Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal and Desalination Properties of the Green Alga Coelastrum morus on a Laboratory Scale
Abstract
1. Introduction
- Higher nutrient content with higher N:P ratio is better for growth and salt tolerance than higher nutrient content with lower ratio, or lower nutrient contents.
- Higher N:P ratios favor more efficient nutrient removal, independently from the initial nutrient contents.
- More favorable growth conditions favor conductivity reduction (salt removal ability).
2. Materials and Methods
2.1. Measurement of the Growth of the Cultures
2.2. Measurement of Nitrate, Phosphate, Conductivity and Chloride
2.3. Statistical Analysis
3. Results
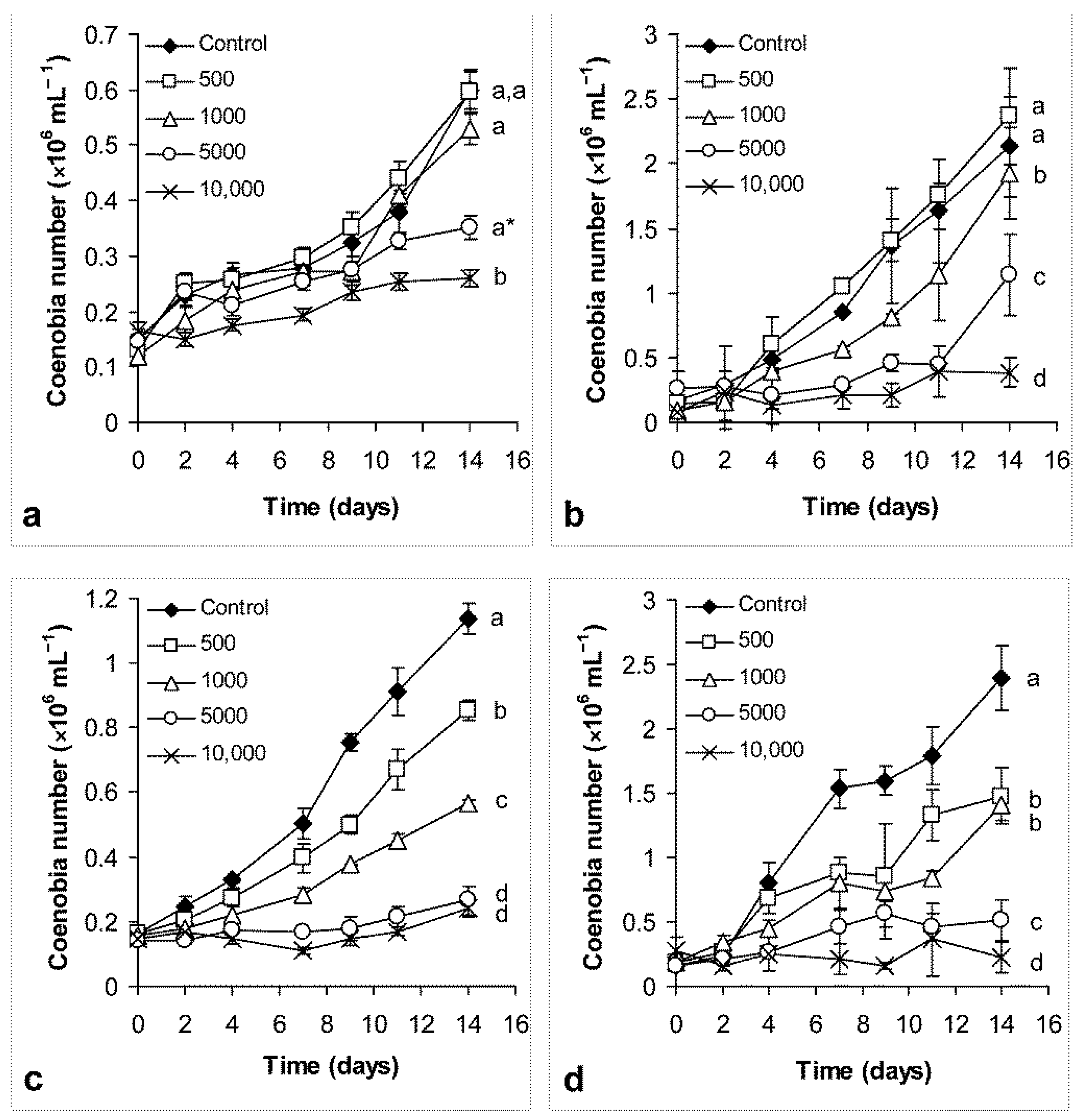
3.1. Growth and Salt Tolerance of the Cultures
3.2. Nutrient (Nitrate and Phosphate) Removal
3.2.1. Nitrate Removal
3.2.2. Phosphate Removal
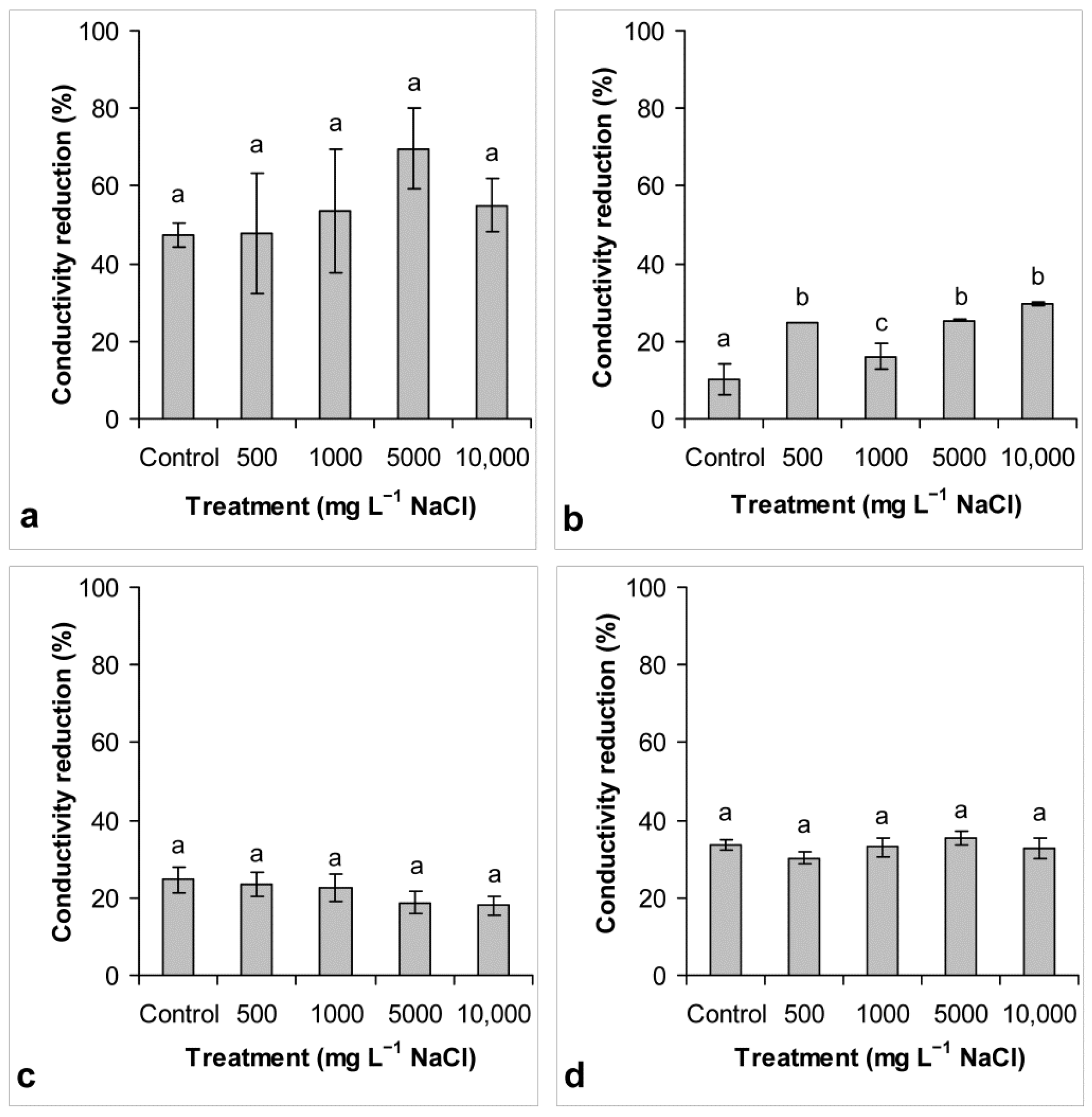
3.3. Conductivity Reduction
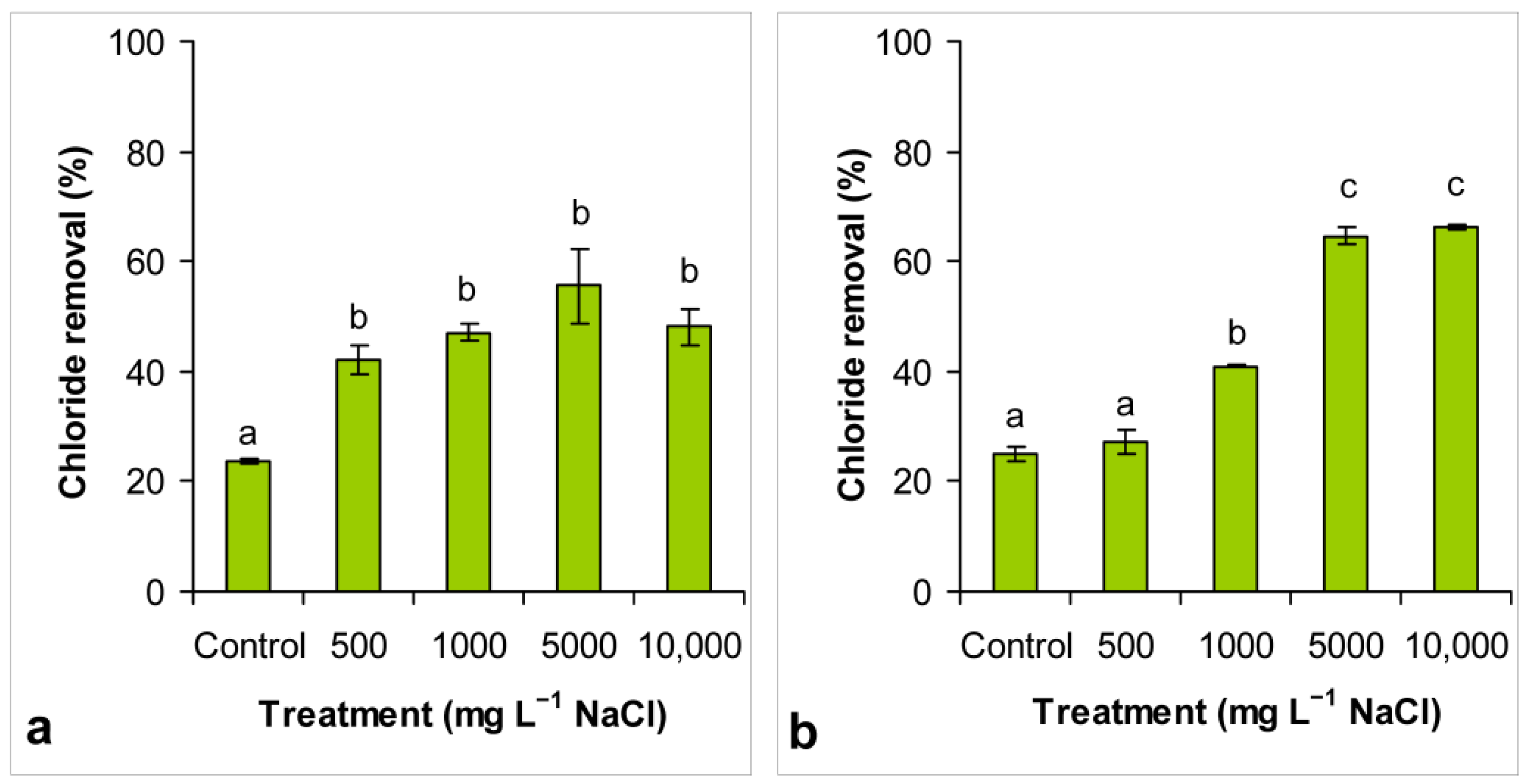
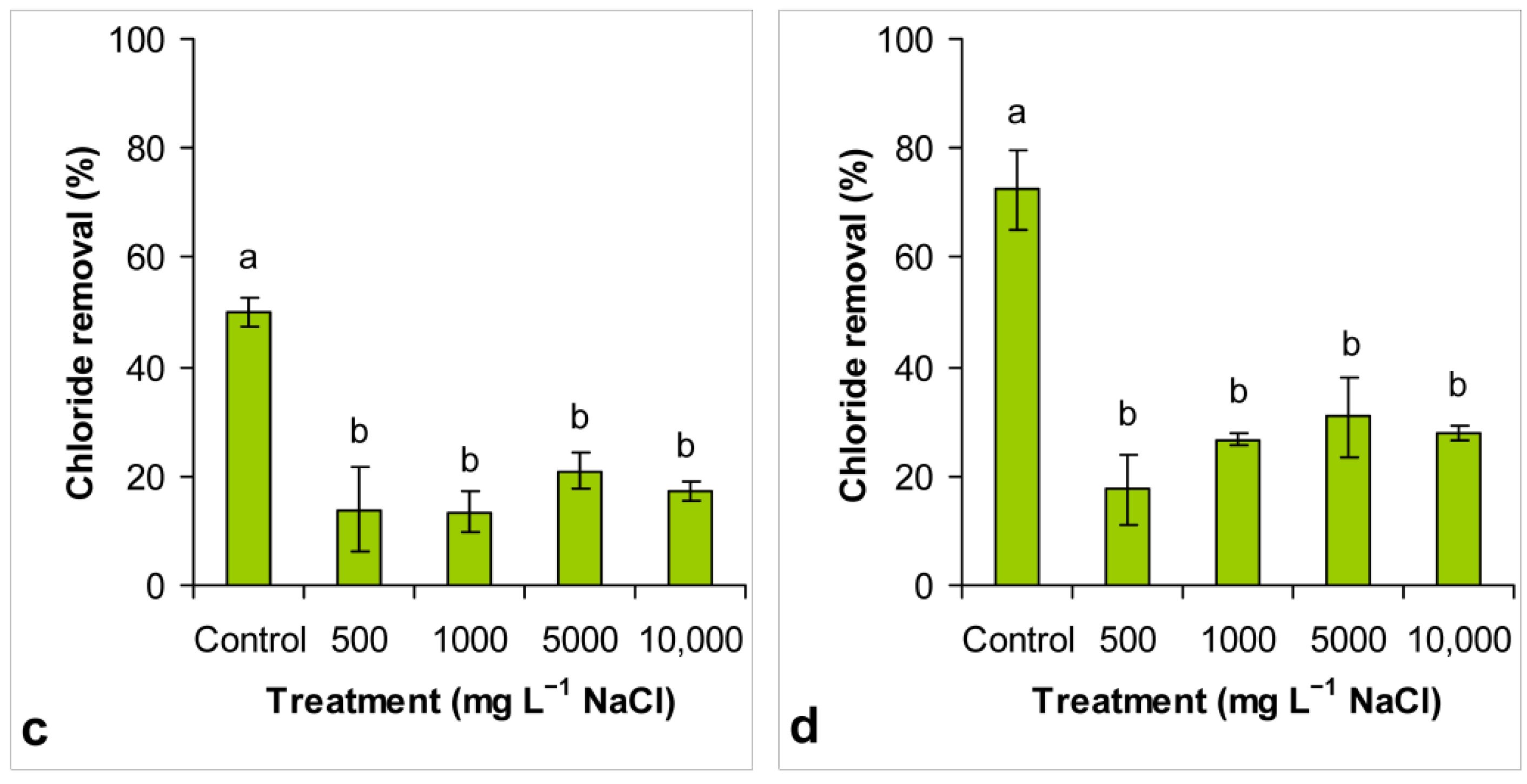
3.4. Chloride Removal
4. Discussion
4.1. Growth and Salt Tolerance
4.2. Nutrient Removal
4.2.1. Nitrate
4.2.2. Phosphate
4.3. Conductivity Reduction and Chloride Removal
