Fabrication and Performance of Micro-Tubular Solid Oxide Cells
Abstract
:1. Introduction
2. Materials and Methods
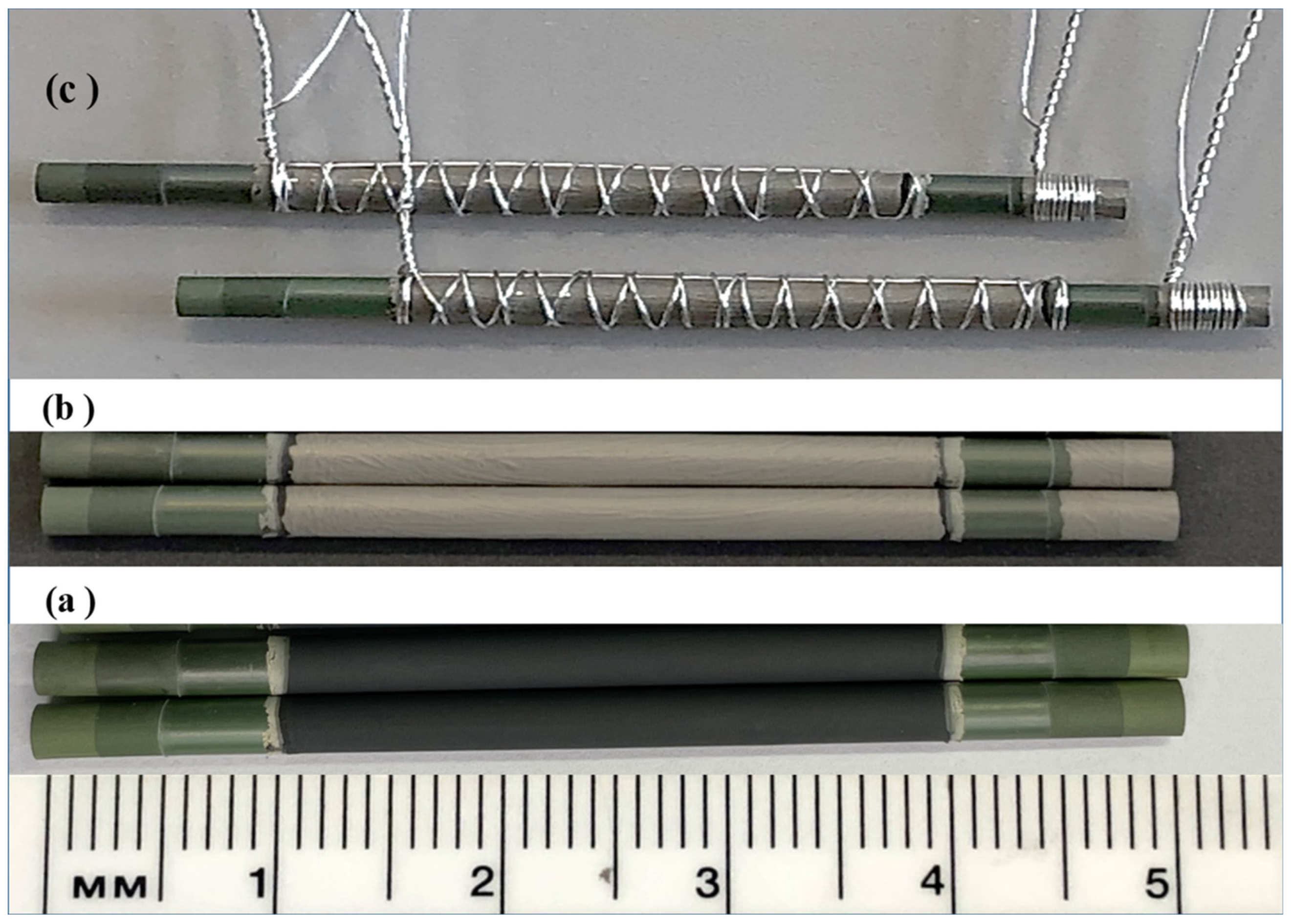
2.1. Cell Fabrication
2.2. Microstructural Characterizations
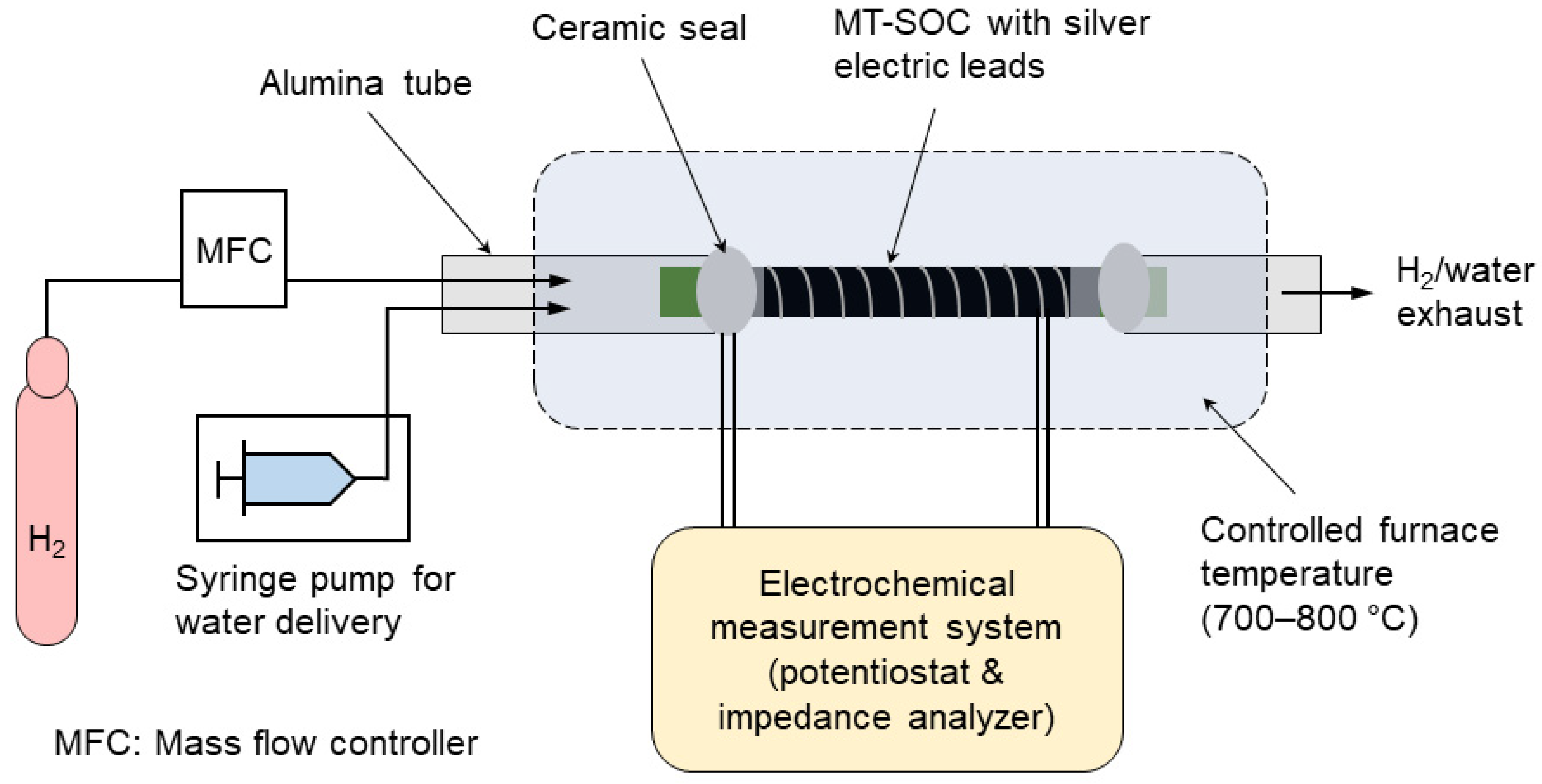
2.3. Electrochemical Testing and Characterizations
3. Results and Discussion
3.1. MT-SOC Fabrication
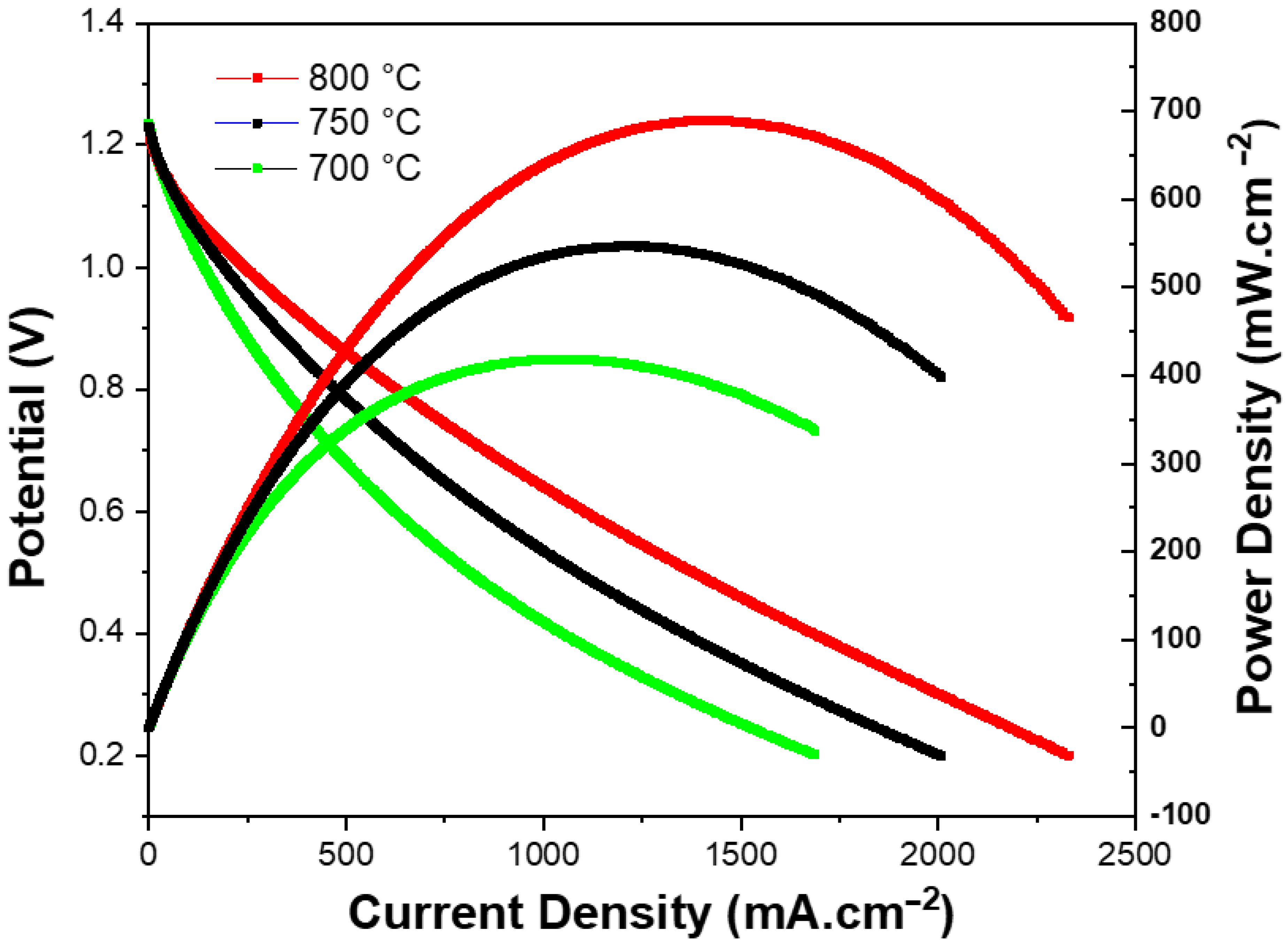
3.2. Electrochemical Performance
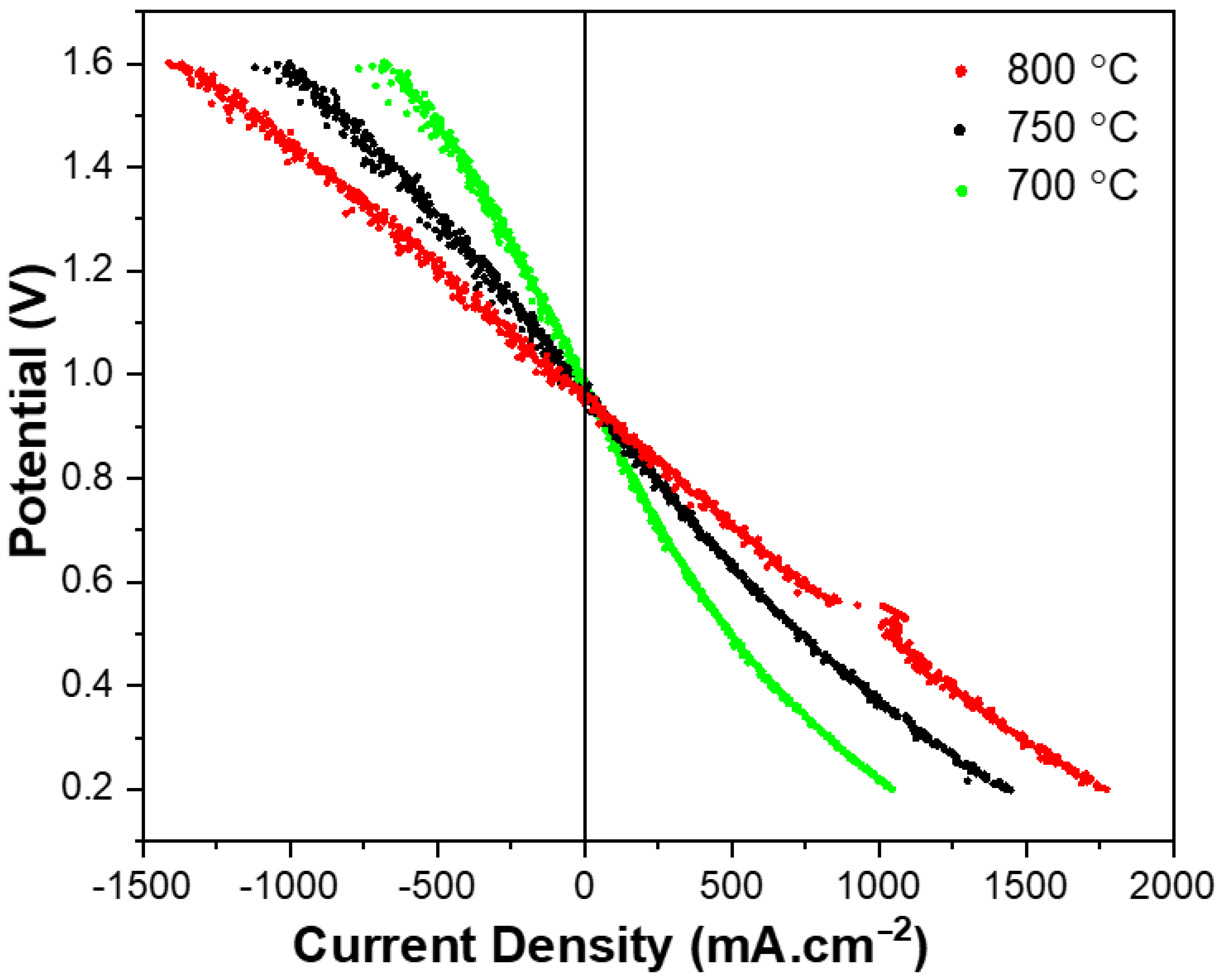
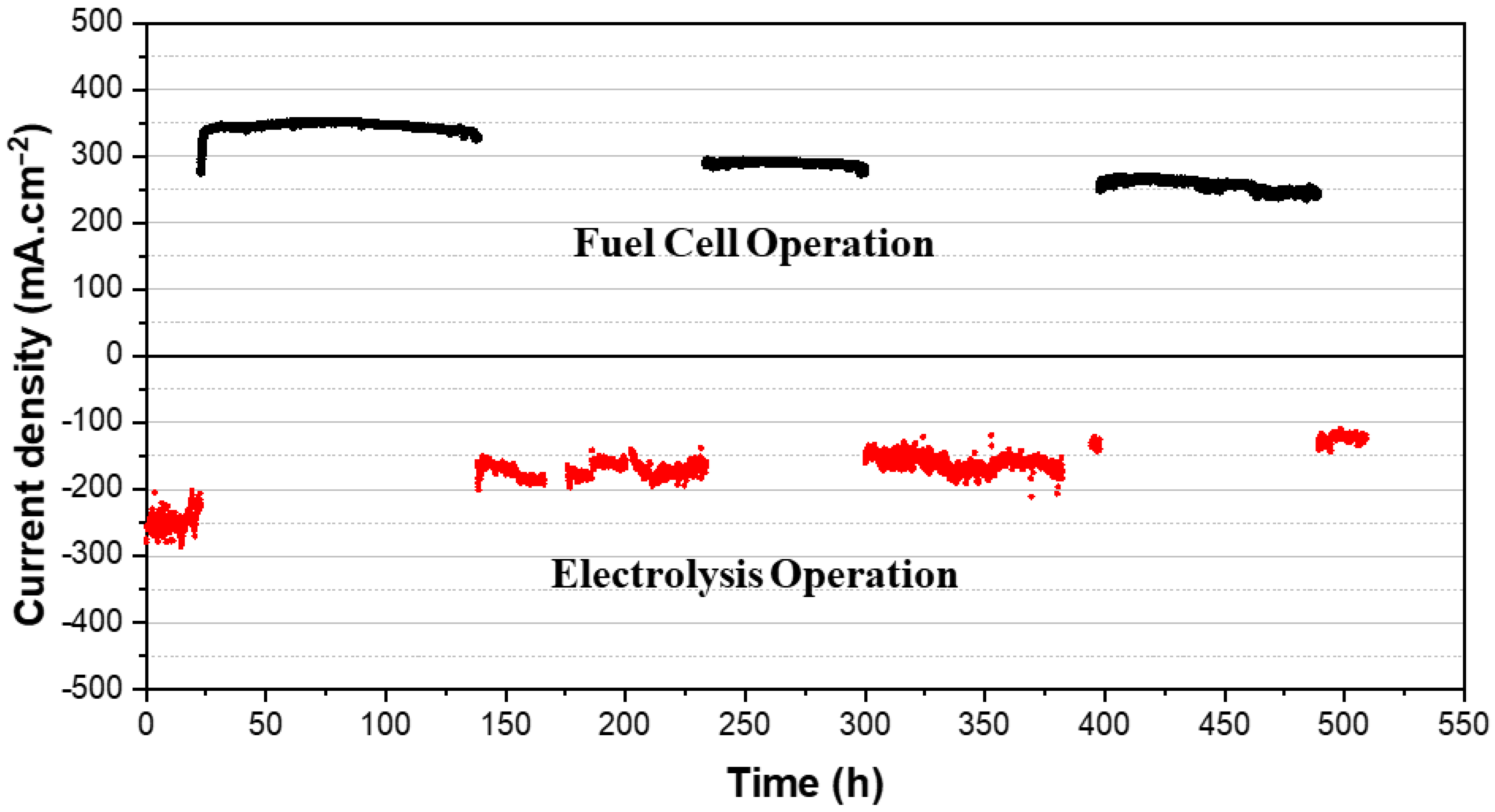
3.3. Reversible Operation
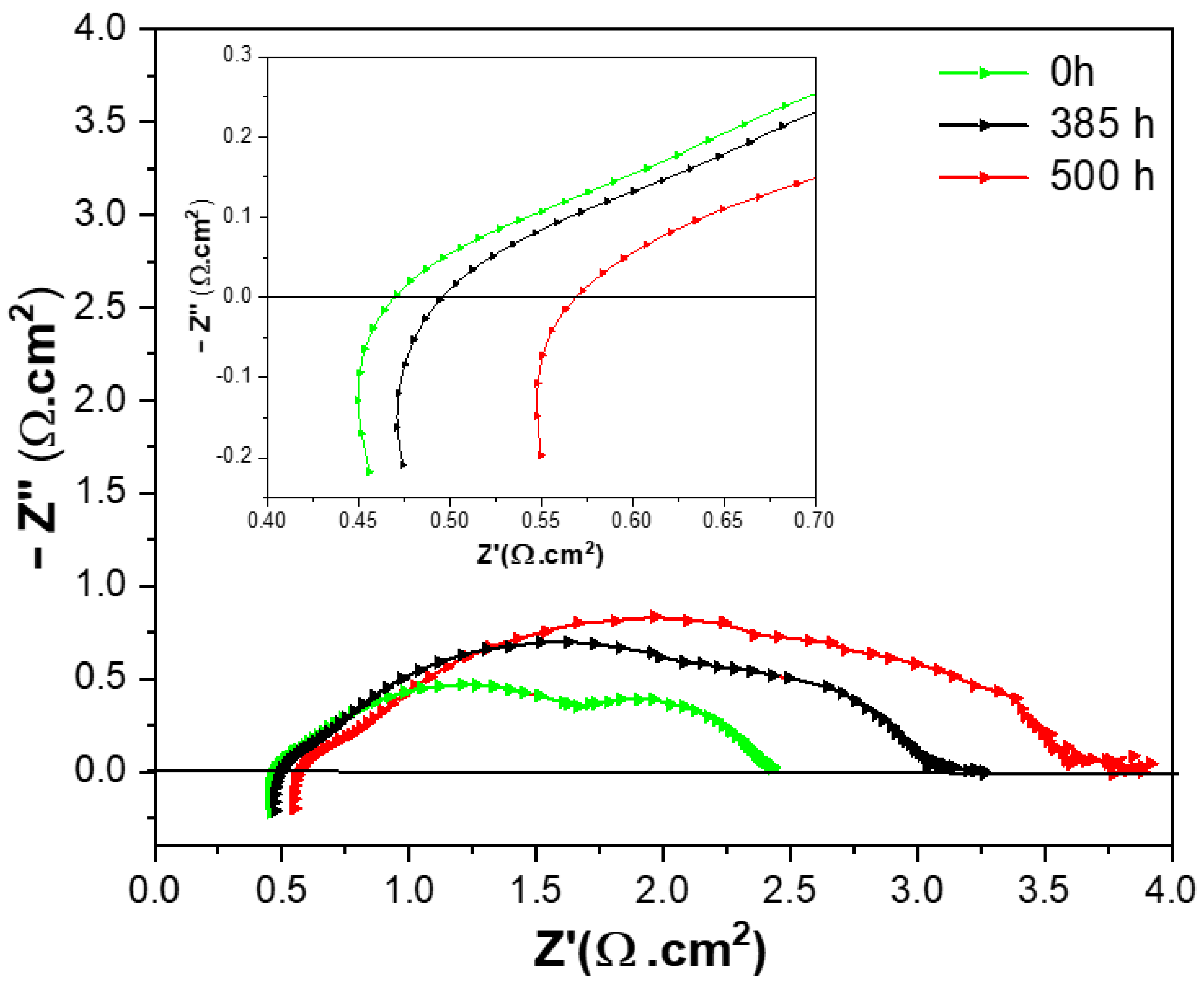
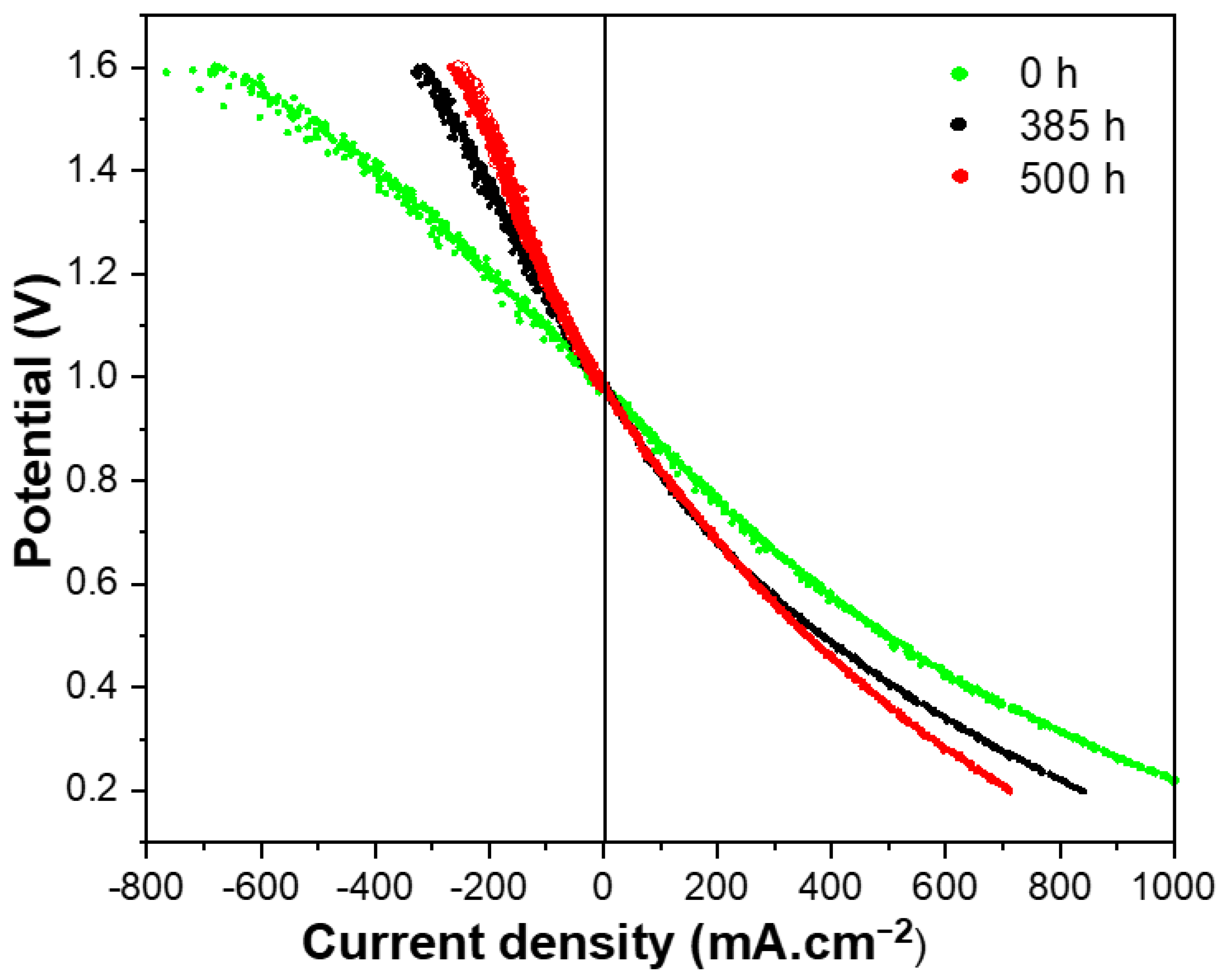
3.4. Durability and Stability Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mogensen, M.; Jensen, S.H.; Hauch, A.; Chorkendorff, I.; Jacobsen, T. Performance of reversible solid oxide cells—A review. In Proceeding European Solid Oxide Fuel Cell (SOFC) Forum; Citeseer: State College, PA, USA, 2006; p. 301. [Google Scholar]
- Mogensen, M.B.; Chen, M.; Frandsen, H.L.; Graves, C.; Hansen, J.B.; Hansen, K.V.; Hauch, A.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; et al. Reversible solid-oxide cells for clean and sustainable energy. Clean Energy 2019, 3, 175–201. [Google Scholar] [CrossRef]
- Myung, J.H.; Neagu, D.; Miller, D.N.; Irvine, J.T. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Torre García, R. Production of Micro-Tubular Solid Oxide Fuel Cells. Ph.D. Thesis, University of Trento, Trento, Italy, 2011. [Google Scholar]
