Charcoal Production in Portugal: Operating Conditions and Performance of a Traditional Brick Kiln
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wood Feedstocks
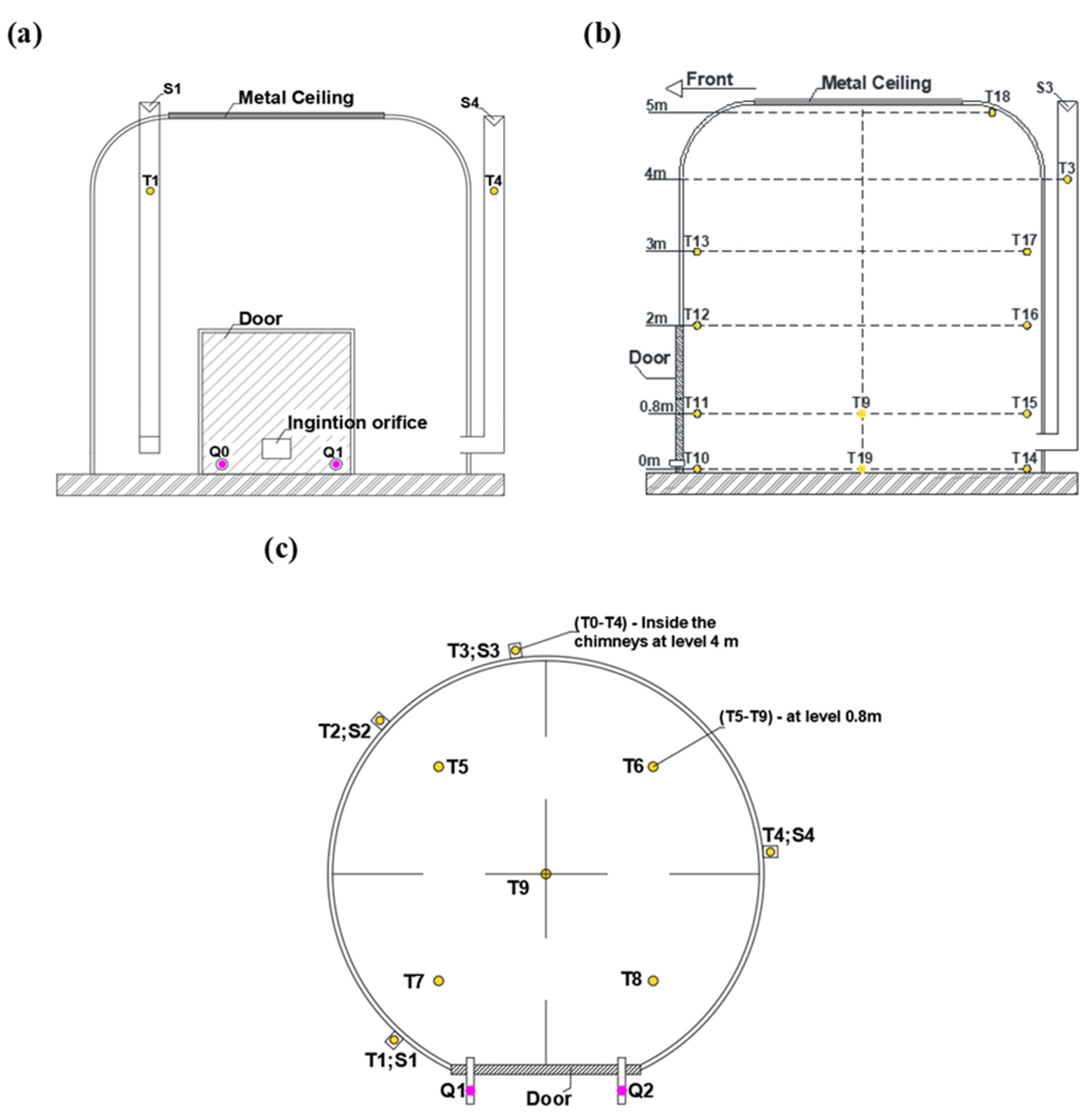
2.2. Carbonization Kiln
2.3. Carbonization Procedure
2.4. Measurements
2.4.1. Mass of the Wood Charge
2.4.2. Temperatures
2.4.3. Airflow Rate
2.4.4. Composition of the Carbonization Gas
2.4.5. Yields and Properties of the Carbonization Solid Products
2.5. Data Acquisition
2.6. Process Conditions
3. Results and Discussion
3.1. Temperature-Time Profiles
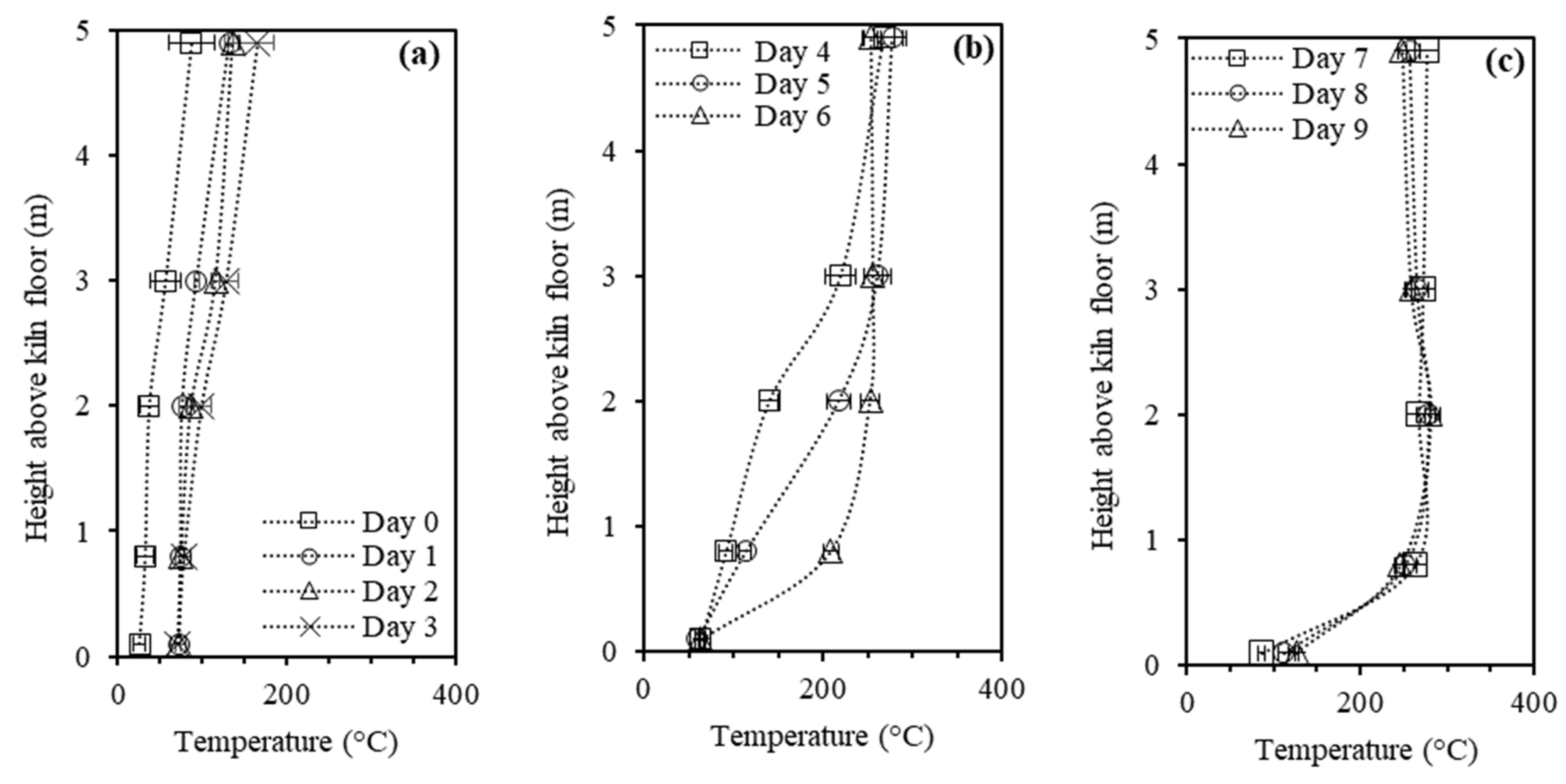
3.2. Vertical Temperature Profiles
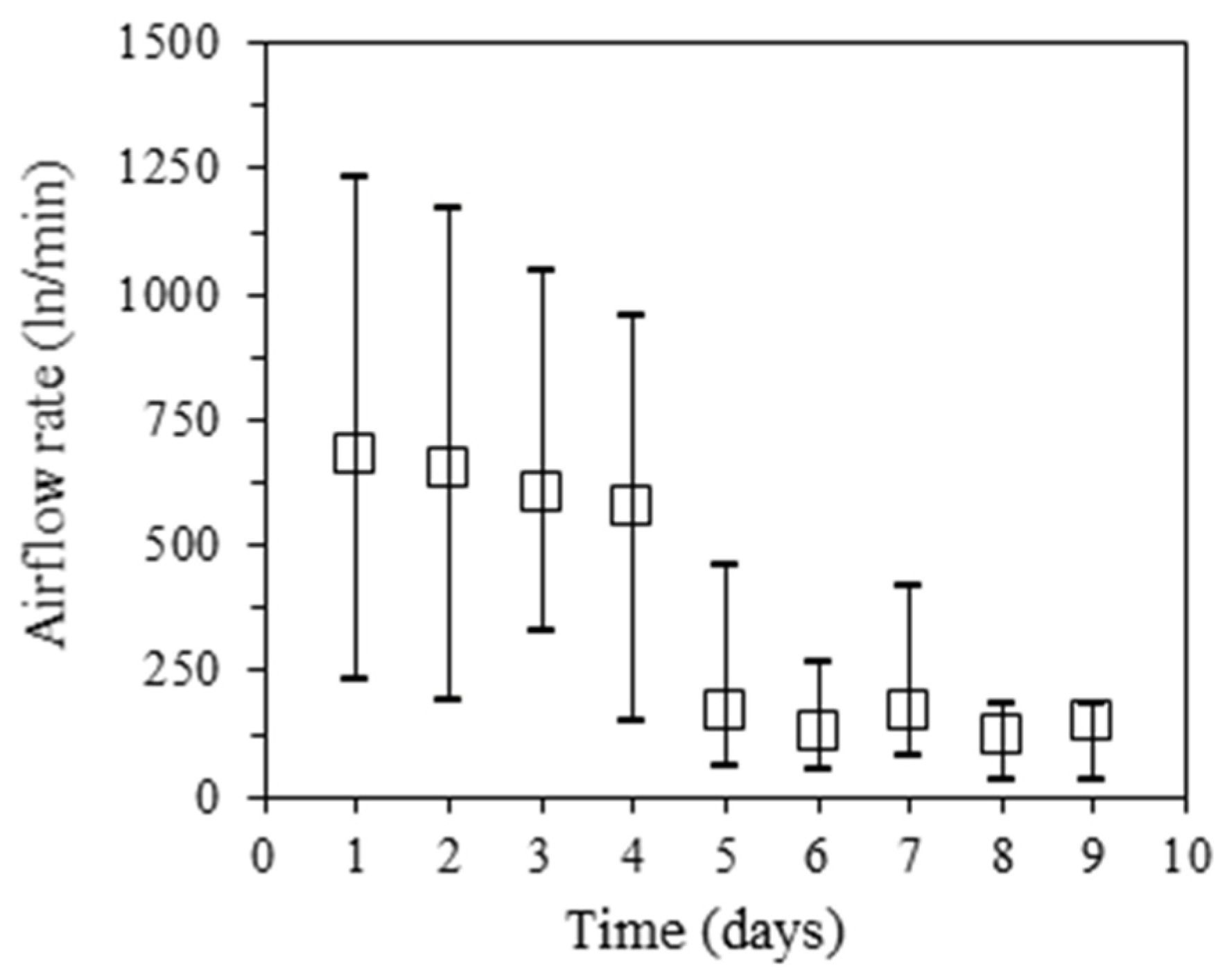
3.3. Intake Airflow Rate
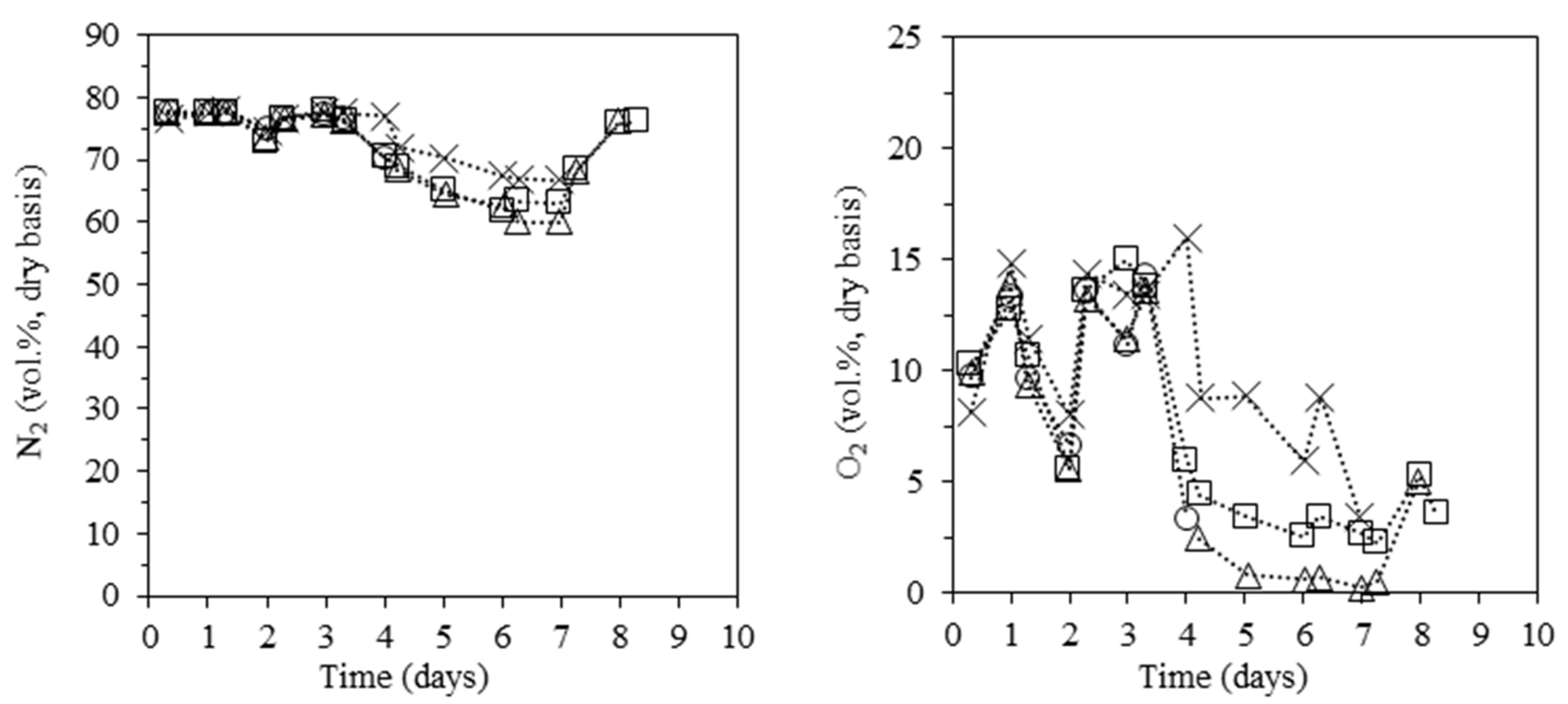
