High Refractive Index Diphenyl Sulfide Photopolymers for Solar Cell Antireflection Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
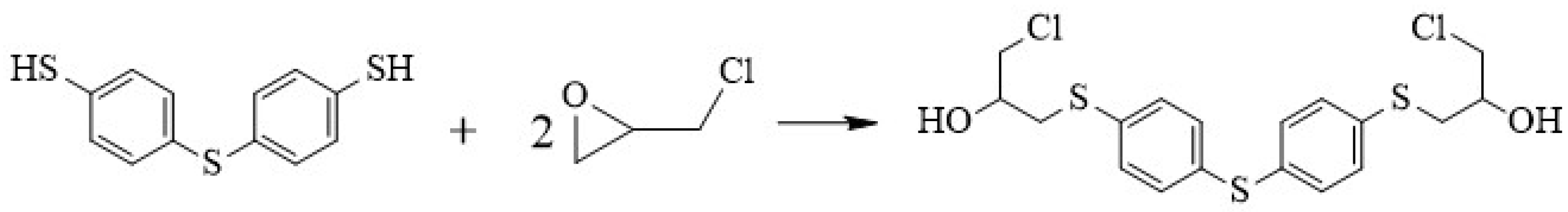
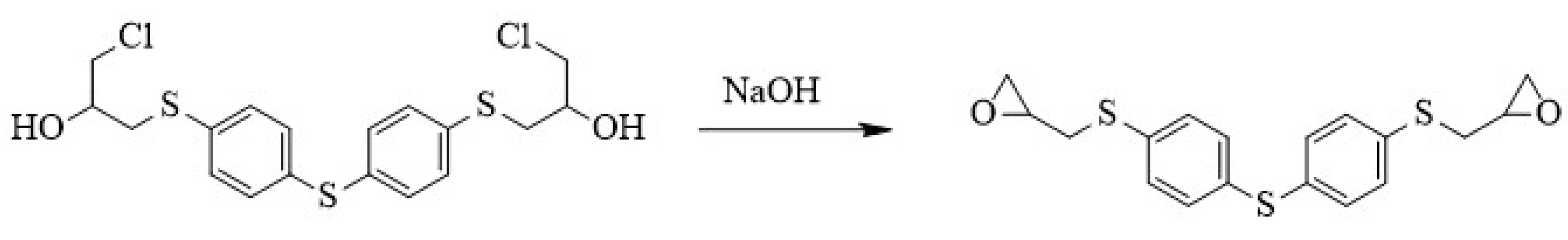
2.2. Synthesis of Thiodibenzenethiol Epoxy Resin
2.3. Synthesis of EA-UV
2.4. Synthesis of AOI-UV
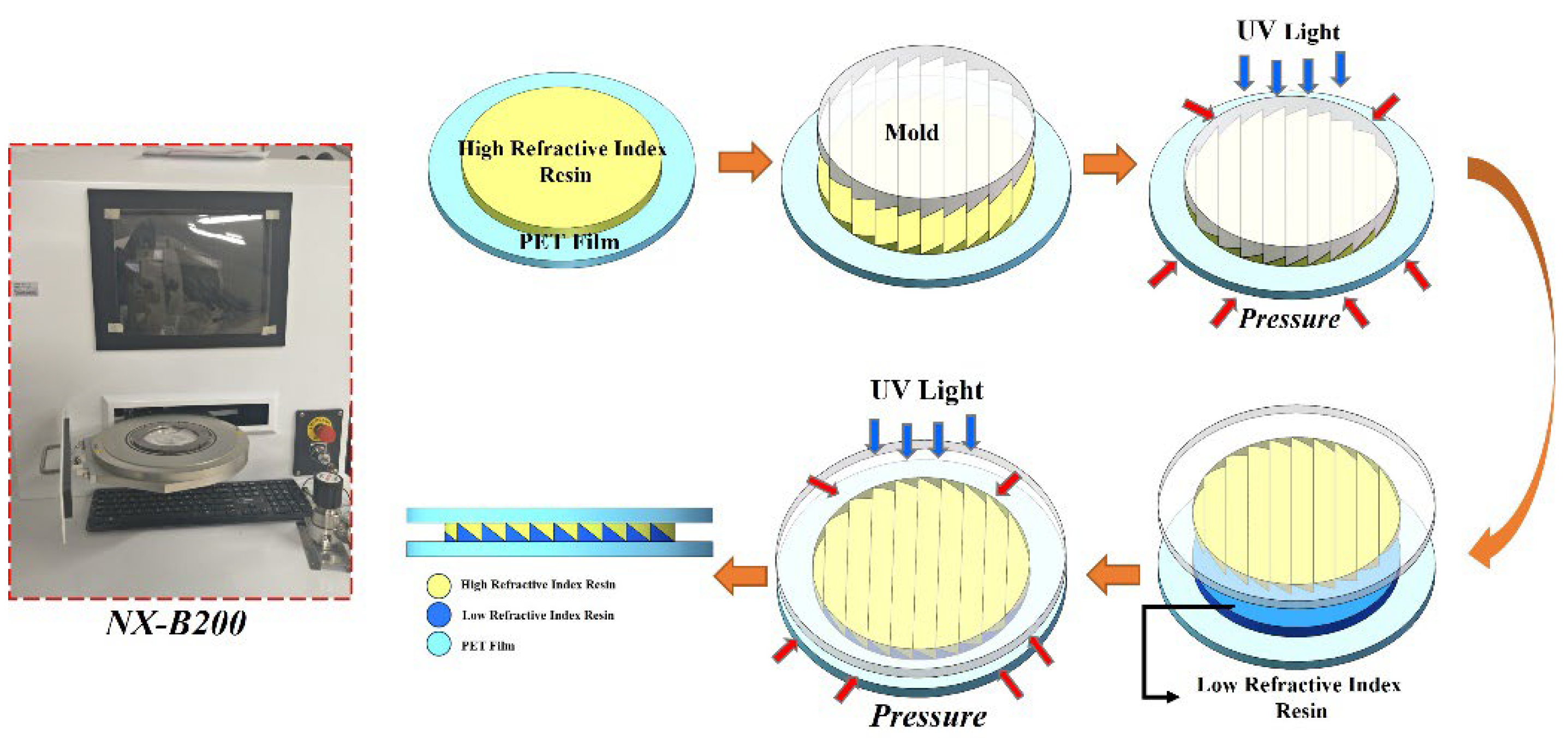
2.5. Preparation of Film
2.6. Preparation of ARCs Film
2.7. Testing and Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of Photopolymers
3.2. Thermal Properties
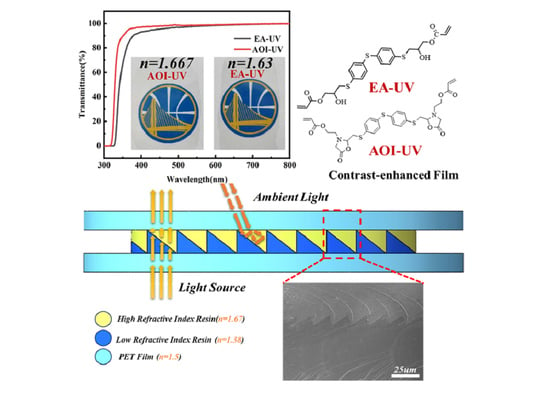
3.3. Optical Properties
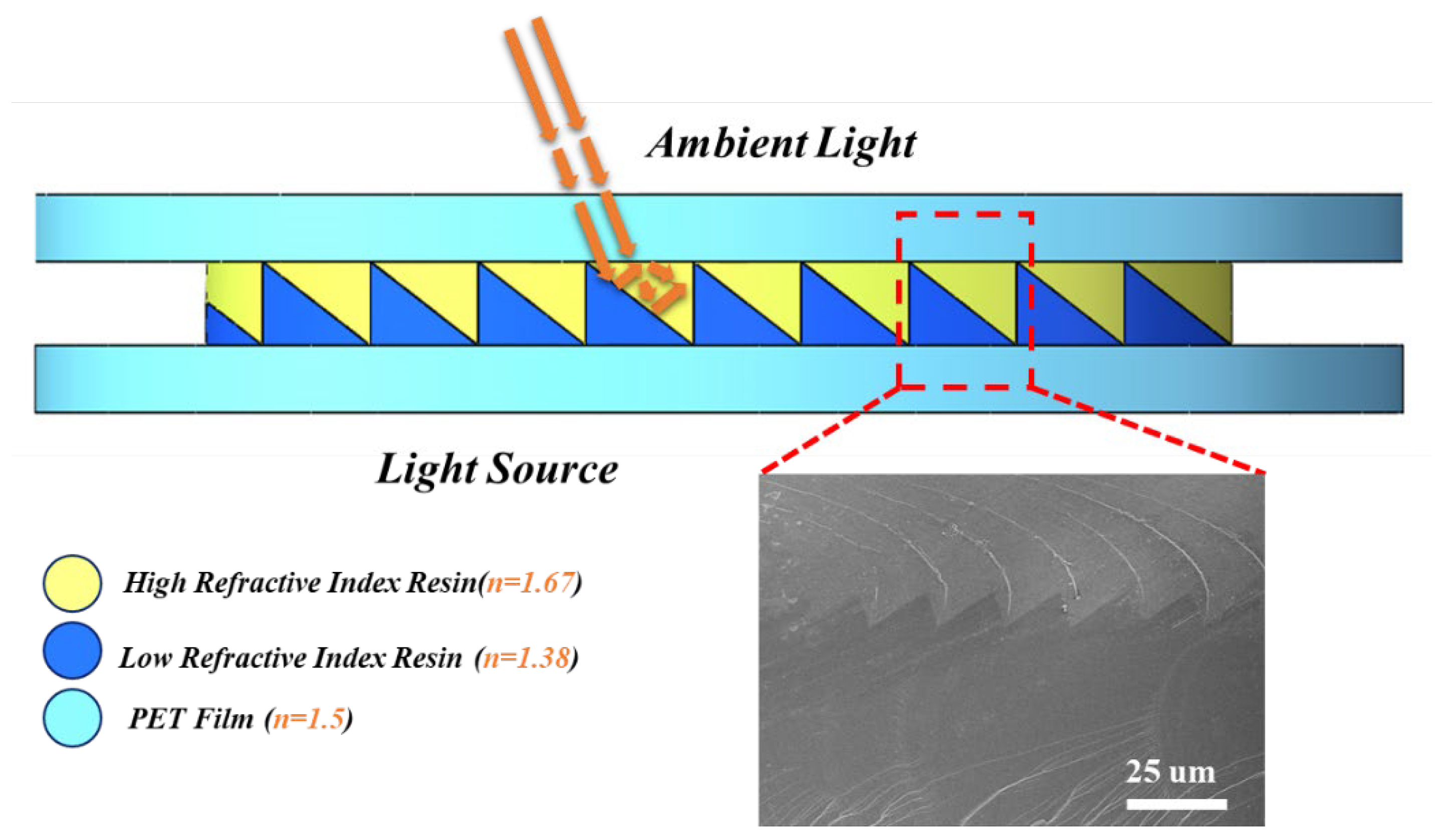
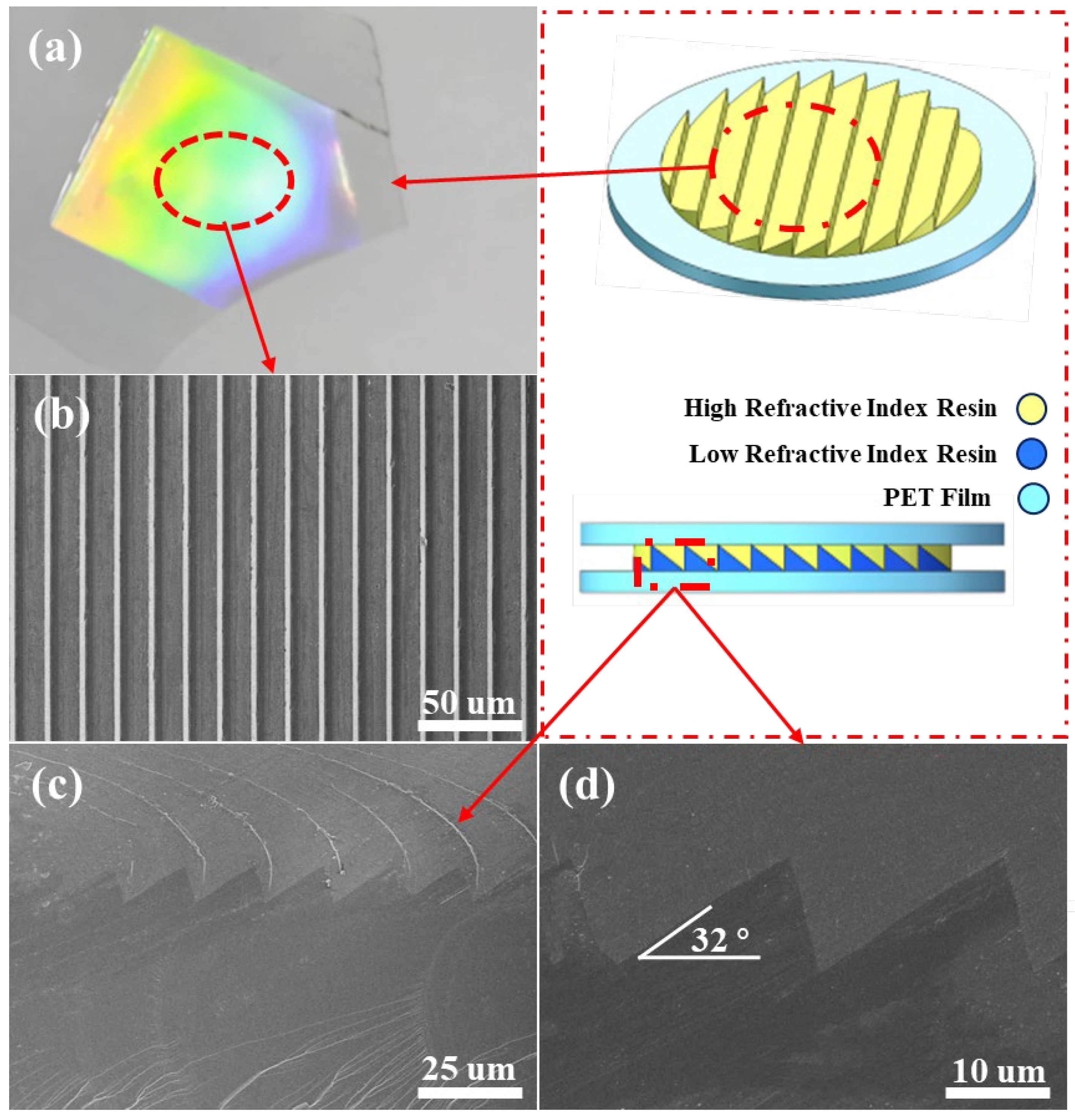
3.4. The Structure of ARCs Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da, Y.; Xuan, Y.; Li, Q. From light trapping to solar energy utilization: A novel photovoltaic–thermoelectric hybrid system to fully utilize solar spectrum. Energy 2016, 95, 200–210. [Google Scholar] [CrossRef]
- Gbashi, K.R. Investigation of morphological, optical and structural properties of multi-layer coatings for solar energy. Appl. Phys. A 2020, 126, 275. [Google Scholar] [CrossRef]
- Leem, J.W.; Choi, M.; Yu, J.S. Multifunctional Microstructured Polymer Films for Boosting Solar Power Generation of Silicon-Based Photovoltaic Modules. ACS Appl. Mater. Interfaces 2015, 7, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-X.; Chen, K.-J.; Chen, P.-Y.; Jan, J.-S. Broadband Antireflection Coatings Based on Low Surface Energy/Refractive Index Silica/Fluorinated Polymer Nanocomposites. ACS Appl. Nano Mater. 2018, 1, 741–750. [Google Scholar] [CrossRef]
- Hou, G.; García, I.; Rey-Stolle, I. High-low refractive index stacks for broadband antireflection coatings for multijunction solar cells. Sol. Energy 2021, 217, 29–39. [Google Scholar] [CrossRef]
