Oxy-Fuel Combustion of Hard Coal, Wheat Straw, and Solid Recovered Fuel in a 200 kWth Calcium Looping CFB Calciner
Abstract
1. Introduction
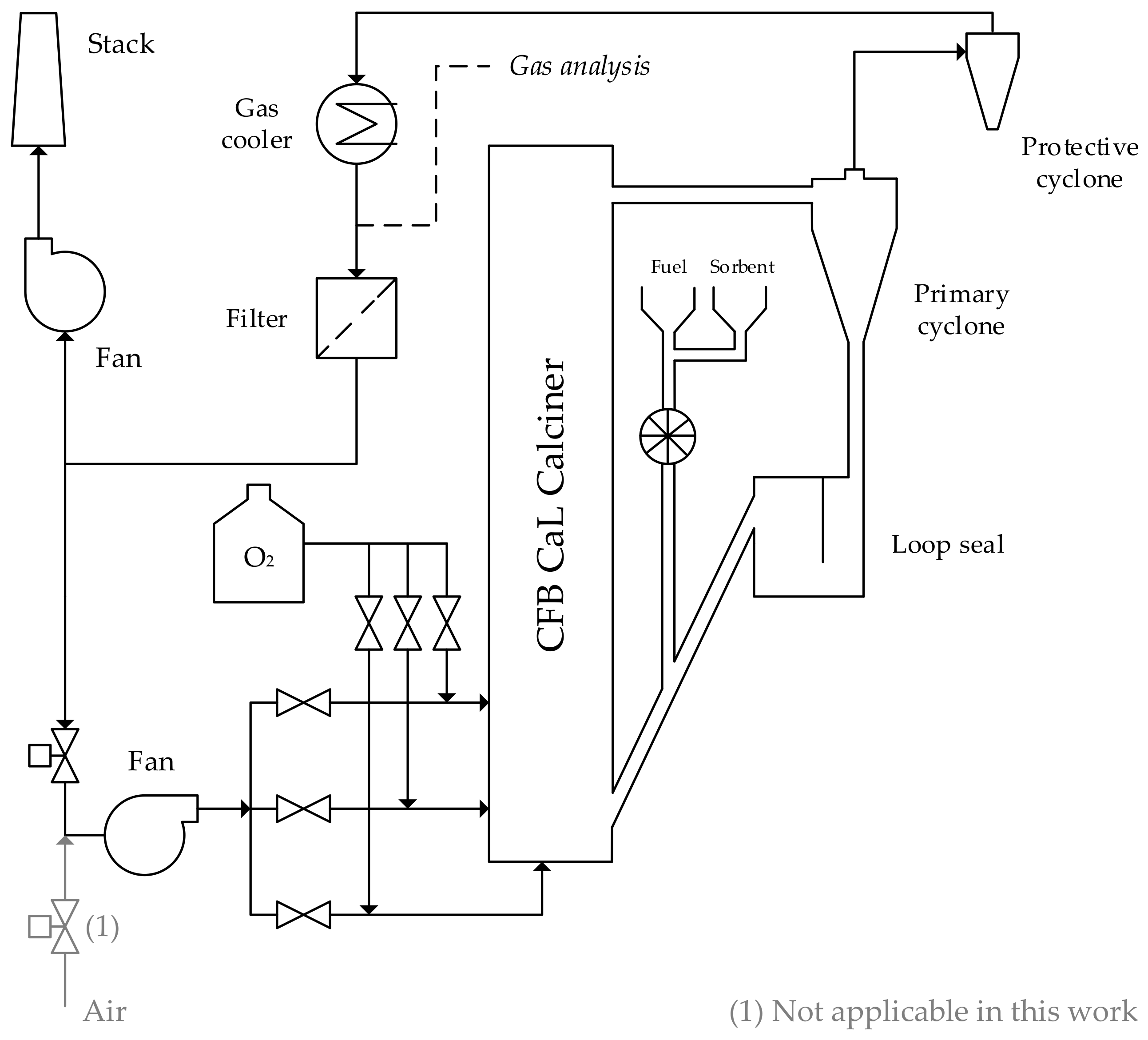
2. Experimental Section
3. Evaluation Methodology
- in ppmv: the volume fractions of NOx, SO2 and HCl measured in the flue gas at standard temperature and pressure (STP) conditions are presented in parts-per-million. and are given in dry conditions, whereas is introduced on a wet basis.
