Bioconversion of the Brown Tunisian Seaweed Halopteris scoparia: Application to Energy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Biomass
2.2. Algal Biomass Characterization and Chemicals Constituents
2.2.1. Carbohydrate Content of the Three Fractions
2.2.2. Phenolic Content
2.2.3. Composition in Chemical Elements
2.2.4. Lipid Extraction
2.2.5. Ash, Moisture, and Protein Contents
2.3. Fermenting Microorganism, Medium Culture, and Production Process
2.3.1. Ethanol Production
2.3.2. Hydrogen and Volatile Fatty Acid Productions
2.3.3. Biodiesel Production
2.4. Statistical Analyses
3. Results and Discussion
3.1. Chemical Constituents
3.1.1. Ash, Moisture, Proteins, Fats, and Phenolic Contents
3.1.2. Composition in Chemical Elements
3.1.3. Sugars’ Contents
3.2. Biofuels’ Production from H. scoparia and By-Products of Extraction
3.2.1. Production of Bioethanol
3.2.2. Production of Hydrogen and Volatile Fatty Acids
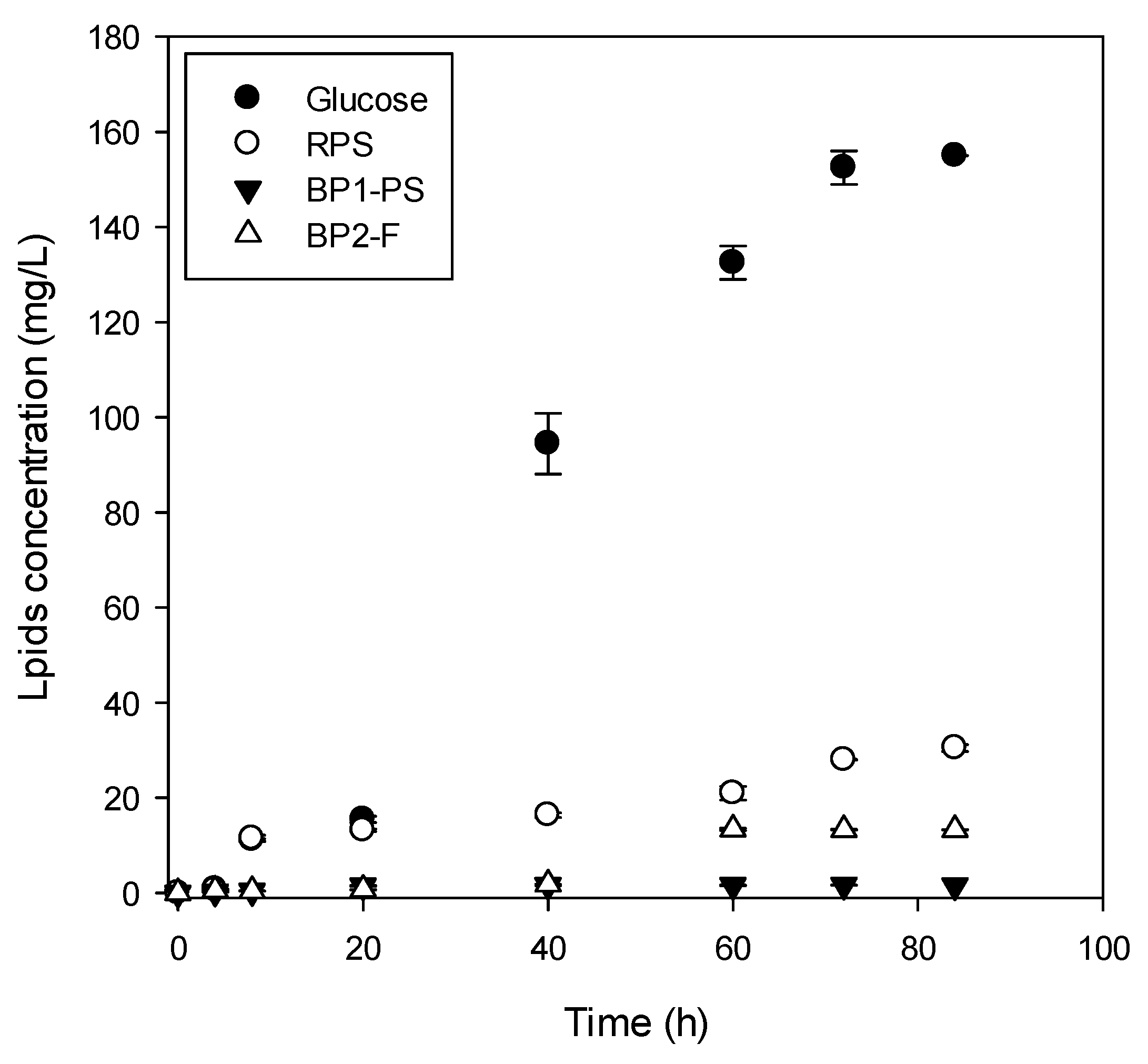
3.2.3. Biodiesel Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godvin Sharmila, V.; Kumar, D.; Pugazhendi, A. Biofuel production from macroalgae: Present scenario and future scope. Bioengineered 2021, 12, 9216–9238. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Gardarin, C.; Desbrières, J.; Le Cerf, D.; Rihouey, C.; Michaud, P.; Abdelkafi, S.; Pierre, G. Structural features and rheological properties of a sulphated xylogalactan-rich fraction isolated from Tunisian red seaweed Jania adhaerens. Appl. Sci. 2020, 10, 1655. [Google Scholar] [CrossRef] [Green Version]
- Biris-Dorhoi, E.; Michiu, D.; Pop, C.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H. Marine seaweed polysaccharides-based engineered cues for the modern biomedical sector. Mar. Drugs 2019, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological investigations of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Food Hydrocoll. 2020, 103, 105631. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Dellai, A.; Clary-Laroche, A.; Ben Said, R.; Robert, J.; Bouraoui, A. Anti-inflammatory and antiproliferative activities of organic fractions from the Mediterranean brown seaweed, Cystoseira compressa. Drug Dev. Res. 2012, 73, 82–89. [Google Scholar] [CrossRef]
- Ktari, L.; Guyot, M. A cytotoxic oxysterol from the marine alga Padina pavonica (L.) Thivy. J. Appl. Phycol. 1999, 11, 511–513. [Google Scholar] [CrossRef]
- El-Shafei, R.; Hegazy, H.; Acharya, B. A review of antiviral and antioxidant activity of bioactive metabolite of macroalgae within an optimized extraction method. Energies 2021, 14, 3092. [Google Scholar] [CrossRef]
- Indira, K.; Balakrishnan, S.; Srinivasan, M.; Bragadeeswaran, S.; Balasubramanian, T. Evaluation of in vitro antimicrobial property of seaweed (Halimeda tuna) from Tuticorin coast, Tamil Nadu, southeast coast of India. Afr. J. Biotechnol. 2013, 12, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Aghbashlo, M.; Karimi, K.; Tabatabaei, M. Shifting fuel feedstock from oil wells to sea: Iran outlook and potential for biofuel production from brown macroalgae (ochrophyta; phaeophyceae). Renew. Sustain. Energy Rev. 2019, 112, 626–642. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I.; Nigam, P.S. A viable technology to generate third-generation biofuel. J. Chem. Technol. Biotechnol. 2011, 86, 1349–1353. [Google Scholar] [CrossRef]
