Operando Analysis of Losses in Commercial-Sized Solid Oxide Cells: Methodology Development and Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
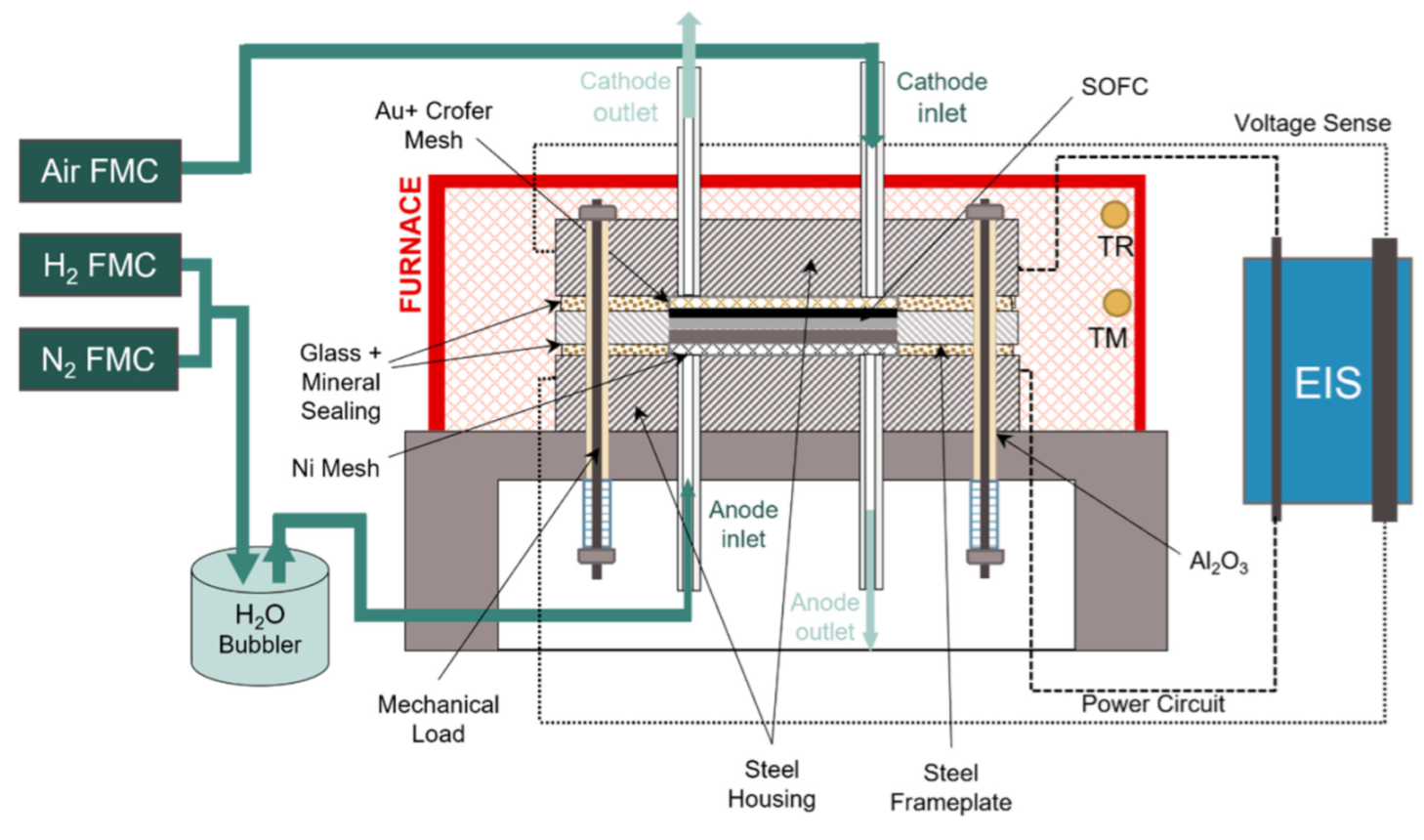
2.2. Methods
2.3. Equivalent Circuit Model and Data Fit
2.4. Test Campaign
3. Results and Discussion
3.1. Optimization of the Regularization Parameter
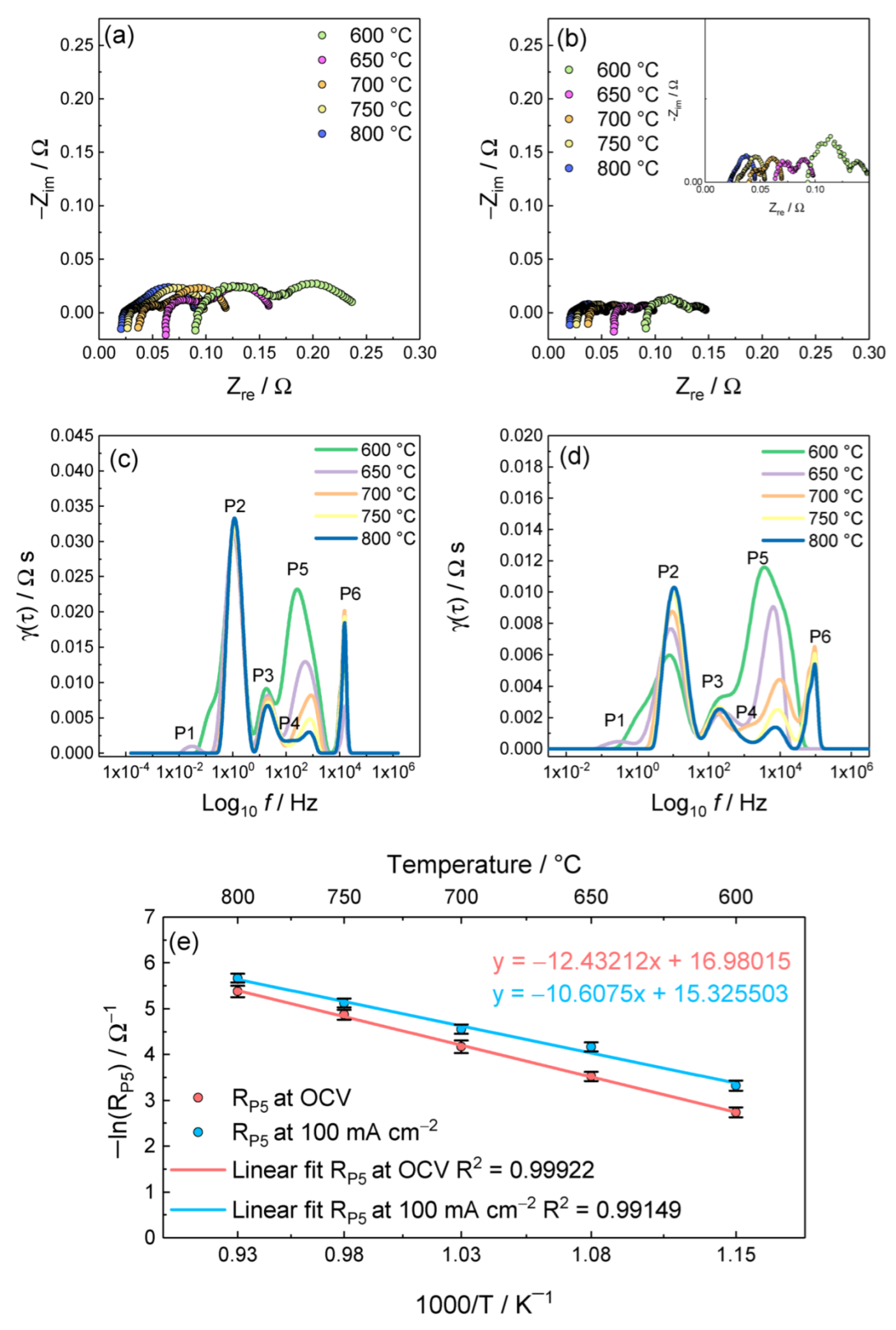
3.2. Effect of the Working Temperature: Detection of the Charge-Transfer Process
3.3. Effect of Fuel Composition: Detection of the Gas Diffusion Process in the Ni-YSZ Substrate
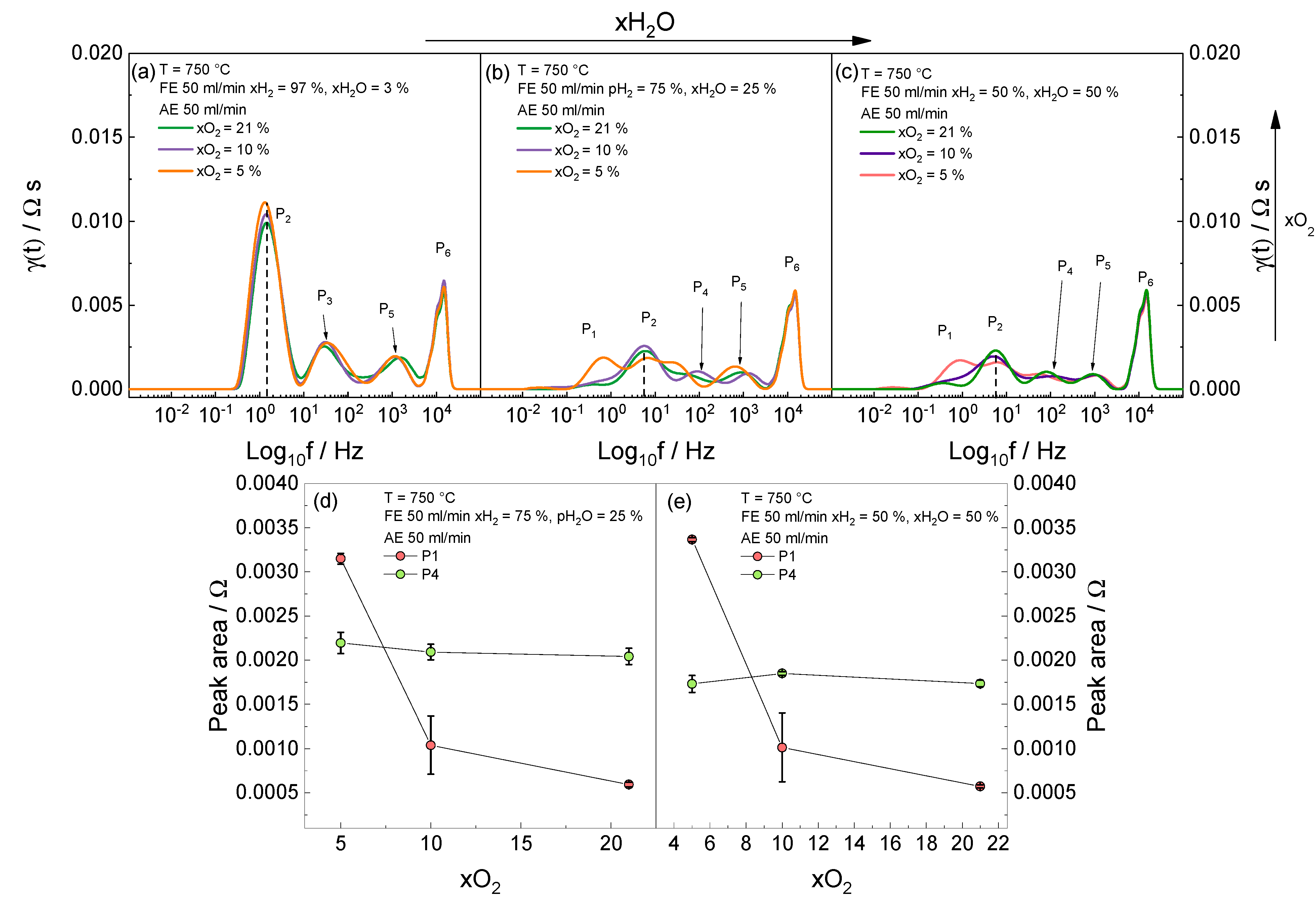
3.4. Effect of Air Electrode Gas Composition
3.5. Equivalent Circuit Model CNLS-Fit
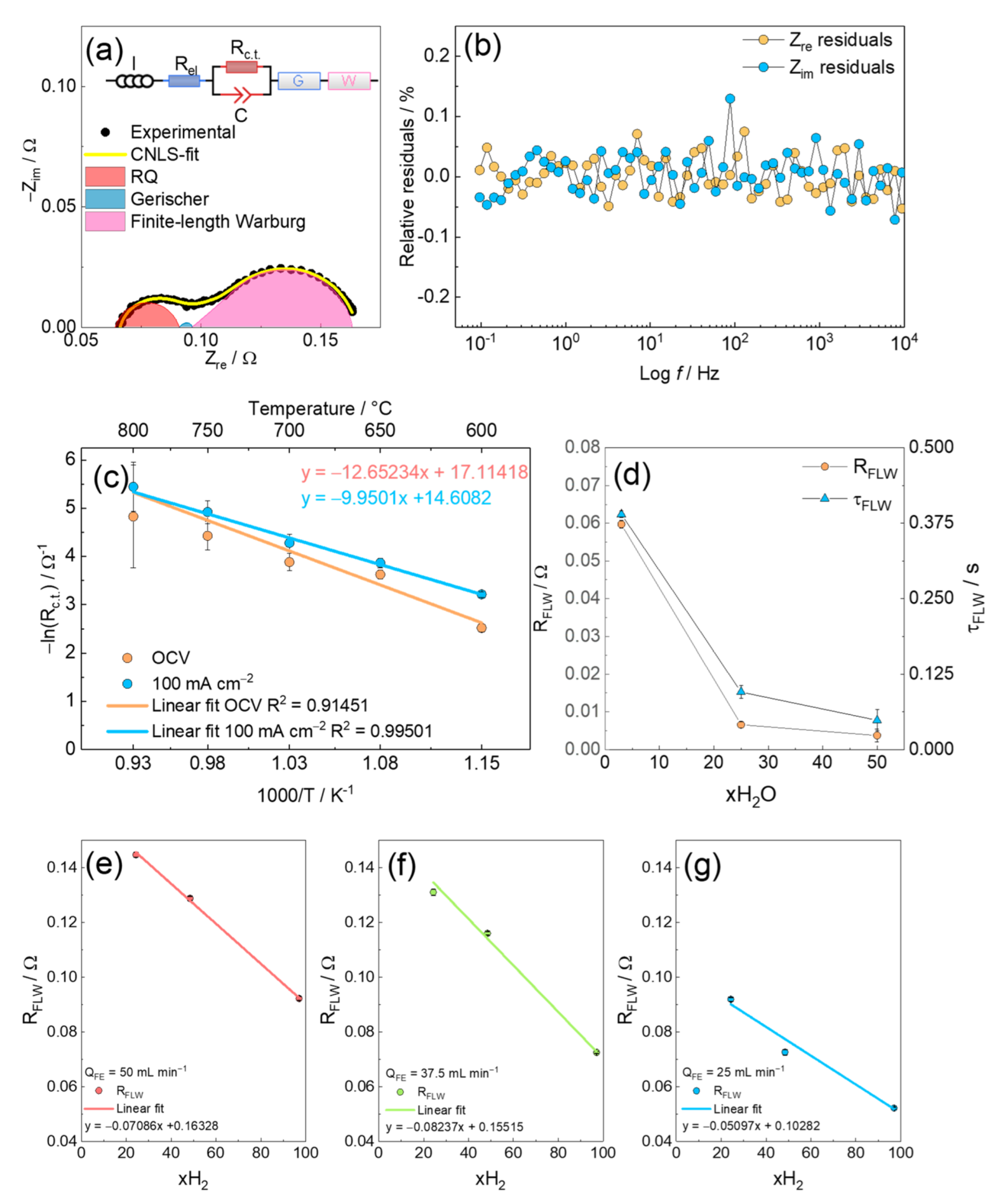
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Alternate Current |
| AEL | Alkaline Electrolysers |
| AFC | Alkaline Fuel Cells |
| CNLS | Complex Non-linear Least Square |
| DRT | Distribution of Relaxation Times |
| ECM | Equivalent Circuit Model |
| EIS | Electrochemical Impedance Spectroscopy |
| KK | Kramers–Kronig |
| MCFC | Molten Carbonate Fuel Cells |
| PAFC | Phosphoric Acid Fuel Cells |
| PEME | Polymer Electrolyte Membrane Electrolysers |
| PEMFC | Polymer Electrolyte Membranes Fuel Cells |
| SOC | Solid Oxide Cells |
| SOE | Solid Oxide Electrolysers |
| SOFC | Solid Oxide Fuel Cell |
| XRD | X-ray Diffraction Analysis |
References
- Victoria, M.; Zhu, K.; Brown, T.; Andresen, G.B.; Greiner, M. The role of storage technologies throughout the decarbonisation of the sector-coupled European energy system. Energy Convers. Manag. 2019, 201, 111977. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions the European Green Deal; European Commission: Brussels, Belgium, 2020; pp. 106–117. [Google Scholar]
