Effect of Pyrolysis Atmosphere on the Gasification of Waste Tire Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Characteristic
2.2. Methodology of Measurements
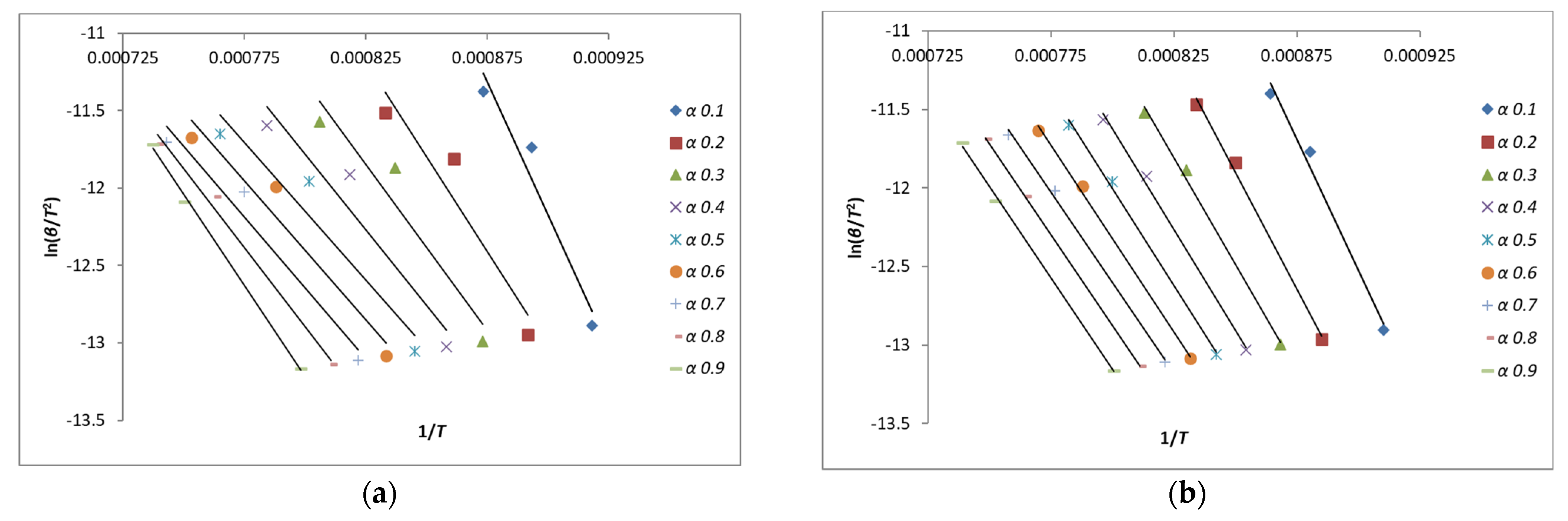
2.3. Methodology of Calculation of Kinetic Parameters
3. Results and Discussion
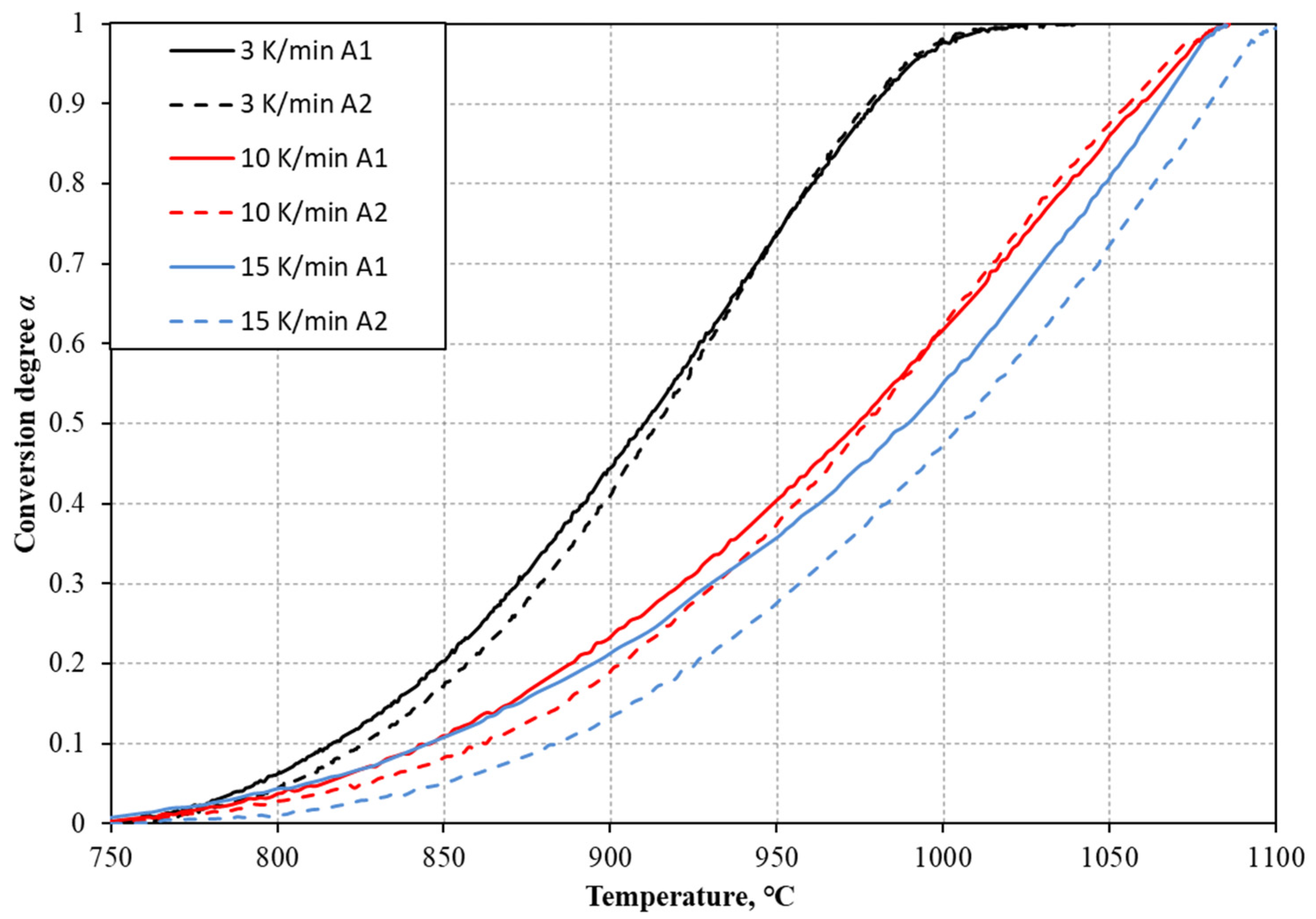
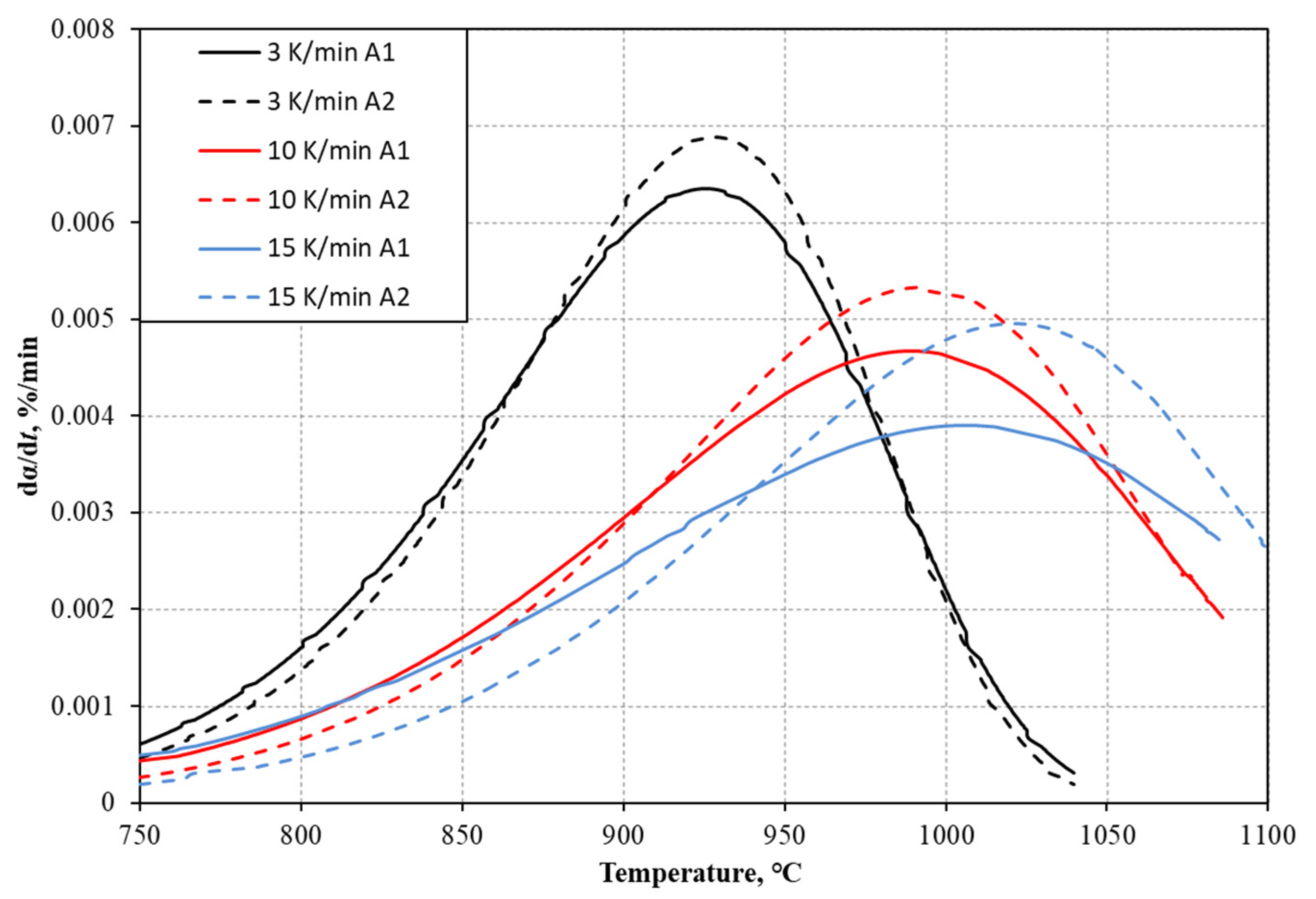
3.1. Analysis of TGA Results
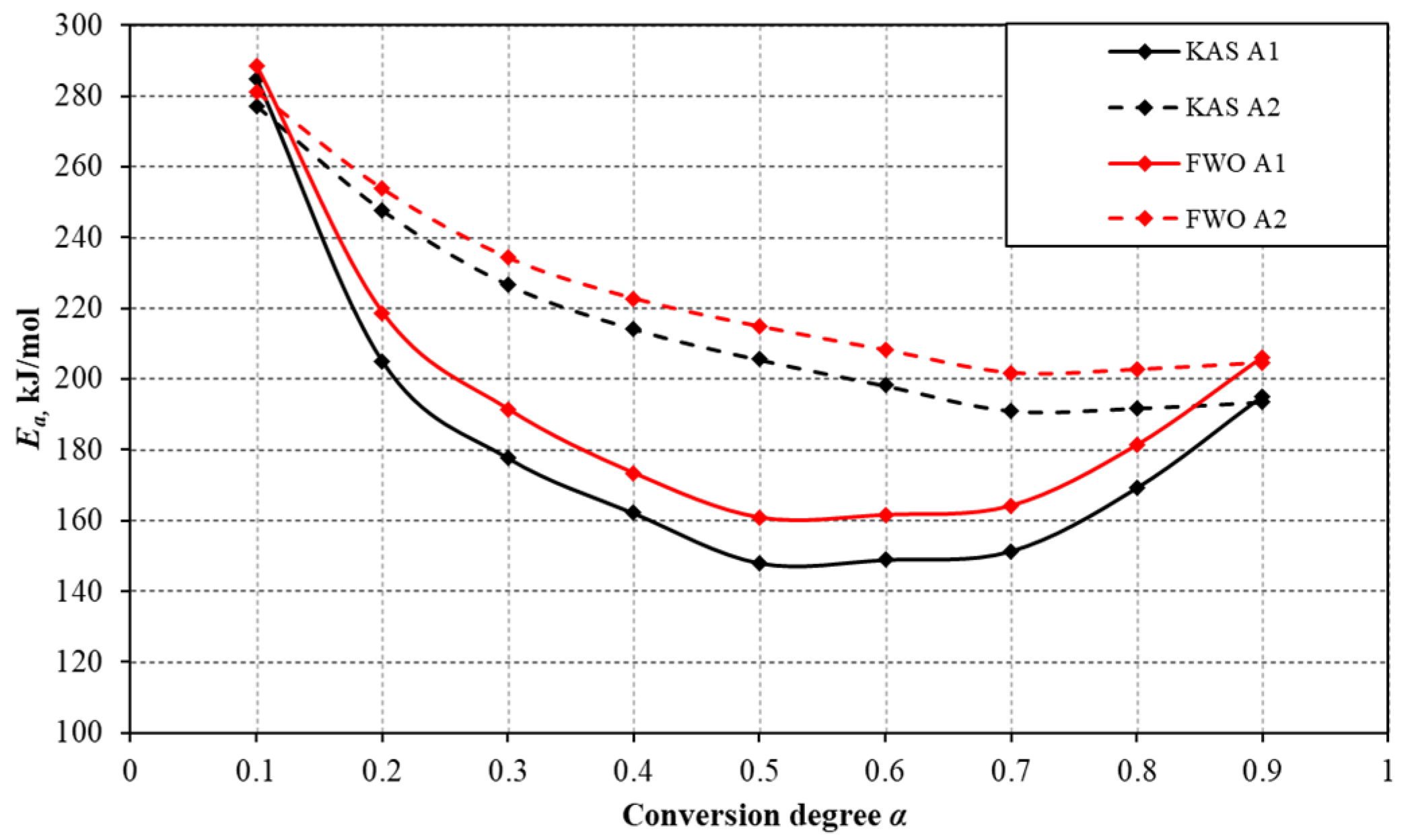
3.2. Analysis of Kinetics of Char Gasification Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nisar, J.; Ali, G.; Ullah, N.; Iftikhar, A.A.; Iqbal, M.; Shah, A.; Sirajuddin Sayed, M.; Mahmood, T.; Khan, M.S. Pyrolysis of waste tire rubber: Influence of temperature on pyrolysates yield. J. Environ. Chem. Eng. 2018, 6, 3469–3473. [Google Scholar] [CrossRef]
- Rowhani, A.; Rainey, J. Scrap Tyre Management Pathways and Their Use as a Fuel—A Review. Energies 2016, 9, 888. [Google Scholar] [CrossRef]
- Labaki, M.; Jeguirim, M. Thermochemical conversion of waste tyres—A review. Environ. Sci. Pollut. Res. 2017, 24, 9962–9992. [Google Scholar] [CrossRef]
- Krawiec, P.; Warguła, Ł.; Małozięć, D.; Kaczmarzyk, P.; Dziechciarz, A.; Czarnecka-Komorowska, D. The Toxicological Testing and Thermal Decomposition of Drive and Transport Belts Made of Thermoplastic Multilayer Polymer Materials. Polymers 2020, 12, 2232. [Google Scholar] [CrossRef]
- Krawiec, P.; Warguła, Ł.; Czarnecka-Komorowska, D.; Janik, P.; Dziechciarz, A.; Kaczmarczyk, P. Chemical compounds released by combustion of polymer composites flat belts. Sci. Rep. 2021, 11, 8269. [Google Scholar] [CrossRef]