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, A.C.; Withers, P.J.A. Linking phosphorus sources to impacts in different types of water body. Soil Use Manag. 2007, 23, 133–143. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi. J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Suárez-Muñoz, M.; Trebuch, L.M.; Verbraak, P.J.; Van de Waal, D.B. Toward an ecologically optimized N:P recovery from wastewater by microalgae. Front. Microbiol. 2017, 8, 1742. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Gunjyal, N.; Ojha, C.S.P.; Asce, F.; Singh, R.P. Review of challenges for algae-based wastewater treatment: Strain selection, wastewater characteristics, abiotic, and biotic factors. J. Hazard. Toxic Radioact. Waste 2021, 25, 03120004. [Google Scholar] [CrossRef]
- Ebrahimian, A.; Kariminia, H.R.; Vosoughi, M. Lipid production in mixotrophic cultivation of Chlorella vulgaris in a mixture of primary and secondary municipal wastewater. Renew. Energy 2014, 71, 502–508. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Chen, P.; Ruan, R. Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour. Technol. 2011, 102, 6909–6919. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.J. Micro-algae and waste-water treatment. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 305–328. [Google Scholar]
- Muñoz, R.; Jacinto, M.; Guieysse, B.; Mattiasson, B. Combined carbon and nitrogen removal from acetonitrile using algal–bacterial bioreactors. Appl. Microbiol. Biotechnol. 2005, 67, 699–707. [Google Scholar] [CrossRef]