- Sunfire takes significant steps to clean energy on demand using solid oxide technology. Fuel Cells Bull. 2016, 2016, 12–14. [CrossRef]
- Keçebaş, A.; Kayfeci, M.; Bayat, M. Electrochemical hydrogen generation. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–317. [Google Scholar]
- Baldinelli, A.; Barelli, L.; Bidini, G.; Di Cicco, A.; Gunnella, R.; Minicucci, M.; Trapananti, A. Advancements regarding in-operando diagnosis techniques for solid oxide cells NiYSZ cermets. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2019; Volume 2191, p. 020012. [Google Scholar]
- Shirbhate, S.; Gaikwad, V.; Acharya, S. Oxygen vacancies disordering and oxy-ion diffusion mechanism in doped ceria electrolytes under IT-SOFC operating conditions. J. Solid State Electrochem. 2022, 26, 133–148. [Google Scholar] [CrossRef]
- Dierickx, S.; Weber, A.; Ivers-Tiffée, E. How the distribution of relaxation times enhances complex equivalent circuit models for fuel cells. Electrochim. Acta 2020, 355, 136764. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Berg, P.; Schönleber, M.; Weber, A.; Ivers-Tiffée, E. The distribution of relaxation times as basis for generalized time-domain models for Li-ion batteries. J. Power Source 2013, 221, 70–77. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, M.; Huang, T.; Yu, A. Detection of lithium plating in lithium-ion batteries by distribution of relaxation times. J. Power Source 2021, 496, 229867. [Google Scholar] [CrossRef]
- Goldammer, E.; Kowal, J. Determination of the Distribution of Relaxation Times by Means of Pulse Evaluation for Offline and Online Diagnosis of Lithium-Ion Batteries. Batteries 2021, 7, 36. [Google Scholar] [CrossRef]
