industries; Electric Power Research Institute: Washington, DC, USA, 2013. [Google Scholar]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the Implementation of Circular Economy in the Wastewater Sector: Challenges and Opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Y.; Chen, J.; Huang, M.; Wang, P.; Wang, G.; Zou, W.; Zhou, G. Assessment of Energy Consumption of Municipal Wastewater Treatment Plants in China. J. Clean. Prod. 2019, 228, 399–404. [Google Scholar] [CrossRef]
- Trapote, A.; Albaladejo, A.; Simón, P. Energy Consumption in an Urban Wastewater Treatment Plant: The Case of Murcia Region (Spain). Civ. Eng. Environ. Syst. 2014, 31, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.bccresearch.com/market-research/membrane-and-separation-technology/membrane-bioreactors.html/ (accessed on 26 May 2022).
- Akkoyunlu, B.; Daly, S.; Casey, E. Membrane Bioreactors for the Production of Value-Added Products: Recent Developments, Challenges and Perspectives. Bioresour. Technol. 2021, 341, 125793. [Google Scholar] [CrossRef]
- Baek, S.H.; Pagilla, K.R. Aerobic and Anaerobic Membrane Bioreactors for Municipal Wastewater Treatment. Water Environ. Res. 2006, 78, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Pagilla, K.R.; Kim, H.-J. Lab-Scale Study of an Anaerobic Membrane Bioreactor (AnMBR) for Dilute Municipal Wastewater Treatment. Biotechnol. Bioproc. E 2010, 15, 704–708. [Google Scholar] [CrossRef]
- Robles, A.; Ruano, M.V.; García-Usach, F.; Ferrer, J. Sub-Critical Filtration Conditions of Commercial Hollow-Fibre Membranes in a Submerged Anaerobic MBR (HF-SAnMBR) System: The Effect of Gas Sparging Intensity. Bioresour. Technol. 2012, 114, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Judd, S. The Status of Membrane Bioreactor Technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.B.; Robles, A.; Carretero, L.; Durán, F.; Ruano, M.V.; Gatti, M.N.; Ribes, J.; Ferrer, J.; Seco, A. Experimental Study of the Anaerobic Urban Wastewater Treatment in a Submerged Hollow-Fibre Membrane Bioreactor at Pilot Scale. Bioresour. Technol. 2011, 102, 8799–8806. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Anaerobic Membrane Bioreactors (AnMBRs) for Municipal Wastewater Treatment- Potential Benefits, Constraints, and Future Perspectives: An Updated Review. Sci. Total Environ. 2022, 802, 149612. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.-Y.; Shu, L. Treatment of Textile Wastewater with Membrane Bioreactor: A Critical Review. Bioresour. Technol. 2016, 204, 202–212. [Google Scholar] [CrossRef]
- Pretel, R.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. The Operating Cost of an Anaerobic Membrane Bioreactor (AnMBR) Treating Sulphate-Rich Urban Wastewater. Sep. Purif. Technol. 2014, 126, 30–38. [Google Scholar] [CrossRef]
- Martin-Garcia, I.; Monsalvo, V.; Pidou, M.; Le-Clech, P.; Judd, S.J.; McAdam, E.J.; Jefferson, B. Impact of Membrane Configuration on Fouling in Anaerobic Membrane Bioreactors. J. Membr. Sci. 2011, 382, 41–49. [Google Scholar] [CrossRef]
- Grossman, A.D.; Yang, Y.; Yogev, U.; Camarena, D.C.; Oron, G.; Bernstein, R. Effect of Ultrafiltration Membrane Material on Fouling Dynamics in a Submerged Anaerobic Membrane Bioreactor Treating Domestic Wastewater. Environ. Sci. Water Res. Technol. 2019, 5, 1145–1156. [Google Scholar] [CrossRef]
- Gharibian, S.; Hazrati, H. Towards Practical Integration of MBR with Electrochemical AOP: Improved Biodegradability of Real Pharmaceutical Wastewater and Fouling Mitigation. Water Res. 2022, 218, 118478. [Google Scholar] [CrossRef]
- Xiao, Y.; Yaohari, H.; De Araujo, C.; Sze, C.C.; Stuckey, D.C. Removal of Selected Pharmaceuticals in an Anaerobic Membrane Bioreactor (AnMBR) with/without Powdered Activated Carbon (PAC). Chem. Eng. J. 2017, 321, 335–345. [Google Scholar] [CrossRef]
- Sun, J.; Kosaki, Y.; Kawamura, K.; Watanabe, N. Operational Load Enhancement for an Anaerobic Membrane Bioreactor through Ethanol Fermentation Pretreatment of Food Waste. Energy Convers. Manag. 2021, 249, 114840. [Google Scholar] [CrossRef]
- Akca, M.S.; Bostancı, O.; Aydin, A.K.; Koyuncu, I.; Altinbas, M. BioH2 Production from Food Waste by Anaerobic Membrane Bioreactor. Int. J. Hydrog. Energy 2021, 46, 27941–27955. [Google Scholar] [CrossRef]
- Yurtsever, A.; Sahinkaya, E.; Çınar, Ö. Performance and Foulant Characteristics of an Anaerobic Membrane Bioreactor Treating Real Textile Wastewater. J. Water Process Eng. 2020, 33, 101088. [Google Scholar] [CrossRef]
- Yurtsever, A.; Sahinkaya, E.; Aktaş, Ö.; Uçar, D.; Çınar, Ö.; Wang, Z. Performances of Anaerobic and Aerobic Membrane Bioreactors for the Treatment of Synthetic Textile Wastewater. Bioresour. Technol. 2015, 192, 564–573. [Google Scholar] [CrossRef]
- Deschamps, L.; Merlet, D.; Lemaire, J.; Imatoukene, N.; Filali, R.; Clément, T.; Lopez, M.; Theoleyre, M.-A. Excellent Performance of Anaerobic Membrane Bioreactor in Treatment of Distillery Wastewater at Pilot Scale. J. Water Process Eng. 2021, 41, 102061. [Google Scholar] [CrossRef]
- Chen, H.; Chang, S.; Guo, Q.; Hong, Y.; Wu, P. Brewery Wastewater Treatment Using an Anaerobic Membrane Bioreactor. Biochem. Eng. J. 2016, 105, 321–331. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane Bioreactor for Wastewater Treatment: A Review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Tetteh, E.K.; Armah, E.K. Membrane Bioreactors for Produced Water Treatment: A Mini-Review. Membranes 2022, 12, 275. [Google Scholar] [CrossRef]
