Numerical Simulation of Premixed Methane–Air Explosion in a Closed Tube with U-Type Obstacles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical Model and Mesh
2.2. Combustion Model
2.3. Boundary Conditions and Initial Conditions
2.4. Numerical Details
2.5. Numerical Verification
3. Results and Discussion
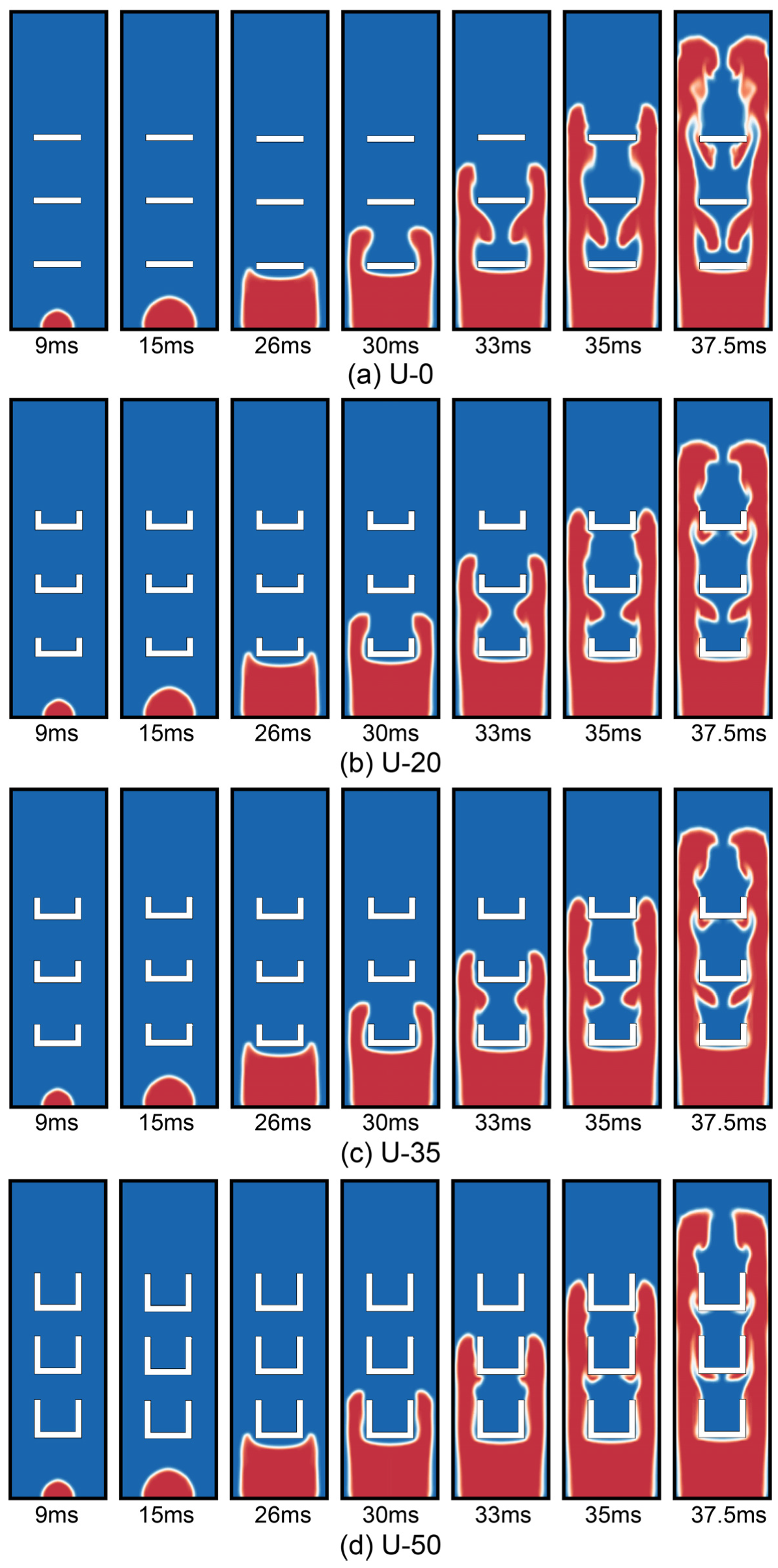
3.1. The Flame Structure
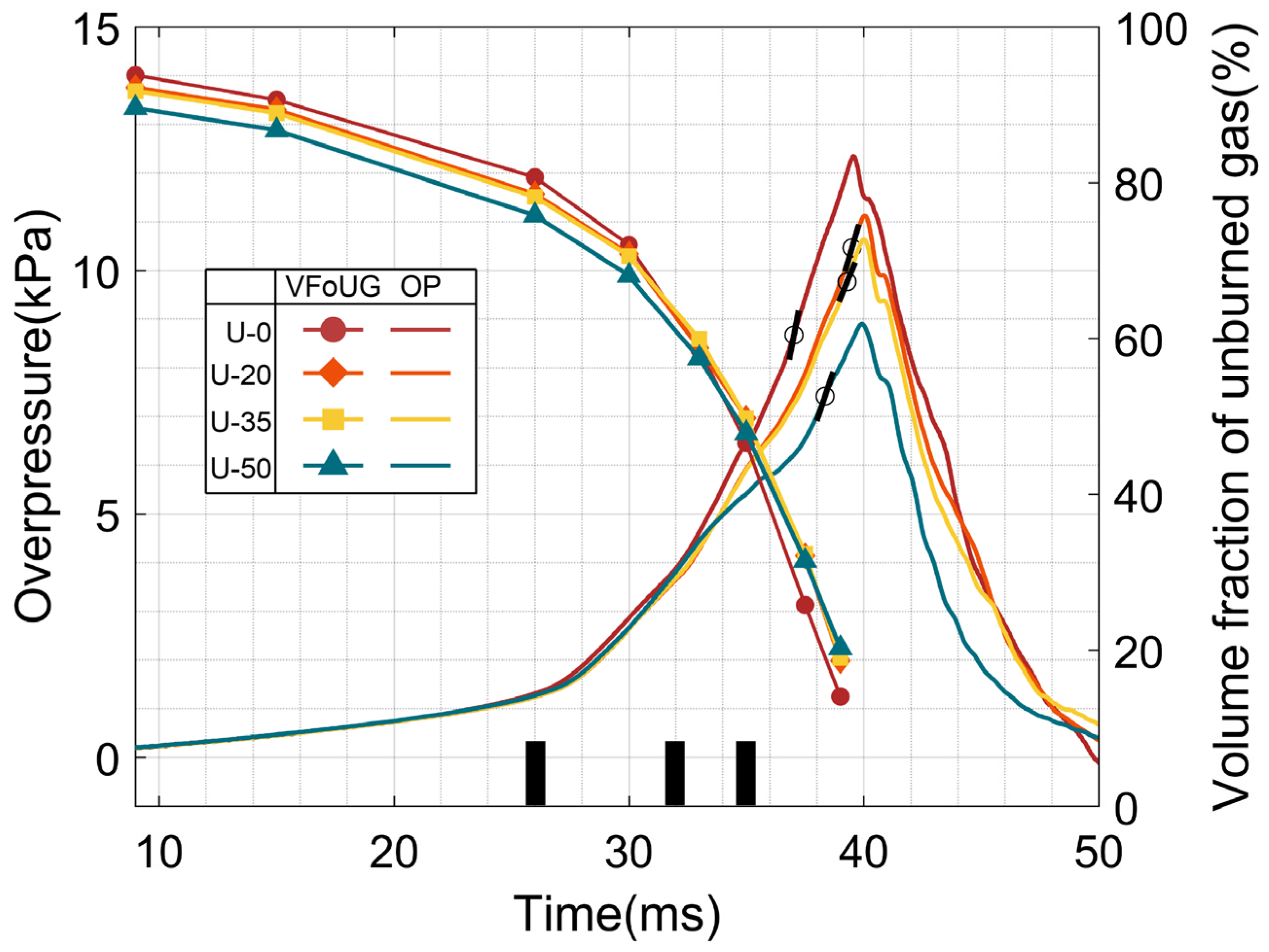
3.2. The Relation of the Flame Propagation, Velocity and Pressure
3.3. The Relation of the Unburned Premixed Gas and Pressure
4. Conclusions
- (1)
- In the process of combustion and explosion, the flame front surface that has been burned cannot access the gap between the adjacent plates in time after increasing the height of U-type obstacles, which causes the unburned premixed gas between the adjacent plates to not be burned in time. Furthermore, it also makes the burning premixed gas volume fraction in the whole tube decrease and eventually leads to an explosion overpressure value lower than that in the complete combustion.
- (2)
- The faster the volume fraction of unburned premixed gas decreases in the process of discharging explosion, the faster the explosion overpressure rises, and the maximum overpressure growth rate also appears in the period that the flame front tip completely breaks out of the tube.