- Perales-Vela, H.V.; Peña-Castro, J.M.; Cañizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Kang, D.; Wu, C.; Wu, Y. Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 717–735. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.; Li, W.; Rahaman, M.H.; Zhao, Y.; Wei, B.; Wei, H. Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol. Eng. 2017, 108, 83–92. [Google Scholar] [CrossRef]
- Singh, P.; Jain, K.; Desai, C.; Tiwari, O.; Madamwar, D. Microbial community dynamics of extremophiles/extreme environment. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 323–332. [Google Scholar]
- Ventosa, A.; de la Haba, R.R.; Sánchez-Porro, C.; Papke, R.T. Microbial diversity of hypersaline environments: A metagenomic approach. Curr. Opin. Microbiol. 2015, 25, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The influence of organisms on the composition of seawater. In The Sea, 2nd ed.; Hill, M.H., Ed.; Wiley: Hoboken, NJ, USA, 1963. [Google Scholar]
- Martiny, A.C.; Vrugt, J.A.; Lomas, M.W. Concentrations and ratios of particulate organic carbon, nitrogen, and phosphorus in the global ocean. Sci. Data 2014, 1, 140048. [Google Scholar] [CrossRef]
- Schreurs, H. Cyanobacterial Dominance, Relation to Eutrophication and Lake Morphology. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1992. [Google Scholar]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Influence of the N:P supply ratioon biomass productivity and time-resolved changes in elemental and bulk biochemical composition of Nannochloropsis sp. Bioresour. Technol. 