1. Introduction
The technology of converting chemical energy stored in a wide range of organic matter and wastewater into electrical energy assisted by the biocatalytic activity of microorganisms has attracted the interest of academic researchers in recent decades. The technology for this energy conversion is achieved by microbial fuel cells. A microbial fuel cell (MFC) is a bioreactor capable of treating wastewater by removing pollutants and simultaneously generating electricity using electrochemically active microorganisms. The feedstock for MFC operation to generate electricity can be selected from a wide variety of wastewaters, from simple to complex. MFCs used for wastewater treatment have been studied for the removal of chemical and biological oxygen demand (COD and BOD, respectively), heavy metals, and ammonia (NH
3) using biological degradation of organic matter to produce electricity [
1]. In order to increase the sustainability of MFC technology for large-scale operations, the recovery of high-value products (such as silver or chromium) from wastewaters from various industries, such as the jewellery, photography, medical/pharmaceutical, personal care product (PCP) and electronic sectors, has also become an essential application [
2].
In general, a microbial fuel cell consists of anode and cathode chambers that are separated physically by a cation/proton exchange membrane. Microorganisms present in wastewater act as biocatalysts in the anode chamber, oxidise organic matters, and produce electrons and protons. The produced protons then travel to the cathode chamber through a proton exchange membrane. Whereas, electrons flow to the cathode through an external circuit containing a resistor or a load. In the cathode chamber, protons and electrons react to produce water. By considering acetic acid in the anode chamber as a substrate or a sole carbon source in the anode chamber, the biochemical reaction that occurs in the anode chamber can be represented by [
3]:
The reaction in the cathode chamber will be:
From the biochemical reactions, it is apparent that the presence of oxygen will inhibit the production of electricity. Therefore, MFC should be designed to make the anode chamber anoxic.
Based on the transfer of produced electrons by active microorganisms from media to the anode, two types of MFCs exist: MFCs with a mediator and mediator-less MFCs. Electron transfer through the membrane (a cytoplasmic membrane envelops microorganisms, and is the primary barrier to the external environment) or nanowires produced by bacteria, is referred to as direct or mediator-less electron transfer. In order to enhance the electron transfer to the anode, chemical mediators, such as neutral red or anthraquinone-2,6-disulfonate (AQDS), can be added to the anode chamber [
4]; this type of transfer is called mediated electron transfer.
In recent decades, MFCs have drawn attention due to their capability to generate electricity while treating wastewater [
5]. Other than electricity generation, they can be used for many different applications, including desalination, chemical production, biosensors and biohydrogen production [
