Abstract
The impact of attrition ball-mill pretreatment on food waste particle size, soluble chemical oxygen demand (SCOD), biochemical methane potential, and microbial community during anaerobic digestion was investigated based on milling speed and time. The uniformity of particle size improved with increasing milling speed and time. The SCOD of the pretreated samples increased to 4%, 7%, and 17% at the speeds of 150, 225, and 300 rpm, respectively, compared to the control. Milling time did not significantly change the SCOD. The cumulative methane productions of 430, 440, and 490 mL/g-VS were observed at the speeds of 150, 225, and 300 rpm, respectively, while the untreated sample exhibited the cumulative methane production of 390 mL/g-VS. Extended milling time did not improve methane production much. When the milling times of 10, 20, and 30 min were applied with the milling speed fixed at 300 rpm, the methane productions of 490, 510, and 500 mL/g-VS were observed respectively. Ball-mill pretreatment also increased the total volatile fatty acids. During the anaerobic digestion (AD) of ball-mill treated food waste, acetoclastic methanogens predominated, with a relative abundance of 48–49%. Interestingly, hydrogenotrophic methanogens were 1.6 times higher in the pretreated samples than those in the control. These results showed the potential of attrition ball milling as a food waste pretreatment for improving methane production.
1. Introduction
Globally, more than a billion tons of essential, nutritious, and life-supporting food is wasted every year [1]. Food waste is not only an economic waste, but also a direct cause of environmental pollution. In landfills, greenhouse gases, such as methane, are exhausted. The Waste and Resource Behavior Program estimated that the annual amount of greenhouse gases generated by landfilled food waste worldwide is approximately 3.3 billion tons, which accounts for 7% of the total global greenhouse gas emission [2]. In this situation, the problem of disposal is emerging because of strengthened regulations on landfills or marine dumping of food waste. Although a variety of regulations are being implemented to reduce the quantity of food waste generated, it is expected to increase by 2030 [3]. To resolve this issue of ever-increasing food waste, research on food waste recycling is being actively conducted by the scientific community.
Over the past decades, anaerobic digestion (AD) has been globally applied in both large and small scales to treat organic wastes because it can degrade the wastes and simultaneously produce energy (i.e., CH4) [4]. The methane produced from the anaerobic digestion plant can be used for generating heat, electricity, and transportation fuel [5,6]. It can also be used as a biochemical like methanol after reforming [7]. In fact, a variety of organic wastes have been used as a feedstock of AD; for example, municipal solid wastes including food waste, agricultural and animal wastes, sewage sludge, and slaughterhouse effluents [8,9,10,11]. Among the various wastes, food waste is an excellent raw material for AD process to produce biogas because of its organic content and wide availability [12,13,14]. AD process is composed of three steps: hydrolysis, acidogenesis, and methanogenesis. Hydrolysis is the rate determination step in the AD process, which is slower than the other steps. As the hydrolysis rate increases, the decomposition of organic matter is accelerated. Therefore, as hydrolysis rate accelerates, anaerobic digestibility increases, finally increasing biogas production. To accelerate the hydrolysis rate, food waste pretreatment is usually performed before AD is applied.