- De Vela, R.J. A Review of the Factors Affecting the Performance of Anaerobic Membrane Bioreactor and Strategies to Control Membrane Fouling. Rev. Environ. Sci. Biotechnol. 2021, 20, 607–644. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Kanafina, D.; Malamis, S.; Katsou, E.; Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E. Anaerobic Membrane Bioreactors for Municipal Wastewater Treatment: A Literature Review. Membranes 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lei, Z.; Dzakpasu, M.; Li, Q.; Li, Y.-Y.; Chen, R. Removal of Trace Organic Contaminants in Municipal Wastewater by Anaerobic Membrane Bioreactor: Efficiencies, Fates and Impact Factors. J. Water Process Eng. 2021, 40, 101953. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A Review of Polymeric Membranes and Processes for Potable Water Reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.-Q.; Leung, K.-T.; Zhao, L.; Chen, J.; Hong, H. Membrane Bioreactors for Industrial Wastewater Treatment: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Kehrein, P.; van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A Critical Review of Resource Recovery from Municipal Wastewater Treatment Plants—Market Supply Potentials, Technologies and Bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, S.; Dosta, J.; Mata-Alvarez, J.; Astals, S. Unravelling the Economics behind Mainstream Anaerobic Membrane Bioreactor Application under Different Plant Layouts. Bioresour. Technol. 2021, 319, 124170. [Google Scholar] [CrossRef]
- Peña, M.; do Nascimento, T.; Gouveia, J.; Escudero, J.; Gómez, A.; Letona, A.; Arrieta, J.; Fdz-Polanco, F. Anaerobic Submerged Membrane Bioreactor (AnSMBR) Treating Municipal Wastewater at Ambient Temperature: Operation and Potential Use for Agricultural Irrigation. Bioresour. Technol. 2019, 282, 285–293. [Google Scholar] [CrossRef]
- Harb, M.; Hong, P.-Y. Anaerobic Membrane Bioreactor Effluent Reuse: A Review of Microbial Safety Concerns. Fermentation 2017, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Rout, P.R.; Bae, J. The Applicability of Anaerobically Treated Domestic Wastewater as a Nutrient Medium in Hydroponic Lettuce Cultivation: Nitrogen Toxicity and Health Risk Assessment. Sci. Total Environ. 2021, 780, 146482. [Google Scholar] [CrossRef]
- Plevri, A.; Mamais, D.; Noutsopoulos, C. Anaerobic MBR Technology for Treating Municipal Wastewater at Ambient Temperatures. Chemosphere 2021, 275, 129961. [Google Scholar] [CrossRef] [PubMed]
- Dereli, R.K.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Jeison, D.; van der Zee, F.; van Lier, J.B. Potentials of Anaerobic Membrane Bioreactors to Overcome Treatment Limitations Induced by Industrial Wastewaters. Bioresour. Technol. 2012, 122, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic Membrane Bioreactors for Wastewater Treatment: Novel Configurations, Fouling Control and Energy Considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Mavukkandy, M.O.; Bilad, M.R.; Giwa, A.; Hasan, S.W.; Arafat, H.A. Leaching of PVP from PVDF/PVP Blend Membranes: Impacts on Membrane Structure and Fouling in Membrane Bioreactors. J. Mater. Sci. 2016, 51, 4328–4341. [Google Scholar] [CrossRef]
- Dvořák, L.; Gómez, M.; Dolina, J.; Černín, A. Anaerobic Membrane Bioreactors—A Mini Review with Emphasis on Industrial Wastewater Treatment: Applications, Limitations and Perspectives. Desalination Water Treat. 2016, 57, 19062–19076. [Google Scholar] [CrossRef] [Green Version]
- Foglia, A.; Akyol, Ç.; Frison, N.; Katsou, E.; Eusebi, A.L.; Fatone, F. Long-Term Operation of a Pilot-Scale Anaerobic Membrane Bioreactor (AnMBR) Treating High Salinity Low Loaded Municipal Wastewater in Real Environment. Sep. Purif. Technol. 2020, 236, 116279. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Ali, S. Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2011; ISBN 978-1-58829-940-6. [Google Scholar]
- Shin, C.; Bae, J. Current Status of the Pilot-Scale Anaerobic Membrane Bioreactor Treatments of Domestic Wastewaters: A Critical Review. Bioresour. Technol. 2018, 247, 1038–1046. [Google Scholar] [CrossRef]
- Mai, D.T.; Kunacheva, C.; Stuckey, D.C. A Review of Posttreatment Technologies for Anaerobic Effluents for Discharge and Recycling of Wastewater. Crit. Rev. Environ. Sci. Technol. 2018, 48, 167–209. [Google Scholar] [CrossRef]
- Jawad, J.; Hawari, A.H.; Javaid Zaidi, S. Artificial Neural Network Modeling of Wastewater Treatment and Desalination Using Membrane Processes: A Review. Chem. Eng. J. 2021, 419, 129540. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A Review of Pressure-Driven Membrane Processes in Wastewater Treatment and Drinking Water Production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The Behavior of Suspensions and Macromolecular Solutions in Crossflow Microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Che Man, H.; Isma Idris, A.; Faezah Yunos, K.; Zainal Abidin, Z. Treatment of Palm Oil Mill Effluent Using Membrane Bioreactor: Novel Processes and Their Major Drawbacks. Water 2018, 10, 1165. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, Z.; Huang, X. Anaerobic Membrane Bioreactors for Sustainable and Energy-Efficient Municipal Wastewater Treatment. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–366. ISBN 978-0-12-819852-0. [Google Scholar]