- (3)
- After increasing the height of the U-type obstacles, the moving direction of the high- pressure areas between the plates is opposite to that of the flame, whereas the high pressure areas in front of the flame moves in the same direction as the flame when the flame propagation passes all obstacles.
- (4)
- The reverse flow structure of flame between the plates is the result of the coupling between the high-pressure areas induced by the combustion of unburned premixed gas and the vortex structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Liu, D.; Shen, T.; Sun, J.; Xiao, H. Effects of obstacle layout and blockage ratio on flame acceleration and DDT in hydrogen-air mixture in a channel with an array of obstacles. Int. J. Hydrogen Energy 2022, 47, 5650–5662. [Google Scholar] [CrossRef]
- Gamezo, V.N.; Bachman, C.L.; Oran, E.S. Flame acceleration and DDT in large-scale obstructed channels filled with methane-air mixtures. Proc. Combust. Inst. 2021, 38, 3521–3528. [Google Scholar] [CrossRef]
- Xu, J.; Ni, Z.; Lu, B.; Xu, J. Experimental study on explosion of gasoline-air mixture and its suppression in pipeline with large length-diameter ratio. J. Saf. Sci. Technol. 2021, 2, 77–83. [Google Scholar]
- Wang, G.; Zhang, J.; Li, D.; Chen, X. Large eddy simulation of impacted obstacles’ effects on premixed flame’s characteristics. Explos. Shock Waves 2017, 1, 68–76. [Google Scholar]
- Li, G.; Wu, J.; Wang, S.; Bai, J.; Wu, D.; Qi, S. Effects of gas concentration and obstacle location on overpressure and flame propagation characteristics of hydrocarbon fuel-air explosion in a semi-confined pipe. Fuel 2021, 285, 119268. [Google Scholar] [CrossRef]
