Numerical Analysis of Thermal Stress for a Stack of Planar Solid Oxide Fuel Cells †
Abstract
1. Introduction
2. Model Description and Governing Equations
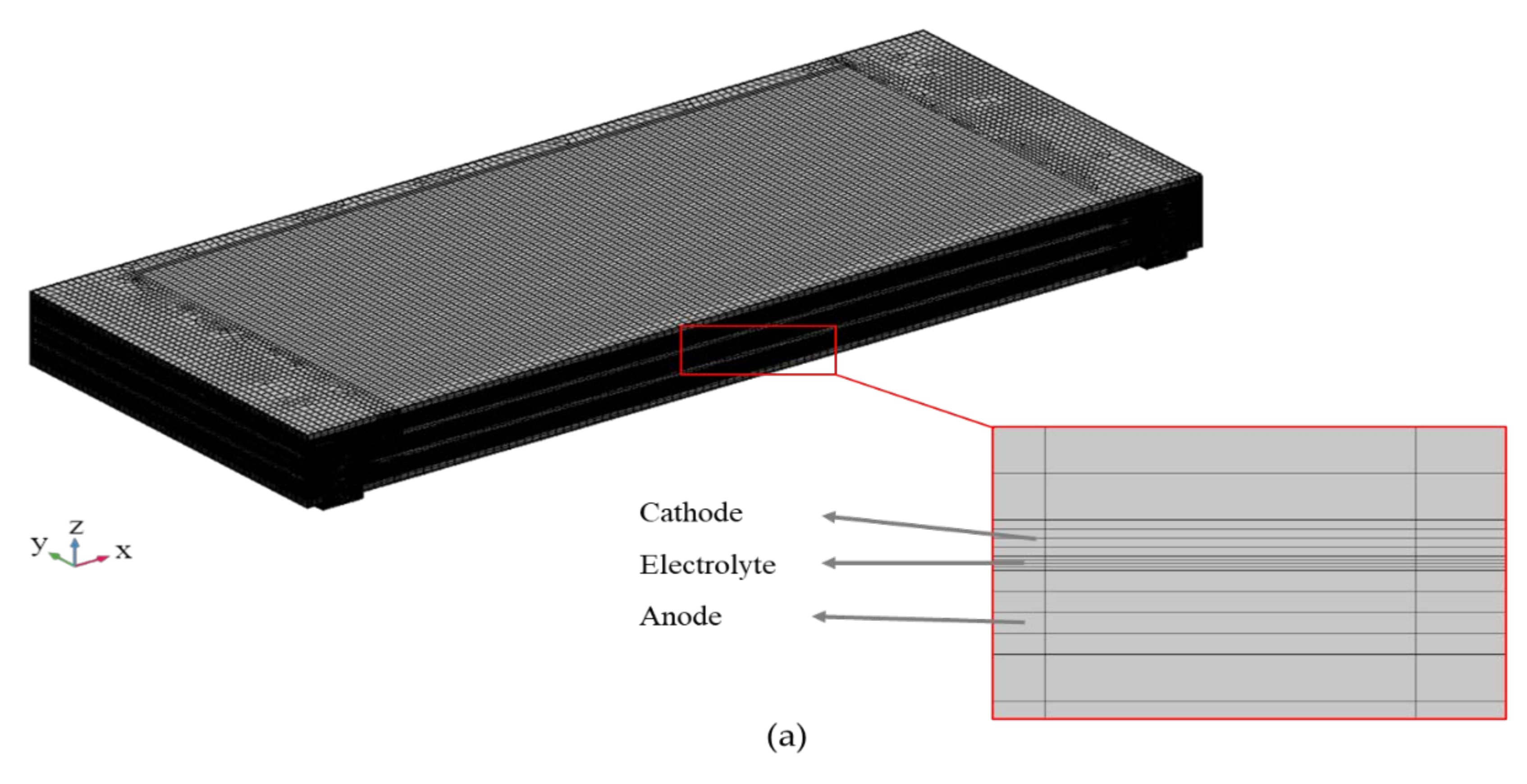
2.1. Model Geometry and Assumptions
2.2. Governing Equations
2.2.1. Electrochemical Reaction Model
2.2.2. Gas Flow
2.2.3. Gas Species Transport
2.2.4. Heat Transfer
2.2.5. Thermal Stress-Strain Relation
2.3. Boundary Condition and Validation of the Model
3. Results and Discussion
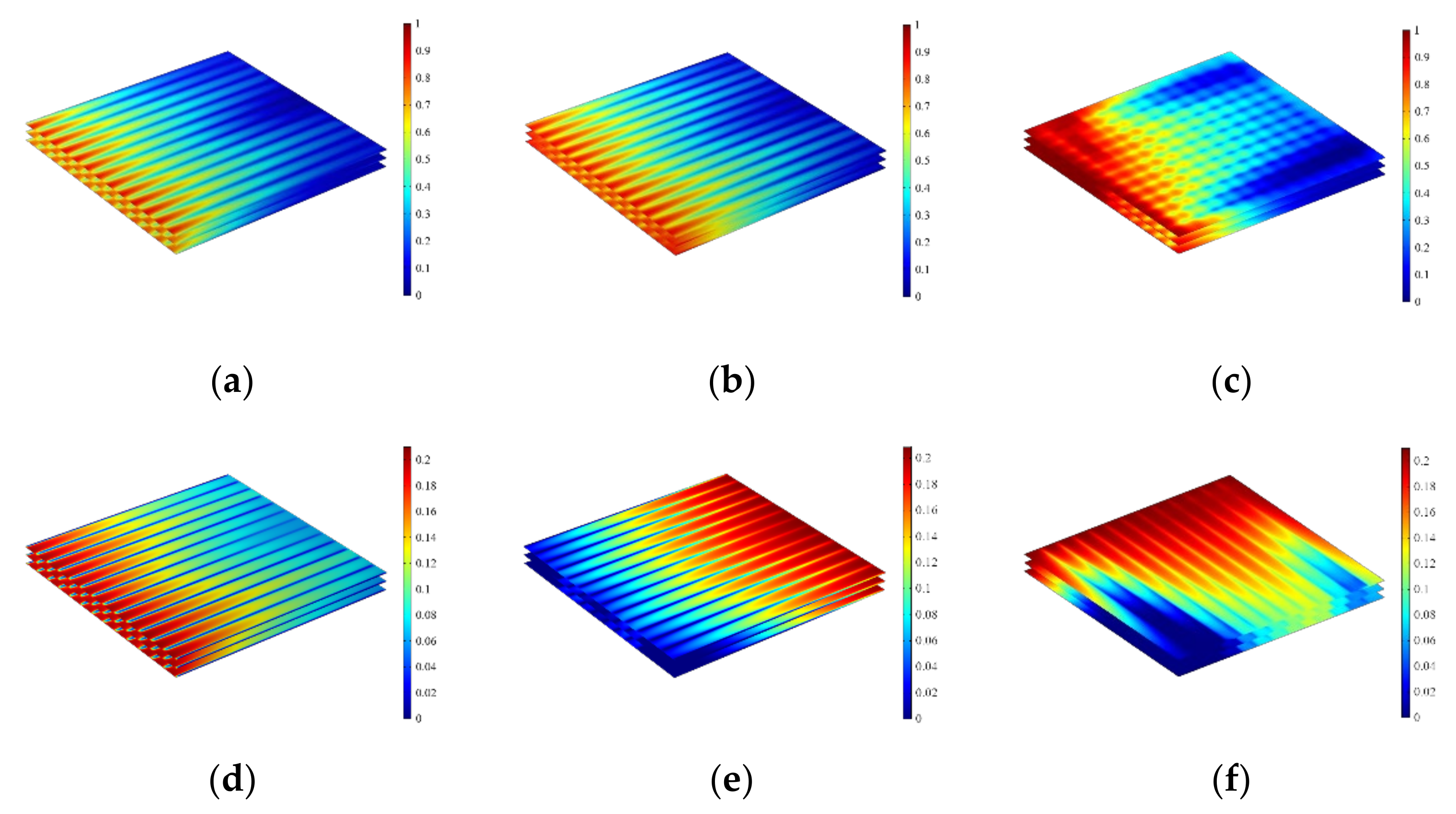
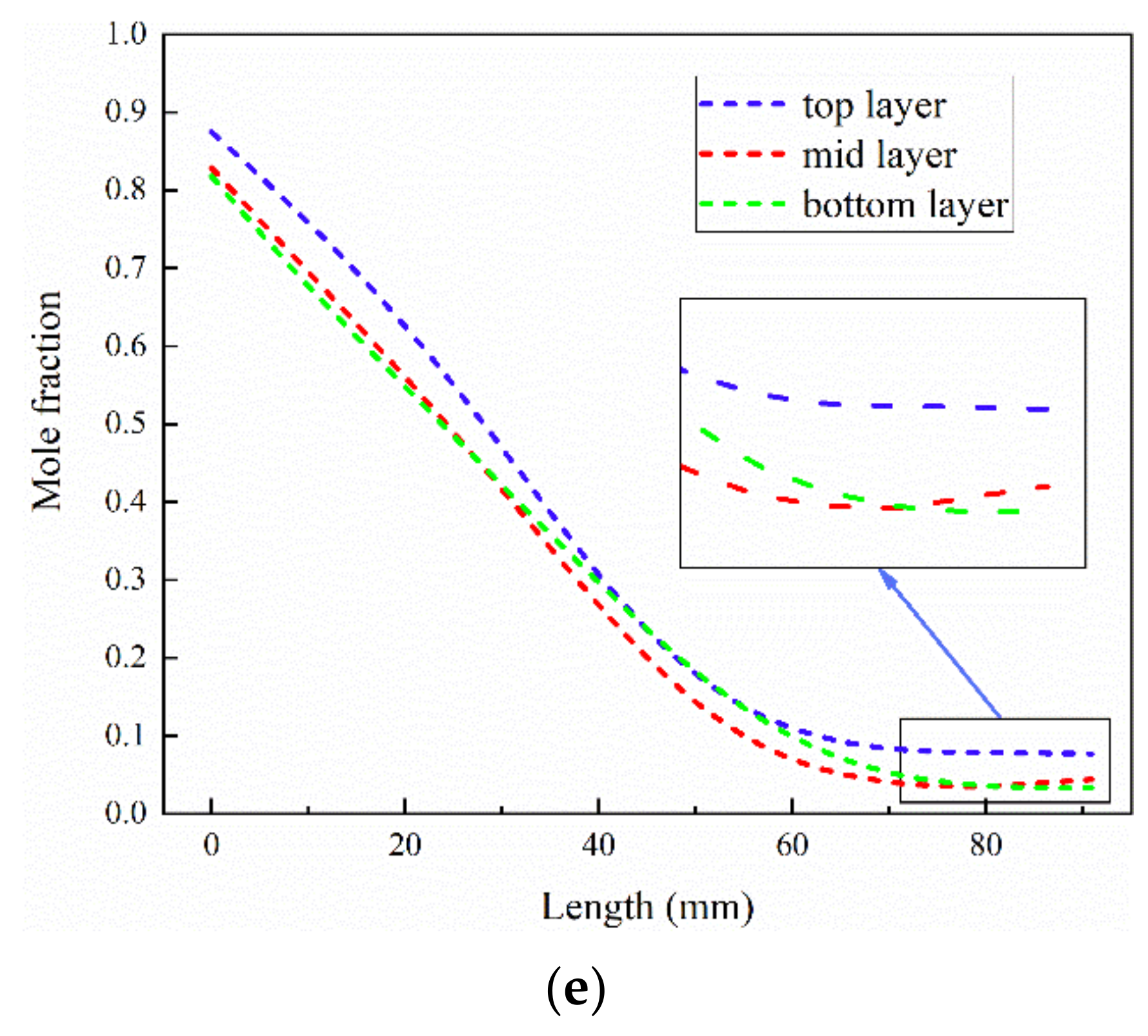
3.1. The Effect of Flow Configurations
3.2. The Effect of Electrolyte Layer Thickness
3.3. The Effect of Sealing Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, C.; Chang, Y.; Hong, C. Multiscale parametric studies on the transport phenomenon of a solid oxide fuel cell. J. Fuel Cell Sci. Technol. 2005, 2, 219–225. [Google Scholar] [CrossRef]
- Khazaee, I.; Rava, A. Numerical simulation of the performance of solid oxide fuel cell with different flow channel geometries. Energy 2017, 119, 235–244. [Google Scholar] [CrossRef]
- Kornely, M.; Leonide, A.; Weber, A.; Ivers-Tiffee, E. Performance limiting factors in anode-supported cells originating from metallic interconnector design. J. Power Sources 2011, 196, 7209–7216. [Google Scholar] [CrossRef]
- Cui, D.; Cheng, M. Thermal stress modeling of anode supported micro-tubular solid oxide fuel cell. J. Power Sources 2009, 192, 400–407. [Google Scholar] [CrossRef]
- Xu, H.; Chen, B.; Tan, P.; Cai, W.; He, W.; Farrusseng, D.; Ni, M. Modeling of all porous solid oxide fuel cells. Appl. Energy 2018, 219, 105–113. [Google Scholar] [CrossRef]
- Jiang, C.; Gu, Y.; Guan, W.; Zheng, J.; Ni, M.; Zhong, Z. 3D thermo-electro-chemo-mechanical coupled modeling of solid oxide fuel cell with double-sided cathodes. Int. J. Hydrog. Energy 2020, 45, 904–915. [Google Scholar] [CrossRef]
