Abstract
An in-depth investigation was performed on a metallic interconnect extracted from an SOFC stack operated for 40,000 h. The characterization was performed on the surface and the cross-section, paying attention to the evolution of the materials due to the interaction with the dual atmosphere of the stack under operating parameters. The interaction between materials (i.e., metal substrate, coatings and atmospheres) and stack components (i.e., current collectors and MIC) generated several modifications that affected the surface and, in some cases, the bulk of the interconnect. The careful metallographic preparation allowed for the performance of an intensive microscopical characterization of the cross-sections all along the interconnect profile, from the inlet to the outlet of the fuel stream. The formation of thermal grown oxides on both sides and their evolution were studied and described. The interconnect, after 40,000 h, was still suitable for operation, but the few bulk changes due to the diffusion of Ni and the TGO that formed at the fuel side suggest the introduction of fuel side coatings to increase the life expectations of the whole stack.
1. Introduction
From the perspective of efficient decarbonization in energy production, the usage of hydrogen as a fuel for power plants seems to be a logical solution [1]. Moreover, green hydrogen production using renewable power sources [2,3] mitigates the issues related to the intermittence of the service and allows for power-on-demand solutions. Hydrogen, as an energy vector [3], would increase the positive impact on the environment of fuel-cell-based power plants, with a sensitive effect on the short and long terms. To reach and sustain such a goal, the scientific community is widely engaged in resolving the issues related to the efficiency and reliability of the long-life of the fuel cell stack [4]. One of the main efforts made by the scientific community in this field is to face the issues related to the incertitude of the stability of cells and stacks in order to speed up the ecological transition to cleaner energy sources demanded by citizens and by the planet itself.
Among the possible solutions in the field of power and gas, solid oxide fuel cells (SOFCs) are favored for their high efficiency in energy conversion once both heat and electrical power are considered [1]. To become a competitive power source, the minimum operating time planned for SOFC stacks working in the range 923–1123 K must be increased from 50,000 to 80,000 h [5]. To reach such a goal, it is of fundamental importance to be aware of the evolution of the stack’s components extending over the longest possible period and to know how they affect the efficiency of the whole system [6,7,8,9]. The materials’ degradation mechanisms are related to both the interaction between parts and the operating parameters [10,11,12]. The present paper focused on the metallic interconnects (MICs) used in SOFC stacks to separate adjacent cells by granting the passage of electrons (from the anode of one cell to the cathode of a neighboring one) while keeping the fuel and the oxidant streams separated. For these reasons, they play a key role in the stack, even though they do not participate in the production of energy [13,14,15,16]. The state-of-the-art combined heat and power (CHP) devices make use of ferritic stainless-steel (FSS) MICs, because they offer (1) high electrical conductivity; (2) relatively simple shaping processes; (3) ease of sealing with the cells to ensure the gas’ tightness between the cathode and anode chambers; (4) a coefficient of thermal expansion (CTE) that matches the electrolyte’s; (5) high mechanical resistance; (6) chemical stability obtained by the formation of electrically conductive oxide at the air side, possibly enhanced by the application of protective coatings [17]; (7) cost effectiveness. Due to both the multiple roles they play in the stack and the direct interaction they have with the cell’s electrodes, the reliability of the MICs is fundamental to offering stacks with trustworthiness. To obtain the correct CTE and the formation of conductive thermally grown oxide (TGO), it is common to use FSS rich in Cr and poor in all other elements. This makes mandatory the presence of coating at the air side to hinder the formation of oxidized, Cr-rich volatile compounds, resulting in cathode poisoning and detrimental degradation of the cell [18]. The coating has the double role of keeping the TGO stable in oxidizing atmospheres and avoiding its direct interaction with the cathode material to hinder the interdiffusion of cations between the electrode and the oxidized scale [19,20].
Due to the high quality of the FSS in use, investigations need extremely long-lasting experiments and the reproduction of “real-life conditions” (i.e., temperature, atmospheres, and materials) to obtain a correct overview of the phenomena. The validation of laboratory tests and the refinement of degradation models can only occur by comparison with components extracted from operated stacks [21,22,23,24]. The project, Ad Astra, offers such an opportunity and several field-operated SOFC stacks over various periods have been studied. The present paper refers to the longest period of operation. The post-mortem characterization of MIC samples extracted from a field stack operated in the fuel cell mode for 40,000 h completed a study carried on identical stacks operated in the range 5000–20,000 h [21,22]. The results are of fundamental importance for the modeling of the stack’s performance evolution and for a better understanding of samples issued by accelerated tests.
The investigation performed completed wider research focused on the metallic interconnects used in SOFC stacks. The research had several targets: (1) to gather information on the alteration of the MIC and its coating after long-term operation; (2) to correlate the observed alterations with local parameters such as the temperature, the atmosphere, and the polarization; (3) to obtain an overview of the behavior in a dual atmosphere to be compared with laboratory samples; (4) to gather data on the TGO growth rate at both the cathode and the anode sides to be used for purposes such modeling, planning AST tests and interpretation of accelerated aging tests. This is the first time that an interconnect operated for 40,000 h was investigated and its characteristics compared with similar interconnects extracted from twin stacks operated for 5000, 9000 and 20,000 h.
2. Materials and Methods
The interconnect submitted for the present study was extracted from an SOFC stack manufactured and operated by the Sunfire company (Sunfire GmbH, Dresden, Germany) according to the disclosable working conditions listed in Table 1.

Table 1.
Operating parameters for the stack.
The fuel composition and the nominal temperature are indicative. They may change with the position between the inlet and the outlet of the anode. The anodic reaction is, in fact, exothermic and generates water vapor during the cell polarization. At the anode side, from inlet to outlet, both the operating temperature and the amount of water in the fuel increase, while the hydrogen content decreases. According to the company’s information, during operation, the temperature, from the inlet to outlet, is in the range ca. 1000 to ca. 1050 K. The PH2/PH2O ratio depends on the fuel consumption and the cell efficiency. The cathode side notices the thermal gradient only.