- Hu, X.; Yao, B.; Liu, J.; Liu, J.; Chen, K.; Yan, M.; Wang, L. Preparation and properties of high refractive index UV-cured titanium hybrid optical materials. Mater. Lett. 2020, 265, 127466. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Chen, Y.; Yang, Z.; Chen, M.; Qin, Z. High-refractive index polythiourethane resin based on 2,3-bis((2-mercaptoethyl) thio)-1-propanethiol and 1,3-bis(isocyanantomethyl) cyclohexane using tertiary amine catalyst. J. Appl. Polym. Sci. 2020, 138, 50278. [Google Scholar] [CrossRef]
- Park, C.Y.; Choi, B. Enhanced Light Extraction from Bottom Emission OLEDs by High Refractive Index Nanoparticle Scattering Layer. Nanomaterials 2019, 9, 1241. [Google Scholar] [CrossRef] [Green Version]
- Cody, D.; Gribbin, S.; Mihaylova, E.; Naydenova, I. Low-Toxicity Photopolymer for Reflection Holography. ACS Appl. Mater. Interfaces 2016, 8, 18481–18487. [Google Scholar] [CrossRef]
- Badur, T.; Dams, C.; Hampp, N. High Refractive Index Polymers by Design. Macromolecules 2018, 51, 4220–4228. [Google Scholar] [CrossRef]
- Koshelev, A.; Calafiore, G.; Pina-Hernandez, C.; Allen, F.I.; Dhuey, S.; Sassolini, S.; Wong, E.; Lum, P.; Munechika, K.; Cabrini, S. High refractive index Fresnel lens on a fiber fabricated by nanoimprint lithography for immersion applications. Opt. Lett. 2016, 41, 3423–3426. [Google Scholar] [CrossRef]
- Bari, G.A.R.; Kim, H. High-refractive-index and high-barrier-capable epoxy-phenoxy-based barrier film for organic electronics. Polym. Adv. Technol. 2020, 31, 1752–1764. [Google Scholar] [CrossRef]
- Tong, L.; Feng, Y.; Sun, X.; Han, Y.; Jiao, D.; Tan, X. High refractive index adamantane-based silicone resins for the encapsulation of light-emitting diodes. Polym. Adv. Technol. 2018, 29, 2245–2252. [Google Scholar] [CrossRef]
- Macdonald, E.K.; Shaver, M.P. Intrinsic high refractive index polymers. Polym. Int. 2015, 64, 6–14. [Google Scholar] [CrossRef]
- Lorentz, H.A. Ueber die Beziehung zwischen der Fortpflanzungsgeschwindigkeit des Lichtes und der Körperdichte. Ann. Phys. 1880, 245, 641–665. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, L. Ueber die refractionsconstante. Ann. Phys. 1880, 247, 70–103. [Google Scholar] [CrossRef] [Green Version]