- Zheng, K.; Song, C.; Yang, X.; Wu, J.; Jiang, J.; Xing, Z. Effect of obstacle location on explosion dynamics of premixed H2/CO/air mixtures in a closed duct. Fuel 2022, 324, 124703. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Huang, F.; Guo, S.; Liu, X. Experimental and LES investigation of premixed methane/air flame propagating in a chamber for three obstacle BR configurations. J. Loss Prev. Process Ind. 2016, 41, 48–54. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, X.; Li, Q.; Rui, S.; Wang, C.; Zhang, A. Explosion venting of hydrogen-air mixture in an obstructed rectangular tube. Fuel 2022, 310, 122473. [Google Scholar] [CrossRef]
- Du, Y.; Li, G.Q.; Wang, S.M.; Qi, S.; Li, Y.C.; Wang, B. Effects of obstacle number on characteristics of vented gasoline-air mixture explosions. CIESC J. 2017, 7, 2946–2955. [Google Scholar]
- Li, Z.; Chen, L.; Yan, H.; Fang, Q.; Zhang, Y.; Xiang, H.; Liu, Y.; Wang, S. Gas explosions of methane-air mixtures in a large-scale tube. Fuel 2021, 285, 119239. [Google Scholar] [CrossRef]
- Li, R.; Luo, Z.; Cheng, F.; Wang, T.; Lin, H.; Liu, H. A comparative investigation of premixed flame propagating of combustible gases-methane mixtures across an obstructed closed tube. Fuel 2021, 289, 119766. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, S.; Zhang, S.; Sun, H.; Huang, M. Large Eddy Simulation of Premixed CH4/Air Deflagration in a Duct with Obstacles at Different Heights. ACS Omega 2021, 6, 27140–27149. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Strebinger, C.; Bogin Jr, G.; Brune, J. A 2D CFD model investigation of the impact of obstacles and turbulence model on methane flame propagation. Process Saf. Environ. Prot. 2021, 146, 95–107. [Google Scholar] [CrossRef]
- Patel, S.; Jarvis, S.; Ibrahim, S.; Hargrave, G. An experimental and numerical investigation of premixed flame deflagration in a semiconfined explosion chamber. Proc. Combust. Inst. 2002, 29, 1849–1854. [Google Scholar] [CrossRef]
