Sewage Sludge Treatment by Hydrothermal Carbonization: Feasibility Study for Sustainable Nutrient Recovery and Fuel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Digested Sewage Sludge
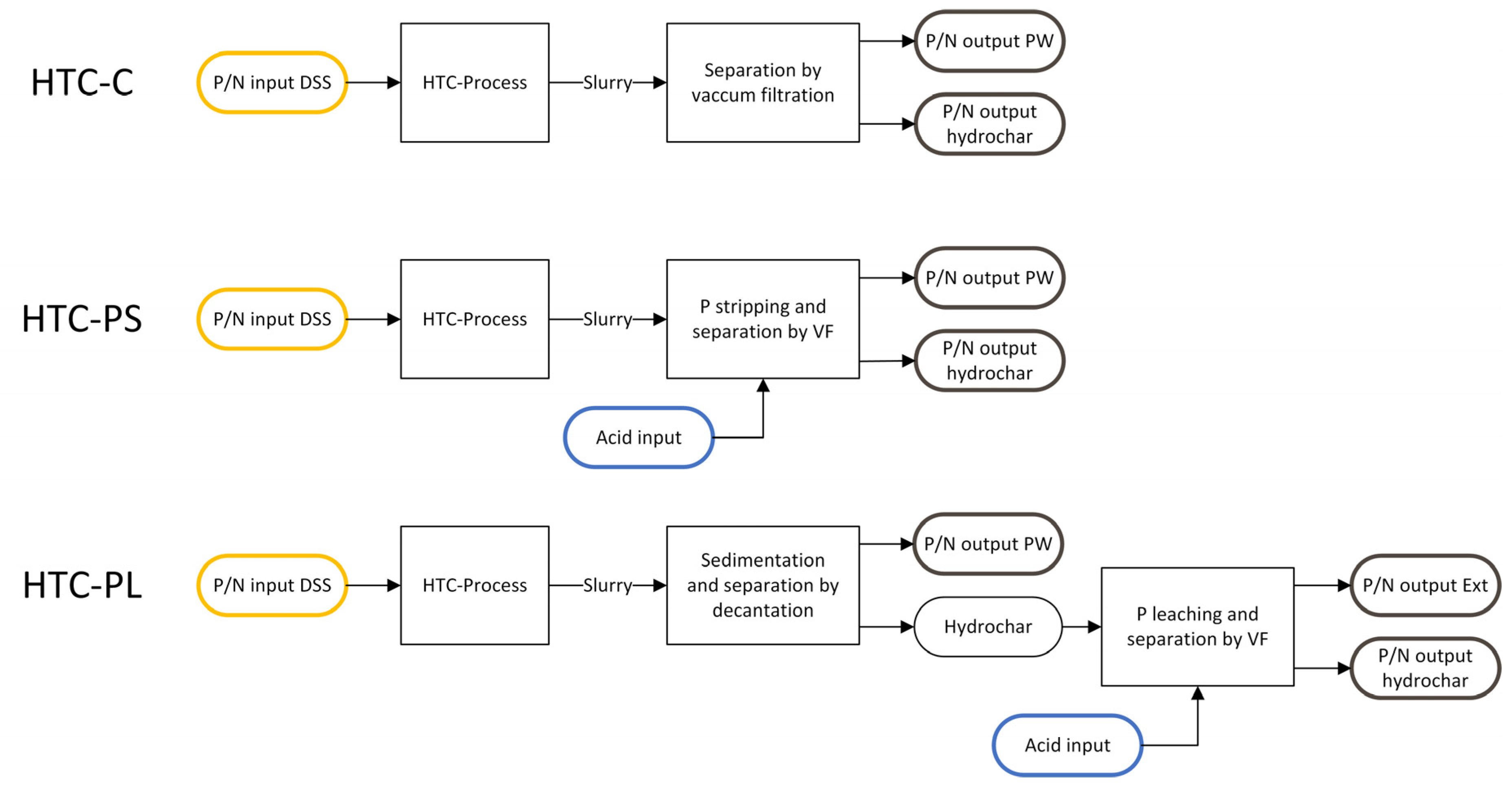
2.2. Hydrothermal Carbonization
2.3. Phosphorus Extraction
2.4. Characterization of Feedstock and HTC-Products
2.5. Economic Feasabiliy of Hydrothermal Carbonization (HTC)
3. Results and Discussion
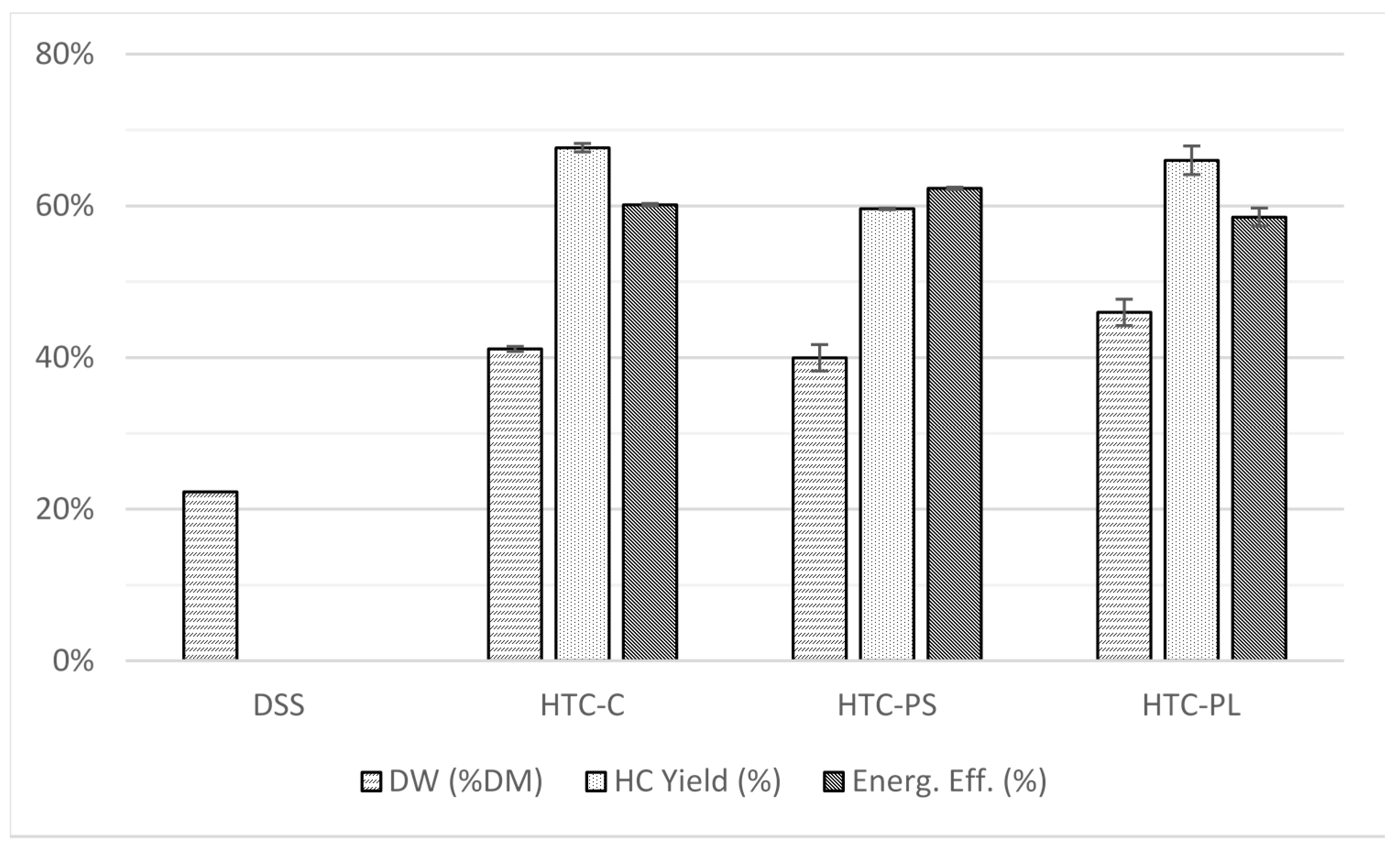
3.1. Carbonization and Separation
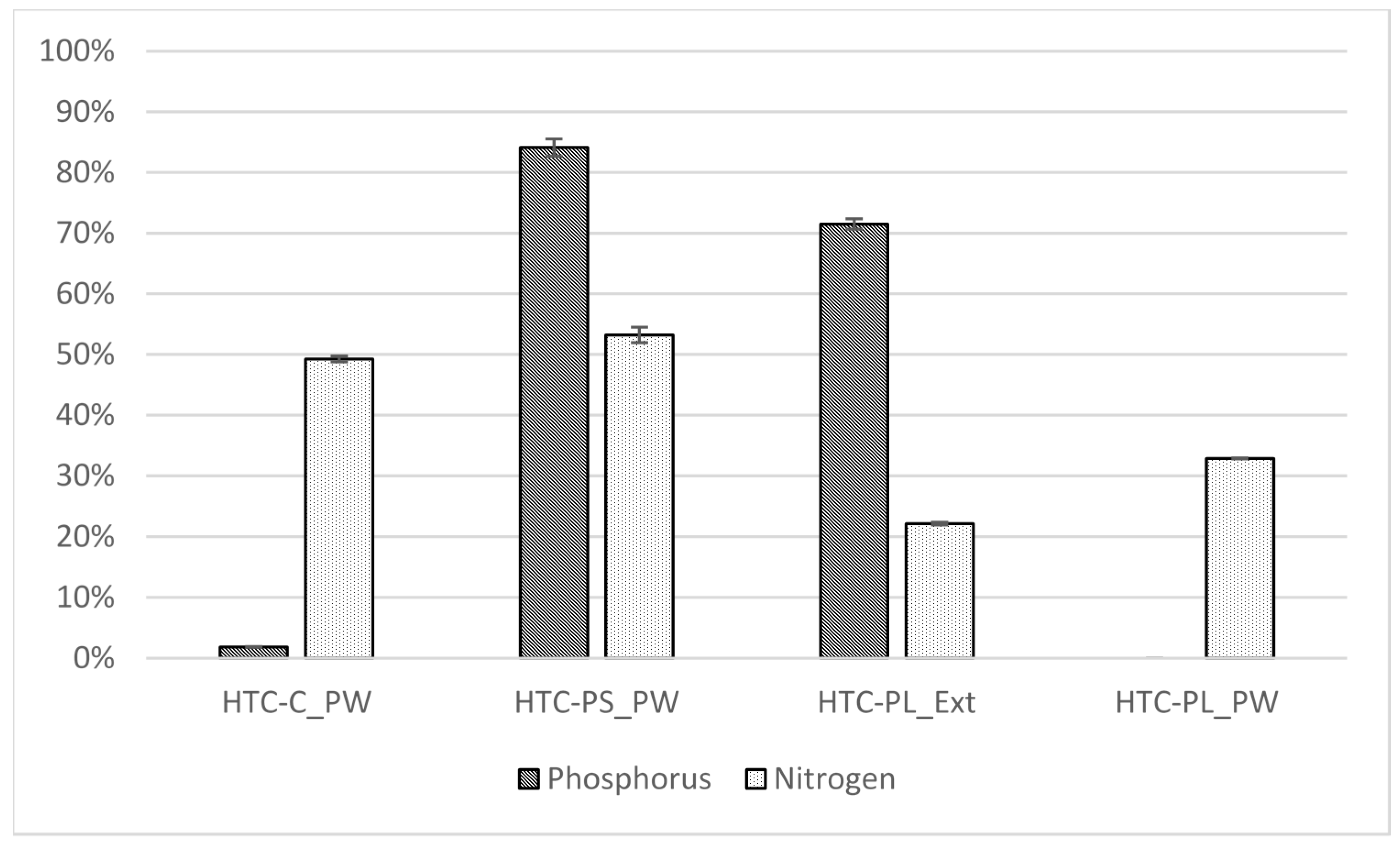
3.2. Nutrient Recovery
3.3. Feasability of Application
3.4. Profitability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathieux, F.; Ardente, F.; Bobba, S.; Nuss, P.; Blengini, G.; Alves Dias, P.; Blagoeva, D.; Torres De Matos, C.; Wittmer, D.; Pavel, C.; et al. Critical Raw Materials and the Circular Economy—Background Report; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; On the Review of the List of Critical Raw Materials for the EU and the Implementation of the Raw Materials Initiative; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Binder, C.R.; de Baan, L.; Wittmer, D. Phosphorflüsse in Der Schweiz. Stand, Risiken Und Handlungsoptionen; Umwelt-Wissen; Bundesamt für Umwelt: Bern, Switzerland, 2009; p. 161. [Google Scholar]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the World’s Phosphorus for a Food Secure Future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Hermann, L. Rückgewinnung von Phosphor aus der Abwassereinigung; Umwelt-Wissen; Bundesamt für Umwelt: Bern, Switzerland, 2009; p. 196. [Google Scholar]
