Synthesis of Donor–Acceptor Copolymers Derived from Diketopyrrolopyrrole and Fluorene via Eco-Friendly Direct Arylation: Nonlinear Optical Properties, Transient Absorption Spectroscopy, and Theoretical Modeling
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Equipment
2.2. Synthesis and Characterization
Synthesis of PFDPP Polymers (General Procedure)
2.3. Elaborating Polymer Nanoparticles by the Reprecipitation Method
2.4. Elaborating Organic Thin Films
3. Results and Discussions
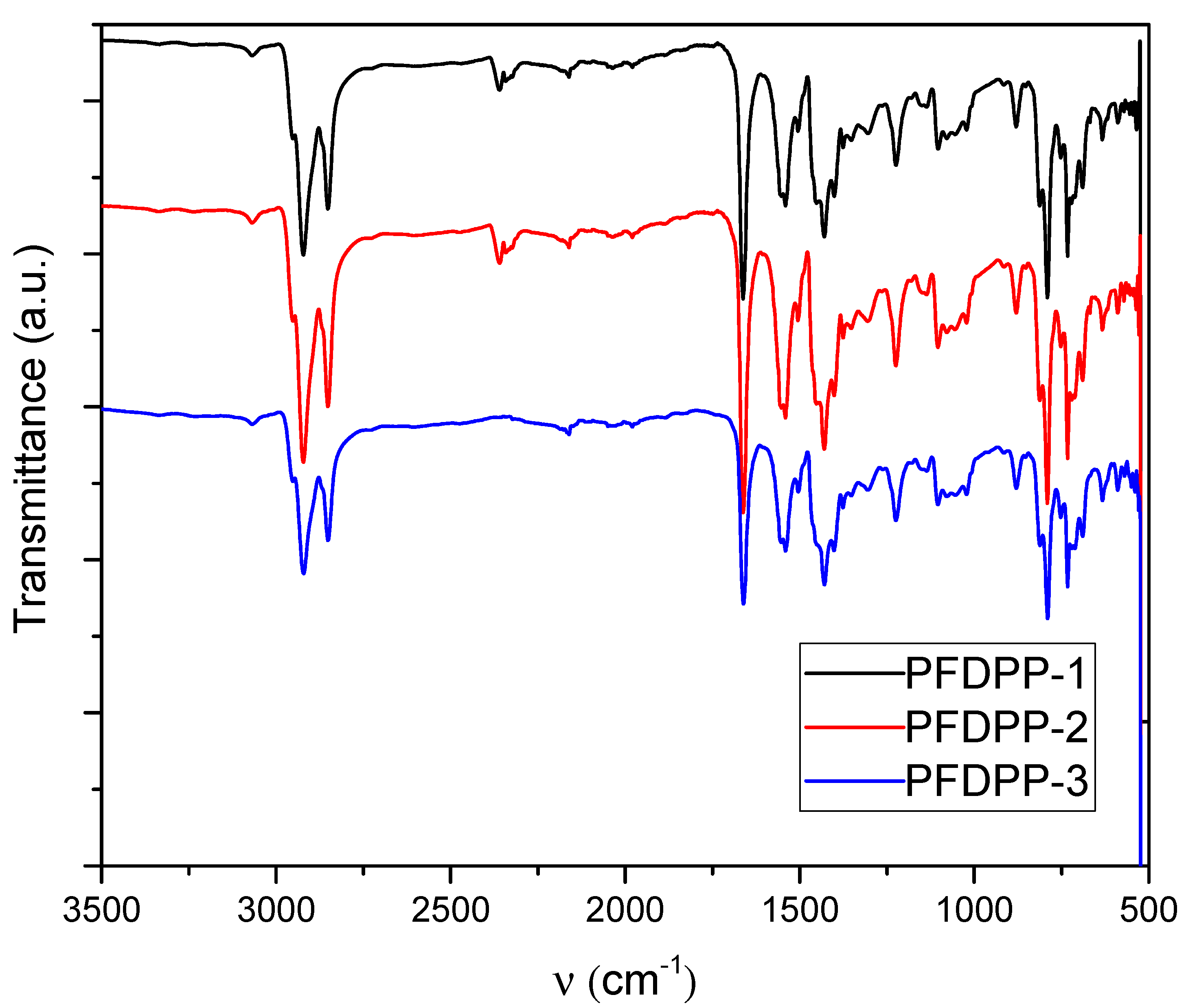
3.1. FT-IR
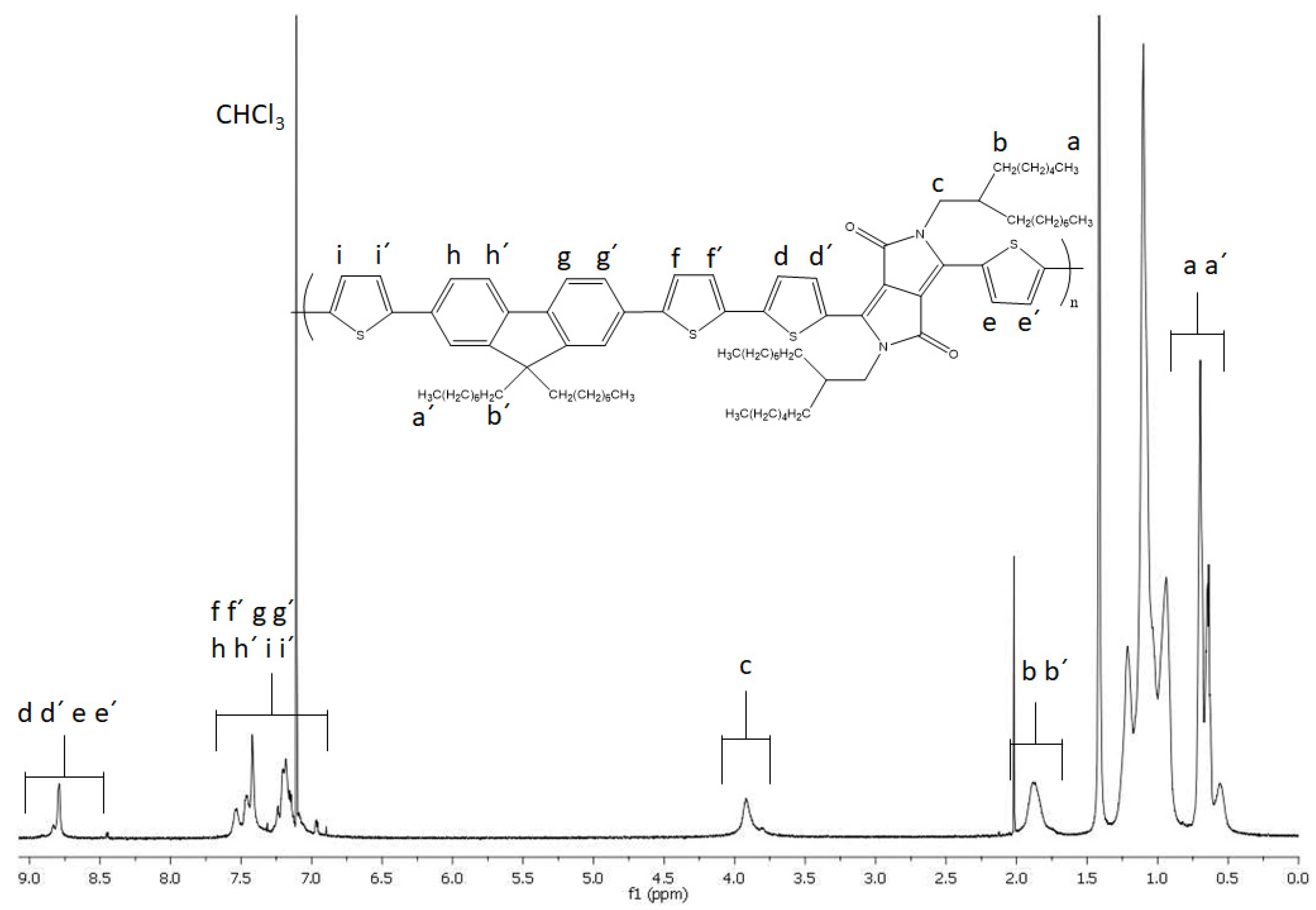
3.2. 1H NMR
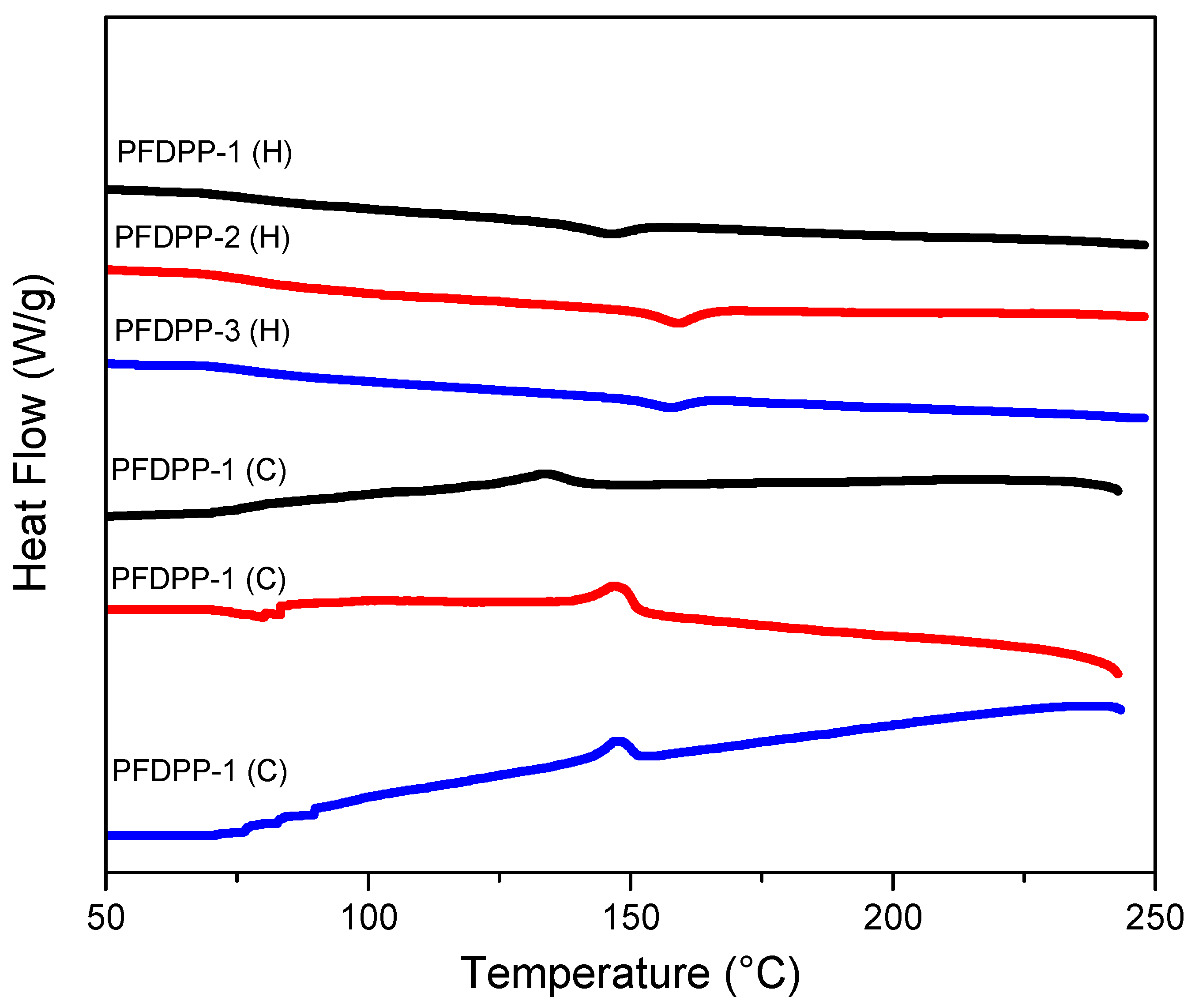
3.3. Differential Scanning Calorimetry and Thermogravimetric Analysis (DSC-TGA)
3.4. Gel Permeation Chromatography (GPC)
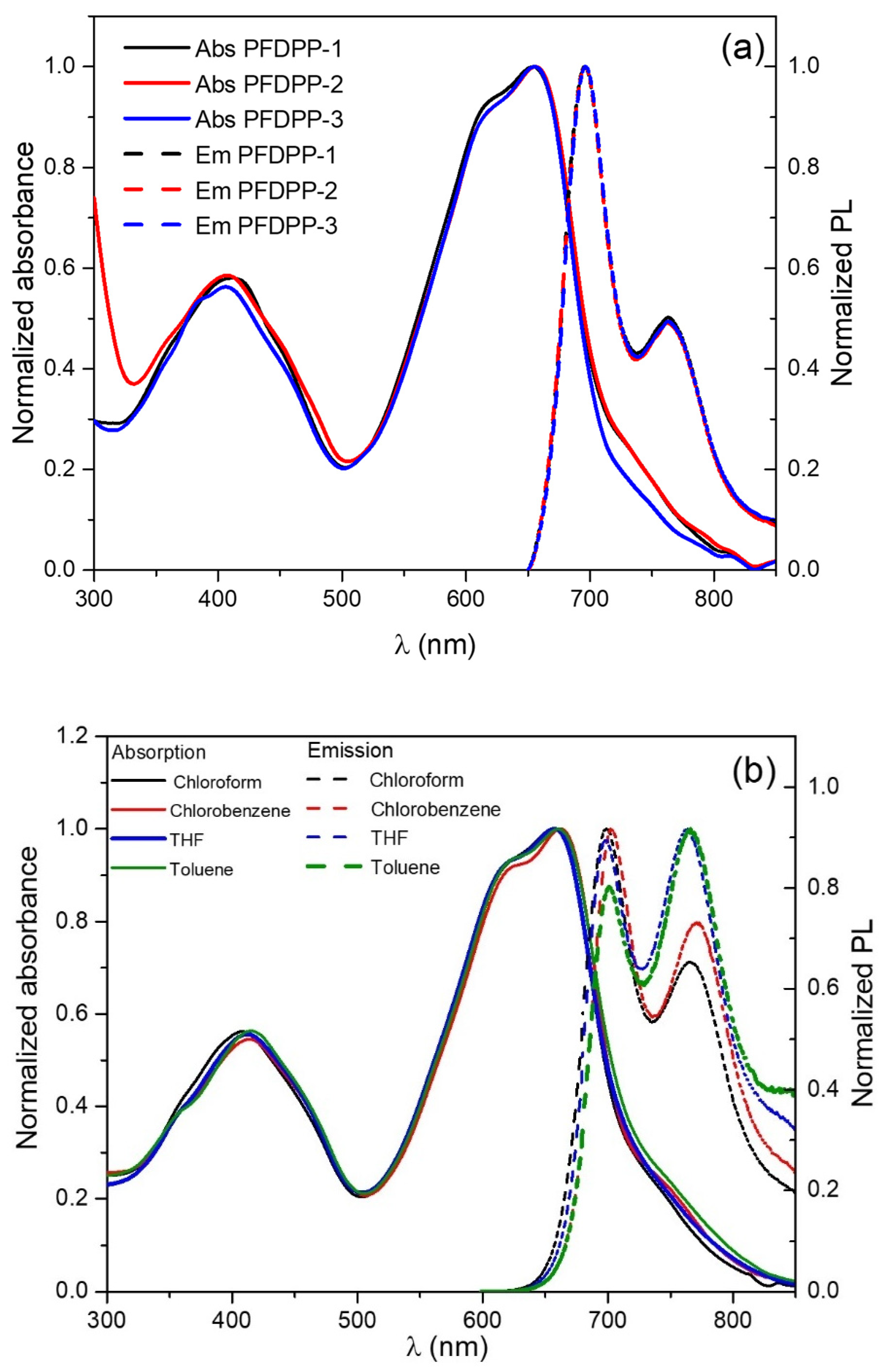
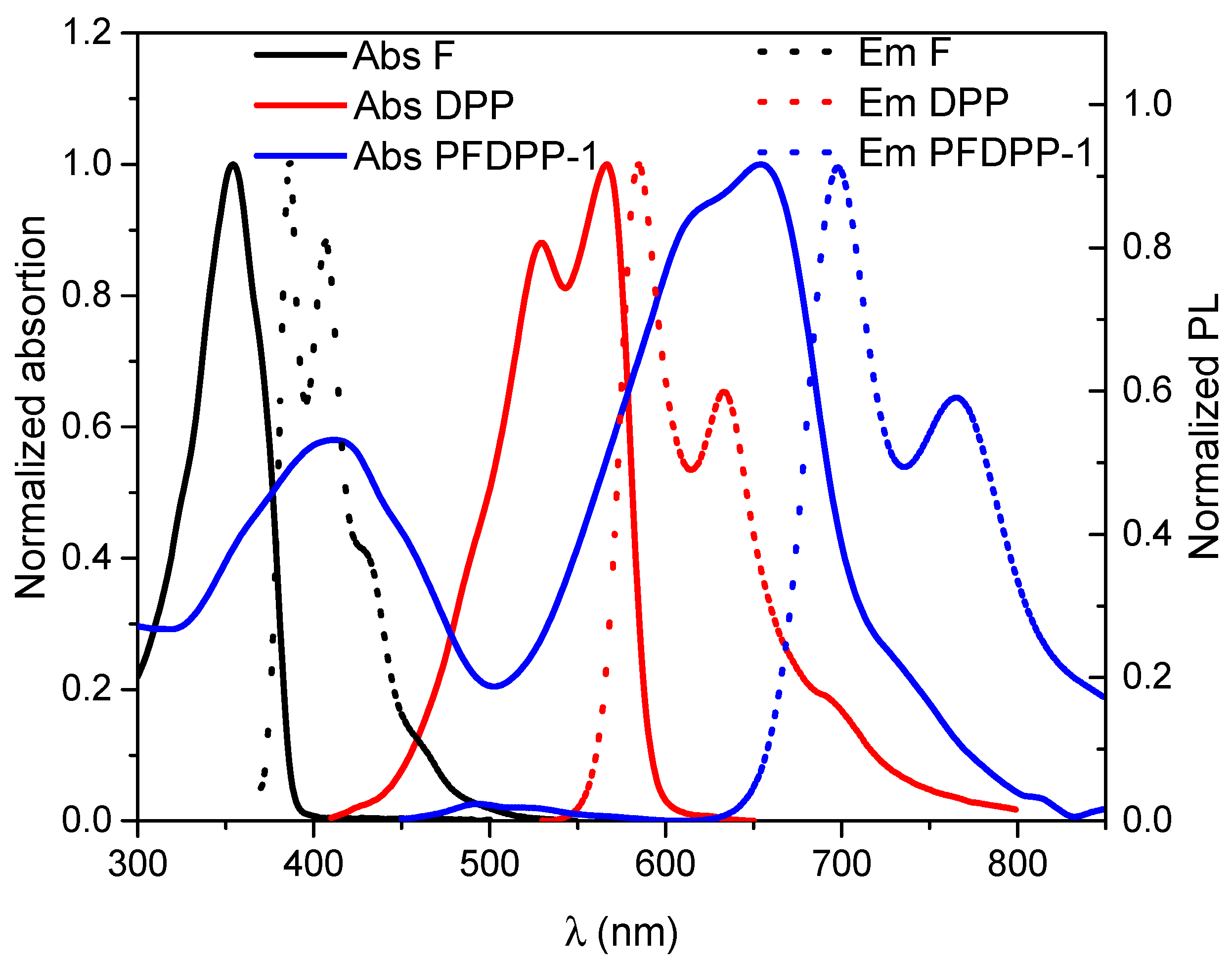
3.5. Photophysical Properties: Absorption and Emission Spectra
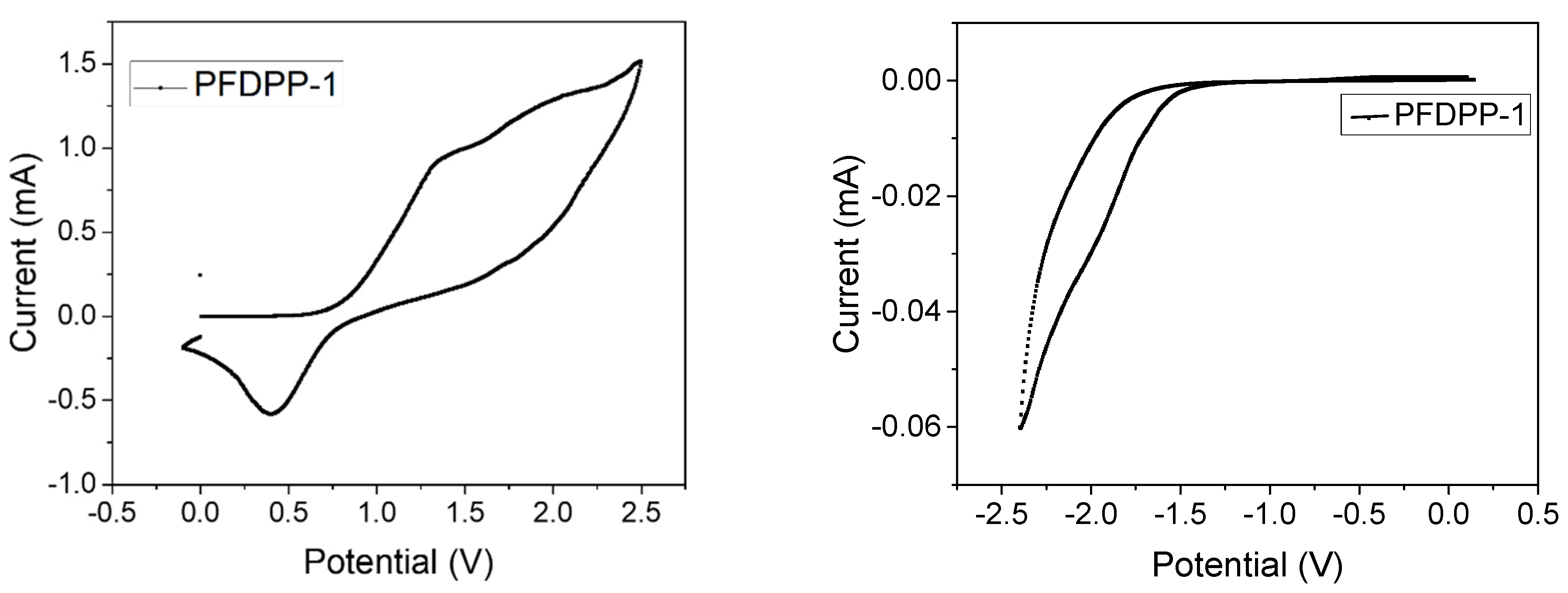
3.6. Electrochemical Properties
3.7. Organic Nanoparticles and Films
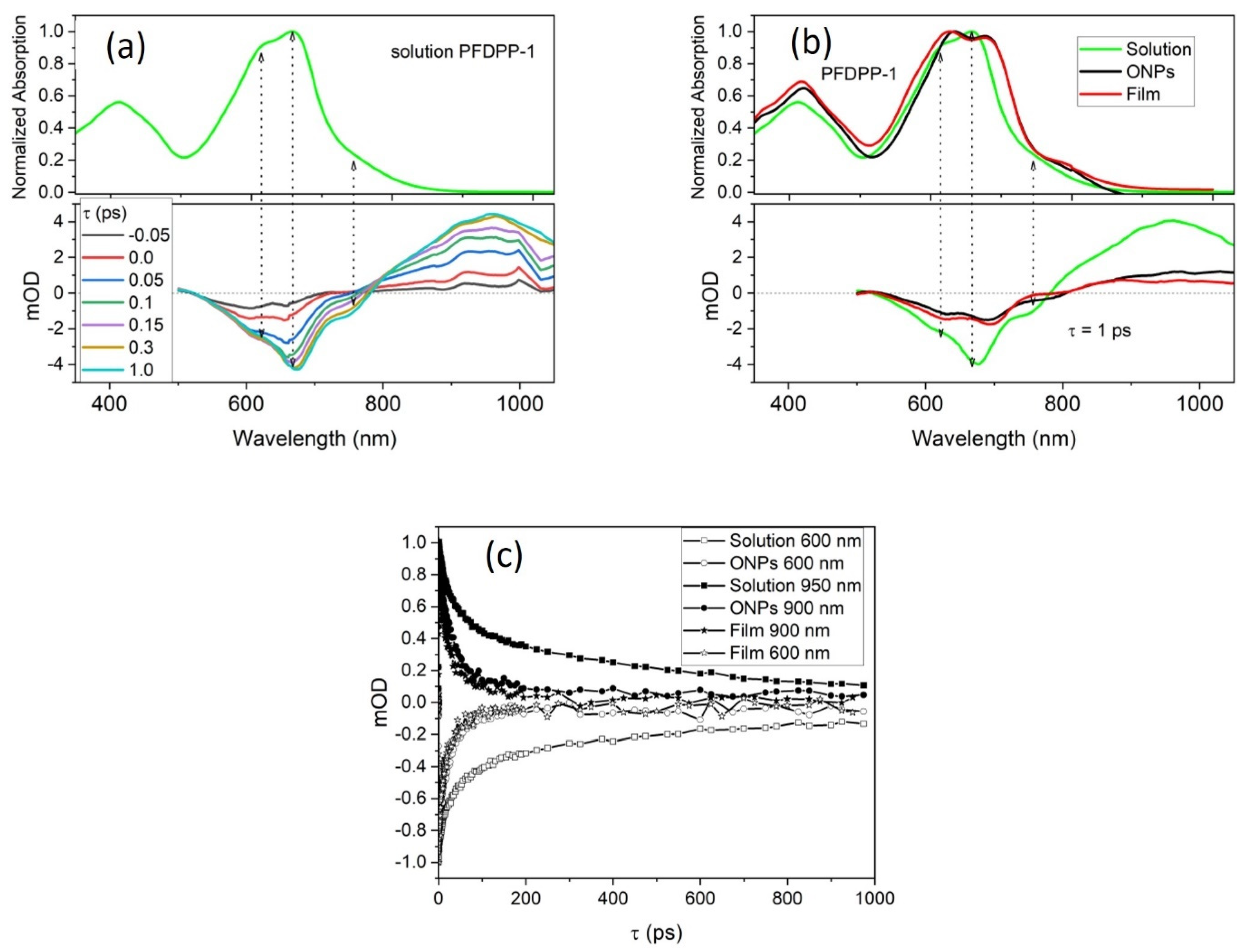
3.8. Transient Absorption
3.9. Nonlinear Optical Properties
3.10. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azadinia, M.; Boroumand, F.; Fathollahi, M.; Mohajerani, E. Polyfluorene copolymer/Al Schottky junction for UV-A photodetector with relatively high stability and photocurrent density. Opt. Commun. 2020, 458, 124809. [Google Scholar] [CrossRef]
- Kim, M.; Ryu, S.; Park, S.; Choi, K.; Kim, T.; Chung, D.; Park, T. Donor–Acceptor-Conjugated Polymer for High-Performance Organic Field-Effect Transistors: A Progress Report. Adv. Funct. Mater. 2019, 30, 1904545. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J. Structures and properties of conjugated Donor-Acceptor copolymers for solar cell applications. J. Mater. Chem. 2012, 22, 4178–4187. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, Y.; Huang, J. Towards high performance organic photovoltaic cells: A review of recent development in organic photovoltaics. Polymers 2014, 6, 2473–2509. [Google Scholar] [CrossRef]