3.4. Composition of the Carbonization Gas
3.5. Properties of the Carbonization Solid Products
3.6. Overall Mass Balance to the Carbonization Process
4. Conclusions
- Charcoal production in traditional brick kilns is a flexible process in terms of the size and properties of the wood raw materials—both small branches and large logs can be used, as well as wood containing bark, inert materials (e.g., soil and stones associated with the stumps), and partially charred materials—which underlines its usefulness in the context of forest waste management.
- Current practices of process control and monitoring are limited to visual observations—e.g., the color of the smoke leaving the chimneys—which makes it difficult to continuously follow the process (e.g., during night periods and weekends) and early detectoperational problems, chiefly combustion hotspots caused by uncontrolled temperatures (sometimes exceeding 800 °C) and air intake, with consequent loss of wood and charcoal.
- Improper design aspects of the kiln further reduce its performance, including (i) lack of insulation in the metal ceiling, (ii) build-up of liquids on the kiln floor because of insufficient drainage, (iii) easy clogging of the air inlets by ash accumulation, (iv) unknown air inlets, including through the chimneys and/or wall cracks, and (v) inadequate number, layout, and size of the chimneys. The large size of the kiln can also hinder productivity as it leads to longer production cycles (>20 days)—associated with the wood drying and heating (>4 days) and charcoal cooling (>10 days) phases—and extended oxidation of charcoal inside the kiln.
- Inappropriate carbonization methods are also in use, of which the following are highlighted given their impact on the charcoal yield: (i) the ignition/heating method that is based on the continuous and uncontrolled combustion of the wood charge inside the kiln, (ii) the kiln charging method that favors the placing of thicker wood pieces on the kiln floor where temperatures are lower and carbonization more difficult, and (iii) the kiln-sealing method that follows an indefinite sequence of closing the chimneys and air intakes (sometimes for two consecutive days) that seems to allow excessive burning of charcoal.
