Effects of Coating on the Electrochemical Performance of a Nickel-Rich Cathode Active Material
Abstract
:1. Introduction
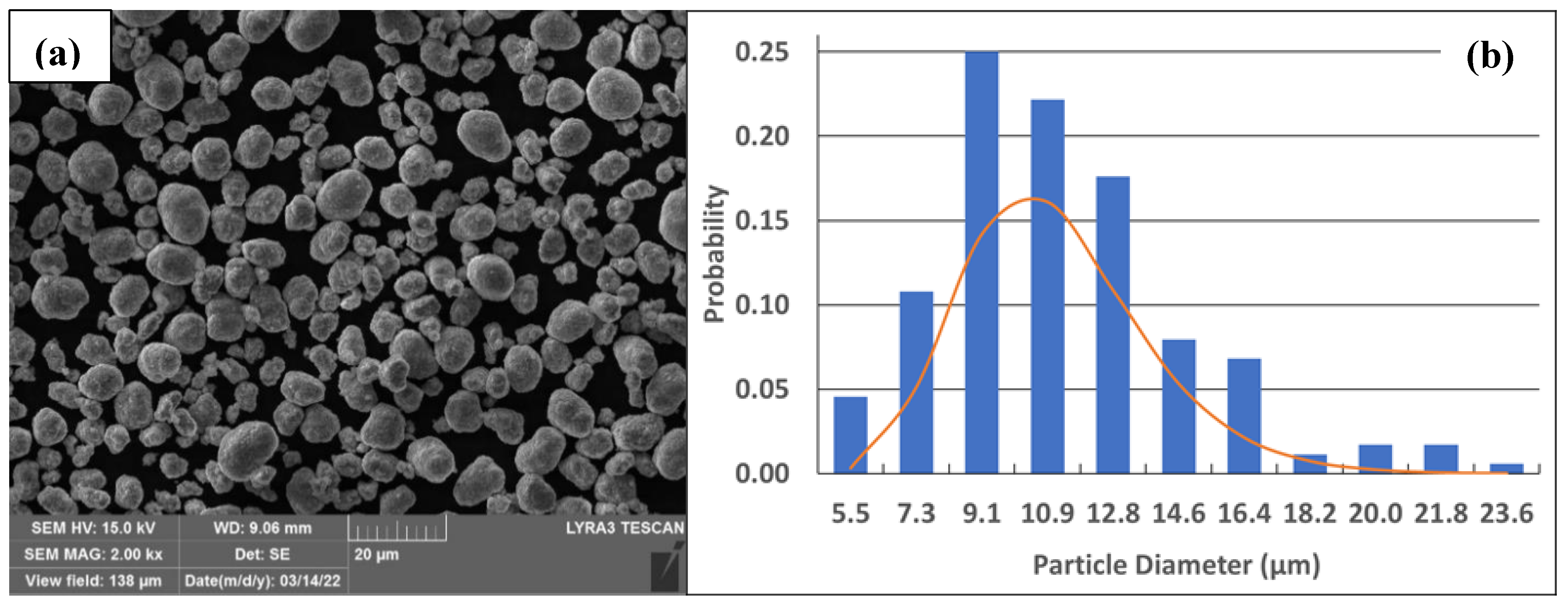
2. Materials and Methods
3. Coating Formulations
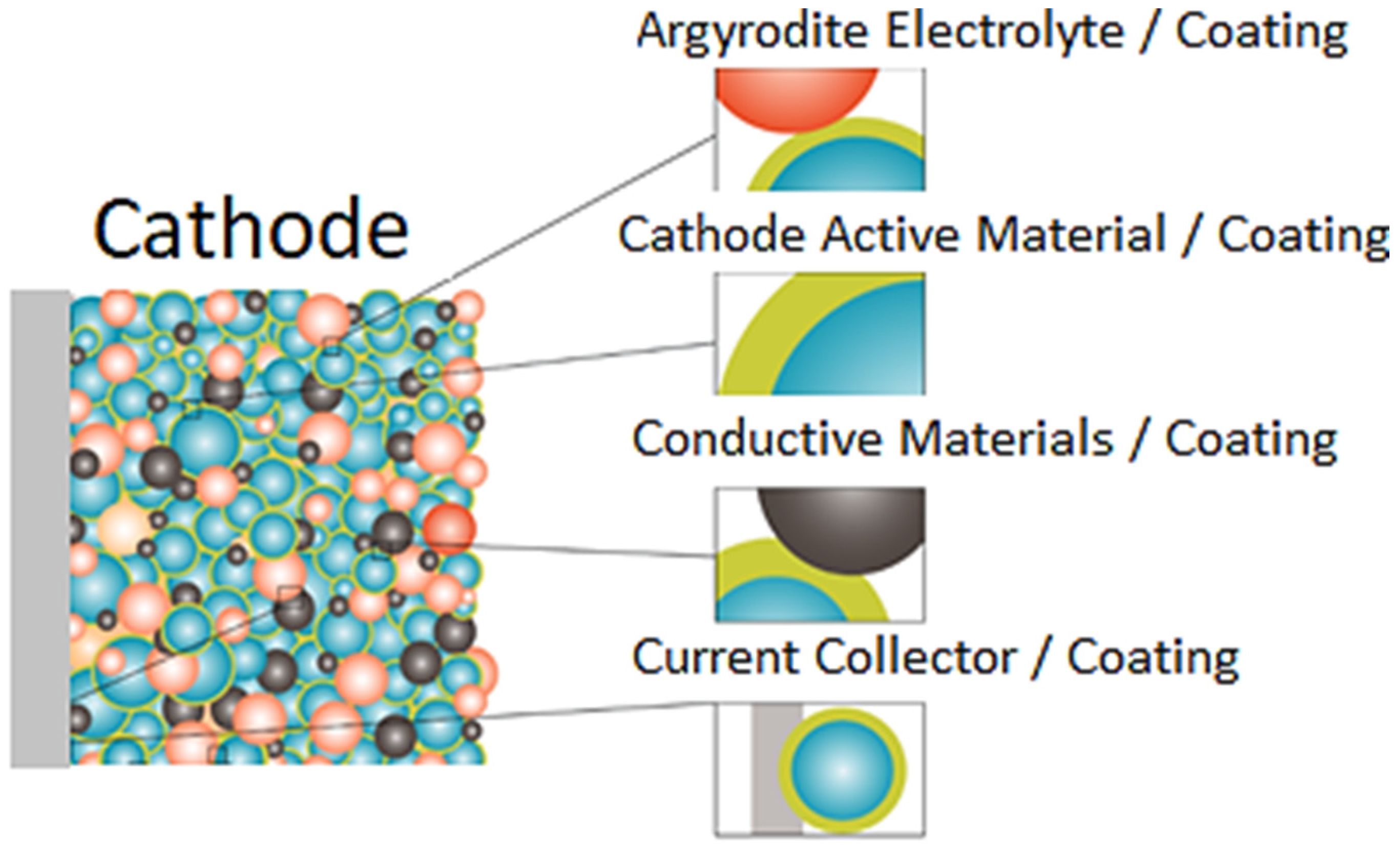
4. Electrode and Cell Fabrication and Testing
5. Results and Discussion
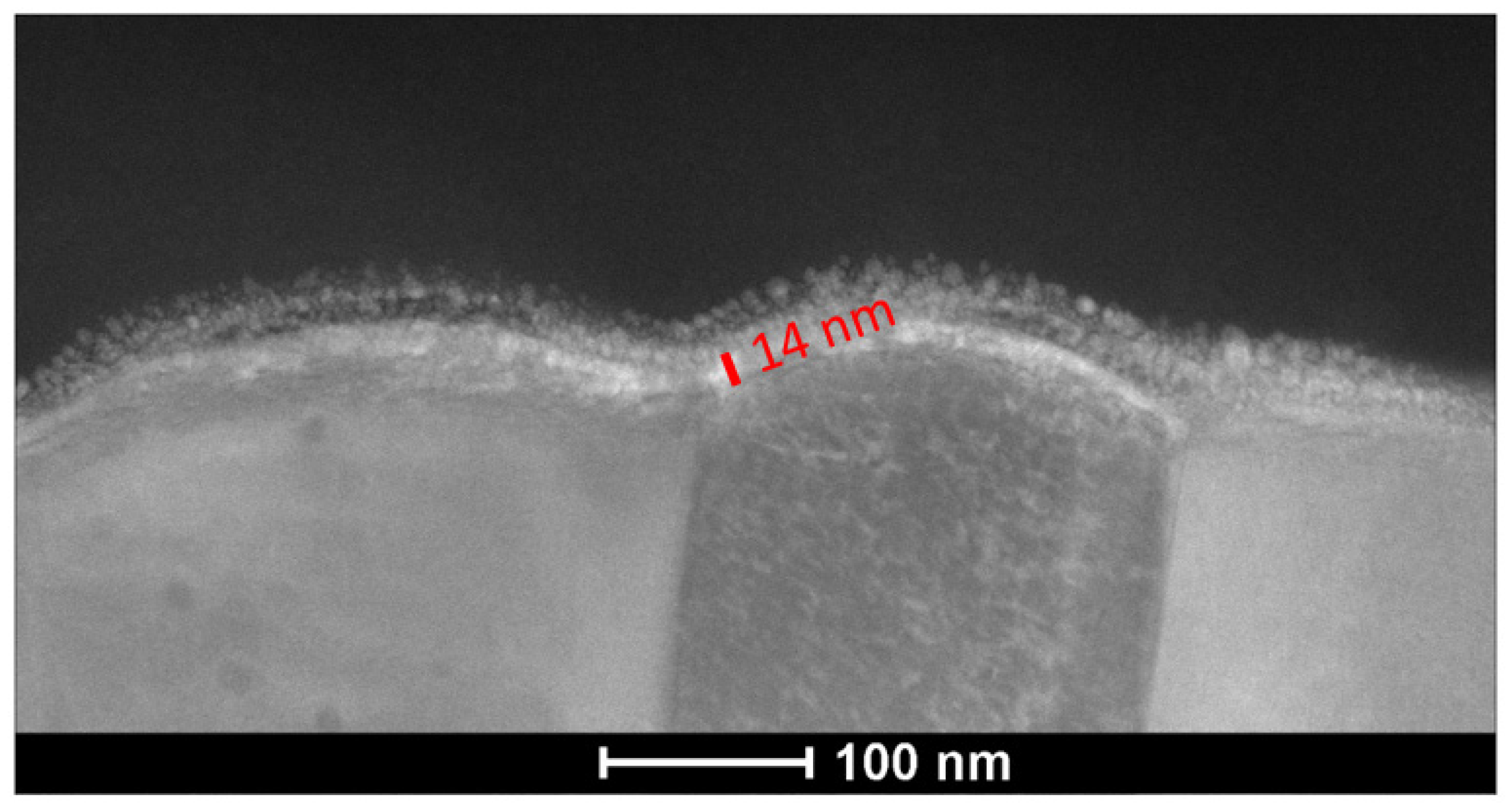
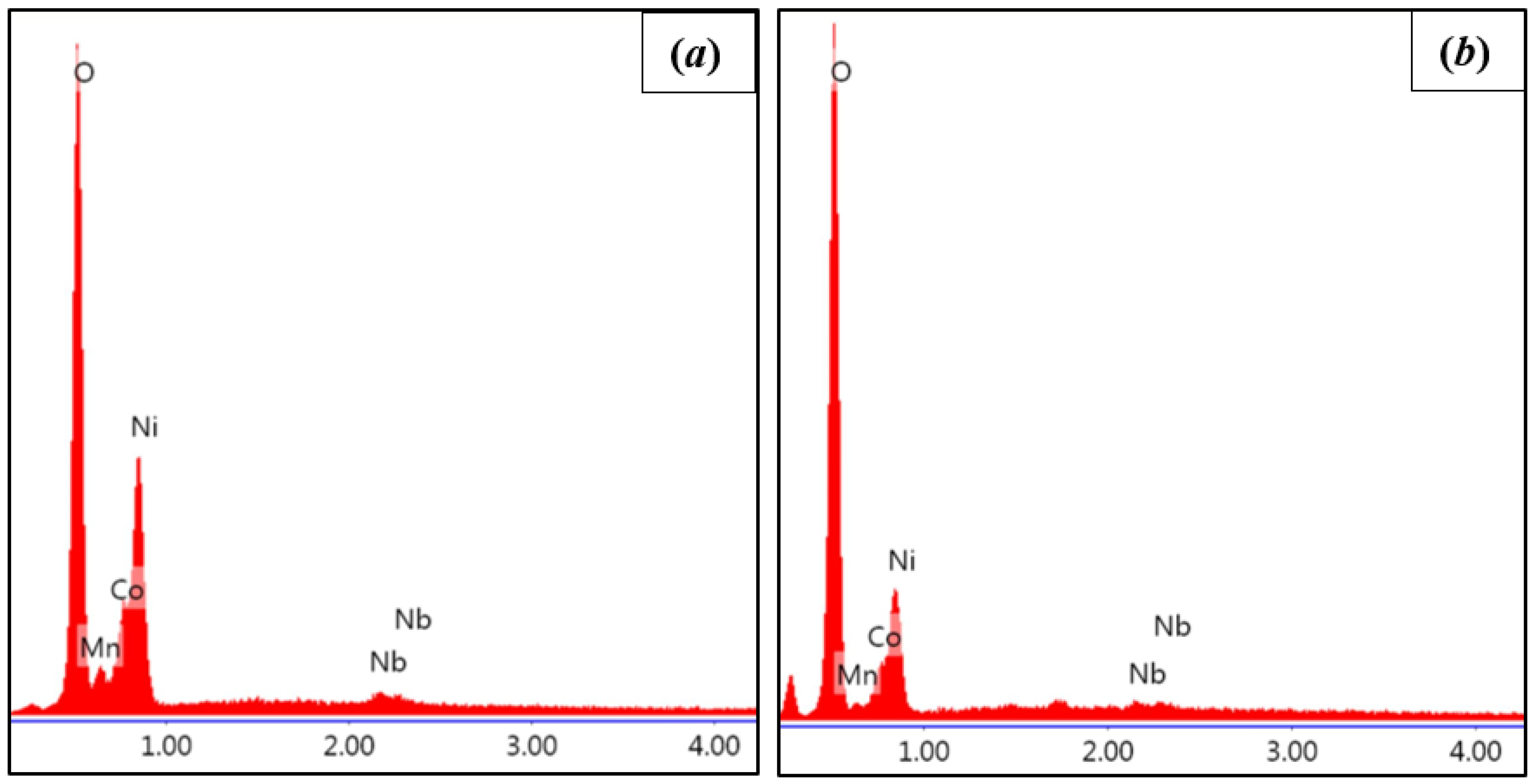
5.1. Coating Formation
5.2. Electrochemical Performance
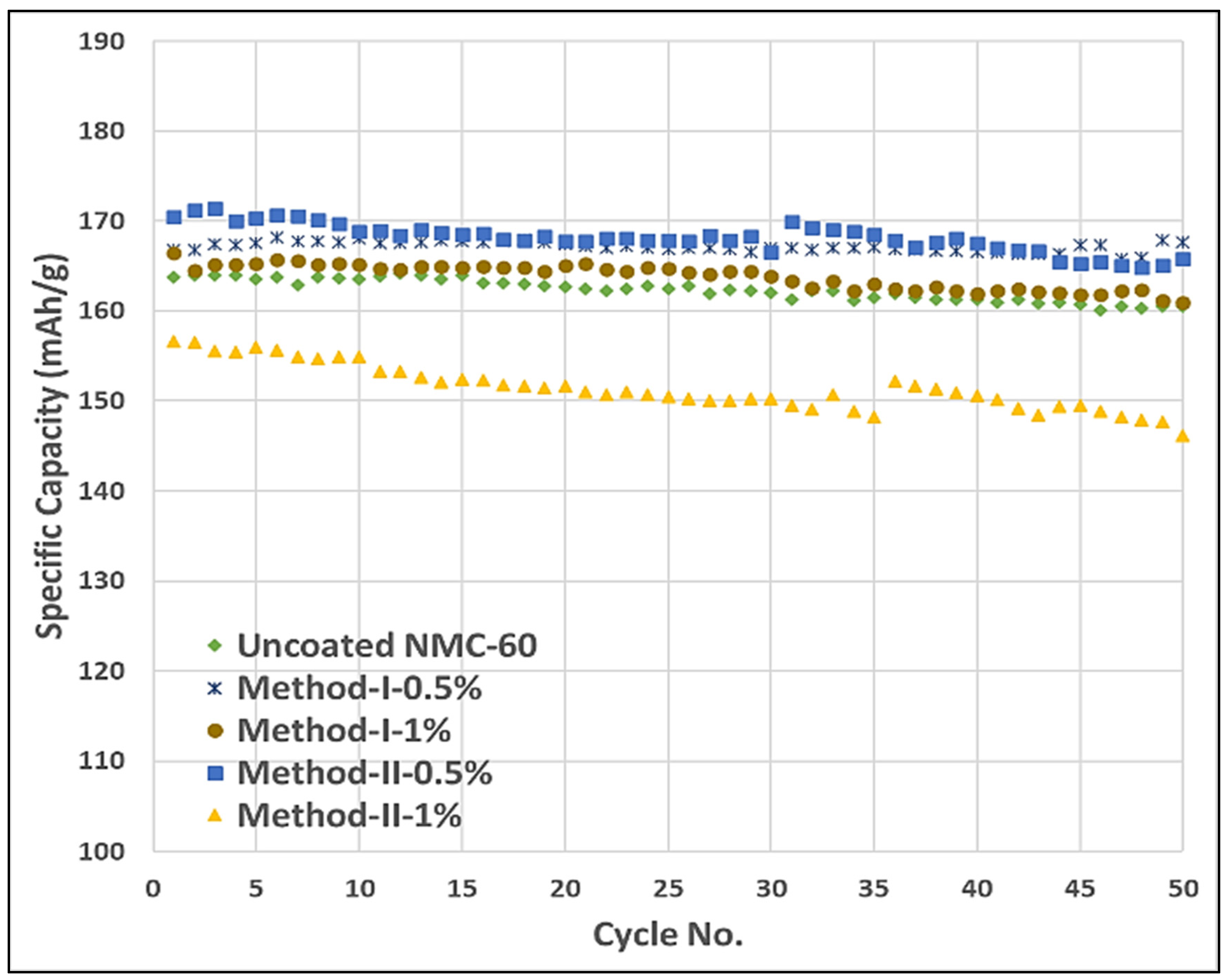
5.2.1. Cycling Performance Test
5.2.2. Rate Performance Test
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chemistry of Materials. Am. Chem. Soc. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Hao, F.; Han, F.; Liang, Y.; Wang, C.; Yao, Y. Architectural Design and Fabrication Approaches for Solid-State Batteries. MRS Bull. 2018, 43, 775–781. [Google Scholar] [CrossRef]
- Zubair, M.; Li, G.; Wang, B.; Wang, L.; Yu, H. Electrochemical Kinetics and Cycle Stability Improvement with Nb Doping for Lithium-Rich Layered Oxides. ACS Appl. Energy Mater. 2018, 2, 503–512. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Xu, Y.; Zhang, G.; Li, S.; Li, C.; Zhang, X.; Sun, X.; Ge, X.; Ma, Y. Strategies to Boost Ionic Conductivity and Interface Compatibility of Inorganic—Organic Solid Composite Electrolytes. Energy Storage Mater. 