- Xu, C.; Cong, L.; Yu, Z.; Song, Z.; Bi, M. Numerical simulation of premixed methane–air deflagration in a semi-confined obstructed chamber. J. Loss Prev. Process Ind. 2015, 34, 218–224. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Guo, H.; Li, X.; Pu, X.; Yan, Z.; Zhang, P. Effects of concentration, temperature, ignition energy and relative humidity on the overpressure transients of fuel-air explosion in a medium-scale fuel tank. Fuel 2020, 259, 116265. [Google Scholar] [CrossRef]
- Wen, X.; Yu, M.; Ji, W.; Yue, M.; Chen, J. Methane–air explosion characteristics with different obstacle configurations. Int. J. Min. Sci. Technol. 2015, 25, 213–218. [Google Scholar] [CrossRef]
- Shiryanpour, I.; Kasiri-Bidhendi, N. Hydrogen flame propagation inside an obstructed chamber. Int. J. Hydrogen Energy 2019, 44, 25031–25041. [Google Scholar] [CrossRef]
- Gubba, S.R.; Ibrahim, S.S.; Malalasekera, W.; Masri, A.R. LES modeling of premixed deflagrating flames in a small-scale vented explosion chamber with a series of solid obstructions. Combust. Sci. Technol. 2008, 180, 1936–1955. [Google Scholar] [CrossRef] [Green Version]
- Na’inna, A.M.; Somuano, G.B.; Phylaktou, H.N.; Andrews, G.E. Flame acceleration in tube explosions with up to three flat-bar obstacles with variable obstacle separation distance. J. Loss Prev. Process Ind. 2015, 38, 119–124. [Google Scholar] [CrossRef]
- Li, G.; Du, Y.; Bai, J.; Wu, J.; Li, M.; Wu, X.; Zhu, L. Effects of flat obstacle channel shapes on characteristics of gasoline-air explosion. CIESC J. 2020, 4, 1912–1921. [Google Scholar]
- Sheng, Z.; Yang, G.; Li, S.; Shen, Q.; Sun, H.; Jiang, Z.; Liao, J.; Wang, H. Modeling of turbulent deflagration behaviors of premixed hydrogen-air in closed space with obstacles. Process Saf. Environ. Prot. 2022, 161, 506–519. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, K.; Chu, T. Gas explosion flame propagation over various hollow-square obstacles. J. Nat. Gas Sci. Eng. 2016, 30, 221–227. [Google Scholar] [CrossRef]