- in mg/MJth: in combustion processes, the emission factor of a gas pollutant ‘i’ is commonly described as the mass of pollutant released per unit of fuel burned [33]:where the mass flow () is calculated as the product of the flue gas volume flow in STP conditions (), and the volume fraction () and standard density () of the desired gas pollutant. The flue gas volume flow is continuously measured using an impeller anemometer, and is accordingly converted to STP conditions. Moreover, represents the thermal duty of a CFB combustor based on the mass flow () and net calorific value () of the fuel.
4. Results
4.1. Combustion of Hard Coal and Wheat Straw
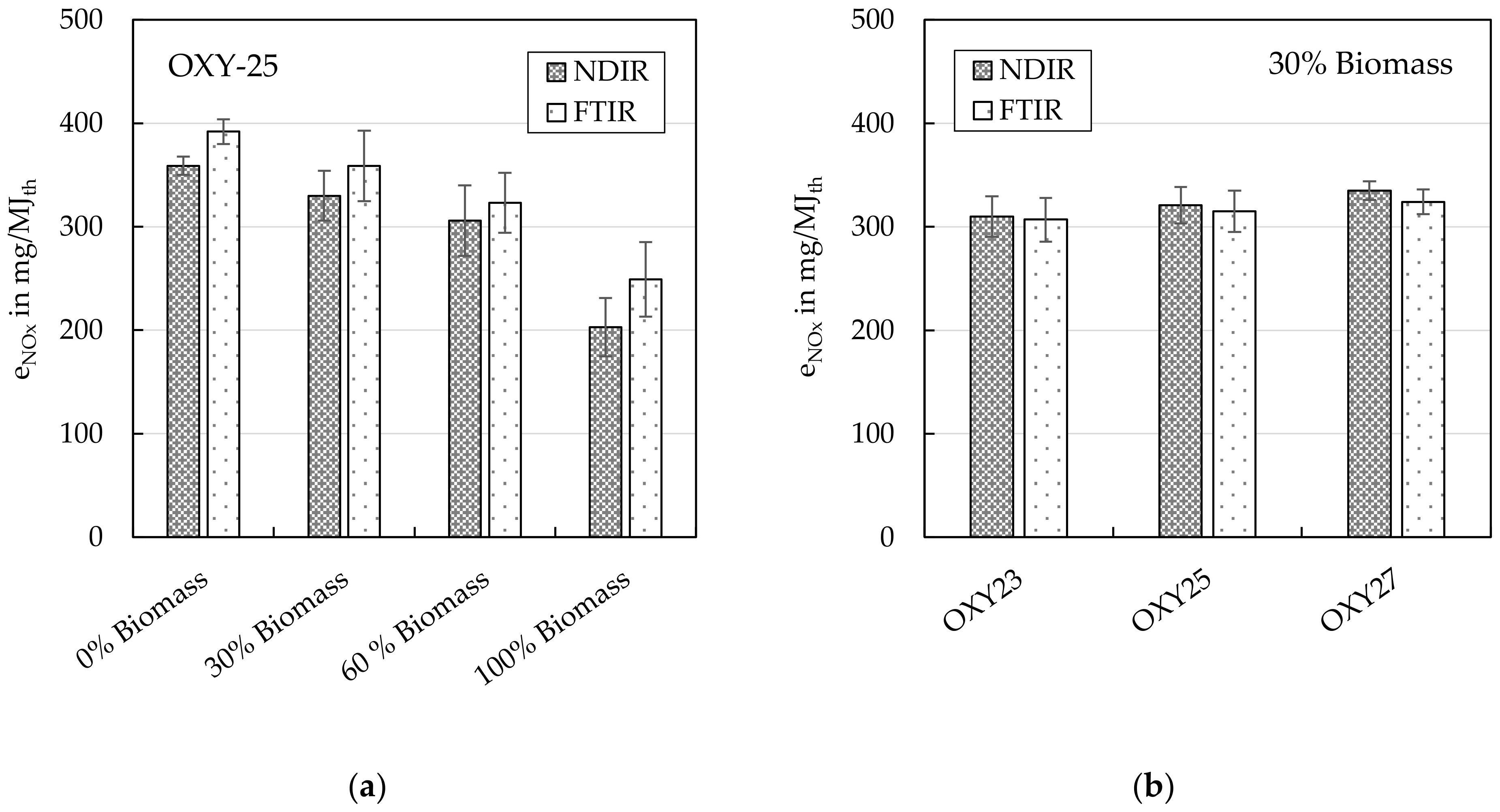
4.1.1. Nitrogen Oxides (NOx)
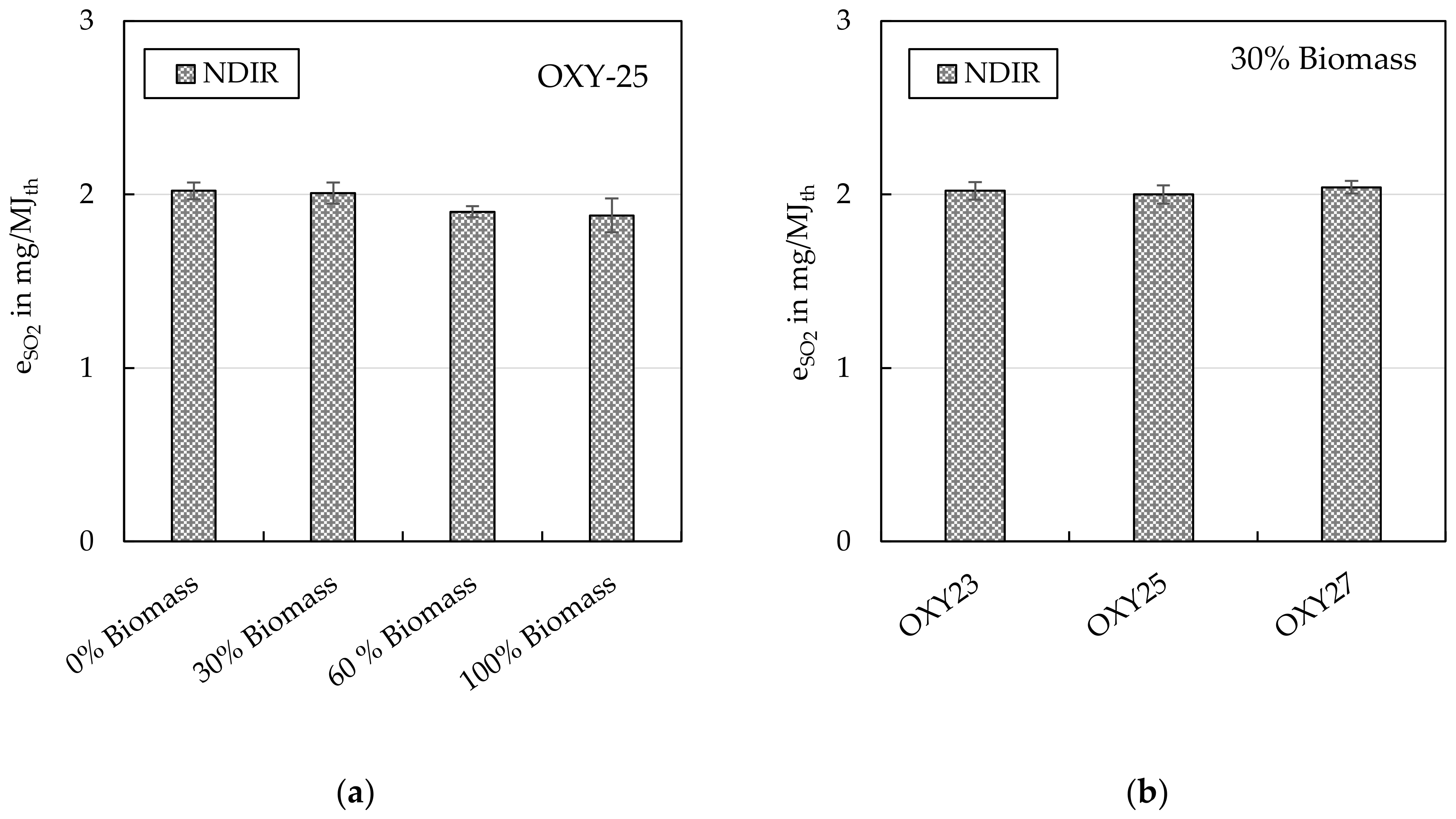
4.1.2. Acidic Gases (SO2 and HCl)
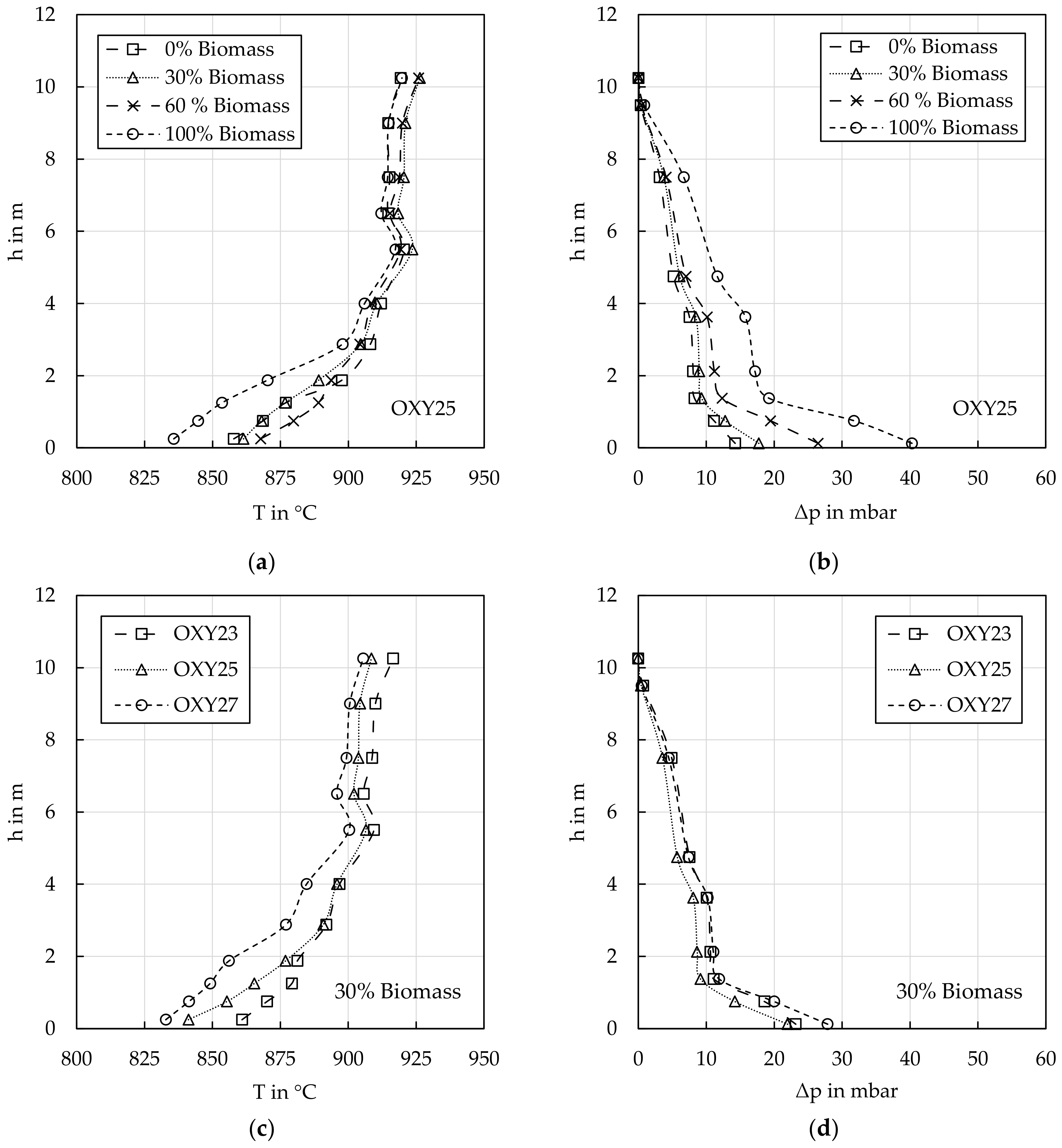
4.1.3. Reactor Profiles
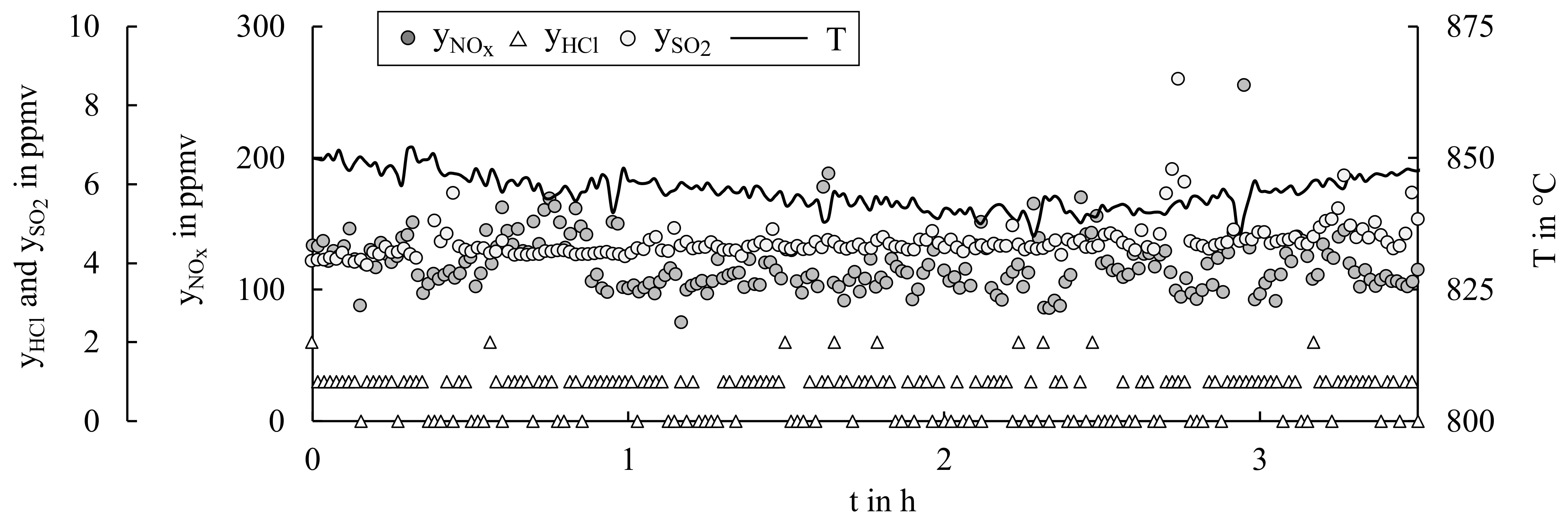
4.2. Mono-Combustion of SRF
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ad | air dried |
| B | fuel |
| BECCS | bio-energy with CCS |
| CaL | calcium looping |
| CCS | carbon capture and storage |
| CFB | circulating fluidized bed |
| CPU | compression and purification unit |
| FBC | fluidized bed combustion |
| FG | flue gas |
| FTIR | fourier-transform infrared spectroscopy |
| MSW | municipal solid waste |
| NDIR | non-dispersive infrared spectroscopy |
| PTFE | polytetrafluoroethylene |
| SRF | solid recovered fuel |
| STP | standard temperature and pressure |
| th | thermal |
| waf | water and ash free |
| wf | water free |
Symbols
| emission factor of gas component i (mg/MJth) | |
| h | height (m) |
| net calorific value (MJ/kg) | |
| mass flow (kg/h or kg/s) | |
| molar mass of component i (kg/kmol) | |
| molar flow (kmol/h or kmol/s) | |
| Δp | differential pressure (mbar) |
| heat flow (kW) | |
| T | temperature (°C) |
| t | time, experimental duration (h) |
| superficial gas velocity (m/s) | |
| volume gas flow (m3/h) | |