- Suzuki, T.; Funahashi, Y.; Yamaguchi, T.; Fujishiro, Y.; Awano, M. Design and fabrication of lightweight, submillimeter tubular solid oxide fuel cells. Electrochem. Solid-State Lett. 2007, 10, A177. [Google Scholar] [CrossRef]
- Ghezel-Ayagh, H. Solid oxide cell technology for supply, recovery, and storage energy. ECS Trans. 2021, 103, 17. [Google Scholar] [CrossRef]
- Orera, V.M.; Laguna-Bercero, M.A.; Larrea, A. Fabrication methods and performance in fuel cell and steam electrolysis operation modes of small tubular solid oxide fuel cells: A review. Front. Energy Res. 2014, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Preininger, M.; Stoeckl, B.; Subotić, V.; Hochenauer, C. Characterization and performance study of commercially available solid oxide cell stacks for an autonomous system. Energy Convers. Manag. 2020, 203, 112215. [Google Scholar] [CrossRef]
- Pandiyan, A.; Uthayakumar, A.; Subrayan, R.; Cha, S.W.; Krishna Moorthy, S.B. Review of solid oxide electrolysis cells: A clean energy strategy for hydrogen generation. Nanomater. Energy 2019, 8, 2–22. [Google Scholar] [CrossRef]
- Jamil, S.M.; Othman, M.H.D.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Li, K. Recent fabrication techniques for micro-tubular solid oxide fuel cell support: A review. J. Eur. Ceram. Soc. 2015, 35, 1–22. [Google Scholar] [CrossRef]