- Bigalke, M.; Ulrich, A.; Rehmus, A.; Keller, A. Accumulation of Cadmium and Uranium in Arable Soils in Switzerland. Environ. Pollut. 2017, 221, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, M.; Schwab, L.; Rehmus, A.; Tondo, P.; Flisch, M. Uranium in Agricultural Soils and Drinking Water Wells on the Swiss Plateau. Environ. Pollut. 2018, 233, 943–951. [Google Scholar] [CrossRef]
- Bigalke, M.; Imseng, M.; Schneider, S.; Schwab, L.; Wiggenhauser, M.; Keller, A.; Müller, M.; Frossard, E.; Wilcke, W. Uranium Budget and Leaching in Swiss Agricultural Systems. Front. Environ. Sci. 2020, 8, 54. [Google Scholar] [CrossRef]
- Schnug, E.; Haneklaus, N. Uranium in phosphate fertilizers—Review and outlook. In Uranium—Past and Future Challenges; Merkel, B.J., Arab, A., Eds.; Springer International Publishing: Cham, Germany, 2015; pp. 123–130. ISBN 978-3-319-11058-5. [Google Scholar]
- Tulsidas, H.; Gabriel, S.; Kiegiel, K.; Haneklaus, N. Uranium Resources in EU Phosphate Rock Imports. Resour. Policy 2019, 61, 151–156. [Google Scholar] [CrossRef]
- Roth, N.; FitzGerald, R. Human and Environmental Impact of Uranium Derived from Mineral Phosphate Fertilizers; Swiss Centre for Applied Human Toxicology: Basel, Switzerland, 2015; p. 50. [Google Scholar]
- Nutrient Source Specifics—Ammonia. Available online: www.ipni.net/specifics (accessed on 19 April 2021).
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An Electrochemical Haber-Bosch Process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Krämer, J. Phosphorrecycling: Wer, Wie, Was?—Umsetzung Einer Iterativen, Zielgruppenorientierten Kommunikationsstrategie; Deutsche Phosphor Plattform: Frankfurt, Germany, 2019; p. 108. [Google Scholar]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, H.; Ge, S.; Yoshikawa, K. Effect of the Hydrothermal Pretreatment for the Reduction of NO Emission from Sewage Sludge Combustion. Appl. Energy 