- Wei, Z.; Xin, F.; Zhang, J.; Wu, M.; Qiu, T.; Lan, Y.; Qiao, S.; Liu, X.; Liu, J. Donor–acceptor conjugated polymer-based nanoparticles for highly effective photoacoustic imaging and photothermal therapy in the NIR-II window. Chem. Commun. 2020, 56, 1093–1096. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.; Xu, T.; Yu, L. Stille Polycondensation for Synthesis of Functional Materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-Coupling Reactions of Organoboranes: An Easy Way to Construct C-C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef]
- Negishi, E. Magical Power of Transition Metals: Past, Present, and Future (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6738–6764. [Google Scholar] [CrossRef]
- Kiriy, A.; Senkovskyy, V.; Sommer, M. Kumada Catalyst-Transfer Polycondensation: Mechanism, Opportunities, and Challenges. Macromol. Rapid Commun. 2011, 32, 1503–1517. [Google Scholar] [CrossRef]
- Grenier, F.; Goudreau, K.; Leclerc, M. Robust Direct (Hetero)arylation Polymerization in Biphasic Conditions. J. Am. Chem. Soc. 2017, 139, 2816–2824. [Google Scholar] [CrossRef]
- Pouliot, J.; Grenier, F.; Terence, J.; Beaupré, S.; Leclerc, M. Direct (Hetero)arylation Polymerization: Simplicity for Conjugated Polymer Synthesis. Chem. Rev. 2016, 116, 14225–14274. [Google Scholar] [CrossRef] [PubMed]
- Matsidik, R.; Komber, H.; Luzio, A.; Caironi, M.; Somme, M. Defect-free Naphthalene Diimide Bithiophene Copolymers with Controlled Molar Mass and High Performance via Direct Arylation Polycondensation. J. Am. Chem. Soc. 2015, 137, 6705–6711. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hendriks, K.; Wienk, M.; Janssen, R. Diketopyrrolopyrrole Polymers for Organic Solar Cells. Acc. Chem. Res. 2016, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bottle, S.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2019, 32, 1903882. [Google Scholar] [CrossRef] [PubMed]
- Chochos, C.L.; Katsouras, A.; Drakopoulou, S.; Miskaki, C.; Krassas, M.; Tzourmpakis, P.; Kakavelakis, G.; Sprau, C.; Colsmann, A.; Squeo, B.; et al. Effects of Alkyl Side Chains Positioning and Presence of Fused Aromatic Units in the Backbone of Low-Bandgap Diketopyrrolopyrrole Copolymers on the Optoelectronic Properties of Organic Solar Cells. J. Polym. Sci. A Polym. Chem. 2018, 56, 138–146. [Google Scholar] [CrossRef]
- Wu, W.; Liu, C.; Chen, W. Synthesis and characterization of new fluorene-acceptor alternating and random copolymers for light-emitting applications. Polymer 2006, 47, 527–538. [Google Scholar] [CrossRef]