- Panthi, D.; Tsutsumi, A. A novel multistep dip-coating method for the fabrication of anode-supported microtubular solid oxide fuel cells. J. Solid State Electrochem. 2014, 18, 1899–1905. [Google Scholar] [CrossRef]
- Panthi, D.; Hedayat, N.; Woodson, T.; Emley, B.J.; Du, Y. Tubular solid oxide fuel cells fabricated by a novel freeze casting method. J. Am. Ceram. Soc. 2020, 103, 878–888. [Google Scholar] [CrossRef]
- Sammes, N.; Du, Y. Fabrication and Characterization of Tubular Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2007, 4, 89–102. [Google Scholar] [CrossRef]
- Zakaria, Z.; Awang Mat, Z.; Abu Hassan, S.H.; Boon Kar, Y. A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 2020, 44, 594–611. [Google Scholar] [CrossRef]
- Tietz, F.; Buchkremer, H.P.; Stöver, D. Components manufacturing for solid oxide fuel cells. Solid State Ion. 2002, 152, 373–381. [Google Scholar] [CrossRef]
- Monzon, H.; Laguna-Bercero, M. Highly stable microtubular cells for portable solid oxide fuel cell applications. Electrochim. Acta 2016, 222, 1622–1627. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.Z.; Wang, S.R.; Huang, B.; Zhao, C.H.; Li, J.L.; Wang, Z.R.; Wen, Z.Y.; Wen, T.L. Dip-coating and co-sintering technologies for fabricating tubular solid oxide fuel cells. J. Solid State Electrochem. 2009, 13, 1905–1911. [Google Scholar] [CrossRef]