6]. In the case of wastewater treatment applications, it is essential to gather more information about BOD and COD characteristics of organic matter to achieve standard effluent wastewater norms. In general, most research studies have concentrated on studying maximum power output instead of COD removal efficiency of MFCs. Few papers have focused on studying the power output using different types of feedstock, including single carbon sources, and artificial as well asnatural wastewater [
7,
8].
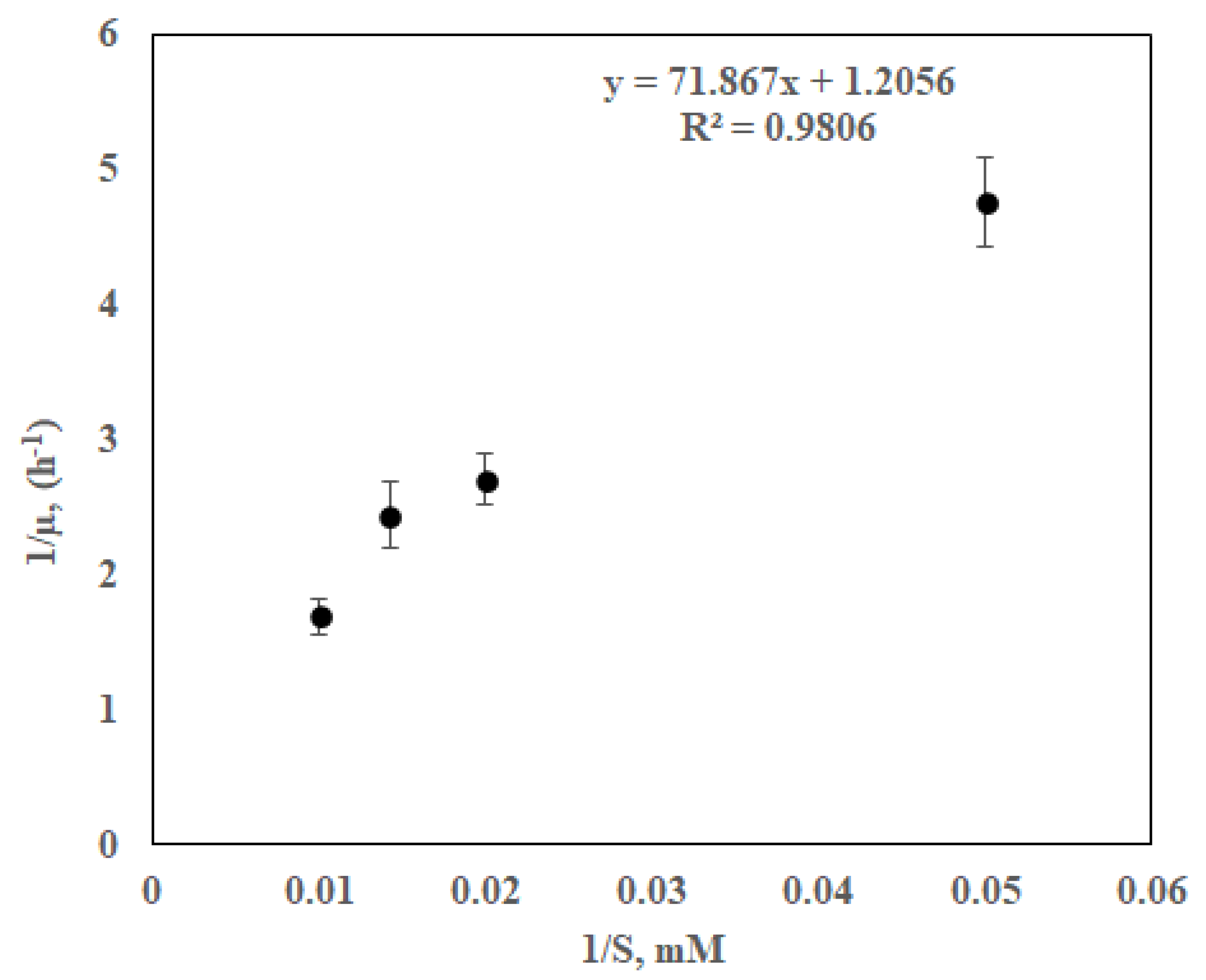
Previously most experiments were performed in batch modes for specific initial COD concentration of feedstock. As expected, these investigations found a proportional correlation between the COD concentration of the feedstock and power output. A Monod-type relationship between power output and substrate concentrations has been obtained earlier [
7,
9,
10].
MFC operation conditions, i.e., flow characteristics and temperature, are essential factors for COD removal rates and the extent of COD removal from wastewater. In the case of isothermal continuous flow operation, the COD removal rates are determined based on the inlet and outlet COD concentrations as a function of the hydraulic retention time (HRT), which is one of the critical operating variables [
11,
12,
13]. A short HRT increases the volumetric loading rate of organic matter to the MFC, resulting in a decrease in COD removal efficiency and power density [
14]. COD removal rates are typically reported for the complete cycle in batch andfed-batch reactors based on initial and final COD concentrations [
15]. The removal rates of COD are not often checked as this can hamper the current generation during the feeding of new solutions [
16].
It was reported that removal efficiency of COD of 94% was achieved by an up-flow tubular MFC treating dairy wastewater with an initial COD of 4300 mg/L after 83–95 days [
17]. The removal efficiency was found to be 96% for the same MFC when treating artificial wastewater, as it contained a minuscule quantity of toxic compounds. The higher concentration of microorganisms in the anode chamber contributes to a better mass transfer between the substrate and microbes, resulting in the enhancement of the contact with the anode surface [
18] for treating domestic wastewater. It was suggested that, for better COD removal, the HRT for a single MFC should be within the range of 72 to 96 h [
17]. The COD concentration of brewery wastewater is 10 times higher than that of domestic wastewater [
19]. Nonetheless, overall coulombic efficiency decreases when brewery wastewater is used as a feedstock for MFC, as found in a previous study [
20].
For long-term continuous flow operation, COD removal of 94.6% was attained for brewery wastewater, and greater removal efficiency was achieved by dilution [
21]. Due to the high fraction of intractable substances present in winery wastewater, only a daily COD removal of 10% was reported [
22], inferring a small amount of organic matter removal. An MFC achieved a high reduction of 83% at 14 days HTR for swine wastewater [
23,
24]. In comparison, for other types, the removal rates were 86.2%, 86.7%, 76.5% and 77.4%, respectively. One explanation for the above findings is that both exoelectrogenic and methanogenic microbes are present in the biomass, and by shifting the balance between these populations in the long-term continuous operation, the power generation efficiency can be modified. Thus, other factors other than the COD concentration of the influent need to be studied to improve an MFC’s coulombic efficiency.