- Lin, C.K.; Huang, L.H.; Chiang, L.K.; Chyou, Y.P. Thermal stress analysis of planar solid oxide fuel cell stacks: Effects of sealing design. J. Power Sources 2009, 192, 515–524. [Google Scholar] [CrossRef]
- Zeng, S.; Xu, M.; Parbey, J.; Yu, G.; Andersson, M.; Li, Q.; Li, B.; Li, T. Thermal stress analysis of a planar anode-supported solid oxide fuel cell: Effects of anode porosity. Int. J. Hydrog. Energy 2017, 42, 20239–20248. [Google Scholar] [CrossRef]
- Villanova, J.; Sicardy, O.; Fortunier, R.; Micha, J.S.; Bleuet, P. Determination of global and local residual stresses in SOFC by X-ray diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2010, 268, 282–286. [Google Scholar] [CrossRef]
- Zhao, Y.; Malzbender, J.; Gross, S.M. The effect of room temperature and high temperature exposure on the elastic modulus, hardness and fracture toughness of glass ceramic sealants for solid oxide fuel cells. J. Eur. Ceram. Soc. 2011, 31, 541–548. [Google Scholar] [CrossRef]
- Fan, P.; Li, G.; Zeng, Y.; Zhang, X. Numerical study on thermal stresses of a planar solid oxide fuel cell. Int. J. Therm. Sci. 2014, 77, 1–10. [Google Scholar] [CrossRef]
- Miao, X.-Y.; Rizvandi, O.B.; Navasa, M.; Frandsen, H.L. Modelling of local mechanical failures in solid oxide cell stacks. Appl. Energy 2021, 293, 116901. [Google Scholar] [CrossRef]
- Xiang, Y.; Da, Y.; Zhong, Z.; Shikazono, N.; Jiao, Z. Thermo-mechanical stress analyses of solid oxide fuel cell anode based on three-dimensional microstructure reconstruction. Int. J. Hydrog. Energy 2020, 45, 19791–19800. [Google Scholar] [CrossRef]
- Serincan, M.F.; Pasaogullari, U.; Sammes, N.M. Effects of operating conditions on the performance of a micro-tubular solid oxide fuel cell (SOFC). J. Power Sources 2009, 192, 414–422. [Google Scholar] [CrossRef]