- Panthi, D.; Choi, B.; Du, Y.; Tsutsumi, A. Lowering the co-sintering temperature of cathode electrolyte bilayers for micro-tubular solid oxide fuel cells. Ceram. Int. 2017, 43, 10698–10707. [Google Scholar] [CrossRef]
- Panthi, D.; Choi, B.; Tsutsumi, A. Fabrication and evaluation of a micro-tubular solid oxide fuel cell with an inert support using scandia-stabilized zirconia electrolyte. J. Electrochem. Soc. 2015, 162, F1555–F1560. [Google Scholar] [CrossRef] [Green Version]
- Hedayat, N.; Panthi, D.; Du, Y. Fabrication of anode-supported microtubular solid oxide fuel cells by sequential dip-coating and reduced sintering steps. Electrochim. Acta 2017, 258, 694–702. [Google Scholar] [CrossRef]
- Hagen, A.; Menon, M.; Rasmousse, S.; Larsen, P.H.; Barfod, R.; Henriksen, P.V. Properties and performance of SOFCs produced on a pre-pilot plant scale. In Proceedings of the Sixth European SOFC Forum, Luzerne, Switzerland, 28 June–2 July 2004; Volume 2, pp. 930–939. [Google Scholar]
- Hedayat, N.; Panthi, D.; Du, Y. Fabrication of tubular solid oxide fuel cells by solvent-assisted lamination and co-firing a rolled multilayer tape cast. Int. J. Appl. Ceram. Technol. 2018, 15, 307–314. [Google Scholar] [CrossRef]