- Kim, D.; Muckley, E.S.; Creange, N.; Wan, T.H.; Ann, M.H.; Quattrocchi, E.; Vasudevan, R.K.; Kim, J.H.; Ciucci, F.; Ivanov, I.N.; et al. Exploring Transport Behavior in Hybrid Perovskites Solar Cells via Machine Learning Analysis of Environmental-Dependent Impedance Spectroscopy. Adv. Sci. 2021, 8, 2002510. [Google Scholar] [CrossRef]
- Hernández-Balaguera, E.; Romero, B.; Najafi, M.; Galagan, Y. Analysis of Light-Enhanced Capacitance Dispersion in Perovskite Solar Cells. Adv. Mater. Interfaces 2022, 9, 2102275. [Google Scholar] [CrossRef]
- Leonide, A.; Sonn, V.; Weber, A.; Ivers-Tiffée, E. Evaluation and Modeling of the Cell Resistance in Anode-Supported Solid Oxide Fuel Cells. J. Electrochem. Soc. 2007, 155, B36. [Google Scholar] [CrossRef]
- Osinkin, D.A. An approach to the analysis of the impedance spectra of solid oxide fuel cell using the DRT technique. Electrochim. Acta 2021, 372, 137858. [Google Scholar] [CrossRef]
- Sumi, H.; Shimada, H.; Yamaguchi, Y.; Yamaguchi, T.; Fujishiro, Y. Degradation evaluation by distribution of relaxation times analysis for microtubular solid oxide fuel cells. Electrochim. Acta 2020, 339, 135913. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, Q.; Blum, L.; Lehnert, W. Performance and degradation of an SOEC stack with different cell components. Electrochim. Acta 2017, 258, 1254–1261. [Google Scholar] [CrossRef]
- Subotić, V.; Königshofer, B.; Juričić, Đ.; Kusnezoff, M.; Schröttner, H.; Hochenauer, C.; Boškoski, P. Detailed insight into processes of reversible solid oxide cells and stacks using DRT analysis. Energy Convers. Manag. 2020, 226, 113509. [Google Scholar] [CrossRef]