- Amiri, A.; Vijay, P.; Tadé, M.O.; Ahmed, K.; Ingram, G.D.; Pareek, V.; Utikar, R. Planar SOFC system modelling and simulation including a 3D stack module. Int. J. Hydrog. Energy 2016, 41, 2919–2930. [Google Scholar] [CrossRef]
- Canavar, M.; Mat, A.; Celik, S.; Timurkutluk, B.; Kaplan, Y. Investigation of temperature distribution and performance of SOFC short stack with/without machined gas channels. Int. J. Hydrog. Energy 2016, 41, 10030–10036. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Z.; Huo, S.; Najmi, A.-U.-H.; Du, Q.; Jiao, K. Modeling and optimization of electrode structure design for solid oxide fuel cell. Int. J. Hydrog. Energy 2018, 43, 14648–14664. [Google Scholar] [CrossRef]
- Chiang, L.K.; Liu, H.C.; Shiu, Y.H.; Lee, C.H.; Lee, R.Y. Thermal stress and thermo-electrochemical analysis of a planar anode-supported solid oxide fuel cell: Effects of anode porosity. J. Power Sources 2010, 195, 1895–1904. [Google Scholar] [CrossRef]
- Lin, C.K.; Chen, T.T.; Chyou, Y.P.; Chiang, L.K. Thermal stress analysis of a planar SOFC stack. J. Power Sources 2007, 164, 238–251. [Google Scholar] [CrossRef]
- Selimovic, A.; Kemm, M.; Torisson, T.; Assadi, M. Steady state and transient thermal stress analysis in planar solid oxide fuel cells. J. Power Sources 2005, 145, 463–469. [Google Scholar] [CrossRef]
- Xu, M.; Li, T.; Yang, M.; Andersson, M. Solid oxide fuel cell interconnect design optimization considering the thermal stresses. Sci. Bull. 2016, 61, 1333–1344. [Google Scholar] [CrossRef]
- Peksen, M. A coupled 3D thermofluid–thermomechanical analysis of a planar type production scale SOFC stack. Int. J. Hydrog. Energy 2011, 36, 11914–11928. [Google Scholar] [CrossRef]
- Fang, X.; Lin, Z. Numerical study on the mechanical stress and mechanical failure of planar solid oxide fuel cell. Appl. Energy 2018, 229, 63–68. [Google Scholar] [CrossRef]
- Song, M.; Wang, W.; Du, C.; Wang, B.; Jiang, W. Thermomechanical Behavior of Planar Solid Oxide Fuel Cell. J. Chin. Ceram. Soc. 2021, 49, 476–482. [Google Scholar] [CrossRef]