- Dammer, L.; Carus, M.; Piotrowski, S.; Puente, A.; Breitmayer, E.; de Beus, N.; Liptow, C. Sustainable first and second-generation bioethanol for Europe: A sustainability assessment in the context of the European Commission’s REDII proposal. Ind. Biotechnol. 2017, 13, 6. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Barberio, G. Utilization of macro-algae for enhanced CO2 fixation and biofuels production: Development of a computing software for an lca study. Fuel Process. Technol. 2005, 86, 1679–1693. [Google Scholar] [CrossRef]
- Levine, R.B.; Pinnarat, T.; Savage, P. Biodiesel production from wet algal biomass through in situ lipid hydrolysis and supercritical transesterification. Energy Fuels 2010, 24, 5235–5243. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Konda, N.V.S.N.; Singh, S.; Simmons, B.; Klein-Marcuschamer, D. An investigation on the economic feasibility of macroalgae as a potential feedstock for biorefineries. Bioenergy Res. 2015, 8, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Soliman, R.M.; Younis, S.A.; El-gendy, N. Batch bioethanol production via the biological and chemical saccharification of some Egyptian marine macroalgae. J. Appl. Microbiol. 2018, 125, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Aboulkas, A.; Hammani, H.; El Achaby, M. Valorization of algal waste via pyrolysis in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, D.; Fakhry, E. Lipid content and fatty acid composition of Mediterranean macro-algae as dynamic factors for biodiesel production. Oceanologia 2015, 57, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, M.; Kawai, S.; Murata, K. Strategies for the production of high concentrations of bioethanol from seaweeds: Production of high concentrations of bioethanol from seaweeds. Bioengineered 2013, 4, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Béligon, V.; Noblecourt, A.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Proof of concept for biorefinery approach aiming at two bioenergy production compartments, hydrogen and biodiesel, coupled by an external membrane. Biofuels 2018, 9, 163–174. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin extractions of brown seaweeds and analysis of their lipid fraction in methanol. Food Sci. Technol. Res. 2012, 18, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT Food Sci. Technol. 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Chakou, F.Z.; Boual, Z.; Ould El Hadj, M.D.; Belkhalfa, H.; Bachari, K.; El Alaoui-Talibi, Z.; El Modafar, C.; Hadjkacem, F.; Fendri, I.; Abdelkafi, S.; et al. Pharmacological investigations in traditional utilization of Alhagi maurorum Medik. in Saharan Algeria: In vitro study of anti-inflammatory and antihyperglycemic activities of water-soluble polysaccharides extracted from the seeds. Plants 2021, 10, 2658. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Waffenschmidt, S.; Jaenicke, L. Assay of reducing sugars in the nanomole range with 2,2′-bicinchoninate. Anal. Biochem. 1987, 165, 337–340. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujr, P.C. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef]
- Ramola, B.; Kumar, V.; Nanda, M.; Mishra, Y.; Tyagi, T.; Gupta, A.; Sharma, N. Evaluation, comparison of different solvent extraction, cell disruption methods and hydrothermal liquefaction of oedogonium macroalgae for biofuel production. Biotechnol. Rep. 2019, 22, e00340. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Bahry, H.; Pons, A.; Abdallah, R.; Pierre, G.; Delattre, C.; Fayad, N.; Taha, S.; Vial, C. Valorization of carob waste: Definition of a second-generation bioethanol production process. Bioresour. Technol. 2017, 235, 25–34. [Google Scholar] [CrossRef]