2014, 169, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Rasdi, N.W.; Qin, J. Effect of N:P ratio on growth and chemical composition of Nannochloropsis oculata and Tisochrysis lutea. J. Appl. Psychol. 2015, 27, 2221–2230. [Google Scholar] [CrossRef]
- Figler, A.; B-Béres, V.; Dobronoki, D.; Márton, K.; Nagy, S.A.; Bácsi, I. Salt tolerance and desalination abilities of nine common green microalgae isolates. Water 2019, 11, 2527. [Google Scholar] [CrossRef]
- Valdez-Ojeda, R.; Gonzalez-Munoz, M.; Us-Vazquez, R.; Narvaez-Zapata, J.; Chavarria-Hernandez, J.C.; Lopez-Adrian, S.; Barahona-Perez, F.; Toledano-Thompson, T.; Garduno-Solorzano, G.; Medrano, R.M.E.G. Characterization of five fresh water microalgae with potential for biodiesel production. Algal Res. 2014, 7, 33–44. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhao, Y.; Liu, Z.Y.; Liu, C.F.; Hu, Z.P.; Hou, Y.Y. A Mathematical Model of Neutral Lipid Content in terms of Initial Nitrogen Concentration and Validation in Coelastrum sp. HA-1 and Application in Chlorella sorokiniana. BioMed Res. Int. 2017, 9253020. Available online: https://www.hindawi.com/journals/bmri/2017/9253020/ (accessed on 8 April 2021). [CrossRef]