- Kawano, Y.; Ito, Y.; Ito, S.; Tanaka, K.; Chujo, Y. π-Conjugated Copolymers Composed of Boron Formazanate and Their Application for a Wavelength Converter to Near-Infrared Light. Macromolecules 2021, 54, 1934–1942. [Google Scholar] [CrossRef]
- Xie, C.; Zhou, W.; Zeng, Z.; Fan, Q.; Pu, K. Grafted Semiconducting Polymer Amphiphiles for Multimodal Optical Imaging and Combination Phototherapy. Chem. Sci. 2020, 11, 10553–10570. [Google Scholar] [CrossRef]
- Mukhopadhyaya, T.; Wagner, J.; Fan, H.; Katz, H. Design and Synthesis of Air-Stable p-Channel-Conjugated Polymers for High Signal-to-Drift Nitrogen Dioxide and Ammonia Sensing. ACS Appl. Mater. Interfaces 2020, 12, 21974–21984. [Google Scholar] [CrossRef]
- Mukhopadhyaya, T.; Katz, H. Trap-dominated nitrogen dioxide and ammonia responses of air-stable p-channel conjugated polymers from detailed bias stress analysis. J. Mater. Chem. C 2021, 9, 3531–3545. [Google Scholar] [CrossRef]
- Kaur, M.; Lee, D.; Yang, D.; Um, H.; Cho, M.; Kang, J.; Cho, D. Diketopyrrolopyrrole-bitellurophene containing a conjugated polymer and its high performance thin-film transistor sensor for bromine detection. Chem. Commun. 2014, 50, 14394–14396. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lin, W.; Huang, B.; Zhang, J.; Long, X.; Zhanga, W.; Zhang, Q. The design strategies and applications for organic multi-branched two-photon absorption chromophores with novel cores and branches: A recent review. J. Mater. Chem. C 2021, 9, 1520–1536. [Google Scholar] [CrossRef]
- Shen, X.; Li, L.; Chow, A.; Gao, N.; Yao, S.; Xu, Q. Water-Soluble Conjugated Polymers for Simultaneous Two-Photon Cell Imaging and Two-Photon Photodynamic Therapy. Adv. Opt. Mater. 2013, 1, 92–99. [Google Scholar] [CrossRef]
- Belfield, K.; Yao, S.; Bondar, M. Two-photon Absorbing Photonic Materials: From Fundamentals to Applications. Adv. Polym. Sci. 2008, 213, 97–156. [Google Scholar] [CrossRef]
- Bao, W.W.; Li, R.; Dai, Z.C.; Tang, J.; Shi, X.; Geng, J.T.; Deng, Z.F.; Hua, J. Diketopyrrolopyrrole (DPP)-Based Materials and Its Applications: A Review. Front. Chem. 2020, 8, 679. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Breazu, C.; Costas, A.; Petre, G.; Stanculescu, A.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G. Organic Thin Films Based on DPP-DTT:C60 Blends Deposited by MAPLE. Nanomaterials 2020, 10, 2366. [Google Scholar] [CrossRef]