- Seferis, J.; Brandrup, J.; Immergut, E. Polymer Handbook; Wiley: New York, NY, USA, 1989; Volume 3, p. 45. [Google Scholar]
- Hadis, M.A.; Tomlins, P.H.; Shortall, A.C.; Palin, W.M. Dynamic monitoring of refractive index change through photoactive resins. Dent. Mater. 2010, 26, 1106–1112. [Google Scholar] [CrossRef]
- Su, M.; Sun, Y.; Chen, B.; Zhang, Z.; Yang, X.; Chen, S.; Pan, Q.; Zuev, D.; Belov, P.; Song, Y. A fluid-guided printing strategy for patterning high refractive index photonic microarrays. Sci. Bull. 2021, 66, 250–256. [Google Scholar] [CrossRef]
- Tokushita, Y.; Watanabe, A.; Torii, A.; Nakabayashi, K.; Samitsu, S.; Mori, H. Photocurable selenophene/maleimide-based high-refractive-index copolymers obtained via radical copolymerization. React. Funct. Polym. 2021, 165, 104960. [Google Scholar] [CrossRef]
- Okutsu, R.; Suzuki, Y.; Ando, S.; Ueda, M. Poly (thioether sulfone) with high refractive index and high Abbe’s number. Macromolecules 2008, 41, 6165–6168. [Google Scholar] [CrossRef]
- Pan, Z.; Zeng, K.; Huang, B.; Zhu, L. Synthesis of Hydrogen-Containing Methyl Phenyl Silicone Resins with a High Refractive Index for LED Encapsulation. J. Electron. Mater. 2020, 49, 4816–4821. [Google Scholar] [CrossRef]
- Wadi, V.S.; Jena, K.K.; Halique, K.; Brigita, R.; Cmok, L.; Tzitzios, V.; Alhassan, S.M. Scalable High Refractive Index polystyrene-sulfur nanocomposites via in situ inverse vulcanization. Sci. Rep. 2020, 10, 14924. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Choi, K.; Choi, J.S.; Pyun, J.; Lim, J.; Char, K.; Im, S.G. One-step vapor-phase synthesis of transparent high refractive index sulfur-containing polymers. Sci. Adv. 2020, 6, eabb5320. [Google Scholar]
