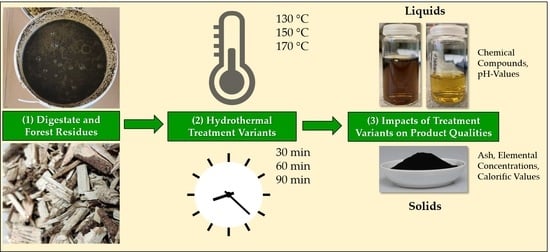
Hydrothermal Treatment of Residual Forest Wood (Softwood) and Digestate from Anaerobic Digestion—Influence of Temperature and Holding Time on the Characteristics of the Solid and Liquid Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Processing, Dry Matter and Ash Concentration
2.3. Hydrothermal Treatment
2.4. C, H, N and O Concentration and Calorific Value Calculation
2.5. Gas Chromatography-Mass Spectrometry
2.6. Inductively Coupled Plasma-Optical Emission Spectroscopy
2.7. pH Value of the Process Water and Severity Factor
3. Results and Discussion
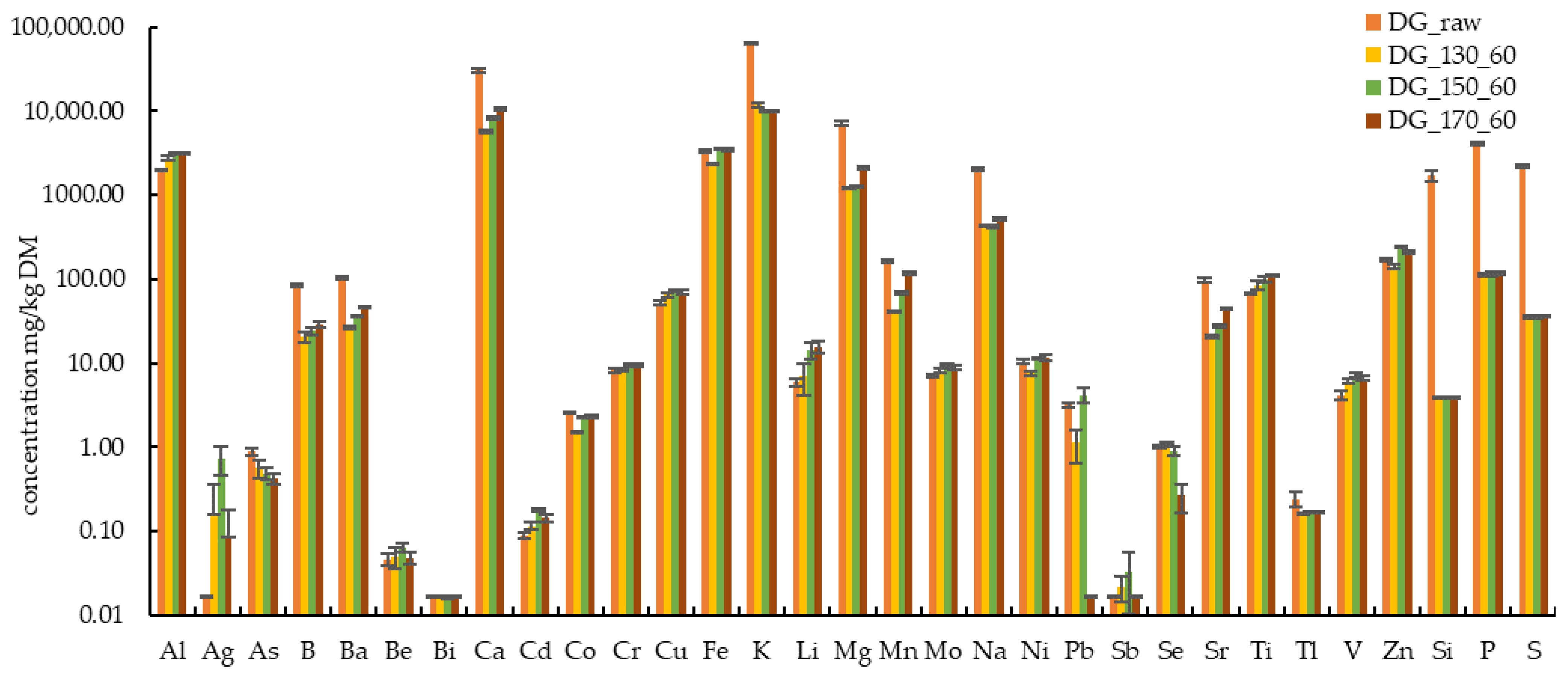
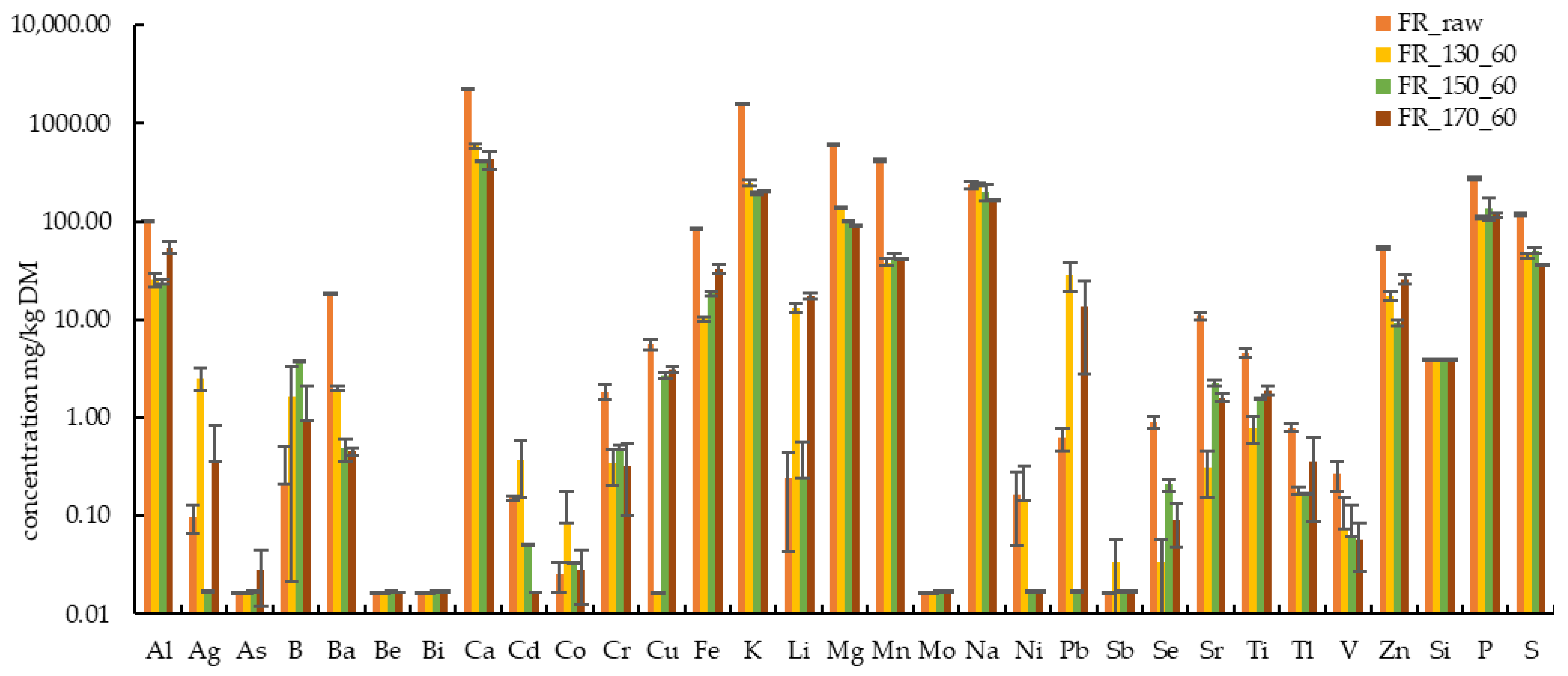
3.1. Basic Characteristics of Raw Biomasses and Hydrothermally Treated Solids
3.2. Characteristics of Liquid Products from Hydrothermal Treatment
3.3. Influence of Hydrothermal Treatment Temperature on Characteristics of Solids and Liquids
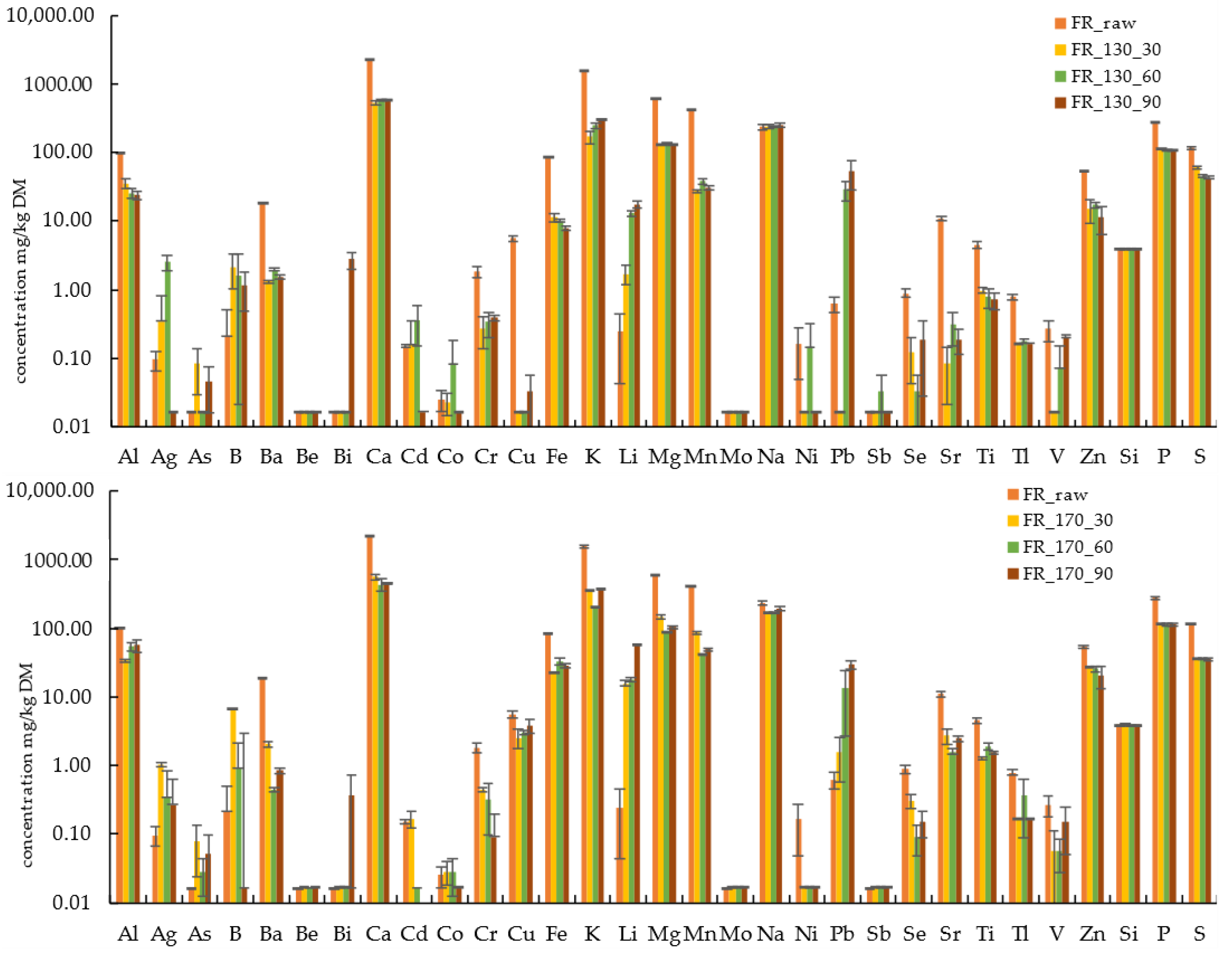
3.4. Influence of Holding Time on Characteristics of Hydrothermally Treated Solids and Liquids
4. Conclusions
- (1)
- In general, the physicochemical characteristics of alleged low-value biomasses can be strongly influenced by low-temperature HTT. Depending on the type of feedstock, the effects of the HTT process on the material properties of solid and liquid HTT products vary. Certainly, each biomass—or at least certain biomass types (e.g., lignocellulosic)—reacts differently to the HTT treatment and thus has to be considered individually.
- (2)
- It is possible to generate FR- and DG-based solids with optimized characteristics while simultaneously producing liquids with a variety of different chemical compounds. In this study, the influence of low-temperature HTT with various parameters as an energy-efficient process on ash and element concentrations as well as on calorific values (solids) and chemical compounds (liquids) was demonstrated.
- (3)
- Depending on the HTT process parameters and on the designated application (e.g., HTT solids as a fuel in combustion), HTT might lead to positive or negative impacts. Thus, a general statement on the optimum HTT conditions is not possible. Major variables for an optimized HTT process are the reaction conditions (e.g., temperature or holding time), feedstock (e.g., biomass type) and designated application (e.g., fuel).