- Shiryanpour, I.; Kasiri, N. Effects of sphericity coefficient and fuel type on flame propagation inside an obstructed chamber. Eur. Phys. J. Plus 2019, 134, 240. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, S.; Mi, H.; Khan, F. Analysis of obstacle shape on gas explosion characteristics. Process Saf. Environ. Prot. 2022, 161, 78–87. [Google Scholar] [CrossRef]
- Li, G.; Du, Y.; Qi, S.; Wang, S.; Zhang, P.; Wei, S.; Li, M. Effects of obstacle position and gas concentration on gasoline-air explosion venting. CIESC J. 2017, 5, 2327–2336. [Google Scholar]
- Li, G.; Du, Y.; Qi, S.; Wang, S.; Li, M.; Li, R. Large eddy simulation on the vented gasoline-air mixture explosions in a semi-confined pipe with continuous circular hollow obstacles. Explos. Shock Waves 2018, 6, 1286–1294. [Google Scholar]
- Qin, J.; Tan, Y.; Wang, Z.; Pan, P. Experiment Study on Obstacle Shape in Pipeline Affected to Gas Explosion. Coal Sci. Technol. 2012, 2, 60–62. [Google Scholar]
- Li, X.; Wang, T.; Chen, B.; Zhou, N. Detonation characteristics of hydrogen-air premixed gas in barrier-filled pipelines. Oil Gas Storage Transp. 2021, 1–8. [Google Scholar]
- Han, S.; Yu, M.; Yang, X.; Li, H.; Ma, Z. Flame propagation mode transition of premixed syngas-air mixtures in a closed duct. Fuel 2022, 318, 123649. [Google Scholar] [CrossRef]
- Luo, G.; Tu, J.-Q.; Qian, Y.-L.; Jin, K.-K.; Ye, T.-J.; Bai, Y.; Gao, S. Impacts of Rectangular Obstacle Lengths on Premixed Methane–Air Flame Propagation in a Closed Tube. Combust. Explos. Shock Waves 2022, 58, 10–21. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, X. Study on the dynamic process of in-duct hydrogen-air explosion flame propagation under different blocking rates. Int. J. Hydrogen Energy 2022, 47, 18857–18876. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Huang, F.; Zhang, Y. LES approach to premixed methane/air flame propagation in the closed duct with a square-hole obstacle. Explos. Shock Waves 2017, 1, 21–26. [Google Scholar]