- Liu, X.; Kong, L.; Du, H.; Zhang, Y.; Zhao, J.; Xie, Y. Synthesis and electrochromic properties of electrochromic polymers based on propylenedioxythiophene, diketopyrrolopyrrole and benzodithiophene units. Org. Electron. 2019, 64, 223–235. [Google Scholar] [CrossRef]
- Gora, M.; Krzywiec, W.; Mieczkowski, J.; Maia, E.C.R.; Louarn, G.; Zagorska, M.; Pron, A. Alternating copolymers of diketopyrrolopyrrole or benzothiadiazole and alkoxy-substituted oligothiophenes: Spectroscopic, electrochemical and spectroelectrochemical investigations. Electrochim. Acta 2014, 144, 211–220. [Google Scholar] [CrossRef]
- Sui, Y.; Shi, Y.; Deng, Y.; Li, R.; Bai, J.; Wang, Z.; Dang, Y.; Han, Y.; Kirby, N.; Ye, L.; et al. Direct Arylation Polycondensation of Chlorinated Thiophene Derivatives to High-Mobility Conjugated Polymers. Appl. Polym. Mater. 2021, 3, 4223–4233. [Google Scholar] [CrossRef]
- Lu, C.; Che, W. Diketopyrrolopyrrole Thiophene-Based Acceptor-Donor-Acceptor Conjugated Materials for High-Performance Field-Effect Transistors. Chem. Asian J. 2013, 8, 2813–2821. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Q.; Zeng, W.; Han, S.; Peng, J.; Liu, S. Synthesis and Characterization of Novel Red-Emitting Alternating Copolymers Based on Fluorene and Diketopyrrolopyrrole Derivatives. Polym. Chem. 2006, 44, 2395–2405. [Google Scholar] [CrossRef]
- Zen, A.; Saphiannikova, M.; Neher, D.; Grenzer, J.; Grigorian, S.; Pietsch, U.; Asawapirom, U.; Janietz, S.; Scherf, U.; Lieberwirth, I.; et al. Effect of molecular weight on the structure and crystallinity of poly(3-hexylthiophene). Macromolecules 2006, 39, 2162–2171. [Google Scholar] [CrossRef]
- Kim, J.; Song, C.; Kim, B.; Kang, I.; Shin, W.; Hwang, D.-H. Thieno[3,2-b]thiophene-Substituted Benzo[1,2-b:4,5-b′]dithiophene as a Promising Building Block for Low Bandgap Semiconducting Polymers for High-Performance Single and Tandem Organi Photovoltaic Cells. Chem. Mater. 2014, 26, 1234–1242. [Google Scholar] [CrossRef]