| standard molar volume (22.4 l/mol) | |
| cross-sectional area based solid inventory (kg/m2) | |
| mass fraction of component i (kg/kg) | |
| outlet gas volume fraction of component i (ppmv) | |
| inlet gas volume fraction of component i (m3/m3) | |
| retention rate of component i (mol/mol) | |
| fuel mass fraction of component i (kg/kg) | |
| standard density of component i (kg/m3) |
References
- RAL-GZ 724. Sekundärbrennstoffe-Gütesicherung; RAL Deutsches Institut für Gütesicherung und Kennzeichnung e. V.: Münster, Germany, 2012. [Google Scholar]
- Sarc, R.; Lorber, K.E.; Pomberger, R. Manufacturing of Solid Recovered Fuels (SRF) for Energy Recovery Processes. Waste Manag. 2016, 6, 401–416. [Google Scholar]
- Iacovidou, E.; Hahladakis, J.; Deans, I.; Velis, C.; Purnell, P. Technical properties of biomass and solid recovered fuel (SRF) co-fired with coal: Impact on multi-dimensional resource recovery value. Waste Manag. 2018, 73, 535–545. [Google Scholar] [CrossRef] [PubMed]
- United Nations Treaty Collection. The Paris Agreement; Chapter XXVII 7 d; United Nations Framework Convention on Climate Change (UNFCCC): Paris, France, 2015. [Google Scholar]
- Bui, M.; Fajardy, M.; Mac Dowell, N. Thermodynamic Evaluation of Carbon Negative Power Generation: Bio-energy CCS (BECCS). Energy Procedia 2017, 114, 6010–6020. [Google Scholar] [CrossRef]
- Gough, C.; Upham, P. Biomass energy with carbon capture and storage (BECCS or Bio-CCS). Greenh. Gases Sci. Technol. 2011, 1, 324–334. [Google Scholar] [CrossRef]
- Ditaranto, M.; Becidan, M.; Stuen, J. Opportunities for CO2 Capture in the Waste-to-Energy Sector. Waste Manag. 2019, 9, 319–328. [Google Scholar]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Shimizu, T.; Hirama, T.; Hosoda, H.; Kitano, K.; Inagaki, M.; Tejima, K. A Twin Fluid-Bed Reactor for Removal of CO2 from Combustion Processes. Chem. Eng. Res. Des. 1999, 77, 62–68. [Google Scholar] [CrossRef]