- Šourková, M.; Adamcová, D.; Winkler, J.; Vaverková, M.D. Phytotoxicity of Tires Evaluated in Simulated Conditions. Environments 2021, 8, 49. [Google Scholar] [CrossRef]
- Adhikari, B.; De, D.; Maiti, S. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Seghar, S.; Hocine, N.A. Recycling of rubber wastes by devulcanization. Resour. Conserv. Recycl. 2018, 133, 250–262. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopolus, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Jamil, M.A.; Murtaza, D.; Qubeissi, M.; UI Hassan, M.; Mujtaba, M.A. Current Status and Potential of Tire Pyrolysis Oil Production as an Alternative Fuel in Developing Countries. Sustainability 2021, 13, 3214. [Google Scholar] [CrossRef]
- Guohao, Z.; Feng, C.; Yuhao, Z.; Liang, Z.; Jingye, C.; Liyuan, C.; Jinsen, G.; Chunming, X. Properties and utilization of waste tire pyrolysis oil: A mini review. Fuel Process. Technol. 2021, 211, 106582. [Google Scholar]
- Gurai, C. Gasification Kinetics of Blends of Waste Tire and Typical South African Coals. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2015. [Google Scholar]
- Zabaniotou, A.A.; Stavropoulos, G. Pyrolysis of used automobile tires and residual char utilisation. J. Anal. Appl. Pyrolysis 2003, 70, 711–722. [Google Scholar] [CrossRef]
- Babinszki, B.; Sebestyén, Z.; Jakab, E.; Kőhalmi, L.; Bozi, J.; Várhegyi, G.; Wang, L.; Skreiberg, Ø.; Czégény, Z. Effect of slow pyrolysis conditions on biocarbon yield and properties: Characterization of the volatiles. Bioresour. Technol. 2021, 338, 125567. [Google Scholar] [CrossRef]