Pretreatment technologies applicable for biomass like food waste can be categorized into four types depending on their mode: biological, chemical, thermal, and mechanical. Biological pretreatment usually involves the use of specially designed microorganisms (e.g., Bacillus licheniformis) [15] or enzymes (e.g., mushroom compost extract) [16]. Often activated sludge is added to improve the AD of food waste [17]. In chemical pretreatment, alkalis (e.g., NaOH, KOH) or oxidants (e.g., ozone) are used [16,18,19]. Usually, alkali treatment is used with thermal or mechanical treatment [20]. Alkali pretreatment has been applied for pretreating pulp and paper sludge [21]. When it is applied for food waste, alkali pretreatment can increase soluble organics and AD of food waste. Ozone breaks down the cell wall and increases soluble chemical oxygen demand (SCOD), resulting in improved sludge digestion [22]. Thermo-oxidative pretreatment is a popular method and has been applied to municipal waste activated sludge [23]. Food waste pretreatments should improve surface area for enhancing microbial accessibility. Sometimes, food waste contains toxic (e.g., aflatoxins) [24] or hardly degradable compounds (e.g., lignin or cellulose rich compounds) [25], and thus pretreatments should reduce the effects of toxic compounds during AD. The composition of food waste varies depending on the region, which determines its characteristics. Therefore, an all-round pretreatment applicable for all types of food wastes is not possible. However, the mechanical reduction of particle size is the most important step for improving AD of food waste regardless of food waste composition [26]. Reducing the particle size of food waste increases its surface area and microbial accessibility. However, most pretreatments previously studied have been performed at lab-scale and are not applicable in scale-up processes because of their low treatment capacity and high operational cost [27,28].
Previously, we used an attrition ball-mill to pretreat lignocellulosic biomass to improve fermentable sugar production [29]. Attrition mill is a principle in which grinding balls are moved by rotating the impeller, and biomass is crushed by the impact and shear actions exerted by the grinding balls. Crushing and dispersion times are significantly faster than those of general ball mills. In particular, attrition-type mills can be rather easily scalable for industrial application [29]. The objective of this study was to investigate the effects of ball-mill pretreatment on food waste particle size, the content of soluble COD and total volatile fatty acids, and methane production. Moreover, to clarify the effects of the ball-mill on the AD of food waste, the microbial community was also analyzed. Ultimately, this study evaluated the feasibility of applying the ball-mill process for food waste pretreatment on an industrial scale.
2. Materials and Methods
2.1. Preparation of Food Waste and Seed Sludge
In order to objectively evaluate the effects of the ball-mill pretreatment, standard food waste was used in this study [30]. The composition of the standard food waste used in this study is presented in Table 1. Standard foods consisted of vegetables 50, fruits 18, fish meat 16, and cereals 16 wt%. The total solid (TS) content of the food waste samples was adjusted to 20 wt% by adding the appropriate amount of tap water. Vegetables, fish meat, and cereals were heated at 100 °C for 10 min in an autoclave (Daihan Scientific Co. Ltd., Seoul, Korea) before use, and fruits were used without heating. The food waste was ground for 30 s using a home blender.

Table 1.
Composition of food waste used in this study.
Digested sludge was used as an inoculum source and was obtained from a local wastewater treatment plant (WWTP) located in Seoul, Korea. This sludge was directly used without any further treatment and was stored at 4 °C prior to use.
2.2. Attrition Mill Pretreatment
Food waste was pretreated using an attrition-type ball-mill device (Korea Pulverizing Machinery, Incheon, Korea). The device had a 2.4 L inner jar where steel balls (Φ = 10 mm) crushed the food waste particles. Based on a previous study [31], 70% of the total volume of the jar was filled with food waste and grinding steel balls in equal volumes. The balls were forced to randomly move inside the jar by rotating the impeller. Rotation speed and milling time were set to 150, 225, and 300 rpm, at 10, 20 and 30 min, respectively. During the milling, the temperature inside the jar was not controlled. After crushing the food waste, it was transferred to a shaking sieve (Analysette3, Fritsch GmbH, Idar-Oberstein, Germany).
2.3. Biochemical Methane Potential (BMP) Test
The methane production potentials of each treatment were measured using an Automatic Methane Potential Test System (AMPTS II) (Bioprocess Control AB, Lund, Sweden). Inoculum and substrate were mixed at a ratio of 2.67 (inoculum/substrate). The mixture pH was adjusted to 7.0 ± 0.2 using 6 M NaOH and 1 M HCl. The mixture was then added to batch test bottles with 400 mL working volume and 100 mL headspace. The bottles were purged with N2 for 5 min at 1 bar to create anaerobic conditions. AD was performed at 37 °C with the stirring rate of 80 rpm. The blank contained only sewage sludge without food waste. The total amount of methane produced from a treatment was subtracted with that of the control to give the actual methane production of the treatment. All samples were processed in triplicates.