- Abanades, J.C.; Anthony, E.J.; Wang, J.; Oakey, J.E. Fluidized Bed Combustion Systems Integrating CO2 Capture with CaO. Environ. Sci. Technol. 2005, 39, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
- Haaf, M.; Hilz, J.; Peters, J.; Unger, A.; Ströhle, J.; Epple, B. Operation of a 1 MWth calcium looping pilot plant firing waste-derived fuels in the calciner. Powder Technol. 2020, 372, 267–274. [Google Scholar] [CrossRef]
- Arias, B.; Diego, M.; Abanades, J.; Lorenzo, M.; Diaz, L.; Martínez, D.; Alvarez, J.; Sánchez-Biezma, A. Demonstration of steady state CO2 capture in a 1.7MWth calcium looping pilot. Int. J. Greenh. Gas Control. 2013, 18, 237–245. [Google Scholar] [CrossRef]
- Haaf, M.; Peters, J.; Hilz, J.; Unger, A.; Ströhle, J.; Epple, B. Combustion of solid recovered fuels within the calcium looping process—Experimental demonstration at 1 MWth scale. Exp. Therm. Fluid Sci. 2020, 113, 110023. [Google Scholar] [CrossRef]
- Vorrias, I.; Atsonios, K.; Nikolopoulos, A.; Nikolopoulos, N.; Grammelis, P.; Kakaras, E. Calcium looping for CO2 capture from a lignite fired power plant. Fuel 2013, 113, 826–836. [Google Scholar] [CrossRef]
- Dieter, H.; Bidwe, A.R.; Varela-Duelli, G.; Charitos, A.; Hawthorne, C.; Scheffknecht, G. Development of the calcium looping CO2 capture technology from lab to pilot scale at IFK, University of Stuttgart. Fuel 2014, 127, 23–37. [Google Scholar] [CrossRef]
- Shah, M.; Degenstein, N.; Zanfir, M.; Kumar, R.; Bugayong, J.; Burgers, K. Near zero emissions oxy-combustion CO2 purification technology. Energy Procedia 2011, 4, 988–995. [Google Scholar] [CrossRef]
- Scheffknecht, G.; Al-Makhadmeh, L.; Schnell, U.; Maier, J. Oxy-fuel coal combustion—A review of the current state-of-the-art. Int. J. Greenh. Gas Control. 2011, 5, S16–S35. [Google Scholar] [CrossRef]
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control. 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Pang, L.; Shao, Y.; Zhong, W.; Gong, Z.; Liu, H. Experimental study of NOx emissions in a 30 kWth pressurized oxy-coal fluidized bed combustor. Energy 2020, 194, 116756. [Google Scholar] [CrossRef]