- Phounglamcheik, A.; Wang, L.; Romar, H.; Kienzl, N.; Broström, M.; Ramser, K.; Skreiberg, Ø.; Umeki, K. Effects of Pyrolysis Conditions and Feedstocks on the Properties and Gasification Reactivity of Charcoal from Woodchips. Energy Fuels 2020, 34, 8353–8365. [Google Scholar] [CrossRef]
- Kim, R.G.; Hwang, C.W.; Jeon, C.H. Kinetics of coal char gasification with CO2: Impact of internal/external diffusion at high temperature and elevated pressure. Appl. Energy 2014, 129, 299–307. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gokalp, I. Effect of char generation method on steam, CO2 and blended mixture gasification of high ash turkish coals. Fuel 2015, 153, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-Q.; Wang, Y.-G.; Chen, Z.-D.; Chen, X.-J.; Zhang, H.-Y.; Bai, L.; Zhang, S. Variations in char structure and reactivity due to the pyrolysis and in-situ gasification using Shengli brown coal. J. Anal. Appl. Pyrolysis 2015, 115, 233–241. [Google Scholar] [CrossRef]
- Alonso, M.J.G.; Borrego, A.G.; Alvarez, D.; Parra, J.B.; Menendez, R. Influence of pyrolysis temperature on char optical texture and reactivity. J. Anal. Appl. Pyrolysis 2001, 58, 887–909. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Dong, L.; Li, C.-Z. Effects of gasification atmosphere and temperature on char structural evolution during the gasification of Collie sub-bituminous coal. Fuel 2014, 117, 1190–1195. [Google Scholar] [CrossRef]
- Czerski, G.; Zubek, K.; Grzywacz, P.; Porada, S. Effect of char preparation conditions on gasification in a carbon dioxide atmosphere. Energy Fuels 2017, 31, 815–823. [Google Scholar] [CrossRef]