- Groetsch, C.W. The Theory of Tikhonov Regularization for Fredholm Equations of the First Kind (C. W. Groetsch). Siam Rev. 1984, 28, 116–118. [Google Scholar]
- Horlin, T. Deconvolution and maximum entropy in impedance spectroscopy of noninductive systems. Solid State Ion. 1998, 107, 241–253. [Google Scholar] [CrossRef]
- Hörlin, T. Maximum entropy in impedance spectroscopy of non-inductive systems. Solid State Ion. 1993, 67, 85–96. [Google Scholar] [CrossRef]
- Boukamp, B.A. Fourier transform distribution function of relaxation times: Application and limitations. Electrochim. Acta 2015, 154, 35–46. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Rolle, A. Analysis and Application of Distribution of Relaxation Times in Solid State Ionics. Solid State Ion. 2017, 302, 12–18. [Google Scholar] [CrossRef]
- Saccoccio, M.; Wan, T.H.; Chen, C.; Ciucci, F. Optimal Regularization in Distribution of Relaxation Times applied to Electrochemical Impedance Spectroscopy: Ridge and Lasso Regression Methods-A Theoretical and Experimental Study. Electrochim. Acta 2014, 147, 470–482. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Staffolani, A.; Baldinelli, A.; Barelli, L.; Bidini, G.; Nobili, F. Early-Stage Detection of Solid Oxide Cells Anode Degradation by Operando Impedance Analysis. Processes 2021, 9, 848. [Google Scholar] [CrossRef]
- Boukamp, B.A. Derivation of a Distribution Function of Relaxation Times for the (fractal) Finite Length Warburg. Electrochim. Acta 2017, 252, 154–163. [Google Scholar] [CrossRef]
- Caliandro, P.; Nakajo, A.; Diethelm, S.; Van Herle, J. Model-assisted identification of solid oxide cell elementary processes by electrochemical impedance spectroscopy measurements. J. Power Sources 2019, 436, 226838. [Google Scholar] [CrossRef]