- Li, W.; Mori, T.; Michinobu, T. Perovskite solar cells based on hole-transporting conjugated polymers by direct arylation polycondensation. MRS Commun. 2018, 8, 1244–1253. [Google Scholar] [CrossRef]
- Zhu, Y.; Rabindranath, A.R.; Beyerlein, T.; Tieke, B. Highly Luminescent 1,4-Diketo-3,6-diphenylpyrrolo[3,4-c]pyrrole-(DPP-) Based Conjugated Polymers Prepared Upon Suzuki Coupling. Macromolecules 2007, 40, 6981–6989. [Google Scholar] [CrossRef]
- SambathKumar, B.; Varathan, E.; Subramanian, V.; Somanathan, N. Design of medium bandgap random terpolymers containing fluorene linked diketopyrrolopyrrole and thiophene co-monomers: An experimental and theoretical study. New J. Chem. 2016, 40, 1377–1386. [Google Scholar] [CrossRef]
- Li, W.; Michinobu, T. Electrochromic Behavior of Donor-Acceptor Polymers Containing Diketopyrrolopyrrole Unit. J. Photopolym. Sci. Technol. 2017, 30, 495–499. [Google Scholar] [CrossRef][Green Version]
- Bura, T.; Beaupre, S.; Ibraikulov, O.; Légaré, M.-A.; Quinn, J.; Lévêque, P.; Heiser, T.; Li, Y.; Leclerc, N.; Leclerc, M. New Fluorinated Dithienyldiketopyrrolopyrrole Monomers and Polymers for Organic Electronics. Macromolecules 2017, 50, 7080–7090. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Deng, J.; Huang, Q.; Peng, Q. Synthesis and photovoltaic performance of low bandgap copolymers based on diketopyrrolopyrrole and tetrathienoacene with different conjugated bridges. J. Mater. Chem. A 2014, 2, 653–662. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, W.M. Macyk, How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Beyerlein, T.; Tieke, B.; Forero, S.; Brutting, W. Red electroluminescence from a 1,4-diketopyrrolo[3,4-c]pyrrole (DPP)-based conjugated polymer. Synth. Met. 2002, 130, 115–119. [Google Scholar] [CrossRef]
- Ruckebusch, C.; Sliwa, M.; Pernot, P.; de Juan, A.; Tauler, R. Comprehensive data analysis of femtosecond transient absorption spectra: A review. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 1–27. [Google Scholar] [CrossRef]
- Wood, S.; Wade, J.; Shahid, M.; Collado-Fregoso, E.; Bradley, D.D.C.; Durrant, J.R.; Heeney, M.; Kim, J.-S. Natures of optical absorption transitions and excitation energy dependent photostability of diketopyrrolopyrrole (DPP)-based photovoltaic copolymers. Energy Environ. Sci. 2015, 8, 3222–3232. [Google Scholar] [CrossRef]