- Úbeda, B.; Galvez, J.A.; Michel, M.; Bartual, A. Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour. Technol. 2016, 228, 210–217. [Google Scholar] [CrossRef]
- Mousavi, S.; Najafpour, G.D.; Mohammadi, M.; Seifi, M.H. Cultivation of newly isolated microalgae Coelastrum sp. in wastewater for simultaneous CO2 fixation, lipid production and wastewater treatment. Bioprocess Biosyst. Eng. 2018, 41, 519–530. [Google Scholar] [CrossRef]
- Rauytanapanit, M.; Janchot, K.; Kusolkumbot, P.; Sirisattha, S.; Waditee-Sirisattha, R.; Praneenararat, T. Nutrient Deprivation-Associated Changes in Green Microalga Coelastrum sp. TISTR 9501RE Enhanced Potent Antioxidant Carotenoids. Mar. Drugs 2019, 17, 328. [Google Scholar] [CrossRef]
- Valdez-Ojeda, R.A.; Serrano-Vazquez, M.G.D.; Toledano-Thompson, T.; Chavarria-Hernandez, J.C.; Barahona-Perez, L.F. Effect of media composition and culture time on the lipid profile of the green microalga Coelastrum sp. and its suitability for Biofuel Production. Bioenergy Res. 2021, 14, 241–253. [Google Scholar] [CrossRef]
- Tharek, A.; Yahya, A.; Salleh, M.M.; Jamaluddin, H.; Yoshizaki, S.; Dolah, R.; Hara, H.; Iwamoto, K.; Mohamad, S.E. Improvement of Astaxanthin Production in Coelastrum sp. by Optimization Using Taguchi Method. Appl. Food. Biotechnol. 2020, 7, 205–214. [Google Scholar]
- CCAP Media Recipes a. Available online: https://www.ccap.ac.uk/media/documents/BB.pdf (accessed on 5 February 2021).