2.4. Analytical Methods
The particle size of the pretreated food waste was analyzed using a particle size analyzer (LA-960; Horiba, Kyoto, Japan). Total chemical oxygen demand (TCOD), SCOD, TS, and VS were measured as per the standard methods [32]. Volatile fatty acids (VFAs) were analyzed using a gas chromatograph (6890N, Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector and peak column (RESTEK stabilwax). Total volatile fatty acid (TVFA) was calculated using conversion factors of 1.07, 1.51, and 1.82 for acetic acid, propionic acid, and butyric acid [33]. Nitrogen was used as the carrier gas at 2 mL/min. Injector, oven, and detector temperatures were set at 250, 180, and 250 °C, respectively.
To analyze the changes in the microbial community, DNA was extracted from the samples using a PowerMax soil DNA isolation kit (Qiagen, Hilden, Germany). The extracted DNA was used as a template to amplify the V3–V4 region of the bacterial 16S rRNA gene using a primer set (341F and 805R) [34]. The specific primer set, Arch519F (5′-CAGCCGCCGCGGTAA-3′) and Arch934R (5′-GTGCTCCCCCGCCAATTC-3′), was used to detect the methanogen species [35]. Illumina library generation methods were subsequently used to generate DNA sequences [36] and conducted by ChunLab, Inc. (Seoul, Korea) using an Illumina/MiSeq platform (San Diego, CA, USA) following the protocol specified by the manufacturer. Sequence similarity was analyzed using the EzBioCloud server (www.ezbiocloud.net, accessed on 10 September 2020) for species level identification (97% cutoff).
3. Results and Discussion
Table 2 shows results of food waste and seed sludge characteristics. Food waste and seed sludge have a similar TCOD. However, seed sludge has a lower SCOD than the food waste. The VS/TS was 93.8 and 71.5% for food waste and seed sludge, respectively. The food waste contained more volatile matter compared to the seed sludge, indicating that the food waste was more biodegradable than the seed sludge.

Table 2.
Chemical characteristics of food waste and seed sludge.
3.1. Effects of Ball-Mill Pretreatment on Food Waste Properties
Attrition ball-milling mainly reduces the size of food waste and, subsequently, improves microbial accessibility. Table 3 presents the mean particle sizes of the food waste after ball milling. Two variables, i.e., milling speed and time, were used to investigate their effects on size reduction. The untreated food waste had a mean diameter of 834 μm. As the milling speed increased, the mean particle size decreased. Milling time is a critical parameter for reducing particle sizes. As the milling time was increased at 300 rpm, the mean diameter of the food waste was reduced. As the milling time was increased by 10 min, the particle size of the food waste was reduced by approximately 12~17%. As shown in Table 3, the smallest particle size was obtained when milling speed and time were 300 rpm and 30 min, respectively. However, BMP of the feedstock was not much improved by enhancing the milling speed and/or extending milling time, which is more discussed below.

Table 3.
Mean particle sizes of food waste depending on pretreatment conditions.
As expected, the uniformity of particle size improved with increasing milling speed and time. Figure 1 presents the distribution of the pretreated food waste sizes based on the milling conditions. Particle distribution of untreated food waste showed a bimodal curve, with the highest peak being observed at approximately 1754 µm. Interestingly, the highest peak in the particle size distribution occurred at the same size (260 µm) regardless of the rotational speed (Figure 1a). However, the peak that occurred at larger particle diameters gradually disappeared when the speed was raised. After the milling, the peak around 1754 µm reduced and shifted to 260 µm as rotation speed increased. At 300 rpm, the peak at 1754 µm completely disappeared. From this result, it was concluded that the food waste had become more homogenized by the ball-milling. Figure 1b shows the effects of milling time on the particle size distribution of food wastes; in this specific experiment, the milling speed was set at 300 rpm. Unlike the case of milling speed, the length of milling time did not result in much difference in the particle size distribution. All the milling times showed similar single modal profiles. It may be due to the milling speed applied for this specific experiment; for all the milling times, the same milling speed of 300 rpm was used. From this observation, it is hypothesized that food waste can be well homogenized with a short milling time if a sufficient milling speed is applied.