The stacks, manufactured according to the company’s standards, used electrolyte-supported cells based on 3YSZ (3 mol % yttria-stabilized zirconia) as the electrolyte, with electrodes made of LSM (lanthanum strontium manganite) as the cathode and Ni/GDC cermet as the anode. Both electrodes were connected to the MIC by contacting layers acting as current collectors: lanthanum-strontium-manganite-doped cobalt (LSCM) and nickel metal foam at the cathode and anode, respectively. To offer an even distribution of the gases, the MIC was shaped in a proprietary design with “ribs and channels” (Figure 1) on both sides. To simplify the identification of the investigated areas on the MIC cross-sections, two main positions are conventionally defined: the ribs, as the part of the plate in contact with the cathode, and the channels, which allow for the air flow from the inlet to outlet.
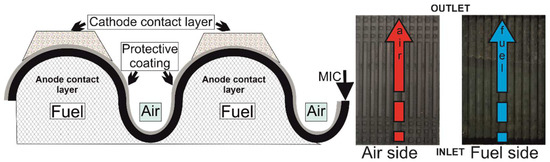
Figure 1.
Schematic representation of the MIC cross-section and overall views of the air side (left) and the fuel side (right) with the inlet–outlet positions indicating the co-flow direction of the streaming.
The MIC was made of Crofer 22 APU® ferritic stainless steel of commercial grade (Table 2) and fully coated with (Co, Mn, Fe)3O4 spinel oxide (MCO) on the side exposed to the cathode. The lateral sides were joined to the cell on the electrolyte by a proprietary glass–ceramic sealant.

Table 2.
Crofer22APU chemical composition (wt. %) according to the ThyssenKrupp datasheet [25].
The CROFER 22 APU© used in this paper is the first ferritic stainless steel formulated to be used as an interconnect in an SOFC stack and can be considered to be state of the art and a reference [26,27,28]. The amount of Cr and the presence of reactive elements, such as La and Mn, have key roles in promoting the formation of a stable and conductive spinel oxide layer on top of the chromia scale. The external layer lowers the risk of chromium evaporation, even though the usage of protective coatings is mandatory (such as the one based on Co and Mn used in the present case) to fight against the chromium poisoning [29,30]. Other stainless-steel compositions suitable for the usage as interconnects [31,32,33] are commercially available, but none can work without a coating. In this paper, the selection of the steel was made by the company according to their best-practice principles. A number of stacks manufactured according to internal standards were put under operation and stopped after a fixed number of hours before any failure. Two previous papers [21,22] were published to describe the status of the interconnect after operation up to 20,000 h, and the present paper was meant to complete the study by describing the results gathered after 40,000 h of working life as an SOFC.
The LSCM was present on top of the MCO and was placed at the ribs only. At the anode, Ni foam was used as a contact layer within the MIC, and the anode was present in the whole volume between the MIC and the electrode, where it has the additional role of evenly distributing the fuel on the anode. Therefore, at this side, there is no distinction between the ribs and channels (Figure 1).
The MCO protective coating covered the whole surface following the ribs/channels profile with a constant thickness; it was applied on the bare metal with an initial porosity and Fe content unknown due to the proprietary application method.
The interconnect was, therefore, divided into four samples (nearly 25 mm long each) distributed all along the flow stream and denominated as: inlet, center-inlet, center-outlet and outlet. Each sample was mounted in epoxy resin and polished with diamond suspensions from 6 μm to 250 nm in granulometry to allow for investigation of the cross-section. The cross-sections were characterized by a scanning electron microscope (SEM) (ZEISS EVO 40) coupled with an energy-dispersive X-ray spectroscope (EDS) (OXFORD PENTAFET) calibrated with a pure Co reference. The EDS data were treated by INCA software (Oxford) with ZAF 5 correction to achieve quantitative information on the metals and semiquantitative data on the oxygen.
3. Results and Discussions
The air side only is described, differentiating channels (only coated with MCO) and ribs connected to the cathode by the contacting layer. The fuel side had the same shape but was considered as a uniform surface due to the presence of the Ni layer contacting the whole surface.
According to the operating parameters listed in Table 1, the fuel at the inlet contained 34% hydrogen and 4% water vapor with a ratio 4/(34 + 4), equivalent to a 10.5% partial pressure of water in hydrogen. The remaining gases were considered inert towards the metal substrate. The working temperature was nominally 1123 K with a gradient between the inlet and outlet of approximately 50–60 K. The total operating time was 40,000 h in the SOFC mode (µCHP) with a switch from reformed methane (field mode) to a mix of hydrogen and nitrogen (lab mode) after 30,000 h of operations.
The macroscopic observation of the surfaces highlighted a visible alteration over all of the fuel side, while at the air side, the channels were affected by the presence of large black crystals only. The fuel side showed two main features separated by a visible border positioned a few centimeters after the center of the interconnect, where a sudden change in color and roughness was noticed (Figure 1). Ghiara et al. [21] suggest that the oxidation of the MIC at the anode side was due to the interaction of the bare metal substrate with the water vapor present in the stream. The H2/H2O ratio evolving from inlet to outlet together with the temperature were the main parameters in charge for such a dual zone observed on the MIC.
To better present the gathered results and for the sake of clarity, the two sides (i.e., the cathode or air side and the anode or fuel side) are hereafter treated separately.
3.1. The Air Side
The ribs were covered by a regular and homogeneous layer of LSCM (Figure 2) with a few small zones showing agglomerates with a very low amount of chromium (i.e., below 1 at. %). The channels, on the contrary, revealed the presence of black crystals on top of the protective layer of the MCO (Figure 3).
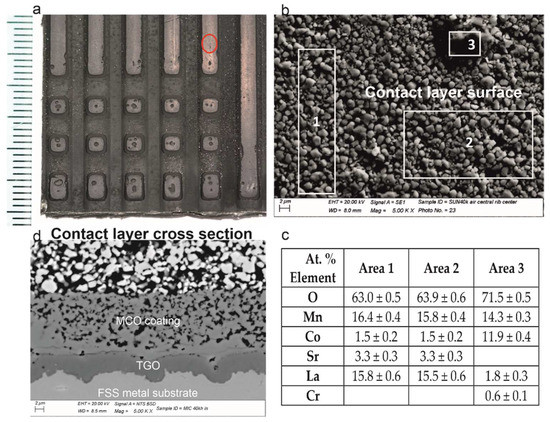
Figure 2.