- Hong, J.; Bhardwaj, A.; Bae, H.; Kim, I.; Song, S.-J. Electrochemical Impedance Analysis of SOFC with Transmission Line Model Using Distribution of Relaxation Times (DRT). J. Electrochem. Soc. 2020, 167, 114504. [Google Scholar] [CrossRef]
- Dierickx, S.; Joos, J.; Weber, A.; Ivers-Tiffée, E. Advanced impedance modelling of Ni/8YSZ cermet anodes. Electrochim. Acta 2018, 265, 736–750. [Google Scholar] [CrossRef]
- Grolig, J.G.; Alnegren, P.; Froitzheim, J.; Svensson, J.-E. Copper Iron Conversion Coating for Solid Oxide Fuel Cell Interconnects. J. Power Source 2015, 297, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Baldinelli, A.; Staffolani, A.; Bidini, G.; Barelli, L.; Nobili, F. An extensive model for renewable energy electrochemical storage with Solid Oxide Cells based on a comprehensive analysis of impedance deconvolution. J. Energy Storage 2020, 33, 102052. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Barsoukov, E.; Ross Macdonald, J. Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Klotz, D.; Weber, A.; Ivers-Tiffée, E. Practical Guidelines for Reliable Electrochemical Characterization of Solid Oxide Fuel Cells. Electrochim. Acta 2017, 227, 110–126. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Linear Kronig-Kramers Transform Test for Immittance Data Validation. J. Electrochem. Soc. 1995, 142, 1885–1894. [Google Scholar] [CrossRef]