- Weil, K.; Koeppel, B. Thermal stress analysis of the planar SOFC bonded compliant seal design. Int. J. Hydrog. Energy 2008, 33, 3976–3990. [Google Scholar] [CrossRef]
- Huang, K.; Goodenough, J.B. Materials for solid oxide fuel cells (SOFCs). Solid Oxide Fuel Cell Technol. 2009, 50, 220–268. [Google Scholar] [CrossRef]
- Laurencin, J.; Delette, G.; Usseglio-Viretta, F.; Di Iorio, S. Creep behaviour of porous SOFC electrodes: Measurement and application to Ni-8YSZ cermets. J. Eur. Ceram. Soc. 2011, 31, 1741–1752. [Google Scholar] [CrossRef]
- Molla, T.T.; Kwok, K.; Frandsen, H.L. Efficient modeling of metallic interconnects for thermo-mechanical simulation of SOFC stacks: Homogenized behaviors and effect of contact. Int. J. Hydrog. Energy 2016, 41, 6433–6444. [Google Scholar] [CrossRef][Green Version]
- Molla, T.T.; Kwok, K.; Frandsen, H.L. Transient deformational properties of high temperature alloys used in solid oxide fuel cell stacks. J. Power Sources 2017, 351, 8–16. [Google Scholar] [CrossRef]
- Nguyen, X.-V.; Chang, C.-T.; Jung, G.-B.; Chan, S.-H.; Lee, W.-T.; Chang, S.-W.; Kao, I.C. Study of sealants for SOFC. Int. J. Hydrog. Energy 2016, 41, 21812–21819. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Zhao, J.; Wu, Y.; Shu, C.; Miao, H.; Wang, F.; Ye, W.; Yuan, J. CFD modeling and performance comparison of solid oxide fuel cell and electrolysis cell fueled with syngas. Int. J. Energy Res. 2018, 43, 2656–2677. [Google Scholar] [CrossRef]
- Patcharavorachot, Y.; Arpornwichanop, A.; Chuachuensuk, A. Electrochemical study of a planar solid oxide fuel cell: Role of support structures. J. Power Sources 2008, 177, 254–261. [Google Scholar] [CrossRef]