- Porada, S.; Czerski, G.; Grzywacz, P.; Makowska, D.; Dziok, T. Comparison of gasification of coals and their chars with CO2 based on the formation kinetics of gaseous products. Thermochim. Acta 2017, 653, 97–110. [Google Scholar] [CrossRef]
- Czerski, G.; Grzywacz, P. Evaluation of gasification of coal density fractions. Przemysl Chemiczny 2018, 97, 1392–1397. [Google Scholar]
- Čepić, Z.; Mihajlović, V.; Đurić, S.; Milotić, M.; Stošić, M.; Stepanov, B.; Ilić Mićunović, M. Experimental Analysis of Temperature Influence on Waste Tire Pyrolysis. Energies 2021, 14, 5403. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Simon, P. Isoconversional methods—Fundamentals, meaning and application. J. Therm. Anal. Calorim. 2004, 76, 123–132. [Google Scholar] [CrossRef]
- Kongkaew, N.; Pruksakit, W.; Patumsawad, S. Thermogravimetric Kinetic Analysis of the Pyrolysis of Rice Straw. Energy Procedia 2015, 79, 663–670. [Google Scholar] [CrossRef] [Green Version]
- House, J.E. Principles of Chemical Kinetics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 271–275. [Google Scholar]
- Lizzio, A.A.; Jiang, H.; Radovic, L.R. On the kinetics of carbon (char) gasification: Reconciling models with experiments. Carbon 1990, 28, 7–19. [Google Scholar] [CrossRef]
- Huttinger, K.J.; Fritz, O.W. The carbon-carbon dioxide reaction: An extended treatment of the active-site concept. Carbon 1991, 29, 1113–1118. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Y.; Lin, X.; Wang, G.; Zheng, P.; Yang, Y.; Mochida, I. Structure and CO2 Gasification Reactivity of Char Derived through Pressured Hydropyrolysis from Low-Rank Coal. Energy Fuels 2019, 33, 8032–8039. [Google Scholar] [CrossRef]