2013, 111, 199–205. [Google Scholar] [CrossRef]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-Efficient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling Hydrothermal Carbonization and Anaerobic Digestion for Sewage Digestate Management: Influence of Hydrothermal Treatment Time on Dewaterability and Bio-Methane Production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef] [PubMed]
- Marin-Batista, J.D.; Mohedano, A.F.; Rodríguez, J.J.; de la Rubia, M.A. Energy and Phosphorous Recovery through Hydrothermal Carbonization of Digested Sewage Sludge. Waste Manag. 2020, 105, 566–574. [Google Scholar] [CrossRef]
- Maurizio, V.; Luca, F.; Fabio, M.; Antonio, M. Andreottola Gianni Hydrothermal Carbonization as an Efficient Tool for Sewage Sludge Valorization and Phosphorous Recovery. Chem. Eng. Trans. 2020, 80, 199–204. [Google Scholar] [CrossRef]
- Becker, G.C.; Wüst, D.; Köhler, H.; Lautenbach, A.; Kruse, A. Novel Approach of Phosphate-Reclamation as Struvite from Sewage Sludge by Utilising Hydrothermal Carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Zessner, M. Phosphorrückgewinnung aus dem Abwasser; Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft, Sektion VIIWasser: Vienna, Austria, 2014; p. 323. [Google Scholar]
- Fux, C.; Theiler, M.; Irzan, T. Studie Phosphorrückgewinnung aus Abwasser und Klärschlamm; TBF+Partner AG: Zürich, Switzerland, 2015; p. 92. [Google Scholar]
- Blöhse, D. Anaerobe Verwertung von HTC-Prozesswässern. In Proceedings of the Biokohle im Blick—Herstellung, Einsatz und Bewertung, 73. Symposium des ANS e.V., Berlin, Germany, 20 September 2012. [Google Scholar]