- Khajavi, P. Improving the Mechanical Properties and Stability of Solid Oxide Fuel and Electrolysis Cells. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2018. [Google Scholar]
- Ni, D.W.; Charlas, B.; Kwok, K.; Molla, T.T.; Hendriksen, P.V.; Frandsen, H.L. Influence of temperature and atmosphere on the strength and elastic modulus of solid oxide fuel cell anode supports. J. Power Sources 2016, 311, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Frandsen, H.L.; Makowska, M.; Greco, F.; Chatzichristodoulou, C.; Ni, D.W.; Curran, D.J.; Strobl, M.; Kuhn, L.T.; Hendriksen, P.V. Accelerated creep in solid oxide fuel cell anode supports during reduction. J. Power Sources 2016, 323, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Laguna-Bercero, M.A.; Campana, R.; Larrea, A.; Kilner, J.A.; Orera, V.M. Performance and aging of microtubular YSZ-based solid oxide regenerative fuel cells. Fuel Cells 2011, 11, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Campana, R.; Merino, R.I.; Larrea, A.; Villarreal, I.; Orera, V.M. Fabrication, electrochemical characterization and thermal cycling of anode supported microtubular solid oxide fuel cells. J. Power Sources 2009, 192, 120–125. [Google Scholar] [CrossRef]