- Krzywanski, J.; Czakiert, T.; Shimizu, T.; Majchrzak-Kuceba, I.; Shimazaki, Y.; Zylka, A.; Grabowska, K.; Sosnowski, M. NOx Emissions from Regenerator of Calcium Looping Process. Energy Fuels 2018, 32, 6355–6362. [Google Scholar] [CrossRef]
- Hofbauer, G. Experimentelle Untersuchung der Oxy-Fuel-Verbrennung von Steinkohle in einer zirkulierenden Wirbelschichtfeuerung. Univ. Stuttg. 2017. [Google Scholar] [CrossRef]
- Liu, H.; Gibbs, B. The influence of calcined limestone on NOx and N2O emissions from char combustion in fluidized bed combustors. Fuel 2001, 80, 1211–1215. [Google Scholar] [CrossRef]
- Hornberger, M.; Moreno, J.; Schmid, M.; Scheffknecht, G. Experimental investigation of the calcination reactor in a tail-end calcium looping configuration for CO2 capture from cement plants. Fuel 2021, 284, 118927. [Google Scholar] [CrossRef]
- Haaf, M.; Müller, A.; Unger, A.; Ströhle, J.; Epple, B. Combustion of solid recovered fuels in a semi-industrial circulating fluid-ized bed pilot plant—Implications of bed material and combustion atmosphere on gaseous emissions. VGB PowerTech. 2020, 3, 51–56. [Google Scholar]
- Lupiáñez, C.; Mayoral, M.C.; Díez, L.I.; Pueyo, E.; Espatolero, S.; Andrés, J.M. The role of limestone during fluidized bed oxy-combustion of coal and biomass. Appl. Energy 2016, 184, 670–680. [Google Scholar] [CrossRef]
- Spliethoff, H. Power Generation from Solid Fuels; Springer: Berlin, Germany, 2010; ISBN 9783642028557. [Google Scholar]
- Partanen, J.; Backman, P.; Backman, R.; Hupa, M. Absorption of HCl by limestone in hot flue gases. Part I: The effects of temperature, gas atmosphere and absorbent quality. Fuel 2005, 84, 1664–1673. [Google Scholar] [CrossRef]
- Partanen, J.; Backman, P.; Backman, R.; Hupa, M. Absorption of HCl by limestone in hot flue gases. Part II: Importance of calcium hydroxychloride. Fuel 2005, 84, 1674–1684. [Google Scholar] [CrossRef]