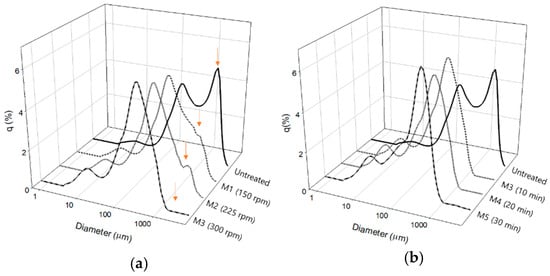
Figure 1.
Size distributions of pretreated food waste based on (a) milling speed and (b) milling time.
Ball-mill pretreatment also affected the SCOD. Untreated sample was found to have 19.1 g O2/L SCOD (Table 3). All pretreated samples exhibited an increased SCOD compared to the control. The SCOD of samples ball-milled at 150, 225, and 300 rpm showed 4%, 7%, and 17% higher values, respectively, comparing to that of the control. However, no significant difference was found in the SCOD values of the samples ball-milled at a fixed speed of 300 rpm for different milling times (i.e., 10, 20, and 30 min). It appeared that milling speed is more important than milling duration in the case of food waste.
3.2. Effect of Ball-Mill Pretreatment on the BMP of Food Waste
The ultimate purpose of pretreatment is to increase methane production by improving solubility of food waste and the microbe accessibility to it. Figure 2 shows the BMPs of food waste pretreated under the milling conditions. Samples without pretreatment exhibited a cumulative methane production of 390 mL/g-VS after 10-d incubation. As the case of SCOD, ball-mill pretreated food waste showed higher methane production than the control. The cumulative methane productions of 430, 440, and 490 mL/g-VS were observed for M1, M2, and M3, respectively (Figure 2a); methane productions of M1, M2, M3 were 8%, 10%, and 22%, respectively, higher than that of the control. The increased BMP values of the ball-milled food waste can be explained with their increased SCOD and particle-size uniformity after ball-milling, comparing to the control. Figure 2b presents the effect of milling time on the BMP of food waste. The milling times of 10, 20, and 30 min at 300 rpm resulted in methane productions of 490, 510, and 500 mL/g-VS, respectively. This result indicates that an extended milling-hour might not be necessary for food waste as long as a sufficient milling speed is applied. Nonetheless, the methane production could be increased by 27% through the ball-milling process (Figure 2b). This result demonstrates the applicability of the attrition ball-mill for improving the methane production from food waste. However, energy consumption and process capacity should be considered and optimized before applying on the industrial scale.
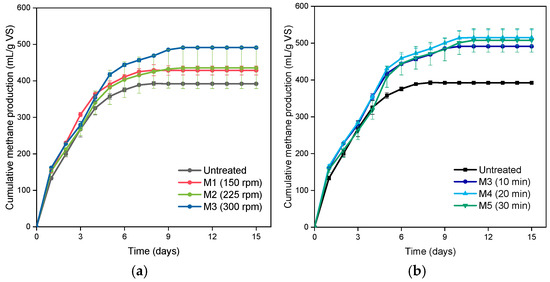
Figure 2.
Cumulative methane productions from the food waste based on (a) milling speed and (b) milling time. The error bars indicate standard deviation.