2021, 36, 291–308. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Hamilton, D.; Manjunatha, K.N.; Minakshi, M. Design, Development and Thermal Analysis of Reusable Li-Ion Battery Module for Future Mobile and Stationary Applications. Energies 2020, 13, 1477. [Google Scholar] [CrossRef] [Green Version]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational Design on Materials for Developing next Generation Lithium-Ion Secondary Battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Bo, S.H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding Interface Stability in Solid-State Batteries. Nat. Rev. Mater. 2020, 5, 105–126. [Google Scholar] [CrossRef]
- Shi, J.L.; Qi, R.; Zhang, X.D.; Wang, P.F.; Fu, W.G.; Yin, Y.X.; Xu, J.; Wan, L.J.; Guo, Y.G. High-Thermal- and Air-Stability Cathode Material with Concentration-Gradient Buffer for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42829–42835. [Google Scholar] [CrossRef]
- Ryu, W.G.; Shin, H.S.; Park, M.S.; Kim, H.; Jung, K.N.; Lee, J.W. Mitigating Storage-Induced Degradation of Ni-Rich LiNi0.8 Co0.1Mn0.1O2 Cathode Material by Surface Tuning with Phosphate. Ceram. Int. 2019, 45, 13942–13950. [Google Scholar] [CrossRef]
