Self-Sustaining Bioelectrochemical Cell from Fungal Degradation of Lignin-Rich Agrowaste
Abstract
1. Introduction
2. Materials and Methods
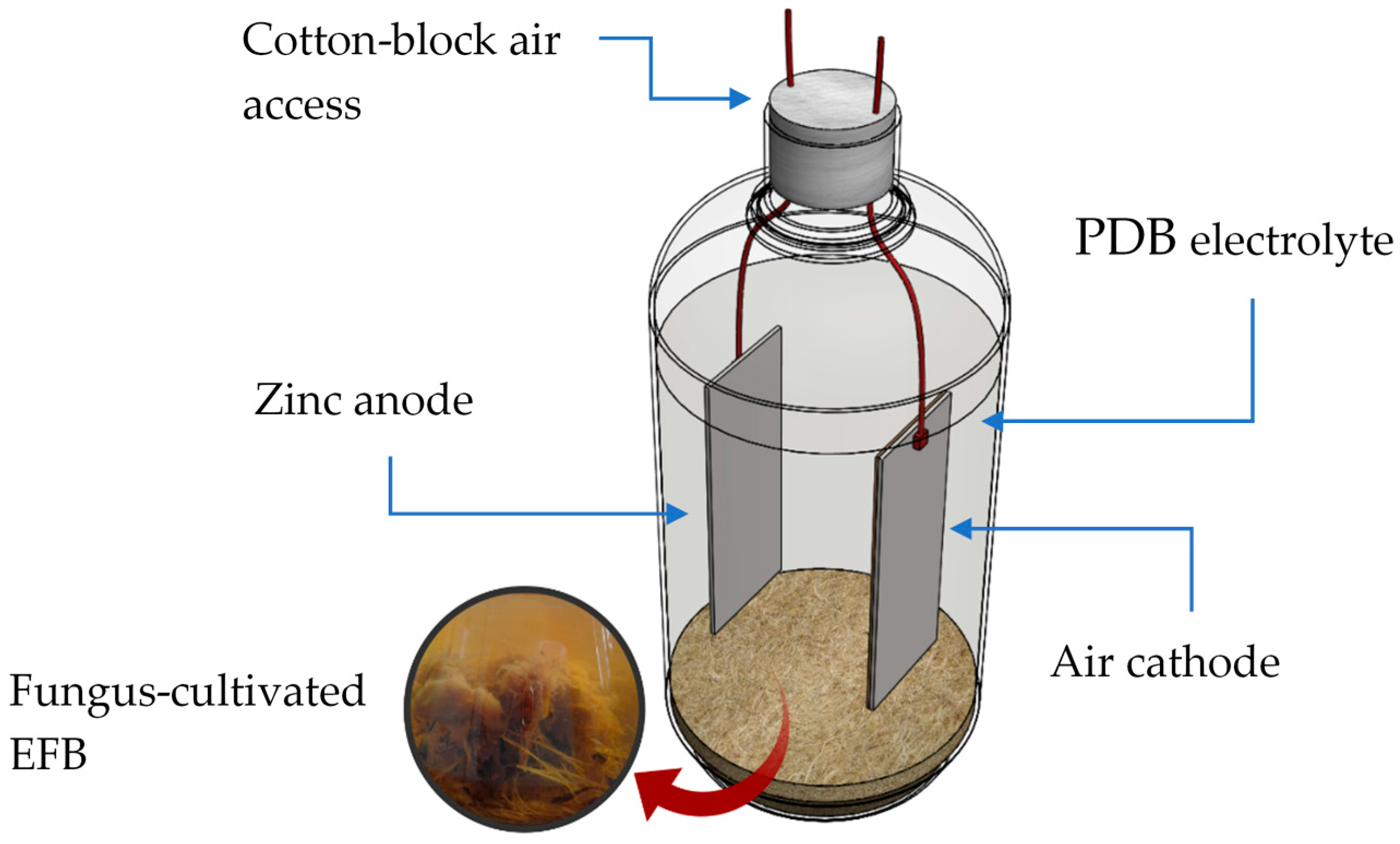
2.1. Cell Components and Design
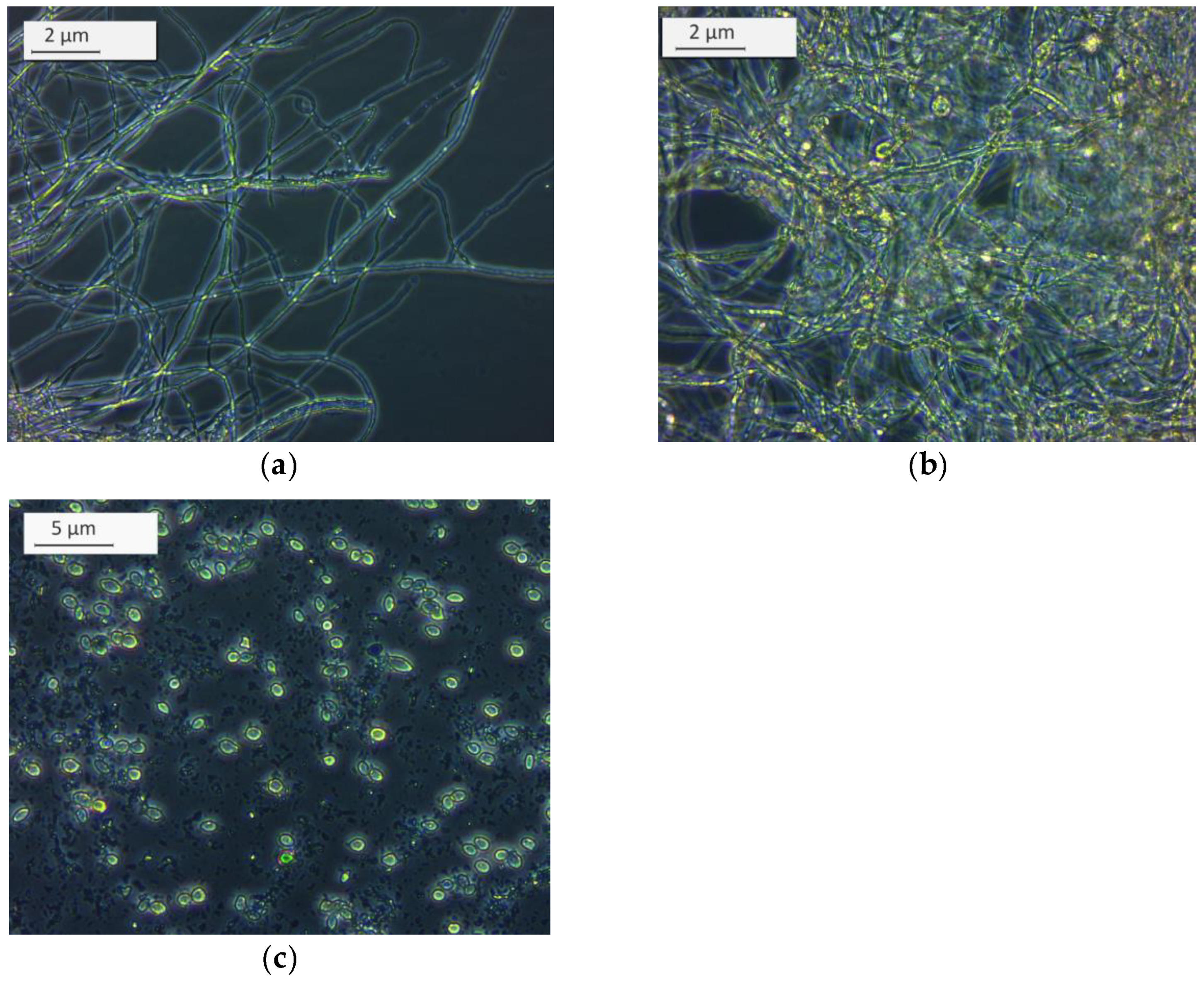
2.2. Microbes and Organic Substrates
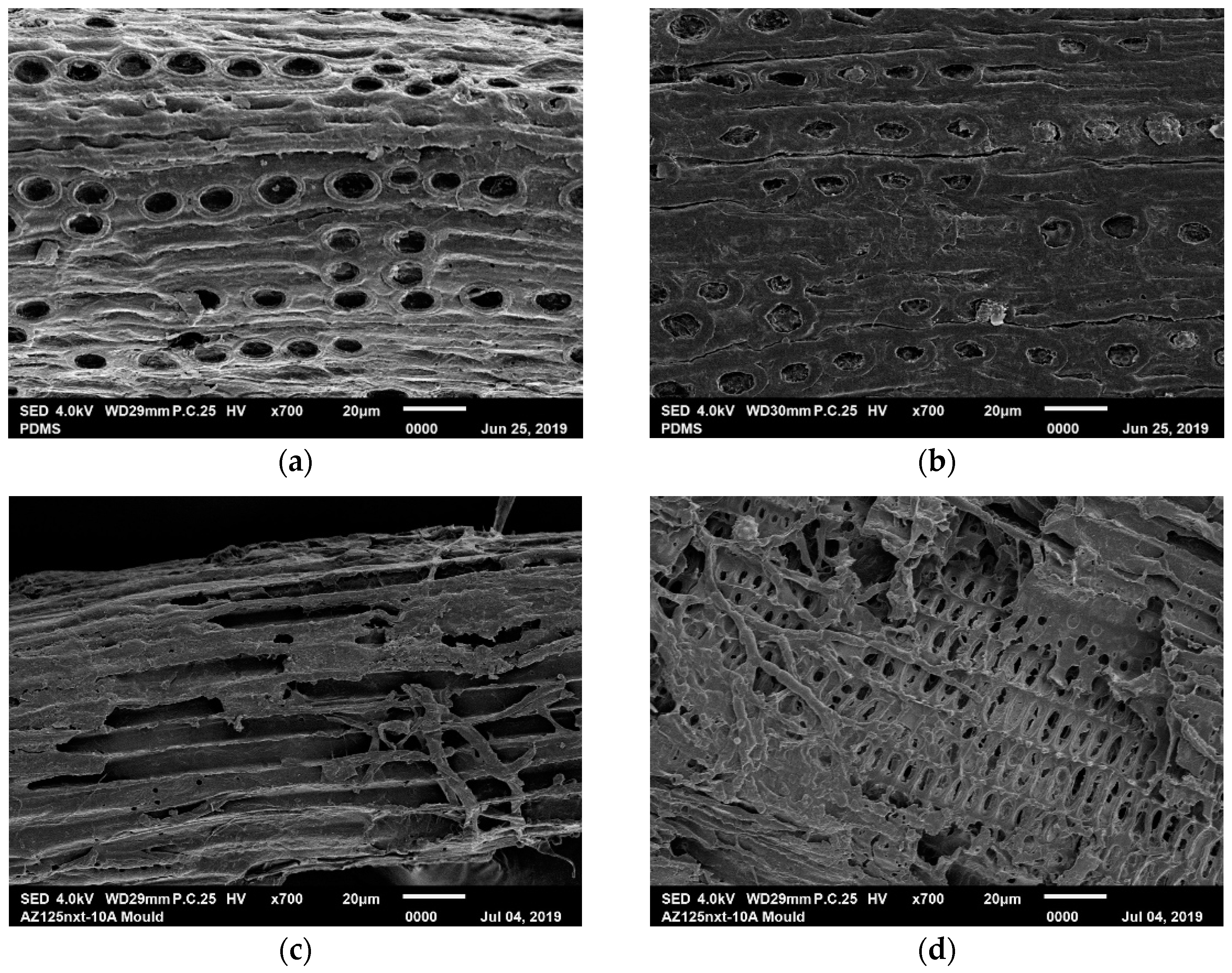
2.3. MFC and Specimen Characterisations
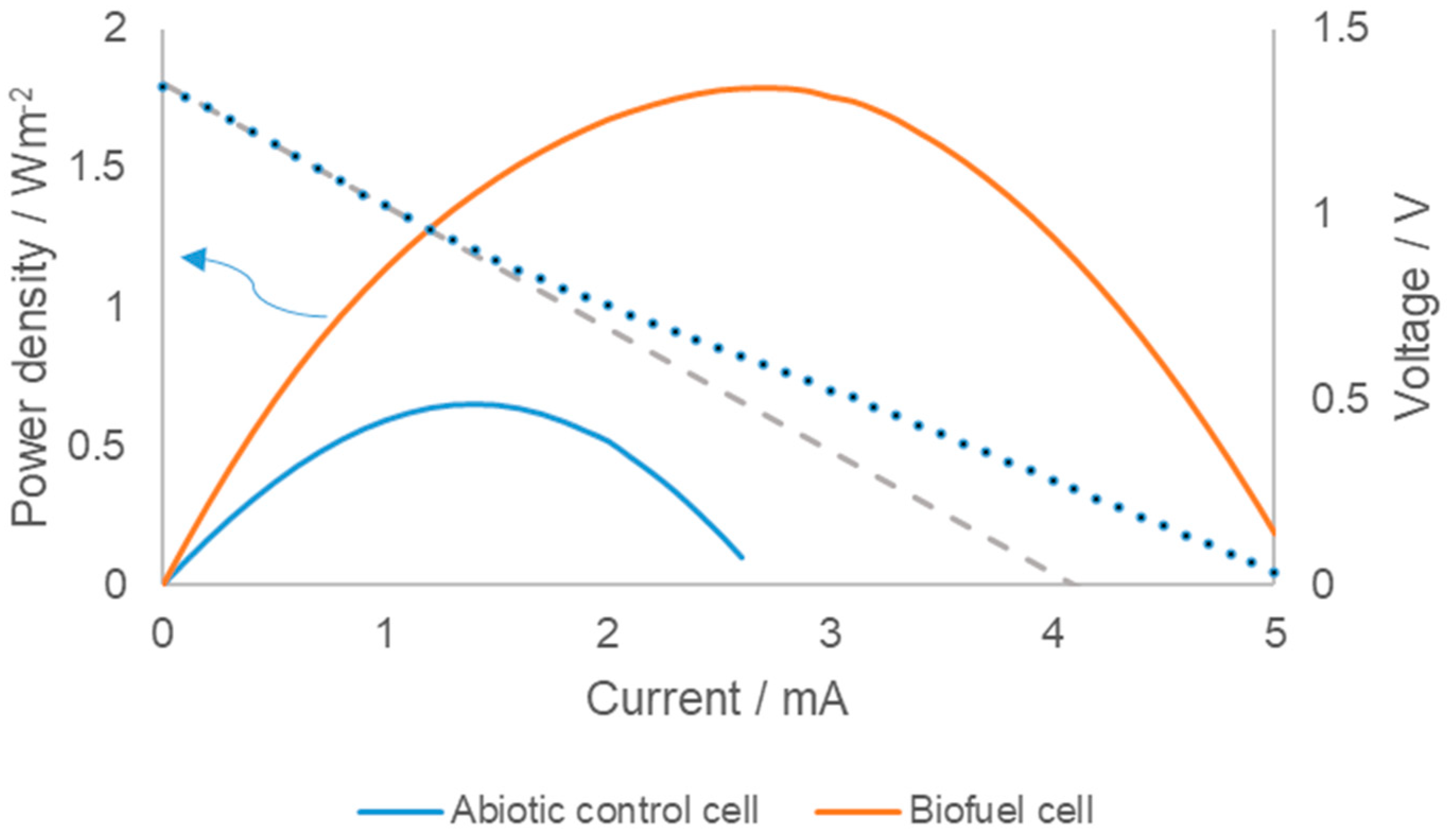
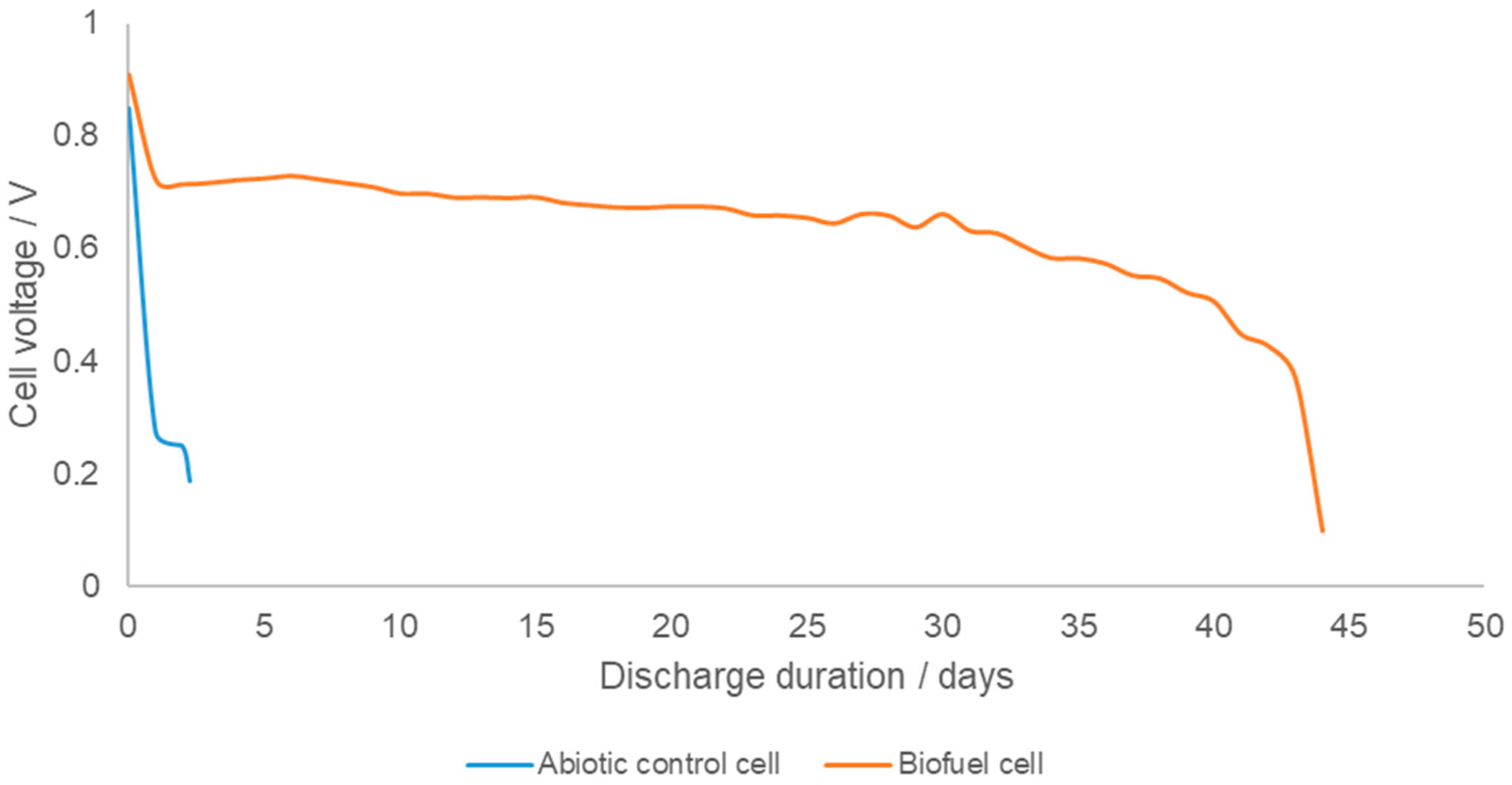
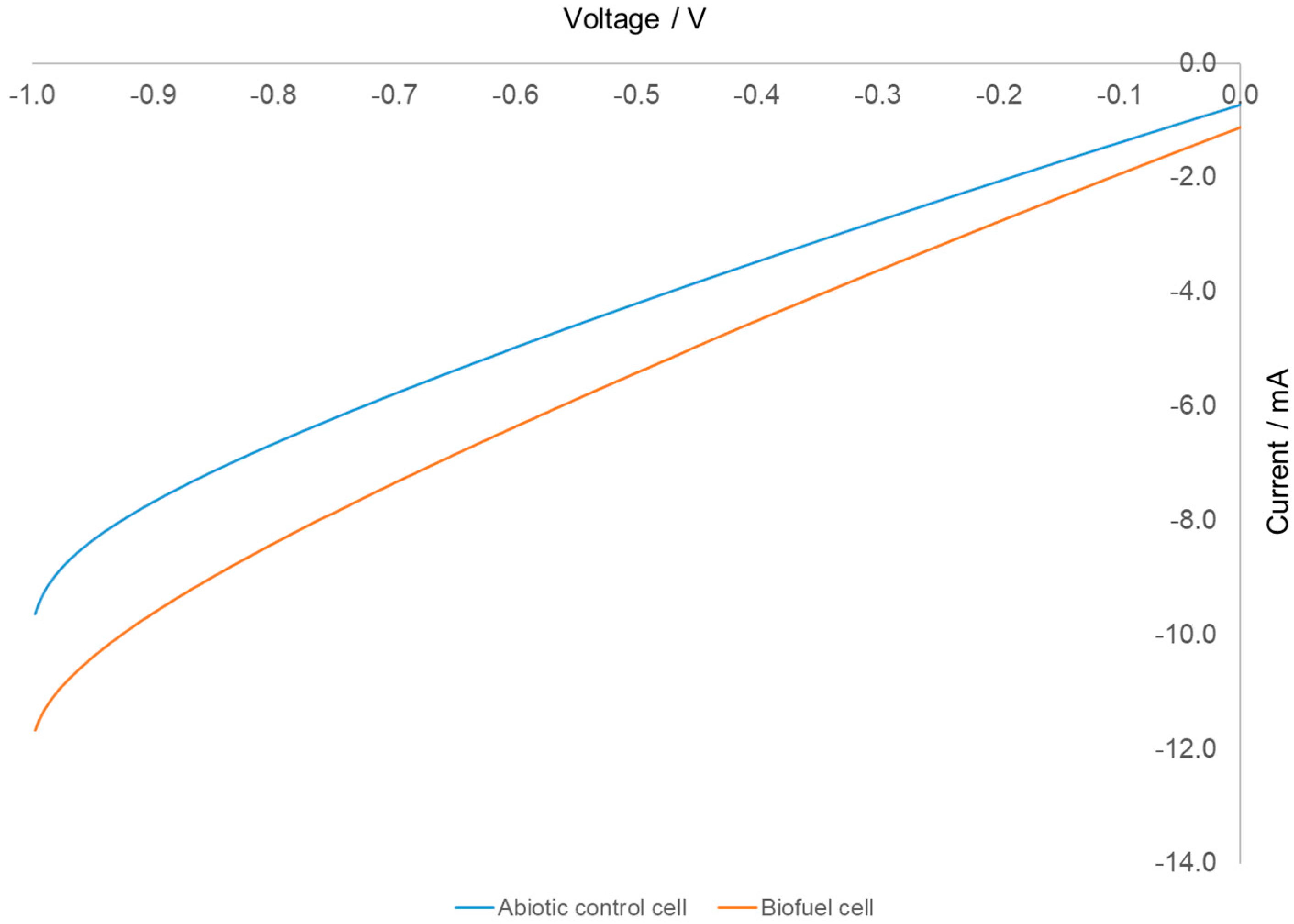
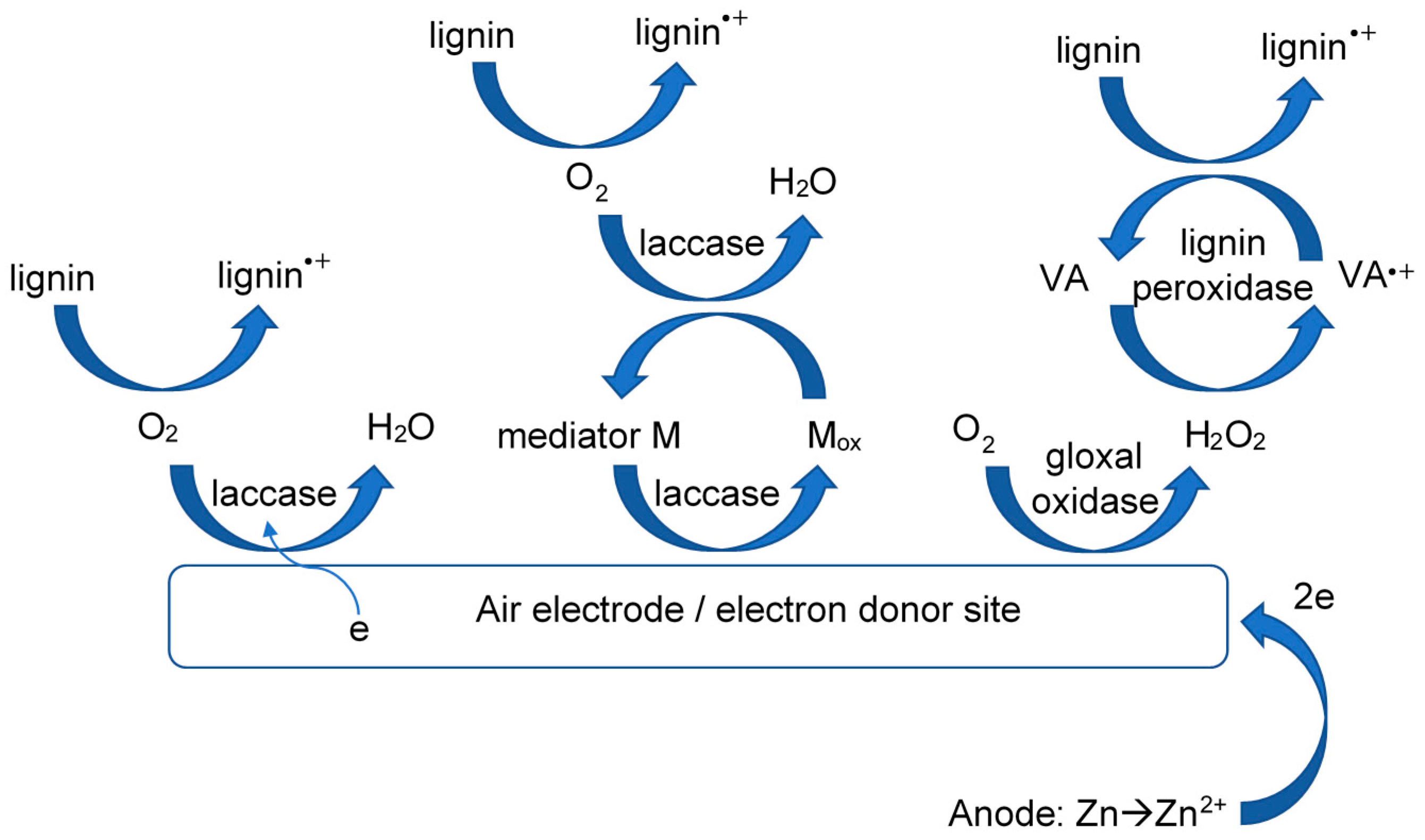
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Choi, H.S.; Yang, X.; Kim, D.S.; Yang, J.H.; Han, S.O.; Park, C.; Kim, S.W. Power generation from cheese whey using enzymatic fuel cell. J. Clean. Prod. 2020, 254, 120181. [Google Scholar] [CrossRef]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yan, M.; Li, H.; Zhang, L.; Peng, B.; Sun, J.; Liu, D.; Liu, S. FeS2 Nanoparticles Decorated Graphene as Microbial-Fuel-Cell Anode Achieving High Power Density. Adv. Mater. 2018, 30, 1–7. [Google Scholar]