- Monzón, H.; Laguna-Bercero, M.A.; Larrea, A.; Arias, B.I.; Várez, A.; Levenfeld, B. Design of industrially scalable microtubular solid oxide fuel cells based on an extruded support. Int. J. Hydrogen Energy 2014, 39, 5470–5476. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Hasan, Z.; Funahashi, Y.; Yamaguchi, T.; Fujishiro, Y.; Awano, M. Impact of anode microstructure on solid oxide fuel cells. Science 2009, 325, 852–855. [Google Scholar] [CrossRef]
- Suzuki, T.; Hasan, Z.; Yamaguchi, T.; Fujishiro, Y.; Awano, M.; Sammes, N. Fabrication of micro-tubular solid oxide fuel cells with a single-grain thick yttria stabilized zirconia electrolyte. J. Power Sources 2010, 195, 7825–7828. [Google Scholar] [CrossRef]
- Suzuki, T.; Sugihara, S.; Hamamoto, K.; Yamaguchi, T.; Fujishiro, Y. Energy efficiency of a microtubular solid-oxide fuel cell. J. Power Sources 2011, 196, 5485–5489. [Google Scholar] [CrossRef]
- Sarkar, P.; Yamarte, L.; Rho, H.; Johanson, L. Anode-supported tubular micro-solid oxide fuel cell. Int. J. Appl. Ceram. Technol. 2007, 4, 103–108. [Google Scholar] [CrossRef]
- Dhir, A.; Kendall, K. Microtubular SOFC anode optimisation for direct use on methane. J. Power Sources 2008, 181, 297–303. [Google Scholar] [CrossRef]
- Kato, T.; Sakaki, N.; Negishi, A.; Honda, T.; Nguyen, L.; Tanaka, Y. Development of tubular solid oxide electrolysis cells for hydrogen production. ECS Trans. 2009, 25, 1015. [Google Scholar] [CrossRef]
