Sensitivity Analysis of High-Pressure Methanol—Steam Reformer Using the Condensation Enthalpy of Water Vapor
Abstract
:1. Introduction
2. Computational Model and Validation
2.1. Model Description
- Two-dimensional axisymmetric, ideal gas, laminar flow;
- Constant values at inlets and nonslip conditions at walls;
- Gaseous state in the inner pipe;
- Constant properties at solid materials.
2.2. Governing Equations of the Methanol–Steam-Reforming Reactions with Phase Change Heat Transfer
2.3. Validation of the Two-Phase Flow Model
3. Results and Discussion
3.1. Performance of the Methanol–Steam-Reforming Reactions Using Two Different Types of Heat Sources
3.2. SA of the Methanol–Steam-Reforming Reactions with a Latent-Heat Source
3.2.1. Parameter Descriptions
3.2.2. Sensitivity of the Reformer Performance to Various Parameters
4. Conclusions
- An MSR that utilizes the phase change energy of steam can supply thermal energy at a uniform and higher temperature than an MSR that employs only the sensible heat of a gas. Moreover, it has a better performance in terms of hydrogen production.
- The thermal–fluid parameters of the reactor are more affected by the change in the boundary conditions of the inner pipe, where the methanol–steam-reforming reactions occur. This is because the wall temperature remains almost uniform owing to the phase change energy supplied by the water vapor.
- Increasing the temperature and velocity through the outer pipe can increase the heat transfer to the inner pipe and reduce the liquid mass flow rate. However, the temperature of the wall is kept almost uniform, so it does not have a significant effect on the methanol–steam-reforming reaction.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Definitions of Symbols | |
| B | Dimensionless permeability |
| C | Concentration |
| Pressure jump coefficient | |
| Dh | Hydraulic diameter |
| Di,m | Mass diffusion coefficient |
| DT,i | Thermal diffusion coefficient |
| E | Total energy |
| Ea | Activation energy |
| External force | |
| fi | Friction coefficient |
| fl | Fanning friction factor |
| h | Enthalpy |
| hsf | Superficial heat transfer coefficient |
| I | Inner pipe |
| J | Mass diffusivity |
| Diffusion flux of species | |
| Ki | Adsorption equilibrium coefficient for i |
| k0 | Pre-exponential factor |
| kb,r | Backward rate constant |
| Effective thermal conductivity | |
| kf,r | Forward rate constant |
| Mass flow rate | |
| O | Outer pipe |
| P | Pressure |
| R | Ideal gas constant |
| Ri | Net rate of production |
| Interaction force between phases | |
| Arrhenius molar rate of creation/destruction of species i in reaction r | |
| Si | Rate of creation source term |
| Fluid enthalpy source term | |
| T | Temperature |
| t | Time |
| Velocity vector | |
| Stoichiometric coefficient for reactant i in reaction r | |
| Stoichiometric coefficient for product i in reaction r | |
| Yi | Local mass fraction |
| Z | Function of thermodynamic vapor gravity and pressure |
| Greek Letters | |
| Void fraction | |
| Porosity | |
| Rate exponent for reactant species j in reaction r | |
| Rate exponent for product species j in reaction r | |
| Density | |
| Stress tensor | |
| Subscripts | |
| f | Fluid |
| i, j | Species |
| l | Liquid |
| p, q | Phases |
| s | Solid |
| sat | Saturation |
| v | Vapor |
References
- De-Troya, J.J.; Alvarez, C.; Fernández-Garrido, C.; Carral, L. Analysing the possibilities of using fuel cells in ships. Int. J. Hydrogen Energy 2016, 41, 2853–2866. [Google Scholar] [CrossRef]
- Psoma, A.; Gunter, S. Fuel cell systems for submarines: From the first idea to serial production. J. Power Sources 2002, 106, 381–383. [Google Scholar] [CrossRef]
- Krummrich, S.; Javier, L. Methanol reformer–The next milestone for fuel cell powered submarines. Int. J. Hydrog. Energy 2015, 40, 5482–5486. [Google Scholar] [CrossRef]
- Sa, S.; Jose, M.S.; Adelio, M. Steam reforming of methanol over a CuO/ZnO/Al2O3 catalyst, part I: Kinetic modelling. Chem. Eng. Sci. 2011, 66, 4913–4921. [Google Scholar] [CrossRef]
- Palo, D.R.; Robert, A.D.; Jamie, D.H. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Garcia, G.; Arriola, E.; Chen, W.H.; De Luna, M.D. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability. Energy 2020, 217, 119384. [Google Scholar] [CrossRef]