- Ferrentino, R.; Merzari, F.; Fiori, L.; Andreottola, G. Coupling Hydrothermal Carbonization with Anaerobic Digestion for Sewage Sludge Treatment: Influence of HTC Liquor and Hydrochar on Biomethane Production. Energies 2020, 13, 6262. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Zhou, Y.; Guo, W.; Jiang, H.; Xu, Q. Characterization of Hydrothermal Carbonization Products (Hydrochars and Spent Liquor) and Their Biomethane Production Performance. Bioresour. Technol. 2018, 267, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and Comparison of Product Yields and Bio-Methane Potential in Sewage Digestate Following Hydrothermal Treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Merzari, F.; Langone, M.; Andreottola, G.; Fiori, L. Methane Production from Process Water of Sewage Sludge Hydrothermal Carbonization. A Review. Valorising Sludge through Hydrothermal Carbonization. Crit. Rev. Environ. Sci. Technol. 2019, 49, 947–988. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal Conversion of Dewatered Sewage Sludge: Focusing on the Transformation Mechanism and Recovery of Phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal Carbonization of Wet Biomass from Nitrogen and Phosphorus Approach: A Review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Harada, H.; Ohto, K.; Kawakita, H. Leaching of Phosphorus from Incinerated Sewage Sludge Ash by Means of Acid Extraction Followed by Adsorption on Orange Waste Gel. J. Environ. Sci. 2009, 21, 1753–1760. [Google Scholar] [CrossRef]
- BBodSch, V. Federal Soil Protection and Contaminated Sites Ordinance; German Federal Council: Berlin, Germany, 1999; p. 1554.
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. (Eds.) Energie aus Biomasse: Grundlagen, Techniken und Verfahren; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-47438-9. [Google Scholar]