- Wang, Z.; Mori, M.; Araki, T. Steam electrolysis performance of intermediate-temperature solid oxide electrolysis cell and efficiency of hydrogen production system at 300 Nm3 h-1. Int. J. Hydrogen Energy 2010, 35, 4451–4458. [Google Scholar] [CrossRef]
- Jin, C.; Yang, C.H.; Chen, F.L. Novel micro-tubular high temperature solid oxide electrolysis cells. ECS Trans. 2011, 35, 2987–2995. [Google Scholar] [CrossRef]
- Shao, L.; Qian, J.; Ye, X.; Wen, T. Optimization of the electrode-supported tubular solid oxide cells for application on fuel cell and steam electrolysis. Int. J. Hydrogen Energy 2013, 38, 4272–4280. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Campana, R.; Larrea, A.; Kilner, J.A.; Orera, V.M. Steam electrolysis using a microtubular solid oxide fuel cell. J. Electrochem. Soc. 2010, 6, B852–B855. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, S.; Liu, Y.; Mori, M.; Funahashi, Y.; Fujishiro, Y. Study of steam electrolysis using a microtubular ceramic reactor. Int. J. Hydrogen Energy 2009, 34, 1159–1165. [Google Scholar] [CrossRef]
- Yang, C.; Jin, C.; Chen, F. Performances of micro-tubular solid oxide cell with novel asymmetric porous hydrogen electrode. Electrochim. Acta 2010, 56, 80–84. [Google Scholar] [CrossRef]
- Duffy, M.; Harrison, K.; Sheahen, T. Measurement of Hydrogen Production Rate Based on Dew Point Temperatures: Independent Review; NREL/MP-150-42237; U.S. Department of Energy Hydrogen Program: Golden, CO, USA, 2007.