From top-left clockwise: (a) the interconnect aspect at the inlet with defects on the rib’s surface; (b) SEM-BSE details of the contact layer surface on top of the rib; (c) EDXS analyses of the top of the rib; (d) cross-sectional features representative of the three layers (i.e., from top to bottom: contact layer, MCO and TGO).
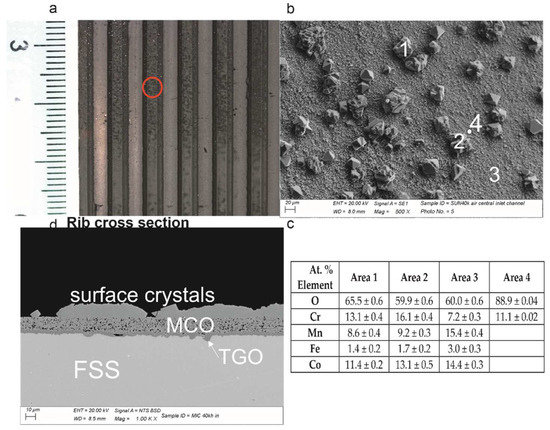
Figure 3.
From top-left clockwise: (a) the interconnect aspect at the inlet with dark crystals on top of the MCO at the channel; (b) SEM-BSE details of the channel with crystals on top; (c) EDXS analyses; (d) cross-sectional features representative of the three layers (i.e., from top to bottom: surface crystals, MCO and TGO).
The crystals formed on top of the MCO were spinel oxides containing Cr, Mn and Co. The analyses performed on the MCO indicate that the areas far from the crystals corresponded to the original elements (i.e., Mn, Co and Fe) plus the Cr diffused from the metal substrate, while the areas below the spinel oxides contained Cr oxide only (Figure 3). This suggests that the formation of the large crystal originated from a local depletion of Co and Mn in the protective coating. The cross-section of this zone evidenced the absence of further changes.
The SEM observations of the cross-sections revealed the formation of an interphase between the metal substrate and the protective coating composed of chromia (Cr2O3) with small amounts of manganese concentrated at the FSS interface at the ribs only, while in the channels, small traces of Mn were found distributed in the TGO (Figure 4). The concentration of Mn was expected on top of the TGO, as it is always observed on FSS exposed to air at high temperatures [20] without polarization. The differences in Mn between the ribs and channels can be explained by the following hypotheses: (1) the polarization of the stack generated an electric field in the oxidized layer affecting the solid-state diffusion of Mn across the TGO; (2) the presence of both layers, the MCO and the LSCM, on top of the ribs limited the amount of oxygen able to react with the FSS surface; (3) the further diffusion of Cr into the MCO generated a depletion in the coating that acted as a booster for the mobility of Cr.
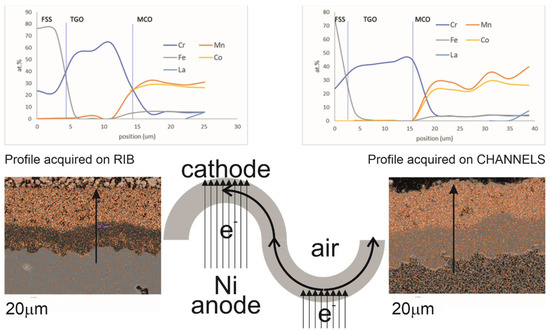
Figure 4.
The figure presents on the left the distribution of the main elements in both the TGO and MCO differentiating the rib (on the left of the drawing) and the channel (on the right of the drawing. The sketch helps to understand the path followed by the electrons while the cell stack operates (i.e., the electrons generated at the anode of one cells moves through the interconnect to the cathode of the neighbouring cell). The X-ray maps represent the distribution of Mn only.
The protective layer and the contacting LSCM on top of the ribs had good stability all over the surface of the interconnect. Neither their thickness nor their morphology changed significantly from inlet to outlet. The ribs’ cross-sections revealed that the LSCM layer was made of smooth and rounded grains with quite homogeneous compositions and the near complete absence of Cr infiltrations from the substrate (Figure 2).
The neighboring rib and channel positions showed TGO thicknesses that were very similar, while the value changed with the distance from the inlet. Considering the four zones previously identified from inlet to outlet, it is possible to indicate the average values of the TGO thickness within a range with a minimum and a maximum. The variation of such values (resulting from the average of more than ten zones of interest per area) as a function of the position from inlet to outlet, depends on the local parameters (e.g., temperature and oxidant concentration). Table 3 refers to the average of the values of each zone from inlet to outlet.

Table 3.
TGO thicknesses measured at the air side.
After comparing the interconnects operated between 5000 and 40,000 h, it is important to highlight that the major difference was found at the fuel side. The investigation performed on the cathode before cutting the cross-section highlighted the persistence of the MCO coating with limited amounts of change (e.g., the formation of spinel oxides on top). In addition, this second layer of LSCM placed on the ribs was unaffected by the operating time. The absence of Cr in the cathode contact layer confirmed the efficacy of the protective coating towards the solid-state diffusion of MIC alloying elements into the cell [21]. On the other hand, the cross-section shows the presence of a TGO formed within the MCO and the metal substrate with the chemical composition corresponding to Cr2O3 with small amounts of Mn. This layer was comparable among the samples extracted from twin stacks operated for differing numbers of hours [21]. Other studies performed on the same kind of steel protected with a coating confirm the formation of a TGO with a thickness that increases with time [34,35,36,37]. In this specific case, it was possible to detect the impact of parameters, such as the polarization and the temperature, on the Mn diffusion. The thickness of the TGO depended on time and temperature, but only above 1123 K, and the scale growth continued up to 40,000 h.