- Gao, Y.; Yu, B.; Wu, K.; Yuan, Q.; Wang, X.; Chen, H. Physicochemical, Pyrolytic, and Combustion Characteristics of Hydrochar Obtained by Hydrothermal Carbonization of Biomass. BioResources 2016, 11, 4113–4133. [Google Scholar] [CrossRef]
- Li, Z.; Yi, W.; Li, Z.; Tian, C.; Fu, P.; Zhang, Y.; Zhou, L.; Teng, J. Preparation of Solid Fuel Hydrochar over Hydrothermal Carbonization of Red Jujube Branch. Energies 2020, 13, 480. [Google Scholar] [CrossRef]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef]
- Luftreinhalte-Verordnung; Swiss Federal Council: Bern, Switzerland, 1985; (Status as of 1 April 2020); p. 82.
- Liu, M.; Duan, Y.; Bikane, K.; Zhao, L. The Migration and Transformation of Heavy Metals in Sewage Sludge during Hydrothermal Carbonization Combined with Combustion. BioMed Res. Int. 2018, 2018, 1913848. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/Flocculation in Dewatering of Sludge: A Review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Böhler, M.; Siegrist, H.; Pinnow, D.; Müller, D.; Krauss, W.; Brauchli, H. Neue Presstechnologie zur verbesserten Klärschlammentwässerung; EAWAG: Dübendorf, Switzerlnd, 2003; p. 42. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Gu, G. Effect of Acid and Surfactant Treatment on Activated Sludge Dewatering and Settling. Water Res. 2001, 35, 2615–2620. [Google Scholar] [CrossRef]
- Laube, A.; Vonplon, A. Klärschlammentsorgung in Der Schweiz—Mengen- Und Kapazitätserhebung; Umwelt-Materialien; BUWAL: Bern, Switzerland, 2004; p. 47. [Google Scholar]
- Schlamm Aus Klärwerken—Preise Für Die Anlieferung von Schlamm Aus Klärwerken. Available online: www.stadt-zuerich.ch/ted/de/index/entsorgung_recycling/sauberes_wasser/entsorgen/schlamm_aus_klaerwerken.html (accessed on 21 January 2021).