- The overall performance of the kiln is poor in terms of charcoal yield (≈23 wt.%) and quality, the latter mainly evidenced by its low fixed carbon contents (values as low as 60 wt.% are possible). This follows from key limitations in the kiln: the low maximum temperatures observed in most of the carbonization chamber (typically less than 300 °C), the inability to raise the temperature at the kiln floor above 130 °C, and the high O2 levels inside the kiln (often above 5 vol.%). Then significant amounts of byproducts are obtained: partially charred wood, charcoal fines, permanent gases, and liquids. While the partially charred wood is reused in the process and the charcoal fines marketed (mainly for briquette manufacturing), the gases and liquids are currently discharged into the atmosphere and soil. The gas composition is most favorable between days 4 and 7 of the cycle, where the concentration of combustible species (mainly, H2, CH4, and CO) and the LHV of the gas is higher; at this stage, the carbonization gas can approach the quality of gases from direct biomass gasifiers. Otherwise, the gas quality is very low, which can halt valorization, although it needs some kind of treatment before discharge into the atmosphere.
- Charcoal consumers are increasingly aware of the quality and environmental issues associated with this activity and initiatives addressing both production certification and charcoal quality requirements are rising. It is then important to take urgent action to modernize the charcoal production activities and the market suitability of both charcoal and byproducts. The focus should be on research and technological development, funding, training, and administrative and legal frameworks to find solutions tailored to current activities and available infrastructure. These will be decisive to prevent charcoal production from vanishing from Portugal and other EU countries, where it is still an important activity, both for economic reasons and for forest biomass management reasons.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Emrich, W. Handbook of Charcoal Making: The Traditional and Industrial; D. Reidel: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Sun, M.; Zhang, J.; Li, K.; Barati, M.; Liu, Z. Co-gasification characteristics of coke blended with hydro-char and pyro-char from bamboo. Energy 2022, 241, 122890. [Google Scholar] [CrossRef]
- Dufourny, A.; Van De Steene, L.; Humbert, G.; Guibal, D.; Martin, L.; Blin, J. Influence of pyrolysis conditions and the nature of the wood on the quality of charcoal as a reducing agent. J. Anal. Appl. Pyrolysis 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Hammani, H.; Hrioua, A.; Aghris, S.; Lahrich, S.; Saqrane, S.; Bakasse, M.; El Mhammedi, M.A. Activated charcoal as a capture material for dopamine, paracetamol and salicylic acid in human blood and pharmaceutical formulations. Mater. Chem. Phys. 2020, 240, 122111. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Jelonek, Z.; Drobniak, A.; Mastalerz, M.; Jelonek, I. Environmental implications of the quality of charcoal briquettes and lump charcoal used for grilling. Sci. Total Environ. 2020, 747, 141267. [Google Scholar] [CrossRef]
- Van Swaaij, W.P.M.; Kersten, S.R.A.; Palz, W. (Eds.) Biomass Power for the World, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2015; ISBN 9780429183836. [Google Scholar]
- Vicente, E.D.; Vicente, A.; Evtyugina, M.; Carvalho, R.; Tarelho, L.A.C.; Oduber, F.I.; Alves, C. Particulate and gaseous emissions from charcoal combustion in barbecue grills. Fuel Process. Technol. 2018, 176, 296–306. [Google Scholar] [CrossRef]
- WorldWildlife Fund (WWF). The Dirty Business of Barbecue Charcoal; WWF Germany: Berlin, Germany, 2018; Available online: https://www.wwf.de/fileadmin/fm-wwf/Publikationen-PDF/WWF_Market_analysis_barbecue_charcoal_2018.pdf (accessed on 28 January 2022).
- Knapp, H.; Nelle, O.; Kirleis, W. Charcoal usage in medieval and modern times in the Harz Mountains Area, Central Germany: Wood selection and fast overexploitation of the woodlands. Quat. Int. 2015, 366, 51–69. [Google Scholar] [CrossRef]
- Carrari, E.; Ampoorter, E.; Bottalico, F.; Chirici, G.; Coppi, A.; Travaglini, D.; Verheyen, K.; Selvi, F. The old charcoal kiln sites in Central Italian forest landscapes. Quat. Int. 2017, 458, 214–223. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Forestry Production and Trade—Wood Charcoal. Available online: http://www.fao.org/faostat/en/#data/FO (accessed on 31 January 2022).