- Sharma, T.; Mohana Reddy, A.L.; Chandra, T.S.; Ramaprabhu, S. Development of carbon nanotubes and nanofluids based microbial fuel cell. Int. J. Hydrogen Energy 2008, 33, 6749–6754. [Google Scholar] [CrossRef]
- Nasar, A.; Perveen, R. Applications of enzymatic biofuel cells in bioelectronic devices—A review. Int. J. Hydrogen Energy 2019, 44, 15287–15312. [Google Scholar] [CrossRef]
- O’Loughlin, E.J. Effects of electron transfer mediators on the bioreduction of lepidocrocite (γ-FeOOH) by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2008, 42, 6876–6882. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chang, Q.; Gao, Y.; Huang, W.; Sun, Z.; Yan, M.; Guo, C. High performance of microbial fuel cell afforded by metallic tungsten carbide decorated carbon cloth anode. Electrochim. Acta 2020, 330, 135243. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Paramjeet, S.; Manasa, P.; Korrapati, N. Biofuels: Production of fungal-mediated ligninolytic enzymes and the modes of bioprocesses utilizing agro-based residues. Biocatal. Agric. Biotechnol. 2018, 14, 57–71. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Alam, M.Z.; Kabbashi, N.A.; Ahsan, A. Effective composting of oil palm industrial waste by filamentous fungi: A review. Resour. Conserv. Recycl. 2012, 58, 69–78. [Google Scholar] [CrossRef]
- Singh, D.; Chen, S. The white-rot fungus Phanerochaete chrysosporium: Conditions for the production of lignin-degrading enzymes. Appl. Microbiol. Biotechnol. 2008, 81, 399–417. [Google Scholar] [CrossRef]
- Lovley, D.R.; Fraga, J.L.; Blunt-Harris, E.L.; Hayes, L.A.; Phillips, E.J.P.; Coates, J.D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim. Hydrobiol. 1998, 26, 152–157. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.H.; Shah, A.A.; Walsh, F.C. Recent progress and continuing challenges in bio-fuel cells. Part I: Enzymatic cells. Biosens. Bioelectron. 2011, 26, 3087–3102. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera De Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Fact. 2019, 18, 1–33. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Huang, L.; Logan, B.E. Scale-up of membrane-free single-chamber microbial fuel cells. J. Power Sources 2008, 179, 274–279. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ortiz, J.; Flores, R.; Vazquez-Duhalt, R. Molecular design of laccase cathode for direct electron transfer in a biofuel cell. Biosens. Bioelectron. 2011, 26, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Smolander, M.; Boer, H.; Valkiainen, M.; Roozeman, R.; Bergelin, M.; Eriksson, J.E.; Zhang, X.C.; Koivula, A.; Viikari, L. Development of a printable laccase-based biocathode for fuel cell applications. Enzym. Microb. Technol. 2008, 43, 93–102. [Google Scholar] [CrossRef]
- Yun, S.H.; Woo, J.J.; Seo, S.J.; Yang, T.H.; Moon, S.H. Estimation of approximate activation energy loss and mass transfer coefficient from a polarization curve of a polymer electrolyte fuel cell. Korean J. Chem. Eng. 2012, 29, 1158–1162. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Zhou, X.; Liang, P.; Zhang, X.; Jiang, Y.; Huang, X. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res. 2016, 98, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Wang, K.; Lv, S.; Wang, X.; Zhuo, L.; Zhang, J. Effects of concentration variations on the performance and microbial community in microbial fuel cell using swine wastewater. Energies 2020, 13, 2231. [Google Scholar] [CrossRef]
- Das, M.; Barbora, L.; Das, P.; Goswami, P. Biofuel cell for generating power from methanol substrate using alcohol oxidase bioanode and air-breathed laccase biocathode. Biosens. Bioelectron. 2014, 59, 184–191. [Google Scholar] [CrossRef]
- Clauwaert, P.; Van Der Ha, D.; Boon, N.; Verbeken, K.; Verhaege, M.; Rabaey, K.; Verstraete, W. Open air biocathode enables effective electricity generation with microbial fuel cells. Environ. Sci. Technol. 2007, 41, 7564–7569. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, X.W.; Li, W.W.; Sheng, G.P.; Zang, G.L.; Cheng, Y.Y.; Shen, N.; Yang, Y.P.; Yu, H.Q. A white-rot fungus is used as a biocathode to improve electricity production of a microbial fuel cell. Appl. Energy 2012, 98, 594–596. [Google Scholar] [CrossRef]