- Cho, D.H.; Jo, C.H.; Cho, W.; Kim, Y.J.; Yashiro, H.; Sun, Y.K.; Myung, S.T. Effect of Residual Lithium Compounds on Layer Ni-Rich Li[Ni0.7 Mn0.3]O2. J. Electrochem. Soc. 2014, 161, A920–A926. [Google Scholar] [CrossRef]
- Meng, K.; Wang, Z.; Guo, H.; Li, X.; Wang, D. Improving the Cycling Performance of LiNi0.8Co0.1Mn0.1O2 by Surface Coating with Li2TiO3. Electrochim. Acta 2016, 211, 822–831. [Google Scholar] [CrossRef]
- Song, B.; Li, W.; Oh, S.M.; Manthiram, A. Long-Life Nickel-Rich Layered Oxide Cathodes with a Uniform Li2ZrO3 Surface Coating for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 9718–9725. [Google Scholar] [CrossRef] [PubMed]
- Srur-Lavi, O.; Miikkulainen, V.; Markovsky, B.; Grinblat, J.; Talianker, M.; Fleger, Y.; Cohen-Taguri, G.; Mor, A.; Tal-Yosef, Y.; Aurbach, D. Studies of the Electrochemical Behavior of LiNi0.80Co0.15Al0.05O2 Electrodes Coated with LiAlO2. J. Electrochem. Soc. 2017, 164, A3266–A3275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.J.; Chou, S.L.; Gu, Q.F.; Liu, H.K.; Li, H.J.; Ozawa, K.; Wang, J.Z. Enhancing the High Rate Capability and Cycling Stability of LiMn2O4 by Coating of Solid-State Electrolyte LiNbO3. ACS Appl. Mater. Interfaces 2014, 6, 22155–22165. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Zhou, H.; Chen, X.; Zuba, M.; Chernova, N.; Zhou, G.; Whittingham, M.S. Li–Nb–O Coating/Substitution Enhances the Electrochemical Performance of the LiNi0.8Mn0.1Co0.1O2 (NMC 811) Cathode. ACS Appl. Mater. Interfaces 2019, 11, 34889–34894. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-Coated LiCoO2 as Cathode Material for All Solid-State Lithium Secondary Batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Gabrielli, G.; Axmann, P.; Diemant, T.; Behm, R.J.; Wohlfahrt-Mehrens, M. Combining Optimized Particle Morphology with a Niobium-Based Coating for Long Cycling-Life, High-Voltage Lithium-Ion Batteries. ChemSusChem 2016, 9, 1670–1679. [Google Scholar] [CrossRef]
- Nakamura, H.; Kawaguchi, T.; Masuyama, T.; Sakuda, A.; Saito, T.; Kuratani, K.; Ohsaki, S.; Watano, S. Dry Coating of Active Material Particles with Sulfide Solid Electrolytes for an All-Solid-State Lithium Battery. J. Power Sources 2020, 448, 227579. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Banis, M.N.; Lushington, A.; Li, R.; Cai, M.; Sun, X. Atomic Layer Deposition of Solid-State Electrolyte Coated Cathode Materials with Superior High-Voltage Cycling Behavior for Lithium Ion Battery Application. Energy Environ. Sci. 2014, 7, 768–778. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-Coated LiNi0.8Co0.1Mn0.1O2 Cathode with High Discharge Capacity and Rate Performance for All-Solid-State Lithium Battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, H.; Choi, W.; Park, M.S. Bifunctional Surface Coating of LiNbO3 on High-Ni Layered Cathode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 35098–35104. [Google Scholar] [CrossRef] [PubMed]
- Mereacre, V.; Stüble, P.; Ghamlouche, A.; Binder, J.R. Enhancing the Stability of Lini0.5Mn1.5O4 by Coating with LinbO3 Solid-State Electrolyte: Novel Chemically Activated Coating Process versus Sol-Gel Method. Nanomaterials 2021, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Mastali, M.; Samadani, E.; Farhad, S.; Fraser, R.; Fowler, M. Three-Dimensional Multi-Particle Electrochemical Model of LiFePO4 Cells Based on a Resistor Network Methodology. Electrochim. Acta 2016, 190, 574–587. [Google Scholar] [CrossRef]