- Chen, P.; Luo, G.; Sun, Y.; Lv, Q. Impacts of plate slits on flame acceleration of premixed methane/air in a closed tube. J. Energy Inst. 2018, 91, 563–572. [Google Scholar] [CrossRef]
- Wen, X.; Yu, M.; Liu, Z.; Li, G.; Ji, W.; Xie, M. Effects of cross-wise obstacle position on methane–air deflagration characteristics. J. Loss Prev. Process Ind. 2013, 26, 1335–1340. [Google Scholar] [CrossRef]
- Xiao, H.; Shen, X.; Sun, J. Experimental study and three-dimensional simulation of premixed hydrogen/air flame propagation in a closed duct. Int. J. Hydrogen Energy 2012, 37, 11466–11473. [Google Scholar] [CrossRef]
- Zimont, V.; Battaglia, V. Joint RANS/LES approach to premixed flame modelling in the context of the TFC combustion model. Flow Turbul. Combust. 2006, 77, 305–331. [Google Scholar] [CrossRef]
- Charlette, F.; Meneveau, C.; Veynante, D. A power-law flame wrinkling model for LES of premixed turbulent combustion Part I: Non-dynamic formulation and initial tests. Combust. Flame 2002, 131, 159–180. [Google Scholar] [CrossRef]
- Ou, Y.; Li, R.; Yuan, G.; Li, G. Experimental and numerical simulation of gasoline-air mixture explosion characteristics in semi-confined space. CIESC J. 2017, 11, 4437–4444. [Google Scholar]
- Zhou, N.; Wang, W.; Zhang, G.; Wang, Z.; Zhao, H.; Yuan, X.; Huang, W. Research on Flow Field Characteristics of Propane Air Explosion in a Long Tube with Obstacles. Ind. Saf. Environ. Prot. 2018, 3, 1–4, 55. [Google Scholar]
- Wang, S.; Li, X.; Cai, Y.; Li, G.; Qi, S. Explosion venting dynamics of fuel vapor-air premixed gas based on small scale experiments. CIESC J. 2021, 9, 4961–4972. [Google Scholar]