- Amin, S.K.; Abdallah, H.A.M.; Roushdy, M.H.; El-Sherbiny, S.A. An Overview of Production and Development of Ceramic Membranes. Int. J. Appl. Eng. Res 2016, 11, 15. [Google Scholar]
- Radjenović, J.; Matošić, M.; Mijatović, I.; Petrović, M.; Barceló, D. Membrane Bioreactor (MBR) as an Advanced Wastewater Treatment Technology. In Emerging Contaminants from Industrial and Municipal Waste; Barceló, D., Petrovic, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5S/2, pp. 37–101. ISBN 978-3-540-79209-3. [Google Scholar]
- Le-Clech, P. Membrane Bioreactors and Their Uses in Wastewater Treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A Review on Anaerobic Membrane Bioreactors: Applications, Membrane Fouling and Future Perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Anjum, F.; Khan, I.M.; Kim, J.; Aslam, M.; Blandin, G.; Heran, M.; Lesage, G. Trends and Progress in AnMBR for Domestic Wastewater Treatment and Their Impacts on Process Efficiency and Membrane Fouling. Environ. Technol. Innov. 2021, 21, 101204. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces Cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef]
- Green Technologies for Sustainable Water Management; Ngo, H.H.; Guo, W.; Surampalli, R.Y.; Zhang, T.C. (Eds.) American Society of Civil Engineers: Reston, VA, USA, 2016; ISBN 978-0-7844-1442-2. [Google Scholar]
- Judd, S.J. The Status of Industrial and Municipal Effluent Treatment with Membrane Bioreactor Technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Sonawane, S.; Thakur, P.; Sonawane, S.H.; Bhanvase, B.A. Nanomaterials for Membrane Synthesis: Introduction, Mechanism, and Challenges for Wastewater Treatment. In Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 537–553. ISBN 978-0-12-821496-1. [Google Scholar]
- Muthukumaraswamy Rangaraj, V.; Wahab, M.A.; Reddy, K.S.K.; Kakosimos, G.; Abdalla, O.; Favvas, E.P.; Reinalda, D.; Geuzebroek, F.; Abdala, A.; Karanikolos, G.N. Metal Organic Framework—Based Mixed Matrix Membranes for Carbon Dioxide Separation: Recent Advances and Future Directions. Front. Chem. 2020, 8, 534. [Google Scholar] [CrossRef]
- Mohd Sabee, M.M.S. Materials and Applications for Functional Polymer Membranes. In Materials Research Foundations; Materials Research Forum LLC: Millersville, PA, USA, 2022; Volume 120, pp. 72–110. ISBN 978-1-64490-181-6. [Google Scholar]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric Antimicrobial Membranes Enabled by Nanomaterials for Water Treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Karasu, K.; Glennon, N.; Lawrence, N.D.; Stevens, G.W.; O’Connor, A.J.; Barber, A.R.; Yoshikawa, S.; Kentish, S.E. A Comparison between Ceramic and Polymeric Membrane Systems for Casein Concentrate Manufacture. Int. J. Dairy Technol. 2010, 63, 284–289. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Ranil Wickramasinghe, S.; Banat, F. Recent Developments in Porous Ceramic Membranes for Wastewater Treatment and Desalination: A Review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric Membranes Incorporated with Metal/Metal Oxide Nanoparticles: A Comprehensive Review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Koyuncu, I.; Sengur-Tasdemir, R.; Ersahin, M.E.; Ozgun, H.; Kose-Mutlu, B.; Turken, T.; Kaya, R.; Yavuzturk-Gul, B. Applications of Ceramic Membrane Bioreactors in Water Treatment. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–176. ISBN 978-0-12-816822-6. [Google Scholar]
- Chen, J.; Lv, Q.; Meng, Q.; Liu, X.; Xiao, X.; Li, X.; Liu, Y.; Zhang, X.; Gao, P. Study on Treatment of Low Concentration Oily Wastewater Using Alumina Ceramic Membranes. Crystals 2022, 12, 127. [Google Scholar] [CrossRef]
- Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules 2021, 26, 3331. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Ismail, A.F. A Review on Inorganic Membranes for Desalination and Wastewater Treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Credibility of Polymeric and Ceramic Membrane Filtration in the Removal of Bacteria and Virus from Water: A Review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, T.C.A.; Bao, Y.; Ng, H.Y.; Tan, S.C.; Wang, J. Developing Better Ceramic Membranes for Water and Wastewater Treatment: Where Microstructure Integrates with Chemistry and Functionalities. Chem. Eng. J. 2022, 428, 130456. [Google Scholar] [CrossRef]
- Sandhya Rani, S.L.; Kumar, R.V. Insights on Applications of Low-Cost Ceramic Membranes in Wastewater Treatment: A Mini-Review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The Application of Pressure-Driven Ceramic Membrane Technology for the Treatment of Industrial Wastewater—A Review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Cross-Flow Microfiltration of Glycerol Fermentation Broths with Citrobacter Freundii. Membranes 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; Sánchez, E. Low-Cost Ceramic Membranes: A Research Opportunity for Industrial Application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Lew, B.; Tarre, S.; Beliavski, M.; Dosoretz, C.; Green, M. Anaerobic Membrane Bioreactor (AnMBR) for Domestic Wastewater Treatment. Desalination 2009, 243, 251–257. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Yuniarto, A.; Olsson, G. Membrane Bioreactor: Applications and Limitations in Treating High Strength Industrial Wastewater. Chem. Eng. J. 2013, 225, 109–119. [Google Scholar] [CrossRef]