| C | H | N | S | Odiff | Ash | H/C | O/C | HHV | |
|---|---|---|---|---|---|---|---|---|---|
| [wt.%] | [wt.%] | [wt.%] | [wt.%] | [wt.%] | [wt.%] | MJ/kg | |||
| DSS | 30.2 | 4.4 | 4.2 | 1.0 | 17.3 | 42.8 | 1.7 | 0.4 | 12.7 |
| HTC-C_HC | 27.4 | 3.5 | 2.4 | 0.9 | 10.1 | 55.6 | 1.5 | 0.3 | 11.3 |
| HTC-PS_HC | 30.8 | 3.9 | 3.1 | 8.7 | 8.4 | 45.1 | 1.5 | 0.2 | 13.3 |
| HTC-PL_HC | 27.0 | 3.7 | 2.7 | 9.2 | 9.6 | 47.7 | 1.6 | 0.3 | 11.3 |
| Nutrient Content | Heavy Metal Content | |||||||
|---|---|---|---|---|---|---|---|---|
| P [mg kg−1] | N [wt.%] | Pb [mg kg−1] | Cd [mg kg−1] | Cu [mg kg−1] | Ni [mg kg−1] | Zn [mg kg−1] | Hg [mg kg−1] | |
| DSS | 34,128 | 4.2 | 24.3 | BLD | 262.8 | 21.9 | 700.8 | 0.9 |
| HTC-C_HC | 49,505 * | 2.4 | 20.8 | BLD | 337.5 | 33.2 | 900.5 | 0.5 |
| HTC-PS_HC | 9100 * | 3.0 | 39.0 | BLD | 433.5 | 38.9 | 1031.9 | 1.0 |
| HTC-PL_HC | 14,745 * | 2.7 | 25.9 | BLD | 367.8 | 43.5 | 894.0 | 1.0 |
| Nutrient Content | Heavy Metal Content | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P [mg L−1] | N [mg L−1] | Pb [mg L−1] | Cd [mg L−1] | Cu [mg L−1] | Ni [mg L−1] | Zn [mg L−1] | Hg [mg L−1] | ||
| HTC-C_PW | 208 | 6900 | BLD | BLD | BLD | BLD | BLD | BLD | 7.1 |
| HTC-PS_PW | 8113 | 6386 | BLD | BLD | BLD | BLD | 21.3 | BLD | 2.1 |
| HTC-PL_Ext | 16,050 | 6195 | BLD | 0.9 | BLD | BLD | 42.9 | BLD | 2.0 |
| HTC-PL_PW | BLD | 6960 | BLD | BLD | BLD | BLD | BLD | BLD | 7.3 |
| Annual Throughput of DSS | Tons (15%DM) | 11,200 |
|---|---|---|
| Tons (db) | 1680 | |
| Investment costs | USD | 4,332,460.00 |
| Annual fixed costs | USD/a | 1,022,674.00 |
| Capital costs | USD/a | 482,317.00 |
| Operation costs | USD/a | 540,357.00 |
| Electrical energy | USD/a | 88,704.00 |
| Heat energy | USD/a | 72,441.00 |
| Rent | USD/a | 74,250.00 |
| Labor costs | 1 Person (USD) | 110,000.00 |
| Maintenance | 2.5% of Invest. (USD) | 108,312.00 |
| Provision | 1% of Invest. (USD) | 43,325.00 |
| Contingency insurance | 1% of Invest. (USD) | 43,325.00 |
| Specific overall costs | USD per ton DSS (db) | 608.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerner, G.; Meyer, L.; Wanner, R.; Keller, T.; Krebs, R. Sewage Sludge Treatment by Hydrothermal Carbonization: Feasibility Study for Sustainable Nutrient Recovery and Fuel Production. Energies 2021, 14, 2697. https://doi.org/10.3390/en14092697
Gerner G, Meyer L, Wanner R, Keller T, Krebs R. Sewage Sludge Treatment by Hydrothermal Carbonization: Feasibility Study for Sustainable Nutrient Recovery and Fuel Production. Energies. 2021; 14(9):2697. https://doi.org/10.3390/en14092697
Chicago/Turabian StyleGerner, Gabriel, Luca Meyer, Rahel Wanner, Thomas Keller, and Rolf Krebs. 2021. "Sewage Sludge Treatment by Hydrothermal Carbonization: Feasibility Study for Sustainable Nutrient Recovery and Fuel Production" Energies 14, no. 9: 2697. https://doi.org/10.3390/en14092697
APA StyleGerner, G., Meyer, L., Wanner, R., Keller, T., & Krebs, R. (2021). Sewage Sludge Treatment by Hydrothermal Carbonization: Feasibility Study for Sustainable Nutrient Recovery and Fuel Production. Energies, 14(9), 2697. https://doi.org/10.3390/en14092697