- Zhong, M.; Gao, S.; Zhou, Q.; Yue, J.; Ma, F.; Xu, G. Characterization of char from high temperature fluidized bed coal pyrolysis in complex atmospheres. Particuology 2016, 25, 59–67. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, S.-H.; Roh, J.-S. Analysis of Activation Process of Carbon Black Based on Structural Parameters Obtained by XRD Analysis. Crystals 2021, 11, 153. [Google Scholar] [CrossRef]
- Betancur, M.; Arenas, C.N.; Martínez, J.D.; Navarro, M.V.; Murillo, R. CO2 gasification of char derived from waste tire pyrolysis: Kinetic models comparison. Fuel 2020, 273, 117745. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.D. Gasification kinetics of waste tire-char with CO2 in a thermobalance reactor. Energy 1996, 21, 343–352. [Google Scholar] [CrossRef]
- Czerski, G.; Śpiewak, K.; Grzywacz, P.; Wierońska-Wiśniewska, F. Assessment of the catalytic effect of various biomass ashes on CO2 gasification of tire char. J. Energy Inst. 2021, 99, 170–177. [Google Scholar] [CrossRef]

| Group of Analysis | Parameter | Value, % |
|---|---|---|
| Ultimate analysis | C daf | 89.9 |
| H daf | 7.48 | |
| S dt | 1.78 | |
| Proximate analysis | W ad | 0.90 |
| A ad | 6.1 | |
| V daf | 75.28 | |
| FC ad | 23.0 | |
| Ash composition | SiO2 | 59.9 |
| Al2O3 | 0.68 | |
| Fe2O3 | 5.73 | |
| CaO | 3.54 | |
| MgO | 0.74 | |
| SO3 | 3.08 | |
| ZnO | 22.97 | |
| K2O | 1.27 | |
| P2O5 | 1.06 | |
| TiO2 | 0.11 | |
| Co3O4 | 0.44 | |
| CuO | 0.14 |
| Conversion Degree (-) | Approach | |||||
|---|---|---|---|---|---|---|
| I | II | |||||