- CCME (Canadian Council of Ministers of the Environment). Canadian Water Quality Guidelines for the Protection of Aquatic Life—Dissolved Oxygen (Freshwater); CCME: Winnipeg, MB, Canada, 1999; Excerpt from Publication No. 1299; ISBN 1-896997-34-1. [Google Scholar]
- Ramaswamy, N.; Mukerjee, S. Fundamental mechanistic understanding of electrocatalysis of oxygen reduction on Pt and non-Pt surfaces: Acid versus alkaline media. Adv. Phys. Chem. 2012, 2012, 491604. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Othman, R.; Yusof, F.; Wahab, M.F.A. A hybrid enzymatic zinc-air fuel cell. Sains Malays. 2014, 43, 459–465. [Google Scholar]
- Ahmad, A.A.; Othman, R.; Yusof, F.; Wahab, M.F.A. Zinc-Laccase Biofuel Cell. IIUM Eng. J. 2011, 12, 153–160. [Google Scholar] [CrossRef][Green Version]
- Cho, N.; Leonowicz, A.; Rogalski, J.; Wesenberg, D.; Luterek, J.; Hofrichter, M.; Matuszewska, A.; Wilkolazka, A. Fungal laccase: Properties and activity on lignin. J. Basic Microbiol. 2001, 41, 185–227. [Google Scholar]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar]