- Carstensen, F.; Marx, C.; André, J.; Melin, T.; Wessling, M. Reverse-Flow Diafiltration for Continuous in Situ Product Recovery. J. Membr. Sci. 2012, 421–422, 39–50. [Google Scholar] [CrossRef]
- Jain, M. Anaerobic Membrane Bioreactor as Highly Efficient and Reliable Technology for Wastewater Treatment—A Review. ACES 2018, 8, 82–100. [Google Scholar] [CrossRef] [Green Version]
- Uman, A.E.; Bair, R.A.; Yeh, D.H. Assessment of an Anaerobic Membrane Bioreactor (AnMBR) Treating Medium-Strength Synthetic Wastewater under Cyclical Membrane Operation. Membranes 2021, 11, 415. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hiasa, M.; Mahmood, T.; Matsuo, T. Direct Solid-Liquid Separation Using Hollow Fiber Membrane in an Activated Sludge Aeration Tank. In Water Pollution Research and Control Brighton; Elsevier: Amsterdam, The Netherlands, 1988; pp. 43–54. ISBN 978-1-4832-8439-2. [Google Scholar]
- Hu, Y.; Cheng, H.; Ji, J.; Li, Y.-Y. A Review of Anaerobic Membrane Bioreactors for Municipal Wastewater Treatment with a Focus on Multicomponent Biogas and Membrane Fouling Control. Environ. Sci. Water Res. Technol. 2020, 6, 2641–2663. [Google Scholar] [CrossRef]
- Liao, B.-Q.; Kraemer, J.T.; Bagley, D.M. Anaerobic Membrane Bioreactors: Applications and Research Directions. Crit. Rev. Environ. Sci. Technol. 2006, 36, 489–530. [Google Scholar] [CrossRef]
- Zhen, G.; Pan, Y.; Lu, X.; Li, Y.-Y.; Zhang, Z.; Niu, C.; Kumar, G.; Kobayashi, T.; Zhao, Y.; Xu, K. Anaerobic Membrane Bioreactor towards Biowaste Biorefinery and Chemical Energy Harvest: Recent Progress, Membrane Fouling and Future Perspectives. Renew. Sustain. Energy Rev. 2019, 115, 109392. [Google Scholar] [CrossRef]
- Gouveia, J.; Plaza, F.; Garralon, G.; Fdz-Polanco, F.; Peña, M. Long-Term Operation of a Pilot Scale Anaerobic Membrane Bioreactor (AnMBR) for the Treatment of Municipal Wastewater under Psychrophilic Conditions. Bioresour. Technol. 2015, 185, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cicek, N.; Ilg, J. State-of-the-Art of Membrane Bioreactors: Worldwide Research and Commercial Applications in North America. J. Membr. Sci. 2006, 270, 201–211. [Google Scholar] [CrossRef]
- Seib, M.D.; Berg, K.J.; Zitomer, D.H. Low Energy Anaerobic Membrane Bioreactor for Municipal Wastewater Treatment. J. Membr. Sci. 2016, 514, 450–457. [Google Scholar] [CrossRef]
- Gouveia, J.; Plaza, F.; Garralon, G.; Fdz-Polanco, F.; Peña, M. A Novel Configuration for an Anaerobic Submerged Membrane Bioreactor (AnSMBR). Long-Term Treatment of Municipal Wastewater under Psychrophilic Conditions. Bioresour. Technol. 2015, 198, 510–519. [Google Scholar] [CrossRef]
- Coutte, F.; Lecouturier, D.; Firdaous, L.; Kapel, R.; Bazinet, L.; Cabassud, C.; Dhulster, P. Recent Trends in Membrane Bioreactors. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–311. ISBN 978-0-444-63663-8. [Google Scholar]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in Membrane Bioreactors Used in Wastewater Treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoo, G.Y.; Yoo, C.K. Development of Combined Fouling Model in a Membrane Bioreactor. Asia-Pac. J. Chem. Eng. 2011, 6, 423–432. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Rout, P.R.; Aslam, M.; Fuwad, A.; Choi, Y.; Banu, J.R.; Park, J.H.; Kumar, G. A Brief Review of Anaerobic Membrane Bioreactors Emphasizing Recent Advancements, Fouling Issues and Future Perspectives. J. Environ. Manag. 2020, 270, 110909. [Google Scholar] [CrossRef]
- Charfi, A.; Ben Amar, N.; Harmand, J. Analysis of Fouling Mechanisms in Anaerobic Membrane Bioreactors. Water Res. 2012, 46, 2637–2650. [Google Scholar] [CrossRef]
- Hermia, J. Blocking Filtration. Application to Non-Newtonian Fluids. In Mathematical Models and Design Methods in Solid-Liquid Separation; Rushton, A., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 83–89. ISBN 978-94-010-8751-3. [Google Scholar]
- Tomczak, W.; Gryta, M. Application of Ultrafiltration Ceramic Membrane for Separation of Oily Wastewater Generated by Maritime Transportation. Sep. Purif. Technol. 2021, 261, 118259. [Google Scholar] [CrossRef]
- Iorhemen, O.; Hamza, R.; Tay, J. Membrane Bioreactor (MBR) Technology for Wastewater Treatment and Reclamation: Membrane Fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Tian, Y.; Li, Z.; Zuo, W.; Zhang, J. A Comprehensive Study into Fouling Properties of Extracellular Polymeric Substance (EPS) Extracted from Bulk Sludge and Cake Sludge in a Mesophilic Anaerobic Membrane Bioreactor. Bioresour. Technol. 2015, 192, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Nie, Y.; Hu, Y.; Miao, R.; Utashiro, T.; Li, Q.; Xu, M.; Li, Y.-Y. Fouling Behaviour of Soluble Microbial Products and Extracellular Polymeric Substances in a Submerged Anaerobic Membrane Bioreactor Treating Low-Strength Wastewater at Room Temperature. J. Membr. Sci. 2017, 531, 1–9. [Google Scholar] [CrossRef]
- Xu, B.; Ng, T.C.A.; Huang, S.; Ng, H.Y. Effect of Quorum Quenching on EPS and Size-Fractioned Particles and Organics in Anaerobic Membrane Bioreactor for Domestic Wastewater Treatment. Water Res. 2020, 179, 115850. [Google Scholar] [CrossRef]