- Kashkooli, A.G.; Amirfazli, A.; Farhad, S.; Lee, D.U.; Felicelli, S.; Park, H.W.; Feng, K.; De Andrade, V.; Chen, Z. Representative Volume Element Model of Lithium-Ion Battery Electrodes Based on X-Ray Nano-Tomography. J. Appl. Electrochem. 2017, 47, 281–293. [Google Scholar] [CrossRef]
- Farhad, S.; Nazari, A. Introducing the Energy Efficiency Map of Lithium-Ion Batteries. Int. J. Energy Res. 2019, 43, 931–944. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Esmaeeli, R.; Aliniagerdroudbari, H.; Alhadri, M.; Hashemi, S.R.; Nadkarni, G.; Farhad, S. Dual-Purpose Cooling Plate for Thermal Management of Prismatic Lithium-Ion Batteries during Normal Operation and Thermal Runaway. Appl. Therm. Eng. 2019, 160, 114106. [Google Scholar] [CrossRef]
- Frisone, D.; Amiriyan, M.; Hassan, E.; Dunham, J.; Farahati, R.; Farhad, S. Effect of LiNbO3 Coating on Capacity and Cycling of Nickle-Rich NMC Cathode Active Material. In Proceedings of the International Mechanical Engineering Congress and Exposition, Virtual, Online, 4 November 2021. [Google Scholar]








| Initial Capacity (mAhg−1) | Capacity at 2C (mAhg−1) | Capacity Retention at 2C (%) | Capacity upon Return to C/10 (mAhg−1) | Capacity Retention at C/10 (%) | |
|---|---|---|---|---|---|
| Uncoated NMC-60 | 167.6 | 146.1 | 87.2 | 172.6 | 103.1 |
| Method-I-0.5% | 167.3 | 140.0 | 83.7 | 175.6 | 105.0 |