- Andersson, M.; Yuan, J.L.; Sunden, B. SOFC modeling considering electrochemical reactions at the active three phase boundaries. Int. J. Heat Mass Transf. 2012, 55, 773–788. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, D.; Yang, G.; Chen, J.; Zheng, Y.; Miao, H.; Wang, W.; Yuan, J.; Huang, N. Three-dimensional CFD modeling of transport phenomena in multi-channel anode-supported planar SOFCs. Int. J. Heat Mass Transf. 2015, 84, 942–954. [Google Scholar] [CrossRef]
- Nakajo, A.; Kuebler, J.; Faes, A.; Vogt, U.F.; Schindler, H.J.; Chiang, L.-K.; Modena, S.; Van herle, J.; Hocker, T. Compilation of mechanical properties for the structural analysis of solid oxide fuel cell stacks. Constitutive materials of anode-supported cells. Ceram. Int. 2012, 38, 3907–3927. [Google Scholar] [CrossRef]
- Nakajo, A.; Van herle, J.; Favrat, D. Sensitivity of Stresses and Failure Mechanisms in SOFCs to the Mechanical Properties and Geometry of the Constitutive Layers. Fuel Cells 2011, 11, 537–552. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.; Cheng, M.; Ma, Y.; Zhang, X.; Li, G. Transient thermomechanical analysis of a solid oxide fuel cell stack based on 3D multiphysical field model. Mod. Phys. Lett. B 2020, 34, 2050158. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, Y.; Diercks, J.; Deutschmann, O. Hierarchical modeling of solid oxide cells and stacks producing syngas via H2O/CO2 Co-electrolysis for industrial applications. Appl. Energy 2018, 230, 996–1013. [Google Scholar] [CrossRef]
- Frandsen, H.L.; Makowska, M.; Greco, F.; Chatzichristodoulou, C.; Ni, D.W.; Curran, D.J.; Strobl, M.; Kuhn, L.T.; Hendriksen, P.V. Accelerated creep in solid oxide fuel cell anode supports during reduction. J. Power Sources 2016, 323, 78–89. [Google Scholar] [CrossRef]
- Jiang, T.L.; Chen, M.H. Thermal-stress analyses of an operating planar solid oxide fuel cell with the bonded compliant seal design. Int. J. Hydrog. Energy 2009, 34, 8223–8234. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Jiang, W.; Tu, S.-T.; Wang, C.-L.; Chen, C. Effect of operating temperature on creep and damage in the bonded compliant seal of planar solid oxide fuel cell. Int. J. Hydrog. Energy 2018, 43, 4492–4504. [Google Scholar] [CrossRef]