There was a visible difference regarding the thicknesses from the inlet to outlet, and the thermal gradient was identified as the key parameter affecting the growth rate, because the oxygen availability was the same all over the interconnect. As mentioned in the Section 1, this paper corresponds to the final step of a wider research activity focused on the characterization of interconnects extracted from SOFC stacks operated for various periods of time. The composition of both the alloy, the protective coating and the contact layers were the same for all stacks. They operated in comparable working conditions, and the time was the sole variable. The cells were studied by other partners of the AdAstra consortium, and the results are published elsewhere. It is therefore reasonable to integrate the results gathered from the 40,000 h operated interconnect with those previously published in [21,22]. They correspond to investigations carried out on interconnects extracted from stacks operated in the range 5000–20,000 h. In terms of composition and features, the TGO formed at the interface between the FSS and MCO perfectly corresponded to the one here described, and this makes it possible to trace the graphs shown in Figure 5, where the evolution of the TGOs’ thicknesses as a function of time and position is presented. All samples showed a range of thicknesses for the TGO, and to simplify the graphical description, only the minimum and maximum values are reported. It is worth noting that the measurements corresponded to a statistical number of acquisitions.
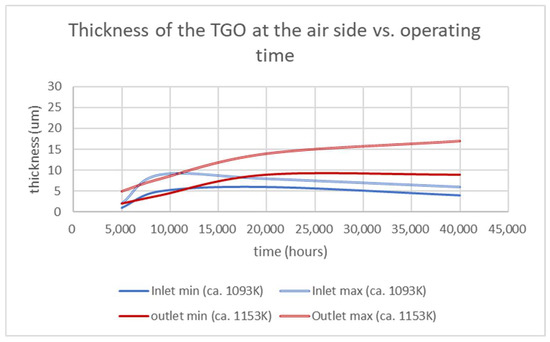
Figure 5.
Graphical representation of the lower and higher thicknesses of the TGOs measured on interconnects extracted from similar stacks operated for differing periods (i.e., 5000; 9000; 20,000; 40,000 h).
At the inlet, the TGO grew in the first 10,000 h with an increasing gap between the thinner and thicker layers. After, the TGO stopped increasing and started to decrease, with a more visible impact on the maximum thickness values. The TGO at the outlet side, on the contrary, continued to grow until 20,000 h for the smaller layer and up to 40,000 h for the larger one. This strongly suggests that, for further studies on accelerated and stressed tests on interconnects (at the air side and in the presence of a protective coating), it is important to use aging temperatures higher than 1123 K.
The shrinkage of the TGO thickness observed at the inlet in the time range 10,000–40,000 h indicates several, probably coexisting, mechanisms: the densification of the oxide layer caused by the high temperature over a longer period; the limited amount of oxygen ions passing through the coating; the diffusion of chromium into the MCO and, in the channels only, outside the MCO to form complex spinel oxides with Co, Mn, Cr and Fe. Additionally, in this range of temperatures, the shrinkage rate was higher than the TGOs’ growth. The outlet zone, where the temperature reached over 1150 K, showed a differing trend with a widening of the thickness values. This indicates diffusion rates of oxygen across the MCO and of Cr across the TGO that were higher than the abovementioned shrinkage rate.
3.2. The Fuel Side
The fuel side showed a surface visibly affected by the interaction with the fuel stream. The metal substrate in the first half of the sample (i.e., inlet to center) was covered by a homogeneous scale of dark gray color further decorated with greenish spots distributed all over the surface (Figure 6).
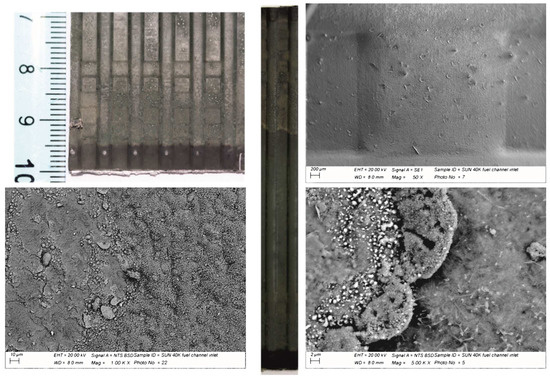
Figure 6.
A general and detailed view of an MIC surface at the fuel side. The green spots visible at the macroscopic level correspond to the footprint of the Ni contact layer between the metal substrate and the anode of the cell.
The SEM observations allowed for the interpretation of such spots as the zones of contact between the Ni foam and the interconnect (Figure 6). The spots appeared as a grey, nearly metallic color in the remaining half of the metal plate (i.e., from the center to the outlet). This highlights the intimate bond between the metal substrate and the Ni foam. The complete characterization of the surface highlighted a wide variety of oxidation features from very small crystals forming a uniform layer of thermally grown oxide to needle like oxides surrounded by amorphous phases of various shapes (Figure 7).
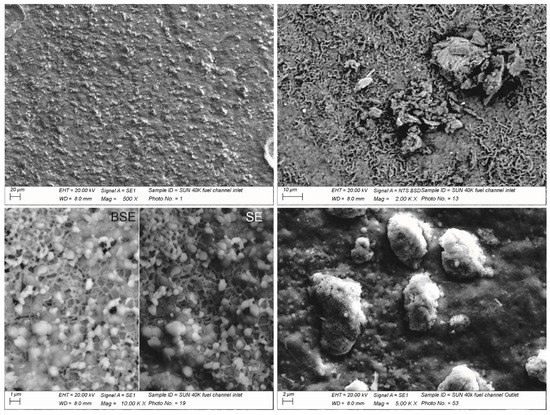
Figure 7.
SEM pictures with details of the various oxide features found on top of the fuel side.
In a cross-section (Figure 8), the TGO appeared well adhered to the metal substrate without gaps or cracks. It penetrated the pores and other pre-existing defects. A tiny and diffused porosity appeared in the TGO. Such small pores grew and tended to concentrate halfway between the metal substrate and the external edge in the second half of the MIC (Figure 9a).