- (4)
- Both the HTT temperature and holding time have major impacts on the material properties of DG and FR. The effect of HTT at 130 °C with a 30 min holding time might deliver better results than HTT at 170 °C with a 90 min holding time in terms of costs and benefits as well as the intended use. Thus, optimized HTT processes at lower temperatures with more suitable product qualities could also increase the overall efficiency of HTT.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| DG | digestate |
| DM | dry matter |
| FR | forest residue |
| GC-MS | gas chromatography-mass spectrometry |
| HHV | higher heating value |
| HTC | hydrothermal carbonization |
| HTT | hydrothermal treatment |
| ICP-OES | inductively coupled plasma-optical emission spectroscopy |
| LHV | lower heating value |
| PTFE | polytetrafluoroethylene |
| SF | severity factor |
| TEs | trace elements |
Appendix A


| Property Class | Unit | TA1 | TA2 |
|---|---|---|---|
| Ash, A, ISO 18122 | w-% dry | A5.0 ≤ 5.0 | A10.0 ≤ 10.0 |
| Net calorific value, Q, ISO 18125 | MJ/kg or kWh/kg as received | Q18 ≥ 18 or Q5.0 ≥ 5.0 Value to be stated | Q17 ≥ 17 or Q4.7 ≥ 4.7 Value to be stated |
| Nitrogen, N, ISO 16948 | w-% dry | N1.5 ≤ 1.5 | N2.0 ≤ 2.0 |
| Sulfur, S, ISO 16994 | w-% dry | S0.05 ≤ 0.05 | S0.1 ≤ 0.1 |
| Arsenic, As, ISO 16968 | mg/kg dry | ≤2 | ≤2 |
| Cadmium, Cd, ISO 16968 | mg/kg dry | ≤1 | ≤1 |
| Chromium, Cr, ISO 16968 | mg/kg dry | ≤50 | ≤50 |
| Copper, Cu, ISO 16968 | mg/kg dry | ≤20 | ≤20 |
| Lead, Pb, ISO 16968 | mg/kg dry | ≤10 | ≤10 |
| Nickel, Ni, ISO 16968 | mg/kg dry | ≤10 | ≤10 |
| Zinc, Zn, ISO 16968 | mg/kg dry | ≤200 | ≤200 |
| Code | Al | Ag | As | B | Ba | Be | Bi | Ca | Cd |
|---|---|---|---|---|---|---|---|---|---|
| DG Raw ± SD | 1972 ± 45 | 0.07 ± 0.08 | 0.91 ± 0.11 | 82.98 ± 2.34 | 101.1 ± 3.7 | 0.04 ± 0.00 | <0.02 | 29,564 ± 1189 | 0.09 ± 0.01 |
| DG_130_30 ± SD | 2727 ± 135 | 0.08 ± 0.08 | 0.56 ± 0.07 | 21.73 ± 2.00 | 58.68 ± 4.03 | 0.08 ± 0.02 | <0.02 | 12,596 ± 981 | 0.22 ± 0.01 |
| DG_130_60 ± SD | 2762 ± 126 | 0.16 ± 0.20 | 0.57 ± 0.14 | 20.56 ± 2.84 | 26.65 ± 1.09 | 0.05 ± 0.01 | <0.02 | 5682 ± 160 | 0.12 ± 0.01 |
| DG_130_90 ± SD | 2983 ± 26 | 0.14 ± 0.18 | 0.44 ± 0.20 | 16.09 ± 3.02 | 50.91 ± 3.30 | 0.07 ± 0.02 | 0.02 | 7923 ± 661 | 0.20 ± 0.01 |
| DG_150_60 ± SD | 3235 ± 25 | 0.73 ± 0.27 | 0.48 ± 0.08 | 24.16 ± 2.52 | 37.17 ± 0.27 | 0.06 ± 0.01 | <0.02 | 8360 ± 402 | 0.18 ± 0.01 |
| DG_170_30 ± SD | 3300 ± 90 | 0.36 ± 0.18 | 0.43 ± 0.04 | 24.72 ± 1.25 | 28.14 ± 0.52 | 0.04 ± 0.00 | <0.02 | 7080 ± 243 | 0.13 ± 0.00 |
| DG_170_60 ± SD | 3123 ± 101 | 0.09 ± 0.10 | 0.42 ± 0.06 | 28.93 ± 2.47 | 46.20 ± 1.71 | 0.05 ± 0.01 | <0.02 | 10,467 ± 455 | 0.14 ± 0.01 |
| DG_170_90 ± SD | 3115 ± 66 | 0.08 ± 0.10 | 0.42 ± 0.06 | 28.84 ± 2.09 | 46.09 ± 1.13 | 0.05 ± 0.01 | <0.02 | 17,171 ± 586 | 0.14 ± 0.01 |
| FR Raw ± SD | 102.1 ± 0.9 | 0.10 ± 0.03 | <0.02 | 0.04 ± 0.04 | 18.65 ± 0.39 | <0.02 | <0.02 | 2269 ± 65 | 0.15 ± 0.01 |
| FR_130_30 ± SD | 35.99 ± 6.2 | 0.34 ± 0.46 | 0.08 ± 0.05 | 2.17 ± 1.14 | 1.31 ± 0.08 | <0.02 | <0.02 | 540.2 ± 33.4 | 0.16 ± 0.18 |
| FR_130_60 ± SD | 25.44 ± 4.08 | 2.52 ± 0.67 | <0.02 | 1.64 ± 1.62 | 1.97 ± 0.1 | <0.02 | <0.02 | 585.7 ± 23.1 | 0.37 ± 0.21 |
| FR_130_90 ± SD | 23.96 ± 3.07 | 0.02 ± 0.00 | 0.04 ± 0.03 | 1.14 ± 0.67 | 1.54 ± 0.13 | <0.02 | 2.77 ± 0.77 | 581.2 ± 7.80 | 0.02 |
| FR_150_60 ± SD | 24.27 ± 1.26 | <0.02 | <0.02 | 3.73 ± 0.11 | 0.48 ± 0.12 | <0.02 | <0.02 | 415.3 ± 6.00 | 0.05 ± 0.00 |
| FR_170_30 ± SD | 33.98 ± 1.12 | 1.03 ± 0.09 | 0.08 ± 0.06 | 6.76 ± 0.18 | 2.03 ± 0.17 | <0.02 | <0.02 | 570.2 ± 53.8 | 0.17 ± 0.05 |
| FR_170_60 ± SD | 53.93 ± 7.94 | 0.35 ± 0.47 | 0.03 ± 0.02 | 0.93 ± 1.19 | 0.45 ± 0.03 | <0.02 | <0.02 | 432.8 ± 91.2 | <0.02 |
| FR_170_90 ± SD | 56.84 ± 12.05 | 0.27 ± 0.36 | 0.05 ± 0.05 | <0.02 | 0.84 ± 0.08 | <0.02 | 0.37 ± 0.35 | 455.0 ± 13.6 | * |
| Code | Co | Cr | Cu | Fe | K | Li | Mg | Mn | Mo |
|---|---|---|---|---|---|---|---|---|---|
| DG Raw ± SD | 2.51 ± 0.06 | 7.96 ± 0.33 | 50.28 ± 1.85 | 3273 ± 105 | 62,915 ± 1906 | 5.72 ± 0.51 | 7053 ± 438 | 163.1 ± 5.20 | 6.96 ± 0.05 |
| DG_130_30 ± SD | 2.26 ± 0.12 | 7.24 ± 0.39 | 59.40 ± 5.19 | 3602 ± 241 | 14,447 ± 879 | 5.83 ± 2.22 | 1770 ± 79 | 102.8 ± 6.4 | 7.11 ± 0.63 |
| DG_130_60 ± SD | 1.47 ± 0.03 | 8.45 ± 0.36 | 64.30 ± 3.64 | 2319 ± 43 | 11,772 ± 567 | 6.98 ± 2.86 | 1202 ± 29 | 42.29 ± 0.38 | 8.13 ± 0.52 |
| DG_130_90 ± SD | 2.09 ± 0.10 | 8.64 ± 0.65 | 64.68 ± 5.47 | 3715 ± 209 | 9354 ± 635 | 8.18 ± 3.59 | 1244 ± 44 | 66.20 ± 3.64 | 7.85 ± 0.58 |
| DG_150_60 ± SD | 2.22 ± 0.05 | 9.63 ± 0.12 | 73.20 ± 2.60 | 3632 ± 55 | 10,139 ± 190 | 14.37 ± 3.45 | 1262 ± 27 | 68.54 ± 2.84 | 9.32 ± 0.45 |
| DG_170_30 ± SD | 1.96 ± 0.03 | 10.16 ± 0.24 | 72.84 ± 3.47 | 3076 ± 38 | 12,628 ± 238 | 13.15 ± 1.34 | 1327 ± 15 | 55.07 ± 1.96 | 9.48 ± 0.10 |
| DG_170_60 ± SD | 2.34 ± 0.10 | 9.40 ± 0.39 | 69.15 ± 3.99 | 3449 ± 145 | 10,501 ± 86 | 15.74 ± 2.42 | 2142 ± 85 | 115.4 ± 5.0 | 8.83 ± 0.46 |