- de la Garza-Rubí, R.M.A.; Güizado-Rodríguez, M.; Mayorga-Cruz, D.; Basurto-Pensado, M.A.; Guerrero-Alvarez, J.A.; Ramos-Ortiz, G.; Rodríguez, M.; Maldonado, J.L. Polythiophene derivative functionalized with disperse red 1 chromophore: Its third-order nonlinear optical properties through Z-scan technique under continuous and femtosecond irradiation. Opt. Mat. 2015, 46, 366–372. [Google Scholar] [CrossRef]
- Vishnumurthy, K.A.; Girish, K.H.; Adhikari, A.V. Synthesis, physicochemical properties and computational study of donor-acceptor polymer for optical limiting application. SN Appl. Sci. 2020, 2, 1727. [Google Scholar] [CrossRef]
- Vazquez, R.J.; Kim, H.; Kobilka, B.M.; Hale, B.J.; Jeffries-EL, M.; Zimmerman, P.M.; Goodson, T. Evaluating the Effect of Heteroatoms on the Photophysical Properties of Donor-Acceptor Conjugated Polymers Based on 2,6-di(thiophen-2-yl) benzo[1,2-b:4,5-b′] difuran: Two-Photon Cross-Section and Ultrafast Time-Resolved Spectroscopy. J. Phys. Chem. C 2017, 121, 14382–14392. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.; Xu, Q. Polyfluorene based conjugated polymer nanoparticles for two-photon live cell imaging. Sci. China Chem. 2018, 61, 88–96. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron -Density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Köster, A.M.; Calaminici, P.; Casida, M.E.; Flores-Moreno, R.; Geudtner, G.; Goursot, A.; Heine, T.; Ipatov, A.; Janetzko, F.; del Campo, J.M.; et al. deMon2k, version 4.4.1; The deMon Developers: Cinvestav, Mexico, 2010; Available online: http://www.deMon-software.com (accessed on 1 May 2022).
- Geudtner, G.; Calaminici, P.; Carmona-Espindola, J.; del Campo, J.M.; Domínguez-Soria, V.D.; Flores-Moreno, R.; Gamboa, G.U.; Goursot, A.; Köster, A.M.; Reveles, J.U.; et al. deMon2k Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 548–555. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian type basis sets for local spin-density functional calculations. 1. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Dunlap, B.I.; Connolly, J.W.D.; Sabin, J.R. On some approximations in applications of X-alpha theory. J. Chem. Phys. 1979, 71, 3396–3402. [Google Scholar] [CrossRef]