- Hayd, J.; Ivers-Tiffée, E. Detailed Electrochemical Study on Nanoscaled La 0.6 Sr 0.4 CoO 3-δ SOFC Thin-Film Cathodes in Dry, Humid and CO 2 -Containing Atmospheres. J. Electrochem. Soc. 2013; 160, F1197–F1206. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Y.; Chena, F. In-situ quantification of solid oxide fuel cell electrode microstructure by electrochemical impedance spectroscopy. J. Power Source 2015, 277, 277–285. [Google Scholar] [CrossRef]
- Pisani, L. Multi-component gas mixture diffusion through porous media: A 1D analytical solution. Int. J. Heat Mass Transf. 2008, 51, 650–660. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Papurello, D.; Menichini, D.; Lanzini, A. Distributed relaxation times technique for the determination of fuel cell losses with an equivalent circuit model to identify physicochemical processes. Electrochim. Acta 2017, 258, 98–109. [Google Scholar] [CrossRef]



| Ref | f < 1 Hz | 1 Hz < f < 10 Hz | 10 Hz < f < 100 Hz | 0.1 kHz < f < 0.5 kHz | 0.5 kHz < f < 100 kHz | 5 kHz < f < 200 Hz |
|---|---|---|---|---|---|---|
| [27] | Conversion diffusion at low pO2 transport in reforming mixture | Gas conversion | Fuel electrode diffusion/oxygen electrode reaction + solid state diffusion | Secondary peaks (fuel and oxygen electrode transport) | Fuel electrode charge transfer | Unidentified high-frequency peak |
| Dependencies | pO2 | Ftot > pH2 > i > pO2 > T | pH2O > i > Ftot > pO2 > T | pH2O > T > pO2 | T > I > pH2O | T |
| 0.1 Hz < f < 1 Hz | 1 Hz < f < 10 Hz | 10 Hz < f < 100 Hz | 0.1 kHz < f < 1 kHz | 1 kHz < f < 100 kHz 5 kHz < f < 200 Hz | ||
| [28] | Gas diffusion or polarisation at the air electrode | Air electrode gas diffusion (low pO2) or surface reaction on the cathode (high pO2) | Fuel electrode gas diffusion (low pH2O) or surface reaction on the fuel electrode | Bulk or surface diffusion at the fuel electrode | ||