| DG_170_90 ± SD | 2.33 ± 0.07 | 9.37 ± 0.25 | 68.98 ± 3.36 | 3440 ± 96 | 17,440 ± 654 | 15.68 ± 2.24 | 2137 ± 53 | 115.2 ± 4.00 | 8.83 ± 0.40 |
| FR Raw ± SD | 0.02 ± 0.01 | 1.79 ± 0.35 | 5.76 ± 0.59 | 84.69 ± 2.61 | 1562 ± 46 | 0.32 ± 0.18 | 605.3 ± 14.1 | 417.1 ± 13.8 | 0.02 ± 0.00 |
| FR_130_30 ± SD | <0.02 ± 0.01 | 0.27 ± 0.13 | <0.02 | 11.36 ± 1.74 | 170.2 ± 33.7 | 1.72 ± 0.55 | 128.0 ± 2.90 | 27.54 ± 1.64 | <0.02 |
| FR_130_60 ± SD | 0.08 ± 0.09 | 0.34 ± 0.14 | <0.02 | 10.11 ± 0.57 | 246.3 ± 17.8 | 13.07 ± 1.38 | 136.2 ± 1.70 | 38.25 ± 3.20 | <0.02 |
| FR_130_90 ± SD | <0.02 | 0.39 ± 0.04 | 0.03 ± 0.02 | 7.79 ± 0.57 | 307.1 ± 10.4 | 17.48 ± 1.78 | 131.4 ± 3.70 | 30.14 ± 2.15 | <0.02 |
| FR_150_60 ± SD | <0.02 | 0.50 ± 0.03 | 2.70 ± 0.18 | 18.4 ± 1.10 | 190.9 ± 6.1 | 0.24 ± 0.32 | 99.57 ± 1.86 | 43.78 ± 2.42 | <0.02 |
| FR_170_30 ± SD | 0.03 ± 0.01 | 0.45 ± 0.03 | 2.56 ± 0.79 | 22.45 ± 0.40 | 362.4 ± 6.8 | 15.80 ± 1.53 | 147.0 ± 10.7 | 86.21 ± 3.11 | <0.02 |
| FR_170_60 ± SD | 0.03 ± 0.02 | 0.32 ± 0.22 | 3.06 ± 0.22 | 32.68 ± 3.57 | 200.7 ± 4.4 | 17.58 ± 1.26 | 89.22 ± 1.82 | 41.78 ± 0.26 | <0.02 |
| FR_170_90 ± SD | <0.02 | 0.09 ± 0.11 | 3.80 ± 0.86 | 28.78 ± 2.55 | 376.1 ± 11.8 | 58.23 ± 1.96 | 104.1 ± 4.30 | 49.36 ± 2.05 | <0.02 |
| Code | Na | Ni | Pb | Sb | Se | Sr | Ti | Tl | V |
|---|---|---|---|---|---|---|---|---|---|
| DG Raw ± SD | 2014 ± 88 | 10.40 ± 0.66 | 3.23 ± 0.10 | <0.02 | 0.98 ± 0.04 | 93.70 ± 4.15 | 67.85 ± 1.47 | 0.27 ± 0.01 | 4.19 ± 0.58 |
| DG_130_30 ± SD | 479.5 ± 23.1 | 8.84 ± 0.71 | 1.46 ± 0.26 | <0.02 | 0.56 ± 0.08 | 39.20 ± 3.22 | 78.98 ± 8.51 | <0.17 | 5.51 ± 0.42 |
| DG_130_60 ± SD | 424.3 ± 7.5 | 7.58 ± 0.40 | 1.12 ± 0.49 | 0.02 ± 0.01 | 1.09 ± 0.04 | 20.51 ± 0.64 | 84.57 ± 9.93 | <0.17 | 6.06 ± 0.31 |
| DG_130_90 ± SD | 394.6 ± 21.6 | 10.20 ± 0.77 | 2.81 ± 0.78 | <0.02 | 0.70 ± 0.02 | 25.75 ± 1.50 | 89.16 ± 14.91 | <0.17 | 5.89 ± 0.83 |
| DG_150_60 ± SD | 422.6 ± 13.7 | 11.5 ± 0.25 | 4.20 ± 0.86 | 0.03 ± 0.02 | 0.90 ± 0.12 | 27.31 ± 1.16 | 98.19 ± 8.19 | <0.17 | 7.16 ± 0.43 |
| DG_170_30 ± SD | 448.0 ± 8.4 | 10.48 ± 0.33 | 5.29 ± 1.51 | <0.02 | 1.01 ± 0.05 | 23.69 ± 0.88 | 124.3 ± 5.8 | <0.17 | 6.95 ± 0.45 |
| DG_170_60 ± SD | 516.5 ± 15.2 | 11.51 ± 0.89 | <0.02 | <0.02 | 0.26 ± 0.10 | 44.58 ± 1.52 | 110.5 ± 2.50 | <0.17 | 6.53 ± 0.41 |
| DG_170_90 ± SD | 515.3 ± 8.1 | 11.50 ± 0.73 | <0.02 | <0.02 | 0.27 ± 0.10 | 44.48 ± 1.2 | 110.2 ± 0.7 | <0.17 | 6.52 ± 0.39 |
| FR Raw ± SD | 244.7 ± 16.4 | 0.16 ± 0.13 | 0.60 ± 0.19 | <0.02 | 0.89 ± 0.15 | 11.34 ± 0.86 | 4.46 ± 0.53 | 0.77 ± 0.06 | 0.22 ± 0.06 |
| FR_130_30 ± SD | 241.0 ± 13.8 | <0.02 | <0.02 | <0.02 | 0.12 ± 0.08 | 0.08 ± 0.06 | 0.97 ± 0.10 | <0.17 | <0.02 |
| FR_130_60 ± SD | 237.5 ± 7.1 | 0.14 ± 0.18 | 28.59 ± 9.14 | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.31 ± 0.15 | 0.79 ± 0.25 | 0.18 ± 0.02 | 0.07 ± 0.08 |
| FR_130_90 ± SD | 250.3 ± 16.1 | <0.02 | 52.96 ± 24.57 | <0.02 | 0.19 ± 0.16 | 0.19 ± 0.08 | 0.72 ± 0.20 | <0.17 | 0.21 ± 0.01 |
| FR_150_60 ± SD | 197.4 ± 36.1 | <0.02 | <0.02 | <0.02 | 0.21 ± 0.03 | 2.22 ± 0.16 | 1.56 ± 0.04 | <0.17 | 0.06 ± 0.06 |
| FR_170_30 ± SD | 170.2 ± 5.0 | <0.02 | 1.60 ± 1.02 | <0.02 | 0.31 ± 0.07 | 2.72 ± 0.69 | 1.28 ± 0.04 | <0.17 | 0.06 ± 0.06 |
| FR_170_60 ± SD | 166.8 ± 2.7 | <0.02 | 13.71 ± 10.97 | <0.02 | 0.09 ± 0.04 | 1.59 ± 0.14 | 1.89 ± 0.19 | 0.36 ± 0.27 | 0.06 ± 0.03 |
| FR_170_90 ± SD | 193.7 ± 11.8 | <0.02 | 29.35 ± 3.53 | <0.02 | 0.15 ± 0.06 | 2.47 ± 0.28 | 1.56 ± 0.06 | 0.16 ± 0.01 | 0.15 ± 0.10 |
| Code | Zn | Si | P | S |
|---|---|---|---|---|
| DG Raw ± SD | 169.13 ± 4.88 | 1737 ± 296 | 4110 ± 220 | 2192 ± 62 |
| DG_130_30 ± SD | 233.9 ± 6.80 | <3.87 ± 0.01 | 112.9 ± 5.10 | 34.95 ± 1.29 |
| DG_130_60 ± SD | 139.0 ± 8.60 | <3.85 ± 0.04 | 113.0 ± 4.30 | 35.01 ± 1.16 |
| DG_130_90 ± SD | 235.0 ± 13.1 | 3.90 ± 0.10 | 115.5 ± 4.20 | 35.78 ± 1.28 |
| DG_150_60 ± SD | 241.4 ± 4.70 | <3.89 ± 0.05 | 113.9 ± 5.90 | 35.28 ± 1.42 |
| DG_170_30 ± SD | 195 4 ± 3.60 | <3.91 ± 0.01 | 115.2 ± 3.80 | 35.69 ± 0.85 |
| DG_170_60 ± SD | 210.0 ± 8.80 | <3.98 ± 0.03 | 116.3 ± 4.90 | 36.02 ± 1.13 |
| DG_170_90 ± SD | 209.5 ± 6.60 | <3.98 ± 0.03 | 116.1 ± 5.50 | 35.96 ± 1.46 |
| FR Raw ± SD | 53.96 ± 2.32 | <3.94 ± 0.01 | 279.0 ± 10.2 | 116.4 ± 4.20 |
| FR_130_30 ± SD | 15.20 ± 5.73 | <3.92 ± 0.03 | 114.7 ± 1.50 | 61.20 ± 2.46 |
| FR_130_60 ± SD | 17.39 ± 1.67 | <3.93 ± 0.02 | 108.8 ± 2.60 | 44.78 ± 1.99 |
| FR_130_90 ± SD | 11.21 ± 4.78 | <3.94 ± 0.04 | 107.3 ± 3.6 | 43.23 ± 1.93 |
| FR_150_60 ± SD | 9.31 ± 0.59 | <3.99 ± 0.01 | 135.7 ± 35.7 | 50.47 ± 3.04 |
| FR_170_30 ± SD | 27.56 ± 0.11 | <3.97 ± 0.03 | 117.1 ± 3.90 | 36.26 ± 0.98 |
| FR_170_60 ± SD | 25.82 ± 2.69 | <3.97 ± 0.02 | 116.0 ± 5.10 | 35.92 ± 1.22 |
| FR_170_90 ± SD | 20.56 ± 7.50 | <3.96 ± 0.03 | 114.2 ± 6.60 | 35.37 ± 1.73 |
References