- Sengupta, A.; Jebur, M.; Kamaz, M.; Wickramasinghe, S.R. Removal of Emerging Contaminants from Wastewater Streams Using Membrane Bioreactors: A Review. Membranes 2021, 12, 60. [Google Scholar] [CrossRef]
- Maneewan, P.; Sajomsang, W.; Singto, S.; Lohwacharin, J.; Suwannasilp, B.B. Fouling Mitigation in an Anaerobic Membrane Bioreactor via Membrane Surface Modification with Tannic Acid and Copper. Environ. Pollut. 2021, 291, 118205. [Google Scholar] [CrossRef]
- Cao, P.; Shi, J.; Zhang, J.; Wang, X.; Jung, J.T.; Wang, Z.; Cui, Z.; Lee, Y.M. Piezoelectric PVDF Membranes for Use in Anaerobic Membrane Bioreactor (AnMBR) and Their Antifouling Performance. J. Membr. Sci. 2020, 603, 118037. [Google Scholar] [CrossRef]
- Joshi, P.; Parker, W. Effect of Pretreatment Using Ultrasound and Hydrogen Peroxide on Digestion of Waste Activated Sludge in an Anaerobic Membrane Bioreactor. Environ. Prog. Sustain. Energy 2015, 34, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Padmasiri, S.I.; Fitch, M.; Norddahl, B.; Raskin, L.; Morgenroth, E. Influence of Cleaning Frequency and Membrane History on Fouling in an Anaerobic Membrane Bioreactor. Desalination 2007, 207, 153–166. [Google Scholar] [CrossRef]
- Mei, X.; Wang, Z.; Miao, Y.; Wu, Z. Recover Energy from Domestic Wastewater Using Anaerobic Membrane Bioreactor: Operating Parameters Optimization and Energy Balance Analysis. Energy 2016, 98, 146–154. [Google Scholar] [CrossRef]
- Ahn, Y.T.; Kang, S.T.; Chae, S.R.; Lee, C.Y.; Bae, B.U.; Shin, H.S. Simultaneous High-Strength Organic and Nitrogen Removal with Combined Anaerobic Upflow Bed Filter and Aerobic Membrane Bioreactor. Desalination 2007, 202, 114–121. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Khanzada, N.K.; Farid, M.U.; Kim, J.; Jeong, S.; An, A.K. Membrane Distillation Bioreactor (MDBR) for Wastewater Treatment, Water Reuse, and Resource Recovery: A Review. J. Water Process Eng. 2022, 47, 102687. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Ercolei, L.; Moulin, P. Membrane-Based Processes Used in Municipal Wastewater Treatment for Water Reuse: State-Of-The-Art and Performance Analysis. Membranes 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Sorme, L.; Lagerkvist, R. Sources of Heavy Metals in Urban Wastewater in Stockholm. Sci. Total Environ. 2002, 298, 131–145. [Google Scholar] [CrossRef]
- Mattsson, A.; Finnson, A.; I’Ons, D. Heavy Metal Content of Swedish Municipal Wastewater Sludge—Status and Goals. Water Sci. Technol. 2017, 76, 869–876. [Google Scholar] [CrossRef]
- Lewkowska, P.; Cieślik, B.; Dymerski, T.; Konieczka, P.; Namieśnik, J. Characteristics of Odors Emitted from Municipal Wastewater Treatment Plant and Methods for Their Identification and Deodorization Techniques. Environ. Res. 2016, 151, 573–586. [Google Scholar] [CrossRef]
- Hang, Y.D. Determination of Oxygen Demand. In Food Analysis; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 503–507. ISBN 978-3-319-45774-1. [Google Scholar]
- Mara, D. Domestic Wastewater Treatment in Developing Countries; Routledge: Oxfordshire, UK, 2013; ISBN 978-1-136-56792-6. [Google Scholar]
- Ji, J.; Ni, J.; Ohtsu, A.; Isozumi, N.; Hu, Y.; Du, R.; Chen, Y.; Qin, Y.; Kubota, K.; Li, Y.-Y. Important Effects of Temperature on Treating Real Municipal Wastewater by a Submerged Anaerobic Membrane Bioreactor: Removal Efficiency, Biogas, and Microbial Community. Bioresour. Technol. 2021, 336, 125306. [Google Scholar] [CrossRef]
- Ji, J.; Du, R.; Ni, J.; Chen, Y.; Hu, Y.; Qin, Y.; Hojo, T.; Li, Y.-Y. Submerged Anaerobic Membrane Bioreactor Applied for Mainstream Municipal Wastewater Treatment at a Low Temperature: Sludge Yield, Energy Balance and Membrane Filtration Behaviors. J. Clean. Prod. 2022, 355, 131831. [Google Scholar] [CrossRef]
- Ji, J.; Sakuma, S.; Ni, J.; Chen, Y.; Hu, Y.; Ohtsu, A.; Chen, R.; Cheng, H.; Qin, Y.; Hojo, T.; et al. Application of Two Anaerobic Membrane Bioreactors with Different Pore Size Membranes for Municipal Wastewater Treatment. Sci. Total Environ. 2020, 745, 140903. [Google Scholar] [CrossRef]
- Rong, C.; Wang, T.; Luo, Z.; Hu, Y.; Kong, Z.; Qin, Y.; Hanaoka, T.; Ito, M.; Kobayashi, M.; Li, Y.-Y. Pilot Plant Demonstration of Temperature Impacts on the Methanogenic Performance and Membrane Fouling Control of the Anaerobic Membrane Bioreactor in Treating Real Municipal Wastewater. Bioresour. Technol. 2022, 354, 127167. [Google Scholar] [CrossRef] [PubMed]
- Seco, A.; Mateo, O.; Zamorano-López, N.; Sanchis-Perucho, P.; Serralta, J.; Martí, N.; Borrás, L.; Ferrer, J. Exploring the Limits of Anaerobic Biodegradability of Urban Wastewater by AnMBR Technology. Environ. Sci. Water Res. Technol. 2018, 4, 1877–1887. [Google Scholar] [CrossRef]
- Saddoud, A.; Ellouze, M.; Dhouib, A.; Sayadi, S. Anaerobic Membrane Bioreactor Treatment of Domestic Wastewater in Tunisia. Desalination 2007, 207, 205–215. [Google Scholar] [CrossRef]
- Calderón, K.; Rodelas, B.; Cabirol, N.; González-López, J.; Noyola, A. Analysis of Microbial Communities Developed on the Fouling Layers of a Membrane-Coupled Anaerobic Bioreactor Applied to Wastewater Treatment. Bioresour. Technol. 2011, 102, 4618–4627. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, J.; Rong, C.; Wang, T.; Li, L.; Luo, Z.; Ji, J.; Hanaoka, T.; Sakemi, S.; Ito, M.; et al. Large Pilot-Scale Submerged Anaerobic Membrane Bioreactor for the Treatment of Municipal Wastewater and Biogas Production at 25 °C. Bioresour. Technol. 2021, 319, 124123. [Google Scholar] [CrossRef]
