Comprehensive Study of the Action of Corrosion Inhibitors Based on Quaternary Ammonium Compounds in Solutions of Hydrochloric and Sulfamic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Objects of This Study
2.2. Experimental Details
2.2.1. Determination of Interfacial Tension
2.2.2. Determination of Corrosion Rate by Gravimetric Method
2.2.3. Determination of Corrosion Rate by Electrochemical Method
2.2.4. Determination of the Contact Angle
2.2.5. Determination of Gibbs Adsorption Energy
3. Results and Discussion
3.1. Determination of the Critical Concentration of Micelle Formation of Inhibitors in the Studied Acids
3.2. Corrosion Rate Study
3.2.1. Gravimetric Studies
3.2.2. Electrochemical Research
3.3. Determination of the Protective Effect
3.3.1. Gravimetric Studies
3.3.2. Electrochemical Research
3.4. Determination of Adsorption
3.4.1. Study of Interfacial Tension
3.4.2. Study of the Contact Angle of Wetting
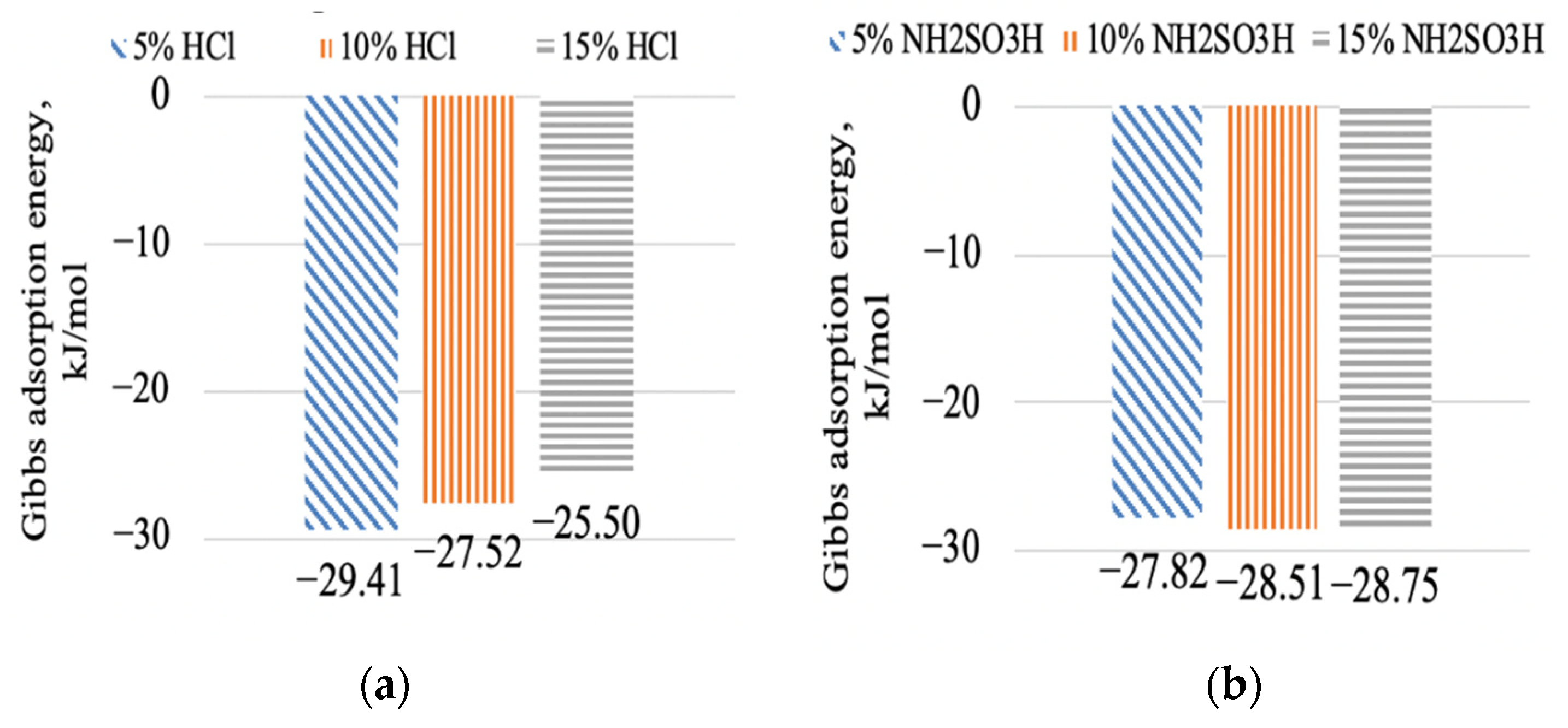
3.4.3. Gibbs Adsorption Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdollahi, R.; Shadizadeh, S.R. The Effect of Spent Acid on Carbonate Rock Wettability During a Matrix Acidizing Treatment. Pet. Sci. Technol. 2013, 32, 450–454. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Wang, Y.; Ma, G. Numerical evaluation of a fracture acidizing treatment in a three-dimensional fractured carbonate reservoir. J. Nat. Gas Sci. Eng. 2020, 81, 103440. [Google Scholar] [CrossRef]
- Shirazi, M.M.; Ayatollahi, S.; Ghotbi, C. Damage evaluation of acid-oil emulsion and asphaltic sludge formation caused by acidizing of asphaltenic oil reservoir. J. Pet. Sci. Eng. 2019, 174, 880–890. [Google Scholar] [CrossRef]
- Lohrasb, S.; Junin, R. Pore volumes to breakthrough estimation in carbonate acidizing with hydrochloric acid by using an analytical derivation method. Petroleum 2020, 6, 362–367. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Hossain, M.E.; Shamim, J.A. Corrosion and its control in crude oil refining process. In Proceedings of the 6th International Mechanical Engineering & 14th Conference Annual Paper Meet (6IMEC&14APM), Dhaka, Bangladesh, 1–5 September 2012. [Google Scholar]
- Shamsheera, K.O.; Prasad, A.R.; Jaseela, P.K.; Joseph, A. Development of self-assembled monolayer of stearic acid grafted chitosan on mild steel and inhibition of corrosion in hydrochloric acid. Chem. Data Collect. 2020, 28, 100402. [Google Scholar] [CrossRef]
- Silin, M.A.; Magadova, L.A.; Davletshina, L.F.; Davletov, Z.R.; Poteshkina, K.A. Particularities of sulfamic acid properties increasing the effectiveness of acid treatments. Neftyanoe Khozyaystvo-Oil Ind. 2021, 1, 44–47. [Google Scholar] [CrossRef]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry, 2nd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 191–212. [Google Scholar]
- Kamburova, K.; Boshkova, N.; Boshkova, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surfaces A: Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Winston Revie, R. (Ed.) Corrosion Inhibitors. In Uhlig’s Corrosion Handbook, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 51, pp. 1021–1047. [Google Scholar]