- Di Gruttola, F.; Borello, D. Analysis of the EU Secondary Biomass Availability and Conversion Processes to Produce Advanced Biofuels: Use of Existing Databases for Assessing a Metric Evaluation for the 2025 Perspective. Sustainability 2021, 13, 7882. [Google Scholar] [CrossRef]
- Antczak, A.; Szadkowski, J.; Szadkowska, D.; Zawadzki, J. Assessment of the effectiveness of liquid hot water and steam explosion pretreatments of fast-growing poplar (Populus trichocarpa) wood. Wood Sci. Technol. 2022, 56, 87–109. [Google Scholar] [CrossRef]
- Akus-Szylberg, F.; Antczak, A.; Zawadzki, J. Effects of soaking aqueous ammonia pretreatment on chemical composition and enzymatic hydrolysis of corn stover. Ann. WULS—SGGW For. Wood Technol. 2021, 115, 29–36. [Google Scholar] [CrossRef]
- Stobernack, N.; Mayer, F.; Malek, C.; Bhandari, R.; Himanshu, H. Hydrothermal carbonization of biowaste as an alternative treatment path to current waste management practices in Germany. Energy Convers. Manag. 2021, 244, 114433. [Google Scholar] [CrossRef]
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valor. 2020, 12, 1–28. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Appl. Energy 2014, 114, 857–864. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Sermyagina, E.; Saari, J.; Silva de Jesus, M.; Cardoso, M.; Matheus de Almeida, G.; Vakkilainen, E. Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Brosowski, A.; Adler, P.; Erdmann, G.; Stinner, W.; Thrän, D.; Mantau, U. Biomassepotenziale von Rest—Und Abfallstoffen: Status Quo in Deutschland; Fachagentur Nachwachsende Rohstoffe e.V. (FNR): Gülzow, Germany, 2015; ISBN 9783942147293. [Google Scholar]
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. (Eds.) Energie aus Biomasse: Grundlagen, Techniken und Verfahren, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-47438-9. [Google Scholar]
- Krutul, D.; Szadkowski, J.A.N.; Antczak, A.; Drożdżek, M.; Radomski, A.; Karpiński, S. The concentration of selected heavy metals in poplar wood biomass and liquid fraction obtained after high temperature pretreatment. Wood Res. 2021, 66, 39–48. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Yokoyama, S.M. The Asian Biomass Handbook a Guide for Biomass Production and Utilization; The Japan Institute of Energy, 2008. [Google Scholar]
- Zhou, L.; Hülsemann, B.; Cui, Z.; Merkle, W.; Sponagel, C.; Zhou, Y.; Guo, J.; Dong, R.; Müller, J.; Oechsner, H. Operating Performance of Full-Scale Agricultural Biogas Plants in Germany and China: Results of a Year-Round Monitoring Program. Appl. Sci. 2021, 11, 1271. [Google Scholar] [CrossRef]
- Parmar, K.R.; Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Fletcher, L.A.; Ross, A.B. Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality. Energies 2022, 15, 1418. [Google Scholar] [CrossRef]
- González, R.; Ellacuriaga, M.; Aguilar-Pesantes, A.; Carrillo-Peña, D.; García-Cascallana, J.; Smith, R.; Gómez, X. Feasibility of Coupling Anaerobic Digestion and Hydrothermal Carbonization: Analyzing Thermal Demand. Appl. Sci. 2021, 11, 11660. [Google Scholar] [CrossRef]
- Bergius, F. Holz und Kohle, chemische und wirtschaftliche Betrachtungen. Z. Angew. Chem. 1928, 41, 707–711. [Google Scholar] [CrossRef]
- Satira, A.; Paone, E.; Bressi, V.; Iannazzo, D.; Marra, F.; Calabrò, P.S.; Mauriello, F.; Espro, C. Hydrothermal Carbonization as Sustainable Process for the Complete Upgrading of Orange Peel Waste into Value-Added Chemicals and Bio-Carbon Materials. Appl. Sci. 2021, 11, 10983. [Google Scholar] [CrossRef]
- Han, S.; Bai, L.; Chi, M.; Xu, X.; Chen, Z.; Yu, K. Conversion of Waste Corn Straw to Value-Added Fuel via Hydrothermal Carbonization after Acid Washing. Energies 2022, 15, 1828. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Miliotti, E.; Casini, D.; Rosi, L.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Lab-scale pyrolysis and hydrothermal carbonization of biomass digestate: Characterization of solid products and compliance with biochar standards. Biomass Bioenergy 2020, 139, 105593. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Bioref. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Cao, X.; Ro, K.S.; Libra, J.A.; Kammann, C.I.; Lima, I.; Berge, N.; Li, L.; Li, Y.; Chen, N.; Yang, J.; et al. Effects of biomass types and carbonization conditions on the chemical characteristics of hydrochars. J. Agric. Food Chem. 2013, 61, 9401–9411. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Blöhse, D.; Echterhof, T.; Eichenauer, S.; Heger, S.; Kammann, C.; Kruse, A.; Quicker, P.; Schulten, M.-A.; Weber, K. Biokohle: Herstellung, Eigenschaften und Verwendung von Biomassekarbonisaten; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2017; ISBN 9783658036898. [Google Scholar]