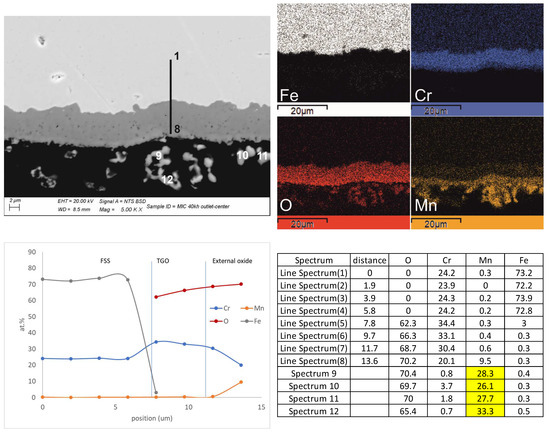
Figure 8.
Quantitative line profile (spectra from 1 to 8 along the black line) and elemental X-ray mapping with a related original micrograph and table showing the increase in Mn content and decrease in Cr content from the metal substrate to the external oxides. Analyses from 9 to 12 were acquired on the external layer only. The highlighted values show that the average content of Mn is close to 30 at. % therefore related to the MnO2 stoichiometry.
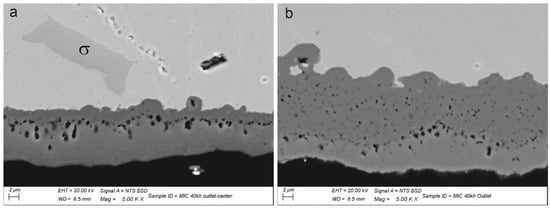
Figure 9.
(a) SEM micrograph representative of the porosity present in the TGO in the center of the stream flow; (b) SEM micrograph representative of the top layer rich in Mn and without porosity in the outlet zone of the interconnect.
Advancing towards the outlet and starting from the center, the scale showed evidence of a double layer (Figure 9b). The one in direct contact with the metal substrate was characterized by a diffused porosity, while the one on top was stiff and dense. This second layer was irregular in thickness (Figure 9b). It represented the edge of the MIC profile with, on top, the footprints of the Ni foam and some external crystals mainly in the form of clusters (Figure 8). According to the performed analyses, the main TGO layer corresponded to Cr3O4 doped with Mn and, more rarely, with Fe (Figure 8). The Mn content at the interface with the FSS was very low, and it grew by moving closer to the outer edge, where the highest values were expected. The top layer, often extremely thin, revealed a drastic change in composition with a stoichiometry close to Mn1-xCrxO2 (Figure 8).
The small crystals found on top of the layer (Figure 8) were mainly composed of Mn oxide (MnO2). They were quite frequent at the outlet but absent in the inlet zone, where a simple enrichment in Mn of the TGO was observed. They were found in addition to the presence of outgrowths observed on top of the TGO surface. The cross-section of such zones confirmed their nature as bubbles, most probably originating from the formation of Cr-rich volatile compounds.
Special attention was paid to the Ni foam footprint on top of the metal plate (Figure 10). The Ni results were intimately joined to the metal substrate with a deep diffusion into the interconnect alloy.
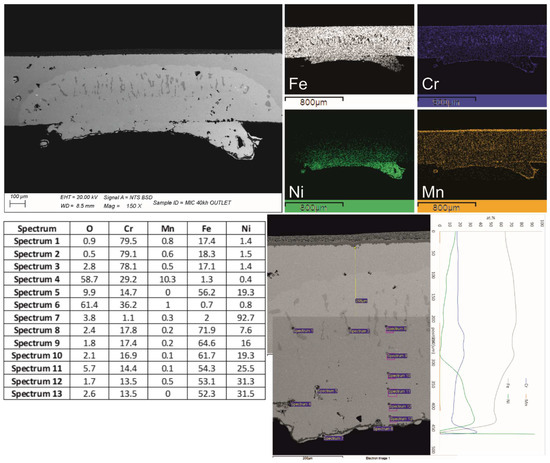
Figure 10.
Cross-section of the footprint of interaction between the Ni-contacting layer and the interconnect with elemental distribution X-ray mapping and a table of analyses.
To explain the Ni diffusion in the interconnect, two main hypotheses are suggested: (a) the observed zone corresponded to the molten pool of the spot-welding phenomenon that occurred at the interface of the Ni layer–interconnect; (b) solid-state diffusion occurred during the operating period of the stack. In both cases, the Ni diffusion front would have changed the steel composition. Both the shape of the Ni-rich zone outside the MIC profile and the presence of a Cr-rich second phase formed nearby the front of diffusion support the hypothesis of the formation of a molten pool because of a spot-welding event. The dark gray precipitate was recognized by the chemical composition as the sigma phase of the ternary equilibrium diagram of Cr-Fe-Ni [38,39]. Its presence, size, distribution and morphology in this second phase recall the epitaxial grains oriented in the direction of heat release during the solidification of a molten alloy. The operating time and temperatures erased the heterogeneity of the solid solution typical of an as-solidified microstructure but left the sigma phase unaffected. Moreover, the rare cases of a Cr-Fe sigma phase found in other zones of the same interconnect had a more polygonal shape (Figure 9a) and no traces of Ni inside or in the surrounding metal matrix. The surface surrounding the Ni layer footprint showed the presence of numerous spheroidal compounds rich in nickel, recalling the welding sprouts and drops. The tips present in the foam facing the FSS concentrate the electrons during the polarization and can generate sparks with partial melting of both the steel and the Ni. Once a bond is created, the electron’s flow generates an increase in temperature due to the resistance of the bottleneck that favors the diffusion process. This results in the formation of an austenitic ternary alloy (pale gray zone in Figure 10). The ternary sigma phase, the austenitic matrix and the surrounding ferritic stainless steel differ in the coefficient of thermal expansion, mechanical properties and chemical properties. These aspects represent potential sources of failure for operating times extending over 40,000 h.
The overall thickness increased with the distance from the inlet; as for the air side, a range of thicknesses with a minimum and a maximum was defined by the investigation of at least 10 zones of interest for each investigated sample (Table 4).