- Ozgun, H.; Tao, Y.; Ersahin, M.E.; Zhou, Z.; Gimenez, J.B.; Spanjers, H.; van Lier, J.B. Impact of Temperature on Feed-Flow Characteristics and Filtration Performance of an Upflow Anaerobic Sludge Blanket Coupled Ultrafiltration Membrane Treating Municipal Wastewater. Water Res. 2015, 83, 71–83. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Liang, P.; Zhang, X.; Kimura, K.; Huang, X. Distinction between Polymeric and Ceramic Membrane in AnMBR Treating Municipal Wastewater: In Terms of Irremovable Fouling. J. Membr. Sci. 2019, 588, 117229. [Google Scholar] [CrossRef]
- Elmaleh, S.; Abdelmoumni, L. Cross-Flow Filtration of an Anaerobic Methanogenic Suspension. J. Membr. Sci. 1997, 131, 261–274. [Google Scholar] [CrossRef]
- An, Y.; Wang, Z.; Wu, Z.; Yang, D.; Zhou, Q. Characterization of Membrane Foulants in an Anaerobic Non-Woven Fabric Membrane Bioreactor for Municipal Wastewater Treatment. Chem. Eng. J. 2009, 155, 709–715. [Google Scholar] [CrossRef]
- Wang, K.M.; Soares, A.; Jefferson, B.; McAdam, E.J. Comparable Membrane Permeability Can Be Achieved in Granular and Flocculent Anaerobic Membrane Bioreactor for Sewage Treatment through Better Sludge Blanket Control. J. Water Process Eng. 2019, 28, 181–189. [Google Scholar] [CrossRef]
- Ho, J.; Sung, S. Methanogenic Activities in Anaerobic Membrane Bioreactors (AnMBR) Treating Synthetic Municipal Wastewater. Bioresour. Technol. 2010, 101, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Bowen, E.J.; Dolfing, J.; Davenport, R.J.; Read, F.L.; Curtis, T.P. Low-Temperature Limitation of Bioreactor Sludge in Anaerobic Treatment of Domestic Wastewater. Water Sci. Technol. 2014, 69, 1004–1013. [Google Scholar] [CrossRef]
- Lin, Q.; He, G.; Rui, J.; Fang, X.; Tao, Y.; Li, J.; Li, X. Microorganism-Regulated Mechanisms of Temperature Effects on the Performance of Anaerobic Digestion. Microb Cell Fact 2016, 15, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, E.; He, P.; Zhang, H.; Hao, L.; Shao, L.; Lü, F. How Does Temperature Regulate Anaerobic Digestion? Renew. Sustain. Energy Rev. 2021, 150, 111453. [Google Scholar] [CrossRef]
- McKeown, R.M.; Hughes, D.; Collins, G.; Mahony, T.; O’Flaherty, V. Low-Temperature Anaerobic Digestion for Wastewater Treatment. Curr. Opin. Biotechnol. 2012, 23, 444–451. [Google Scholar] [CrossRef]
- Gao, D.; Tao, Y.; An, R.; Fu, Y.; Ren, N. Fate of Organic Carbon in UAFB Treating Raw Sewage: Impact of Moderate to Low Temperature. Bioresour. Technol. 2011, 102, 2248–2254. [Google Scholar] [CrossRef]
- Gkotsis, P.K.; Zouboulis, A.I. Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling. Molecules 2019, 24, 2867. [Google Scholar] [CrossRef] [Green Version]
- Naidu, G.; Shim, W.G.; Jeong, S.; Choi, Y.; Ghaffour, N.; Vigneswaran, S. Transport Phenomena and Fouling in Vacuum Enhanced Direct Contact Membrane Distillation: Experimental and Modelling. Sep. Purif. Technol. 2017, 172, 285–295. [Google Scholar] [CrossRef]
- Gao, W.J.; Qu, X.; Leung, K.T.; Liao, B.Q. Influence of Temperature and Temperature Shock on Sludge Properties, Cake Layer Structure, and Membrane Fouling in a Submerged Anaerobic Membrane Bioreactor. J. Membr. Sci. 2012, 421–422, 131–144. [Google Scholar] [CrossRef]
- Ho, J.; Sung, S. Anaerobic Membrane Bioreactor Treatment of Synthetic Municipal Wastewater at Ambient Temperature. Water Environ. Res. 2009, 81, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Parker, W.; Dagnew, M. Influence of SRT and HRT on Bioprocess Performance in Anaerobic Membrane Bioreactors Treating Municipal Wastewater. Water Environ. Res. 2016, 88, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Robles, Á.; Durán, F.; Giménez, J.B.; Jiménez, E.; Ribes, J.; Serralta, J.; Seco, A.; Ferrer, J.; Rogalla, F. Anaerobic Membrane Bioreactors (AnMBR) Treating Urban Wastewater in Mild Climates. Bioresour. Technol. 2020, 314, 123763. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-H.; Harb, M.; Amy, G.; Hong, P.-Y.; Leiknes, T. Sustainable Organic Loading Rate and Energy Recovery Potential of Mesophilic Anaerobic Membrane Bioreactor for Municipal Wastewater Treatment. Bioresour. Technol. 2014, 166, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.; Xiong, Y.; Guest, J.; Amy, G.; Hong, P.-Y. Differences in Microbial Communities and Performance between Suspended and Attached Growth Anaerobic Membrane Bioreactors Treating Synthetic Municipal Wastewater. Environ. Sci. Water Res. Technol. 2015, 1, 800–813. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Peláez, M.L.; Morgan-Sagastume, J.M.; Noyola, A. Influence of Hydraulic Retention Time on Fouling in a UASB Coupled with an External Ultrafiltration Membrane Treating Synthetic Municipal Wastewater. Desalination 2011, 277, 164–170. [Google Scholar] [CrossRef]
- Dong, Q.; Parker, W.; Dagnew, M. Impact of FeCl3 Dosing on AnMBR Treatment of Municipal Wastewater. Water Res. 2015, 80, 281–293. [Google Scholar] [CrossRef]
- Mei, X.; Wang, Z.; Miao, Y.; Wu, Z. A Pilot-Scale Anaerobic Membrane Bioreactor under Short Hydraulic Retention Time for Municipal Wastewater Treatment: Performance and Microbial Community Identification. J. Water Reuse Desalination 2018, 8, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Chen, J.; Wang, F.; Ding, L.; Hong, H. Feasibility Evaluation of Submerged Anaerobic Membrane Bioreactor for Municipal Secondary Wastewater Treatment. Desalination 2011, 280, 120–126. [Google Scholar] [CrossRef]