| Ea (kJ/mol) | A (1/min) | R2 | Ea (kJ/mol) | A (1/min) | R2 | |
| 0.1 | 284.8 | 4.553 × 1011 | 0.9460 | 277.2 | 1.369 × 1011 | 0.9857 |
| 0.2 | 205.0 | 5.204 × 107 | 0.9680 | 247.7 | 4.480 × 109 | 0.9955 |
| 0.3 | 177.7 | 2.470 × 106 | 0.9700 | 226.7 | 4.257 × 108 | 0.9957 |
| 0.4 | 162.1 | 4.508 × 105 | 0.9722 | 214.2 | 1.052 × 108 | 0.9964 |
| 0.5 | 148.0 | 9.980 × 104 | 0.9690 | 205.5 | 4.057 × 107 | 0.9971 |
| 0.6 | 149.0 | 1.132 × 105 | 0.9811 | 198.2 | 1.864 × 107 | 0.9977 |
| 0.7 | 151.4 | 1.495 × 105 | 0.9895 | 191.1 | 8.995 × 106 | 0.9971 |
| 0.8 | 169.2 | 9.700 × 105 | 0.9971 | 191.8 | 9.878 × 106 | 0.9999 |
| 0.9 | 195.2 | 1.410 × 107 | 0.9984 | 193.6 | 1.272 × 107 | 0.9984 |
| Mean value | 182.5 | 5.059 × 1010 | 0.9768 | 216.2 | 1.578 × 1010 | 0.9960 |
| Conversion Degree (-) | Approach | |||||
|---|---|---|---|---|---|---|
| I | II | |||||
| Ea (kJ/mol) | A (1/min) | R2 | Ea (kJ/mol) | A (1/min) | R2 | |
| 0.1 | 288.4 | 3.530 × 109 | 0.9523 | 281.3 | 1.138 × 109 | 0.9875 |
| 0.2 | 218.7 | 1.487 × 106 | 0.9823 | 253.8 | 4.918 × 107 | 0.9962 |
| 0.3 | 191.4 | 7.902 × 104 | 0.9833 | 234.2 | 5.783 × 106 | 0.9964 |
| 0.4 | 173.3 | 1.218 × 104 | 0.9810 | 222.7 | 1.651 × 106 | 0.9971 |
| 0.5 | 160.8 | 3.476 × 103 | 0.9801 | 214.8 | 7.101 × 105 | 0.9977 |
| 0.6 | 161.5 | 3.824 × 103 | 0.9882 | 208.1 | 3.593 × 105 | 0.9982 |
| 0.7 | 164.1 | 5.026 × 103 | 0.9941 | 201.6 | 1.909 × 105 | 0.9977 |
| 0.8 | 181.2 | 2.687 × 104 | 0.9982 | 202.6 | 2.130 × 105 | 1.0000 |
| 0.9 | 206.1 | 3.030 × 105 | 0.9986 | 204.5 | 2.763 × 105 | 0.9987 |
| Mean value | 194.0 | 3.924 × 108 | 0.9842 | 224.9 | 1.329 × 108 | 0.9966 |