Table 4.
TGO thicknesses measured at the fuel side.
The TGO at the inlet was up to 8 μm thick and fully dense. Moving across the interconnect from this position to the outlet, there was an increase in thickness and the appearance of the already mentioned porosities concentrated in the middle of the TGO. The two zones separated by the porosity had a similar structure with differing Mn contents. The porosity could be explained by the evaporation of CrVI compounds formed by the reaction of CrIII oxides with the water vapor of the stream, but the position of the pores in a zone still rich in Cr contradicts this explanation. It is more likely that the pores formed due to the Kirkendall effect, explained by different atomic diffusive rates of elements at high temperature [27]. Mn diffuses from the metal substrate to the external edge, and Cr itself diffuses across the TGO, and the operating time and temperature lead to the coalescence of vacancies into the pores. On the other hand, the increased TGO thickness from inlet to outlet was due to the synergistic effect of the thermal gradient and of the PH2/PH2O changes. At the inlet, the wet fuel contained, in total, 34% hydrogen and 4% water; considering that these two gases react with the steel, it is important to estimate their absolute values, which were 89.5% hydrogen and 10.5% water. By the continuous transformation of hydrogen into water, it is possible to reach 80% water at the outlet. Therefore, the oxidizing potential of the stream while moving from inlet to outlet increases with both the water vapor concentration and the temperature rise. The interaction between the MIC and the atmosphere at the anode side was more detrimental, causing the formation of oxidized scale with features changing according to the distance from the inlet manifold. The effect of time enhanced the TGO’s formation and evolution. In the first 5000 h, evidence of a scale of chromia rich in Mn on the top was discussed by Ghiara et al. [22], and the observation of samples exposed for longer periods confirmed the process and support the hypothesis of the formation of Mn oxides on top of the scale. The features shown in this paper testify to the reactivity of the TGO with the water in the fuel stream, causing further oxidation of the chromia into Cr-rich volatile compounds, while the Mn remained on top as manganese oxide. As discussed by Ghiara et al. [22], the overall resistance of the system’s “anode contact layer–MIC” was not affected by the scale as long as the Mn oxides formed elsewhere from the zone of the interface among the nickel foam and the interconnect.
As for the air side, the comparison with previous measurements, acquired from field-operated stacks in the range 5000–20,000 h, offered a very interesting overview of the evolution of the TGO as a function of temperature and time (Figure 11). The growth rate of the TGO at the inlet of the fuel side recalls the one observed at the air side with a maximum at approximately 10,000 h and a constant decrease until the minimum was reached at 40,000 h. A similar explanation as the one previously discussed for the air side could be proposed with some adaptation: the inlet T was, of course, the same but, here, the fuel stream carried a low amount of water. The TGO therefore shrinks for a number of reasons such as sintering, element diffusion (as suggested by the Kirkendall voiding) and chromium evaporation. At the outlet, the TGO formed at higher T (as for the air side) and a higher oxidation potential of the fuel stream (increased PH2O and decreased PH2) resulting in a scale growth mitigated by the increased Cr volatilization (as evidenced by both the outgrowth bubbles and the formation of MnO2 clusters).
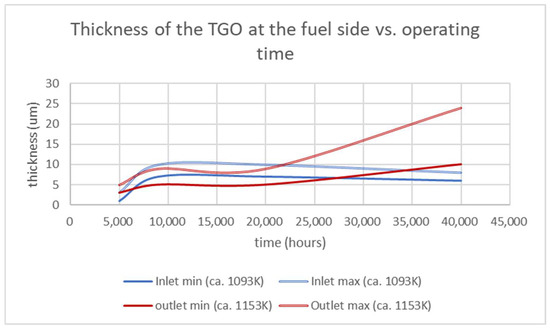
Figure 11.
Graphical representation of the lower and higher thicknesses at the fuel side of the TGOs measured on interconnects extracted from similar stacks operated for differing periods (i.e., 5000; 9000; 20,000; 40,000 h).
The parameters affecting the formation of an oxidized layers were the oxidizing potential of the environment (e.g., neighboring materials and atmosphere), the temperature, the polarization (i.e., current flow) and time. At the air side, the temperature and the polarization played the most important role: T acted on the growth as an accelerator, while the current flow, on top of the ribs only, affected the diffusion of Mn, which concentrated at the interface with the metal substrate. At the fuel side, the polarization favored the increase in Mn content on the external side of the TGO, and the PH2/PH2O of the fuel stream and the temperature enhanced the Cr’s evaporation and accelerated the scale’s growth. The time highlights the differences between the lower temperature of the inlet and the higher temperature at the outlet. After 10,000 h at lower temperature, the scale shrank instead of growing on both sides. This means that competing events occurred at the same time: the diffusion of cations and oxygen ions across the TGO to form new layers of oxide and the densification of the scale (plus the Cr evaporation at the fuel side only). At higher temperature, the scale continued to grow on both sides also after 40,000 h of operation. This suggests that accelerated aging tests on Crofer 22APU© would be simulated at the longest-lasting period using a temperature higher than 1123 K. Another important achievement was the highlighted effect of the water’ partial pressure on the oxidation of the stainless steel at the fuel side and, thus, in the absence of free oxygen. This also acts as an accelerating factor in synergy with temperature. Accelerated stress tests and models on the oxidation of interconnects should be designed taking both T and PH2O into account to obtain a more reliable prediction of events. The effect of polarization was mainly related to the diffusion process and should be considered in lab tests to obtain samples closer to reality.
4. Conclusions
The interconnect survived the 40,000 h of field operation in a stack with the presence of a limited number of changes at the air side and some more issues at the fuel side. The observations enriched and confirmed the information previously gathered from similar samples extracted from stacks operated for shorter periods (i.e., 5000; 9000; 20,000 h).
The air side ribs showed the formation of a TGO at the interface between the protective layer and the metal substrate. The contacting layer was untouched by the presence of Cr or any other issues worthy of notice.