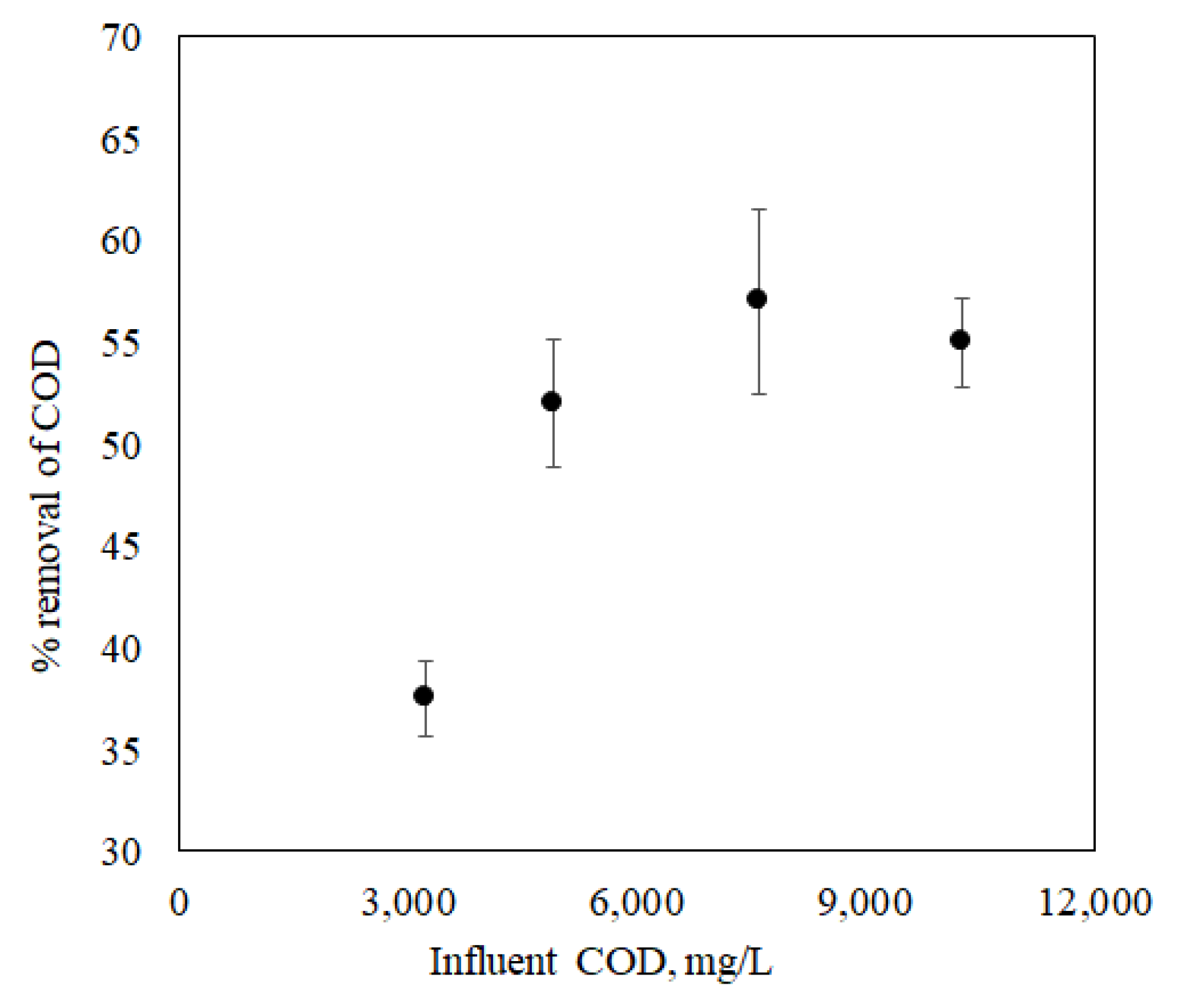
It is essential to acquire a better understanding of the correlation between the current generation and COD removal in the aerobic or anaerobic processes within the MFC so that the MFC system can be designed for maximum energy generation and efficient wastewater treatment. The depletion of organic matter concentration in the MFC reactor depends on the arrangement of the electrical circuit. With a lower resistance, the concentration of organic matter decreases faster and produces higher current densities. In contrast, the depletion rate of organic matter is much slower for an open circuit arrangement. Ren et al., 2014 [
25], reported that the achieved removal of organic matter was 67% after 9 h of operation for an open circuit arrangement, and removal of 88–92% was attained for a closed circuit over the same time. A higher OCV is not a good parameter for evaluating an MFC’s performance as it only measures the potential difference between the two electrodes when no electrons are flowing (current), because there is no transfer of electrons to complete the oxidation-reduction reaction (i.e., formation of water at the cathode). OCV only indicates the extent of activation losses in a cell and compares it with theoretical cell emf (electromotive force), whereas, for energy harvesting, external resistance is necessary. If existing bacteria are primarily exoelectrogenic, the current production starts after some time, based on the bacterial growth rate and reactor condition. Thus, external resistance and organic loading rate both influence the balance between exoelectrogenic activity and coulombic efficiency. To achieve maximum output power density, it is important that the reactor operates at minimum internal resistance and mass transfer losses. Therefore, closed circuit MFC studies are important to establish an optimal operating regime for an MFC for both maximum COD removal and coulombic efficiency.