In this study, the maximum methane production was 510 mL/g-VS at 300 rpm for 20 min. From the methane production point of view, the ball-milling shows a superior performance than other physical pretreatment technologies. Zhang et al. [37] treated food waste with 600 W microwave to increase the temperature of food waste to 100 ℃ and to increase soluble COD. As a result, they obtain the methane production of 316 mL/g-VS (6.4% higher amount than the control). Naran et al. [21] also applied thermal pretreatment to improve BMP of food waste. The thermal condition of 120 °C for 30 min could result in 481 mL/g-VS methane production. They also applied the ultrasonic pretreatment for food waste. Application of 360 kJ/L ultrasonic wave for 30 min showed 424 mL/g-VS methane production.
Total volatile fatty acids (TVFAs) of food waste were also measured before and after pretreatments. After ball-mill pretreatment, the quantity of TVFAs increased, compared to the control (Table 4). This indicated that the ball-mill pretreatment used in this study would have a beneficial impact on hydrolysis of organics to VFAs by increasing particle size uniformity and SCOD. Typically, the TVFAs of ball-mill-treated food waste on day 1 were significantly higher than those of the untreated sample.

Table 4.
TVFAs of food waste based on pretreatment conditions (unit: mg COD/L).
3.3. Microbial Community Analysis
Microbial community was analyzed to understand the differences in the BMP after the attrition ball-mill pretreatment. The microbial composition was similar between the control and treatments. However, microbial abundances between the control and treatments showed an obvious difference at the family level (Figure 3). Clostridiaceae was found to be the predominant family with a relative abundance of 12.6% and 16.9% under the untreated and ball-mill pretreated conditions, respectively, after 1 d of inoculation. Clostridiaceae was reported to be able to anaerobically grow by utilizing solubilized carbohydrates and produce VFAs, such as acetate, propionate, and butyrate [38]. These VFAs may become the substrate of acetoclastic methanogen [39,40]. When Clostridiaceae was scrutinized at the species level, the major species were found to be Clostridium butyrium, C. chromireducens, C. tertium, C. perfrigens, and C. beijerinkii (Figure S1a). In particular, the relative abundance of Clostridium butyricum increased to 7.33% and 5.1% under the pretreated and untreated conditions, respectively, which indicated that ball-mill affected the proliferation of bacteria producing VFAs. As shown in Figure S1, ball pretreatment resulted in a higher TVFA production, which was consistent with the increase observed in the relative abundance of bacteria related to VFA production. On day 7 after inoculation, while the relative abundance of Clostridiaceae family significantly decreased, Anaerolinaceae was observed to be the predominant family, followed by Porphyromonadaceae, with a relative abundance of 18.2–18.8% under both conditions (Table S1). Anaerolinaceae can grow fermentatively from a recalcitrant carbon source and Porphyromonadaceae can grow even under non-fermentative conditions [41,42,43]. Therefore, they were considered to have grown from the remnants after the easy-to-use carbon sources were depleted. Porphyromonadaceae and Anaerolinaceae families have been reported to provide acetate for methanogen, therefore, they were suggested to have syntrophic cooperation with acetoclastic methanogen [44].
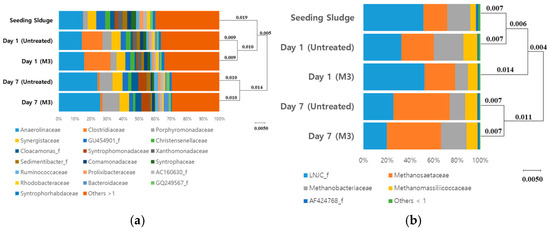
Figure 3.
Microbial community structure of (a) bacteria and (b) Achaea during methane production from ball-mill treated and untreated food wastes. Operational Taxonomic Units (OTUs) were classified at the species level (97% sequence similarity). The data shown were binned at the family level.