- Chen, W.-H.; Bo-Jhih, L. Effect of microwave double absorption on hydrogen generation from methanol steam reforming. Int. J. Hydrog. Energy 2010, 35, 1987–1997. [Google Scholar] [CrossRef]
- Conant, T.; Karim, A.M.; Lebarbier, V.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Datye, A. Stability of bimetallic Pd–Zn catalysts for the steam reforming of methanol. J. Catal. 2008, 257, 64–70. [Google Scholar] [CrossRef]
- Chein, R.; Yen-Cho, C.; Chung, J.N. Axial heat conduction and heat supply effects on methanol-steam reforming performance in micro-scale reformers. Int. J. Heat Mass Transf. 2012, 55, 3029–3042. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Abdollahzadeh, M.; Boaventura, M.; Mendes, A. H2 production with low carbon content via MSR in packed bed membrane reactors for high-temperature polymeric electrolyte membrane fuel cell. Appl. Energy 2017, 188, 409–419. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Ryuji, K.; Koichi, E. Thermodynamic evaluation of methanol steam reforming for hydrogen production. J. Power Sources 2006, 161, 87–94. [Google Scholar] [CrossRef]
- Yun, J.; Ngoc, V.T.; Sangseok, Y. Performance improvement of methanol steam reforming system with auxiliary heat recovery units. Int. J. Hydrogen Energy 2021, 46, 25284–25293. [Google Scholar] [CrossRef]
- Mao, X.; Li, W.; Yuan, Y.; Yang, L. Numerical analysis of methanol steam reforming reactor heated by catalytic combustion for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 14469–14482. [Google Scholar] [CrossRef]
- Srivastava, A.; Parmod, K.; Atul, D. Performance enhancement of methanol reforming reactor through finned surfaces and diffused entry for on-board hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 7478–7490. [Google Scholar] [CrossRef]
- Saltelli, A. Sensitivity analysis for importance assessment. Risk Anal. 2002, 22, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Jinwon, Y.; Sangseok, Y. Thermal design of methane steam reformer with low-temperature non-reactive heat source for high efficiency engine-hybrid stationary fuel cell system. Int. J. Hydrogen Energy 2017, 42, 1469–14707. [Google Scholar] [CrossRef]
- Yun, J.; Sangseok, Y. Transient behavior of 5 kW class shell-and-tube methane steam reformer with intermediate temperature heat source. Int. J. Heat Mass Transf. 2019, 134, 600–609. [Google Scholar] [CrossRef]
- Shi, X.; Che, D.; Agnew, B.; Gao, J. An investigation of the performance of compact heat exchanger for latent heat recovery from exhaust flue gases. Int. J. Heat Mass Transf. 2011, 54, 606–615. [Google Scholar] [CrossRef]
- Osakabe, M.; Ishida, K.; Yagi, K.; Itoh, T.; Ohmasa, K. Condensation heat transfer on tubes in actual flue gas. Heat Transf. Asian Res. Co-Spons. By Soc. Chem. Eng. Jpn. Heat Transf. Div. ASME 2001, 30, 139–151. [Google Scholar] [CrossRef]
- White, M.T.; Abdulnaser, I.S. A new method to identify the optimal temperature of latent-heat thermal-energy storage systems for power generation from waste heat. Int. J. Heat Mass Transf. 2020, 149, 119111. [Google Scholar] [CrossRef]
- Ji, H.; Lee, J.; Choi, E.; Seo, I. Hydrogen production from steam reforming using an indirect heating method. Int. J. Hydrogen Energy 2018, 43, 3655–3663. [Google Scholar] [CrossRef]
- Song, H.; Kim, Y.; Yu, D.; Kim, B.J.; Ji, H.; Yu, S. A computational analysis of a methanol steam reformer using phase change heat transfer. Energies 2020, 13, 4324. [Google Scholar] [CrossRef]
- Yu, D.; Kim, B.; Van Trinh, N.; Ji, H.; Yu, S. Analysis of phase change heat transfer with annulus 1 kW class methanol steam reforming reaction under high-pressure operation. Int. J. Hydrogen Energy 2021, 46, 28775–28788. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl. Catal. A Gen. 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Peppley, B.A. A Comprehensive Kinetic Model of Methanol-Steam Reforming on Cu/ZnO/Al2O3 Catalyst. Ph.D. Thesis, The Royal Military College of Canada, Kingston, ON, Canada, 1998. [Google Scholar]
- Frilund, C. CO2 Hydrogenation to Methanol. Master’s Thesis, Aalto University, Espoo, Finland, 2016. Appendix2 (2/2). Available online: https://aaltodoc.aalto.fi/bitstream/handle/123456789/19938/master_Frilund_Christian_2016.pdf?sequence=1 (accessed on 31 January 2016).