In the present study, the performance of a small-scale air cathode MFC was investigated in terms of its COD removal efficiency. In the previous literature,
Shewanella sp., namely
Shewanella putrefaciens and
Shewanella oneidensis, are commonly used for MFC operation. However,
Shewanella baltica is a newly isolated bacterium whose performance regarding MFC application has not been widely explored. Therefore, a pure culture of
Shewanella baltica 20 was selected for the present investigation for biofilm formation on the anode surface.
Shewanella baltica 20 is a facultative bacterium capable of absorbing significant quantities of Fe(III) from its medium in aerobic conditions and reducing it to Fe(II) in anaerobic conditions. Furthermore,
Shewanella baltica 20 has the potential to oxidise organic matters from the reduction of nitrate and sulphur compounds in anaerobic conditions [
26]. Different lactate concentrations of microbial growth medium for
Shewanella baltica 20 and influent COD concentrations of artificial wastewater were used in this study. The external resistance was varied to investigate various losses and to analyse the power output at different resistances. Thus, the paper outcome presents the wastewater treatment (i.e., COD removal) ability in tandem with the electricity generation capability of the selected air cathode MFC design.
2. Materials and Methods
A small-scale air cathode MFC design was selected for this study. A series of experiments using electrochemically active microorganism Shewanella baltica 20 were conducted. The details of different components of MFC, microbial growth conditions and experimental procedures for MFC operation are provided in the following sections.
2.1. MFC Assembly
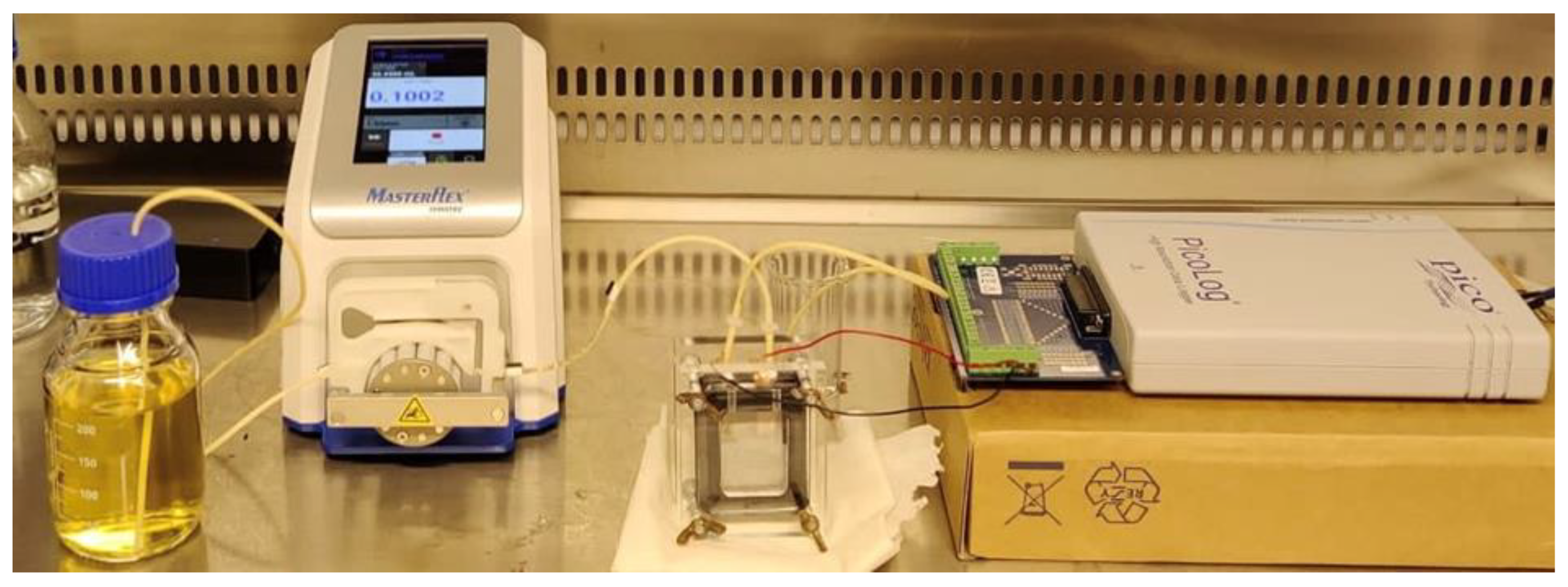
A plexiglass material was used to fabricate the anode and cathode chamber and end plate for both sides of the MFC. The dimension of each chamber was 30 × 40 × 7.5 mm, resulting in a working volume of 9 mL. Inlet and outlet ports were included in the anode chamber to allow the flow of wastewater in and out of the chamber.
The electrode material for both anode and cathode was selected as carbon felt having a dimension of 40 × 50 × 0.2 mm. A proton exchange membrane (PEM; NAFION 117, Sigma–Aldrich) was used to separate the two compartments. In order to prevent leakage, 1 mm rubber gaskets were used for both the anode and cathode sides. The anode and cathode compartments were filled with deionised water when the microbial fuel cell was not in use to maintain the conductivity of the membrane. The components of the MFC are shown in