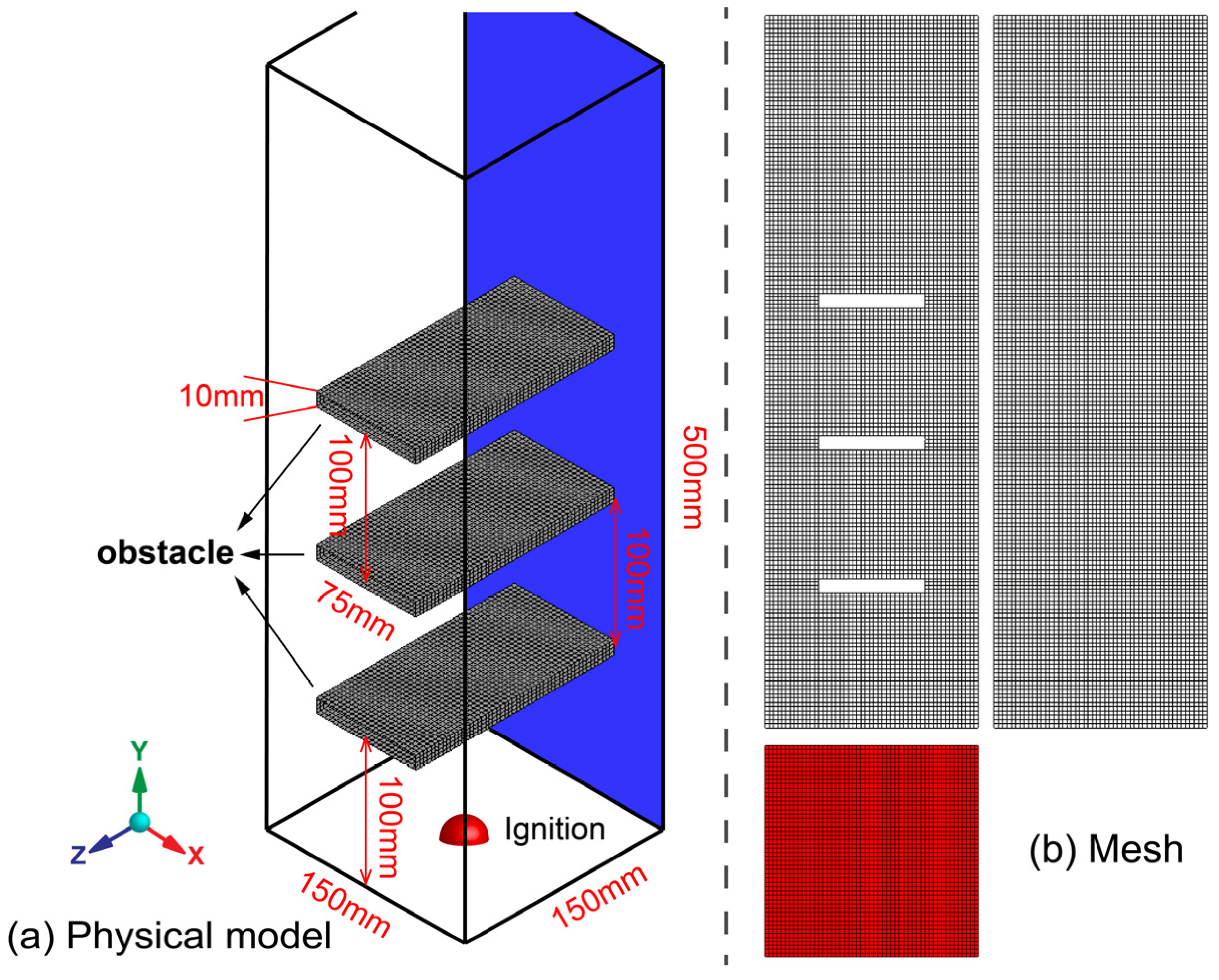
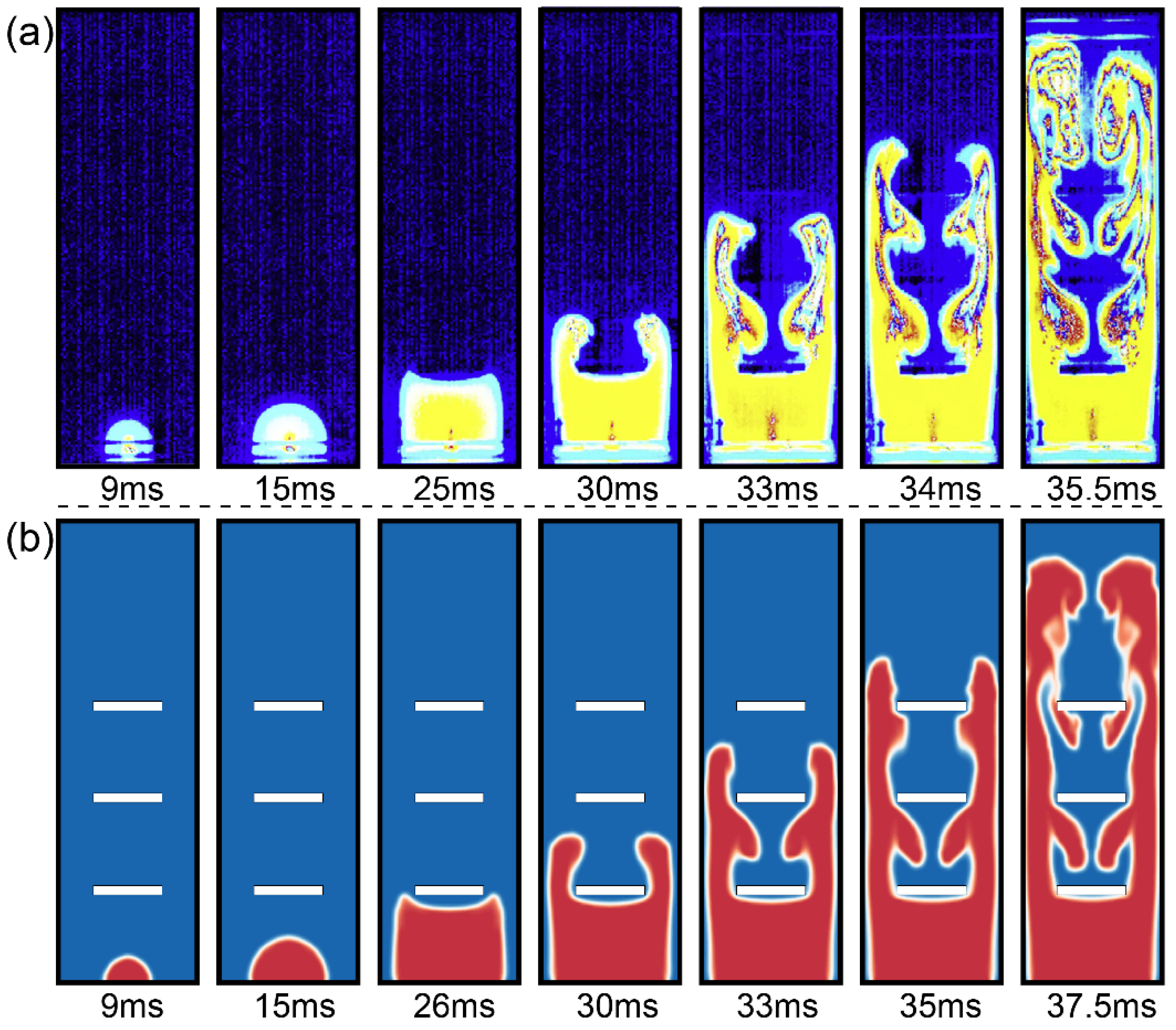
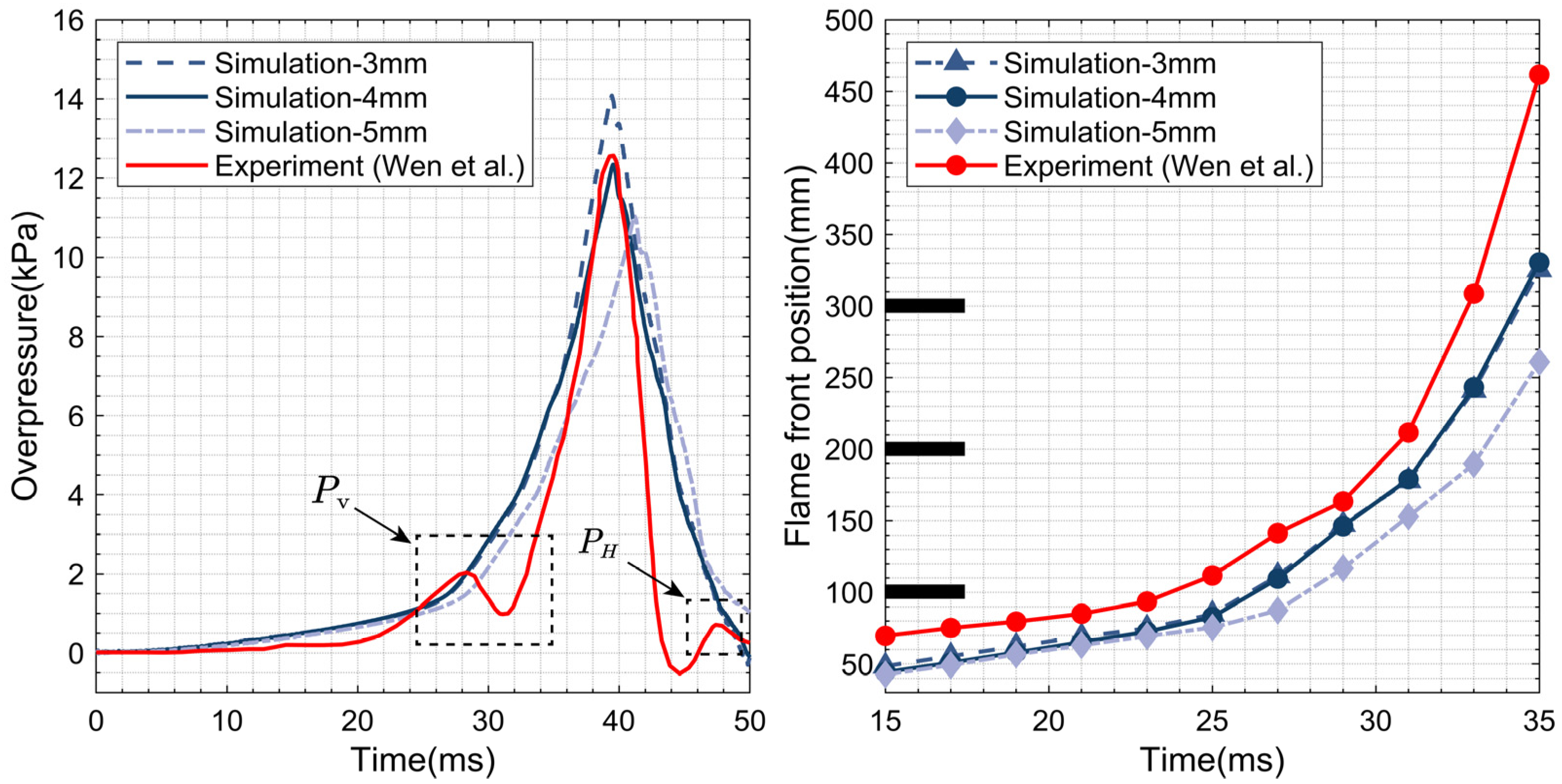
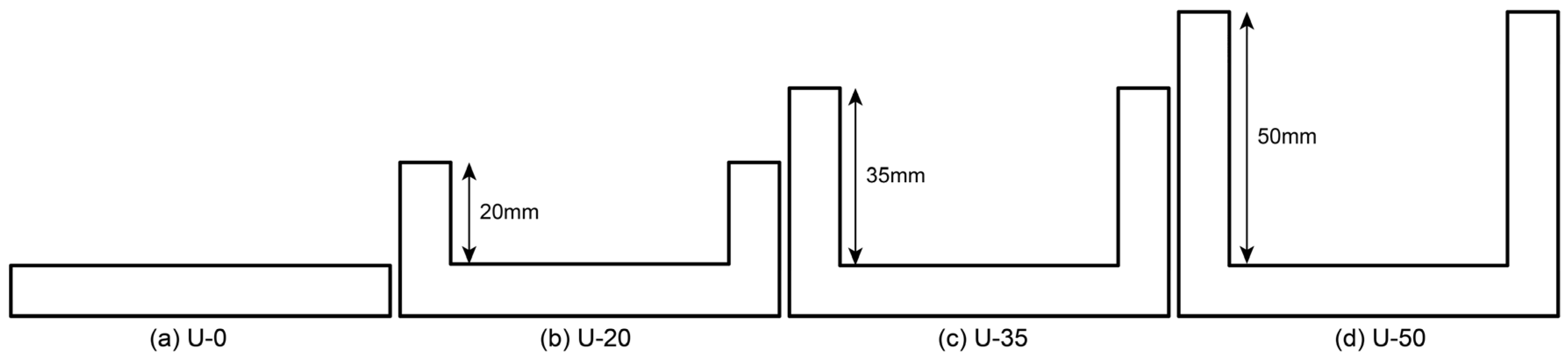

| Parameter | Value | ||
|---|---|---|---|
| Cell size | 3 mm | 4 mm | 5 mm |
| The total of hexahedral grids | 415,950 | 183,198 | 91,740 |
| The total of nodes | 439,824 | 197,028 | 100,564 |
| Angle | 90° | 90° | 90° |
| Aspect ratio | 1.0~1.2 | 1.0~1.18 | 1.0~1.5 |
| Quality | 1 | 1 | 1 |
| Boundary | Momentum | Thermal | Species | Note |
|---|---|---|---|---|
| outlet | 0.0 Pa | 300 K | 0 | Non-Reflection |
| Wall | No Slip | 0 W/m2 | - | Adiabatic |
| Parameters | Value | Parameters | Value |
|---|---|---|---|
| Heat of Combustion (J/kg) | 55,643,750 | Specific Heat (J/kg·K) | Piecewise Polynomial |
| Laminar Flame Speed (m/s) | 0.36 | Viscosity (kg/m·s) | Sutherland’ law |
| Unburnt Fuel Mass Fraction | 0.055 | Initial patch radius (mm) | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, B.; Gao, J.; Guo, B.; Ai, B.; Hong, B.; Jiang, X. Numerical Simulation of Premixed Methane–Air Explosion in a Closed Tube with U-Type Obstacles. Energies 2022, 15, 4909. https://doi.org/10.3390/en15134909
Hao B, Gao J, Guo B, Ai B, Hong B, Jiang X. Numerical Simulation of Premixed Methane–Air Explosion in a Closed Tube with U-Type Obstacles. Energies. 2022; 15(13):4909. https://doi.org/10.3390/en15134909
Chicago/Turabian StyleHao, Bin, Jianfen Gao, Bingang Guo, Bingjian Ai, Bingyuan Hong, and Xinsheng Jiang. 2022. "Numerical Simulation of Premixed Methane–Air Explosion in a Closed Tube with U-Type Obstacles" Energies 15, no. 13: 4909. https://doi.org/10.3390/en15134909
APA StyleHao, B., Gao, J., Guo, B., Ai, B., Hong, B., & Jiang, X. (2022). Numerical Simulation of Premixed Methane–Air Explosion in a Closed Tube with U-Type Obstacles. Energies, 15(13), 4909. https://doi.org/10.3390/en15134909