- Soyez, K. Integrated design of a fermentation plant: The production of Baker’s yeast. Engineering 1995, 15, 209–210. [Google Scholar] [CrossRef]
- Gaudet, G.; Forano, E.; Dauphin, G.; Delort, A.M. Futile cycling of glycogen in fibrobacter succinogenes as shown by in situ 1H-NMR and 13C-NMR investigation. Eur. J. Biochem. 1992, 207, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Noblecourt, A.; Christophe, G.; Larroche, C.; Santa-Catalina, G.; Trably, E.; Fontanille, P. High hydrogen production rate in a submerged membrane anaerobic bioreactor. Int. J. Hydrogen Energy 2017, 42, 24656–24666. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef]
- Christophe, G.; Deo, J.L.; Kumar, V.; Nouaille, V.; Fontanille, P.; Larroche, C. Production of oils from acetic acid by the oleaginous yeast Cryptococcus curvatus. Appl. Biochem. Biotechnol. 2012, 167, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Rosell, K.G.; Srivastava, L.M. Seasonal variation in the chemical constituents of the brown algae Macrocystis integrifolia and Nereocystis luetkeana. Can. J. Bot. 1984, 62, 2229–2236. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-conte, R.; Barriga, A.; Buschmann, A.H.; Maki-Arvela, P.; Mikkola, J.-P.; Linqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Hay, C.H. The distribution of Macrocystis (phaeophyta: Laminariales) as a biological indicator of cool sea surface temperature, with special reference to new Zealand waters. J. R. Soc. N. Z. 1990, 20, 313–336. [Google Scholar] [CrossRef]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical characterization of eight seaweed species and evaluation of their potential use as an alternative for biofuel production and source of bioactive compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef]
- Chkhikvishvili, I.D.; Ramazanov, Z.M. Phenolic substances of brown algae and their antioxidant activity. Appl. Biochem. Microbiol. 2000, 36, 289–291. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Simat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.S.; Mehmood, M.A.; Al Ayed, O.S.; Ye, G.; Luo, H.; Ibrahim, M.; Rashid, U.; Nehdi, I.A.; Qadir, G. Kinetic analyses and pyrolytic behavior of Para grass (Urochloa mutica) for its bioenergy potential. Bioresour. Technol. 2017, 224, 708–713. [Google Scholar] [CrossRef]
- Suutari, M.; Leskinen, E.; Fagerstedt, K.; Kuparinen, J.; Kuuppo, P.; Blomster, J. Macroalgae in biofuel production. Phycol. Res. 2015, 63, 1–18. [Google Scholar] [CrossRef]
- Xia, A.; Jacob, A.; Herrmann, C. Production of hydrogen, ethanol and volatile fatty acids from the seaweed carbohydrate mannitol. Bioresour. Technol. 2015, 193, 488–497. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.; Baroutian, S. Advances in the pretreatment of brown macroalgae for biogas production. Fuel Proc. Technol. 2019, 195, 106151. [Google Scholar] [CrossRef]
- Hou, X.; Hansen, J.H.; Bjerre, A. Integrated bioethanol and protein production from brown seaweed Laminaria digitata. Bioresour. Technol. 2015, 197, 310–317. [Google Scholar] [CrossRef]