| Method-I-1% | 168.9 | 148.7 | 88.0 | 174.1 | 103.1 |
| Method-II-0.5% | 176.9 | 154.8 | 87.5 | 178.8 | 101.1 |
| Method-II-1% | 159.1 | 135.4 | 85.1 | 163.2 | 102.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, E.; Amiriyan, M.; Frisone, D.; Dunham, J.; Farahati, R.; Farhad, S. Effects of Coating on the Electrochemical Performance of a Nickel-Rich Cathode Active Material. Energies 2022, 15, 4886. https://doi.org/10.3390/en15134886
Hassan E, Amiriyan M, Frisone D, Dunham J, Farahati R, Farhad S. Effects of Coating on the Electrochemical Performance of a Nickel-Rich Cathode Active Material. Energies. 2022; 15(13):4886. https://doi.org/10.3390/en15134886
Chicago/Turabian StyleHassan, Eman, Mahdi Amiriyan, Dominic Frisone, Joshua Dunham, Rashid Farahati, and Siamak Farhad. 2022. "Effects of Coating on the Electrochemical Performance of a Nickel-Rich Cathode Active Material" Energies 15, no. 13: 4886. https://doi.org/10.3390/en15134886
APA StyleHassan, E., Amiriyan, M., Frisone, D., Dunham, J., Farahati, R., & Farhad, S. (2022). Effects of Coating on the Electrochemical Performance of a Nickel-Rich Cathode Active Material. Energies, 15(13), 4886. https://doi.org/10.3390/en15134886