- Partanen, J.; Backman, P.; Backman, R.; Hupa, M. Absorption of HCl by limestone in hot flue gases. Part III: Simultaneous absorption with SO2. Fuel 2005, 84, 1685–1694. [Google Scholar] [CrossRef]
- Piao, G.; Aono, S.; Kondoh, M.; Yamazaki, R.; Mori, S. Combustion test of refuse derived fuel in a fluidized bed. Waste Manag. 2000, 20, 443–447. [Google Scholar] [CrossRef]
- Desroches-Ducarne, E.; Marty, E.; Martin, G.; Delfosse, L.; Nordin, A. Effect of Operating Conditions on HCl Emissions from Municipal Solid Waste Combustion in a Laboratory-Scale Fluidized Bed Incinerator. Environ. Eng. Sci. 1998, 15, 279–289. [Google Scholar] [CrossRef]
- DIN EN 14792. In Stationary Source Emissions—Determination of Mass Concentration of Nitrogen Oxides—Standard Reference Method: Chemiluminescence; DIN German Institute for Standardization: Berlin, Germany, 2017.
- Trozzi, C. EMEP/EEA Air Pollution Emission Inventory Guidebook 2019: Energy Industries; European Environment Agency: Copenhagen, Denmark, 2019. [Google Scholar]
- Pu, G.; Zan, H.; Du, J.; Zhang, X. Study on NO Emission in the Oxy-Fuel Combustion of Co-Firing Coal and Biomass in a Bub-bling Fluidized Bed Combustor. BioResources 2016, 12, 1890–1902. [Google Scholar] [CrossRef]
- Riaza, J.; Gil, M.; Álvarez, L.; Pevida, C.; Pis, J.; Rubiera, F. Oxy-fuel combustion of coal and biomass blends. Energy 2012, 41, 429–435. [Google Scholar] [CrossRef]
- De Diego, L.; Rufas, A.; García-Labiano, F.; Obras-Loscertales, M.D.L.; Abad, A.; Gayán, P.; Adánez, J. Optimum temperature for sulphur retention in fluidised beds working under oxy-fuel combustion conditions. Fuel 2013, 114, 106–113. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, Z.; Yan, J.; Zhang, Y.; Chen, Z.; Gao, R.; Zhong, P. Dichlorination in a circulating fluidized-bed incinerator for municipal solid waste incineration system. Waste Dispos. Sustain. Energy 2019, 1, 207–212. [Google Scholar] [CrossRef]