- Ramke, H.-G.; Blöhse, D.; Lehmann, H.-J.; Fettig, J. Hydrothermal Carbonization of Organic Waste. In Proceedings of the Sardinia 2009: Twelfth International Waste Management and Landfill Symposium, Margherita di Pula, Italy, 5–9 October 2009. [Google Scholar]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Bioref. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Bandura, A.V.; Lvov, S.N. The Ionization Constant of Water over Wide Ranges of Temperature and Density. J. Phys. Chem. Ref. Data 2006, 35, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Blöhse, L.R. Verbesserte Energetische Nutzung Organischer Industrieabfälle durch Hydrothermale Carbonsierung: Förderlinie: FHprofUnd2009 Förderkennzeichen:17290X9; Forschungsbericht; Hochschule Ostwestfalen: Höxter, Germany, 2014. [Google Scholar]
- Watanabe, M.; Aida, T.M.; Smith, R.L. Review of Biomass Conversion in High Pressure High Temperature Water (HHW) Including Recent Experimental Results (Isomerization and Carbonization). In Application of Hydrothermal Reactions to Biomass Conversion; Jin, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 249–274. ISBN 978-3-642-54457-6. [Google Scholar]
- Funke, A. Hydrothermale Karbonisierung von Biomasse—Reaktionsmechanismen und Reaktionswärme. Ph.D. Thesis, Der Technischen Universität Berlin, Berlin, Germany, 2012. [Google Scholar]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chemistry 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y.; Oshiki, T.; Kishimoto, T. Low-temperature catalytic hydrothermal treatment of wood biomass: Analysis of liquid products. Chem. Eng. J. 2005, 108, 127–137. [Google Scholar] [CrossRef]
- Vallejoy, M.-E.; Fernando Esteban, F.; Maria Cristina, A. Hydrothermal Treatments Applied to Agro- and Forest-Industrial Waste to Produce High Added-Value Compounds. BioResources 2017, 12, 2058–2080. [Google Scholar]
- Ferrentino, R.; Merzari, F.; Fiori, L.; Andreottola, G. Coupling Hydrothermal Carbonization with Anaerobic Digestion for Sewage Sludge Treatment: Influence of HTC Liquor and Hydrochar on Biomethane Production. Energies 2020, 13, 6262. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Biomethane production using fresh and thermally pretreated Chlorella vulgaris biomass: A comparison of batch and semi-continuous feeding mode. Ecol. Eng. 2015, 84, 273–277. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenès, J.P.; Carrère, H. Effects of thermal treatments on five different waste activated sludge samples solubilisation, physical properties and anaerobic digestion. Chem. Eng. J. 2008, 139, 236–244. [Google Scholar] [CrossRef]
- Yuan, T.; Cheng, Y.; Zhang, Z.; Lei, Z.; Shimizu, K. Comparative study on hydrothermal treatment as pre- and post-treatment of anaerobic digestion of primary sludge: Focus on energy balance, resources transformation and sludge dewaterability. Appl. Energy 2019, 239, 171–180. [Google Scholar] [CrossRef]
- Deng, C.; Kang, X.; Lin, R.; Murphy, J.D. Microwave assisted low-temperature hydrothermal treatment of solid anaerobic digestate for optimising hydrochar and energy recovery. Chem. Eng. J. 2020, 395, 124999. [Google Scholar] [CrossRef]
- Knappe, V.; Paczkowski, S.; Tejada, J.; Diaz Robles, L.A.; Gonzales, A.; Pelz, S. Low temperature microwave assisted hydrothermal carbonization (MAHC) reduces combustion emission precursors in short rotation coppice willow wood. J. Anal. Appl. Pyrolysis 2018, 134, 162–166. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Paczkowski, S.; Comi, J.; Küttner, I.; Knappe, V.; Russ, M.; Robles, L.A.D.; Jaeger, D.; Pelz, S. Hydrothermal treatment (HTT) for improving the fuel properties of biomass residues. Biomass Conv. Bioref. 2022, 136, 61. [Google Scholar] [CrossRef]
- Sailer, G.; Empl, F.; Kuptz, D.; Silberhorn, M.; Ludewig, D.; Lesche, S.; Pelz, S.; Müller, J. Characteristics and Anaerobic Co-Digestion of Press Water from Wood Fuel Preparation and Digested Sewage Sludge. Fermentation 2022, 8, 37. [Google Scholar] [CrossRef]
- Sailer, G.; Knappe, V.; Poetsch, J.; Paczkowski, S.; Pelz, S.; Oechsner, H.; Bosilj, M.; Ouardi, S.; Müller, J. Upgrading the Organic Fraction of Municipal Solid Waste by Low Temperature Hydrothermal Processes. Energies 2021, 14, 3041. [Google Scholar] [CrossRef]
- DIN EN ISO 18122:2016-03; Biogene Festbrennstoffe—Bestimmung des Aschegehaltes (ISO 18122:2015); Deutsche Fassung EN ISO 18122:2015. Beuth Verlag GmbH: Berlin, Germany, 2016.
- DIN EN ISO 16948:2015-09; Biogene Festbrennstoffe—Bestimmung des Gesamtgehaltes an Kohlenstoff, Wasserstoff und Stickstoff (ISO 16948:2015), Deutsche Fassung EN ISO 16948:2015. Beuth Verlag GmbH: Berlin, Germany, 2015.
- Prof. Dr.-Ing.H. Netz, Verbrennung und Gasgewinnung bei Festbrennstoffen, Technischer Verlag Resch KG, Gräfelfing 1982, 196 S., DIN A 5, Preis 38.-DM. Fette Seifen Anstrichm. 1982, 84, 322. [CrossRef]
- DIN EN ISO 11885:2009-09; Wasserbeschaffenheit—Bestimmung von Ausgewählten Elementen durch Induktiv Gekoppelte Plasma-Atom-Emissionsspektrometrie (ICP-OES) (ISO 11885:2007). Deutsche Fassung EN ISO 11885:2009. Beuth Verlag GmbH: Berlin, Germany, 2009.