- Chiu, Y.J.; Chiu, H.C.; Hsieh, R.H.; Jang, J.H.; Jiang, B.Y. Simulations of hydrogen production by methanol steam reforming. Energy Procedia 2019, 156, 38–42. [Google Scholar] [CrossRef]
- Ha, S.J.; Park, C.E.; Kim, K.D.; Ban, C.H. Development of the SPACE code for nuclear power plants. Nucl. Eng. Technol. 2011, 43, 45–62. [Google Scholar] [CrossRef] [Green Version]
- Wallis, G.B. One-Dimensional Two-Phase Flow McGraw-Hill Book Company; McGraw Hill Book Company: New York, NY, USA, 1969. [Google Scholar] [CrossRef]
- Saha, P.; Novak, Z. Point of net vapor generation and vapor void fraction in subcooled boiling. In Proceedings of the International Heat Transfer Conference Digital Library, Tokyo, Japan, 3–7 September 1974. [Google Scholar]
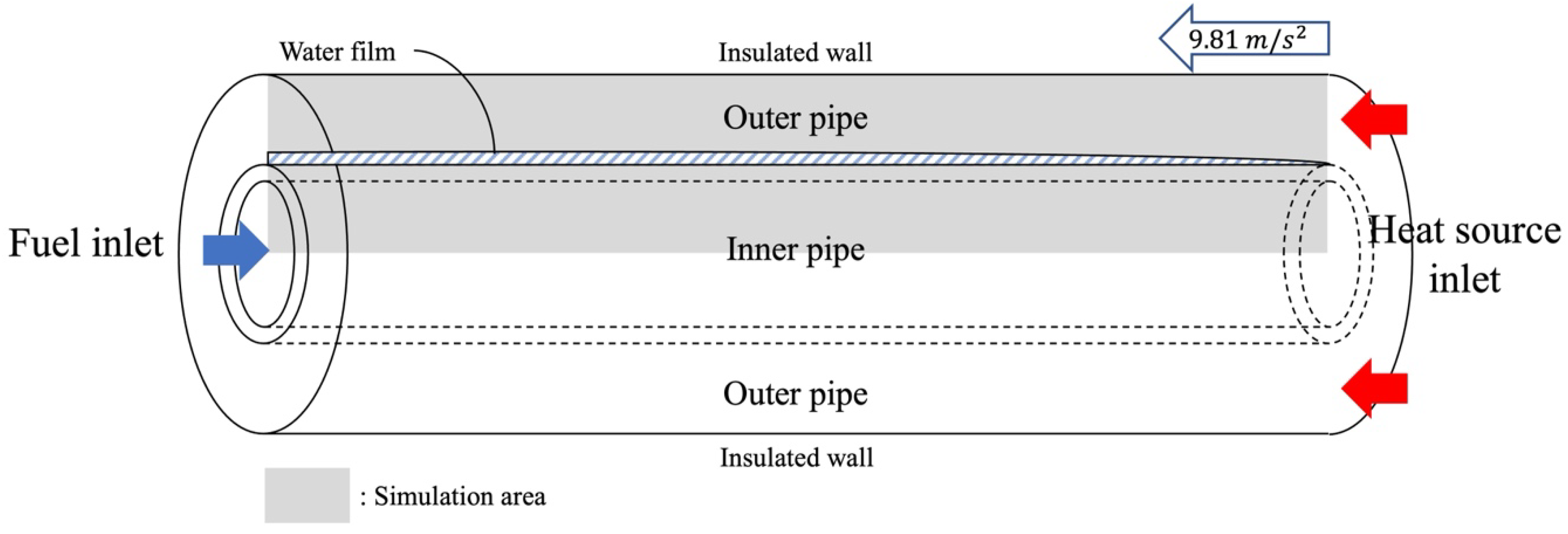
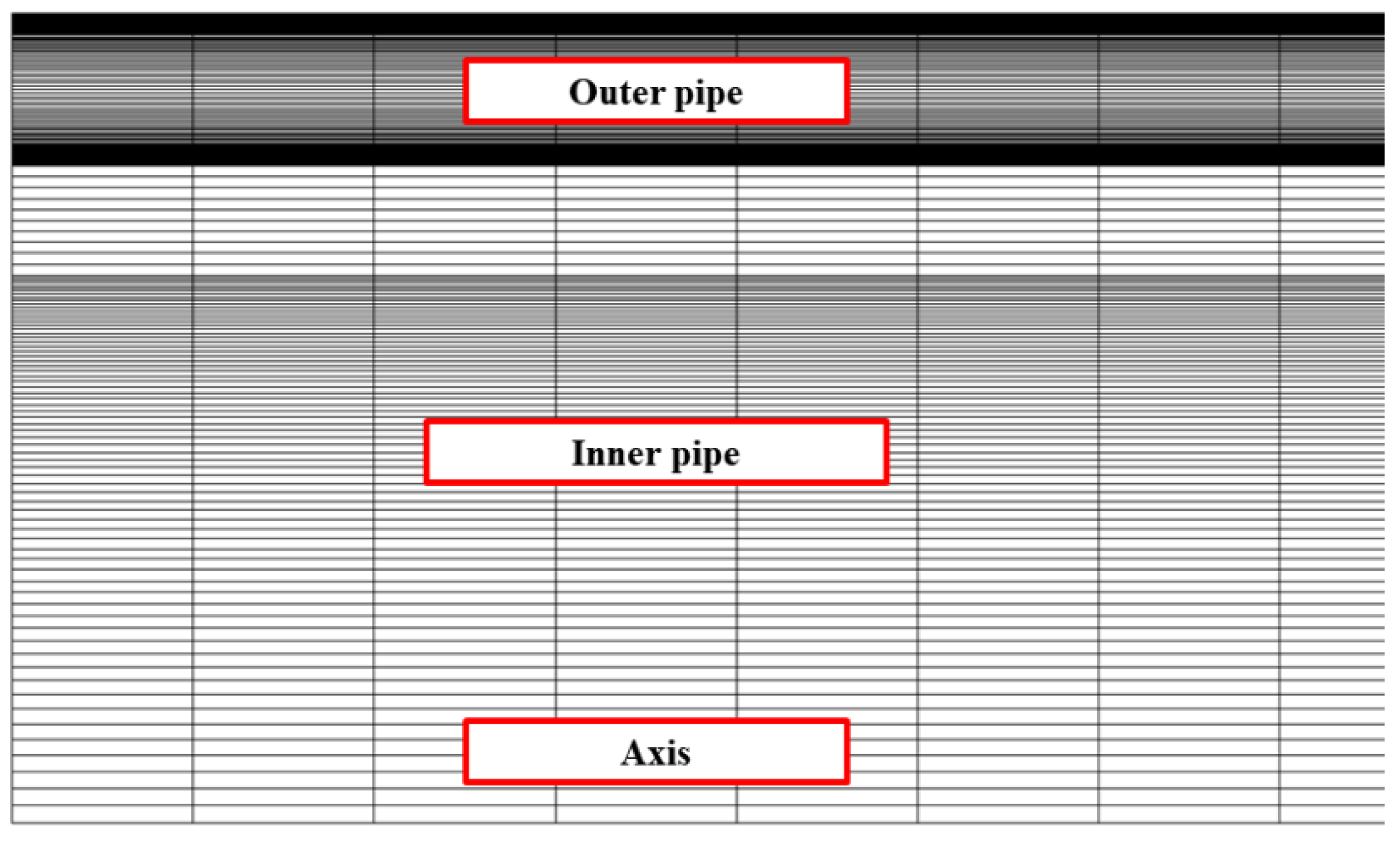
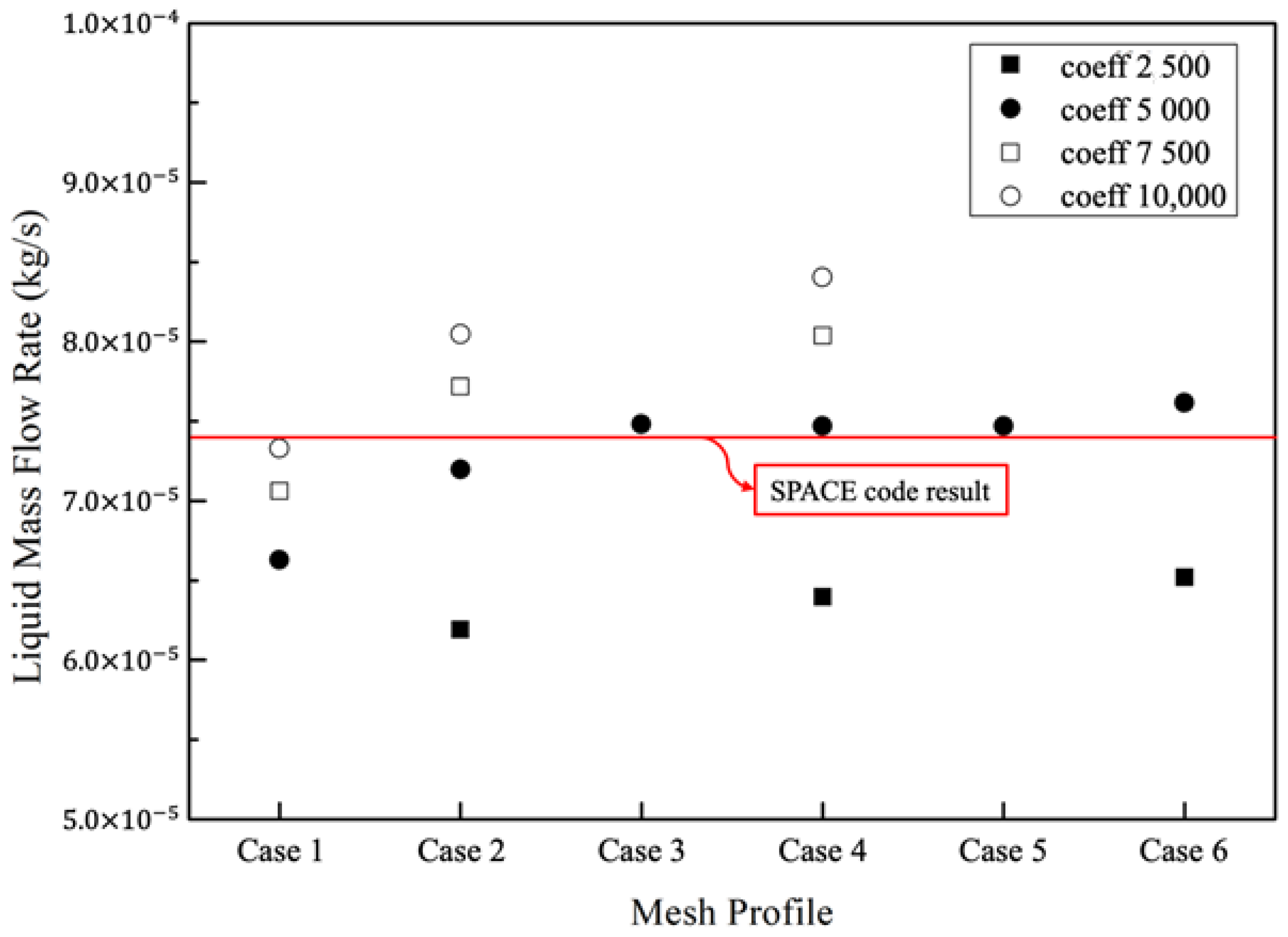



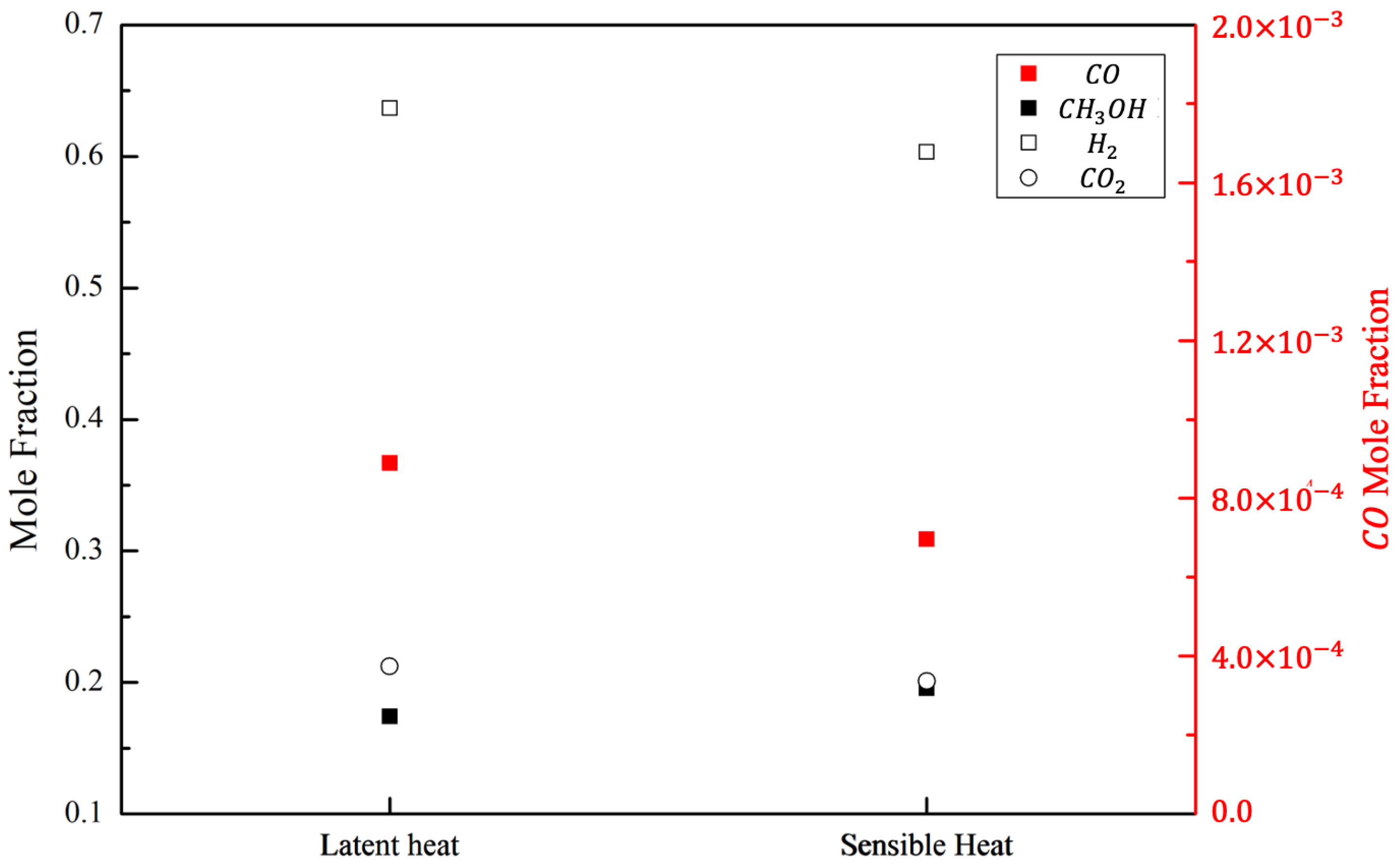

| Parameter | Value |
|---|---|
| I-inner diameter | 21.18 mm |
| I-outer diameter | 25.4 mm |
| O-inner diameter | 31.29 mm |
| Length | 700 mm |
| I-velocity | 0.417 m/s |
| O-velocity | 0.26 m/s |
| I-temperature | 543.15 K |
| O-temperature | 558.76 K |
| I-pressure | 2980 kPa |
| O-pressure | 6550 kPa |
| I-SCR | 1.2 |
| Rate Constants | ||
|---|---|---|
| Division | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Radial | 50 | 100 | 200 | 200 | 200 | 400 |