- Askari, M.; Aliofkhazraei, M.; Jafari, R.; Hamghalam, P.; Hajizadeh, A. Downhole corrosion inhibitors for oil and gas production–A review. Appl. Surf. Sci. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. Fabrication and characterization of layered double hydroxide/silane nanocomposite coatings for protection of mild steel. J. Taiwan Inst. Chem. Eng. 2017, 80, 924–934. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Yi, G. The effects of surfactant concentration, adsorption, aggregation, and solution conditions on steel corrosion inhibition and associated modeling in aqueous media. Corros. Sci. 2016, 102, 233–250. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.; Quraishi, M.A.; Salman, M. Myristic acid based imidazoline derivative as effective corrosion inhibitor for steel in 15% HCl medium. J. Colloid Interface Sci. 2019, 551, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Elabbasy, H.M.; Fouda, A.S. Olive leaf as green corrosion inhibitor for C-steel in Sulfamic acid solution. Green Chem. Lett. Rev. 2019, 12, 332–342. [Google Scholar] [CrossRef]
- Usman, B.J.; Ali, S.A. Carbon dioxide corrosion inhibitors: A review. Arab. J. Sci. Eng. 2018, 43, 1–22. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Gao, K.; Yan, L.; Pang, X. Inhibition of the corrosion of X70 and Q235 steel in CO2-saturated brine by imidazoline-based inhibitor. J. Electroanal. Chem. 2017, 791, 83–94. [Google Scholar] [CrossRef]
- Palumbo, G.; Górny, M.; Banaś, J. Corrosion Inhibition of Pipeline Carbon Steel (N80) in CO2-Saturated Chloride (0.5 M of KCl) Solution Using Gum Arabic as a Possible Environmentally Friendly Corrosion Inhibitor for Shale Gas Industry. J. Mater. Eng. Perform. 2019, 28, 6458–6470. [Google Scholar] [CrossRef]
- Jaal, R.A.; Ismail, M.C.; Ariwahjoedi, B. A Review of CO2 Corrosion Inhibition by Imidazoline-based Inhibitor. MATEC Web Conf. 2014, 13, 05012. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Cho, J.-H. Integrated evaluation of mixed surfactant distribution in water-oil-steel pipe environments and associated corrosion inhibition efficiency. Corros. Sci. 2016, 110, 213–227. [Google Scholar] [CrossRef]
- Nikpour, S.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M. Eriobotrya japonica Lindl leaves extract application for effective corrosion mitigation of mild steel in HCl solution: Experimental and computational studies. Constr. Build. Mater. 2019, 220, 161–176. [Google Scholar] [CrossRef]
- Kashkovskiy, R.; Kuznetsov, Y.; Kazansky, L. Inhibition of hydrogen sulfide corrosion of steel in gas phase by tributylamine. Corros. Sci. 2012, 64, 126–136. [Google Scholar] [CrossRef]
- Ansari, K.R.; Sudheer; Singh, A.; Quraishi, M.A. Some Pyrimidine Derivatives as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. J. Dispers. Sci. Technol. 2014, 36, 908–917. [Google Scholar] [CrossRef]
- Zhu, Y.; FreeM, M.L. Effects of surfactant aggregation and adsorption on steel corrosion inhibition in salt solution. Polymer Sceiences 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Yi, G. Electrochemical measurement, modeling, and prediction of corrosion inhibition efficiency of ternary mixtures of homologous surfactants in salt solution. Corros. Sci. 2015, 98, 417–429. [Google Scholar] [CrossRef]