- Reveles, J.U.; Köster, A.M. Geometry optimization in density functional methods. J. Comput. Chem. 2004, 25, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Vijay, D.; Varathan, E.; Subramanian, V. Theoretical design of core modified (oxa and thia) porphyrin based organic dyes with bridging thiophene linkers. J. Mater. Chem. A 2013, 1, 4358–4369. [Google Scholar] [CrossRef]
- Casida, M.E. Recent Advances in Density Functional Methods, Part I. In Time-Dependent Density-Functional Response Theory for Molecules; Chong, D.P., Ed.; World Scientific: Singapore, 1995; pp. 155–192. [Google Scholar] [CrossRef]




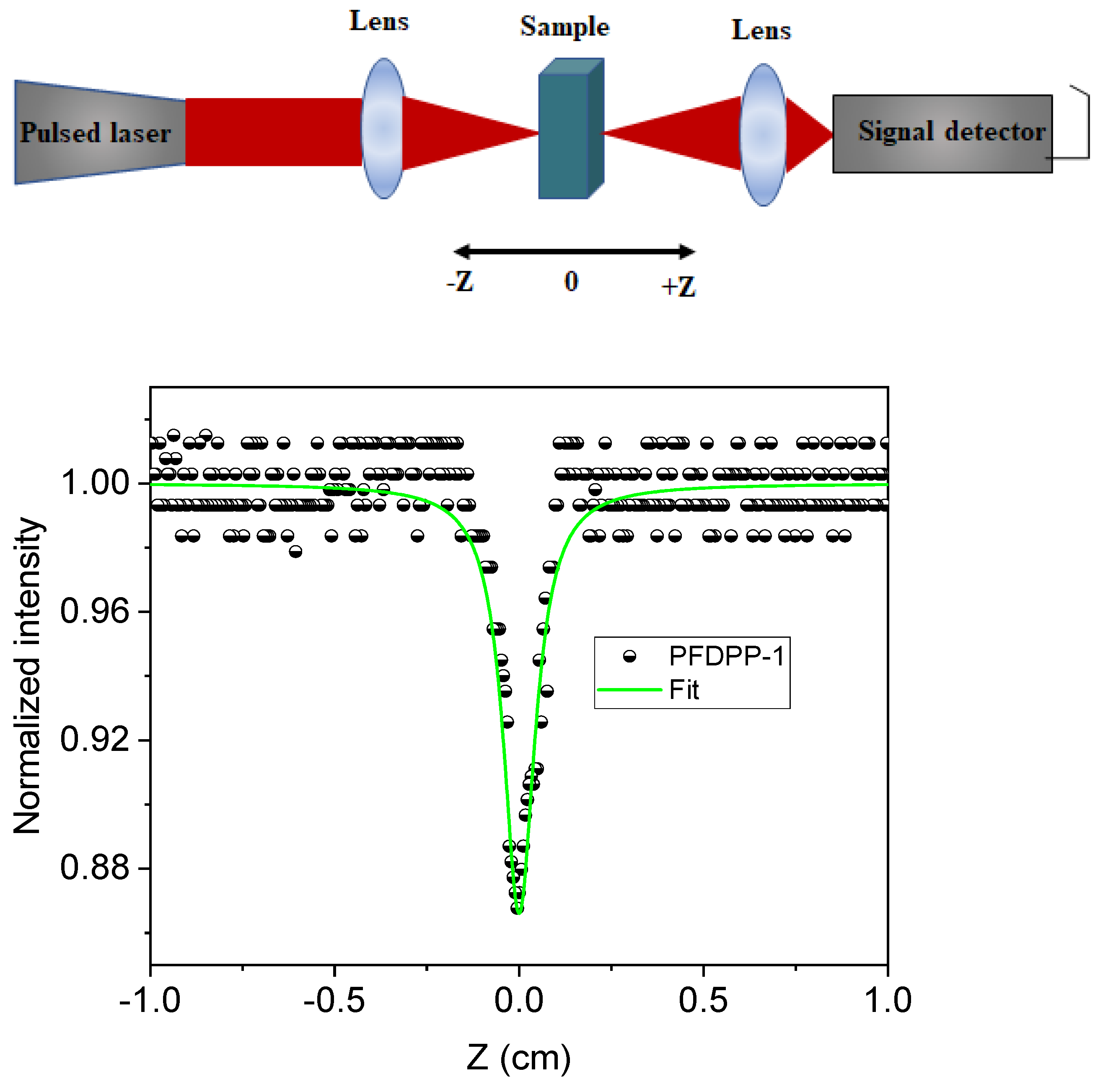
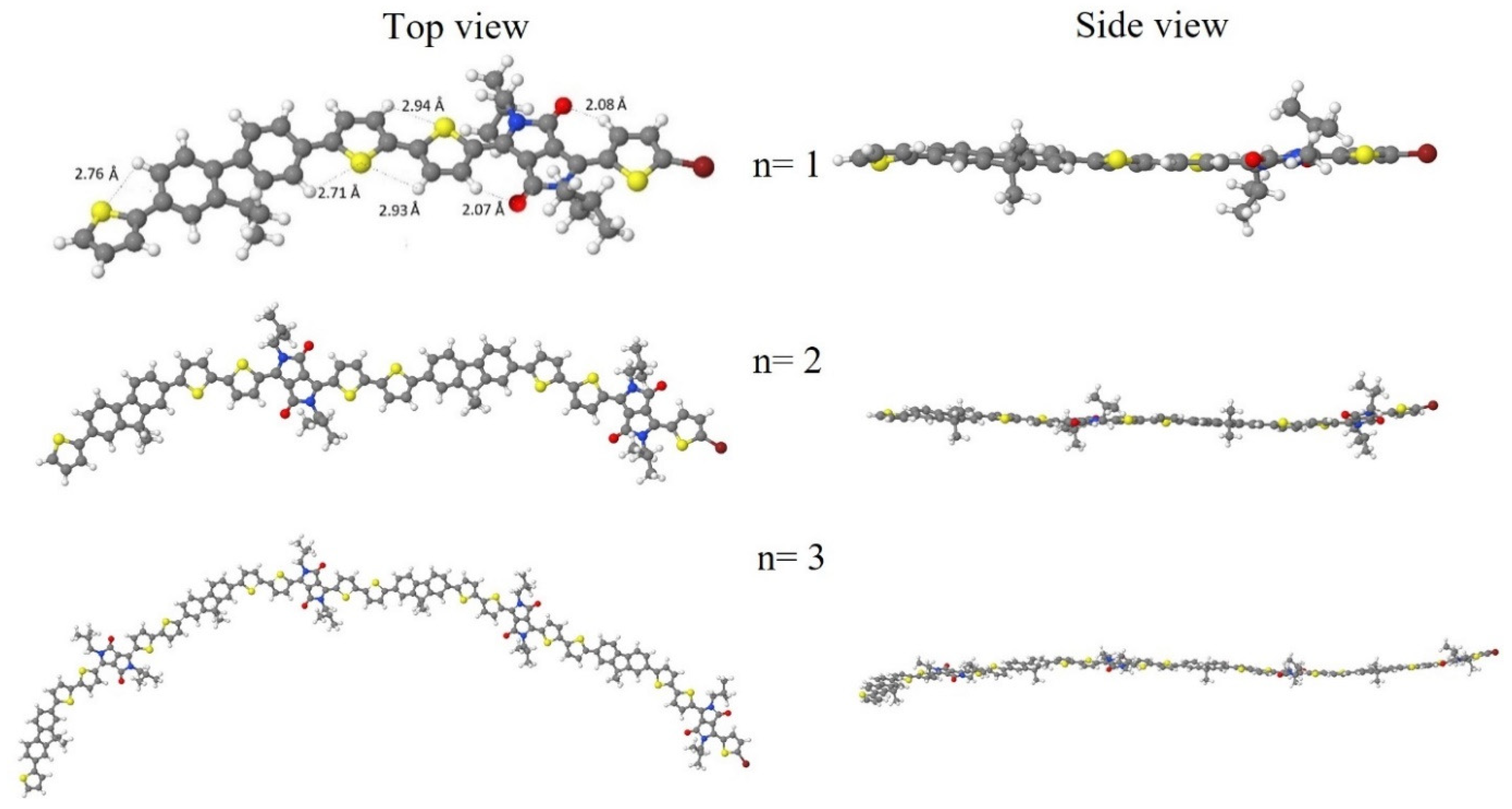
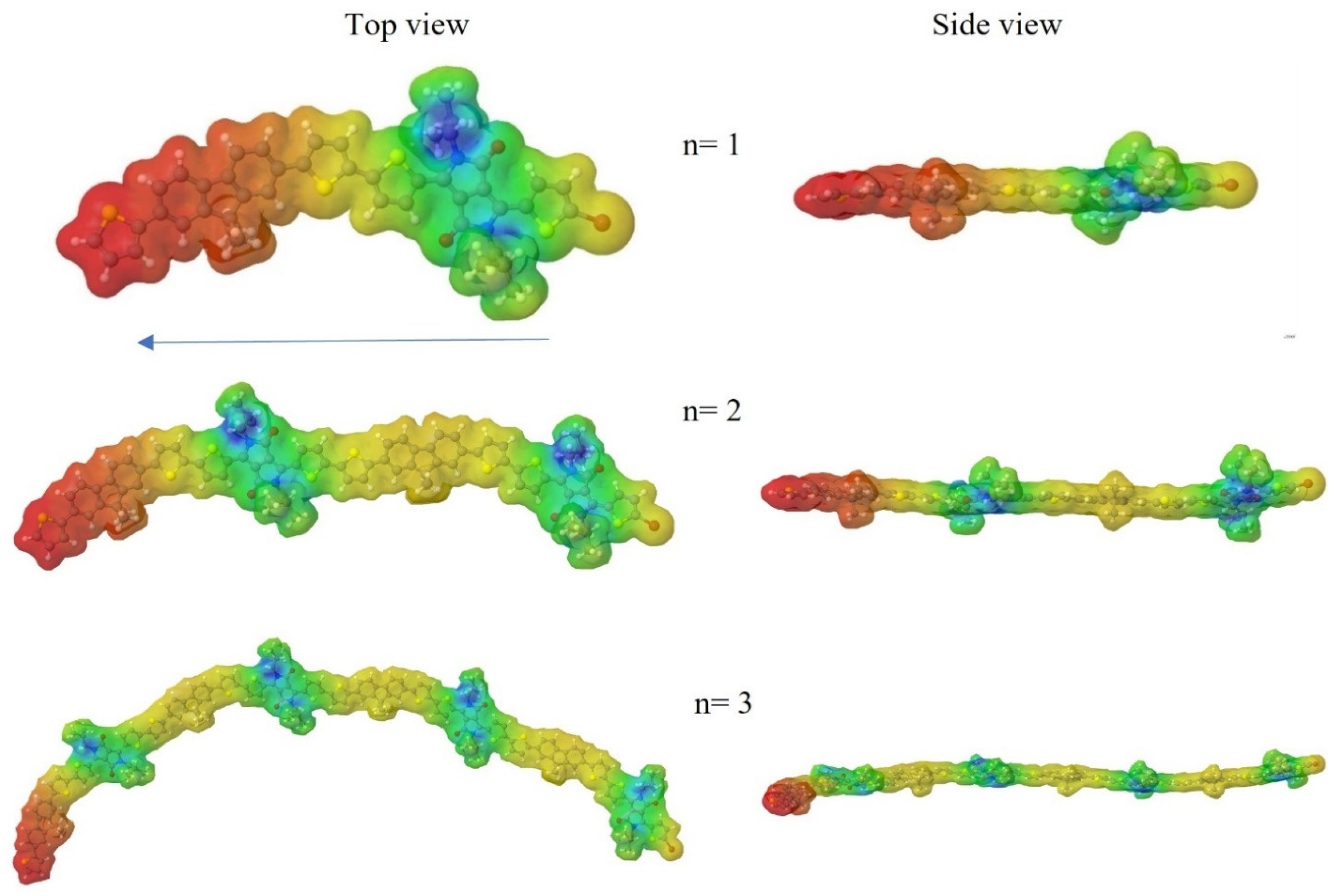
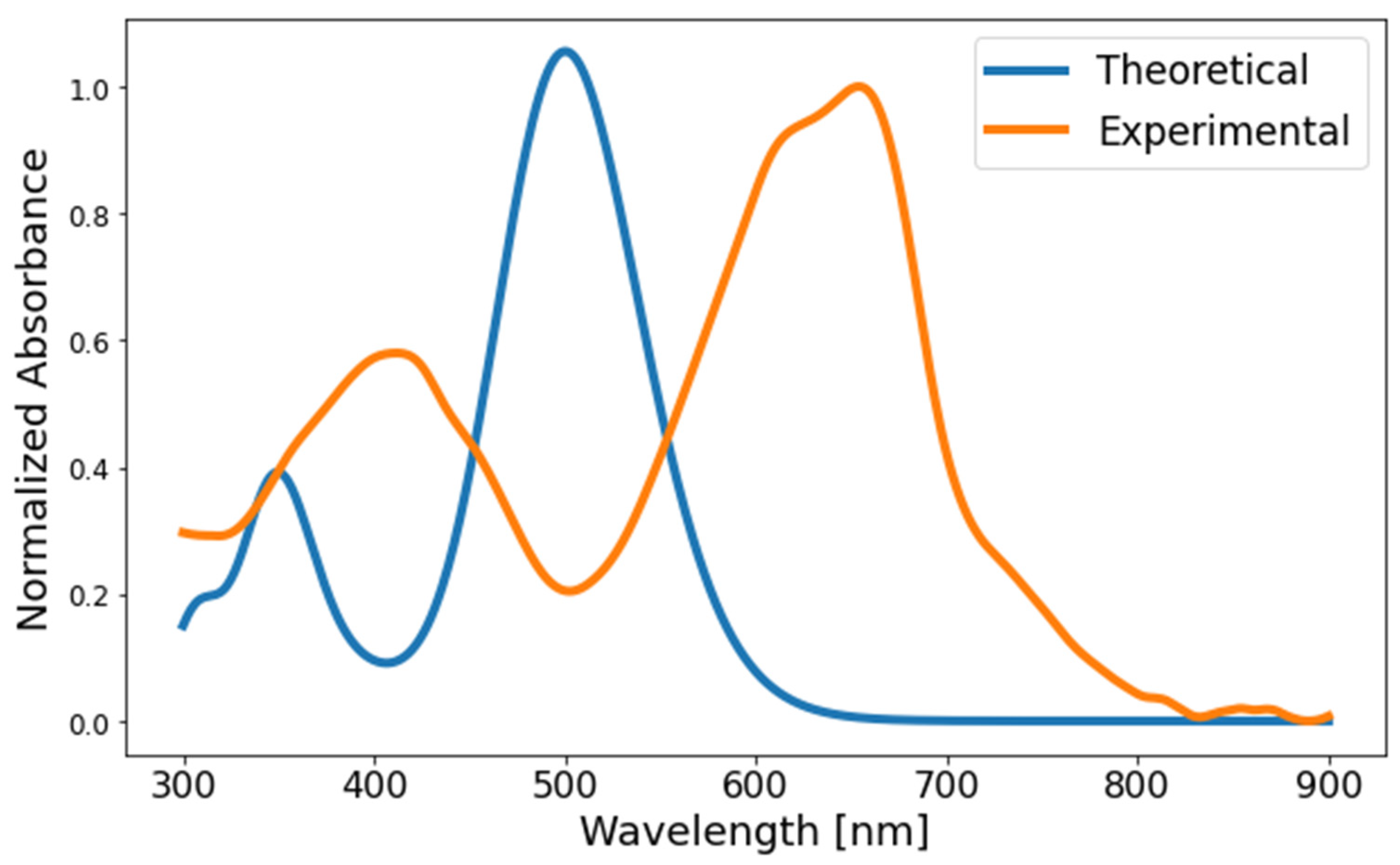
| Polymer | DPP (g, mmol) | Fluorene (g, mmol) | Neodecanic Acid (mg, mmol) | Pd(OAc)2 a (% mol, mmol × 10−2) | K2CO3 (mg, mol) | Time (h) | Yield (% b, % c) |
|---|---|---|---|---|---|---|---|
| PFDPP-1 | 0.20, 0.22 | 0.12, 0.22 | 0.01, 0.06 | 8, 0.017 | 0.09, 0.65 | 24 | 15, 64.2 |
| PFDPP-2 | 0.20, 0.22 | 0.12, 0.22 | 0.01, 0.06 | 12, 0.026 | 0.09, 0.65 | 24 | 0.4, 81.5 |
| PFDPP-3 | 0.25, 0.27 | 0.15, 0.27 | 0.01, 0.06 | 16, 0.034 | 0.09, 0.65 | 24 | 0.1, 41.0 |
| Polymer | Tg (°C) | Tm (°C) | ΔHm (J/g) | TC (°C) | ΔHC (J/g) | Td (°C) | Wc (%) |
|---|---|---|---|---|---|---|---|
| PFDPP-1 | 65 | 146 | 1.27 | 142 | 1.26 | 443 | 60.8 |
| PFDPP-2 | 64 | 159 | 1.59 | 152 | 2.67 | 437 | 69.8 |
| PFDPP-3 | 63 | 157 | 0.89 | 151 | 1.04 | 425 | 65.7 |
| Polymer | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|
| PFDPP-1 | 11,347 ± 3.8% | 34,812 ± 0.5% | 3.07 ± 3.3% |
| PFDPP-2 | 13,848 ± 0.7% | 43,803 ± 1.4% | 3.16 ± 2.2% |
| PFDPP-3 | 13,875 ± 0.7% | 43,011 ± 0.3% | 3.10 ± 0.4% |
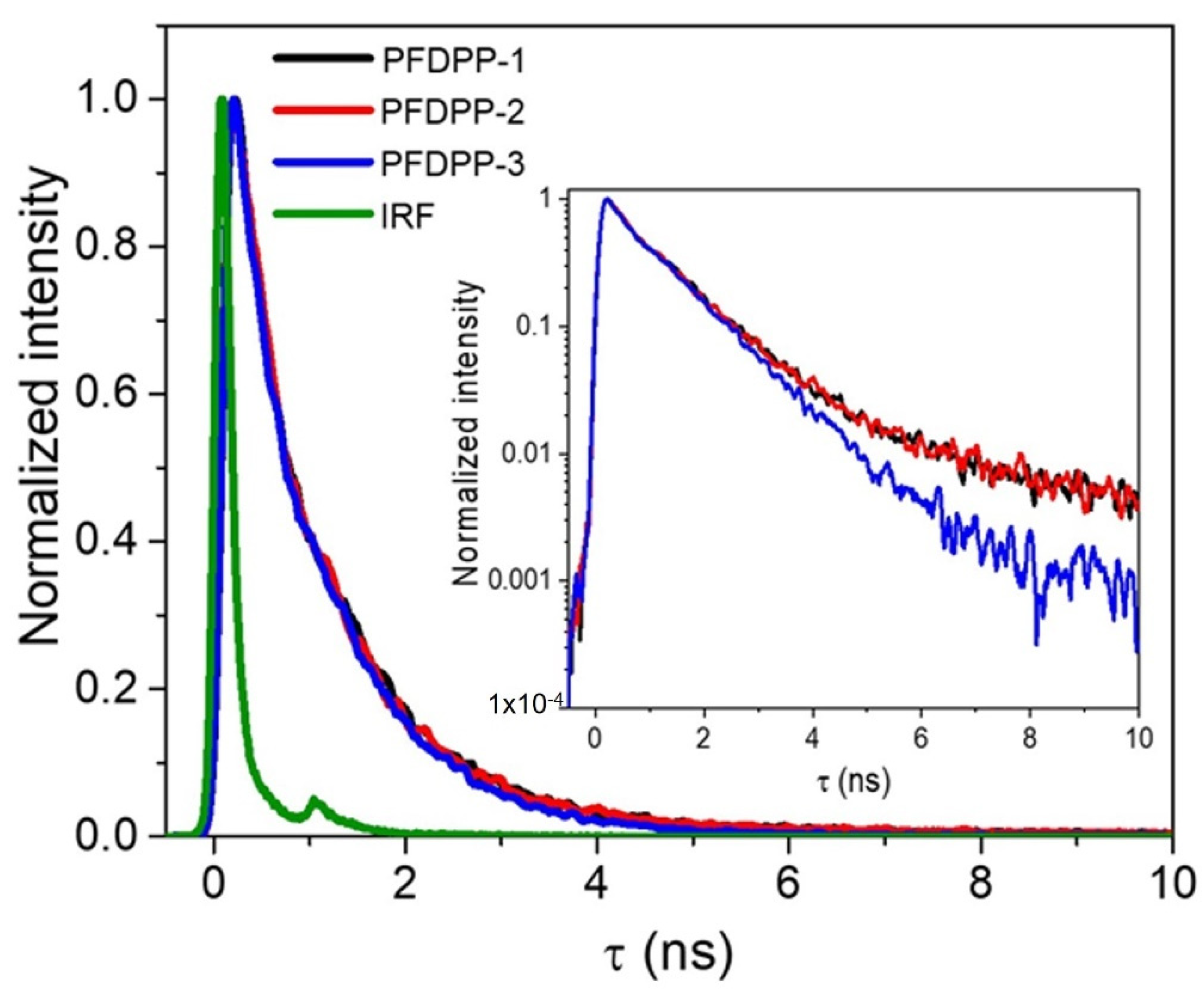
| Polymer | Absorbance λmax (nm) | Emission λmax (nm) | Stokes Shift (nm) | Φ (%) | Lifetime Fluorescence (ns) | Ꜫ (M−1cm−1) | (eV) | |
|---|---|---|---|---|---|---|---|---|
| PFDPP-1 | 412, 655 | 696 | 41 | 43.8 | 0.78 | 486,786 | 1.74 | 2.32 |
| PFDPP-2 | 408, 655 | 696 | 41 | 47.4 | 0.77 | 635,623 | 1.76 | 2.40 |
| PFDPP-3 | 407, 655 | 696 | 41 | 47.9 | 0.70 | 736,762 | 1.77 | 2.52 |
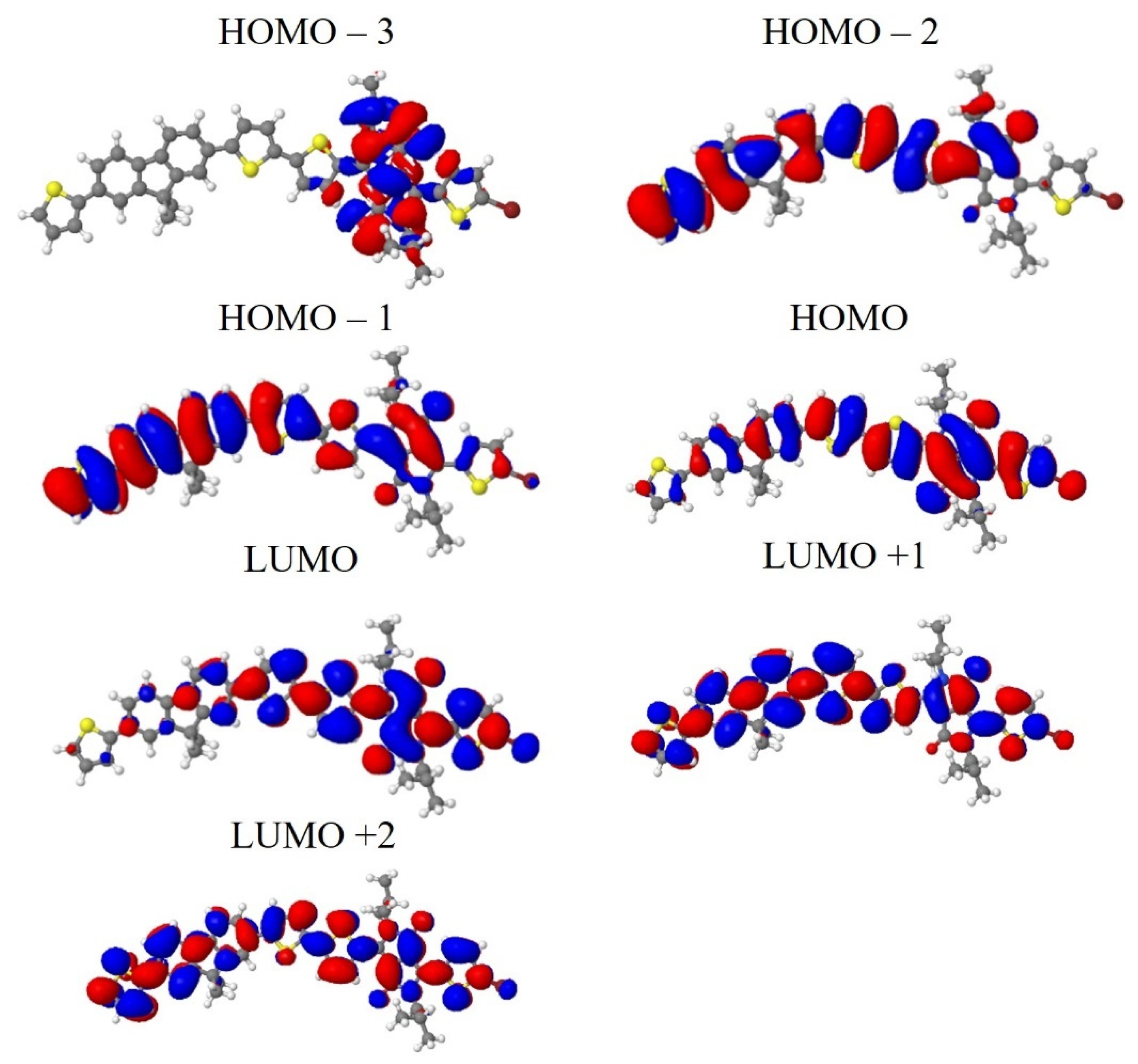
| from | to | Energy Gap (eV) | λ (nm) |
|---|---|---|---|