- Goutianos, S.; Frandsen, H.L.; Sørensen, B.F. Fracture properties of nickel-based anodes for solid oxide fuel cells. J. Eur. Ceram. Soc. 2010, 30, 3173–3179. [Google Scholar] [CrossRef]
- Boccaccini, D.N.; Frandsen, H.L.; Soprani, S.; Cannio, M.; Klemensø, T.; Gil, V.; Hendriksen, P.V. Influence of porosity on mechanical properties of tetragonal stabilized zirconia. J. Eur. Ceram. Soc. 2018, 38, 1720–1735. [Google Scholar] [CrossRef] [Green Version]
- Fleischhauer, F.; Bermejo, R.; Danzer, R.; Mai, A.; Graule, T.; Kuebler, J. High temperature mechanical properties of zirconia tapes used for electrolyte supported solid oxide fuel cells. J. Power Sources 2015, 273, 237–243. [Google Scholar] [CrossRef]
- Chen, M.; Sun, X.; Chatzichristodoulou, C.; Koch, S.; Hendriksen, P.V.; Mogensen, M.B. Thermoneutral operation of solid oxide electrolysis cells in potentiostatic mode. ECS Trans. 2017, 78, 3077. [Google Scholar] [CrossRef] [Green Version]
- Ahlgren, E.; Poulsen, F.W. Thermoelectric power of YSZ. Solid State Ion. 1994, 70, 528–532. [Google Scholar] [CrossRef]
- Sammes, N.M.; Du, Y. The mechanical properties of tubular solid oxide fuel cells. J. Mater. Sci. 2003, 38, 4811–4816. [Google Scholar] [CrossRef]




| Individual Layers | Materials | Targeted Thickness/µm | No. of Dips | Firing Temperature/°C and Duration of Firing |
|---|---|---|---|---|
| fuel electrode support layer | NiO/3YSZ (65/35 wt%) | 400 | 14 | 1000/1 h |
| fuel electrode functional layer | NiO/ScSZ (60/40 wt%) | 15 | 1 | 1000/1 h |
| electrolyte | ScSZ | 10 | 6 | 1400/3 h |
| oxygen electrode protective layer | SDC | 2 | 6 | 1300/2 h |
| oxygen electrode | LSCF | 20 | 2 | 1100/1 h |
| Ref | Cell | Maximum Performance |
|---|---|---|
| This work | Fuel support electrode: Ni-ScSZ, dip-coating, Fuel support electrode + Fuel support electrode functional layer Electrolyte: ScSZ, 10 µm Oxygen electrode: SDC/LSCF 20 µm | 690 mW cm−2, 800 °C |
| Liu et al. [17] | Fuel support electrode: Ni–YSZ, 500–600 µm Electrolyte: ScSZ, 10 µm Oxygen electrode: ScSZ–LSM/LSM, 70 µm | 240 mW cm−2, 800 °C |
| Monzón et al. [29] | Fuel support electrode: Ni–YSZ, Extrusion, 700 µm Electrolyte: YSZ, Dip-coating, 20 µm Oxygen electrode: LSM–YSZ/LSM, dip-coating 30–50 µm | 600 mW cm−2, 850 °C |
| Suzuki et al. [30] | Fuel support electrode: Ni–ScSZ, Extrusion, 400 µm Electrolyte: ScSZ, Dip-coating, 3 µm Oxygen electrode: GDC/LSCF–GDC, 6 µm, dip-coating | 800 mW cm−2, 600 °C |
| Suzuki et al. [31] | Fuel support electrode: Ni–YSZ, Extrusion Electrolyte: YSZ; Dip-coating, <1 µm Oxygen electrode: GDC/LSCF–GDC, Dip-coating, 6 µm | 300 mW cm−2, 600 °C |
| Suzuki et al. [32] | Fuel support electrode: Ni–YSZ, Extrusion Electrolyte: Ni–ScSZ/ScSZ, Dip-coating, 10 µm Oxygen electrode: GDC/LSCF–GDC, Dip-coating, 6 µm | 600 mW cm−2, 780 °C |
| Sarkar et al. [33] | Fuel support electrode: Ni–YSZ, Fuel support electrode + Fuel support functional electrode Electrolyte: YSZ <10 µm Oxygen electrode: YSZ–LSM/LSM | 190 mW cm−2, 800 °C |
| Dhir and Kendall [34] | Fuel support electrode: Ni–YSZ, Extrusion 300 µm Electrolyte: YSZ, 15 µm Oxygen electrode: LSM | 350 mW cm−2, 800 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, S.K.; Panthi, D.; Soliman, I.; Feng, H.; Du, Y. Fabrication and Performance of Micro-Tubular Solid Oxide Cells. Energies 2022, 15, 3536. https://doi.org/10.3390/en15103536
Sahu SK, Panthi D, Soliman I, Feng H, Du Y. Fabrication and Performance of Micro-Tubular Solid Oxide Cells. Energies. 2022; 15(10):3536. https://doi.org/10.3390/en15103536
Chicago/Turabian StyleSahu, Sulata K., Dhruba Panthi, Ibrahim Soliman, Hai Feng, and Yanhai Du. 2022. "Fabrication and Performance of Micro-Tubular Solid Oxide Cells" Energies 15, no. 10: 3536. https://doi.org/10.3390/en15103536
APA StyleSahu, S. K., Panthi, D., Soliman, I., Feng, H., & Du, Y. (2022). Fabrication and Performance of Micro-Tubular Solid Oxide Cells. Energies, 15(10), 3536. https://doi.org/10.3390/en15103536