- Catel, Y.; Angermann, J.; Fassler, P.; Fischer, U.; Schnur, T.; Moszner, N. High refractive index monofunctional monomers as promising diluents for dental composites. Dent. Mater. 2021, 37, 351–358. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Liu, X.; Yang, X.; Zhang, Y.; Yu, R.; Zuo, X.; Huang, W. Synthesis and characterization of thianthrene-based epoxy with high refractive index over 1.7. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 33–40. [Google Scholar] [CrossRef]
- Zuo, J.; Liu, Z.; Luo, C.; Lin, F. Preparation and properties of high refractive index polythioetherimide film based on thiol-ene click chemistry. Prog. Org. Coat. 2021, 151, 106048. [Google Scholar] [CrossRef]
- Hifumi, R.; Tomita, I. Synthesis and high refractive index properties of poly(thiophosphonate)s. Polym. J. 2018, 50, 467–471. [Google Scholar] [CrossRef]
- Mavila, S.; Sinha, J.; Hu, Y.; Podgorski, M.; Shah, P.K.; Bowman, C.N. High Refractive Index Photopolymers by Thiol-Yne “Click” Polymerization. ACS Appl. Mater. Interfaces 2021, 13, 15647–15658. [Google Scholar] [CrossRef]
- Jeong, M.K.; Choi, W.T.; Ahn, B.-H.; Gal, Y.-S.; Lim, K.T. High refractive index organic/inorganic hybrid films prepared by carbazole phenoxy based copolymers and titanium alkoxide. Mol. Cryst. Liq. Cryst. 2019, 686, 45–54. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, D.H.; Goh, M.; Kim, J.G.; You, N.-H. Synthesis and characterization of UV-Curable pyrimidine-based Poly(Acrylate) and zirconium acrylate nanocomposite with high refractive index. Polymer 2021, 227, 123847. [Google Scholar] [CrossRef]
- Iino, S.; Sobu, S.; Nakabayashi, K.; Samitsu, S.; Mori, H. Highly transparent and photopatternable spirobifluorene-based polythioethers with high refractive indices via thiol-ene click chemistry. Polymer 2021, 224, 123725. [Google Scholar] [CrossRef]