- CCAP Media Recipes b. Available online: https://www.ccap.ac.uk/media/documents/JM.pdf (accessed on 5 February 2021).
- European Standard EN 15204: Water Quality—Guidance Sandard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermöhl Technique). Available online: https://standards.iteh.ai/catalog/standards/cen/d9020a71-2bd3-478f-867f-46ea3a0c0620/en-15204-2006 (accessed on 24 May 2019).
- Hungarian Standard MSZ 1484-13: 2009. Water Quality. Part 13: Determination of Nitrate and Nitrite Content by Spectrophotometric Method. Available online: http://www.mszt.hu/web/guest/webaruhaz (accessed on 24 May 2019).
- Hungarian Standard MSZ EN ISO 6878: 2004. Water Quality. Determination of Phosphorus. Ammonium Molybdate Spectrometric Method (ISO 6878:2004). Available online: https://www.iso.org/standard/36917.html (accessed on 24 May 2019).
- Németh, J. Methods of Biological Water Qualification; Institute of Environmental Management, Environmental Protection Information Service: Budapest, Hungary, 1998. [Google Scholar]
- Zar, H. Biostatistical Analysis, 3rd ed.; Prentice-Hall International: Hoboken, NJ, USA, 1996. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 2001, 9. [Google Scholar]
- Shriwastav, A.; Gupta, S.K.; Ansari, F.A.; Rawat, I.; Bux, B. Adaptability of growth and nutrient uptake potential of Chlorella sorokiniana with variable nutrient loading. Bioresour. Technol. 2014, 174, 60–66. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Jia, R. The Optimum Resource Ratio (N:P) for the Growth of Microcystis aeruginosa with Abundant Nutrients. Procedia Environ. Sci. 2011, 10, 2134–2140. [Google Scholar] [CrossRef]
- Terry, K.L. Nitrate and phosphate uptake interactions in a marine prymnesiophyte. J. Phycol. 1982, 18, 79–86. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Mereta, S.T.; Feyisa, F.F. Simultaneous removal of nitrate and phosphate from wastewater using solid waste from factory. Appl. Water Sci. 2019, 9, 28. [Google Scholar] [CrossRef]
- Park, M.H.; Park, C.H.; Sim, Y.B.; Hwang, S.J. Response of Scenedesmus quadricauda (Chlorophyceae) to Salt Stress Considering Nutrient Enrichment and Intracellular Proline Accumulation. Int. J. Environ. Res. Public Health 2020, 17, 3624. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.K.; Dash, R.C.; Panda, S.S.; Mishra, R.K.; Mohanty, R.C. Effects of nutrients at different salinities on growth of the freshwater green alga Scenedesmus bijugatus in water of Narendra Pond, Puri, Orissa. Int. Rev. Hvdrobiol. 1998, 83, 297–304. [Google Scholar] [CrossRef]
- Rai, A.K.; Abraham, G. Relationship of combined nitrogen sources to salt tolerance in freshwater cyanobacterium Anabaena doliolum. J. Appl. Microbiol. 1995, 78, 501–506. [Google Scholar]
- Collos, Y.; Vaquer, A.; Souchu, P. Acclimation of nitrate uptake by phytoplankton to high substrate levels. J. Phycol. 2005, 41, 466–478. [Google Scholar] [CrossRef]