- Silin, M.A.; Magadova, L.A.; Tolstykh, L.I.; Davletshina, L.F.; Vlasova, V.D.; Yunusov, T.I.; Makarova, A.M. Aspects of Interaction of Surfactant—Acid Compositions at Phase Boundary with Hydrocarbons. Russ. J. Appl. Chem. 2019, 92, 1810–1819. [Google Scholar] [CrossRef]
- Silin, M.; Magadova, L.A.; Davletshina, L.F.; Vlasova, V.D.; Yunusov, T.I.; Merzlyakov, K.K. Interfacial Phenomena on the Phase Boundary Between Hydrocarbon Systems and Acids. Chem. Technol. Fuels Oils 2020, 56, 163–172. [Google Scholar] [CrossRef]
- Motamedi, M.; Tehrani-Bagha, A.; Mahdavian, M. A comparative study on the electrochemical behavior of mild steel in sulfamic acid solution in the presence of monomeric and gemini surfactants. Electrochim. Acta 2011, 58, 488–496. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 140–179. [Google Scholar]
- Watanabe, A.; Sugiyama, S. The Effect of various electrolytes on the deposition of ferric oxide particles onto various fabrics. J. Jpn. Soc. Chem. 1973, 11, 2051–2056. [Google Scholar] [CrossRef][Green Version]
- Obot, I.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Philip, D.; Eapen, A.; Aruldhas, G. Vibrational and Surface Enhanced Raman Scattering Spectra of Sulfamic Acid. J. Solid State Chem. 1995, 116, 217–223. [Google Scholar] [CrossRef]
- Baymou, Y.; Bidi, H.; Touhami, M.E.; Allam, M.; Rkayae, M.; Belakhmima, R.A. Corrosion Protection for Cast Iron in Sulfamic Acid Solutions and Studies of the Cooperative Effect Between Cationic Surfactant and Acid Counterions. J. Bio-Tribo-Corros. 2018, 4, 11. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhang, G. Inhibitive effects of inhibitors on the galvanic corrosion between N80 carbon steel and 13Cr stainless steel under dynamic supercritical CO2 conditions. Corros. Sci. 2018, 146, 121–133. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, B.; Yang, W.; Yin, X.; Liu, Y.; Chen, Y. Halogen-substituted imidazoline derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2015, 90, 284–295. [Google Scholar] [CrossRef]
- Bestetti, M.; Franz, S.; Hashempour, M.I.; Vicenzo, A. Calculation of Uniform Corrosion Current Density of Iron in Hydrochloric Acid Solutions based on the Principle of Maximum Entropy Production Rate Applied to Literature Data. Prot. Met. Phys. Chem. Surf. 2018, 54, 673–679. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Foss, M.; Gulbrandsen, E.; Sjoblom, J. Alteration of Wettability of Corroding Carbon Steel Surface by Carbon Dioxide Corrosion Inhibitors—Effect on Carbon Dioxide Corrosion Rate and Contact Angle. Corrosion 2008, 64, 905–919. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J. Corrosion of multiphase flow pipelines: The impact of crude oil. Corros. Rev. 2016, 34, 17–40. [Google Scholar] [CrossRef]
- Babic, M. Role of Interfacial Chemistry on Wettability and Carbon Dioxide Corrosion of Mild Steels. Ph.D. Dissertation, Ohio University, Athens, OH, USA, 2017. [Google Scholar]
- Zhang, C.; Duan, H.; Zhao, J. Synergistic inhibition effect of imidazoline derivative and l -cysteine on carbon steel corrosion in a CO 2 -saturated brine solution. Corros. Sci. 2016, 112, 160–169. [Google Scholar] [CrossRef]