- Fu, M.C.; Ueda, M.; Ando, S.; Higashihara, T. Development of Novel Triazine-Based Poly(phenylene sulfide)s with High Refractive Index and Low Birefringence. ACS Omega 2020, 5, 5134–5141. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Hu, X.; Liu, J.; Chen, K.; Liu, J. Preparation and properties of high refractive index ZrO2 nano-hybrid materials. Mater. Lett. 2020, 261, 126878. [Google Scholar] [CrossRef]
- Lü, C.; Cui, Z.; Wang, Y.; Li, Z.; Guan, C.; Yang, B.; Shen, J. Preparation and characterization of ZnS–polymer nanocomposite films with high refractive index. J. Mater. Chem. 2003, 13, 2189–2195. [Google Scholar] [CrossRef]
- Chen, X.; Fang, L.; Wang, J.; He, F.; Chen, X.; Wang, Y.; Zhou, J.; Tao, Y.; Sun, J.; Fang, Q. Intrinsic High Refractive Index Siloxane–Sulfide Polymer Networks Having High Thermostability and Transmittance via Thiol–Ene Cross-Linking Reaction. Macromolecules 2018, 51, 7567–7573. [Google Scholar] [CrossRef]
- Legrand, S.; Hannu-Kuure, M.; Kärkkäinen, A. Design, preparation, and characterization of new high-refractive index hybrid organic–inorganic polysiloxanes: Innovative coatings for optoelectronic applications. J. Appl. Polym. Sci. 2020, 138, 49877. [Google Scholar] [CrossRef]
- Dan, S.; Gu, H.; Tan, J.; Zhang, B.; Zhang, Q. Transparent epoxy/TiO2 optical hybrid films with tunable refractive index prepared via a simple and efficient way. Prog. Org. Coat. 2018, 120, 252–259. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, M.; Tang, H.; Sun, M.; Liu, P.; Liu, Y.; Chen, L.; Li, D.; Wu, D.; Hao, J.; et al. Enhanced light emission of quantum dot films by scattering of poly(zinc methacrylate) coating CdZnSeS/ZnS quantum dots and high refractive index BaTiO3 nanoparticles. RSC Adv. 2020, 10, 31705–31710. [Google Scholar] [CrossRef]
- Wildner, W.; Drummer, D. Nanofiller materials for transparent polymer composites: Influences on the properties and on the transparency—A review. J. Thermoplast. Compos. Mater. 2019, 32, 1547–1565. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zheng, J.; Lu, M.; Wu, K. Modified epoxy acrylate resin for photocurable temporary protective coatings. Prog. Org. Coat. 2015, 89, 17–25. [Google Scholar] [CrossRef]