- Hu, Y.; Naito, S.; Kobayashi, N.; Hasatani, M. CO2, NOx and SO2 emissions from the combustion of coal with high oxygen concentration gases. Fuel 2000, 79, 1925–1932. [Google Scholar] [CrossRef]
- Wolf, C.; Leino, T.J.; Stephan, A.R.; Aho, M.J.; Spliethoff, H. Online Corrosion Measurements in Combination with Deposit and Aerosol Analysis during the Co-firing of Straw with Coal in Electrically Heated, Small-Scale Pulverized Fuel and Circulating Fluidized Bed Systems. Energy Fuels 2018, 32, 2506–2516. [Google Scholar] [CrossRef]
- Xie, W.; Liu, K.; Pan, W.-P.; Riley, J. Interaction between emissions of SO2 and HCl in fluidized bed combustors. Fuel 1999, 78, 1425–1436. [Google Scholar] [CrossRef]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Enhancement of the Direct Sulfation of Limestone by Alkali Metal Salts, Calcium Chloride, and Hydrogen Chloride. Ind. Eng. Chem. Res. 2007, 46, 5295–5303. [Google Scholar] [CrossRef]
- Addink, R.; Bakker, W.C.M.; Olie, K. Influence of HCl and Cl2 on the Formation of Polychlorinated Diben-zo-p-dioxins/Dibenzofurans in a Carbon/Fly Ash Mixture. Environ. Sci. Technol. 1995, 29, 2055–2058. [Google Scholar] [CrossRef] [PubMed]
- Dieter, H.; Hawthorne, C.; Zieba, M.; Scheffknecht, G. Progress in Calcium Looping Post Combustion CO2 Capture: Successful Pilot Scale Demonstration. Energy Procedia 2013, 37, 48–56. [Google Scholar] [CrossRef]
- Scala, F.; Salatino, P. Modelling fluidized bed combustion of high-volatile solid fuels. Chem. Eng. Sci. 2002, 57, 1175–1196. [Google Scholar] [CrossRef]
- Dri, M.; Canfora, P.; Antonopoulos, I.S.; Gaudillat, P. Best Environmental Management Practice for the Waste Management Sector. JRC Sci. Policy Rep. 2018. [Google Scholar] [CrossRef]
- Bolhàr-Nordenkampf, M.; Nummelin, T.; Luomaharju, T.; Viljanen, J. Operating Experience from the World´s Largest Waste Fired Circulating Fluidized Bed Reactor in Västerås. Waste Manag. 2015, 5, 167–178. [Google Scholar]