- Loto, R.T.; Loto, C.A.; Joseph, O.; Olanrewaju, G. Adsorption and corrosion inhibition properties of thiocarbanilide on the electrochemical behavior of high carbon steel in dilute acid solutions. Results Phys. 2016, 6, 305–314. [Google Scholar] [CrossRef]
- Chakravarthy, M.P.; Mohana, K.N. Adsorption and Corrosion Inhibition Characteristics of Some Nicotinamide Derivatives on Mild Steel in Hydrochloric Acid Solution. ISRN Corros. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mertens, S.; Arshadi, M.R. Synergism and antagonism in mild steel corrosion inhibition by sodium dodecylbenzenesulphonate and hexamethylenetetramine. Corros. Sci. 2003, 45, 1473–1489. [Google Scholar] [CrossRef]
- Pareek, S.; Jain, D.; Hussain, S.; Biswas, A.; Shrivastava, R.; Parida, S.K.; Kisan, H.K.; Lgaz, H.; Chung, I.-M.; Behera, D. A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly Imidazopyrimidine Dye: Experimental and theoretical approach. Chem. Eng. J. 2018, 358, 725–742. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Zhou, Y.; Yang, R.; Yang, Q.; Qing, D.; Niu, Q. A novel imidazoline derivative as corrosion inhibitor for P110 carbon steel in hydrochloric acid environment. Petroleum 2015, 1, 237–243. [Google Scholar] [CrossRef]










| Steel Grade | Content of Chemical Elements, % wt. | ||
|---|---|---|---|
| Carbon | Manganese | Silicon | |
| St3 | 0.14–0.22 | 0.30–0.60 | <0.05 |
| Interfacial Tension, mN/m | |||||
|---|---|---|---|---|---|
| 5% HCl | 10% HCl | 15% HCl | 5% NH2SO3H | 10% NH2SO3H | 15% NH2SO3H |
| 43.97 | 43.97 | 43.97 | 43.97 | 43.97 | 43.97 |
| Time, h | Interfacial Tension, mN/m | |||||
|---|---|---|---|---|---|---|
| 5% HCl | 10% HCl | 15% HCl | 5% NH2SO3H | 10% NH2SO3H | 15% NH2SO3H | |
| IC-1 | IC-2 | |||||
| 0 | 2.37 | 3.91 | 6.38 | 2.82 | 2.47 | 2.06 |
| 3 | 2.38 | 3.90 | 6.40 | 2.80 | 2.48 | 2.05 |
| 6 | 2.35 | 3.91 | 6.39 | 2.81 | 2.46 | 2.05 |
| 18 | 2.31 | 3.95 | 6.40 | 2.79 | 2.47 | 2.06 |
| 24 | 2.40 | 3.92 | 6.38 | 2.83 | 2.47 | 2.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhail, S.; Lyubov, M.; Lucia, D.; Kira, P.; Viktoriia, K.; Alexandra, G. Comprehensive Study of the Action of Corrosion Inhibitors Based on Quaternary Ammonium Compounds in Solutions of Hydrochloric and Sulfamic Acids. Energies 2022, 15, 24. https://doi.org/10.3390/en15010024
Mikhail S, Lyubov M, Lucia D, Kira P, Viktoriia K, Alexandra G. Comprehensive Study of the Action of Corrosion Inhibitors Based on Quaternary Ammonium Compounds in Solutions of Hydrochloric and Sulfamic Acids. Energies. 2022; 15(1):24. https://doi.org/10.3390/en15010024
Chicago/Turabian StyleMikhail, Silin, Magadova Lyubov, Davletshina Lucia, Poteshkina Kira, Kotekhova Viktoriia, and Galkina Alexandra. 2022. "Comprehensive Study of the Action of Corrosion Inhibitors Based on Quaternary Ammonium Compounds in Solutions of Hydrochloric and Sulfamic Acids" Energies 15, no. 1: 24. https://doi.org/10.3390/en15010024
APA StyleMikhail, S., Lyubov, M., Lucia, D., Kira, P., Viktoriia, K., & Alexandra, G. (2022). Comprehensive Study of the Action of Corrosion Inhibitors Based on Quaternary Ammonium Compounds in Solutions of Hydrochloric and Sulfamic Acids. Energies, 15(1), 24. https://doi.org/10.3390/en15010024