- Dam, J.V. The Charcoal Transition: Greening the Charcoal Value Chain to Mitigate Climate Change and Improve Local Livelihoods; Food and Agriculture Organization (FAO): Rome, Italy, 2017; ISBN 9789251096802. [Google Scholar]
- Zhao, C.; Jiang, E.; Chen, A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Pettorali, M.; Nistri, R.; Chiaramonti, D. Mass and energy balances of an autothermal pilot carbonization unit. Biomass Bioenergy 2019, 120, 144–155. [Google Scholar] [CrossRef]
- Demirbaş, A. Relationships between carbonization temperature and pyrolysis products from biomass. Energy Explor. Exploit. 2004, 22, 411–420. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Rodrigues, T.; Braghini Junior, A. Charcoal: A discussion on carbonization kilns. J. Anal. Appl. Pyrolysis 2019, 143, 104670. [Google Scholar] [CrossRef]
- Rodrigues, T.; Braghini Junior, A. Technological prospecting in the production of charcoal: A patent study. Renew. Sustain. Energy Rev. 2019, 111, 170–183. [Google Scholar] [CrossRef]
- Santos, S.D.F.D.O.; Piekarski, C.M.; Ugaya, C.M.L.; Donato, D.B.; Braghini, A.; de Francisco, A.C.; Carvalho, A.M.M.L. Life cycle analysis of charcoal production in masonry kilns with and without carbonization process generated gas combustion. Sustainbility 2017, 9, 1558. [Google Scholar] [CrossRef] [Green Version]
- Pyshyev, S.; Miroshnichenko, D.; Malik, I.; Bautista Contreras, A.; Hassan, N.; Abd Elrasoul, A. State of the art in the production of charcoal: A review. Chem. Chem. Technol. 2021, 15, 61–73. [Google Scholar] [CrossRef]
- Chidumayo, E.N.; Gumbo, D.J. The environmental impacts of charcoal production in tropical ecosystems of the world: A synthesis. Energy Sustain. Dev. 2013, 17, 86–94. [Google Scholar] [CrossRef]
- Sparrevik, M.; Cornelissen, G.; Sparrevik, M.; Adam, C.; Martinsen, V.; Cornelissen, G.; Cornelissen, G. Emissions of gases and particles from charcoal/biochar production in rural areas using medium-sized traditional and improved “retort” kilns. Biomass Bioenergy 2015, 72, 65–73. [Google Scholar] [CrossRef]
- EN1860-2-2005:E; Appliances, Solid Fuels and Firelighters for Barbecueing—Part 2: Barbecue Charcoal and Barbecue Charcoal Briquettes—Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- Bustamante-García, V.; Carrillo-Parra, A.; González-Rodríguez, H.; Ramírez-Lozano, R.G.; Corral-Rivas, J.J.; Garza-Ocañas, F. Evaluation of a charcoal production process from forest residues of Quercus sideroxyla Humb., & Bonpl. in a Brazilian beehive kiln. Ind. Crops Prod. 2013, 42, 169–174. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Carneiro, A.D.C.O.; Pereira, B.L.C.; Vital, B.R.; Carvalho, A.M.M.L.; Trugilho, P.F.; Damásio, R.A.P. Otimização da produção do carvão vegetal por meio do controle de temperaturas de carbonização. Rev. Arvore 2013, 37, 557–566. [Google Scholar] [CrossRef]
- Cardoso, M.T.; Damásio, R.A.P.; Carneiro, A.D.C.O.; Jacovine, L.A.G.; Vital, B.R.; Barcelos, D.C. Construção De Um Sistema De Queima De Gases Da Carbonização Para Redução Da Emissão De Poluentes. Cerne 2010, 16, 115–124. [Google Scholar]
- Bustos-Vanegas, J.D.; Martins, M.A.; Carneiro, A.D.C.O.; Freitas, A.G.; Barbosa, R.C. Thermal inertia effects of the structural elements in heat losses during the charcoal production in brick kilns. Fuel 2018, 226, 508–515. [Google Scholar] [CrossRef]
- Mensah, K.E.; Damnyag, L.; Kwabena, N.S. Analysis of charcoal production with recent developments in Sub-Sahara Africa: A review. Afr. Geogr. Rev. 2022, 41, 35–55. [Google Scholar] [CrossRef]