- Mayala, T.S.; Ngavouka, M.D.N.; Douma, D.H.; Hammerton, J.M.; Ross, A.B.; Brown, A.E.; M’Passi-Mabiala, B.; Lovett, J.C. Characterisation of Congolese Aquatic Biomass and Their Potential as a Source of Bioenergy. Biomass 2022, 2, 1–13. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomass: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.; Ryu, C.; Jeon, J. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Chemical composition and potential use of Fucus vesiculosus from Gulf of Riga. Energy Procedia 2016, 95, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Milla, S.; Elina, L.; Kurt, F.; Jorma, K.; Pirjo, K.; Jaanika, B. Macroalgae in biofuel production. Phycol. Res. 2014. [CrossRef]
- Chen, X.; Zhai, R.; Li, Y. Understanding the structural characteristics of water-soluble phenolic compounds from four pretreatments of corn stover and their inhibitory effects on enzymatic hydrolysis and fermentation. Biotechnol. Biofuels 2020, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Obata, O.; Akunna, J.; Bockhorn, H. Ethanol production from brown seaweed using non-conventional yeasts. Bioethanol 2016, 2, 134–145. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-bonaca, M. Chemical elements in Mediterranean macroalgae. Ecotoxicol. Environ. Saf. 2018, 148, 44–71. [Google Scholar] [CrossRef]
- Ozgun, S.; Turan, F. Biochemical composition of some brown algae from Iskenderun bay, the northeastern Mediterranean coast of turkey. J. Blac Sea Mediterr. Environ. 2015, 21, 125–134. [Google Scholar]
- Manev, Z.; Iliev, A.; Vachkova, V. Chemical characterization of brown seaweed—Cystoseira barbata. Bulg. J. Agric. Sci. 2013, 19, 12–15. [Google Scholar]
- Mutripah, S.; Meinita, m.; Kang, J. Bioethanol production from the hydrolysate of Palmaria palmata using sulfuric acid and fermentation with brewer’s yeast. J. Appl. Phycol. 2014, 26, 687–693. [Google Scholar] [CrossRef]
- Ravanal, M.C.; Sharma, S.; Gimpel, J. The role of alginate lyases in the enzymatic saccharification of brown macroalgae, Macrocystis pyrifera and Saccharina latissima. Algal Res. 2017, 26, 287–293. [Google Scholar] [CrossRef]
- Offei, F.; Mensah, M.; Thygesen, A. Seaweed bioethanol production: A process selection review on hydrolysis and fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Khambhaty, Y.; Upadhyay, D.; Kriplani, Y. Bioethanol from macroalgal biomass: Utilization of marine yeast for production of the same. BioEnergy Res. 2013, 6, 188–195. [Google Scholar] [CrossRef]
- Ra, C.H.; Kim, S.K. Optimization of pretreatment conditions and use of a two-stage fermentation process for the production of ethanol from seaweed, Saccharina japonica. Biotechnol. Bioproc. Eng. 2013, 18, 715–720. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Fattah, A.F.; Hussein, M.M. Composition of some brown algae as influenced by seasonal variation. Phytochemistry 1970, 9, 721–724. [Google Scholar] [CrossRef]
- Khalid, A.; Aslam, M.; Qyyum, M.A.; Faisal, A.; Khan, A.L.; Ahmed, F.; Lee, M.; Kim, J.; Jang, N.; Chang, I.S.; et al. Membrane separation processes for dehydration of bioethanol from fermentation broths: Recent developments, challenges, and prospects. Renew. Sustain. Energy Rev. 2019, 105, 427–443. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A review of seaweed pre-treatment methods for enhanced biofuel production by anaerobic digestion or fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Fasahati, P.; Liu, J.J. Process simulation of bioethanol production from brown algae. IFAC Proceed. Vol. 2012, 45, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Popper, Z.A.; Ralet, M.C.; Domozych, D.S. Plant and algal cell walls: Diversity and functionality. Ann. Bot. 2014, 114, 1043–1048. [Google Scholar] [CrossRef] [Green Version]