- Cheng, F.; Fan, Y.; Zhang, L.; Jiao, X.; Lai, G.; Hua, X.; Yang, X. An Easy Fabrication Method to Prepare Inexpensive UV–Cured Transparent Silicone Modified Polyacrylate Coatings with Good Adhesion and UV Resistance. Coatings 2020, 10, 675. [Google Scholar] [CrossRef]
- Vang, K.C.; Yu, T.H.; David, M. 52.5: Broadband Antireflection (BBAR) Nanostructure Contrast Enhancement (NCEF) Films For Electronic Optical Display Applications; SID Symposium Digest of Technical Papers; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 770–772. [Google Scholar]
- Ostrovsky, N.; Yehuda, D.; Tzadka, S.; Kassis, E.; Joseph, S.; Schvartzman, M. Direct Imprint of Optical Functionalities on Free-Form Chalcogenide Glasses. Adv. Opt. Mater. 2019, 7, 1900652. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhong, M.; Lu, Y.; Jiajie, Y.; Yongmin, Z.; He, X. Synthesis of NSAIDs–Se derivatives as potent anticancer agents. Med. Chem. Res. 2018, 27, 2071–2078. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, C.; Li, Y.; Liang, J.; Liu, J.; Qian, T.; Ding, J.; Cao, Y.C. Preparation of carbon quantum dots based high photostability luminescent membranes. Luminescence 2017, 32, 625–630. [Google Scholar] [CrossRef]
- Tang, S.; Lan, Q.; Xu, L.; Liang, J.; Lou, P.; Liu, C.; Mai, L.; Cao, Y.-C.; Cheng, S. A novel cross-linked nanocomposite solid-state electrolyte with super flexibility and performance for lithium metal battery. Nano Energy 2020, 71, 104600. [Google Scholar] [CrossRef]
- Guo, S.; Lv, Y.; Chen, Y.; Cai, S. Amine-containing olefin copolymer with CO2-switchable oil absorption and desorption. J. Appl. Polym. Sci. 2019, 136, 47439. [Google Scholar] [CrossRef]