- Chiteculo, V.; Lojka, B.; Surový, P.; Verner, V.; Panagiotidis, D.; Woitsch, J. Value chain of charcoal production and implications for forest degradation: Case study of Bié Province, Angola. Environments 2018, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Ekeh, O.; Fangmeier, A.; Müller, J. Quantifying greenhouse gases from the production, transportation and utilization of charcoal in developing countries: A case study of Kampala, Uganda. Int. J. Life Cycle Assess. 2014, 19, 1643–1652. [Google Scholar] [CrossRef]
- Tintner, J.; Preimesberger, C.; Pfeifer, C.; Theiner, J.; Ottner, F.; Wriessnig, K.; Puchberger, M.; Smidt, E. Pyrolysis profile of a rectangular kiln—Natural scientific investigation of a traditional charcoal production process. J. Anal. Appl. Pyrolysis 2020, 146, 104757. [Google Scholar] [CrossRef]
- Tintner, J.; Fierlinger, R.; Gerzabek, H.; Pfeifer, C.; Smidt, E. Pyrolysis profiles of a traditional circular kiln in Austria and a drum kiln in Namibia. J. Anal. Appl. Pyrolysis 2020, 150, 104865. [Google Scholar] [CrossRef]
- Silva, F.T.M.; Silva, J.J.F.; Silva, D.; Tarelho, L.A.C.; Matos, M.A.A.; Neves, D. Charcoal production infrastructure in Portalegre district (Portugal): First assessment from satellite imagery and field observations. In Proceedings of the 3rd Bioenergy International Conference, Portalegre, Portugal, 11–13 September 2019. [Google Scholar]
- Neves, D.; Charvet, F.; Ruivo, L.; Matos, A.; Tarelho, L.; Figueiredo Silva, J. Current practices of charcoal production in Southern Portugal: A case-study un a large production site. In Proceedings of the 29th European Biomass Conference and Exhibition, Virtual, Online, 26–29 April 2021; pp. 1026–1032. [Google Scholar]
- Dias, E.C.; Alejandro, H.; Prais, C. Processo de trabalho e saúde dos trabalhadores na produção artesanal de carvão vegetal em Minas Gerais, Brasil Labor process and workers’ health in charcoal production in Minas Gerais, Brazil. Cadernos de Saúde Pública 2002, 18, 269–277. [Google Scholar] [CrossRef]
- Doggart, N.; Meshack, C. The marginalization of sustainable charcoal production in the policies of a modernizing African Nation. Front. Environ. Sci. 2017, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Tassie, K.; Misganaw, B.; Addisu, S.; Tesfaye, E. Socioeconomic and Environmental Impacts of Charcoal Production Activities of Rural Households in Mecha District, Ethiopia. Adv. Agric. 2021, 2021, 6612720. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Claude-Alain, R.; Foradini, F. Simple and Cheap Air Change Rate Measurement Using CO2 Concentration Decays. Int. J. Vent. 2002, 1, 39–44. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Boles, M.A. Thermodynamics: An Engineering Approach; McGraw-Hill, Inc.: New York, NY, USA, 2006; ISBN 9780070606593. [Google Scholar]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chen, W.H.; Colin, B.; Pétrissans, A.; Lopes Quirino, R.; Pétrissans, M. Thermodegradation characterization of hardwoods and softwoods in torrefaction and transition zone between torrefaction and pyrolysis. Fuel 2022, 310, 122281. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Masson, E.; Celzard, A.; Fierro, V. Modelling the reactions of cellulose, hemicellulose and lignin submitted to hydrothermal treatment. Ind. Crops Prod. 2018, 124, 919–930. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C.; Matos, M.A.A. Characteristics of the gas produced during biomass direct gasification in an autothermal pilot-scale bubbling fluidized bed reactor. Energy 2017, 120, 915–928. [Google Scholar] [CrossRef]
- Neves, D.; Matos, A.; Tarelho, L.; Thunman, H.; Larsson, A.; Seemann, M. Volatile gases from biomass pyrolysis under conditions relevant for fluidized bed gasifiers. J. Anal. Appl. Pyrolysis 2017, 127, 57–67. [Google Scholar] [CrossRef]