- Noblecourt, A.; Christophe, G.; Larroche, C. Hydrogen production by dark fermentation from pre-fermented depackaging food wastes. Bioresour. Technol. 2018, 247, 864–870. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G. Fermentative hydrogen production from macro-algae Laminaria japonica using anaerobic mixed bacteria. Int. J. Hydrog. Energy 2014, 39, 9012–9017. [Google Scholar] [CrossRef]
- Song, M.; Pham, H.D.; Seon, J. Marine brown algae: A conundrum answer for sustainable biofuels production. Renew. Sustain. Energy Rev. 2015, 50, 782–792. [Google Scholar] [CrossRef]
- Jeliani, Z.Z.; Fazelian, N.; Yousefzadi, M. Introduction of macroalgae as a source of biodiesel in Iran: Analysis of total lipid content, fatty acid and biodiesel indices. J. Mar. Biol. Assoc. U. K. 2021, 101, 527–534. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]


| Composition % (w/w) | H. scoparia (RPS) | By-Product after Carbohydrate Extraction (BP1-PS) | By-Products after Fucoxanthin Extraction (BP2-F) |
|---|---|---|---|
| Fat | 2.92 ± 0.28 | 1.5 ± 0.52 | 0.93 ± 0.6 |
| Protein | 6.53 ± 0.56 | 1 ± 0.5 | 0.87 ± 0.71 |
| Ash | 42.63 ± 2.2 | 47.34 ± 1.4 | 35.41 ± 2.5 |
| Moisture | 6.79 ± 0.16 | 7.57 ± 0.16 | 9.06 ± 0.45 |
| Phenolic content | 4.4 ± 0.005 | 0.43 ± 0.0003 | 0.29 ± 0.0005 |
| Chemical Elements | mg/kg DW 1 |
|---|---|
| P | 29.950 ± 0.008 |
| Ca | 19.919 ± 0.003 |
| K | 15.689 ± 0.037 |
| Na | 10.350 ± 0.097 |
| Mg | 8137.5 ± 0.056 |
| Fe | 3946.75 ± 0.023 |
| Mn | 51.5 ± 0.005 |
| Ni | 23.75 ± 0.008 |
| Zn | 23.55 ± 0.012 |
| Cu | 6.95 ± 0.01 |
| Pb | 4 ± 0.01 |
| Samples (g/kg DW) | Total Sugars (g/kg) | Reducing Sugars (g/kg) |
|---|---|---|
| RPS | 133.9 ± 0.12 | 39.5 ± 0.05 |
| BP1-PS | 79.02 ± 0.03 | 15.73 ± 0.09 |
| BP2-F | 113.20 ± 0.04 | 15.26 ± 0.02 |
| Fatty Acids Composition | % |
|---|---|
| Palmitic acid C16:0 | 11 |
| Palmitoleic acid C16:1 | 10 |
| Stearic acid C18:0 | 1 |
| Oleic acid C18:1 | 28 |
| Linoleic acid C18:2 | 51 |
| Linolenic acid C18:3 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjkacem, F.; Pierre, G.; Christophe, G.; Elleuch, J.; Fendri, I.; Boual, Z.; Ould El Hadj, M.D.; El Alaoui-Talibi, Z.; El Modafar, C.; Dubessay, P.; et al. Bioconversion of the Brown Tunisian Seaweed Halopteris scoparia: Application to Energy. Energies 2022, 15, 4342. https://doi.org/10.3390/en15124342
Hadjkacem F, Pierre G, Christophe G, Elleuch J, Fendri I, Boual Z, Ould El Hadj MD, El Alaoui-Talibi Z, El Modafar C, Dubessay P, et al. Bioconversion of the Brown Tunisian Seaweed Halopteris scoparia: Application to Energy. Energies. 2022; 15(12):4342. https://doi.org/10.3390/en15124342
Chicago/Turabian StyleHadjkacem, Farah, Guillaume Pierre, Gwendoline Christophe, Jihen Elleuch, Imen Fendri, Zakaria Boual, Mohamed Didi Ould El Hadj, Zainab El Alaoui-Talibi, Cherkaoui El Modafar, Pascal Dubessay, and et al. 2022. "Bioconversion of the Brown Tunisian Seaweed Halopteris scoparia: Application to Energy" Energies 15, no. 12: 4342. https://doi.org/10.3390/en15124342
APA StyleHadjkacem, F., Pierre, G., Christophe, G., Elleuch, J., Fendri, I., Boual, Z., Ould El Hadj, M. D., El Alaoui-Talibi, Z., El Modafar, C., Dubessay, P., Delattre, C., Michaud, P., & Abdelkafi, S. (2022). Bioconversion of the Brown Tunisian Seaweed Halopteris scoparia: Application to Energy. Energies, 15(12), 4342. https://doi.org/10.3390/en15124342