| The absorption band at 350 nm | Oscillator Strength = 0.70 | ||
| HOMO-2 | LUMO | 3.19 | 389 |
| HOMO-1 | LUMO + 1 | 3.45 | 359 |
| HOMO | LUMO + 1 | 2.86 | 434 |
| HOMO | LUMO + 2 | 3.39 | 366 |
| The absorption band at 502 nm | Oscillator Strength = 2.47 | ||
| HOMO-2 | LUMO | 3.19 | 389 |
| HOMO-1 | LUMO | 2.59 | 479 |
| HOMO | LUMO | 2.00 | 620 |
| HOMO | LUMO + 2 | 3.39 | 366 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rea, J.; Güizado-Rodríguez, M.; Maldonado, J.-L.; Ramos-Ortiz, G.; Reveles, J.U.; Silva, C.; Barba, V.; Saucedo-Salazar, E.M.; Rodríguez Hernández, M.T. Synthesis of Donor–Acceptor Copolymers Derived from Diketopyrrolopyrrole and Fluorene via Eco-Friendly Direct Arylation: Nonlinear Optical Properties, Transient Absorption Spectroscopy, and Theoretical Modeling. Energies 2022, 15, 3855. https://doi.org/10.3390/en15113855
Rodríguez-Rea J, Güizado-Rodríguez M, Maldonado J-L, Ramos-Ortiz G, Reveles JU, Silva C, Barba V, Saucedo-Salazar EM, Rodríguez Hernández MT. Synthesis of Donor–Acceptor Copolymers Derived from Diketopyrrolopyrrole and Fluorene via Eco-Friendly Direct Arylation: Nonlinear Optical Properties, Transient Absorption Spectroscopy, and Theoretical Modeling. Energies. 2022; 15(11):3855. https://doi.org/10.3390/en15113855
Chicago/Turabian StyleRodríguez-Rea, Jonatan, Marisol Güizado-Rodríguez, José-Luis Maldonado, Gabriel Ramos-Ortiz, José Ulises Reveles, Carlos Silva, Victor Barba, Esmeralda Monserrat Saucedo-Salazar, and María Teresa Rodríguez Hernández. 2022. "Synthesis of Donor–Acceptor Copolymers Derived from Diketopyrrolopyrrole and Fluorene via Eco-Friendly Direct Arylation: Nonlinear Optical Properties, Transient Absorption Spectroscopy, and Theoretical Modeling" Energies 15, no. 11: 3855. https://doi.org/10.3390/en15113855
APA StyleRodríguez-Rea, J., Güizado-Rodríguez, M., Maldonado, J.-L., Ramos-Ortiz, G., Reveles, J. U., Silva, C., Barba, V., Saucedo-Salazar, E. M., & Rodríguez Hernández, M. T. (2022). Synthesis of Donor–Acceptor Copolymers Derived from Diketopyrrolopyrrole and Fluorene via Eco-Friendly Direct Arylation: Nonlinear Optical Properties, Transient Absorption Spectroscopy, and Theoretical Modeling. Energies, 15(11), 3855. https://doi.org/10.3390/en15113855