The air side channels presented, on top, large crystals of spinel oxide-containing Cr, Mn, Co and Fe. They might represent a threat to the cathode due to the potential of chromium poisoning. However, this seems unlikely due to the stability of such a complex spinel oxide.
The fuel side was more affected by the oxidation due to the presence of water vapor in the fuel stream. The combination of T and water vapor was deleterious for the steel, and the TGO formed at the outlet was thicker than the one observed at the air side. There were several indications of the formation of volatile Cr-rich compounds carried away by the fuel stream. The Ni-contact layer left numerous traces on the metal surface: fully or partially oxidized footprints and spot-welded zones.
The T confirmed to be a strong accelerating parameter of the oxidation process. This effect was more visible above 1123 K, where after 40,000 h, the TGO’s thickness was still increasing, while below 1123 K, a shrinkage process started after 9000 h.
The water vapor was another important accelerating factor, even in the presence of hydrogen. The main effect was on the TGO’s composition and the formation Mn-rich oxides on top of the oxide scale. Once combined with T, the TGO’s growth rate increased. The synergy between T and PH2O was mainly visible at operating temperatures higher than 1123 K.
These results support the usage of T and water vapor in AST tests to simulate long-lasting operating periods and open a route for further studies on protective coatings to be applied at the fuel side.
Author Contributions
Conceptualization, methodology, data curation and analyses and writing—original draft preparation, P.P.; investigation, writing—review and editing and formal analysis, R.S.; stack manufacturing, stack operations and samples extraction, C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is part of the ADASTRA EU PROJECT, which has received funding from the Fuel Cells and Hydrogen 2 Joint Undertaking under Grant Agreement No. 825027. This Joint Undertaking received support from the European Union’s Horizon 2020 Research and Innovation Program and Hydrogen Europe.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hansen, J.B. Solid oxide electrolysis—A key enabling technology for sustainable energy scenarios. Faraday Discuss. 2015, 182, 9–48. [Google Scholar] [CrossRef] [PubMed]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2011, 37, 1954–1971. [Google Scholar] [CrossRef]
- Tao, Z.; Fu, M.; Liu, Y.; Gao, Y.; Tong, H.; Hu, W.; Lei, L.; Bi, L. High-performing proton-conducting solid oxide fuel cells with triple-conducting cathode of Pr0.5Ba0.5(Co0.7Fe0.3)O3-δ tailored with W. Int. J. Hydrogen Energy 2021, 47, 1947–1953. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipinski, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Yokokawa, H. Report of Five-Year NEDO Project on Durability/Reliability of SOFC Stacks. ECS Trans. 2013, 57, 299–308. [Google Scholar] [CrossRef]
- Al-Khori, K.; Al-Ghamdi, S.; Boulfrad, S.; Koç, M. Life Cycle Assessment for Integration of Solid Oxide Fuel Cells into Gas Processing Operations. Energies 2021, 14, 4668. [Google Scholar] [CrossRef]
- Yokokawa, H.; Horita, T.; Yamaji, K.; Kishimoto, H.; Brito, M.E. Degradation of SOFC Cell/Stack Per-formance in Relation to Materials Deterioration. J. Korean Ceram. Soc. 2012, 49, 11. [Google Scholar] [CrossRef]
- Fang, Q.; de Haart, U.; Schäfer, D.; Thaler, F.; Rangel-Hernandez, V.; Peters, R.; Blum, L. Degradation Analysis of an SOFC Short Stack Subject to 10,000 h of Operation. J. Electrochem. Soc. 2020, 167, 144508. [Google Scholar] [CrossRef]
- Alenazey, F.; Alyousef, Y.; AlOtaibi, B.; Almutairi, G.; Minakshi, M.; Cheng, C.K.; Vo, D.-V.N. Degradation Behaviors of Solid Oxide Fuel Cell Stacks in Steady-State and Cycling Conditions. Energy Fuels 2020, 34, 14864–14873. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrog. Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, Q.; Blum, L.; Lehnert, W. Performance and degradation of an SOEC stack with different cell components. Electrochim. Acta 2017, 258, 1254–1261. [Google Scholar] [CrossRef]
- Chatzichristodoulou, C.; Chen, M.; Hendriksen, P.V.; Jacobsen, T.; Mogensen, M.B. Understanding degradation of solid oxide electrolysis cells through modeling of electrochemical potential profiles. Electrochim. Acta 2015, 189, 265–282. [Google Scholar] [CrossRef] [Green Version]
- The, D.; Grieshammer, S.; Schroeder, M.; Martin, M.; Al Daroukh, M.; Tietz, F.; Schefold, J.; Brisse, A. Microstructural comparison of solid oxide electrolyser cells operated for 6100 h and 9000 h. J. Power Sources 2014, 275, 901–911. [Google Scholar] [CrossRef]
- Riedel, M.; Heddrich, M.; Friedrich, K.A. Analysis of pressurized operation of 10 layer solid oxide electrolysis stacks. Int. J. Hydrog. Energy 2019, 44, 4570–4581. [Google Scholar] [CrossRef]
- Anghilante, R.; Colomar, D.; Brisse, A.; Marrony, M. Bottom-up cost evaluation of SOEC systems in the range of 10–100 MW. Int. J. Hydrog. Energy 2018, 43, 20309–20322. [Google Scholar] [CrossRef]
- Yoo, J.; Woo, S.; Yu, J.; Lee, S.; Park, G. La0.8Sr0.2MnO3 and (Mn1.5Co1.5)O4 double layer coated by electrophoretic deposition on Crofer22 APU for SOEC interconnect applications. Int. J. Hydrog. Energy 2009, 34, 1542–1547. [Google Scholar] [CrossRef]