- Shi, Y.; Chrusciel, L.; Zoulalian, A. Production of charcoal from different wood species. Récents Progrès en Génie des Procédés 2008, 96, 9. [Google Scholar]
- Charvet, F.; Silva, F.; Ruivo, L.; Tarelho, L.; Matos, A.; Figueiredo da Silva, J.; Neves, D. Pyrolysis Characteristics of Undervalued Wood Varieties in the Portuguese Charcoal Sector. Energies 2021, 14, 2537. [Google Scholar] [CrossRef]
- Schure, J.; Pinta, F. Efficiency of charcoal production in Sub-Saharan Africa: Solutions beyond the kiln. Bois et Forêts des Tropiques 2019, 340, 57–70. [Google Scholar] [CrossRef]




| Wood Feedstock (a) | Volatile Matter | Ash | Fixed Carbon | Elemental Carbon | O/C | H/C | LHV (b) |
|---|---|---|---|---|---|---|---|
| [wt.%] | [wt.%] | [wt.%] | [wt.%] | [kg/kg] | [kg/kg] | [MJ/kg] | |
| Holm Oak—branches | 82.7 ± 2.7 | 0.96 ± 0.04 | 16.3 ± 2.7 | 47.3 ± 0.4 | 0.95 ± 0.02 | 0.14 ± 0.004 | 17.1 ± 1.3 |
| Cork Oak—logs and branches | 83.4 ± 0.9 | 0.80 ± 0.03 | 15.8 ± 1.0 | 46.5 ± 0.4 | 0.98 ± 0.02 | 0.14 ± 0.005 | 16.7 ± 1.3 |
| Partially charred wood | 67.8 ± 9.9 | 1.88 ± 0.13 | 30.3 ± 10.1 | 54.4 ± 6.4 | 0.70 ± 0.16 | 0.10 ± 0.02 | 20.0 ± 14.5 |
| Tree stumps | 76.8 ± 0.4 | 1.85 ± 0.14 | 21.3 ± 0.5 | 47.3 ± 0.1 | 0.94 ± 0.01 | 0.13 ± 0.004 | 17.9 ± 1.8 |
| Test 1 | Test 2 | |
|---|---|---|
| Time (days) | ||
| Total cycle (carbonization + cooling) | 22 | 23 |
| Carbonization phase (from ignition up to the start of cooling phase) | 10 | 11 |
| Cooling phase (up to kiln temperatures below 50 °C) (1) | 12 | 12 |
| Total precipitation during cycle (mm) | 3 | 4 |
| Average feedstock moisture content (wt.%, wet basis) | 23 | 12 |
| Dry feedstock input (ton) | ||
| Total feedstock | 21.7 | 21.6 |
| Cork oak wood | 16.8 | 17.5 |
| Holm oak wood | 1.5 | 1.3 |
| Tree stumps | 2.4 | 0.9 |
| Partially charred wood | 1.1 | 1.8 |
| Maximum temperatures (°C) | ||
| Kiln front (T10 to T13) | 807 | 808 |
| Kiln rear (T14 to T18) | 298 | 322 |
| Kiln floor (T14 and T19) | 132 | 135 |
| Chimneys (T1 to T4) | 379 | 211 |
| Solid Products (e) | Fixed Carbon | Ash | Carbon Content | O/C | H/C | LHV (d) |
|---|---|---|---|---|---|---|
| [wt.%] | [wt.%] | [wt.%] | [kg/kg] | [kg/kg] | [MJ/kg] | |
| Charcoal—a (a) | 81.1 ± 2.0 | 6.8 ± 2.0 | 82.7 ± 1.4 | 0.09 ± 0.04 | 0.02 ± 0.01 | 29.9 ± 0.1 |
| Charcoal—b (b) | 64.2 ± 4.7 | 5.3 ± 0.8 | 72.3 ± 3.4 | 0.25 ± 0.06 | 0.04 ± 0.01 | 26.3 ± 1.3 |
| Charcoal fines (c) | 50.5 ± 3.8 | 23.0 ± 4.4 | 60.5 ± 4.9 | 0.25 ± 0.10 | 0.04 ± 0.01 | 21.4 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charvet, F.; Matos, A.; Figueiredo da Silva, J.; Tarelho, L.; Leite, M.; Neves, D. Charcoal Production in Portugal: Operating Conditions and Performance of a Traditional Brick Kiln. Energies 2022, 15, 4775. https://doi.org/10.3390/en15134775
Charvet F, Matos A, Figueiredo da Silva J, Tarelho L, Leite M, Neves D. Charcoal Production in Portugal: Operating Conditions and Performance of a Traditional Brick Kiln. Energies. 2022; 15(13):4775. https://doi.org/10.3390/en15134775
Chicago/Turabian StyleCharvet, Felix, Arlindo Matos, José Figueiredo da Silva, Luís Tarelho, Mariana Leite, and Daniel Neves. 2022. "Charcoal Production in Portugal: Operating Conditions and Performance of a Traditional Brick Kiln" Energies 15, no. 13: 4775. https://doi.org/10.3390/en15134775
APA StyleCharvet, F., Matos, A., Figueiredo da Silva, J., Tarelho, L., Leite, M., & Neves, D. (2022). Charcoal Production in Portugal: Operating Conditions and Performance of a Traditional Brick Kiln. Energies, 15(13), 4775. https://doi.org/10.3390/en15134775