- Wei, Y.; Su, Y.; Liu, C.; Nie, X.; Liu, Z.; Zhang, Y.; Zhang, Y. Two-channel SPR sensor combined application of polymer-and vitreous-clad optic fibers. Sensors 2017, 17, 2862. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Liang, X.; Jiang, Y.; Wang, S.; Qi, Y.; Liu, Y.; Yu, L.; Yang, H.; Zhao, X.Z. High-Efficiency and Reliable Smart Photovoltaic Windows Enabled by Multiresponsive Liquid Crystal Composite Films and Semi-Transparent Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1900720. [Google Scholar] [CrossRef]
- Lv, R.; Bei, Z.; Huang, Y.; Chen, Y.; Zheng, Z.; You, Q.; Zhu, C.; Cao, Y. Mussel-Inspired Flexible, Wearable, and Self-Adhesive Conductive Hydrogels for Strain Sensors. Macromol. Rapid Commun. 2020, 41, 1900450. [Google Scholar] [CrossRef]
- Pan, G.; Du, Z.; Zhang, C.; Li, C.; Yang, X.; Li, H. Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety. Polymer 2007, 48, 3686–3693. [Google Scholar] [CrossRef]
- Yan, R.; Yang, D.; Zhang, N.; Zhao, Q.; Liu, B.; Xiang, W.; Sun, Z.; Xu, R.; Zhang, M.; Hu, W. Performance of UV curable lignin based epoxy acrylate coatings. Prog. Org. Coat. 2018, 116, 83–89. [Google Scholar] [CrossRef]
- Chen, K.; Tian, C.; Cao, F.; Liang, S.; Jia, X.; Wang, J. Preparation and characterization of highly thermostable polyisocyanurate foams modified with epoxy resin. J. Appl. Polym. Sci. 2016, 133, 43085. [Google Scholar] [CrossRef]
- Pal, R.; Sudhi, S.; Raghavan, R. Fabrication and evaluation of structural film adhesive using oxazolidinone modified novolac epoxy resin. J. Appl. Polym. Sci. 2019, 136, 47520. [Google Scholar] [CrossRef]
- Chian, K.; Yi, S. Synthesis and characterization of an isocyanurate–oxazolidone polymer: Effect of stoichiometry. J. Appl. Polym. Sci. 2001, 82, 879–888. [Google Scholar] [CrossRef]
- Pretorius, F.; Focke, W.W.; Androsch, R.; du Toit, E. Estimating binary liquid composition from density and refractive index measurements: A comprehensive review of mixing rules. J. Mol. Liq. 2021, 332, 115893. [Google Scholar] [CrossRef]
- Carnegie, M.R.; Sherine, A.; Sivagami, D.; Sakthivel, S. Anti-reflection coatings with enhanced abrasion and scratch resistance properties. J. Sol-Gel Sci. Technol. 2016, 78, 176–186. [Google Scholar] [CrossRef]