- Zhang, X.; O’Brien, J.E.; O’Brien, R.; Hartvigsen, J.J.; Tao, G.; Housley, G.K. Improved durability of SOEC stacks for high temperature electrolysis. Int. J. Hydrog. Energy 2013, 38, 20–28. [Google Scholar] [CrossRef]
- Yokokawa, H.; Horita, T.; Sakai, N.; Yamaji, K.; Brito, M.E.; Xiong, Y.-P.; Kishimoto, H. Thermodynamic considerations on Cr poisoning in SOFC cathodes. Solid State Ion. 2006, 177, 3193–3198. [Google Scholar] [CrossRef]
- Ardigò, M.R.; Popa, I.; Chevalier, S.; Girardon, P.; Perry, F.; Laucournet, R.; Brevet, A.; Desgranges, C. Effect of coatings on long term behaviour of a commercial stainless steel for solid oxide electrolyser cell interconnect application in H2/H2O atmosphere. Int. J. Hydrog. Energy 2014, 39, 21673–21677. [Google Scholar] [CrossRef]
- Bianco, M.; Ouweltjes, J.P.; Van Herle, J. Degradation analysis of commercial interconnect materials for solid oxide fuel cells in stacks operated up to 18,000 h. Int. J. Hydrog. Energy 2019, 44, 31406–31422. [Google Scholar] [CrossRef]
- Ghiara, G.; Piccardo, P.; Bongiorno, V.; Repetto, L.; Geipel, C.; Spotorno, R. Characterization of metallic interconnects extracted from Solid Oxide Fuel Cell stacks operated up to 20,000 h in real life conditions: The fuel side. Int. J. Hydrog. Energy 2021, 46, 23815–23827. [Google Scholar] [CrossRef]
- Ghiara, G.; Piccardo, P.; Bongiorno, V.; Geipel, C.; Spotorno, R. Characterization of Metallic Interconnects Extracted from Solid Oxide Fuel Cell Stacks Operated up to 20,000 h in Real Life Conditions: The Air Side. Energies 2020, 13, 6487. [Google Scholar] [CrossRef]
- Bianco, M.; Caliandro, P.; Diethelm, S.; Yang, S.; Dellai, A.; Van Herle, J.; Steinberger-Wilckens, R. In-situ experimental benchmarking of solid oxide fuel cell metal interconnect solutions. J. Power Sources 2020, 461, 228163. [Google Scholar] [CrossRef]
- Paravidino, D.; Piccardo, P.; Spotorno, R. A Novel Method for Evaluation of Chromium Evaporation from Solid Oxide Fuel Cells Interconnects: A Feasibility Study. Mater. Sci. Forum 2021, 1016, 1109. [Google Scholar] [CrossRef]
- ThyssenKrupp. VDM, Crofer 22 APU—Material, Data Sheet No 4046. 2010. Available online: https://kipdf.com/vdm-crofer-22-apu-material-data-sheet-no-may-2010-edition_5ab1d0be1723dd339c808799.html (accessed on 5 May 2022).
- Quadakkers, W.J.; Shemet, V.; Singheiser, L. US Patent No.2,003,059,335, 11 September 2003.
- Timurkutluk, B.; Toros, S.; Onbilgin, S.; Korkmaz, H.G. Determination of formability characteristics of Crofer 22 APU sheets as interconnector for solid oxide fuel cells. Int. J. Hydrog. Energy 2018, 43, 14638–14647. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X. Recent Development of SOFC Metallic Interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Jin, Y.; Sheng, J.; Hao, G.; Guo, M.; Hao, W.; Yang, Z.; Xiong, X.; Peng, S. Highly dense (Mn,Co)3O4 spinel protective coating derived from MneCo metal precursors for SOFC interconnect applications. Int. J. Hydrog. Energy 2022, 47, 13960–13968. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrog. Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Spotorno, R.; Paravidino, D.; Delsante, S.; Piccardo, P. Volatilization of chromium from AISI 441 stainless steel: Time and temperature dependence. Surf. Coat. Technol. 2022, 433, 128125. [Google Scholar] [CrossRef]
- Bongiorno, V.; Spotorno, R.; Paravidino, D.; Piccardo, P. On the High-Temperature Oxidation and Area Specific Resistance of New Commercial Ferritic Stainless Steels. Metals 2021, 11, 405. [Google Scholar] [CrossRef]
- Talic, B.; Venkatachalam, V.; Hendriksen, P.V.; Kiebach, R. Comparison of MnCo2O4 coated Crofer 22 H, 441, 430 as interconnects for intermediate-temperature solid oxide fuel cell stacks. J. Alloys Compd. 2020, 821, 153229. [Google Scholar] [CrossRef]
- Przybylski, K.; Brylewski, T.; Durda, E.; Gawel, R.; Kruk, A. Oxidation properties of the Crofer 22 APU steel coated with La0.6Sr0.4Co0.2Fe0.8O3 for IT-SOFC interconnect applications. J. Therm. Anal. 2014, 116, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Si, X.; Wang, D.; Li, C.; Qi, J.; Cao, J. Exploring the role of Mn–Co spinel coating on Crofer 22 APU in adjusting reactions with the Ag based sealant during reactive air brazing. J. Mater. Res. Technol. 2021, 16, 608–618. [Google Scholar] [CrossRef]
- Magdefrau, N.J.; Chen, L.; Sun, E.Y.; Yamanis, J.; Aindow, M. Formation of spinel reaction layers in manganese cobaltite—coated Crofer22 APU for solid oxide fuel cell interconnects. J. Power Sources 2013, 227, 318–326. [Google Scholar] [CrossRef]
- Amendola, R.; Gannon, P.; Ellingwood, B.; Hoyt, K.; Piccardo, P.; Genocchio, P. Oxidation behavior of coated and preoxidized ferritic steel in single and dual atmosphere exposures at 800 °C. Surf. Coat. Technol. 2012, 206, 2173–2180. [Google Scholar] [CrossRef]
- Ansara, I.; Chart, T.; Chevalier, P.Y. Phase Diagrams for FE-CR-NI Based Alloys. In Directorate-General for Research and Innovation (European Commission); Technical Report, EUR 9657; European Commission: Brussels, Belgium, 1996. [Google Scholar]
- Ohring, M. Interdiffusion, Reactions, and Transformations in Thin Films. In Materials Science of Thin Films, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2002; p. 641. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).