Figure 1.
2.2. Bacterial Culture Conditions
An exoelectrogen, gram-negative bacterium, Shewanella baltica 20, was selected for the present investigation. This bacterial strain was received from the department of medical microbiology and immunology, University of Pécs, Hungary. In our preliminary experiments conducted with the biotechnology laboratory of Sintef, Narvik, Norway, Shewanella baltica 20 showed good biofilm formation on both carbon felt and carbon cloths.
Shewanella baltica 20 is a facultative anaerobe, and was grown in Luria Bertani (LB) media at 25 °C for 48 h. After achieving a full-grown microbial population determined by plating, four different batch operations were performed to assess the microbial growth kinetic of Shewanella baltica 20. In each batch, experiments were conducted in an Erlenmeyer flask by varying initial lactate concentrations from 20 to 100 mM in the LB media. At every 3 h interval, samples were collected for further analysis.
2.3. Experimental Procedure
The microbial fuel cell was fed with lactate-rich LB media containing Shewanella baltica 20 with an optical density of 0.3 (at 600 nm) to enrich the anode chamber with the microbial community for the development of biofilm on the anode surface. The two electrodes were connected to a voltage data logger (Picolog), and the data were recorded on a personal computer. The open-circuit voltage was recorded for seven days until a stable voltage output was obtained from the MFC.
After achieving a stable open-circuit voltage, the MFC was fed continuously with lactate-containing LB media without the presence of bacterial strain. In addition, anode and cathode were connected with resistance to obtain the current output. The current outputs were observed for different lactate concentrations of the LB media. The details of the experimental setup are presented in
Figure 2 and shown schematically in
Figure 3.
In a similar manner, the feed for the MFC was replaced with artificial wastewater, mimicking dairy wastewater containing NH4Cl, 40 mg/L; MgCl2, 10 mg/L; CuSO4, 0.1 mg/L; CaCl2, 5 mg/L; MnSO4, 0.1 mg/L; ZnCl2, 0.1 mg/L; phosphate buffer (1.0 mol/L, pH7); in addition, the lactate concentrations were varied. In this case, the current outputs were also observed and recorded. In both cases, the flow rates of LB media and synthetic wastewater were 0.1 mL/min. The samples from the outlet of the anode chamber were collected for each change in lactate concentration to determine the lactate consumption and COD removal by the MFC.