- Martinez-Sosa, D.; Helmreich, B.; Netter, T.; Paris, S.; Bischof, F.; Horn, H. Anaerobic Submerged Membrane Bioreactor (AnSMBR) for Municipal Wastewater Treatment under Mesophilic and Psychrophilic Temperature Conditions. Bioresour. Technol. 2011, 102, 10377–10385. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Mahvi, A.; Vaezi, F.; Naddafi, K. Evaluation of Hollow Fiber Membrane Bioreactor Efficiency for Municipal Wastewater Treatment. Iran. J. Environ. Health Sci. Eng. 2008, 5, 257–268. [Google Scholar]
- Berkessa, Y.W.; Yan, B.; Li, T.; Tan, M.; She, Z.; Jegatheesan, V.; Jiang, H.; Zhang, Y. Novel Anaerobic Membrane Bioreactor (AnMBR) Design for Wastewater Treatment at Long HRT and High Solid Concentration. Bioresour. Technol. 2018, 250, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, S.; Astals, S.; Jaramillo, M.; Mata-Alvarez, J.; Dosta, J. Anaerobic Membrane Bioreactor Performance at Different Wastewater Pre-Concentration Factors: An Experimental and Economic Study. Sci. Total Environ. 2021, 750, 141625. [Google Scholar] [CrossRef] [PubMed]
- Keskin, B.; Ersahin, M.E.; Ozgun, H.; Koyuncu, I. Pilot and Full-Scale Applications of Membrane Processes for Textile Wastewater Treatment: A Critical Review. J. Water Process Eng. 2021, 42, 102172. [Google Scholar] [CrossRef]
- Brepols, C.; Schäfer, H.; Engelhardt, N. Considerations on the Design and Financial Feasibility of Full-Scale Membrane Bioreactors for Municipal Applications. Water Sci. Technol. 2010, 61, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological Insights into Anaerobic Digestion for Biogas, Hydrogen or Volatile Fatty Acids (VFAs): A Review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Ekstrand, E.-M.; Björn, A.; Karlsson, A.; Schnürer, A.; Kanders, L.; Yekta, S.S.; Karlsson, M.; Moestedt, J. Identifying Targets for Increased Biogas Production through Chemical and Organic Matter Characterization of Digestate from Full-Scale Biogas Plants: What Remains and Why? Biotechnol Biofuels 2022, 15, 16. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. ABB 2015, 06, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, J.; Pretel, R.; Durán, F.; Giménez, J.B.; Robles, A.; Ruano, M.V.; Serralta, J.; Ribes, J.; Seco, A. Design Methodology for Submerged Anaerobic Membrane Bioreactors (AnMBR): A Case Study. Sep. Purif. Technol. 2015, 141, 378–386. [Google Scholar] [CrossRef]
- Pretel, R.; Durán, F.; Robles, A.; Ruano, M.V.; Ribes, J.; Serralta, J.; Ferrer, J. Designing an AnMBR-Based WWTP for Energy Recovery from Urban Wastewater: The Role of Primary Settling and Anaerobic Digestion. Sep. Purif. Technol. 2015, 156, 132–139. [Google Scholar] [CrossRef]
- Pretel, R.; Moñino, P.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. Economic and Environmental Sustainability of an AnMBR Treating Urban Wastewater and Organic Fraction of Municipal Solid Waste. J. Environ. Manag. 2016, 179, 83–92. [Google Scholar] [CrossRef] [Green Version]
- van Lier, J.B.; Tilche, A.; Ahring, B.K.; Macarie, H.; Moletta, R.; Dohanyos, M.; Hulshoff Pol, L.W.; Lens, P.; Verstraete, W. New Perspectives in Anaerobic Digestion. Water Sci. Technol. 2001, 43, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Performance of Submerged Anaerobic Membrane Bioreactor at Different SRTs for Domestic Wastewater Treatment. J. Biotechnol. 2013, 164, 82–90. [Google Scholar] [CrossRef]
- Saddoud, A.; Ellouze, M.; Dhouib, A.; Sayadi, S. A Comparative Study on the Anaerobic Membrane Bioreactor Performance During the Treatment of Domestic Wastewaters of Various Origins. Environ. Technol. 2006, 27, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Chen, Y.; Hu, Y.; Ohtsu, A.; Ni, J.; Li, Y.; Sakuma, S.; Hojo, T.; Chen, R.; Li, Y.-Y. One-Year Operation of a 20-L Submerged Anaerobic Membrane Bioreactor for Real Domestic Wastewater Treatment at Room Temperature: Pursuing the Optimal HRT and Sustainable Flux. Sci. Total Environ. 2021, 775, 145799. [Google Scholar] [CrossRef]
- Liu, H.; Gu, J.; Wang, S.; Zhang, M.; Liu, Y. Performance, Membrane Fouling Control and Cost Analysis of an Integrated Anaerobic Fixed-Film MBR and Reverse Osmosis Process for Municipal Wastewater Reclamation to NEWater-like Product Water. J. Membr. Sci. 2020, 593, 117442. [Google Scholar] [CrossRef]
- Saddoud, A.; Abdelkafi, S.; Sayadi, S. Effects of Domestic Wastewater Toxicity on Anaerobic Membrane-bioreactor (MBR) Performances. Environ. Technol. 2009, 30, 1361–1369. [Google Scholar] [CrossRef]
- Yue, X.; Koh, Y.K.K.; Ng, H.Y. Effects of Dissolved Organic Matters (DOMs) on Membrane Fouling in Anaerobic Ceramic Membrane Bioreactors (AnCMBRs) Treating Domestic Wastewater. Water Res. 2015, 86, 96–107. [Google Scholar] [CrossRef]