| Axis | 200 | 200 | 100 | 200 | 300 | 200 |
| Variable | Value | Parameter Change (%) | ||
|---|---|---|---|---|
| SCR | 1.0 | 0.610 | −16.67 | −1.54 |
| 1.2 | 0.619 | 0.00 | 0.00 | |
| 1.5 | 0.628 | 25.00 | 1.37 | |
| 2.0 | 0.634 | 66.67 | 2.44 | |
| 2.5 | 0.637 | 108.33 | 2.78 | |
| GHSV-I | 0.690 | −50.00 | 10.89 | |
| 0.622 | 0.00 | 0.00 | ||
| 0.527 | 100.00 | −15.33 | ||
| GHSV-O | 0.622 | −50.00 | 0.00 | |
| 0.622 | 0.00 | 0.00 | ||
| 0.622 | 100.00 | 0.00 | ||
| Temp.-I | 523.15 K | 0.615 | −3.68 | −1.24 |
| 533.15 K | 0.618 | −1.84 | −0.61 | |
| 543.15 K | 0.622 | 0.00 | 0.00 | |
| 553.15 K | 0.626 | 1.84 | 0.58 | |
| 558.76 K | 0.628 | 2.87 | 0.89 | |
| Temp.-O | 558.76 K | 0.622 | 0.00 | 0.00 |
| 568.76 K | 0.622 | 1.79 | 0.04 | |
| 578.76 K | 0.623 | 3.58 | 0.18 | |
| Length | 0.35 m | 0.527 | −50.00 | −15.34 |
| 0.7 m | 0.622 | 0.00 | 0.00 | |
| 1.4 m | 0.690 | 100.00 | 10.91 | |
| Pressure-I | 1 bara | 0.017 | −96.64 | −97.32 |
| 15 bara | 0.485 | −49.66 | −22.01 | |
| 29.8 bara | 0.622 | 0.00 | 0.00 | |
| 45 bara | 0.679 | 51.01 | 9.05 | |
| 65.5 bara | 0.723 | 119.80 | 16.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Kim, B.; Ji, H.; Yu, S. Sensitivity Analysis of High-Pressure Methanol—Steam Reformer Using the Condensation Enthalpy of Water Vapor. Energies 2022, 15, 3832. https://doi.org/10.3390/en15103832
Yu D, Kim B, Ji H, Yu S. Sensitivity Analysis of High-Pressure Methanol—Steam Reformer Using the Condensation Enthalpy of Water Vapor. Energies. 2022; 15(10):3832. https://doi.org/10.3390/en15103832
Chicago/Turabian StyleYu, Dongjin, Byoungjae Kim, Hyunjin Ji, and Sangseok Yu. 2022. "Sensitivity Analysis of High-Pressure Methanol—Steam Reformer Using the Condensation Enthalpy of Water Vapor" Energies 15, no. 10: 3832. https://doi.org/10.3390/en15103832
APA StyleYu, D., Kim, B., Ji, H., & Yu, S. (2022). Sensitivity Analysis of High-Pressure Methanol—Steam Reformer Using the Condensation Enthalpy of Water Vapor. Energies, 15(10), 3832. https://doi.org/10.3390/en15103832