As observed on day 7, shown in Figure 3, the examination of archaeal community showed Methanosaetaceae as the predominant family. The relative abundance of Methanosaetaceae increased to 48–49% under both conditions (Figure 3). Methanosaeta concilii was the most dominant species observed under both conditions, with its relative abundances increased to 30.2% and 30.3%, respectively. In addition, considerable uncultured Methanosaeta species were observed, and these species also seemed to play a role as a methanogen using an acetoclastic mechanism [45]. Interestingly, the relative abundance of hydrogenotrophic methanogen was observed to be 13.5% in the untreated sample on day 7, whereas for the ball-mill treated sample, it increased up to 21.7% (Figure S2 and Table S1). Most hydrogenotrophic methanogens were composed of Methanobacterium species and this genus can produce methane mainly using carbon dioxide and hydrogen [46]. Carbon dioxide and hydrogen have been considered as the final metabolites of anaerobic bacteria, such as Clostridium, which was considered to increase the growth of hydrogenotrophic methanogen. Consequently, the ball-mill pretreatment increased the activity of methanogenic Archaea using the end-products of bacteria by allowing food waste to be readily available via softening and breaking processes.
4. Conclusions
To improve the AD of food waste, the effects of attrition mill were investigated in this study. The attrition ball-mill reduced the particle size and improved size uniformity and SCOD. Change in food waste characteristics increased the BMP of the food waste, maximally 27%. During AD, the attrition of milled food waste produced higher TVFAs. Microbial community led the ball-mill treatment to allow VFA-producing bacteria and hydrogenotrophic methanogens to proliferate with significantly abundant acetoclastic methanogen during the BMP test. Therefore, we found the attrition mill pretreatment to be effective in improving methane production from food waste. The energy required for the attrition ball-mill was not estimated in this study, since the focus of the current study was evaluating the effects of the attrition ball-milling on the BMP of food waste. In order for the technology to be implemented at full scale, the economic feasibility of the ball-milling will be investigated in future studies.
Supplementary Materials
The following information are available at https://www.mdpi.com/article/10.3390/en14082085/s1, Figure S1: Distribution of species belonging to genera Clostridium (A) and Anaerolinea (B) during the methane production from the food wastes treated and untreated with ball-mill. The data shown were binned at the species level, Figure S2: Comparisons of relative abundance (RA) based on the methane producing mechanism of the methanogen. LNJC_s: Function unknown. Hydrogenotrophic methanogen: Total relative abundances of hydrogenotrophic methanogen, including Methanobacterium, Methanobrevibacter, Methanolinea, and Methanosphaera; acetoclastic methanogen: total relative abundance of acetoclastic methanogen, including Methanosaeta spp. Methaosarcina spp., and Methanomethylovorans spp. Table S1: Comparison of the relative abundance of major microorganisms directly or indirectly affecting methane production.
Author Contributions
Conceptualization, J.H.L. and H.K.; methodology, Y.M.G., S.Y.P. and J.Y.P.; validation, B.-I.S.; formal analysis, Y.M.G., B.S.J. and J.H.L.; investigation, J.H.L. and B.S.J.; data curation, Y.M.G. and S.Y.P.; writing—original draft preparation, Y.M.G.; writing—review and editing, J.H.L., B.S.J. and H.K.; project administration, J.H.L.; funding acquisition, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grant from the Korea Institute of Energy Technology Evaluation and Planning (KETEP) (Grant #20173010092510).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Anaerobic digestion |
| TS | Total solid |
| WWTP | Waste water treatment plant |
| TCOD | Total chemical oxygen demand |
| SCOD | Soluble chemical oxygen demand |
| VS | Volatile solid |
| VFAs | Volatile fatty acids |