| Approach | Heating Rate (K/min) | R2 | |||||
|---|---|---|---|---|---|---|---|
| Reaction Order | |||||||
| 0 | 0.33 | 0.5 | 0.67 | 1 | 2 | ||
| A1 | 3 | 0.9739 | 0.9864 | 0.9867 | 0.9961 | 0.9986 | 0.9867 |
| 10 | 0.9821 | 0.9924 | 0.9768 | 0.9977 | 0.9979 | 0.9768 | |
| 15 | 0.9658 | 0.9789 | 0.9665 | 0.9675 | 0.9829 | 0.9675 | |
| A2 | 3 | 0.9796 | 0.9906 | 0.9946 | 0.9974 | 0.9995 | 0.9852 |
| 10 | 0.9822 | 0.9927 | 0.9962 | 0.9984 | 0.9992 | 0.9804 | |
| 15 | 0.9889 | 0.9950 | 0.9849 | 0.9982 | 0.9984 | 0.9849 | |
| Approach | Heating Rate (K/min) | A (1/min) | Ea (kJ/mol) |
|---|---|---|---|
| A1 | 3 | 6.04 × 106 | 187.0 |
| 10 | 1.37 × 105 | 148.5 | |
| 15 | 2.43 × 103 | 106.7 | |
| A2 | 3 | 3.90 × 107 | 205.1 |
| 10 | 1.64 × 106 | 173.1 | |
| 15 | 9.04 × 105 | 167.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzywacz, P.; Czerski, G.; Gańczarczyk, W. Effect of Pyrolysis Atmosphere on the Gasification of Waste Tire Char. Energies 2022, 15, 34. https://doi.org/10.3390/en15010034
Grzywacz P, Czerski G, Gańczarczyk W. Effect of Pyrolysis Atmosphere on the Gasification of Waste Tire Char. Energies. 2022; 15(1):34. https://doi.org/10.3390/en15010034
Chicago/Turabian StyleGrzywacz, Przemysław, Grzegorz Czerski, and Wojciech Gańczarczyk. 2022. "Effect of Pyrolysis Atmosphere on the Gasification of Waste Tire Char" Energies 15, no. 1: 34. https://doi.org/10.3390/en15010034
APA StyleGrzywacz, P., Czerski, G., & Gańczarczyk, W. (2022). Effect of Pyrolysis Atmosphere on the Gasification of Waste Tire Char. Energies, 15(1), 34. https://doi.org/10.3390/en15010034