| Dependencies | pO2 | pO2 | pH2O | |||
| 0.1 Hz < f < 1 Hz | 1 Hz < f < 50 Hz | 10 Hz < f < 100 Hz | 0.5 kHz < f < 10 kHz | |||
| [7,13,29] | Gas diffusion in the cathode (including contact mesh and flowfield) | Gas diffusion in the anode substrate (including contact mesh and flowfield) | Oxygen surface exchange kinetics & bulk diffusion of O2- | Charge transfer coupled to gas diffusion and ionic transport. | ||
| Dependencies | pO2, T, j | pH2, pH2O, T, j | pO2, T, j | T, pH2, pH2O, T | ||
| T | FE Supply | AE Supply | Operating Currents | ||||||
|---|---|---|---|---|---|---|---|---|---|
| j0 | j1 | ||||||||
| °C | NL/h | %vol,wb | %vol,wb | %vol,wb | NL/h | %vol,db | %vol,db | mA/cm2 | mA/cm2 |
| 750 | 50 | 97.0% | 0% | 3% | 50 | 21% | 79% | 0 | 100 |
| 750 | 50 | 48.5% | 48.5% | 3% | 50 | 21% | 79% | 0 | 100 |
| 750 | 50 | 24.3% | 72.8% | 3% | 50 | 21% | 79% | 0 | 100 |
| 750 | 50 | 97.0% | 0% | 3% | 50 | 10% | 90% | 0 | 100 |
| 750 | 50 | 48.5% | 48.5% | 3% | 50 | 10% | 90% | 0 | 100 |
| 750 | 50 | 24.3% | 72.8% | 3% | 50 | 10% | 90% | 0 | 100 |
| 750 | 50 | 97.0% | 0% | 3% | 50 | 5% | 95% | 0 | 100 |
| 750 | 50 | 48.5% | 48.5% | 3% | 50 | 5% | 95% | 0 | 100 |
| 750 | 50 | 24.3% | 72.8% | 3% | 50 | 5% | 95% | 0 | 100 |
| 750 | 50 | 75% | 0% | 25% | 50 | 21% | 79% | 0 | 100 |
| 750 | 50 | 75% | 0% | 25% | 50 | 10% | 90% | 0 | 100 |
| 750 | 50 | 75% | 0% | 25% | 50 | 5% | 95% | 0 | 100 |
| 750 | 50 | 50% | 0% | 50% | 50 | 21% | 79% | 0 | 100 |
| 750 | 50 | 50% | 0% | 50% | 50 | 10% | 90% | 0 | 100 |
| 750 | 50 | 50% | 0% | 50% | 50 | 5% | 95% | 0 | 100 |
| T | Fuel Electrode Supply | Air Electrode Supply | Operating Currents | ||||||