- Gatternig, B. Predicting Agglomeration in Biomass Fired Fluidized Beds; University of Erlangen-Nürnberg: Erlangen, Germany, 2015. [Google Scholar]




| kg/kg, waf | kg/kg, wf | kg/kg, ad | MJ/kg, ad | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Colombian hard coal | 0.776 | 0.052 | 0.145 | 0.016 | 0.011 | 0.000 | 0.091 | 0.019 | 27.5 |
| German wheat straw | 0.497 | 0.066 | 0.425 | 0.010 | 0.001 | 0.001 | 0.059 | 0.081 | 15.6 |
| Spanish SRF | 0.515 | 0.067 | 0.377 | 0.026 | 0.006 | 0.009 | 0.261 | 0.067 | 14.3 |
| kg/kg, wf | ||||||
|---|---|---|---|---|---|---|
| German limestone | 0.551 | 0.007 | 0.004 | 0.001 | 0.435 | 0.002 |
| Parameter | Symbol | Value/Range | Unit |
|---|---|---|---|
| Temperature | 835–852 | °C | |
| Thermal input | 93–112 | kWth | |
| Superficial gas velocity | 3.9–4.1 | m/s | |
| Solid inventory | 971–1479 | kg/m2 | |
| O2 inlet volume fraction | 0.21–0.22 | m3/m3 | |
| Experimental duration | 3.5 | h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, J.; Hornberger, M.; Schmid, M.; Scheffknecht, G. Oxy-Fuel Combustion of Hard Coal, Wheat Straw, and Solid Recovered Fuel in a 200 kWth Calcium Looping CFB Calciner. Energies 2021, 14, 2162. https://doi.org/10.3390/en14082162
Moreno J, Hornberger M, Schmid M, Scheffknecht G. Oxy-Fuel Combustion of Hard Coal, Wheat Straw, and Solid Recovered Fuel in a 200 kWth Calcium Looping CFB Calciner. Energies. 2021; 14(8):2162. https://doi.org/10.3390/en14082162
Chicago/Turabian StyleMoreno, Joseba, Matthias Hornberger, Max Schmid, and Günter Scheffknecht. 2021. "Oxy-Fuel Combustion of Hard Coal, Wheat Straw, and Solid Recovered Fuel in a 200 kWth Calcium Looping CFB Calciner" Energies 14, no. 8: 2162. https://doi.org/10.3390/en14082162
APA StyleMoreno, J., Hornberger, M., Schmid, M., & Scheffknecht, G. (2021). Oxy-Fuel Combustion of Hard Coal, Wheat Straw, and Solid Recovered Fuel in a 200 kWth Calcium Looping CFB Calciner. Energies, 14(8), 2162. https://doi.org/10.3390/en14082162