| MPS | Mean particle size |
| BMP | Biochemical methane potential |
| TVFAs | Total volatile fatty acids |
References
- Browne, J.; Murphy, J. Assessment of the resource associated with biomethane from food waste. Appl. Energy 2013, 104, 170–177. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2016; Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; p. 200. [Google Scholar]
- WRAP. Strategies to Achieve Economic and Environmental Gains by Reducing Food Waste; WRAP: Banbury, UK, 2015. [Google Scholar]
- Dev, S.; Saha, S.; Kurade, M.B.; Salama, E.; El-Dalatony, M.M.; Ha, G.; Chang, S.W.; Jeon, B. Perspective on anaerobic digestion for biometahanation in cold environments. Renew. Sustain. Energy Rev. 2019, 103, 85–95. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Increasing Profits in Food Waste Biorefinery—A Techno-Economic Analysis. Energies 2018, 11, 1551. [Google Scholar] [CrossRef]
- Ingrao, C.; Bacenetti, J.; Adamczyk, J.; Ferrante, V.; Messineo, A.; Huisingh, D. Investigating energy and environmental issues of agro-biogas derived energy systems; A comprehensive review of Life Cycle Assessments. Renew. Energy 2019, 136, 296–307. [Google Scholar] [CrossRef]
- Amaral, A.F.; Previtali, D.; Bassani, A.; Italiano, C.; Palella, A.; Pino, L.; Vita, A.; Bozzano, G.; Pirola, C.; Manenti, F. Biogas beyond CHP: The HPC (heat, power & chemicals) process. Energy 2020, 203, 117820. [Google Scholar]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Christopoulou, N.; Goumenaki, M. Optimization of biogas production from olive-oil mill wastewater, by codigesting with diluted poultry-manure. Appl. Energy 2007, 84, 646–663. [Google Scholar] [CrossRef]
- Igoni, A.H.; Ayotamuno, M.; Eze, C.; Ogaji, S.; Probert, S. Designs of anaerobic digesters for producing biogas from municipal solid-waste. Appl. Energy 2008, 85, 430–438. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Perkoulidis, G. A multi-criteria ranking of different technologies for the anaerobic digestion for energy recovery of the organic fraction of municipal solid wastes. Bioresour. Technol. 2009, 100, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tang, X.; Zhao, K.; Balan, V.; Zhu, Q. Biogas Production from Anaerobic Co-Digestion of Spent Mushroom Substrate with Different Livestock Manure. Energies 2021, 14, 570. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour. Technol. 2014, 152, 307–315. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Merrylin, J.; Kumar, S.A.; Kaliappan, S.; Yeom, I.; Banu, J.R. Biological pretreatment of non-flocculated sludge augments the biogas production in the anaerobic digestion of the pretreated waste activated sludge. Environ. Technol. 2013, 34, 2113–2123. [Google Scholar] [CrossRef]
- Yunqin, L.; Dehan, W.; Lishang, W. Biological pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. Waste Manag. Res. 2010, 28, 800–810. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Lee, J.; Loh, K.-C.; Dai, Y.; Tong, Y.W. Enhancement of biogas production in anaerobic co-digestion of food waste and waste activated sludge by biological co-pretreatment. Energy 2017, 137, 479–486. [Google Scholar] [CrossRef]
- Cai, M.; Liu, J.; Wei, Y. Enhanced biohydrogen production from sewage sludge with alkaline pretreatment. Environ. Sci. Technol. 2004, 38, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Potassium Hydroxyde Pre-Treatment Enhances Methane Yield from Giant Reed (Arundo donax L.). Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Appels, L.; Van Assche, A.; Willems, K.; Degrève, J.; Van Impe, J.; Dewil, R. Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour. Technol. 2011, 102, 4124–4130. [Google Scholar] [CrossRef]
- Naran, E.; Toor, U.A.; Kim, D.J. Effect of pretreatment and anaerobic co-digestion of food waste and waste activated sludge on stabilization and methane production. Int. Biodeterior. Biodegrad. 2016, 113, 17–21. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Cheng, J.; Hua, J.; Dong, H.; Zhou, J.; Li, Y.-Y. Improving fermentative methane production of glycerol trioleate and food waste pretreated with ozone through two-stage dark hydrogen fermentation and anaerobic digestion. Energy Convers. Manag. 2020, 203, 112225. [Google Scholar] [CrossRef]