|---|---|---|---|---|---|---|---|---|---|
| j0 | j1 | ||||||||
| °C | NL/h | %vol,wb | %vol,wb | %vol,wb | NL/h | %vol,db | %vol,db | mA/cm2 | mA/cm2 |
| 800 | 50 | 97% | 0% | 3% | 50 | 21% | 79% | 0 | 100 |
| 750 | 50 | 97% | 0% | 3% | 50 | 21% | 79% | 0 | 100 |
| 700 | 50 | 97% | 0% | 3% | 50 | 21% | 79% | 0 | 100 |
| 650 | 50 | 97% | 0% | 3% | 50 | 21% | 79% | 0 | 100 |
| 600 | 50 | 97% | 0% | 3% | 50 | 21% | 79% | 0 | 100 |
| Technique | Ea | |
|---|---|---|
| j = OCV | j = 100 mA cm−2 | |
| DRT | Ea = 1.07132 ± 0.0851 eV | Ea = 0.90674 ± 0.07104 eV |
| CNLS-fit | Ea = 1.09029 ± 0.19247 eV | Ea = 0.76992 ± 0.03505 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staffolani, A.; Baldinelli, A.; Bidini, G.; Nobili, F.; Barelli, L. Operando Analysis of Losses in Commercial-Sized Solid Oxide Cells: Methodology Development and Validation. Energies 2022, 15, 4978. https://doi.org/10.3390/en15144978
Staffolani A, Baldinelli A, Bidini G, Nobili F, Barelli L. Operando Analysis of Losses in Commercial-Sized Solid Oxide Cells: Methodology Development and Validation. Energies. 2022; 15(14):4978. https://doi.org/10.3390/en15144978
Chicago/Turabian StyleStaffolani, Antunes, Arianna Baldinelli, Gianni Bidini, Francesco Nobili, and Linda Barelli. 2022. "Operando Analysis of Losses in Commercial-Sized Solid Oxide Cells: Methodology Development and Validation" Energies 15, no. 14: 4978. https://doi.org/10.3390/en15144978
APA StyleStaffolani, A., Baldinelli, A., Bidini, G., Nobili, F., & Barelli, L. (2022). Operando Analysis of Losses in Commercial-Sized Solid Oxide Cells: Methodology Development and Validation. Energies, 15(14), 4978. https://doi.org/10.3390/en15144978