- Datta, A.; Bettermann, A.; Kirk, T.K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Appl. Environ. Microbiol. 1991, 57, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Faison, B.D.; Kirk, T.K.; Farrell, R.L. Role of veratryl alcohol in regulating ligninase activity in Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1986, 52, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Sekrecka-Belniak, A.; Toczyłowska-Maminska, R. Fungi-based microbial fuel cells. Energies 2018, 11, 2827. [Google Scholar] [CrossRef]
- Goswami, R.; Mishra, V.K. A review of design, operational conditions and applications of microbial fuel cells. Biofuels 2018, 9, 203–220. [Google Scholar] [CrossRef]
- Lai, C.Y.; Wu, C.H.; Meng, C.T.; Lin, C.W. Decolorization of azo dye and generation of electricity by microbial fuel cell with laccase-producing white-rot fungus on cathode. Appl. Energy 2017, 188, 392–398. [Google Scholar] [CrossRef]
- Korcan, S.E.; Ciğerci, İ.H.; Konuk, M. White-Rot Fungi in Bioremediation. In Fungi as Bioremediators; Springer: Berlin/Heidelberg, Germany, 2013; pp. 371–390. [Google Scholar]
- Kapich, A.N.; Prior, B.A.; Botha, A.; Galkin, S.; Lundell, T.; Hatakka, A. Effect of lignocellulose-containing substrates on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enzym. Microb. Technol. 2004, 34, 187–195. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukri, A.; Othman, R.; Abd-Wahab, F.; M. Noor, N. Self-Sustaining Bioelectrochemical Cell from Fungal Degradation of Lignin-Rich Agrowaste. Energies 2021, 14, 2098. https://doi.org/10.3390/en14082098
Sukri A, Othman R, Abd-Wahab F, M. Noor N. Self-Sustaining Bioelectrochemical Cell from Fungal Degradation of Lignin-Rich Agrowaste. Energies. 2021; 14(8):2098. https://doi.org/10.3390/en14082098
Chicago/Turabian StyleSukri, Asiah, Raihan Othman, Firdaus Abd-Wahab, and Noraini M. Noor. 2021. "Self-Sustaining Bioelectrochemical Cell from Fungal Degradation of Lignin-Rich Agrowaste" Energies 14, no. 8: 2098. https://doi.org/10.3390/en14082098
APA StyleSukri, A., Othman, R., Abd-Wahab, F., & M. Noor, N. (2021). Self-Sustaining Bioelectrochemical Cell from Fungal Degradation of Lignin-Rich Agrowaste. Energies, 14(8), 2098. https://doi.org/10.3390/en14082098