- Nakajo, A.; Mueller, F.; Brouwer, J.; Van herle, J.; Favrat, D. Mechanical reliability and durability of SOFC stacks. Part II: Modelling of mechanical failures during ageing and cycling. Int. J. Hydrog. Energy 2012, 37, 9269–9286. [Google Scholar] [CrossRef]
- Weil, K.S.; Koeppel, B.J. Comparative finite element analysis of the stress–strain states in three different bonded solid oxide fuel cell seal designs. J. Power Sources 2008, 180, 343–353. [Google Scholar] [CrossRef]
- Stephens, E.V.; Vetrano, J.S.; Koeppel, B.J.; Chou, Y.; Sun, X.; Khaleel, M.A. Experimental characterization of glass–ceramic seal properties and their constitutive implementation in solid oxide fuel cell stack models. J. Power Sources 2009, 193, 625–631. [Google Scholar] [CrossRef]








| Parameter | Cathode | Anode |
|---|---|---|
| 2.35 × 1011 | 6.54 × 1011 | |
| 137 | 140 | |
| 0.3 | 0.3 | |
| 3 | 3 | |
| 1.3 × 105 | 1.3 × 105 |
| Parameter | Porosity | Permeability (m2) | Thermal Conductivity (W·m−1·K−1) | Thermal Capacity (J·kg−1·K−1) |
|---|---|---|---|---|
| Anode layer | 0.3 | 2 × 10−11 | 11 | 450 |
| Cathode layer | 0.3 | 2 × 10−11 | 6 | 430 |
| Electrolyte | - | - | 2.7 | 550 |
| Interconnect | - | - | 44.5 | 475 |
| Seal | - | - | 0.064 | 1050 |
| Parameter | Young’s Modulus (GPa) | CTE (10−6·K−1) | Poisson’s Ratio |
|---|---|---|---|
| Anode | 58.1 | 13.2 | 0.39 |
| Cathode | 98 | 11 | 0.3 |
| Electrolyte | 148.6 | 10.3 | 0.32 |
| Interconnect | 60 | 15.5 | 0.3 |
| Seal | 0.019 | 13.9 | 0 |
| Current Density (A·cm−2) | Experimental Data (Voltage/V) | Simulation Data (Voltage/V) | Relative Error | RMSE |
|---|---|---|---|---|
| 0.0202539 | 1.04981 | 1.05822 | 0.81% | 0.89% |
| 0.0852187 | 1.00029 | 1.0143 | 1.41% | |