2.4. Data Acquisition and Calculations
During the characterisation of microbial growth of Shewanella baltica 20, the biomass concentrations were measured using a spectrophotometer at 600 nm. The obtained viable microbial population was calculated in CFU/mL which is estimated by multiplying colony count by dilution multiple The lactate concentrations were measured by the rapid colourimetric method using the D-Lactate Assay kit (Megazyme). A DR3900 Laboratory spectrophotometer was used for measuring the chemical oxygen demand (COD) of influent and effluent streams of the anode chamber of the MFC. After obtaining a stable current for each change in COD in artificial wastewater, the polarisation curve was obtained by applying external resistance from 5 KΩ to 100 Ω. The output voltages were recorded using Ohm’s law, the output currents were calculated, and the polarisation curves were obtained. The online voltage and current were recorded through PicoLog, a high-resolution data logger. Linear sweep voltammetry (LSV) using a PalmSens4 potentiostat was used to determine MFC’s internal resistance.
4. Conclusions
This study investigated the performance of a small-scale MFC in terms of power output and COD removal efficiency for artificial dairy wastewater. The study also illustrated that external resistance is an important operating parameter for process optimisation to gain maximum power density.
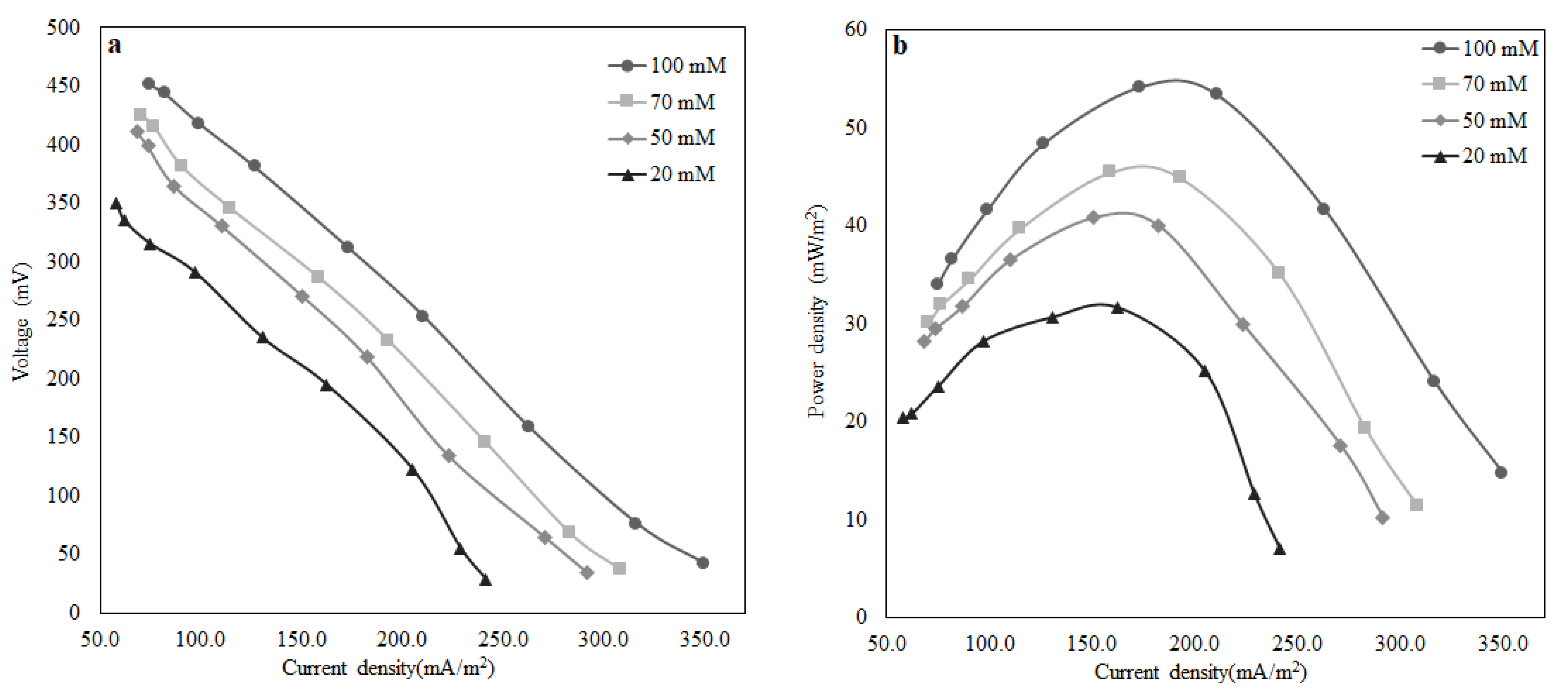
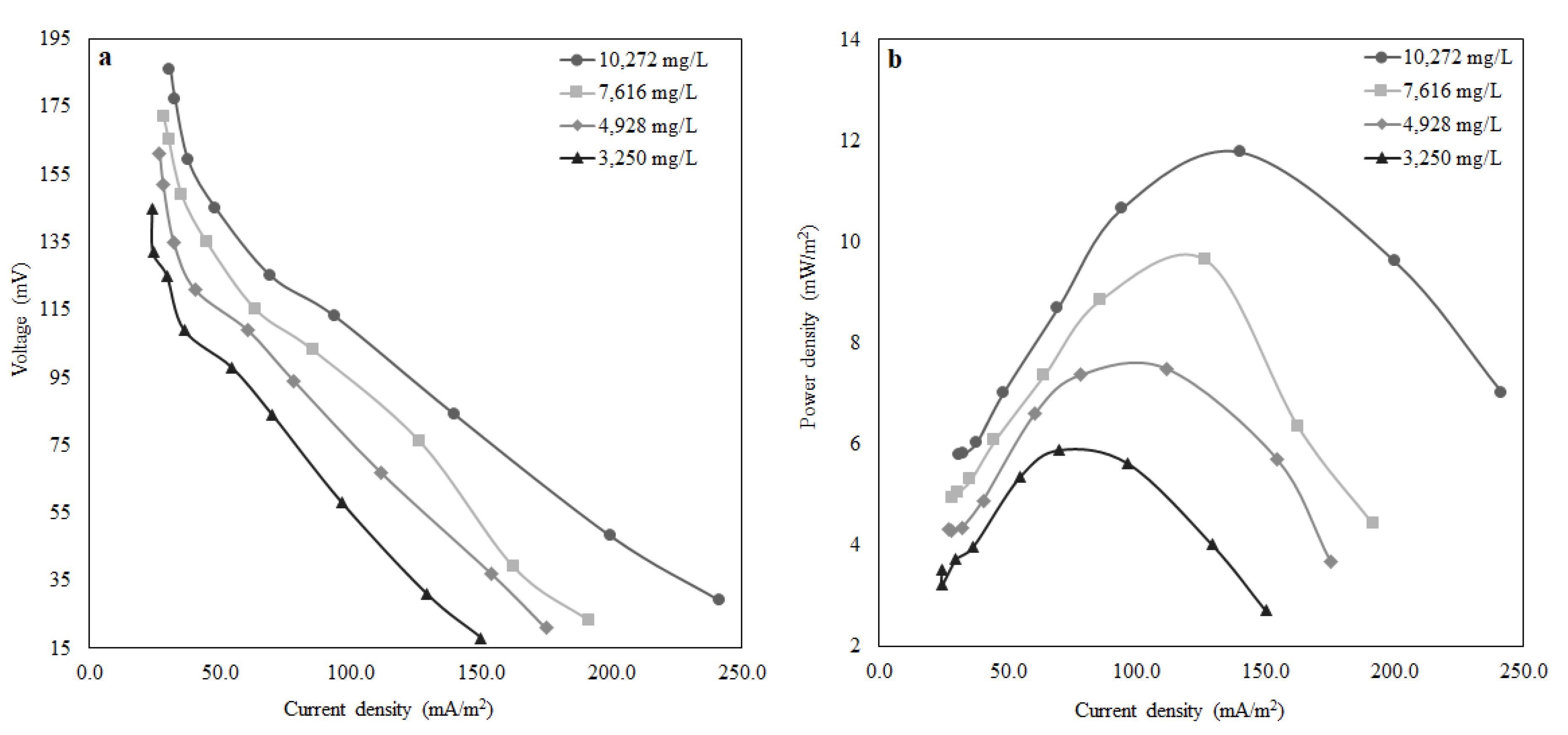
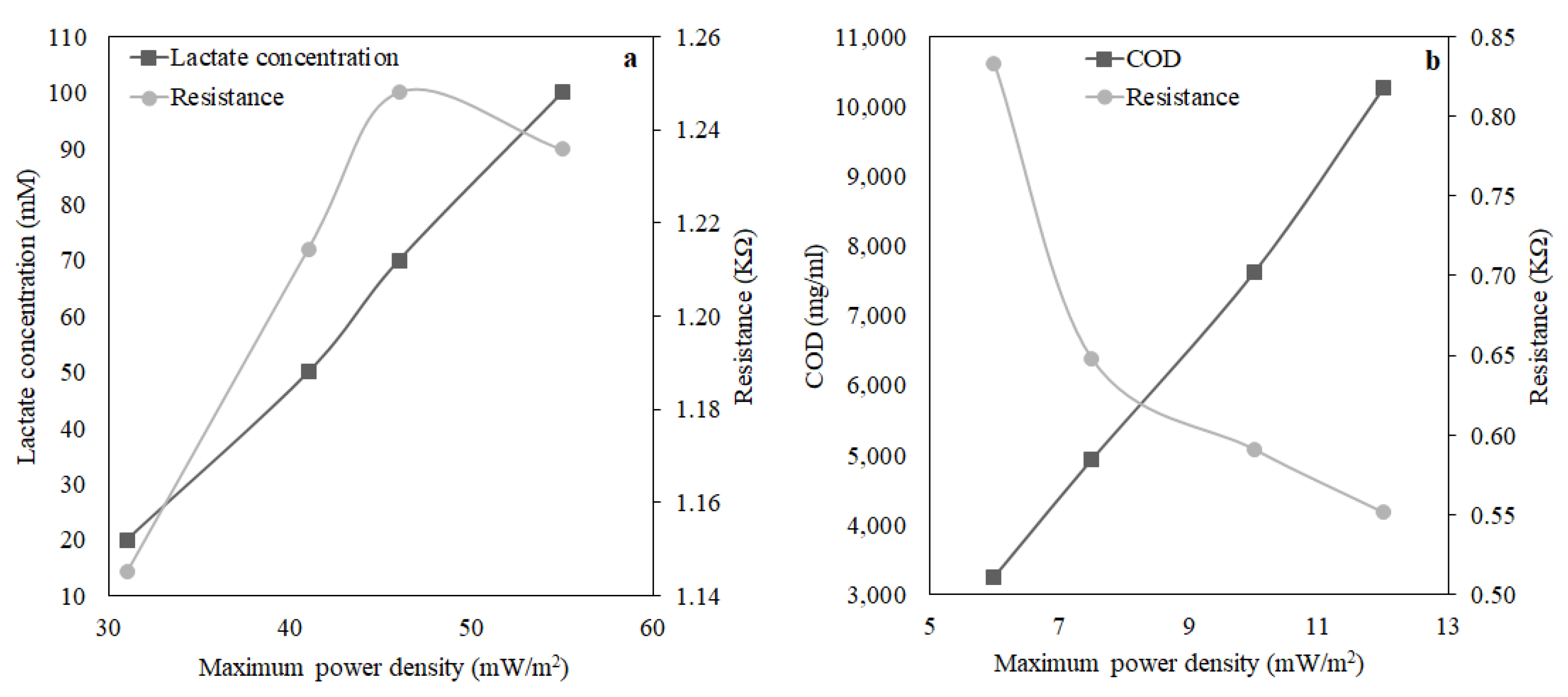
The pure culture of Shewanella baltica 20 indicated a good response to the presence of lactate in the growth medium. A maximum power density of 55 mW/m2 was observed when the MFC was fed with LB media containing 100 mM of lactate at an HRT of 1.5 h. In addition, the performance of MFC using artificial wastewater was examined, and a maximum power density of 12 mW/m2 was obtained, which is relatively low compared to the previous case where the MFC operated with lactate. The reason behind lower power output is that the microbial community takes a relatively long time to regain the electricity generation capacity as the substrate was altered. The MFC achieved a COD removal efficiency of 57% for an HRT of 1.5 h. In the above case, where the MFC was operated to treat wastewater by removing organic matter, it performed well by removing 57% of COD at an HRT of 1.5 h. Working with this small-scale MFC at an HRT higher than 1.5 h is impractical as the MFC operation will be close to a batch system, and there will be no point in considering HRT as an operating variable. Earlier literature reported that at higher HRT, COD removal was more than 94% because there is a direct correlation between the percentage removal of COD and HRT. Furthermore, observing the influence of external resistance on power density and wastewater treatment efficiency (percentage removal of (COD)), it can be inferred that optimised external resistance depends upon the quality of influent fed to the MFC. It was also reported that for maximum energy harvesting, other relevant factors include the supply and consumption of oxygen by the cathode, oxidation of substrates in the anode, efficient electron shuttling from the anode compartment to the anode surface, and the permeability of the proton exchange membrane are accountable. Therefore, additional investigations are required to understand how power production affects the percentage removal of COD and how hydraulic retention time and external resistance can be optimised. The above initial findings confirm that electricity generation by MFC depends on the characteristics of the wastewater along with its microbial community. Thus, the overall performance can be improved by optimising the design aspects of the MFC, i.e., the surface area to volume ratio and the external resistance with respect to wastewater quality.