- Sprokel, G.; Santo, R.; Swalen, J. Determination of the surface tilt angle by attenuated total reflection. Mol. Cryst. Liq. Cryst. 1981, 68, 29–38. [Google Scholar] [CrossRef]



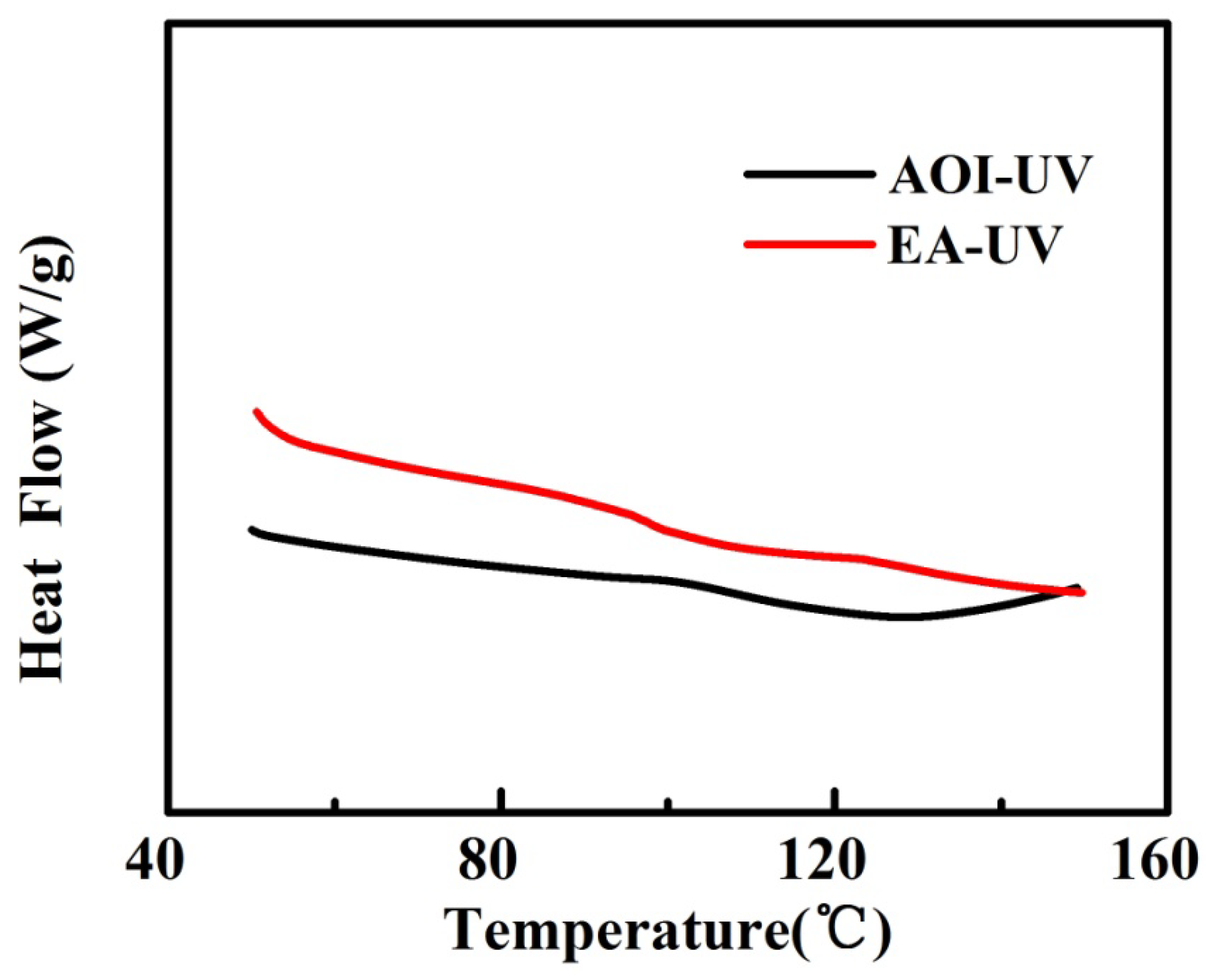
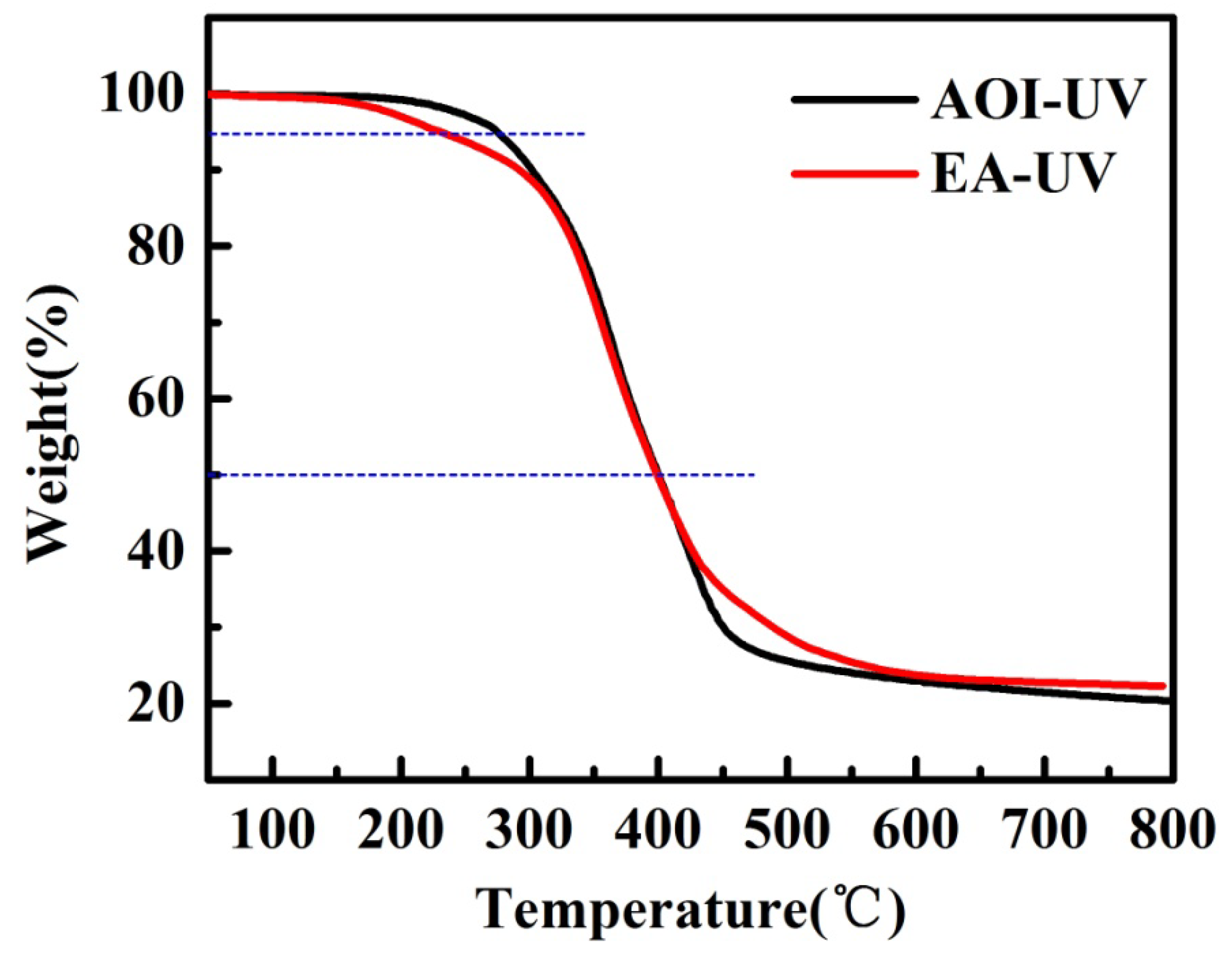
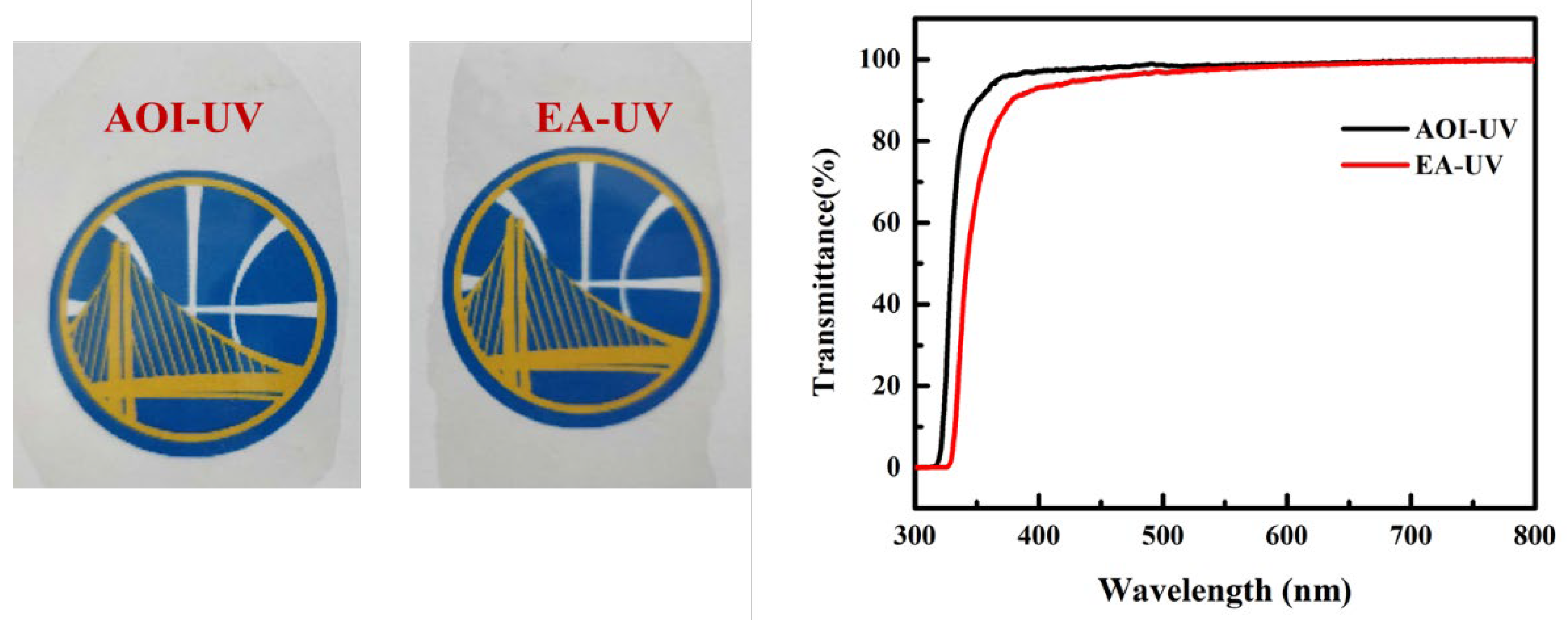
| Film | T1/°C | T5/°C | T10/°C | T50/°C | DTG/°C | Residue @600 °C |
|---|---|---|---|---|---|---|
| EA-UV | 153.60 | 230.21 | 291.70 | 396.39 | 370.73 | 20.36% |
| AOI-UV | 209.40 | 274.42 | 301.80 | 400.31 | 382.38 | 23.00% |
| Film (25 μm) | λcut-off (nm) 1 | T450 (%) 2 | Tmax (%) 3 | Refractive Index |
|---|---|---|---|---|
| EA-UV | 328 | 95.38 | 99.83 | 1.633 |
| AOI-UV | 318 | 97.9 | 99.86 | 1.667 |
| Film | n | Transmittance | Critical Angle of Reflection | |
|---|---|---|---|---|
| PET | 1.5 | ≥93% | 69.639° | 56.758° |
| Yellow Layer | 1.67 | ≥97% | ||
| Blue Layer | 1.38 | ≥93% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, B.; Song, H.; Zhao, C.; Liang, S.; Dong, Z.; Yu, J. High Refractive Index Diphenyl Sulfide Photopolymers for Solar Cell Antireflection Coatings. Energies 2022, 15, 3972. https://doi.org/10.3390/en15113972
Zhang J, Li B, Song H, Zhao C, Liang S, Dong Z, Yu J. High Refractive Index Diphenyl Sulfide Photopolymers for Solar Cell Antireflection Coatings. Energies. 2022; 15(11):3972. https://doi.org/10.3390/en15113972
Chicago/Turabian StyleZhang, Jingran, Baozhu Li, Heran Song, Chen Zhao, Songfeng Liang, Zhurong Dong, and Jie Yu. 2022. "High Refractive Index Diphenyl Sulfide Photopolymers for Solar Cell Antireflection Coatings" Energies 15, no. 11: 3972. https://doi.org/10.3390/en15113972
APA StyleZhang, J., Li, B., Song, H., Zhao, C., Liang, S., Dong, Z., & Yu, J. (2022). High Refractive Index Diphenyl Sulfide Photopolymers for Solar Cell Antireflection Coatings. Energies, 15(11), 3972. https://doi.org/10.3390/en15113972