| 0.146767 | 0.965399 | 0.978482 | 1.36% | |
| 0.211737 | 0.937987 | 0.945463 | 0.80% | |
| 0.340538 | 0.890016 | 0.885344 | 0.53% | |
| 0.404939 | 0.864785 | 0.861982 | 0.33% | |
| 0.46649 | 0.841734 | 0.833635 | 0.97% | |
| 0.53431 | 0.816192 | 0.814011 | 0.27% | |
| 0.598711 | 0.792829 | 0.789091 | 0.48% | |
| 0.663681 | 0.768844 | 0.765418 | 0.45% | |
| 0.728082 | 0.745482 | 0.748285 | 0.38% | |
| 0.792483 | 0.721496 | 0.725234 | 0.52% | |
| 0.858594 | 0.6972 | 0.704364 | 1.03% | |
| 0.921854 | 0.673526 | 0.683182 | 1.44% | |
| 0.990814 | 0.647049 | 0.654836 | 1.21% |
| Mode | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Current density (A/cm2) | 0.71 | 0.76 | 0.72 | 0.78 |
| The first principal thermal stress (MPa) | 120 | 113 | 113 | 112 |
| Case | Case 1 | Case 2 (Baseline) | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Anode | 405 | 400 | 395 | 390 | 385 |
| Electrolyte | 5 | 10 | 15 | 20 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Xiao, L.; Wu, M.; Lang, S.; Zhang, Z.; Chen, M.; Yuan, J. Numerical Analysis of Thermal Stress for a Stack of Planar Solid Oxide Fuel Cells. Energies 2022, 15, 343. https://doi.org/10.3390/en15010343
Zheng J, Xiao L, Wu M, Lang S, Zhang Z, Chen M, Yuan J. Numerical Analysis of Thermal Stress for a Stack of Planar Solid Oxide Fuel Cells. Energies. 2022; 15(1):343. https://doi.org/10.3390/en15010343
Chicago/Turabian StyleZheng, Jianmin, Liusheng Xiao, Mingtao Wu, Shaocheng Lang, Zhonggang Zhang, Ming Chen, and Jinliang Yuan. 2022. "Numerical Analysis of Thermal Stress for a Stack of Planar Solid Oxide Fuel Cells" Energies 15, no. 1: 343. https://doi.org/10.3390/en15010343
APA StyleZheng, J., Xiao, L., Wu, M., Lang, S., Zhang, Z., Chen, M., & Yuan, J. (2022). Numerical Analysis of Thermal Stress for a Stack of Planar Solid Oxide Fuel Cells. Energies, 15(1), 343. https://doi.org/10.3390/en15010343