- Sanchez, L.; Carrier, M.; Cartier, J.; Charmette, C.; Heran, M.; Steyer, J.-P.; Lesage, G. Enhanced Organic Degradation and Biogas Production of Domestic Wastewater at Psychrophilic Temperature through Submerged Granular Anaerobic Membrane Bioreactor for Energy-Positive Treatment. Bioresour. Technol. 2022, 353, 127145. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; McDonald, J.A.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Development of a Predictive Framework to Assess the Removal of Trace Organic Chemicals by Anaerobic Membrane Bioreactor. Bioresour. Technol. 2015, 189, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Cho, K.; Kwon, E.E.; Tsang, Y.F.; Rinklebe, J.; Park, C. Evaluating the Feasibility of Pyrophyllite-Based Ceramic Membranes for Treating Domestic Wastewater in Anaerobic Ceramic Membrane Bioreactors. Chem. Eng. J. 2017, 328, 567–573. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, Y.; Jin, Y.; Hong, S.; Park, C. Comparison of Filtration and Treatment Performance between Polymeric and Ceramic Membranes in Anaerobic Membrane Bioreactor Treatment of Domestic Wastewater. Sep. Purif. Technol. 2018, 199, 182–188. [Google Scholar] [CrossRef]
- Wen, C.; Huang, X.; Qian, Y. Domestic Wastewater Treatment Using an Anaerobic Bioreactor Coupled with Membrane Filtration. Process Biochem. 1999, 35, 335–340. [Google Scholar] [CrossRef]
- Chu, L.-B.; Yang, F.-L.; Zhang, X.-W. Anaerobic Treatment of Domestic Wastewater in a Membrane-Coupled Expended Granular Sludge Bed (EGSB) Reactor under Moderate to Low Temperature. Process Biochem. 2005, 40, 1063–1070. [Google Scholar] [CrossRef]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Anaerobic Membrane Bioreactor Treatment of Domestic Wastewater at Psychrophilic Temperatures Ranging from 15 °C to 3 °C. Environ. Sci. Water Res. Technol. 2015, 1, 56–64. [Google Scholar] [CrossRef]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Psychrophilic Anaerobic Membrane Bioreactor Treatment of Domestic Wastewater. Water Res. 2013, 47, 1655–1665. [Google Scholar] [CrossRef]
- Liu, J.; Tian, H.; Luan, X.; Zhou, X.; Chen, X.; Xu, S.; Kang, X. Submerged Anaerobic Membrane Bioreactor for Low-Concentration Domestic Sewage Treatment: Performance and Membrane Fouling. Environ. Sci. Pollut. Res. 2020, 27, 6785–6795. [Google Scholar] [CrossRef]
- Ni, J.; Ji, J.; Li, Y.-Y.; Kubota, K. Microbial Characteristics in Anaerobic Membrane Bioreactor Treating Domestic Sewage: Effects of HRT and Process Performance. J. Environ. Sci. 2022, 111, 392–399. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Submerged Anaerobic Membrane Bioreactor for Low-Strength Wastewater Treatment: Effect of HRT and SRT on Treatment Performance and Membrane Fouling. Water Res. 2011, 45, 705–713. [Google Scholar] [CrossRef]
- Martin, I.; Pidou, M.; Soares, A.; Judd, S.; Jefferson, B. Modelling the Energy Demands of Aerobic and Anaerobic Membrane Bioreactors for Wastewater Treatment. Environ. Technol. 2011, 32, 921–932. [Google Scholar] [CrossRef]
- Achilli, A.; Marchand, E.A.; Childress, A.E. A Performance Evaluation of Three Membrane Bioreactor Systems: Aerobic, Anaerobic, and Attached-Growth. Water Sci. Technol. 2011, 63, 2999–3005. [Google Scholar] [CrossRef]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Membrane Biofilm Development Improves COD Removal in Anaerobic Membrane Bioreactor Wastewater Treatment. Microb. Biotechnol. 2015, 8, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Zhang, P.; Zhou, Y. Quorum Quenching Altered Microbial Diversity and Activity of Anaerobic Membrane Bioreactor (AnMBR) and Enhanced Methane Generation. Bioresour. Technol. 2020, 315, 123862. [Google Scholar] [CrossRef] [PubMed]

| AnMBR Configuration | Submerged | External |
|---|---|---|
| Energy consumption | Low | High |
| Permeate flux | Low | High |
| Flexibility | Little | Good |
| Packing density | High | Low |
| Biomass density | High | High |
| City, Country | tCOD [mg/L] | sCOD [mg/L] | tBOD [mg/L] | TSS [mg/L] | VSS [mg/L] | TN [mg/L] | Ammonium Nitrogen [mg/L] | TP [mg/L] | pH | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| NI | 338 | NI | 167 | 84 | NI | NI | 35 | NI | NI | [48] |
| Beer-Sheva, Israel | 939.2 ± 53.2 | 706.3 ± 14.1 | NI | 600 ± 210 | NI | 82.4 ± 3.74 | NI | 9.60 ± 0.70 | 7.00 ± 0.2 | [49] |
| Tagajo, Japan | 422 ± 74 | NI | 182 ± 67 | 197 ± 74 | NI | 27.30 ± 1.30 | NI | 2.10 ± 0.27 | 7.16 ± 0.17 | [195] |
| Beijing, China | 269–712 | NI | NI | NI | NI | NI | 21.59–44.65 | 4.17–5.88 | 6.95~7.10 | [196] |
| Tunis, Tunisia | 670 | NI | 180 | 288 | 180 | 49.35 | NI | 10.40 | 7.62 | [194] |
| Tunis, Tunisia | 900 | NI | 280 | 540 | 130 | 57.00 | NI | 16.00 | 7.70 | [194] |
| Tunis, Tunisia | 419 | NI | 160 | 220 | 200 | 51.47 | NI | 52.50 | 7.80 | [194] |
| Ksour Essef, Tunisia | 786 | NI | 315 | 377 | 286 | 166.00 | NI | 11.79 | 7.23 | [194] |
| Tunis, Tunisia | 663 ± 240 | NI | 206.66 ± 64 | 350 ± 160 | 170 ± 36 | 52.6 ± 3.95 | NI | 26.30 ± 22.86 | 7.70 ± 0.09 | [197] |
| Singapore | 330.4 ± 89.8 | 68.2 ± 47.6 | NI | 341.1 ± 94.9 | NI | 68.2 ± 10.2 | 32.7 ± 5.5 | NI | NI | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. Energy-Efficient AnMBRs Technology for Treatment of Wastewaters: A Review. Energies 2022, 15, 4981. https://doi.org/10.3390/en15144981
Tomczak W, Gryta M. Energy-Efficient AnMBRs Technology for Treatment of Wastewaters: A Review. Energies. 2022; 15(14):4981. https://doi.org/10.3390/en15144981
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2022. "Energy-Efficient AnMBRs Technology for Treatment of Wastewaters: A Review" Energies 15, no. 14: 4981. https://doi.org/10.3390/en15144981
APA StyleTomczak, W., & Gryta, M. (2022). Energy-Efficient AnMBRs Technology for Treatment of Wastewaters: A Review. Energies, 15(14), 4981. https://doi.org/10.3390/en15144981