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Suwelack, K.; Dostert, N.; Wüst, D.; Kruse, A. Economics of hydrothermal carbonization of biogas digestate in a hybrid AD-HTC plant. In Proceedings of the 24th European Biomass Conference and Exhibition, Amsterdam, The Netherlands, 6–9 June 2016. [Google Scholar]
- Fang, Z.; Sato, T.; Smith, R.L.; Inomata, H.; Arai, K.; Kozinski, J.A. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water. Bioresour. Technol. 2008, 99, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Williams, P.T. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Garlapalli, R.K.; Wirth, B.; Reza, M.T. Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresour. Technol. 2016, 220, 168–174. [Google Scholar] [CrossRef]
- Ramke, H.-G.; Blöhse, D.; Lehmann, H.-J.; Antoniertti, M.; Fettig, J. Machbarkeitsstudie zur Energiegewinnung aus Organischen Siedlungsabfällen durch Hydrothermale Carbonisierung; Deutsche Bundesstiftung Umwelt (DBU): Höxter, Germany, 2010; Available online: https://www.dbu.de/OPAC/ab/DBU-Abschlussbericht-AZ-25604.pdf (accessed on 28 April 2021).
- Parshetti, G.K.; Kent Hoekman, S.; Balasubramanian, R. Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour. Technol. 2013, 135, 683–689. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273. [Google Scholar] [CrossRef] [Green Version]
- prEN ISO 17225-8:2016; Biogene Festbrennstoffe—Brennstoffspezifikationen und Klassen. Teil 8 Klassifizierung von Thermisch Behandelten und Gepressten Brennstoffen aus Biomasse: Solid Biofuels—Fuel Specifications and Classes, Graded Thermally Treated and Densified Biomass Fuels. Beuth Verlag GmbH: Berlin, Germany, 2016.
- Geffert, A.; Geffertova, J.; Dudiak, M. Direct Method of Measuring the pH Value of Wood. Forests 2019, 10, 852. [Google Scholar] [CrossRef] [Green Version]
- Du, F.-L.; Du, Q.-S.; Dai, J.; Tang, P.-D.; Li, Y.-M.; Long, S.-Y.; Xie, N.-Z.; Wang, Q.-Y.; Huang, R.-B. A comparative study for the organic byproducts from hydrothermal carbonizations of sugarcane bagasse and its bio-refined components cellulose and lignin. PLoS ONE 2018, 13, e0197188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; DOE/GO-102004-1992; National Renewable Energy Laboratory: Golden, CO, USA, 2004. Available online: https://www.osti.gov/biblio/15008859-top-value-added-chemicals-from-biomass-volume-results-screening-potential-candidates-from-sugars-synthesis-gas (accessed on 28 April 2021).
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Vanoye, L.; Fanselow, M.; Holbrey, J.D.; Atkins, M.P.; Seddon, K.R. Kinetic model for the hydrolysis of lignocellulosic biomass in the ionic liquid, 1-ethyl-3-methyl-imidazolium chloride. Green Chem. 2009, 11, 390. [Google Scholar] [CrossRef]
- Jeder, A. Carbon Materials Synthesis by Hydrothermal Carbonization of Olive Stones: Process and Product Characterization; Université de Lorraine Université de Gabès (Tunisie). Ph.D. Thesis, University of Lorraine, Lorraine, France, 2018. [Google Scholar]
- Huang, Y.-B.; Yang, Z.; Dai, J.-J.; Guo, Q.-X.; Fu, Y. Production of high quality fuels from lignocellulose-derived chemicals: A convenient C–C bond formation of furfural, 5-methylfurfural and aromatic aldehyde. RSC Adv. 2012, 2, 11211. [Google Scholar] [CrossRef]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Fierro, V.; Jeder, A.; Ouederni, A.; Masson, E.; Celzard, A. High added-value products from the hydrothermal carbonisation of olive stones. Environ. Sci. Pollut. Res. 2017, 24, 9859–9869. [Google Scholar] [CrossRef]
- Kumar, M.; Myagmardoloonjin, B.; Keshari, S.; Negari, I.P.; Huang, C.-M. 5-methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome. Microorganisms 2019, 7, 239. [Google Scholar] [CrossRef] [Green Version]








| Temperature (°C) | Variant 1 Ramp/Holding Time | Variant 2 Ramp/Holding Time | Variant 3 Ramp/Holding Time |
|---|---|---|---|
| 170 | 5.00 °C × min−1/30 min | 5.00 °C × min−1/60 min | 5 °C × min−1/90 min |
| 150 | - | 4.33 °C × min−1/60 min | - |
| 130 | 3.67 °C × min−1/30 min | 3.67 °C × min−1/60 min | 3.67 °C × min−1/90 min |
| Temperature (°C) | Holding Time (min) | SF |
|---|---|---|
| 130 | 30 | 2.4 |
| 130 | 60 | 2.7 |
| 130 | 90 | 2.8 |
| 150 | 60 | 3.3 |
| 170 | 30 | 3.5 |
| 170 | 60 | 3.8 |
| 170 | 90 | 4.0 |
| Parameter, unit | DG Raw | DG 130 °C|30 min | DG 130 °C|60 min | DG 130 °C|90 min | DG 150 °C|60 min | DG 170 °C|30 min | DG 170 °C|60 min | DG 170 °C|90 min |
|---|---|---|---|---|---|---|---|---|
| mass loss, %DM | - | 26.14 ± 0.18 | 30.69 ± 0.57 | 31.46 ± 0.35 | 35.70 ± 1.44 | 35.84 ± 0.06 | 38.49 ± 0.01 | 39.32 ± 0.96 |
| ash, %DM | 26.67 ± 0.04 | 8.68 ± 0.02 | 8.86 ± 0.03 | 9.82 ± 0.06 | 11.48 ± 0.02 | 11.80 ± 0.04 | 12.17 ± 0.01 | 14.64 ± 0.02 |
| C, %DM | 40.66 ± 0.27 | 48.16 ± 0.71 | 49.31 ± 0.71 | 50.98 ± 0.29 | 51.49 ± 0.20 | 51.53 ± 0.14 | 50.34 ± 0.58 | 49.50 ± 0.44 |
| H, %DM | 5.02 ± 0.03 | 5.72 ± 0.16 | 5.60 ± 0.13 | 6.00 ± 0.18 | 5.87 ± 0.17 | 5.79 ± 0.13 | 5.62 ± 0.11 | 5.44 ± 0.20 |
| O, %DM c | 25.28 ± 0.26 | 34.41 ± 0.85 | 32.82 ± 0.60 | 30.22 ± 0.47 | 28.03 ± 0.29 | 27.96 ± 0.08 | 29.03 ± 0.60 | 27.39 ± 0.61 |