- Liu, J.; Vyverman, W. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Alketife, A.M.; Judd, S.; Znad, H. Synergistic effects and optimization of nitrogen and phosphorus concentrations on the growth and nutrient uptake of a freshwater Chlorella vulgaris. Environ. Technol. 2017, 38, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Laurens, L.M.L.; Sweeney, N.; Pruthi, V.; Poluri, K.M.; Pienkos, P.T. Elucidating the unique physiological responses of halotolerant Scenedesmus sp. cultivated in sea water for biofuel production. Algal Res. 2019, 37, 260–268. [Google Scholar] [CrossRef]
- Lagus, A.; Suomela, J.; Weithoff, G.; Heikkila, K.; Helminen, H.; Sipura, J. Species-specific differences in phytoplankton responses to N and P enrichments and the N:P ratio in the Archipelago Sea, northern Baltic Sea. J. Plankton Res. 2004, 26, 779–798. [Google Scholar] [CrossRef]
- Ruzhitskaya, O.; Gogina, E. Methods for Removing of Phosphates from Wastewater. MATEC Web Conf. 2017, 106, 07006. [Google Scholar] [CrossRef]
- Kirst, G.O. Salinity tolerance of eukaryotic marine algae. Annu. Rev. Plant Physiol. 1989, 41, 21–53. [Google Scholar] [CrossRef]
- Raven, J.A. Chloride: Essential micronutrient and multifunctional beneficial ion. J. Exp. Bot. 2017, 68, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Sahle-Demessie, E.; Aly Hassan, A.; El Badawy, A. Bio-desalination of brackish and seawater using halophytic algae. Desalination 2019, 465, 104–113. [Google Scholar] [CrossRef] [PubMed]


| Medium | Nitrogen | Phosphorous | N:P Ratio (Molar) | ||
|---|---|---|---|---|---|
| mg L−1 | mmol | mg L−1 | mmol | ||
| HC-LR (BBM) | 41.06 | 2.93 | 53 | 1.7 | 1.7 |
| HC-HR (modified BBM) | 41.06 | 2.93 | 15.5 | 0.5 | 5.8 |
| LC-HR (JM) | 15.64 | 1.1 | 5.94 | 0.19 | 5.8 |
| LC-LR (modified JM) | 15.64 | 1.1 | 19.7 | 0.635 | 1.7 |
| NaCl Treatment | Medium (mL) | Alga Inoculum (mL) | dH2O (μL) | 300 g L−1 NaCl Stock Solution (μL) |
|---|---|---|---|---|
| Control | 41.7 | 5 | 3300 | 0 |
| 500 mg L−1 | 41.7 | 5 | 3215 | 85 |
| 1000 mg L−1 | 41.7 | 5 | 3135 | 165 |
| 5000 mg L−1 | 41.7 | 5 | 2475 | 825 |
| 10,000 mg L−1 | 41.7 | 5 | 1650 | 1650 |
| Medium | EC50 (mg L−1 NaCl) | ||
|---|---|---|---|
| Day 4 | Day 7 | Day 14 | |
| HC-LR | n.c. | n.c. | 6720 |
| HC-HR | 3870 | 2790 | 5430 |
| LC-HR | 5300 | 2970 | 1000 |
| LC-LR | 2010 | 1620 | 1860 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figler, A.; Márton, K.; B-Béres, V.; Bácsi, I. Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal and Desalination Properties of the Green Alga Coelastrum morus on a Laboratory Scale. Energies 2021, 14, 2112. https://doi.org/10.3390/en14082112
Figler A, Márton K, B-Béres V, Bácsi I. Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal and Desalination Properties of the Green Alga Coelastrum morus on a Laboratory Scale. Energies. 2021; 14(8):2112. https://doi.org/10.3390/en14082112
Chicago/Turabian StyleFigler, Aida, Kamilla Márton, Viktória B-Béres, and István Bácsi. 2021. "Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal and Desalination Properties of the Green Alga Coelastrum morus on a Laboratory Scale" Energies 14, no. 8: 2112. https://doi.org/10.3390/en14082112
APA StyleFigler, A., Márton, K., B-Béres, V., & Bácsi, I. (2021). Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal and Desalination Properties of the Green Alga Coelastrum morus on a Laboratory Scale. Energies, 14(8), 2112. https://doi.org/10.3390/en14082112