- Shapiro, H.; Siegel, J.B. How ‘the crowd’ is tackling a silent killer. Nature 2018, 562, 7727. [Google Scholar]
- Achinas, S.; Euverink, G.J.W. Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material. Energies 2019, 12, 217. [Google Scholar] [CrossRef]
- Carballa, M.; Manterola, G.; Larrea, L.; Ternes, T.; Omil, F.; Lema, J.M. Influence of ozone pre-treatment on sludge anaerobic digestion: Removal of pharmaceutical and personal care products. Chemosphere 2007, 67, 1444–1452. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Hafez, H.; Nakhla, G.; Ray, M.B. Thermo-oxidative pretreatment of municipal waste activated sludge for volatile sulfur compounds removal and enhanced anaerobic digestion. Chem. Eng. J. 2011, 174, 166–174. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.-K.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Gu, Y.M.; Byun, H.R.; Kim, Y.-H.; Park, D.-Y.; Lee, J.H. Assessing the potential of facile biofuel production from corn stover using attrition mill treatment. Water Energy Nexus 2019, 2, 46–49. [Google Scholar] [CrossRef]
- Kwon, B.G.; Na, S.H.; Lim, H.J.; Lim, C.S. Slurry phase decomposition of food waste by using various microorganisms. J. Korean Soc. Environ. Eng. 2014, 36, 303–310. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, J.H.; Kim, T.H.; Choi, W.I. Impact of planetary ball mills on corn stover characteristics and enzymatic digestibility depending on grinding ball properties. Bioresour. Technol. 2017, 241, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- 5220 B Open Reflux Method. In Standard Methods for the Examinations of Water and Waterwaters; American Pubilic Health Association: Washington, DC, USA, 2005.
- Lettinga, G.; Rebac, S.; Parshina, S.; Nozhevnikova, A.; van Lier, J.; Stams, A.J.M. High-rate anaerobic treatment of wastewater at low temperatures. Appl. Environ. Microbiol. 1999, 65, 1696–1702. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Choi, O.; Pandey, A.; Kim, Y.G.; Joo, J.S.; Sang, B.-I. Simultaneous production of methane and acetate by thermophilic mixed culture from carbon dioxide in bioelectrochemical system. Bioresour. Technol. 2019, 281, 474–479. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.; Jeong, S.; Chandran, K.; Park, K.Y. Interactions between substrate characteristics and microbial communities on biogas production yield and rate. Bioresour. Technol. 2020, 303, 122934. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Castenholz, R.W.; Garrity, G.M. Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Dürre, P. Handbook on Clostridia; Taylor & Francis: Boca Raton, FL, USA, 2005; p. 903. [Google Scholar]
- Kim, B.H.; Gadd, G.M. Bacterial Physiology and Metabolism; Cambridge University Press: Cambridge, NY, USA, 2008; Volume xxii, p. 529. [Google Scholar]
- Kotsyurbenko, O.R.; Chin, K.J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Eyice, O.; Cetecioglu, Z.; Plaza, E. The study of structure of anaerobic granules and methane producing pathways of pilot-scale UASB reactors treating municipal wastewater under sub-mesophilic conditions. Bioresour. Technol. 2019, 290, 121733. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. The Prokaryotes: Deltaproteobacteria and Epsilonproteobacteria, 4th ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Rosenberg, E. The Prokaryotes: Gammaproteobacteria, 4th ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Parawira, W.; Read, J.S.; Mattiasson, B.; Björnsson, L. Energy production from agricultural residues: High methane yields in pilot-scale two-stage anaerobic digestion. Biomass Bioenergy 2008, 32, 44–50. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Y.; Zhao, P.; Li, Q.X.; Guo, S.; Chen, C. Potential and optimization of two-phase anaerobic digestion of oil refinery waste activated sludge and microbial community study. Sci. Rep. 2016, 6, 38245. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).