| N, %DM | 2.37 ± 0.04 | 3.03 ± 0.12 | 3.41 ± 0.16 | 2.98 ± 0.03 | 3.13 ± 0.09 | 2.92 ± 0.06 | 2.84 ± 0.04 | 3.03 ± 0.04 |
| S, %DM | 0.22 ± 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| H/C | 1.47 | 1.41 | 1.35 | 1.40 | 1.36 | 1.34 | 1.33 | 1.31 |
| O/C | 0.47 | 0.54 | 0.50 | 0.45 | 0.41 | 0.41 | 0.43 | 0.42 |
| HHV, MJ/kgDM a | 16.55 | 19.38 | 19.86 | 20.64 | 20.82 | 20.77 | 20.20 | 19.83 |
| LHV, MJ/kgDM b | 16.31 | 18.60 | 19.08 | 20.30 | 20.60 | 20.53 | 19.84 | 19.57 |
| Parameter | Fr Raw | FR 130 °C|30 min | FR 130 °C|60 min | FR 130 °C|90 min | FR 150 °C|60 min | FR 170 °C|30 min | FR 170 °C|60 min | FR 170 °C|90 min |
|---|---|---|---|---|---|---|---|---|
| mass loss, %DM | 26.94 ± 3.19 | 34.65 ± 0.72 | 35.43 ± 2.50 | 37.44 ± 0.84 | 38.00 ± 1.45 | 41.29 ± 0.80 | 43.80 ± 0.81 | |
| ash, %DM | 0.95 ± 0.02 | 0.68 ± 0.02 | 0.65 ± 0.03 | 0.64 ± 0.02 | 0.58 ± 0.01 | 0.65 ± 0.03 | 0.62 ± 0.03 | 0.74 ± 0.04 |
| C, %DM | 51.00 ± 0.07 | 50.57 ± 0.22 | 51.44 ± 0.48 | 50.12 ± 0.38 | 50.70 ± 0.09 | 50.85 ± 0.26 | 51.68 ± 0.25 | 51.78 ± 0.30 |
| H, %DM | 6.31 ± 0.01 | 5.90 ± 0.06 | 5.93 ± 0.20 | 5.86 ± 0.11 | 5.94 ± 0.06 | 5.77 ± 0.17 | 5.81 ± 0.07 | 5.75 ± 0.21 |
| O, %DM c | 41.32 ± 0.08 | 42.25 ± 0.24 | 41.21 ± 0.72 | 42.47 ± 0.40 | 41.82 ± 0.14 | 41.71 ± 0.22 | 40.90 ± 0.29 | 40.59 ± 0.46 |
| N, %DM | 0.43 ± 0.01 | 0.61 ± 0.08 | 0.76 ± 0.06 | 0.91 ± 0.07 | 0.97 ± 0.04 | 1.02 ± 0.04 | 0.98 ± 0.02 | 1.13 ± 0.03 |
| S, %DM | 0.012 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| H/C | 1.47 | 1.39 | 1.37 | 1.39 | 1.40 | 1.35 | 1.34 | 1.32 |
| O/C | 0.61 | 0.63 | 0.60 | 0.64 | 0.62 | 0.62 | 0.59 | 0.59 |
| HHV, MJ/kgDM a | 20.46 | 20.12 | 20.52 | 20.10 | 20.24 | 20.24 | 20.60 | 20.63 |
| LHV, MJ/kgDM b | 19.23 | 18.61 | 19.06 | 18.56 | 18.76 | 18.67 | 19.09 | 19.11 |
| 130 °C 30 min | 130 °C 60 min | 130 °C 90 min | 150 °C 60 min | 170 °C 30 min | 170 °C 60 min | 170 °C 90 min | |
|---|---|---|---|---|---|---|---|
| DG | 4.56 | 3.56 | 3.54 | 3.56 | 3.29 | 3.37 | 2.96 |
| FR | 1.41 | 2.06 | 1.98 | 2.49 | 2.62 | 2.55 | 2.51 |
| Peak No. | Compound | Mean Retention Time |
|---|---|---|
| 1 | Acetic acid | 5.23 |
| 2 | Hexanal | 6.73 |
| 3 | Furfural | 7.51 |
| 4 | Benzaldehyde | 10.52 |
| 5 | 5-Methylfurfural | 10.58 |
| 6 | Heptanonitrile | 10.97 |
| 7 | Benzonitrile | 11.16 |
| 8 | 2-Octanol, (R)- | 11.40 |
| 9 | Cyclohexanone,2,2,6-trimethyl | 12.27 |
| 10 | Benzeneacetaldehyde | 12.49 |
| 11 | Guaiacol | 13.50 |
| 12 | .alpha.-Campholenal | 13.66 |
| 13 | 1-Nonanol or 1-Heptanol,6-methyl | 14.36 |
| 14 | Camphor | 14.81 |
| 15 | Bicyclo[3.1.1]heptan-3-one,2,6,6-trimethyl(1.alpha.,2.alpha.,5.alpha)- | 15.16 |
| 16 | Ethanone, 1-(4-methylphenyl) or Ethanone, 1-(2-methylphenyl) or Ethanone, 1-(3-methylphenyl) | 15.61 |
| 17 | Carvenone | 17.15 |
| 18 | Nonanoic acid | 17.20 |
| 19 | Octanenitrile | 17.68 |
| 20 | 2-Methoxy-4-vinylphenol or Phenol,5-ethyl-,2-methoxy or 4-hydroxy-2-methylacetophenone | 18.20 |
| 21 | Phenol, 2-methoxy-3(2-propenyl) or Eugenol or trans-isoeugenol or 3-Allyl-6-methoxyphenol | 19.16 |
| 22 | Ethanol, 2-(2-butoxyethoxy)-acetate | 19.19 |
| 23 | Vanillin | 20.22 |
| 24 | 2(4H)-Benzofuranone,5,6,7,7a-tetrahydro-4,4,7a-trimethyl-,(R)- | 24.18 |
| 25 | 4-Nitroguajacol or 5-Nitroguajacol | 24.28 |
| 26 | Butyrovanillone | 25.08 |
| 27 | Phenol,2-4,dinitro-6-methoxy | 27.25 |
| 28 | n-Hexadecanoic acid | 28.53 |
| 29 | Squalene | 28.67 |
| 30 | Naphthol[2.1-b]furan-2(1H)-one,decahydro-3a,6,6,9a-tetramethyl-[3as-(3a.alpha.,5a.alpha.,9a.beta.,9b.alpha)] | 29.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sailer, G.; Comi, J.; Empl, F.; Silberhorn, M.; Heymann, V.; Bosilj, M.; Ouardi, S.; Pelz, S.; Müller, J. Hydrothermal Treatment of Residual Forest Wood (Softwood) and Digestate from Anaerobic Digestion—Influence of Temperature and Holding Time on the Characteristics of the Solid and Liquid Products. Energies 2022, 15, 3738. https://doi.org/10.3390/en15103738
Sailer G, Comi J, Empl F, Silberhorn M, Heymann V, Bosilj M, Ouardi S, Pelz S, Müller J. Hydrothermal Treatment of Residual Forest Wood (Softwood) and Digestate from Anaerobic Digestion—Influence of Temperature and Holding Time on the Characteristics of the Solid and Liquid Products. Energies. 2022; 15(10):3738. https://doi.org/10.3390/en15103738
Chicago/Turabian StyleSailer, Gregor, Julian Comi, Florian Empl, Martin Silberhorn, Valeska Heymann, Monika Bosilj, Siham Ouardi, Stefan Pelz, and Joachim Müller. 2022. "Hydrothermal Treatment of Residual Forest Wood (Softwood) and Digestate from Anaerobic Digestion—Influence of Temperature and Holding Time on the Characteristics of the Solid and Liquid Products" Energies 15, no. 10: 3738. https://doi.org/10.3390/en15103738
APA StyleSailer, G., Comi, J., Empl, F., Silberhorn, M., Heymann, V., Bosilj, M., Ouardi, S., Pelz, S., & Müller, J. (2022). Hydrothermal Treatment of Residual Forest Wood (Softwood) and Digestate from Anaerobic Digestion—Influence of Temperature and Holding Time on the Characteristics of the Solid and Liquid Products. Energies, 15(10), 3738. https://doi.org/10.3390/en15103738